Abstract
Since its first description in Drosophila by Drs Nusslein-Volhard and Wieschaus in 1980, hedgehog (Hh) signaling has been implicated in regulation of cell differentiation, proliferation, tissue polarity, stem cell maintenance, and carcinogenesis. The first link of Hh signaling to cancer was established through studies of Gorlin syndrome in 1996 by two independent teams. Later, it was shown that Hh signaling may be involved in many types of cancer, including skin, leukemia, lung, brain, and gastrointestinal cancers. In early 2012, the US Food and Drug Administration approved the clinical use of Hh inhibitor Erivedge/vismodegib for treatment of locally advanced and metastatic basal cell carcinomas. With further investigation, it is possible to see more clinical applications of Hh signaling inhibitors. In this review, we will summarize major advances in the last 3 years in our understanding of Hh signaling activation in human cancer, and recent developments in preclinical and clinical studies using Hh signaling inhibitors.
Keywords: hedgehog, smoothened, PTCH1, cancer, signal transduction, clinical trials, animal model
Introduction
Remarkable progress has been made since the hedgehog (Hh) mutant phenotype was first described in fruit fly in 1980.1 Three vertebrate homologues of Hh and their receptors were identified in the 1990s.2–6 As an essential pathway during development, the Hh pathway is critical for maintaining tissue polarity and stem cell population. The first link between Hh signaling and cancer was shown in tumor-prone Gorlin syndrome in 1996.7–11 In early 2012, Hh signaling inhibitor GDC-0449 (Erivedge/vismodegib; Hoffmann-La Roche Ltd, Basel, Switzerland) was approved by the US Food and Drug Administration for treatment of locally advanced and metastatic basal cell carcinomas (BCCs).
The general signaling mechanisms of the Hh pathway are conserved from flies to humans.12 Mammalian Hh signaling molecules include ligands (sonic Hh, Indian Hh, and desert Hh), patched receptors (PTCH1, PTCH2), signal transducer smoothened (SMO), and transcription factors (Gli1, Gli2, Gli3) (see Figure 1). In the absence of ligands, SMO serves as the key signal transducer, whose function is inhibited by another transmembrane protein patched (PTCH1). Upon binding of an active Hh ligand, this inhibition is released, allowing SMO to signal downstream, eventually leading to activation of Gli transcription factors. Gli molecules can bind the specific consensus sequences located in the promoter region of the target genes to regulate target gene expression.13,14
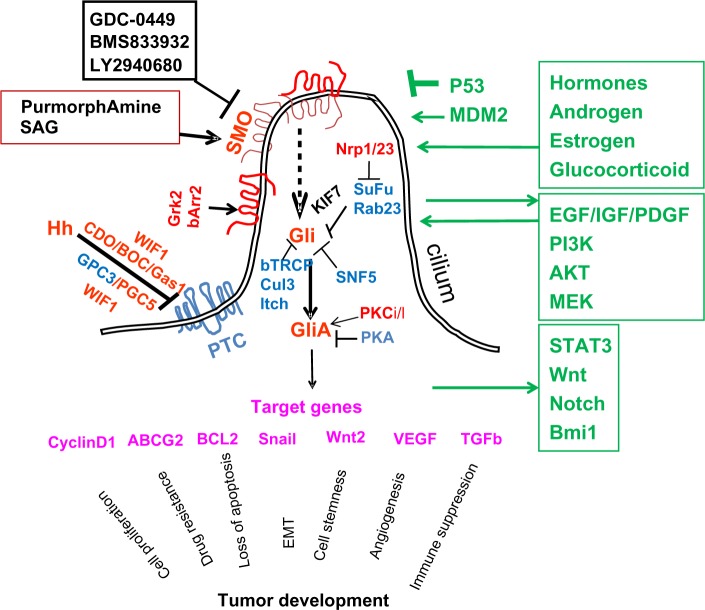
Figure 1.
A diagram of hedgehog (Hh) signaling in mammalian cells. Smoothened (SMO) is the key signal transducer of the Hh pathway. In the absence of the Hh ligands, Hh receptor patched (PTC) is thought to be localized in the cilium to inhibit SMO signaling. Coreceptors of Hh include CDO (cell adhesion molecule-related/down regulated by oncogenes), brother of CDO (BOC), Gas1, glypican 3, (GPC3), and GPC5. Wnt inhibitory factor-1 (WIF1) can also regulate Hh signaling through association with CDO, BOC, or GPC5. Gli molecules are processed with the help of suppressor of fused (SuFu)/KIF7, β-TRCP molecules into repressor forms, which turn off the Hh signaling pathway. Other negative regulators of Gli molecules include Rab23, protein kinase A (PKA), SuFu, tumor suppressor sucrose nonfermenting 5 (SNF5), Culin 3 (Cul3), and itchy E3 ubiquitin ligase (Itch) through regulation Gli protein modifications, nuclear–cytoplasm shuttling, as well as transcriptional activities. In the presence of Hh, PTC is thought to be shuttled out of cilium and is unable to inhibit SMO. The ciliary localization of SMO is thought to require β-arrestin 2 (βArr2), and G protein coupled receptor kinase 2 (GRK2). Hh reception promotes SMO conformational changes to form dimers. Gli molecules are now processed to active forms (GliA), which will activate the Hh target genes. This process can be inhibited by KIF7 and SuFu. Protein kinase C isoform ι/λ0 (PKCι/λ) is known to positively regulate Gli transcriptional activity. Positive regulators are in red, negative regulators are in blue, and target genes are in pink. KIF7 can function (in black) as a negative regulator or a positive regulator. The interacting pathways with the Hh pathway are in green. Although the role of cilium for Hh signaling during embryonic development is well established, cancer cells generally lack cilia. It has been demonstrated that lack of cilia prevents development of basal cell carcinomas in mice. It is not clear whether this is true for all other types of Hh signaling-associated cancer.
Abbreviations: EGF, epidermal growth factor; EMT, epithelial–mesenchymal transition; IGF, insulin-like growth factor; PDGF, platelet-derived growth factor; TGFβ, transforming growth factor β; VEGF, vascular endothelial growth factor; GDC0449, synthetic small molecules targeting at SMO signaling; BMS833932, synthetic small molecules targeting at SMO signaling; LY2940680 synthetic small molecules targeting at SMO signaling; SAG, smoothened agonist; MDM2, Mouse double minute 2 homolog; PI3K, Phosphatidylinositide 3-kinases; AKT, homolog of viral oncogene v-AKT; MEK, MAPK or ERK kinase; Stat3, signal transducer and activator of transcription 3; Wnt wingless homolog; ABCG2, ATP-binding cassette sub-family G member 2; BCL2, B-cell lymphoma 2; bTRCP, beta-transducin repeat containing protein.
In the last 3 years, there has been significant progress regarding Hh signaling and its significance in cancer development and therapeutics. The total number of publications on Hh signaling in the last 3 years is close to 30% of all Hh-related publications, and progress has been made in the following areas: 1) better understanding of Hh signal transduction and the associated target genes, 2) more reliable mouse models linking Hh signaling to human malignancies, 3) better understanding of Hh signaling mechanisms during cancer development and metastasis, 4) an increasing number of clinical and preclinical studies on cancer treatment using Hh signaling inhibitors, and 5) emerging evidence of Hh signaling in supporting residual cancer cells and cancer stem cells.
Signal transduction of the Hh pathway
All Hh proteins are secreted molecules, functioning at short range on nearby cells or at long range to distant cells during development.15–17 Hh protein precursors undergo post-translational modifications (autocleavage to release the N-terminal fragment [HhN], covalently binding to a cholesterol moiety at the C-terminal end, and palmitoylation by a palmitoylacyltransferase at the N-terminus of HhN).18–21 Molecules involved in Hh protein transport and distribution include the transmembrane transporter-like protein dispatched (Disp),22–24 metalloproteinases,25 the heparan sulfate proteoglycans Dally-like (Dlp) and Dally26,27 or their regulators,28 as well as enzymes such as sulfateless and tout velu.29–31
Figure 1 shows the mammalian Hh signaling pathway with major players in the diagram. Several molecules are engaged in reception of Hh ligands, with patched (PTC, one PTC in fly, and two PTCs in vertebrates: PTCH1 and PTCH2) as the major receptor.32 Studies from cultured cells indicate that PTC inhibits SMO at a substochiometric concentration.33 Hh-interacting protein (HIP) can compete with PTC on Hh binding, resulting in negative regulation of Hh signaling.34 On the other hand interference Hh or its vertebrate homologues cell adhesion molecule related/downregulated by oncogenes (CDO) and BOC (brother of CDO), GAS1, and glypican-3 (GPC3) serve as coreceptors of Hh.35–42 In contrast to the inhibitory effect of glypican-3, glypican-5 (GPC5) and other heparan sulfate proteoglycans are shown to stimulate Hh signaling by promoting binding of sonic Hh to PTCH1.43,44 The effect of GPC5 and interference Hh homologues requires another secreted extracellular molecule: Wnt inhibitory factor-1 (WIF1).45,46 It is still not entirely clear how binding of Hh proteins results in the pathway activation. It is proposed that PTC limits SMO signaling via transporting endogenous small molecules specifically targeted to SMO. Candidates of these small molecules include PI4P, lipoproteins, and provitamin D3.47–50 It is currently not very clear how these molecules regulate SMO signaling.
