Abstract
♦ Background and Objectives: Although cardiovascular disease (CVD) is an important cause of morbidity and mortality in patients with end-stage renal disease, non-CVD causes account for more than 50% of total deaths. We previously showed that, compared with men, women starting dialysis— both hemodialysis and peritoneal dialysis (PD)—have higher non-CVD mortality rates. Here, we evaluate sex-specific outcomes in a large cohort of incident PD patients.
♦ Methods: Incident de novo PD patients from the Andalusian SICATA Registry for 1999 - 2010, with follow-up until 31 December 2010 or up to 5 years, were investigated for fatal outcomes. Causes of death were extracted from medical records. The analysis used traditional and competing-risk Cox models for all-cause and cause-specific mortality in men and women, correcting in the competing-risk models for the events of kidney transplantation and transfer to hemodialysis.
♦ Results: A total of 1458 patients (57% men; mean overall age: 55.3 ± 17.0 years) initiated PD in Andalusia during the study period. During follow-up, 350 deaths, 355 renal transplantation procedures, and 331 transfers to hemodialysis were recorded. Vascular disease and diabetic nephropathy were the most frequent causes of kidney failure in men; other causes were more common in women. In the traditional Cox model, both sexes showed a similar all-cause mortality risk [crude hazard ratio (HR): 0.90; 95% confidence interval (CI): 0.72 to 1.12]. However, with respect to specific causes of death, women showed a borderline lower risk of both CVD (crude HR: 0.71; 95% CI: 0.50 to 0.99) and non-CVD mortality from other than infection (crude HR: 0.81; 95% CI: 0.57 to 1.15). In contrast, the risk of death from infection was almost doubled in women compared with men (crude HR: 1.92; 95% CI: 1.15 to 3.20), a finding that held true after multivariate adjustment for age, primary renal disease, period of inclusion, and initial PD modality (adjusted HR: 1.76; 95% CI: 1.03 to 3.01). This result was confirmed even taking into consideration the competing events of kidney transplantation and transfer to hemodialysis.
♦ Conclusions: Compared with men starting PD, women starting PD are at higher risk of mortality from infection. More stringent screening measures and corrective efforts in women might be indicated.
Keywords: Hormones, sex, gender, female, male
Chronic dialysis patients have a markedly increased mortality rate, with approximately 20% of both hemodialysis (HD) and peritoneal dialysis (PD) patients dying annually (1). The increase is a result of a generally exacerbated mortality risk from both cardiovascular disease (CVD) and non-CVD causes of death (2). Together, CVD and infections account for up to 70% of all deaths in dialysis patients (3).
At the general population level, women have a longer life expectancy and lower incidence of CVD than men do (4,5), but end-stage renal disease cancels this survival advantage in women (6). Consequently, and unlike the case with other diseases and across the general population, men and women die at equal rates when starting dialysis (7,8).
In a study based on the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) registry, we recently demonstrated that, despite equal all-cause mortality rates in men and women initiating dialysis, specific causes of death differed (9), making it important to identify and potentially target sex-specific risks (10). Specifically, compared with men, incident dialysis women had an increased non-CVD mortality risk (9). Among the non-CVD problems associated with dialysis therapy, infections (mainly in the forms of peritonitis and septicemia) are important influencers of survival, technique failure, and transplantation rates in PD patients (3,11,12). Against this background, we set out in the present study to study sex-specific outcomes in a large cohort of incident PD patients, with an emphasis on fatal infections.
Methods
Study Population
The study cohort consisted of incident PD patients from SICATA (the information system of the Andalusian Transplant Autonomic Coordination Registry) who started PD between 1 January 1999 and 31 December 2010. The registry includes all 12 PD units in Andalusia: Almería (Torrecárdenas Hospital), Cádiz (Puerto Real, Puerta del Mar, and Jerez de la Frontera hospitals), Córdoba (Reina Sofia Hospital), Granada (Virgen de las Nieves and San Cecilio hospitals), Huelva (Juan Ramón Jiménez Hospital), Jaén (Médico-Quirúrgico Hospital), Málaga (Carlos Haya Hospital), and Sevilla (Virgen del Rocío and Virgen de Macarena hospitals). The cohort protocol was approved by the ethics committees of the participating hospitals, and patients gave written consent to send their data to the SICATA registry in an anonymous form. Details concerning the registry, its data collection, and its representativeness have previously been published (13). For the present study, we included all patients who started PD therapy de novo (not coming from a preceding failed kidney transplant or from HD) in Andalusia, with the additional exclusion of pediatric patients (13 years old or younger).
