Abstract
♦ Background: Encapsulating peritoneal sclerosis (EPS) is a rare but devastating complication of peritoneal dialysis (PD), with clinical signs of abdominal pain, bowel obstruction, and weight loss in late stages.
♦ Methods: We retrospectively analyzed all patients who were diagnosed with EPS between March 1998 and October 2011 in our department of nephrology. We focused on the 24 EPS patients who underwent surgery because of symptomatic late-stage EPS. We identified 3 different macroscopic phenotypes of EPS that we categorized as types I - III. We correlated histologic findings with those macroscopic phenotypes of EPS. The postoperative and long-term outcomes were evaluated by macroscopic phenotype.
♦ Results: Duration of PD was longer in type III than in types I and II EPS (p = 0.05). We observed no other statistically significant differences between the groups in baseline characteristics, except for operation time, which was longer in the type I than in the type III group (p = 0.02). Furthermore, we observed no statistically significant difference between the groups with respect to the onset of complaints before surgery (7.8 ± 5.9 months vs 7.0 ± 7.0 months vs 6.5 ± 5.3 months). Concerning patient outcomes, there was no evidence that any of the macroscopic EPS types was associated with more major or minor complications after surgery. For all study patients, follow-up was at least 3 years, with 19 patients still being alive, and 16 having no or very mild complaints. The typical histologic findings of EPS were present in all macroscopic types; only fibrin deposits were more prominent in type II than in type III.
♦ Conclusions: We describe 3 subtypes of EPS based on macroscopic findings. Postoperative treatment should probably not be influenced by the macroscopic EPS phenotype. Whether the different phenotypes represent different pathophysiologic processes remains unclear and has to be further evaluated.
Keywords: Encapsulating peritoneal sclerosis, macroscopic phenotype
Encapsulating peritoneal sclerosis (EPS) is a rare but devastating complication of peritoneal dialysis (PD), with clinical signs of abdominal pain, bowel obstruction, and weight loss in late stages. Exudations of fibrin and chronic inflammation of the peritoneal membrane lead to adhesions and the development of a fibrous cocoon covering the intestines, causing further symptoms of bowel obstruction (1,2). Earlier stages of the disease are difficult to detect, and the clinical symptoms and histologic findings are nonspecific. Changes in transport status and ultrafiltration failure are common (3). The incidence of EPS increases with time on PD, peritonitis rate, male sex, younger age, smoking, and glucose exposure (3-11). The clinical features result from underlying pathogenic processes, particularly ileus, inflammation, and peritoneal adhesions (12).
Encapsulating peritoneal sclerosis can occur when a patient is still on PD, but often becomes apparent after renal transplantation or a switch to hemodialysis (13-17). The three diagnostic hallmarks of EPS are clinical symptoms, radiologic findings, and histologic criteria (18).
Medical and surgical treatment options for EPS are still under debate. Data concerning medical treatment options for the disease are limited (19,20). Patients in the advanced stages of EPS, with signs of bowel obstruction, should undergo surgery. Whether surgery should be performed in earlier stages of the disease is still not well established. Overall morbidity and mortality in EPS remain high (25% - 55%), and the recurrence rate in the first year after diagnosis is 18% (3,4,13,14,16). In patients with advanced disease, enterolysis and peritonectomy (PEEL) are the surgical options of choice (21-23).
We retrospectively analyzed patients who were diagnosed with EPS between March 1998 and October 2011 in our department of nephrology. We focused on the 24 EPS patients who underwent major surgery with PEEL for late-stage EPS. We identified 3 different macroscopic phenotypes of EPS, and we correlated histologic findings with those macroscopic types. We also analyzed postoperative and long-term outcomes by macroscopic phenotype.
Methods
In 24 patients with clinically, radiologically, and histologically proven advanced-stage EPS, PEEL was conducted with the intent to eliminate the typical chronic small-intestinal ileus. For diagnosis, we used the clinical criteria set out by Nakamoto et al. (12), the radiologic criteria set out by Vlijm et al. (24), and the histologic criteria set out by Braun et al. (5) and Honda et al. (25).