It is now known that glucocorticoid molecules can modulate SMO signaling through regulating its ciliary localization.51 Several recent reports support SMO to G protein coupling,52–55 but the physiological relevance of the G protein coupling of SMO in carcinogenesis has not been convincingly demonstrated. Gα can also regulate Gli proteins independent of SMO.56 It is quite clear that two important events occur during SMO signaling in mammalian cells. First, SMO protein undergoes conformational change to favor SMO signaling,57 although the regulatory mechanism underlying this conformational change is not clear. Second ciliary translocation of mammalian SMO protein is critical for Hh signaling.58–63 Several reports now link neuropilin 1/2 (Nrp1/2) to SMO signaling.64–67
Several molecules are identified to be genetically downstream of SMO in Drosophila, including COS2, suppressor of fused (SuFu), and fused. A COS2 homologue, kinesin like-protein KIF7, functions in the Hh pathway but not directly associated with SMO,68–72 suggesting that KIF7 does not contain all COS2 functions in vertebrates. In contrast, the phenotype of fused−/− mice is very different from Shh null mice,73–75 indicating that fused is not critical for Hh signaling during early embryonic development in mice.
In addition to the Drosophila homologues, mammalian cells have several novel cytoplasmic regulators of Hh signaling, including Rab2376 and tectonic.77 Rab23 and tectonic are all negative regulators downstream of SMO. We have shown that Rab23 is involved in Gli–SuFu interaction78 (see Figure 1). Unlike many Rab proteins, we found that Rab23 is localized both in the nucleus and in cytoplasm,79 suggesting that Rab23 may have other unrevealed functions apart from membrane trafficking.
The ultimate effect of Hh signaling is activation of downstream Gli transcription factors, which regulate target genes by directly binding a consensus binding site (5′-tgggtggtc-3′) in the promoter.13,14,80,81 The activity of Gli transcription factors can be regulated at several levels. First, nuclear–cytoplasmic shuttling of Gli molecules is tightly regulated.82–85 Protein kinase A can retain Gli1 protein in the cytoplasm via a protein kinase A site in the nuclear localization signal domain,83 whereas activated Ras signaling induces Gli nuclear localization.85 Second ubiquitination, acetylation, and protein degradation of Gli molecules are regulated by several distinct mechanisms, including β-TRCP-, cul3/BTB-, and numb/itch-mediated Gli ubiquitination, sumoylation, and acetylation.86–93 In addition, Gli3 (Gli2 to a lesser extent) can be processed into transcriptional repressors, which may be mediated by the β-TRCP E3 ligase.88,94 SuFu not only prevents nuclear translocation of Gli molecules but also inhibits Gli1-mediated transcriptional activity.95–97 Other mechanisms to modify Gli functions include interaction with a negative regulator sucrose nonfermenting 5 (SNF5)98 and a positive regulator protein kinase C isoform ι/λ.99
Several feedback regulatory loops exist in this pathway to maintain a certain level of Hh signaling in a given cell. PTC, HIP, GAS1, neuropilins, and Gli1 are components, as well as the target genes of this pathway. PTC and HIP provide negative feedback regulation, whereas Gli1 and Nrp1/2 form positive regulatory loops. On the other hand GAS1 is downregulated by the Hh pathway but is a positive regulator for Hh signaling.100 Alterations of these loops would lead to abnormal signaling of this pathway, such as inactivation of PTCH1 in BCCs.
Activation of the Hh pathway in human cancer
The initial link between Hh signaling and human cancers was made from the discovery that mutations of human PTCH1 are associated with a rare and hereditary form of BCC, basal cell nevus syndrome (BCNS) (also Gorlin syndrome).101–103 Gorlin syndrome is a rare autosomal genetic disease with two distinct sets of phenotypes: an increased risk of developing cancers such as BCCs, medulloblastomas, rhabdomyosarcomas, and meningiomas, as well as developmental defects such as bifid ribs and ectopic calcification.104
Almost all BCCs and about 30% of medulloblastomas have activated Hh signaling via gene mutations in PTCH1, SMO, or other Hh pathway molecules.105–109 In addition, cancers associated with Gorlin syndrome, including rhabdomyosarcoma110,111 and meningiomas,112–114 are reported to have gene mutations in the Hh signaling pathway or elevated Hh target gene expression. Activated Hh signaling has been detected in a variety of human cancer types, either in the tumor or in the stroma.100,115–117
Genetically engineered mice with Ptch1 and Smo genes have generated more convincing evidence for the critical role of Hh signaling in cancer. In addition to BCCs and medulloblastomas, rhabdomyosarcomas develop in mice with expression of oncogenic SmoM2 or knockout of Ptch1.118–121 One surprising finding from tissue-specific Ptch1 knockout is the development of gastrointestinal stromal-like tumors (GIST),122 suggestive of a role of Hh signaling in GIST. Even in the situation of a small cell lung cancer (SCLC) mouse model, expression of oncogenic SmoM2 increases the tumor number, whereas Smo knockout reduces the tumor number.123 Recent study of Barrett’s esophagus indicates that sonic Hh expression in the epithelium of the esophagus can lead to stromal expression of Hh signaling target genes, which is similar to the human situation.124,125 In contrast, tissue-specific expression of oncogenic Smo molecule SmoM2 has no effects on K-Ras-induced pancreatic cancer126 or on prostate cancer.127 The negative data, however, do not rule out the promoting effects of Hh signaling for tumor metastasis, a major factor for cancer mortality. Currently, there are only a limited number of mouse models for cancer metastasis. Even for the available mouse models for cancer metastasis, several variable factors make cancer metastasis models less robust, and these factors include mouse genetic backgrounds, low incidence, and long duration to observe metastasis in mice.
Hh signaling in tumor initiation, promotion, and metastases
Hh signaling plays different roles in different types of cancer.100 Based on the published data, we attempt to divide the functions of Hh signaling during cancer development into three types: the tumor driver, the tumor promoter, or the regulator for residual cancer cells after therapy. For example, activated Hh signaling can drive development of BCCs, medulloblastomas, rhabdomyosarcoma, GIST, and Barrett’s esophagus,118,119,122,124,128,129 and Hh signaling in these lesions serves as the tumor driver, at least in the mouse models. For SCLC, Hh signaling can promote cancer development but is not sufficient to drive tumor formation, and thus serves as a tumor promoter.123 In pancreatic cancer, inhibition of Hh signaling does not affect tumor formation but can promote tumor metastasis.130–137 For other cancer types, Hh signaling may regulate the number of cancer stem cells or the tumor microenvironment, such as leukemia and liver cancer.138,139 As more in vivo data are available, we predict more revelation of the tumor promoting role of Hh signaling. Tumor recurrence after therapy is a major issue in clinical care of cancer patients, such as chemotherapy or radiotherapy resistance, and will be discussed in “Hh signaling, cancer stem cell, and residual cancer cells.” For some cancer types, Hh signaling may not have any roles to play.
Activation of Hh signaling does not work in isolation but rather crosstalks with other signaling pathways during cancer development and metastasis. Earlier studies indicated that Ptch1+/− mice with P53 knock out all developed medulloblastomas, whereas <30% of Ptch1+/− mice (with wild-type P53) had this type of tumor.140 We have shown that skin-specific knockout of Stat3 or its upstream activator Il11ra significantly reduced Hh signaling-mediated BCC formation.141 Increasing data have indicated close collaboration between Hh signaling and growth factor signaling pathways. Our earlier work indicated that platelet-derived growth factor α (PDGFRα) is regulated by Hh signaling and is responsible for cell proliferation in BCCs.142 Now more links are reported between Hh and other pathways, including epidermal growth factor, insulin growth factor, transforming growth factor β (TGFβ), mTOR/S6K1, RACK1, notch, and protein kinase C.100,143–151 Although some of these molecules are involved in regulation of tumor microenvironment, such as TGFβ, others are known to regulate cancer stem cells, such as PDGFRα and notch. We will have more discussion on cancer stem cells in “Hh signaling, cancer stem cell, and residual cancer cells.”
Increasing evidence indicates that Hh signaling plays an important role during tumor metastasis in several types of cancer, such as pancreatic and breast cancers.135,152 Studies from many groups indicate activation of Hh signaling in the stromal as well as tumor compartments in metastatic pancreatic cancer.130,133–137,153 In fact, Hh signaling inhibitors are effective in suppressing tumor metastases of pancreatic cancer.135 Hh signaling also regulates bone homeostasis as well as bone metastasis in breast cancer independent of the Hh ligands.143 During tumor metastasis, Hh signaling activation is observed both in the tumor compartment and in the stroma.135 The molecules mediating Hh’s metastatic functions remain largely untested, but there are reports to indicate the following molecules: snail, TGFβ, Wnt, HGF, and muc5 AC.135,154–157 Further studies will be needed to understand the molecular basis by which Hh signaling mediates cancer metastases.
Hh signaling inhibitors: preclinical and clinical studies
More than 200 compounds have been disclosed to have inhibitory effects on Hh signaling. Of these, eight have been used for clinical trials (see Table 1 for the list). There are three major targeting sites for Hh signaling inhibitors identified so far: Hh molecules (Shh neutralizing antibodies, small molecule Robotnikinin), SMO protein (cyclopamine and its derivatives IPI-926 and CycT, and synthetic compounds GDC-0449, XL-139/BMS833923, LDE-225, PF04449913, and LY2940680), and Gli inhibitors (HPI-1, HPI-2, GANT-56, and GANT-61).100 The major advances include successful clinical trials using GDC-0449 and US Food and Drug Administration approval of GDC-0449 for treatment of locally advanced and metastatic BCCs. However, combination of Hh signaling inhibitors with gemcitabine or Hh signaling inhibitors alone did not show any improvements in the outcomes of pancreatic cancer patients. We summarize these data below.
Table 1.
