Encapsulating peritoneal sclerosis (EPS), a serious but uncommon complication of peritoneal dialysis (PD), initially well described in the Japanese patient population (1-3), is now widely recognized. Because of the severe morbidity and mortality attached, EPS has the potential to negatively affect perceptions of the value of PD as a long-term therapy and so affect the take-on rate and increase early transfer to HD. It is now well established that EPS is strongly associated with duration of PD therapy (4,5), with ultrafiltration failure associated with membrane fibrosis (6,7), and also, paradoxically, with stopping PD. Nevertheless, significant deficits in the understanding of EPS remain. That lack of understanding is implicit in nonspecific diagnostic criteria and an uncertain association with peritonitis, issues that arise in a study by Wong et al. (8) in this issue of Peritoneal Dialysis International.
What are the Diagnostic Criteria Currently, and how have they been Applied?
The current International Society for Peritoneal Dialysis diagnostic criteria are set out in a review by Kawaguchi et al. (9) and are based on two factors: symptoms suggestive of bowel obstruction, and either surgical or radiologic evidence of peritoneal thickening or cocooning as a cause of the symptoms. Disturbance of normal intestinal function was emphasized, but clear obstruction was not specified as a necessity. Symptoms mentioned included abdominal pain, nausea, vomiting, weight loss, fullness, and anorexia, although the severity of these manifestations can be variable. Although the diagnosis at laparotomy is usually apparent, the radiologic criteria have subsequently been refined and evaluated in numerous studies. It is apparent from the published literature that application of these diagnostic criteria has varied considerably.
The largest case series of EPS comes from a retrospective London-wide “Pan-Thames” study, in which 33% of 111 patients were reported to have obstruction; 67%, abdominal pain; 59%, vomiting; and 39%, ascites. Only 20% were reported to have weight loss, but 39 of the 111 were receiving parenteral nutrition. Mortality was 53%, with a median time to death of 7 months (10).
Summers et al. (11) published a single-center retrospective case series from what is now one of the two designated UK surgical centers for EPS. Of 27 patients, 16 had severe disease, with 6 cases of complete obstruction. The remaining 11 were classified as having “mild to moderate” disease, but the authors explicitly state that the division was arbitrary. Among the latter 11 patients, 3 had features of EPS on computed tomography (CT), but with no symptoms; the others had symptoms not requiring surgery.
To minimize diagnostic heterogeneity in a Dutch series of 62 patients, only patients with persistent or recurrent obstruction together with radiologic or macroscopic evidence were included (12). In the single-center Stoke PD cohort, 2 of 11 patients with EPS were considered to present slightly differently, their EPS being associated with early and severe infectious peritonitis (6).
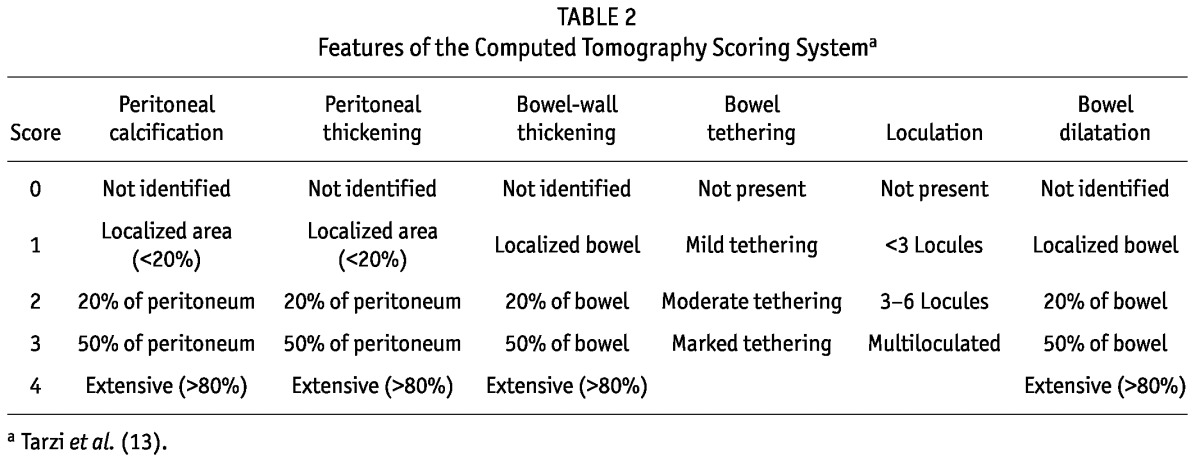
Based on a comparative analysis of CT images from 27 EPS patients, 15 hemodialysis patients, and 20 PD patients with a mean dialysis duration of 4.0 years, a scoring system was developed based on quantifying the degree of 6 different findings. The resulting scores differentiated the groups well and produced good interobserver agreement; however, CT scores did not necessarily reflect disease activity (13).