Only patients who underwent major surgery with the intent to eliminate ileus were included. Patients who underwent procedures such as biopsy or surgery for other reasons were excluded. All patients had given informed consent for a scientific work-up of tissues taken during surgery.
Histopathologic Analysis
Biopsies obtained from the peritoneum in the 24 late-stage EPS patients were formalin-fixed and paraffin-embedded using routine protocols (5). Each slide underwent hematoxylin and eosin staining for morphology analysis:
Fibrosis: absent (score 0); 1% - 10% per low-power field [LPF (3.2 mm2)] (score 1); 11% - 50% per LPF (score 2); >51% per LPF (score 3)
Fibroblast-like cells: absent (score 0); 1 in 5 high-power fields [HPFs (0.26 mm2)] (score 1); 2 - 4 in 5 HPFs (score 2); >5 in 5 HPFs (score 3)
Exudation: absent (score 0); 1 small area in 1 medium-power field [MPF (0.91 mm2)] (score 1); area < 50% in 1 MPF (score 2); area > 50% in 1 MPF (score 3)
Cellularity: 1 - 2 nuclei per HPF (score 0); 3 - 5 nuclei per HPF (score 1); 6 - 20 nuclei per HPF (score 2); >20 nuclei per HPF (score 3)
Vessel density (in the submesothelial cell layer): absent (score 0); 1 - 5 per HPF (score 1); 6 - 10 per HPF (score 2); >10 per HPF (score 3)
Acute inflammation (neutrophils): absent (score 0); 1 per HPF (score 1); 2 - 5 per HPF (score 2); >5 per HPF (score 3)
Chronic inflammation (round cells): absent (score 0); 1 - 5 per HPF (score 1); 6 - 20 per HPF (score 2); >20 per HPF (score 3)
Hemorrhage (extravasal erythrocytes): absent (score 0); 1 area < 10% per 5 LPFs (score 1); 2 + 3 areas per 5 LPFs or 1 area of 11% - 30% per LPF (score 2); 4 + 5 areas per 5 LPFs or 1 area of >30% per LPF (score 3)
Fibrin deposits (eosinophilic area): absent (score 0); 1 area < 5% per 5 MPFs (score 1); 1 area 6% - 20% per 5 MPFs (score 2); 1 area > 20% per 5 MPFs (score 3)
Presence of vasculopathy: thickening of vessel walls or inflammation of the vessel wall, or both (score 0 or 1)
Mesothelial denudation: no visible mesothelium (score 0 or 1)
Presence of acellular areas (score 0 or 1)
Fibroblast-like cells were defined as elongated cells separated from vessel lumen, with a vesicular nucleus and 1 - 3 nucleoli. Acute inflammatory reaction was defined as the presence of neutrophilic granulocytes. Chronic inflammatory reaction was defined as the presence of round cells, without taking into consideration further subclasses such as lymphocytes, plasma cells, monocytes, and histiocytes.
Because of the small sample size in each group, we reduced the initial 4-step scale to a 2-step scale. This approach compares only very severe histologic changes with less severe changes and simplifies the histologic analysis.
Follow-Up, Outcomes, and Surgical Complications
Baseline for the present study was 1 December 2011. Physicians, either in person or during telephone contact, reviewed the outcomes of all EPS patients. Current complaints were requested and, for comparison, were analyzed on a scale of 0, no complaints; 1, rare complaints; 2, frequent complaints; and 3, continuous complaints throughout the day.
Additionally, the onset of complaints related to EPS (abdominal pain, bowel obstruction, weight loss, anorexia) was evaluated retrospectively.
Complications were categorized into minor complications resolved without surgical or radiologic intervention (for example, wound infection, postoperative small-bowl paralysis) and major complications requiring surgical intervention (for example, small-bowl fistulas, severe postoperative bleeding, or complete wound rupture).
Statistical Analysis
Two observers blinded to the diagnosis performed the histopathologic analysis. Variables were classified as either binary (present or absent) or ordinal. The ordinal variables were discriminated as absent (score 0), or low- (score 1), moderate- (2), or high-grade (score 3).