A list of hedgehog signaling inhibitors in clinical trials (from http://clinicaltrials.gov)a
| Molecule | Other names | Phase | Tumor types | FDA approval | Company |
|---|---|---|---|---|---|
| GDC-0449 | Vismodegib/erivedge | I/II/III | BCCs and solid tumors | BCCs | Hoffmann-La Roche Ltd |
| IPI-926 | I/II | Solid tumors | Infinity Pharmaceuticals, Inc. | ||
| LDE225 | I/II | Leukemia and solid tumors | Novartis AG | ||
| LEQ506 | I | Solid tumors | Novartis AG | ||
| PF-04449913 | I/II | Leukemia and solid tumors | Pfizer, Inc. | ||
| TAK-441 | I | Solid tumors | Millennium Pharmaceuticals, Inc. | ||
| BMS833923 | XL-139 | I/II | SCLC and solid tumors | Bristol-Myers Squibb | |
| LY2940680 | I/II | SCLC/advanced cancer | Eli Lilly and Company |
Notes:
All small molecules target smoothened molecule. GDC-0449 has been approved by the FDA to treat locally advanced and metastatic BCCs. There are no ongoing clinical trials for LEQ506, TAK-441, and BMS833923.
Abbreviations: BCC, basal cell carcinoma; FDA, US Food and Drug Administration; SCLC, small cell lung cancer.
Table 1 shows the list of Hh signaling inhibitors in clinical trials, with all eight small molecules targeting SMO. Clinical trials with GDC-0449 in BCCs are the most successful. The successful Phase II clinical trials were preceded with a remarkable Phase I clinical trial in patients with metastatic BCCs.158 This drug is well tolerated by patients.159–161 Two independent groups used GDC-0449 to treat BCNS patients and sporadic BCCs, respectively, via oral administration. Although the overall outcomes were very encouraging, the responses of two groups of patients were quite different. Although BCNS patients had virtually a 100% response rate, sporadic BCCs had only a 33% response rate. Previous studies in mouse models indicate that tumors acquire somatic mutations in Smo or other signaling pathways following GDC-0449 administration,162 which may explain why not all sporadic BCCs responded well. A more rational way to treat sporadic BCCs is topical application. Two groups (one from Novartis AG and one from Hoffmann-La Roche Ltd/Genentech) indeed tested that possibility with BCNS patients and obtained impressive responses.163,164 Mechanisms to Smo antagonist resistance include mutations in the target SMO gene or alterations in the PI3K pathway.165,166 Several ways have been explored to mitigate drug resistance to SMO antagonists, such as itraconazole and arsenic trioxide, polymeric nanoparticle-encapsulated Hh signaling inhibitors, or vitamin D3.167–170 Hopefully, some of these combined treatments will provide benefits to BCC patients.
Studies in animal models demonstrated significant inhibition of Hh signaling inhibitors on medulloblastoma development. For example, oral administration of IPI-926 or PF-5274857 can reduce tumor development, leading to a longer lifespan in mouse medulloblastoma models.171,172 However, an early clinical trial on a medulloblastoma patient using GDC-0449 yielded only a transient therapeutic effect, due to an SMO mutation occurring soon after treatment.173 The outcome data of current medulloblastoma clinical trials are not available, but there is still a high expectation.
There is evidence to support that rhabdomyosarcoma is very responsive to Hh signaling inhibitors. First, gene expression analyses revealed elevated Hh target gene expression in embryonic rhabdomyosarcomas.111 Second, preliminary studies used forskolin or SMO inhibitor to shrink tumors in mouse models.174 In addition, evidence for Hh signaling in meningiomas and SCLC is quite clear, and Hh signaling inhibitors should be effective in these tumor types as well.
Hh signaling, cancer stem cell, and residual cancer cells
Increasing evidence indicates that Hh signaling is critical for cancer stem cell maintenance and function.138,175,176 For example, leukemia stem cell maintenance and expansion are dependent on Hh signaling.138,175 The effect of Hh signaling on a normal hematopoietic stem cell population, however, is still quite controversial, with some showing effects but others with no effects.138,177–180 Based on cancer stem cell theory, it is anticipated that Hh signaling activation exerts resistance to cancer chemotherapy and radiotherapy.181 Several studies have indeed shown that Hh signaling activation is associated with chemotherapy or radiotherapy resistance.182–183 Hh signaling inhibitor IPI-926 enhances delivery of the chemotherapeutical drug gemcitabine in a mouse model of pancreatic cancer. Relevance to the cancer stem cell theory is the link between Hh signaling activation and cancer relapse from drug resistance.
Based on the published data, we propose that Hh signaling may help maintain the stemness of cancer stem cells, which are generally insensitive to chemotherapy and radiotherapy. There is evidence to indicate that Hh signaling regulates expression of cancer stem cell-related markers, such as aldehyde dehydrogenase, Bmi1, snail, Wnt2, PDGFRα, jagged-1, CD44, and c-MET.135,155,184–188 The level of Hh expression is often higher in the cancer stem cell population in several cancer types.189–193 Thus, we have reasons to believe that inhibition of Hh signaling may be effective in reducing the number of cancer stem cells, which may play an important role in chemotherapy and radiotherapy resistance.
Chemotherapy and radiotherapy play an important role in the clinical care of cancer patients, but resistance to these treatments remains a major obstacle in cancer patient care. Recent studies revealed a few examples for the role of Hh signaling in chemotherapy and radiotherapy resistance. Resistance to docetaxel is a major clinical challenge for prostate cancer patients. A recent study revealed an important role of Hh signaling on docetaxel resistance in prostate cancer.194 Combination of notch and Hh signaling inhibitors was able to reverse docetaxel resistance both in cultured cells and in xenografts. Activation of Hh signaling via PI3 K is also reported in tamoxifen-resistant breast cancer,195 and a combination of Hh signaling inhibitor GDC-0449 with tamoxifen significantly reduced cell colony formation and tumor development in xenografts. In addition, activated Hh signaling is shown to be responsible for drug resistance in ovarian cancer, cervical cancer, and myeloid leukemic cells.196–198 A recent study also suggests that Hh signaling may be associated with antiepidermal growth factor receptor therapy (targeted therapy) resistance observed in head and neck cancer.144 The exact mechanisms by which Hh signaling activation confers drug resistance are not entirely clear, but it is reported that Hh signaling can regulate expression of several drug resistance-related genes such as ABCG2 and MDR.198,199 The cancer stem cell theory can also explain some of the mechanisms.
Overcoming recurrence to radiotherapy is also very challenging, but recent studies suggest that inhibiting Hh signaling may help mitigate radiotherapy resistance in pancreatic and head/neck cancer. For pancreatic cancer, we found that a combination of Hh signaling inhibitor BMS833932 (see Table 1 for details) and radiation could significantly reduce the number of lymph node metastasis.135 Similarly, high expression of Gli1 is reported to be associated with lymph node metastases and tumor progression after radiotherapy in squamous cell carcinomas of the head/neck.200
Summary and future perspectives
In summary, the link of Hh signaling activation to a variety of human cancer implies the relevance of studying Hh signaling to human health. Rapid advancement in the discovery of novel Hh signaling inhibitors has provided many opportunities for developing novel cancer therapeutic strategies. It is not surprising to learn that several major challenges still exist to prevent the use of Hh signaling inhibitors in clinics. These challenges include a lack of basic understanding of the molecular mechanisms by which Hh signaling mediates carcinogenesis; no clear criteria to identify the right tumors for therapeutic application; only a few reliable, physiologically relevant, and reproducible mouse models for cancer metastases to test and optimize drug dosages in order to minimize side effects; and a lack of clear strategies to mitigate drug resistance. Over the last 3 years, research in this area has greatly improved, as indicated in this review. It is anticipated that additional novel therapeutic strategies will be developed for cancer clinical trials using Hh signaling inhibitors in the next few years.