In a review from Amsterdam of CT images, 15 patients with EPS were compared with 16 control subjects with a mean PD duration of 62 months (14). Of the control subjects, 10% or more showed peritoneal enhancement; 12.5% or more, peritoneal calcification; and 18.7% or more, peritoneal thickening. Only 1 (6.3%) had obstruction. Notably, the authors considered non-resolving peritonitis to be an indicator of EPS. In another study of 20 EPS patients by Goodlad et al. (15), CT images from several months before a formal EPS diagnosis were reviewed. Compared with asymptomatic patients, those who were symptomatic had a significantly higher validated EPS CT score, but neither group was considered at the time to have EPS.
Several further studies have clearly identified patients with an abnormal pathologic process (sometimes associated with symptoms), among whom at least some have gone on to develop what the study authors consider to be “full-blown” EPS. However, because of the lack of more-specific diagnostic criteria, it is not possible to compare these groups because each study relied on the authors’ own perceptions of what constitutes “full-blown” EPS.
What is the Role of Peritonitis in EPS?
Early publications frequently cite peritonitis as a risk factor for EPS, but the potential role that peritonitis might play in EPS is complex. First, peritonitis is a common reason for stopping PD, and stopping PD appears to be a risk factor. Second, a high frequency of peritonitis over a prolonged period might contribute to membrane damage accrued during long-term PD. Third, peritonitis might act as an acute precipitant of EPS (a “second hit”) in patients with fibrosed, damaged membranes. Fourth, EPS might present as a sterile peritonitis (an “inflammatory stage”), with or without an infectious trigger. And finally, EPS patients with ascites might develop spontaneous bacterial peritonitis.
The first issue is suggested by the fact that many case series report EPS more often developing after PD has stopped (10-12), possibly because of local accumulation of inflammatory or fibrotic signaling molecules. However, no studies have examined whether typical infectious peritonitis is over- or under-represented as a reason for stopping PD in patients who subsequently developed EPS compared with other patients without EPS matched for duration of PD.
The second issue has been examined most robustly in an ANZDATA study, in which peritonitis was not associated with EPS (16). That finding was replicated in other recent case series (6,11), with the EPS group actually having a significantly lower overall peritonitis rate in some cases (12). The likely reason for this counterintuitive finding may be the risk associated with stopping PD early if the peritonitis risk is higher.
The third issue has been only partially addressed in the past. A small study by Bilgic et al. (17) suggested that patients stopping PD for peritonitis were more likely to develop intra-abdominal collections, which themselves are associated with systemic inflammation; however, that study did not specifically address EPS. Another study by Szeto et al. (18) addressed intra-abdominal collections after peritonitis requiring catheter removal. In 24 patients with available CT imaging, features compatible with EPS were present: peritoneal enhancement in 12.5%, peritoneal thickening in 45.8%, peritoneal calcification in 4.2%, bowel-wall thickening in 12.5%, adhesions in 58.3%, and signs of obstruction in 16.7%. None of the patients were considered to have EPS despite the fact that 2 were subsequently admitted with obstruction.
Very few published data are available to inform the fourth and final questions, aside from inferences from histologic work to address the fourth question.
What does this Latest Study add?
The single-center retrospective study by Wong et al. of 62 patients who had catheter removal for refractory peritonitis identified 39 “high risk” patients who were considered to have a persistent sterile peritonitis and 23 others in whom peritonitis resolved after catheter removal and who acted as a control group. Evidence of persistent peritonitis included continued systemic inflammation and the presence of intra-abdominal collections that were often bloodstained and always sterile on multiple paracenteses.
One important weakness of the study is its failure to report leukocyte counts in the collections (in EPS, blood-stained ascites is usually free of leucocytes). Compared with the control group, the group designated high risk also had other features typically expected of an EPS cohort, with longer duration of PD, faster peritoneal solute transport rates, and most importantly, CT features and symptoms of EPS. Of the high-risk group, 12 went on to develop “full-blown” EPS at a mean of 37 days after catheter removal, and those 12 were compared with the rest of the high-risk group. No significant differences were found, although a trend toward faster solute transport and ultrafiltration failure was observed in this study with very small numbers to support such an analysis.