Data Collection
The SICATA registry records basic demographic and clinical data such as age, sex, body mass index, primary kidney disease, glomerular filtration rate, and initial PD modality (continuous ambulatory PD or automated PD). The present analysis was performed using variables that constitute part of the mandatory data reported to the registry. Certain additional variables (such as urea kinetic analysis) are optional data in the registry and were excluded from our analysis because of missing values. We also excluded the Charlson comorbidity index, which was implemented as mandatory data several years after initiation of the registry. Glomerular filtration rate was calculated as the mean of urinary creatinine and urea clearances, adjusted for body surface area.
Follow-Up Data
The SICATA registry records dates and causes of technique failure (transfer to HD), peritonitis episodes, death, and kidney transplantation. Causes of death are registered using ERA-EDTA codes. Cardiovascular mortality was defined as death attributable to myocardial ischemia, infarction, heart failure, cardiac arrest because of other or unknown causes, or cerebrovascular accident (ERA-EDTA codes 11, 14 - 16, 18, and 22). Infectious mortality was defined as death because of peritonitis, lung infection, sepsis or septicemia, tuberculosis, viral infection, or liver infection from hepatitis B or other viral hepatitis (ERA-EDTA codes 39, 100 - 102, 31 - 33, 35, 36, 37, 38, 41, 42). Other non-CVD and noninfectious deaths included suicide or refusal of treatment, withdrawal, cachexia, malignancies, and miscellaneous (ERA-EDTA codes 12, 13, 17, 21, 23 - 29, 43 - 46, 51 - 54, 62 - 64, 66 - 73, 81, 82, and 99 - 102). Unknown (ERA-EDTA code 0) or missing causes of death were defined as “unknown or missing” and were not included in the analysis. Only 6 deaths (1.7% of all deaths) fell into that category. Causes of death were extracted from medical records and were not necessarily confirmed by autopsy.
For the present analysis, patients were followed for a maximum of 5 years from the start of dialysis (day 1) until death or censoring. Censored events included transplantation, transfer to HD, end of follow-up, or end of study (31 December 2010).
Statistical Analysis
Statistical analyses were performed using the Stata statistical software application (version 12: StataCorp LP, College Station, TX, USA). Tests were two-tailed, and p < 0.05 was considered significant. Values are expressed as mean ± standard deviation, median and interquartile range, or a percentage of the total, as appropriate. Comparisons between men and women were tested using the Student t-test or chi-square test.
For the study of incident mortality rates, person-time at risk was calculated for each individual patient as the time between the start of dialysis and censoring or death. Total person-time at risk was calculated as the sum of the individual person-times, allowing for the calculation of all-cause and specific (CVD, infectious, and other non-CVD) mortality rates (per 100 person-years). Incidence rate ratios were calculated to assess differences between men and women by the chi-square test.
Traditional Cox regression models were used to study the associations between sex and cause-specific mortality, reporting hazard ratios (HRs). Patients were censored if transplanted or transferred to HD or at the end of follow-up. Because kidney transplantation, transfer to HD, and death before transplantation are mutually exclusive events (that is, the occurrence of any one prevents the occurrence of the others), traditional Cox regressions may be biased. We therefore used a competing-risks approach to perform two sensitivity analyses, calculating the cumulative incidence of death before kidney transplantation or before the composite of transplantation plus transfer to HD (14). In the first sensitivity analysis, patients were censored if transferred to HD or at the end of follow-up. In the second sensitivity analysis, patients were censored if at the end of follow-up. Data are presented as crude and adjusted sub-HRs, with 95% confidence intervals (CIs). Multivariate adjustments were made for age (per year of increase), period of inclusion (using the period 2005 - 2010 as the reference category), modality of PD (using continuous ambulatory PD as the reference category), and primary renal disease (using glomerulonephritis as the reference category). The primary renal disease adjustment was taken as an indirect proxy for comorbidities (15).