Acknowledgments
Current research in the authors’ laboratory is supported by grants from the National Cancer Institute (CA94160, CA155086), Riley Children’s Foundation, and Wells Center for Pediatric Research. Due to space limit, we could not include many important findings in this review but want to take this opportunity to thank all the investigators in this field for their work.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287(5785):795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 2.Krauss S, Concordet JP, Ingham PW. A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell. 1993;75(7):1431–1444. doi: 10.1016/0092-8674(93)90628-4. [DOI] [PubMed] [Google Scholar]
- 3.Echelard Y, Epstein DJ, St-Jacques B, et al. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75(7):1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- 4.Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75(7):1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- 5.Chang DT, López A, von Kessler DP, et al. Products, genetic linkage and limb patterning activity of a murine hedgehog gene. Development. 1994;120(11):3339–3353. doi: 10.1242/dev.120.11.3339. [DOI] [PubMed] [Google Scholar]
- 6.Roelink H, Augsburger A, Heemskerk J, et al. Floor plate and motor neuron induction by vhh-1, a vertebrate homolog of hedgehog expressed by the notochord. Cell. 1994;76(4):761–775. doi: 10.1016/0092-8674(94)90514-2. [DOI] [PubMed] [Google Scholar]
- 7.Epstein EH. Basal cell carcinomas: attack of the hedgehog. Nat Rev Cancer. 2008;8(10):743–754. doi: 10.1038/nrc2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie J. Hedgehog signaling in prostate cancer. Future Oncol. 2005;1(3):331–338. doi: 10.1517/14796694.1.3.331. [DOI] [PubMed] [Google Scholar]
- 9.Xie J. Hedgehog signaling pathway: development of antagonists for cancer therapy. Curr Oncol Rep. 2008;10(2):107–113. doi: 10.1007/s11912-008-0018-7. [DOI] [PubMed] [Google Scholar]
- 10.Xie J. Molecular biology of basal and squamous cell carcinomas. Adv Exp Med Biol. 2008;624:241–251. doi: 10.1007/978-0-387-77574-6_19. [DOI] [PubMed] [Google Scholar]
- 11.Jiang J, Hui CC. Hedgehog signaling in development and cancer. Dev Cell. 2008;15(6):801–812. doi: 10.1016/j.devcel.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ingham PW, Placzek M. Orchestrating ontogenesis: variations on a theme by sonic hedgehog. Nat Rev Genet. 2006;7(11):841–850. doi: 10.1038/nrg1969. [DOI] [PubMed] [Google Scholar]
- 13.Sasaki H, Hui C, Nakafuku M, Kondoh H. A binding site for Gli proteins is essential for HNF-3beta floor plate enhancer activity in transgenics and can respond to Shh in vitro. Development. 1997;124(7):1313–1322. doi: 10.1242/dev.124.7.1313. [DOI] [PubMed] [Google Scholar]
- 14.Kinzler KW, Vogelstein B. The Gli gene encodes a nuclear protein which binds specific sequences in the human genome. Mol Cell Biol. 1990;10(2):634–642. doi: 10.1128/mcb.10.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMahon AP, Ingham PW, Tabin CJ. Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol. 2003;53:1–114. doi: 10.1016/s0070-2153(03)53002-2. [DOI] [PubMed] [Google Scholar]
- 16.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15(23):3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 17.Taipale J, Beachy PA. The hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411(6835):349–354. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- 18.Lee JJ, Ekker SC, von Kessler DP, Porter JA, Sun BI, Beachy PA. Autoproteolysis in hedgehog protein biogenesis. Science. 1994;266(5190):1528–1537. doi: 10.1126/science.7985023. [DOI] [PubMed] [Google Scholar]
- 19.Porter JA, Young KE, Beachy PA. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996;274(5285):255–259. doi: 10.1126/science.274.5285.255. [DOI] [PubMed] [Google Scholar]
- 20.Porter JA, von Kessler DP, Ekker SC, et al. The product of hedgehog autoproteolytic cleavage active in local and long-range signalling. Nature. 1995;374(6520):363–366. doi: 10.1038/374363a0. [DOI] [PubMed] [Google Scholar]
- 21.Buglino JA, Resh MD. Hhat is a palmitoylacyltransferase with specificity for N-palmitoylation of sonic hedgehog. J Biol Chem. 2008;283(32):22076–22088. doi: 10.1074/jbc.M803901200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawakami T, Kawcak T, Li YJ, Zhang W, Hu Y, Chuang PT. Mouse dispatched mutants fail to distribute hedgehog proteins and are defective in hedgehog signaling. Development. 2002;129(24):5753–5765. doi: 10.1242/dev.00178. [DOI] [PubMed] [Google Scholar]
- 23.Ma Y, Erkner A, Gong R, et al. Hedgehog-mediated patterning of the mammalian embryo requires transporter-like function of dispatched. Cell. 2002;111(1):63–75. doi: 10.1016/s0092-8674(02)00977-7. [DOI] [PubMed] [Google Scholar]
- 24.Caspary T, García-García MJ, Huangfu D, et al. Mouse dispatched homolog1 is required for long-range, but not juxtacrine, Hh signaling. Curr Biol. 2002;12(18):1628–1632. doi: 10.1016/s0960-9822(02)01147-8. [DOI] [PubMed] [Google Scholar]
- 25.Dierker T, Dreier R, Petersen A, Bordych C, Grobe K. Heparan sulfate-modulated, metalloprotease-mediated sonic hedgehog release from producing cells. J Biol Chem. 2009;284(12):8013–8022. doi: 10.1074/jbc.M806838200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beckett K, Franch-Marro X, Vincent JP. Glypican-mediated endocytosis of hedgehog has opposite effects in filies and mice. Trends Cell Biol. 2008;18(8):360–363. doi: 10.1016/j.tcb.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Lum L, Yao S, Mozer B, et al. Identification of hedgehog pathway components by RNAi in Drosophila cultured cells. Science. 2003;299(5615):2039–2045. doi: 10.1126/science.1081403. [DOI] [PubMed] [Google Scholar]
- 28.Baena-Lopez LA, Rodriguez I, Baonza A. The tumor suppressor genes dachsous and fat modulate different signalling pathways by regulating dally and dally-like. Proc Natl Acad Sci U S A. 2008;105(28):9645–9650. doi: 10.1073/pnas.0803747105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toyoda H, Kinoshita-Toyoda A, Fox B, Selleck SB. Structural analysis of glycosaminoglycans in animals bearing mutations in sugarless, sulfateless, and tout-velu. Drosophila homologues of vertebrate genes encoding glycosaminoglycan biosynthetic enzymes. J Biol Chem. 2000;275(29):21856–21861. doi: 10.1074/jbc.M003540200. [DOI] [PubMed] [Google Scholar]
- 30.Bellaiche Y, The I, Perrimon N. Tout-velu is a Drosophila homologue of the putative tumour suppressor EXT-1 and is needed for Hh diffusion. Nature. 1998;394(6688):85–88. doi: 10.1038/27932. [DOI] [PubMed] [Google Scholar]
- 31.Koziel L, Kunath M, Kelly OG, Vortkamp A. Ext1-dependent heparan sulfate regulates the range of Ihh signaling during endochondral ossification. Dev Cell. 2004;6(6):801–813. doi: 10.1016/j.devcel.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Stone DM, Hynes M, Armanini M, et al. The tumour-suppressor gene patched encodes a candidate receptor for sonic hedgehog. Nature. 1996;384(6605):129–134. doi: 10.1038/384129a0. [DOI] [PubMed] [Google Scholar]
- 33.Taipale J, Cooper MK, Maiti T, Beachy PA. Patched acts catalytically to suppress the activity of smoothened. Nature. 2002;418(6900):892–897. doi: 10.1038/nature00989. [DOI] [PubMed] [Google Scholar]
- 34.Chuang PT, McMahon AP. Vertebrate hedgehog signalling modulated by induction of a hedgehog-binding protein. Nature. 1999;397(6720):617–621. doi: 10.1038/17611. [DOI] [PubMed] [Google Scholar]
- 35.Martinelli DC, Fan CM. Gas1 extends the range of hedgehog action by facilitating its signaling. Genes Dev. 2007;21(10):1231–1243. doi: 10.1101/gad.1546307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seppala M, Depew MJ, Martinelli DC, Fan CM, Sharpe PT, Cobourne MT. Gas1 is a modifier for holoprosencephaly and genetically interacts with sonic hedgehog. J Clin Invest. 2007;117(6):1575–1584. doi: 10.1172/JCI32032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allen BL, Tenzen T, McMahon AP. The hedgehog-binding proteins Gas1 and Cdo cooperate to positively regulate Shh signaling during mouse development. Genes Dev. 2007;21(10):1244–1257. doi: 10.1101/gad.1543607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okada A, Charron F, Morin S, et al. Boc is a receptor for sonic hedgehog in the guidance of commissural axons. Nature. 2006;444(7117):369–373. doi: 10.1038/nature05246. [DOI] [PubMed] [Google Scholar]
- 39.Tenzen T, Allen BL, Cole F, Kang JS, Krauss RS, McMahon AP. The cell surface membrane proteins Cdo and Boc are components and targets of the hedgehog signaling pathway and feedback network in mice. Dev Cell. 2006;10(5):647–656. doi: 10.1016/j.devcel.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Zhang W, Kang JS, Cole F, Yi MJ, Krauss RS. Cdo functions at multiple points in the sonic hedgehog pathway, and Cdo-deficient mice accurately model human holoprosencephaly. Dev Cell. 2006;10(5):657–665. doi: 10.1016/j.devcel.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 41.Yao S, Lum L, Beachy P. The ihog cell-surface proteins bind hedgehog and mediate pathway activation. Cell. 2006;125(2):343–357. doi: 10.1016/j.cell.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 42.Capurro MI, Xu P, Shi W, Li F, Jia A, Filmus J. Glypican-3 inhibits hedgehog signaling during development by competing with patched for hedgehog binding. Dev Cell. 2008;14(5):700–711. doi: 10.1016/j.devcel.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 43.Li F, Shi W, Capurro M, Filmus J. Glypican-5 stimulates rhabdomyosarcoma cell proliferation by activating hedgehog signaling. J Cell Biol. 2011;192(4):691–704. doi: 10.1083/jcb.201008087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Witt RM, Hecht ML, Pazyra-Murphy MF, et al. Heparan sulfate proteoglycans containing a glypican 5 core and 2-O-sulfo-iduronic acid function as sonic hedgehog co-receptors to promote proliferation. J Biol Chem. 2013;288(36):26275–26288. doi: 10.1074/jbc.M112.438937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanchez-Hernandez D, Sierra J, Ortigao-Farias JR, Guerrero I. The WIF domain of the human and Drosophila Wif-1 secreted factors confers specificity for Wnt or hedgehog. Development. 2012;139(20):3849–3858. doi: 10.1242/dev.080028. [DOI] [PubMed] [Google Scholar]