The authors have therefore partly addressed the fourth question concerning the role of peritonitis in EPS by describing, with greater clarity than previously, a distinct presentation of EPS in which a non-resolving peritonitis is presumably precipitated by infectious peritonitis requiring catheter removal. Their patient group also had a relatively fast transition to obstructed EPS after catheter removal. The mean onset of 37 days is a finding similar to that in the London case series, in which the overall mean time to EPS after PD catheter removal was 5.5 months, but in which the progression to EPS occurred within 3 months for 17 of 20 patients whose catheter was removed for peritonitis.
Unfortunately, Wong et al. missed an opportunity to compare either their high-risk group or the “full blown” EPS subgroup to the rest of the EPS patients at their center, which would have helped to define how distinct their study group was. They could also have confirmed the culture-negative rate of the precipitating—presumed infectious—peritonitis.
What they have also done, however, is to also illustrate the variability in the diagnosis of EPS. The high-risk group as a whole had a mean Tarzi CT score of 7.69, and all had symptoms compatible with EPS. Nevertheless, the authors felt that these factors were not sufficient to diagnose EPS. Based on existing case series, it seems highly likely that other authors would have described these patients as having EPS. As such, this study by Wong and colleagues could also be viewed as examining EPS patients for predictors of subsequent obstruction.
TABLE 2.
Features of the Computed Tomography Scoring Systema
What can we do about the Diagnostic Variability?
Bearing in mind the large number of unanswered questions concerning EPS, there is a clear need to standardize the reporting of EPS cases so that it is clear which group of patients a study has incorporated and therefore how to interpret the findings. The easiest way of ensuring that cases of EPS are unequivocal is to stipulate that cases must have a severe form of obstruction—a strategy used by at least two recent studies, but likely to lead to under-reporting of the problem, leaving many of the remaining questions concerning EPS unaddressed. For studies to be comparable and consistent, standardized reporting of details is necessary.
We suggest that studies should report all the findings covering the main features of EPS: that is, radiologic, surgical, and histologic findings; symptoms; the presence (or not) of peritonitis as defined by standard criteria; and local and systemic inflammation. Table 1 sets out one potential structure that could be used. The structures for macroscopic appearance of encapsulation and intra-abdominal extent are based on work reported in this issue of Peritoneal Dialysis International (19,20), the radiologic structure is based on work by Tarzi et al. (10) and Vlijm et al. (14), and the histologic criteria are based on work by Braun et al. (21). As more data emerge, the structure can be refined iteratively.
TABLE 1.
Suggested Structure for Reporting of Encapsulating Peritoneal Sclerosis (EPS)
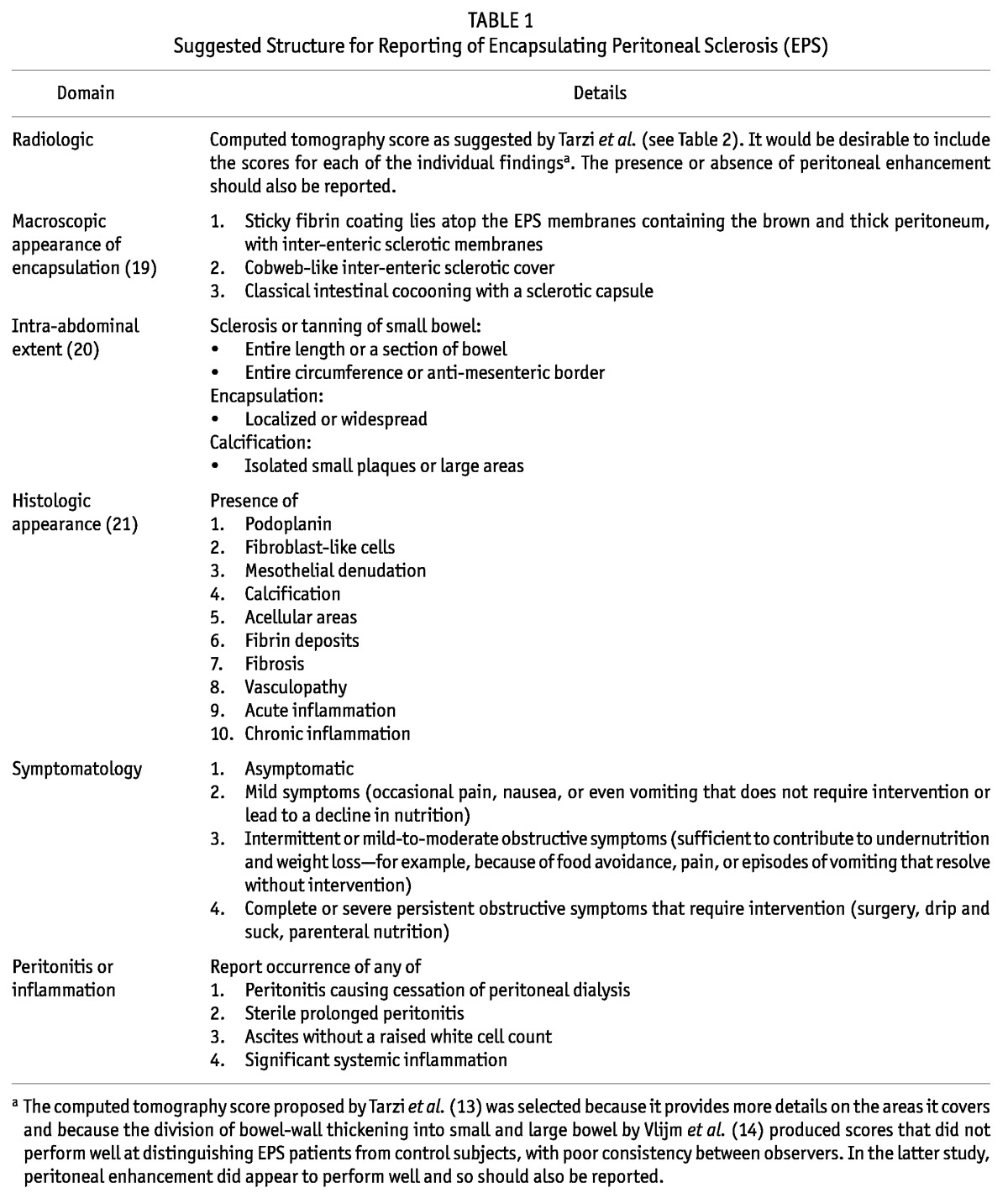
