Encapsulating peritoneal sclerosis (EPS) is an uncommon complication of peritoneal dialysis (PD), associated with prolonged PD duration and repeated or severe episodes of peritoneal infection. Variations in the physical appearance of the disease and in interpretations of the underlying pathology have, over the years, clouded the terminology used.
The first published report of the condition in 1980 highlighted the underlying peritoneal sclerosis and described encapsulation as “loops of bowel... bound together and shortened by a dense casing” (1). The condition was subsequently designated “sclerosing peritonitis” (2), reflecting some similarities with practolol-induced sclerosing peritonitis (3-5); however, there is now general agreement that the condition should be termed EPS, although encapsulating fibrosis with peritoneal sclerosis would be more accurate. The condition presents with many different physical appearances, but all probably reflect varying manifestations of the peritoneum’s stereotypical response to acute-on-chronic injury.
In this edition of Peritoneal Dialysis International, two papers describe the varying manifestations of EPS at the time of surgery (6,7). One describes a localized form of the disease; the other suggests classifying EPS into three patterns based on its appearance, but interestingly does not distinguish between localized and generalized disease. These two papers underscore the difficulty in describing the condition because of its varying manifestations. Those difficulties have not been helped by confusion over the nature of the surgical treatment applied. Some reports describe enterolysis combined with peritonectomy and partial bowel resection, analogous to the Sugarbaker procedure for pseudomyxoma peritonei (8,9). However, the largest reported experience describes enterolysis accompanied by simple removal of the encasing fibrous tissue from the peritoneal surfaces (10), a procedure more akin to decortication of empyema of the lung.
To understand the various manifestations of EPS, it is helpful to consider the reaction of the peritoneum to injury. Adjacent loops of small bowel adhere to an area of inflammation, effectively walling it off from the rest of the peritoneal cavity. This mechanism is the same one by which appendicitis evolves into an appendix mass. In the same way, inflammation of the serosal surfaces of the bowel in response to PD fluid or peritoneal infection results in adjacent segments of intestine becoming adherent. Where this adherence occurs, the anti-mesenteric surfaces of the intestine remain exposed to PD and become sclerotic and tanned. The sides of the intestine that are hidden from continued PD exposure are spared, either because they were not exposed to PD for long enough, or if they were already sclerotic when they became adherent, the process reversed over time. The overall appearance is similar to the skin of the fingers: the palmar skin is thickened; the non-exposed interdigital skin is soft and thin.
In other cases, the bowel remains adhesion-free until PD stops, and in consequence, the visceral peritoneum is circumferentially sclerotic. The exposed peritoneal surface produces a fibrin-rich exudate when PD ceases, resulting in fibrin deposition, which organizes into a fibrous sheet covering the bowel. As the inflammatory exudative state subsides, the exudate is reabsorbed, bringing inflamed surfaces together, and fibrin organizes into fibrous tissue encapsulating the bowel. On top of this inflammation, dystrophic calcification may occur.
Latus et al. (6) propose categorizing EPS based on three different physical appearances; however, those appearances do not cover the full range of presentations, others of which are described by Habib et al. (7). The appearance of the peritoneal cavity in EPS that is encountered at surgery can be described in terms of each of the following elements:
Distribution of sclerosis and tanning: affecting the entire length of the small bowel or just a section; affecting the entire circumference of the bowel or just the anti-mesenteric border
Appearance of the encapsulating fibrotic sheet: a fibrous cocoon encapsulating concertinaed loops of small intestine, or a dense sheet anterior to the small bowel
Distribution of encapsulation: localized or distributed throughout the peritoneum
Presence and distribution of calcification: isolated deposits on the surface of the bowel akin to atherosclerotic plaques, or larger areas of plaque
The large bowel may be affected by sclerosis and tanning, but is rarely obstructed. Likewise, the duodenum (being extraperitoneal for most of its course) is rarely affected, and the stomach, although subject to sclerosis on its anterior surface, is rarely constrained by encapsulation. Each of the elements outlined here may affect the presentation of EPS, its radiologic appearances, and its response to surgical intervention.