- 46.Avanesov A, Honeyager SM, Malicki J, Blair SS. The role of glypicans in Wnt inhibitory factor-1 activity and the structural basis of Wif1’s effects on Wnt and hedgehog signaling. PLoS Genetics. 2012;8(2):e1002503. doi: 10.1371/journal.pgen.1002503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yavari A, Nagaraj R, Owusu-Ansah E, et al. Role of lipid metabolism in smoothened derepression in hedgehog signaling. Dev Cell. 2010;19(1):54–65. doi: 10.1016/j.devcel.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khaliullina H, Panakova D, Eugster C, Riedel F, Carvalho M, Eaton S. Patched regulates smoothened trafficking using lipoprotein-derived lipids. Development. 2009;136(24):4111–4121. doi: 10.1242/dev.041392. [DOI] [PubMed] [Google Scholar]
- 49.Callejo A, Culi J, Guerrero I. Patched, the receptor of hedgehog, is a lipoprotein receptor. Proc Natl Acad Sci U S A. 2008;105(3):912–917. doi: 10.1073/pnas.0705603105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bijlsma MF, Spek CA, Zivkovic D, van de Water S, Rezaee F, Peppelenbosch MP. Repression of smoothened by patched-dependent (pro-)vitamin D3 secretion. PLoS Biol. 2006;4(8):e232. doi: 10.1371/journal.pbio.0040232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y, Davidow L, Arvanites AC, et al. Glucocorticoid compounds modify smoothened localization and hedgehog pathway activity. Chem Bio. 2012;19(8):972–982. doi: 10.1016/j.chembiol.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Philipp M, Fralish GB, Meloni AR, et al. Smoothened signaling in vertebrates is facilitated by a G protein-coupled receptor kinase. Mol Biol Cell. 2008;19(12):5478–5489. doi: 10.1091/mbc.E08-05-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ogden SK, Fei DL, Schilling NS, Ahmed YF, Hwa J, Robbins DJ. G protein Galphai functions immediately downstream of smoothened in hedgehog signalling. Nature. 2008;456(7224):967–970. doi: 10.1038/nature07459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Molnar C, Holguin H, Mayor F, Jr, Ruiz-Gomez A, de Celis JF. The G protein-coupled receptor regulatory kinase GPRK2 participates in hedgehog signaling in Drosophila. Proc Natl Acad Sci U S A. 2007;104(19):7963–7968. doi: 10.1073/pnas.0702374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Riobo NA, Saucy B, Dilizio C, Manning DR. Activation of heterotrimeric G proteins by smoothened. Proc Natl Acad Sci U S A. 2006;103(33):12607–12612. doi: 10.1073/pnas.0600880103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Douglas AE, Heim JA, Shen F, et al. The alpha subunit of the G protein G13 regulates activity of one or more Gli transcription factors independently of smoothened. J Biol Chem. 2011;286(35):30714–30722. doi: 10.1074/jbc.M111.219279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao Y, Tong C, Jiang J. Hedgehog regulates smoothened activity by inducing a conformational switch. Nature. 2007;450(7167):252–258. doi: 10.1038/nature06225. [DOI] [PubMed] [Google Scholar]
- 58.Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate smoothened functions at the primary cilium. Nature. 2005;437(7061):1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 59.Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intrafagellar transport proteins. Nature. 2003;426(6962):83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- 60.May SR, Ashique AM, Karlen M, et al. Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev Biol. 2005;287(2):378–389. doi: 10.1016/j.ydbio.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 61.Huangfu D, Anderson KV. Cilia and hedgehog responsiveness in the mouse. Proc Natl Acad Sci U S A. 2005;102(32):11325–11330. doi: 10.1073/pnas.0505328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Q, Davenport JR, Croyle MJ, Haycraft CJ, Yoder BK. Disruption of IFT results in both exocrine and endocrine abnormalities in the pancreas of Tg737(orpk) mutant mice. Lab Invest. 2005;85(1):45–64. doi: 10.1038/labinvest.3700207. [DOI] [PubMed] [Google Scholar]
- 63.Hoover AN, Wynkoop A, Zeng H, Jia J, Niswander LA, Liu A. C2cd3 is required for cilia formation and hedgehog signaling in mouse. Development. 2008;135(24):4049–4058. doi: 10.1242/dev.029835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hillman RT, Feng BY, Ni J, et al. Neuropilins are positive regulators of hedgehog signal transduction. Genes Dev. 2011;25(22):2333–2346. doi: 10.1101/gad.173054.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Snuderl M, Batista A, Kirkpatrick ND, et al. Targeting placental growth factor/neuropilin 1 pathway inhibits growth and spread of medulloblastoma. Cell. 2013;152(5):1065–1076. doi: 10.1016/j.cell.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cao Y, Wang L, Nandy D, et al. Neuropilin-1 upholds dedifferentiation and propagation phenotypes of renal cell carcinoma cells by activating Akt and sonic hedgehog axes. Cancer Res. 2008;68(21):8667–8672. doi: 10.1158/0008-5472.CAN-08-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parra LM, Zou Y. Sonic hedgehog induces response of commissural axons to Semaphorin repulsion during midline crossing. Nat Neurosci. 2010;13(1):29–35. doi: 10.1038/nn.2457. [DOI] [PubMed] [Google Scholar]
- 68.Cheung HO, Zhang X, Ribeiro A, et al. The kinesin protein Kif7 is a critical regulator of Gli transcription factors in mammalian hedgehog signaling. Sci Signal. 2009;2(76):ra29. doi: 10.1126/scisignal.2000405. [DOI] [PubMed] [Google Scholar]
- 69.Endoh-Yamagami S, Evangelista M, Wilson D, et al. The mammalian Cos2 homolog Kif7 plays an essential role in modulating Hh signal transduction during development. Curr Biol. 2009;19(15):1320–1326. doi: 10.1016/j.cub.2009.06.046. [DOI] [PubMed] [Google Scholar]
- 70.Law KK, Makino S, Mo R, Zhang X, Puviindran V, Hui CC. Antagonistic and cooperative actions of Kif7 and Sufu define graded intracellular Gli activities in hedgehog signaling. PLoS One. 2012;7(11):e50193. doi: 10.1371/journal.pone.0050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li ZJ, Nieuwenhuis E, Nien W, et al. Kif7 regulates Gli2 through Sufu-dependent and -independent functions during skin development and tumorigenesis. Development. 2012;139(22):4152–4161. doi: 10.1242/dev.081190. [DOI] [PubMed] [Google Scholar]
- 72.Hsu SH, Zhang X, Yu C, et al. Kif7 promotes hedgehog signaling in growth plate chondrocytes by restricting the inhibitory function of Sufu. Development. 2011;138(17):3791–3801. doi: 10.1242/dev.069492. [DOI] [PubMed] [Google Scholar]
- 73.Wilson CW, Nguyen CT, Chen MH, et al. Fused has evolved divergent roles in vertebrate hedgehog signalling and motile ciliogenesis. Nature. 2009;459(7243):98–102. doi: 10.1038/nature07883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Merchant M, Evangelista M, Luoh SM, et al. Loss of the serine/threonine kinase fused results in postnatal growth defects and lethality due to progressive hydrocephalus. Mol Cell Biol. 2005;25(16):7054–7068. doi: 10.1128/MCB.25.16.7054-7068.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen MH, Gao N, Kawakami T, Chuang PT. Mice deficient in the fused homolog do not exhibit phenotypes indicative of perturbed hedgehog signaling during embryonic development. Mol Cell Biol. 2005;25(16):7042–7053. doi: 10.1128/MCB.25.16.7042-7053.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eggenschwiler JT, Espinoza E, Anderson KV. Rab23 is an essential negative regulator of the mouse sonic hedgehog signalling pathway. Nature. 2001;412(6843):194–198. doi: 10.1038/35084089. [DOI] [PubMed] [Google Scholar]
- 77.Reiter JF, Skarnes WC. Tectonic, a novel regulator of the hedgehog pathway required for both activation and inhibition. Genes Dev. 2006;20(1):22–27. doi: 10.1101/gad.1363606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li C, Chi S, Xie J. Hedgehog signaling in skin cancers. Cell Signal. 2011;23(8):1235–1243. doi: 10.1016/j.cellsig.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang S, Yang L, An Y, et al. Expression of hedgehog signaling molecules in lung cancer. Acta Histochem. 2011;113(5):564–569. doi: 10.1016/j.acthis.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 80.Kinzler KW, Ruppert JM, Bigner SH, Vogelstein B. The Gli gene is a member of the Kruppel family of zinc finger proteins. Nature. 1988;332(6162):371–374. doi: 10.1038/332371a0. [DOI] [PubMed] [Google Scholar]
- 81.Ruppert JM, Kinzler KW, Wong AJ, et al. The Gli-Kruppel family of human genes. Mol Cell Biol. 1988;8(8):3104–3113. doi: 10.1128/mcb.8.8.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barnfield PC, Zhang X, Thanabalasingham V, Yoshida M, Hui CC. Negative regulation of Gli1 and Gli2 activator function by suppressor of fused through multiple mechanisms. Differentiation. 2005;73(8):397–405. doi: 10.1111/j.1432-0436.2005.00042.x. [DOI] [PubMed] [Google Scholar]