Results
General Description
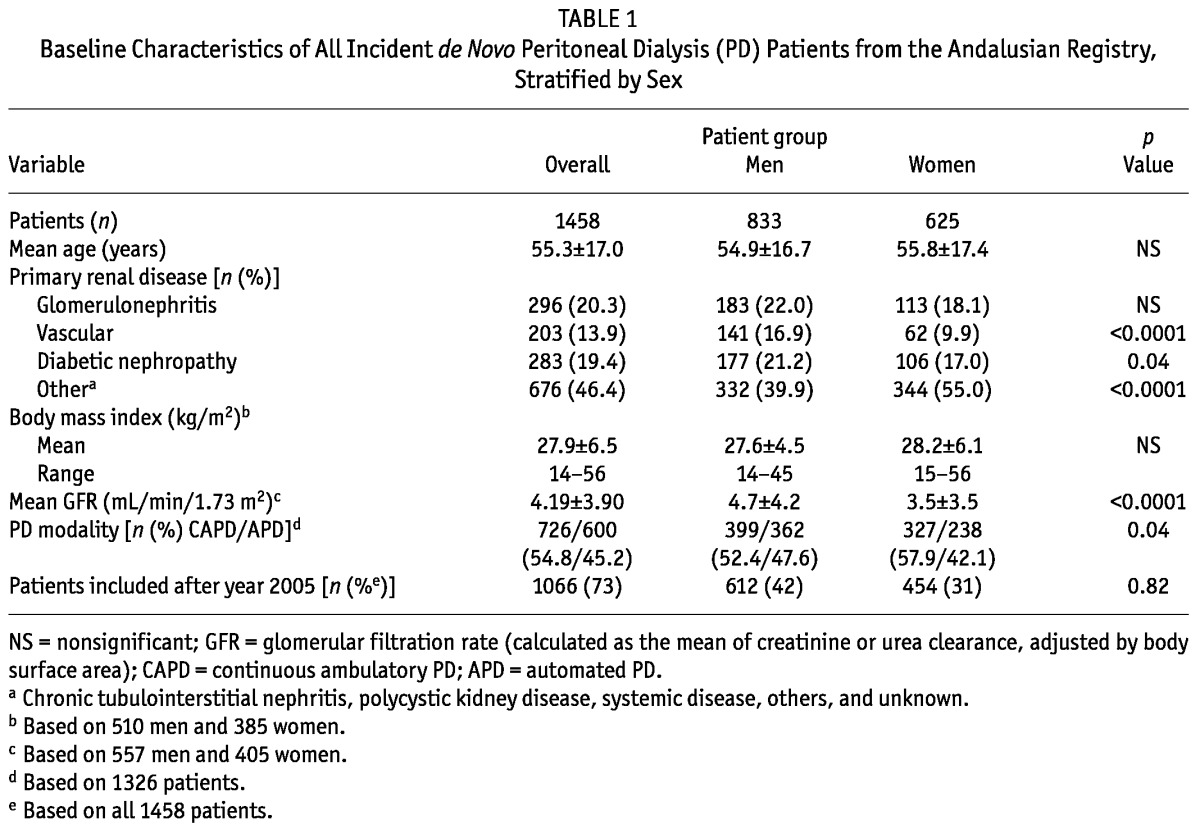
As many as 18 patients (1.2%) recovered renal function after PD initiation and were censored from the subsequent analyses. Table 1 shows a basic description of the remaining 1458 incident patients who started PD therapy between 1999 and 2010 in Andalusia, stratified by sex. Mean age of the patients at the start of dialysis was 55.3 ± 17.0 years (range: 14 - 90 years), with a male sex prevalence of 57.1%. The most common primary renal diseases were chronic glomerulonephritis (20.3%) and diabetic nephropathy (19.4%). In the rest of patients, renal failure had other or unknown causes (46.4%). Men more often had vascular disease or diabetic nephropathy as their primary renal disease. Women more often had other causes of renal disease. At start of PD therapy, the glomerular filtration rate tended to be higher in men than in women. In women, the intention-to-treat PD modality was more often continuous ambulatory PD.
TABLE 1.
Baseline Characteristics of All Incident de Novo Peritoneal Dialysis (PD) Patients from the Andalusian Registry, Stratified by Sex
Follow-Up Data
Table 2 shows the outcomes recorded for the study patients. Mean follow-up time was 21.5 ± 17.5 months. The causes of death were CVD in 145 patients (9.9% of all patients, 41.4% of all deaths), infection in 65 patients (4.5% of all patients, 18.6% of all deaths), and other non-CVD causes in 134 patients (9.2% of all patients, 38.3% of all deaths).