We also suggest that studies should be quite clear, based on the foregoing features, which criteria they used to diagnose EPS: that is, the CT score or the criteria used if radiologic findings were considered important, how the presence of peritonitis was viewed, the level of symptoms that were considered necessary, and whether surgical findings were considered important, with a clear description of what was considered EPS based on the outlined structure. Furthermore, given that the natural history of EPS is still not exactly clear, we recommend that studies stipulate whether the diagnostic criteria were applied based on initial presentation or subsequent progression. If the diagnosis was not made at initial presentation, then information on which of the EPS features were present at that time should also be provided, if available.
We believe that this kind of approach will allow for a clearer picture of the natural history of EPS to emerge and for better comparability between studies. It will also provide a structure to investigate issues such as the role of inflammation.
Disclosures
ML has no financial conflicts of interest to declare. NB is supported by a grant from Baxter Healthcare and the Robert-Bosch Foundation. SJD has received research funding and honoraria for lectures from Baxter Healthcare and Fresenius Medical Care.
References
- 1. Nomoto Y, Kawaguchi Y, Kubo H, Hirano H, Sakai S, Kurokawa K. Sclerosing encapsulating peritonitis in patients undergoing continuous ambulatory peritoneal dialysis: a report of the Japanese Sclerosing Encapsulating Peritonitis Study Group. Am J Kidney Dis 1996; 28:420–7 [DOI] [PubMed] [Google Scholar]
- 2. Kawanishi H, Kawaguchi Y, Fukui H, Hara S, Imada A, Kubo H, et al. Encapsulating peritoneal sclerosis in Japan: a prospective, controlled, multicenter study. Am J Kidney Dis 2004; 44:729–37 [PubMed] [Google Scholar]
- 3. Honda K, Nitta K, Horita S, Tsukada M, Itabashi M, Nihei H, et al. Histologic criteria for diagnosing encapsulating peritoneal sclerosis in continuous ambulatory peritoneal dialysis patients. Adv Perit Dial 2003; 19:169–75 [PubMed] [Google Scholar]
- 4. Rigby RJ, Hawley CM. Sclerosing peritonitis: the experience in Australia. Nephrol Dial Transplant 1998; 13:154–9 [DOI] [PubMed] [Google Scholar]
- 5. Brown MC, Simpson K, Kerssens JJ, Mactier RA. on behalf of the Scottish Renal Registry. Encapsulating peritoneal sclerosis in the new millennium: a national cohort study. Clin J Am Soc Nephrol 2009; 4:1222–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lambie ML, John B, Mushahar L, Huckvale C, Davies SJ. The peritoneal osmotic conductance is low well before the diagnosis of encapsulating peritoneal sclerosis is made. Kidney Int 2010; 78:611–8 [DOI] [PubMed] [Google Scholar]
- 7. Sampimon DE, Coester AM, Struijk DG, Krediet RT. The time course of peritoneal transport parameters in peritoneal dialysis patients who develop encapsulating peritoneal sclerosis. Nephrol Dial Transplant 2011; 26:291–8 [DOI] [PubMed] [Google Scholar]