- 83.Sheng T, Chi S, Zhang X, Xie J. Regulation of Gli1 localization by the cAMP/protein kinase A signaling axis through a site near the nuclear localization signal. J Biol Chem. 2006;281(1):9–12. doi: 10.1074/jbc.C500300200. [DOI] [PubMed] [Google Scholar]
- 84.Kogerman P, Grimm T, Kogerman L, et al. Mammalian suppressor-of-fused modulates nuclear-cytoplasmic shuttling of Gli-1. Nat Cell Biol. 1999;1(5):312–319. doi: 10.1038/13031. [DOI] [PubMed] [Google Scholar]
- 85.Stecca B, Mas C, Clement V, et al. Melanomas require hedghog-Gli signaling regulated by interactions between Gli1 and the RAS-MEK/AKT pathways. Proc Natl Acad Sci U S A. 2007;104(14):5895–5900. doi: 10.1073/pnas.0700776104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pan Y, Bai CB, Joyner AL, Wang B. Sonic hedgehog signaling regulates Gli2 transcriptional activity by suppressing its processing and degradation. Mol Cell Biol. 2006;26(9):3365–3377. doi: 10.1128/MCB.26.9.3365-3377.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huntzicker EG, Estay IS, Zhen H, Lokteva LA, Jackson PK, Oro AE. Dual degradation signals control Gli protein stability and tumor formation. Genes Dev. 2006;20(3):276–281. doi: 10.1101/gad.1380906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang B, Li Y. Evidence for the direct involvement of βTrCP in Gli3 protein processing. Proc Natl Acad Sci U S A. 2006;103(1):33–38. doi: 10.1073/pnas.0509927103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Di Marcotullio L, Ferretti E, Greco A, et al. Numb is a suppressor of hedgehog signalling and targets Gli1 for Itch-dependent ubiquitination. Nat Cell Biol. 2006;8(12):1415–1423. doi: 10.1038/ncb1510. [DOI] [PubMed] [Google Scholar]
- 90.Jiang J. Regulation of Hh/Gli signaling by dual ubiquitin pathways. Cell Cycle. 2006;5(21):2457–2463. doi: 10.4161/cc.5.21.3406. [DOI] [PubMed] [Google Scholar]
- 91.Canettieri G, Di Marcotullio L, Greco A, et al. Histone deacetylase and Cullin3-REN(KCTD11) ubiquitin ligase interplay regulates hedgehog signalling through Gli acetylation. Nat Cell Biol. 2010;12(2):132–142. doi: 10.1038/ncb2013. [DOI] [PubMed] [Google Scholar]
- 92.Coni S, Antonucci L, D’Amico D, et al. Gli2 acetylation at lysine 757 regulates hedgehog-dependent transcriptional output by preventing its promoter occupancy. PLoS One. 2013;8(6):e65718. doi: 10.1371/journal.pone.0065718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Han L, Pan Y, Wang B. Small ubiquitin-like modifier (SUMO) modification inhibits Gli2 protein transcriptional activity in vitro and in vivo. J Biol Chem. 2012;287(24):20483–20489. doi: 10.1074/jbc.M112.359299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang Q, Shi Q, Chen Y, et al. Multiple Ser/Thr-rich degrons mediate the degradation of Ci/Gli by the Cul3-HIB/SPOP E3 ubiquitin ligase. Proc Natl Acad Sci U S A. 2009;106(50):21191–21196. doi: 10.1073/pnas.0912008106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cheng SY, Bishop JM. Suppressor of fused represses Gli-mediated transcription by recruiting the SAP18-mSin3 corepressor complex. Proc Natl Acad Sci U S A. 2002;99(8):5442–5447. doi: 10.1073/pnas.082096999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang C, Pan Y, Wang B. Suppressor of fused and Spop regulate the stability, processing and function of Gli2 and Gli3 full-length activators but not their repressors. Development. 2010;137(12):2001–2009. doi: 10.1242/dev.052126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li ZJ, Mack SC, Mak TH, Angers S, Taylor MD, Hui CC. Evasion of p53 and G/M checkpoints are characteristic of Hh-driven basal cell carcinoma. Oncogene. doi: 10.1038/onc.2013.212. Epub June 10, 2013. [DOI] [PubMed] [Google Scholar]
- 98.Jagani Z, Mora-Blanco EL, Sansam CG, et al. Loss of the tumor suppressor Snf5 leads to aberrant activation of the hedgehog-Gli pathway. Nat Med. 2010;16(12):1429–1433. doi: 10.1038/nm.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Atwood SX, Li M, Lee A, Tang JY, Oro AE. Gli activation by atypical protein kinase C iota/lambda regulates the growth of basal cell carcinomas. Nature. 2013;494(7438):484–488. doi: 10.1038/nature11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang L, Xie G, Fan Q, Xie J. Activation of the hedgehog-signaling pathway in human cancer and the clinical implications. Oncogene. 2010;29(4):469–481. doi: 10.1038/onc.2009.392. [DOI] [PubMed] [Google Scholar]
- 101.Hahn H, Wicking C, Zaphiropoulous PG, et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85(6):841–851. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- 102.Johnson RL, Rothman AL, Xie J, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272(5268):1668–1671. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- 103.Epstein E., Jr Genetic determinants of basal cell carcinoma risk. Med Pediatr Oncol. 2001;36(5):555–558. doi: 10.1002/mpo.1129. [DOI] [PubMed] [Google Scholar]
- 104.Gorlin RJ. Nevoid basal-cell carcinoma syndrome. Medicine. 1987;66(2):98–113. doi: 10.1097/00005792-198703000-00002. [DOI] [PubMed] [Google Scholar]
- 105.Xie J, Murone M, Luoh SM, et al. Activating smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391(6662):90–92. doi: 10.1038/34201. [DOI] [PubMed] [Google Scholar]
- 106.Lam CW, Xie J, To KF, et al. A frequent activated smoothened mutation in sporadic basal cell carcinomas. Oncogene. 1999;18(3):833–836. doi: 10.1038/sj.onc.1202360. [DOI] [PubMed] [Google Scholar]
- 107.Reifenberger J, Wolter M, Knobbe CB, et al. Somatic mutations in the PTCH, SMOH, SUFUH and TP53 genes in sporadic basal cell carcinomas. Br J Dermatol. 2005;152(1):43–51. doi: 10.1111/j.1365-2133.2005.06353.x. [DOI] [PubMed] [Google Scholar]
- 108.Reifenberger J, Wolter M, Weber RG, et al. Missense mutations in SMOH in sporadic basal cell carcinomas of the skin and primitive neuroectodermal tumors of the central nervous system. Cancer Res. 1998;58(9):1798–1803. [PubMed] [Google Scholar]
- 109.Couve-Privat S, Bouadjar B, Avril MF, Sarasin A, Daya-Grosjean L. Significantly high levels of ultraviolet-specific mutations in the smoothened gene in basal cell carcinomas from DNA repair-deficient xeroderma pigmentosum patients. Cancer Res. 2002;62(24):7186–7189. [PubMed] [Google Scholar]
- 110.Tostar U, Malm CJ, Meis-Kindblom JM, Kindblom LG, Toftgard R, Unden AB. Deregulation of the hedgehog signalling pathway: a possible role for the PTCH and SUFU genes in human rhabdomyoma and rhabdomyosarcoma development. J Pathol. 2006;208(1):17–25. doi: 10.1002/path.1882. [DOI] [PubMed] [Google Scholar]
- 111.Pressey JG, Anderson JR, Crossman DK, Lynch JC, Barr FG. Hedgehog pathway activity in pediatric embryonal rhabdomyosarcoma and undifferentiated sarcoma: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2011;57(6):930–938. doi: 10.1002/pbc.23174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Clark VE, Erson-Omay EZ, Serin A, et al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science. 2013;339(6123):1077–1080. doi: 10.1126/science.1233009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Aavikko M, Li SP, Saarinen S, et al. Loss of SUFU function in familial multiple meningioma. Am J Hum Genet. 2012;91(3):520–526. doi: 10.1016/j.ajhg.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kijima C, Miyashita T, Suzuki M, Oka H, Fujii K. Two cases of nevoid basal cell carcinoma syndrome associated with meningioma caused by a PTCH1 or SUFU germline mutation. Fam Cancer. 2012;11(4):565–570. doi: 10.1007/s10689-012-9548-0. [DOI] [PubMed] [Google Scholar]
- 115.Fei DL, Sanchez-Mejias A, Wang Z, et al. Hedgehog signaling regulates bladder cancer growth and tumorigenicity. Cancer Res. 2012;72(17):4449–4458. doi: 10.1158/0008-5472.CAN-11-4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rodriguez-Blanco J, Schilling NS, Tokhunts R, et al. The hedgehog processing pathway is required for NSCLC growth and survival. Oncogene. 2013;32(18):2335–2345. doi: 10.1038/onc.2012.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shin K, Lee J, Guo N, et al. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature. 2011;472(7341):110–114. doi: 10.1038/nature09851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hahn H, Wojnowski L, Zimmer AM, Hall J, Miller G, Zimmer A. Rhabdomyosarcomas and radiation hypersensitivity in a mouse model of Gorlin syndrome. Nat Med. 1998;4(5):619–622. doi: 10.1038/nm0598-619. [DOI] [PubMed] [Google Scholar]
- 119.Hatley ME, Tang W, Garcia MR, et al. A mouse model of rhabdomyosarcoma originating from the adipocyte lineage. Cancer Cell. 2012;22(4):536–546. doi: 10.1016/j.ccr.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ignatius MS, Chen E, Elpek NM, et al. In vivo imaging of tumor-propagating cells, regional tumor heterogeneity, and dynamic cell movements in embryonal rhabdomyosarcoma. Cancer Cell. 2012;21(5):680–693. doi: 10.1016/j.ccr.2012.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nitzki F, Zibat A, Frommhold A, et al. Uncommitted precursor cells might contribute to increased incidence of embryonal rhabdomyosarcoma in heterozygous patched1-mutant mice. Oncogene. 2011;30(43):4428–4436. doi: 10.1038/onc.2011.157. [DOI] [PubMed] [Google Scholar]