TABLE 2.
Total and Sex-Specific Outcomes and Incidence Rates Recorded During Follow-Up
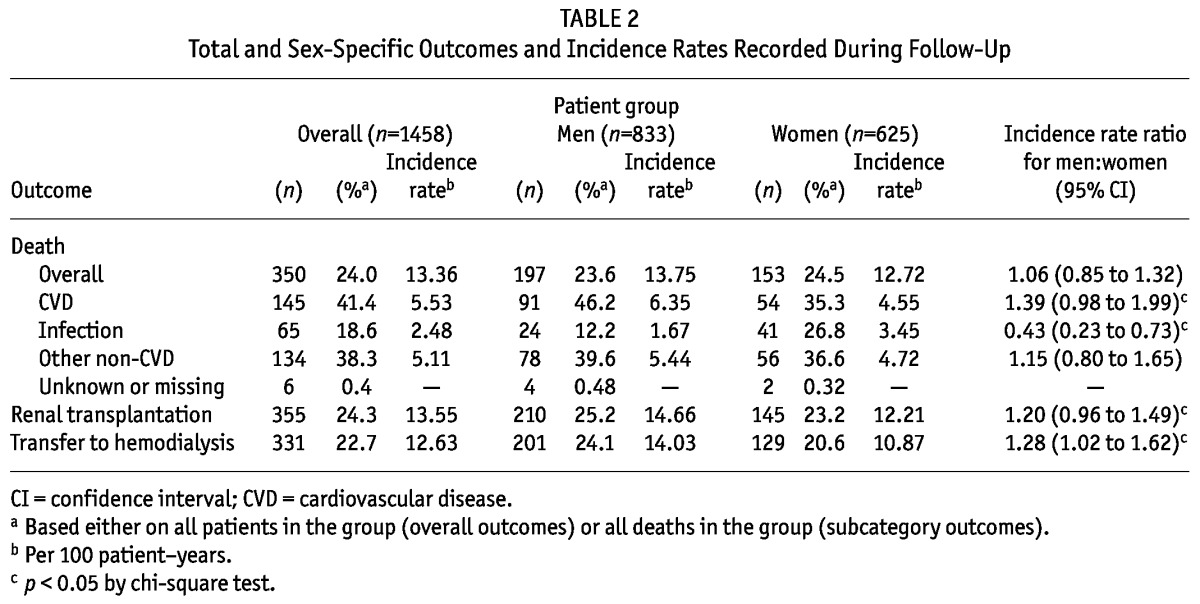
Table 2 also depicts deaths and incident mortality rates stratified by sex. The number of deaths and the all-cause incident mortality rates were similar for the sexes. However, compared with men, women had a significantly higher number of deaths and a higher incident mortality rate for deaths from infection, but fewer CVD deaths and a lower incident CVD mortality rate and lower rates of transfer to HD or renal transplantation.
Hazard Ratios and Relative Risks
Table 3 reports mortality risks by sex (with men as the reference category). The all-cause mortality risk was similar for both sexes (crude HR: 0.90; 95% CI: 0.72 to 1.12). However, with respect to specific causes of death, women showed, before transplantation, a borderline significantly lower risk of CVD (HR: 0.71; 95% CI: 0.50 to 0.99) and a similar risk of non-CVD mortality from other than infection (HR: 0.81; 95% CI: 0.57 to 1.15). Conversely, the risk of death from infection was almost doubled in women (HR: 1.92; 95% CI: 1.15 to 3.20) compared with men. In multivariate analysis, adjustment for potential confounders abrogated the association between female sex and CVD mortality, but the increased risk of death from infection remained (adjusted HR: 1.76; 95% CI: 1.03 to 3.01).
TABLE 3.