All data were processed using the GraphPad Prism software program (version 5: La Jolla, CA, USA). Comparisons between the groups were made using analysis of variance and the Fisher exact test. Significance was defined at the level of p < 0.05, with p < 0.01 indicating a high level of significance, and p < 0.001 being highly significant. “Trend” was defined as a p value between 0.05 and 0.10. Data are reported as mean ± standard deviation unless otherwise specified.
Results
For the 24 EPS patients who required major surgery with PEEL, the intraoperative situs was assigned to 1 of 3 macroscopic EPS categories. Type I is peritoneum with a sticky fibrin coating on top of the EPS membranes containing the brown and thickened peritoneum, with rare inter-enteric sclerotic membranes [Figure 1(A)]. Type II is characterized by a resoluble cobweb-like inter-enteric sclerotic cover on top of the brown EPS membrane [Figure 1(B)]. Type III is the classic type, with intestinal cocooning and a sclerotic capsule enclosing the whole intestine, having a tendency of shrinking and being accompanied by inter-enteric sclerotic capsules [Figure 1(C)].
Figure 1 —
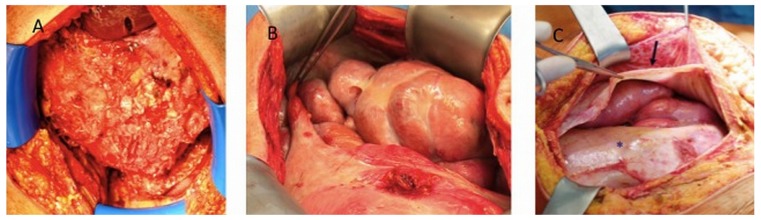
(A) In type I encapsulating peritoneal sclerosis (EPS), a sticky fibrin coating lies on top of the EPS membranes containing the brown and thick peritoneum, with inter-enteric sclerotic membranes. (B) Type II EPS is characterized by a cobweb-like inter-enteric sclerotic cover. (C) Type III EPS is the classic intestinal cocooning in which a sclerotic capsula encloses the entire intestine, with a tendency to shrink (arrow).