- 122.Pelczar P, Zibat A, van Dop WA, et al. Inactivation of patched1 in mice leads to development of gastrointestinal stromal-like tumors that express PDGFRα but not kit. Gastroenterology. 2013;144(1):134–144. doi: 10.1053/j.gastro.2012.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Park KS, Martelotto LG, Peifer M, et al. A crucial requirement for hedgehog signaling in small cell lung cancer. Nat Med. 2011;17(11):1504–1508. doi: 10.1038/nm.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang DH, Clemons NJ, Miyashita T, et al. Aberrant epithelial-mesenchymal hedgehog signaling characterizes Barrett’s metaplasia. Gastroenterology. 2010;138(5):1810–1822. doi: 10.1053/j.gastro.2010.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang L, Wang LS, Chen XL, et al. Hedgehog signaling activation in the development of squamous cell carcinoma and adenocarcinoma of esophagus. Int J Biochem Mol Biol. 2012;3(1):46–57. [PMC free article] [PubMed] [Google Scholar]
- 126.Tian H, Callahan CA, DuPree KJ, et al. Hedgehog signaling is restricted to the stromal compartment during pancreatic carcinogenesis. Proc Natl Acad Sci U S A. 2009;106(11):4254–4259. doi: 10.1073/pnas.0813203106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mao J, Ligon KL, Rakhlin EY, et al. A novel somatic mouse model to survey tumorigenic potential applied to the hedgehog pathway. Cancer Res. 2006;66(20):10171–10178. doi: 10.1158/0008-5472.CAN-06-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Aszterbaum M, Epstein J, Oro A, et al. Ultraviolet and ionizing radiation enhance the growth of BCCs and trichoblastomas in patched heterozygous knockout mice. Nat Med. 1999;5(11):1285–1291. doi: 10.1038/15242. [DOI] [PubMed] [Google Scholar]
- 129.Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277(5329):1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 130.Feldmann G, Dhara S, Fendrich V, et al. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer Res. 2007;67(5):2187–2196. doi: 10.1158/0008-5472.CAN-06-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bailey JM, Swanson BJ, Hamada T, et al. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin Cancer Res. 2008;14(19):5995–6004. doi: 10.1158/1078-0432.CCR-08-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chang Q, Foltz WD, Chaudary N, Hill RP, Hedley DW. Tumor-stroma interaction in orthotopic primary pancreatic cancer xenografts during hedgehog pathway inhibition. Int J Cancer. 2013;133(1):225–234. doi: 10.1002/ijc.28006. [DOI] [PubMed] [Google Scholar]
- 133.Tang SN, Fu J, Nall D, Rodova M, Shankar S, Srivastava RK. Inhibition of sonic hedgehog pathway and pluripotency maintaining factors regulate human pancreatic cancer stem cell characteristics. Int J Cancer. 2012;131(1):30–40. doi: 10.1002/ijc.26323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Feldmann G, Fendrich V, McGovern K, et al. An orally bioavailable small-molecule inhibitor of hedgehog signaling inhibits tumor initiation and metastasis in pancreatic cancer. Mol Cancer Ther. 2008;7(9):2725–2735. doi: 10.1158/1535-7163.MCT-08-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gu D, Liu H, Su GH, et al. Combining hedgehog signaling inhibition with focal irradiation on reduction of pancreatic cancer metastasis. Mol Cancer Ther. 2013;12(6):1038–1048. doi: 10.1158/1535-7163.MCT-12-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bailey JM, Mohr AM, Hollingsworth MA. Sonic hedgehog paracrine signaling regulates metastasis and lymphangiogenesis in pancreatic cancer. Oncogene. 2009;28(40):3513–3525. doi: 10.1038/onc.2009.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324(5933):1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhao C, Chen A, Jamieson CH, et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009;458(7239):776–779. doi: 10.1038/nature07737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Boyd AL, Salci KR, Shapovalova Z, McIntyre BA, Bhatia M. Nonhematopoietic cells represent a more rational target of in vivo hedgehog signaling affecting normal or acute myeloid leukemia progenitors. Exp Hematol. doi: 10.1016/j.exphem.2013.05.287. Epub June 6, 2013. [DOI] [PubMed] [Google Scholar]
- 140.Romer JT, Kimura H, Magdaleno S, et al. Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/−)p53(−/−) mice. Cancer Cell. 2004;6(3):229–240. doi: 10.1016/j.ccr.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 141.Gu D, Fan Q, Zhang X, Xie J. A role for transcription factor STAT3 signaling in oncogene smoothened-driven carcinogenesis. J Biol Chem. 2012;287(45):38356–38366. doi: 10.1074/jbc.M112.377382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xie J, Aszterbaum M, Zhang X, et al. A role of PDGFRalpha in basal cell carcinoma proliferation. Proc Natl Acad Sci U S A. 2001;98(16):9255–9259. doi: 10.1073/pnas.151173398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Johnson RW, Nguyen MP, Padalecki SS, et al. TGF-beta promotion of Gli2-induced expression of parathyroid hormone-related protein, an important osteolytic factor in bone metastasis, is independent of canonical hedgehog signaling. Cancer Res. 2011;71(3):822–831. doi: 10.1158/0008-5472.CAN-10-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Keysar SB, Le PN, Anderson RT, et al. Hedgehog signaling alters reliance on EGF receptor signaling and mediates anti-EGFR therapeutic resistance in head and neck cancer. Cancer Res. 2013;73(11):3381–3392. doi: 10.1158/0008-5472.CAN-12-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Eberl M, Klingler S, Mangelberger D, et al. Hedgehog-EGFR cooperation response genes determine the oncogenic phenotype of basal cell carcinoma and tumour-initiating pancreatic cancer cells. EMBO Mol Med. 2012;4(3):218–233. doi: 10.1002/emmm.201100201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fan Q, He M, Sheng T, et al. Requirement of TGFbeta signaling for SMO-mediated carcinogenesis. J Biol Chem. 2010;285(47):36570–36576. doi: 10.1074/jbc.C110.164442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang Y, Ding Q, Yen CJ, et al. The crosstalk of mTOR/S6K1 and hedgehog pathways. Cancer Cell. 2012;21(3):374–387. doi: 10.1016/j.ccr.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shi S, Deng YZ, Zhao JS, et al. RACK1 promotes non-small-cell lung cancer tumorigenicity through activating sonic hedgehog signaling pathway. J Biol Chem. 2012;287(11):7845–7858. doi: 10.1074/jbc.M111.315416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hsieh A, Ellsworth R, Hsieh D. Hedgehog/Gli1 regulates IGF dependent malignant behaviors in glioma stem cells. J Cell Physiol. 2011;226(4):1118–1127. doi: 10.1002/jcp.22433. [DOI] [PubMed] [Google Scholar]
- 150.Mainwaring LA, Kenney AM. Divergent functions for eIF4E and S6 kinase by sonic hedgehog mitogenic signaling in the developing cerebellum. Oncogene. 2011;30(15):1784–1797. doi: 10.1038/onc.2010.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fernandez LA, Squatrito M, Northcott P, et al. Oncogenic YAP promotes radioresistance and genomic instability in medulloblastoma through IGF2-mediated Akt activation. Oncogene. 2012;31(15):1923–1937. doi: 10.1038/onc.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Heller E, Hurchla MA, Xiang J, et al. Hedgehog signaling inhibition blocks growth of resistant tumors through effects on tumor microenvironment. Cancer Res. 2012;72(4):897–907. doi: 10.1158/0008-5472.CAN-11-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Abbruzzese JL, National Institutes of Health (US) Are there new targets for pancreatic cancer therapeutics? 2007Available from: http://videocast.nih.gov/launch.asp?13834Accessed September 10, 2013
- 154.Inaguma S, Kasai K, Ikeda H. Gli1 facilitates the migration and invasion of pancreatic cancer cells through MUC5AC-mediated attenuation of E-cadherin. Oncogene. 2011;30(6):714–723. doi: 10.1038/onc.2010.459. [DOI] [PubMed] [Google Scholar]
- 155.Joost S, Almada LL, Rohnalter V, et al. Gli1 inhibition promotes epithelial-to-mesenchymal transition in pancreatic cancer cells. Cancer Res. 2012;72(1):88–99. doi: 10.1158/0008-5472.CAN-10-4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li X, Deng W, Lobo-Ruppert SM, Ruppert JM. Gli1 acts through snail and E-cadherin to promote nuclear signaling by beta-catenin. Oncogene. 2007;26(31):4489–4498. doi: 10.1038/sj.onc.1210241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Javelaud D, Pierrat MJ, Mauviel A. Crosstalk between TGF-beta and hedgehog signaling in cancer. FEBS Lett. 2012;586(14):2016–2025. doi: 10.1016/j.febslet.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 158.Von Hoff DD, LoRusso PM, Rudin CM, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009 Sep 17;361(12):1164–1172. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- 159.Graham RA, Lum BL, Cheeti S, et al. Pharmacokinetics of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with locally advanced or metastatic solid tumors: the role of alpha-1-acid glycoprotein binding. Clin Cancer Res. 2011;17(8):2512–2520. doi: 10.1158/1078-0432.CCR-10-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.LoRusso PM, Rudin CM, Reddy JC, et al. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res. 2011;17(8):2502–2511. doi: 10.1158/1078-0432.CCR-10-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lorusso PM, Jimeno A, Dy G, et al. Pharmacokinetic dose-scheduling study of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with locally advanced or metastatic solid tumors. Clin Cancer Res. 2011;17(17):5774–5782. doi: 10.1158/1078-0432.CCR-11-0972. [DOI] [PubMed] [Google Scholar]