Crude and Adjusted Cause-Specific Mortality Risks Using Traditional and Competing-Risk Models in Incident de Novo Peritoneal Dialysis (PD) Patients
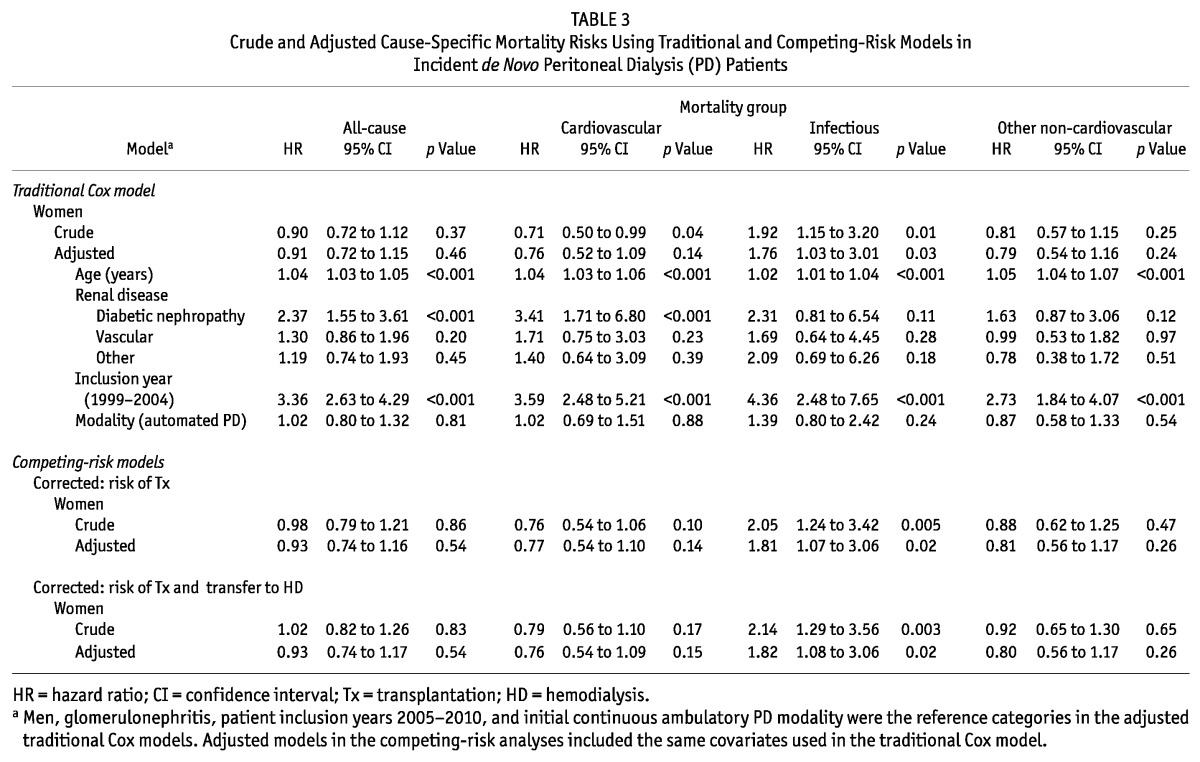
Table 3 also shows the output of sensitivity analyses using competing-risk models to take into account the competing event of renal transplantation or the composite competing event of transplantation or transfer to HD. In both cases, the results were similar.
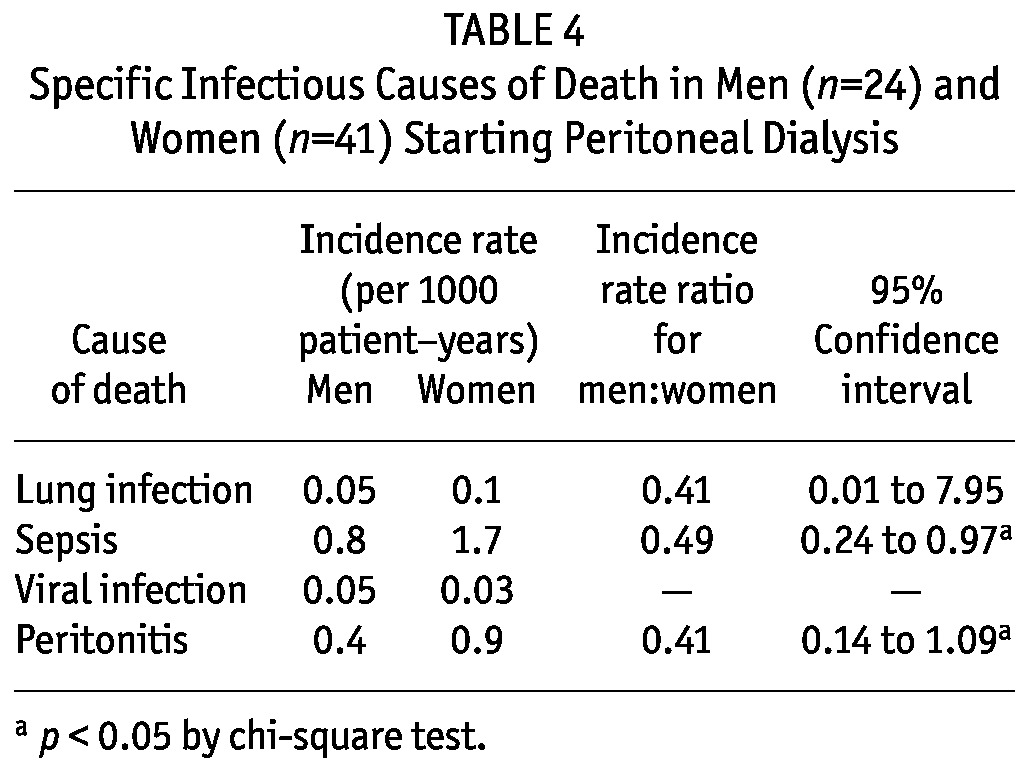
Table 4 shows specific causes of infectious death. The incidence rates for peritonitis and sepsis were significantly higher in women than in men.
TABLE 4.
Specific Infectious Causes of Death in Men (n=24) and Women (n=41) Starting Peritoneal Dialysis
Discussion
Our study shows that, although overall mortality is similar between the sexes, causes of death differ for men and women starting PD. Specifically, women are at higher risk of mortality from infection, expanding our previous observations in the ERA-EDTA registry (9). Overall, our results show that, in both dialysis modalities, women are at increased risk of fatal infection. Those findings might suggest that stringent screening measures and corrective efforts should be applied in women and that there is a need to better understand the reasons behind this increased susceptibility.
In a European population, de Jager et al. (2) reported that the proportions of CVD and non-CVD mortality in patients starting dialysis were 44% and 56% respectively. Those proportions agree with those observed in our cohort. Infections are the second most frequent cause of morbidity and mortality in dialysis patients (1,16-19) and, in our study, accounted for 18.6% of total deaths. Factors potentially contributing to susceptibility to infection include altered host defense system because of uremia, advanced age, comorbid conditions such as diabetes mellitus, the dialysis procedure itself, the transcutaneous access necessary for both HD and PD, and poor nutrition (1,3,16-19). Our analysis identifies female sex as a novel factor predisposing to this problem, with women being almost twice as likely as men to die from infection.