- 8. Wong YY, Wong PN, Mak SK, Chan SF, Cheuk YY, Ho LY, et al. Persistent sterile peritoneal inflammation after catheter removal for refractory bacterial peritonitis predicts full-blown encapsulating peritoneal sclerosis. Perit Dial Int 2013; 33:507–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kawaguchi Y, Saito A, Kawanishi H, Nakayama M, Miyazaki M, Nakamoto H, et al. Recommendations on the management of encapsulating peritoneal sclerosis in Japan, 2005: diagnosis, predictive markers, treatment, and preventive measures. Perit Dial Int 2005; 25(Suppl 4):S83–95 [PubMed] [Google Scholar]
- 10. Balasubramaniam G, Brown EA, Davenport A, Cairns H, Cooper B, Fan SL, et al. The Pan-Thames EPS study: treatment and outcomes of encapsulating peritoneal sclerosis. Nephrol Dial Transplant 2009; 24:3209–15 [DOI] [PubMed] [Google Scholar]
- 11. Summers AM, Clancy MJ, Syed F, Harwood N, Brenchley PE, Augustine T, et al. Single-center experience of encapsulating peritoneal sclerosis in patients on peritoneal dialysis for end-stage renal failure. Kidney Int 2005; 68:2381–8 [DOI] [PubMed] [Google Scholar]
- 12. Korte MR, Sampimon DE, Lingsma HF, Fieren MW, Looman CW, Zietse R, et al. on behalf of the Dutch Multicenter EPS Study. Risk factors associated with encapsulating peritoneal sclerosis in Dutch EPS study. Perit Dial Int 2011; 31:269–78 [DOI] [PubMed] [Google Scholar]
- 13. Tarzi RM, Lim A, Moser S, Ahmad S, George A, Balasubramaniam G, et al. Assessing the validity of an abdominal CT scoring system in the diagnosis of encapsulating peritoneal sclerosis. Clin J Am Soc Nephrol 2008; 3:1702–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vlijm A, Stoker J, Bipat S, Spijkerboer AM, Phoa SS, Maes R, et al. Computed tomographic findings characteristic for encapsulating peritoneal sclerosis: a case-control study. Perit Dial Int 2009; 29:517–22 [PubMed] [Google Scholar]
- 15. Goodlad C, Tarzi R, Gedroyc W, Lim A, Moser S, Brown EA. Screening for encapsulating peritoneal sclerosis in patients on peritoneal dialysis: role of CT scanning. Nephrol Dial Transplant 2011; 26:1374–9 [DOI] [PubMed] [Google Scholar]
- 16. Johnson DW, Cho Y, Livingston BE, Hawley CM, McDonald SP, Brown FG, et al. Encapsulating peritoneal sclerosis: incidence, predictors, and outcomes. Kidney Int 2010; 77:904–12 [DOI] [PubMed] [Google Scholar]
- 17. Bilgic A, Sezer S, Ozdemir FN, Akgul A, Arat Z, Haberal M. Clinical outcome after transfer from peritoneal dialysis to hemodialysis. Adv Perit Dial 2006; 22:94–8 [PubMed] [Google Scholar]
- 18. Szeto CC, Kwan BC, Chow KM, Pang WF, Kwong VW, Leung CB, et al. Persistent symptomatic intra-abdominal collection after catheter removal for PD-related peritonitis. Perit Dial Int 2011; 31:34–8 [DOI] [PubMed] [Google Scholar]
- 19. Latus J, Ulmer C, Fritz P, Rettenmaier B, Biegger D, Lang T, et al. Phenotypes of encapsulating peritoneal sclerosis—macroscopic appearance, histologic findings, and outcome. Perit Dial Int 2013; 33:495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Watson CJE, Butle AJ, Bradley JA. Classification of encapsulating peritoneal sclerosis is important, but must encapsulate the entire spectrum of the disease. Perit Dial Int 2013; 33:479–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Braun N, Fritz P, Ulmer C, Latus J, Kimmel M, Biegger D, et al. Histological criteria for encapsulating peritoneal sclerosis—a standardized approach. PLoS One 2012; 7:e48647 [DOI] [PMC free article] [PubMed] [Google Scholar]