Macroscopic type I EPS was diagnosed in 10 patients, type II in 5, and type III in 9. All patients fulfilled the computed tomography criteria (24) and clinical criteria (12) for EPS. Table 1 summarizes the clinical data for the patient cohort. No statistically significant differences in baseline characteristics were evident between the groups. The duration of PD was longer in patients with type III than with types I and II EPS, and each group included only 1 woman (Table 2). Because of the “inflammatory” nature of type I, we analyzed episodes of peritonitis in the groups. We observed no differences in the number of episodes of peritonitis or in the organisms responsible (Table 2). Additionally, no correlation between the timing of peritonitis and the requirement for major surgery were evident in patients with type I EPS.
TABLE 1.
Clinical Data for the Study Patients by Macroscopic Type of Encapsulating Peritoneal Sclerosis (EPS)
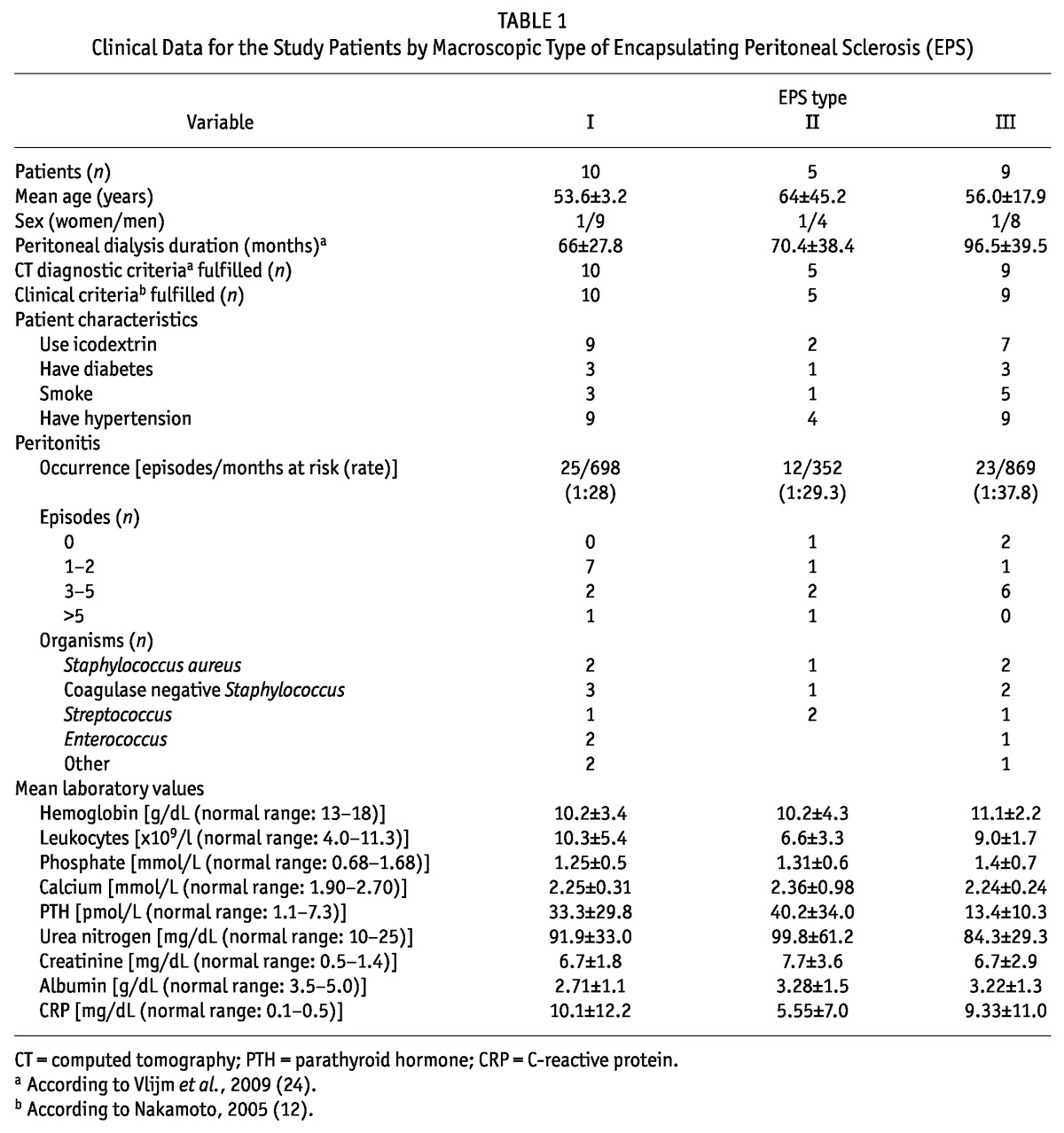
TABLE 2.
Onset of Complaints and Outcome After Surgery by Macroscopic Type of Encapsulating Peritoneal Sclerosis (EPS)
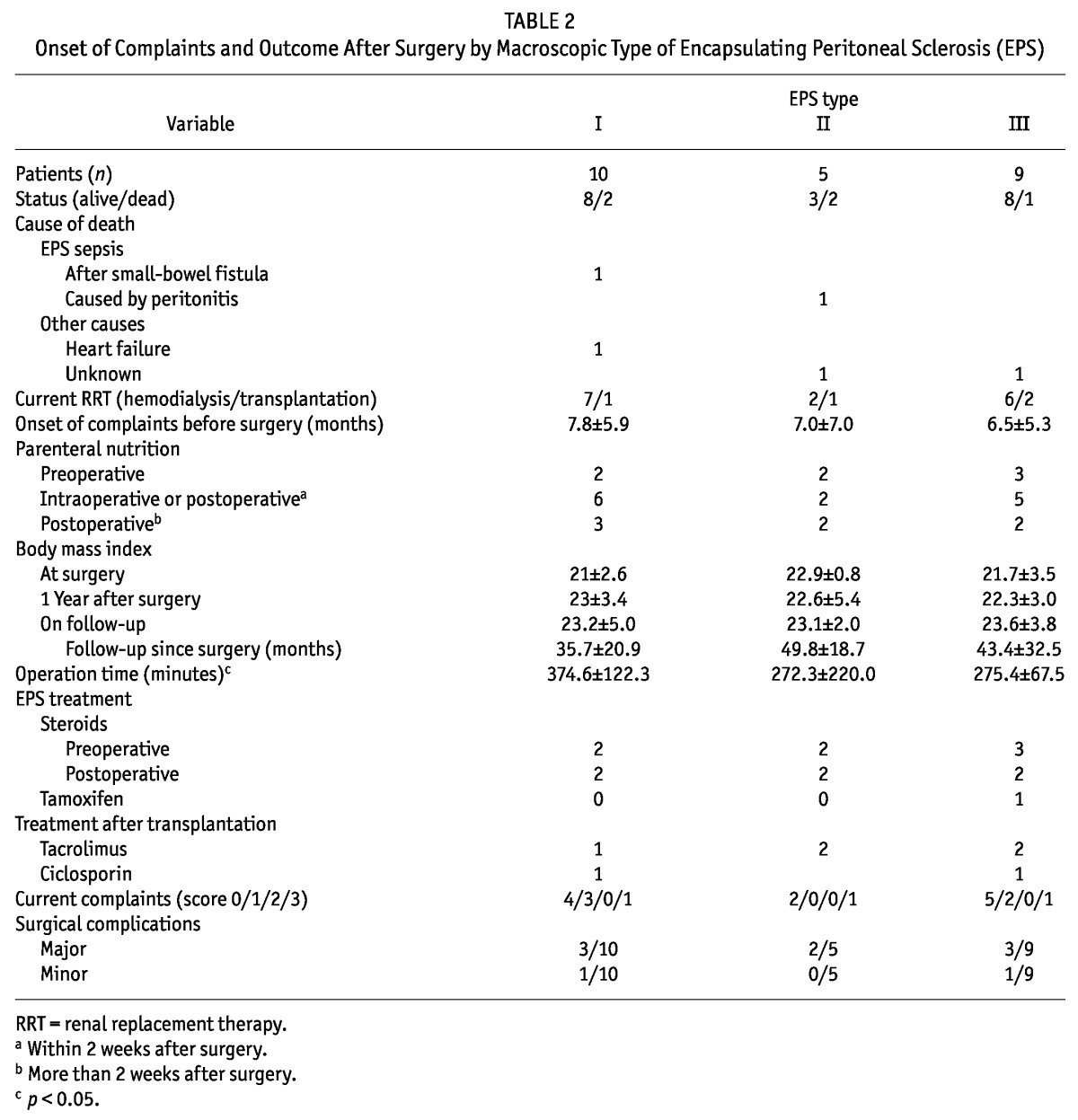
The proportions of smokers and patients with hypertension were not different between the groups. Levels of C-reactive protein were markedly elevated in all EPS types, but were not significantly different between the groups (p < 0.05). No correlation could be found between computed tomography findings and macroscopic phenotype.
Furthermore, we observed no statistically significant difference between the groups with respect to the onset of complaints before surgery (7.8 ± 5.9 months vs 7.0 ± 7.0 months vs 6.5 ± 5.3 months) or to major (3 vs 2 vs 3) or minor surgical complications (1 vs 0 vs 1). Operation time was significantly longer in type I than in type III patients (p = 0.02). Most of the patients who had major complications after surgery developed small-bowel fistulas and had to undergo another operation.
We observed no differences between the groups in parenteral nutrition or in body mass index at time of diagnosis or of follow-up. Additionally, treatment with steroids and immunosuppressive drugs was not different between the groups.
Outcome after surgery and time from onset of complaints to surgery could not be analyzed in 5 patients because they died too close to baseline. Neither the duration of follow-up after surgery nor current complaints were statistically significantly different between the groups (Table 2). Only 1 patient in each group expressed moderate or severe complaints associated with EPS throughout the day. The most frequent current complaints were nausea, diarrhea, and abdominal pain.