- 162.Metcalfe C, de Sauvage FJ. Hedgehog fights back: mechanisms of acquired resistance against smoothened antagonists. Cancer Res. 2011;71(15):5057–5061. doi: 10.1158/0008-5472.CAN-11-0923. [DOI] [PubMed] [Google Scholar]
- 163.Tang T, Tang JY, Li D, et al. Targeting superficial or nodular basal cell carcinoma with topically formulated small molecule inhibitor of smoothened. Clin Cancer Res. 2011;17(10):3378–3387. doi: 10.1158/1078-0432.CCR-10-3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Skvara H, Kalthoff F, Meingassner JG, et al. Topical treatment of basal cell carcinomas in nevoid basal cell carcinoma syndrome with a smoothened inhibitor. J Invest Dermatol. 2011;131(8):1735–1744. doi: 10.1038/jid.2011.48. [DOI] [PubMed] [Google Scholar]
- 165.Buonamici S, Williams J, Morrissey M, et al. Interfering with resistance to smoothened antagonists by inhibition of the PI3K pathway in medulloblastoma. Sci Transl Med. 2010;2(51):51ra70. doi: 10.1126/scitranslmed.3001599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dijkgraaf GJ, Alicke B, Weinmann L, et al. Small molecule inhibition of GDC-0449 refractory smoothened mutants and downstream mechanisms of drug resistance. Cancer Res. 2011;71(2):435–444. doi: 10.1158/0008-5472.CAN-10-2876. [DOI] [PubMed] [Google Scholar]
- 167.Kim J, Aftab BT, Tang JY, et al. Itraconazole and arsenic trioxide inhibit hedgehog pathway activation and tumor growth associated with acquired resistance to smoothened antagonists. Cancer Cell. 2013;23(1):23–34. doi: 10.1016/j.ccr.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kim J, Tang JY, Gong R, et al. Itraconazole, a commonly used anti-fungal that inhibits hedgehog pathway activity and cancer growth. Cancer Cell. 2010;17(4):388–399. doi: 10.1016/j.ccr.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tang JY, Xiao TZ, Oda Y, et al. Vitamin D3 inhibits hedgehog signaling and proliferation in murine basal cell carcinomas. Cancer Prev Res (Phila) 2011;4(5):744–751. doi: 10.1158/1940-6207.CAPR-10-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chenna V, Hu C, Pramanik D, et al. A polymeric nanoparticle encapsulated small-molecule inhibitor of hedgehog signaling (NanoHHI) bypasses secondary mutational resistance to smoothened antagonists. Mol Cancer Ther. 2012;11(1):165–173. doi: 10.1158/1535-7163.MCT-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rohner A, Spilker ME, Lam JL, et al. Effective targeting of hedgehog signaling in a medulloblastoma model with PF-5274857, a potent and selective smoothened antagonist that penetrates the blood-brain barrier. Mol Cancer Ther. 2012;11(1):57–65. doi: 10.1158/1535-7163.MCT-11-0691. [DOI] [PubMed] [Google Scholar]
- 172.Lee MJ, Hatton BA, Villavicencio EH, et al. Hedgehog pathway inhibitor saridegib (IPI-926) increases lifespan in a mouse medulloblastoma model. Proc Natl Acad Sci U S A. 2012;109(20):7859–7864. doi: 10.1073/pnas.1114718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rudin CM, Hann CL, Laterra J, et al. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med. 2009;361(12):1173–1178. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fulda S. Molecular targeted therapies for rhabdomyosarcoma: focus on hedgehog and apoptosis signaling. Klinische Padiatrie. 2013;225(3):115–119. doi: 10.1055/s-0032-1331762. [DOI] [PubMed] [Google Scholar]
- 175.Dierks C, Beigi R, Guo GR, et al. Expansion of Bcr-Abl-positive leukemic stem cells is dependent on hedgehog pathway activation. Cancer Cell. 2008;14(3):238–249. doi: 10.1016/j.ccr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 176.Read TA, Fogarty MP, Markant SL, et al. Identification of CD15 as a marker for tumor-propagating cells in a mouse model of medulloblastoma. Cancer Cell. 2009;15(2):135–147. doi: 10.1016/j.ccr.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hofmann I, Stover EH, Cullen DE, et al. Hedgehog signaling is dispensable for adult murine hematopoietic stem cell function and hematopoiesis. Cell Stem Cell. 2009;4(6):559–567. doi: 10.1016/j.stem.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gao J, Graves S, Koch U, et al. Hedgehog signaling is dispensable for adult hematopoietic stem cell function. Cell Stem Cell. 2009;4(6):548–558. doi: 10.1016/j.stem.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Siggins SL, Nguyen NY, McCormack MP, et al. The hedgehog receptor patched1 regulates myeloid and lymphoid progenitors by distinct cell-extrinsic mechanisms. Blood. 2009;114(5):995–1004. doi: 10.1182/blood-2009-03-208330. [DOI] [PubMed] [Google Scholar]
- 180.Merchant A, Joseph G, Wang Q, Brennan S, Matsui W. Gli1 regulates the proliferation and differentiation of HSCs and myeloid progenitors. Blood. 2010;115(12):2391–2396. doi: 10.1182/blood-2009-09-241703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 182.Sims-Mourtada J, Izzo JG, Apisarnthanarax S, et al. Hedgehog: an attribute to tumor regrowth after chemoradiotherapy and a target to improve radiation response. Clin Cancer Res. 2006;12(21):6565–6572. doi: 10.1158/1078-0432.CCR-06-0176. [DOI] [PubMed] [Google Scholar]
- 183.Yoshikawa R, Nakano Y, Tao L, et al. Hedgehog signal activation in oesophageal cancer patients undergoing neoadjuvant chemoradiotherapy. Br J Cancer. 2008;98(10):1670–1674. doi: 10.1038/sj.bjc.6604361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Takebe N, Harris PJ, Warren RQ, Ivy SP. Targeting cancer stem cells by inhibiting Wnt, notch, and hedgehog pathways. Nat Rev Clin Oncol. 2011;8(2):97–106. doi: 10.1038/nrclinonc.2010.196. [DOI] [PubMed] [Google Scholar]
- 185.Song Z, Yue W, Wei B, et al. Sonic hedgehog pathway is essential for maintenance of cancer stem-like cells in human gastric cancer. PLoS One. 2011;6(3):e17687. doi: 10.1371/journal.pone.0017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tanaka H, Nakamura M, Kameda C, et al. The hedgehog signaling pathway plays an essential role in maintaining the CD44+CD24−/low subpopulation and the side population of breast cancer cells. Anticancer Res. 2009;29(6):2147–2157. [PubMed] [Google Scholar]
- 187.Liu S, Dontu G, Mantle ID, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66(12):6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Takahashi T, Kawakami K, Mishima S, et al. Cyclopamine induces eosinophilic differentiation and upregulates CD44 expression in myeloid leukemia cells. Leuk Res. 2011;35(5):638–645. doi: 10.1016/j.leukres.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 189.Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67(3):1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 190.Visbal AP, LaMarca HL, Villanueva H, et al. Altered differentiation and paracrine stimulation of mammary epithelial cell proliferation by conditionally activated smoothened. Dev Biol. 2011;352(1):116–127. doi: 10.1016/j.ydbio.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bar EE, Chaudhry A, Lin A, et al. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25(10):2524–2533. doi: 10.1634/stemcells.2007-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. Hedgehog-Gli1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007;17(2):165–172. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Su W, Meng F, Huang L, Zheng M, Liu W, Sun H. Sonic hedgehog maintains survival and growth of chronic myeloid leukemia progenitor cells through β-catenin signaling. Exp Hematol. 2012;40(5):418–427. doi: 10.1016/j.exphem.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 194.Domingo-Domenech J, Vidal SJ, Rodriguez-Bravo V, et al. Suppression of acquired docetaxel resistance in prostate cancer through depletion of notch- and hedgehog-dependent tumor-initiating cells. Cancer Cell. 2012;22(3):373–388. doi: 10.1016/j.ccr.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ramaswamy B, Lu Y, Teng KY, et al. Hedgehog signaling is a novel therapeutic target in tamoxifen-resistant breast cancer aberrantly activated by PI3K/AKT pathway. Cancer Res. 2012;72(19):5048–5059. doi: 10.1158/0008-5472.CAN-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Steg AD, Bevis KS, Katre AA, et al. Stem cell pathways contribute to clinical chemoresistance in ovarian cancer. Clin Cancer Res. 2012;18(3):869–881. doi: 10.1158/1078-0432.CCR-11-2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chaudary N, Pintilie M, Hedley D, et al. Hedgehog pathway signaling in cervical carcinoma and outcome after chemoradiation. Cancer. 2012;118(12):3105–3115. doi: 10.1002/cncr.26635. [DOI] [PubMed] [Google Scholar]
- 198.Queiroz KC, Ruela-de-Sousa RR, Fuhler GM, et al. Hedgehog signaling maintains chemoresistance in myeloid leukemic cells. Oncogene. 2010;29(48):6314–6322. doi: 10.1038/onc.2010.375. [DOI] [PubMed] [Google Scholar]
- 199.Singh RR, Kunkalla K, Qu C, et al. ABCG2 is a direct transcriptional target of hedgehog signaling and involved in stroma-induced drug tolerance in diffuse large B-cell lymphoma. Oncogene. 2011;30(49):4874–4886. doi: 10.1038/onc.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lin SH, George TJ, Ben-Josef E, et al. Opportunities and challenges in the era of molecularly targeted agents and radiation therapy. J Natl Cancer Inst. 2013;105(10):686–693. doi: 10.1093/jnci/djt055. [DOI] [PMC free article] [PubMed] [Google Scholar]