Localized disease is typically found in the distal small bowel, where it forms a smaller peritoneal cavity surrounding the PD catheter in the pelvis. If the proximal bowel is not involved, it can dilate and produce the typical radiologic features of intestinal obstruction (Figure 1). Localized disease is the most straightforward to treat and least likely to recur. Generalized disease, particularly when associated with a sheet of fibrous tissue overlaying the bowel and encasing an inflammatory collection (Figure 2), is the most difficult to treat and more likely to be associated with EPS recurrence or postoperative adhesive obstruction because of the more extensive surgery involved.
Figure 1 —
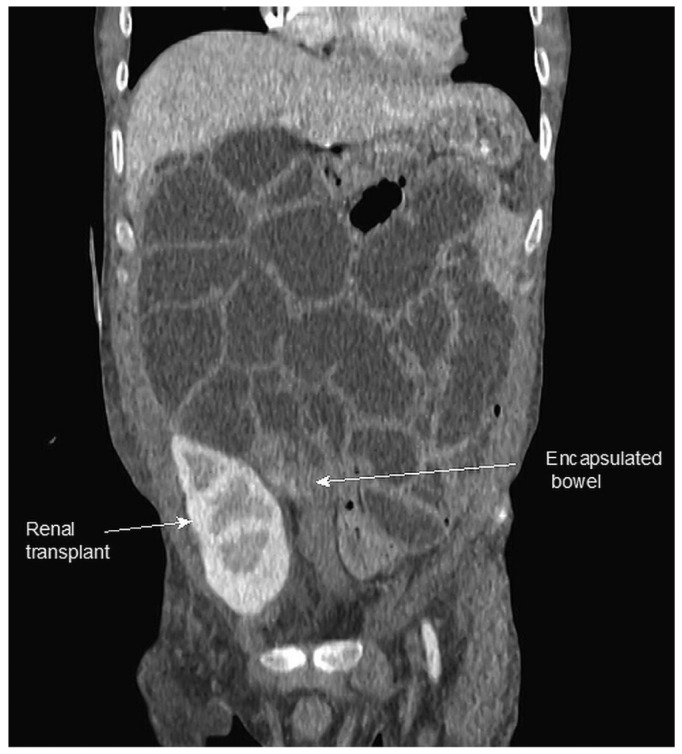
Computed tomography image demonstrating localized encapsulating peritoneal sclerosis, with encapsulation of distal small bowel adjacent to a kidney graft and dilatation of the proximal bowel.
Figure 2 —
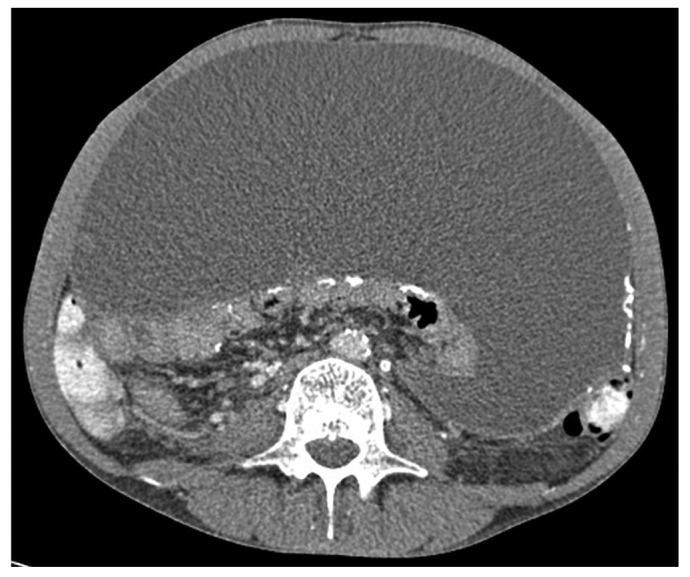
Computed tomography image demonstrating a posterior fibrous sheet encasing small bowel, with an anteriorly placed collection.
Classifying the varying clinical phenotypes of EPS is important. The diverse physical appearances of EPS are associated with different presentations and pose different surgical challenges with varying outcomes. More research is needed into the therapeutic options to prevent and treat EPS. The optimal surgical procedure is a matter of some confusion, with misinterpretation of descriptions and terms, as highlighted by the unambiguous reference in the Latus article to the work of Kawanishi and colleagues as “peritonectomy” and its advocation of resection. Kawanishi et al. (11) actually refer to “decortication” and avoidance of resection and primary anastomosis.