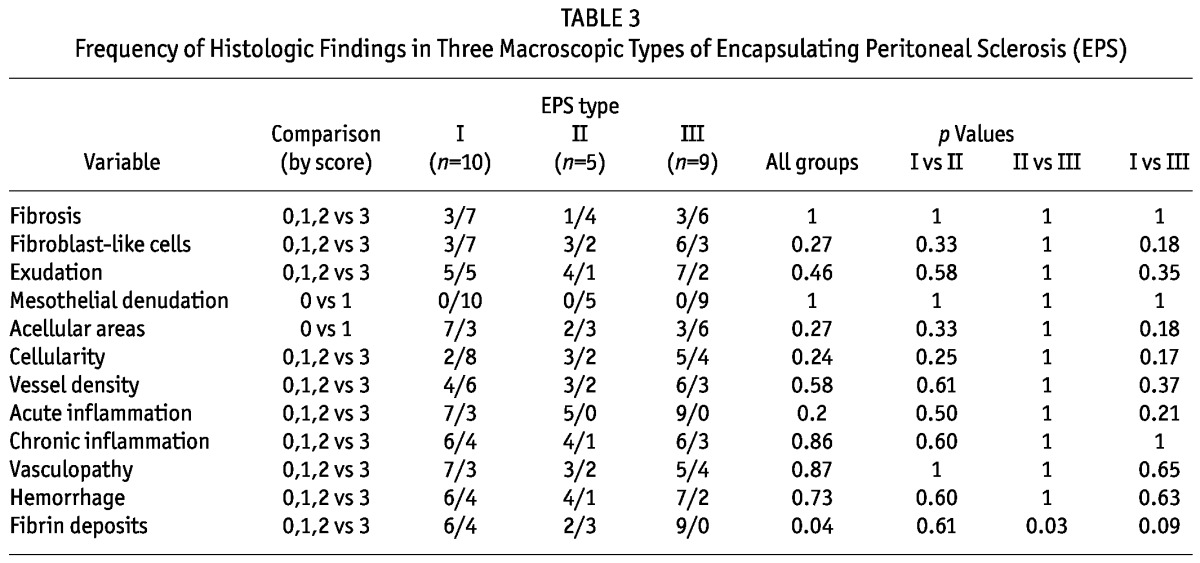
Table 3 summarizes the frequency of histologic findings in the different macroscopic EPS types. All the typical histologic findings of EPS were analyzed and were present in all 3 EPS types. Denudation was present in all biopsies of peritoneum. Fibrin deposits were more pronounced in EPS type II than in EPS type III, and we observed a trend toward more pronounced fibrin deposition in type I than in type III. The other histologic findings were not significantly different between or within the various macroscopic EPS types (Table 3).
TABLE 3.
Frequency of Histologic Findings in Three Macroscopic Types of Encapsulating Peritoneal Sclerosis (EPS)
Discussion
We identified 3 different macroscopic phenotypes of EPS and hypothesized that they might influence outcome. We therefore correlated the macroscopic types with histologic, radiologic, and clinical parameters and also with the outcome of EPS.
Type I EPS is a very active macroscopic pattern with inflammation, exudations, ascites, and adhesions. Type III EPS shows the typical cocooning with sparse exudations of the sclerotic capsula, and type II is somewhat between types I and III. One obvious potential explanation for these different macroscopic characteristics is that these phenotypes are various stages in the same pathologic process (from an inflammatory type I to the fibrotic type III). Adding past medical history, especially the duration from onset of complaints to surgery, we observed no differences between the macroscopic types of EPS, suggesting that the 3 types are not different stages of the same pathologic process, but are different presentations of the same disease.
Time on PD was somewhat longer in type III than in types I and II EPS, but the difference was not statistically significant (p = 0.05). Those findings might have two explanations. The first is that the progression through the stages might be fast—and variable from patient to patient—obscuring the differences within the groups. A second explanation is that the course of EPS has different pathophysiologic processes, which of course requires further investigation in larger cohorts.
We hypothesized that, compared with type III EPS, type I may be a more “inflammatory” type of the disease. Levels of C-reactive protein were markedly elevated in all types of EPS, which accords with the histologic findings of acute and chronic inflammation. The expected increased infiltration of inflammatory cells in type I compared with type III EPS could not be identified. Additionally, there were no differences in the number of episodes and the organisms responsible for peritonitis in the various groups, and no correlation between the timing of peritonitis and the requirement for major surgery in type I disease.