Complementing our findings, the report of Kotsanas et al. (20) showed that the risk of peritonitis is increased by a factor of 2 in women compared with men, and Pérez Fontan et al. (11) reported a higher incidence of severe peritonitis, but not all-cause peritonitis, in women. It has been proposed that infections ascending from genitourinary tract, which in women are caused mostly by gram-negative bacilli, might explain this higher-severity peritonitis (20).
An alternative explanation for our findings might be sex-specific differences in the immune system response to infections. In general, female sex hormones strengthen the immune response, and estrogen is considered a proinflammatory mediator—for instance, increasing the production of tumor necrosis factor, interacting with the interferon γ promoter, enhancing antigen-specific CD4+ T-cell response, and inducing the T helper 1 inflammatory response (21,22). Those observations might explain why women in general have higher incidences of autoimmune diseases and also conditions that lead to chronic kidney disease (23). However, a general state of hypogonadism prevails among uremic patients, and most women undergoing dialysis are in the postmenopausal age range (10). Among others, prolactin retention further inhibits sex hormone production (24), with estrogen and estradiol levels both being much lower in women on dialysis than in the general population (25,26). Taken together, the foregoing factors would promote a lower immune response in women undergoing dialysis that might explain the observed increased risk of fatal infections. In support of that hypothesis, the incidence rates of fatal peritonitis and sepsis were significantly higher in women compared with men. However, we must acknowledge that our sample size was relatively small to specifically address that issue. In contrast, a slightly lower incidence rate of transfer to HD was observed for women compared with men in our study, which may seem counterintuitive, because peritonitis is an important cause of transfer to HD. Nevertheless, a sensitivity analysis correcting for the competing risk of technique failure did not modify our results.