Some evidence has been advanced that medical treatment comprising steroids or tamoxifen may have a role in the treatment of EPS (12-14), but those agents are probably best introduced early in the course of the disease and may be most appropriate if started after surgery to prevent EPS recurrence, at the time of kidney transplantation, or when a patient at high risk of EPS is switched from PD to hemodialysis.
Classifying the underlying disease at the time of EPS surgery may be particularly useful in trials of interventions that prevent recurrence, such as the use of Nobel plication or tamoxifen (10).
Disclosures
The authors have no financial conflicts of interest in relation to this article.
References
- 1. Gandhi VC, Humayun HM, Ing TS, Daugirdas JT, Jablokow VR, Iwatsuki S, et al. Sclerotic thickening of the peritoneal membrane in maintenance peritoneal dialysis patients. Arch Intern Med 1980; 140:1201–3 [PubMed] [Google Scholar]
- 2. Bradley JA, Hamilton DN, McWhinnie DL, Briggs JD, Junor BJ. Sclerosing peritonitis after CAPD. Lancet 1983; 2:572–3 [DOI] [PubMed] [Google Scholar]
- 3. Brown P, Baddeley H, Read AE, Davies JD, McGarry J. Sclerosing peritonitis, an unusual reaction to a beta-adrenergic-blocking drug (practolol). Lancet 1974; 2:1477–81 [DOI] [PubMed] [Google Scholar]
- 4. Eltringham WK, Espiner HJ, Windsor CW, Griffiths DA, Davies JD, Baddeley H, et al. Sclerosing peritonitis due to practolol: a report on 9 cases and their surgical management. Br J Surg 1977; 64:229–35 [DOI] [PubMed] [Google Scholar]
- 5. Jackson BT. Surgical treatment of sclerosing peritonitis caused by practolol. Br J Surg 1977; 64:255–7 [DOI] [PubMed] [Google Scholar]
- 6. Latus J, Ulmer C, Fritz P, Rettenmaier B, Biegger D, Lang T, et al. Phenotypes of encapsulating peritoneal sclerosis—macroscopic appearance, histologic findings, and outcome. Perit Dial Int 2013; 33:495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Habib SM, Hagen SM, Korte MR, Zietse R, Dor FJ, Betjes MG. Localized encapsulating peritoneal sclerosis constricting the terminal ileum—an unusual appearance requiring surgical intervention. Perit Dial Int 2013; 33:503–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sugarbaker PH, Kern K, Lack E. Malignant pseudomyxoma peritonei of colonic origin. Natural history and presentation of a curative approach to treatment. Dis Colon Rectum 1987; 30:772–9 [DOI] [PubMed] [Google Scholar]
- 9. Ulmer C, Braun N, Rieber F, Latus J, Hirschburger S, Emmel J, et al. Efficacy and morbidity of surgical therapy in late-stage encapsulating peritoneal sclerosis. Surgery 2013; 153:219–24 [DOI] [PubMed] [Google Scholar]
- 10. Kawanishi H, Shintaku S, Moriishi M, Dohi K, Tsuchiya S. Seventeen years’ experience of surgical options for encapsulating peritoneal sclerosis. Adv Perit Dial 2011; 27:53–8 [PubMed] [Google Scholar]
- 11. Kawanishi H, Watanabe H, Moriishi M, Tsuchiya S. Successful surgical management of encapsulating peritoneal sclerosis. Perit Dial Int 2005; 25(Suppl 4):S39–47 [PubMed] [Google Scholar]
- 12. Allaria PM, Giangrande A, Gandini E, Pisoni IB. Continuous ambulatory peritoneal dialysis and sclerosing encapsulating peritonitis: tamoxifen as a new therapeutic agent? J Nephrol 1999; 12:395–7 [PubMed] [Google Scholar]
- 13. Guest S. Tamoxifen therapy for encapsulating peritoneal sclerosis: mechanism of action and update on clinical experiences. Perit Dial Int 2009; 29:252–5 [PubMed] [Google Scholar]
- 14. Korte MR, Fieren MW, Sampimon DE, Lingsma HF, Weimar W, Betjes MG. on behalf of the investigators of the Dutch Multicentre EPS Study. Tamoxifen is associated with lower mortality of encapsulating peritoneal sclerosis: results of the Dutch Multicentre EPS Study. Nephrol Dial Transplant 2011; 26:691–7 [DOI] [PubMed] [Google Scholar]