Some limitations should be taken into consideration when interpreting these results. First, the observational nature of our analysis does not allow for conclusions about causality. Second, there are several problems inherent to large registries and data reporting. For instance, mortality data were collected from medical history and death records, which may convey bias that probably lowers the actual associations or even introduce spurious findings. However, the fact that our results accord with previous observations in the ERA-EDTA registry (9) may be suggestive of a true finding. We also do not have detailed data concerning peritonitis rates and severity, which would have been interesting to analyze in relation to sex. Third, the limited set of variables does not allow us to control for all potential confounders. The strengths of our analysis are the relatively large sample size of incident de novo PD patients and the use of competing-risk models to control for high rates of kidney transplantation and transfer to HD. Overall, we are limited by the observational nature of the study and residual confounding, and thus our data should be regarded as hypothesis-generating, warranting replication. If confirmed, further investigation concerning the underlying mechanisms behind our observation would be warranted.
Conclusions
Altogether, our study emphasizes that the outcomes of chronic kidney disease are different in men and women, and consequently that targeted sex-specific care is likely necessary. Stringent screening measures and corrective efforts with respect to infection may be indicated in women on dialysis.
Disclosures
Baxter Novum is the result of a grant from Baxter Healthcare Corporation to Karolinska Institutet. BL is employed by Baxter Healthcare Corporation. SR was supported by a Progress and Health Foundation grant for a short-term stay at the Divisions of Renal Medicine and Baxter Novum in Sweden. JJC acknowledges grant support from the Swedish Research Council, the Kvinnor och Hälsa, and the Westman Foundation.
Acknowledgments
We acknowledge the patients and centers contributing to the Andalusian registry, and we are especially grateful to the people contributing to data collection: J.M. Gil Cunquero, N. Areste Fosalba, A. Ruiz Fernández, D. Toran Monserrat, F. Tejuca Marenco, M.J. Espigares Huete, E. Martínez Benavides, L. González Burdiel, F. Fernández Girón, and F.J. Camacho Guerrero. We are also grateful to SICATA for database management and coordination.
References
- 1. Kato S, Chmielewski M, Honda H, Pecoits-Filho R, Matsuo S, Yuzawa Y, et al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol 2008; 3:1526–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Jager DJ, Grootendorst DC, Jager KJ, van Dijk PC, Tomas LM, Ansell D, et al. Cardiovascular and noncardiovacular mortality among patients starting dialysis. JAMA 2009; 302:1782–9 [DOI] [PubMed] [Google Scholar]
- 3. Sarnak MJ, Jaber BL. Mortality caused by sepsis in patients with end-stage renal disease compared with the general population. Kidney Int 2000; 58:1758–64 [DOI] [PubMed] [Google Scholar]
- 4. Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet 1997; 349:1269–76 [DOI] [PubMed] [Google Scholar]
- 5. Kardys I, Vliegenthart R, Oudkerk M, Hofman A, Witteman JC. The female advantage in cardiovascular disease: do vascular beds contribute equally? Am J Epidemiol 2007; 166:403–12 [DOI] [PubMed] [Google Scholar]
- 6. Turin TC, Tonelli M, Manns BJ, Ravani P, Ahmed SB, Hemmelgarn BR. Chronic kidney disease and life expectancy. Nephrol Dial Transplant 2012; 27:3182–6 [DOI] [PubMed] [Google Scholar]
- 7. Carrero JJ, de Mutsert R, Axelsson J, Dekkers OM, Jager KJ, Boeschoten EW, et al. Sex differences in the impact of diabetes on mortality in chronic dialysis patients. Nephrol Dial Transplant 2011; 26:270–6 [DOI] [PubMed] [Google Scholar]
- 8. Villar E, Remontet L, Labeeuw M, Ecochard R. Effect of age, gender, and diabetes on excess death in end-stage renal failure. J Am Soc Nephrol 2007; 18:2125–34 [DOI] [PubMed] [Google Scholar]
- 9. Carrero JJ, de Jager DJ, Verduijn M, Ravani P, De Meester J, Heaf JG, et al. Cardiovascular and noncardiovascular mortality among men and women starting dialysis. Clin J Am Soc Nephrol 2011; 6:1722–30 [DOI] [PubMed] [Google Scholar]
- 10. Carrero JJ. Gender differences in chronic kidney disease: underpinnings and therapeutic implications. Kidney Blood Press Res 2010; 33:383–92 [DOI] [PubMed] [Google Scholar]
- 11. Pérez Fontan M, Rodríguez-Carmona A, García-Naveiro R, Rosales M, Villaverde P, Valdés F. Peritonitis-related mortality in patients undergoing chronic peritoneal dialysis. Perit Dial Int 2005; 25:274–84 [PubMed] [Google Scholar]
- 12. Jager KJ, Merkus MP, Dekker FW, Boeschoten EW, Tijssen JG, Stevens P, et al. Mortality and technique failure in patients starting chronic peritoneal dialysis: results of the Netherlands Cooperative Study on the Adequacy of Dialysis. Kidney Int 1999; 55:1476–85 [DOI] [PubMed] [Google Scholar]
- 13. Remón Rodríguez C, Quirós Ganga PL, Gil Cunquero JM, Ros Ruiz S, Aresté Fosalba N, Ruiz Fernández A, et al. Ten years of peritoneal dialysis in Andalusia (1999-2008): epidemiologic data, types of treatment, peritonitis, comorbidity and survival in patients and technique. Nefrologia 2010; 30:46–53 20098470 [Google Scholar]
- 14. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Am J Stat Assoc 1999; 94:496–509 [Google Scholar]
- 15. van Manen JG, van Dijk PC, Stel VS, Dekker FW, Clèries M, Conte F, et al. Confounding effect of comorbidity in survival studies in patients on renal replacement therapy. Nephrol Dial Transplant 2007; 22:187–95 [DOI] [PubMed] [Google Scholar]
- 16. Powe NR, Jaar B, Furth SL, Hermann J, Briggs W. Septicemia in dialysis patients: incidence, risk factors, and prognosis. Kidney Int 1999; 55:1081–90 [DOI] [PubMed] [Google Scholar]
- 17. Foley RN. Infectious complications in chronic dialysis patients. Perit Dial Int 2008; 28(Suppl 3):S167–71 [PubMed] [Google Scholar]
- 18. O’Seaghdha CM, Foley RN. Septicemia, access, cardiovascular disease, and death in dialysis patients. Perit Dial Int 2005; 25:534–40 [PubMed] [Google Scholar]
- 19. Bloembergen WE, Port FK, Mauger EA, Wolfe RA. Causes of death in dialysis patients: racial and gender differences. J Am Soc Nephrol 1994; 5:1231–42 [DOI] [PubMed] [Google Scholar]
- 20. Kotsanas D, Polkinghorne KR, Korman TM, Atkins RC, Brown F. Risk factors for peritoneal dialysis-related peritonitis: can we reduce the incidence and improve patient selection? Nephrology (Carlton) 2007; 12:239–45 [DOI] [PubMed] [Google Scholar]
- 21. Snider H, Lezama-Davila C, Alexander J, Satoskar AR. Sex hormones and modulation of immunity against leishmaniasis. Neuroimmunomodulation 2009; 16:106–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McClelland EE, Smith JM. Gender specific differences in the immune response to infection. Arch Immunol Ther Exp (Warsz) 2011; 59:203–13 [DOI] [PubMed] [Google Scholar]
- 23. Silbiger S, Neugarten J. Gender and human chronic renal disease. Gend Med 2008; 5(Suppl A):S3–10 [DOI] [PubMed] [Google Scholar]
- 24. Carrero JJ, Kyriazis J, Sonmez A, Tzanakis I, Qureshi AR, Stenvinkel P, et al. Prolactin levels, endothelial dysfunction, and the risk of cardiovascular events and mortality in patients with CKD. Clin J Am Soc Nephrol 2012; 7:207–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kramer HM, Curhan G, Singh A. on behalf of the HELP Study Group. Hemodialysis and Estrogen Levels in Postmenopausal (HELP) patients: the Multicenter HELP Study. Am J Kidney Dis 2003; 41:1240–6 [DOI] [PubMed] [Google Scholar]
- 26. Weisinger JR, Bellorin-Font E. Outcomes associated with hypogonadism in women with chronic kidney disease. Adv Chron Kidney Dis 2004; 11:361–70 [PubMed] [Google Scholar]