We compared histologic findings in the three groups, expecting decreased cellularity and pronounced fibrosis in type III compared with type I EPS, and more fibrin deposits and inflammation in type I compared with type III EPS. Inflammatory cells, cellularity, and fibrosis were not significantly different between the groups. Fibrin deposition was significantly different between the groups. None of the patients with type III disease showed pronounced fibrin deposition, which accords with the macroscopic appearance. Despite dramatic differences in the macroscopic phenotypes, we found only a weak correlation between the macroscopic and microscopic characteristics, which is in itself an important finding.
Simple fibrosis induced by PD is well described in the literature (26,27). The excessive fibrotic peritoneal thickening in EPS is, in some respects, histologically different from simple fibrosis (5,28). However, we still do not know whether EPS is an independent disease or the result of a long-lasting disease process aggravated by a so-called second hit (29,30).
We also investigated whether there were correlations between macroscopic EPS type and outcomes in our cohort. Outcome during a follow-up of at least 3 years was not different between the three EPS groups. Additionally, we observed no differences between the groups with respect to major or minor surgical complications; however, the time after surgery was statistically longer in type I than in type III EPS.
The surgical procedure in EPS is very challenging, especially in type I, because there is just a diffuse fibrin membrane and not marked capsula covering the intestine. To release the intestine, the membranes and the capsules must be resected. Interestingly, the number of patients who required small-bowel resection because of inseparable conglomerates did not differ between the groups. Additionally, the number of bowel fistulae was not different between the groups. Despite the small number of EPS patients in each group, the foregoing results suggest that the macroscopic EPS type should not influence postoperative treatment, that none of the macroscopic types is associated with more frequent surgical or postoperative complications, and that outcomes are not different between the groups. It must be highlighted that, during the development of EPS, the possible presence of unrecognized risk factors may be more relevant to clinical outcome in late-stage EPS patients after surgery. The current literature provides no data concerning risk factors for recurrence of EPS after major surgery.
Conclusions
Our study is the first to define 3 different macroscopic types of EPS. Whether these different phenotypes represent different pathophysiologic processes remains unclear and requires further evaluation. Currently, there is no evidence that any of the described macroscopic types is associated with a worse postoperative or long-term outcome and should therefore be differently treated.
Disclosures
The authors declare that they have no financial conflicts of interest. They also declare that this manuscript has not been published previously except in abstract form.
Acknowledgments
This work was supported by the Robert-Bosch Foundation and the Sabine Dörges Foundation.
References
- 1. Alscher DM, Braun N, Biegger D, Fritz P. Peritoneal mast cells in peritoneal dialysis patients, particularly in encapsulating peritoneal sclerosis patients. Am J Kidney Dis 2007; 49:452–61 [DOI] [PubMed] [Google Scholar]
- 2. Kawaguchi Y, Kawanishi H, Mujais S, Topley N, Oreopoulos DG. Encapsulating peritoneal sclerosis: definition, etiology, diagnosis, and treatment. International Society for Peritoneal Dialysis Ad Hoc Committee on Ultrafiltration Management in Peritoneal Dialysis. Perit Dial Int 2000; 20(Suppl 4):S43–55 [PubMed] [Google Scholar]
- 3. Brown MC, Simpson K, Kerssens JJ, Mactier RA. Encap su lating peritoneal sclerosis in the new millennium: a national cohort study. Clin J Am Soc Nephrol 2009; 4:1222–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johnson DW, Cho Y, Livingston BE, Hawley CM, McDonald SP, Brown FG, et al. Encapsulating peritoneal sclerosis: incidence, predictors, and outcomes. Kidney Int 2010; 77:904–12 [DOI] [PubMed] [Google Scholar]
- 5. Braun N, Alscher MD, Kimmel M, Amann K, Buttner M. Encapsulating peritoneal sclerosis—an overview. Nephrol Ther 2011; 7:162–71 [DOI] [PubMed] [Google Scholar]
- 6. Guest S. Hypothesis: gender and encapsulating peritoneal sclerosis. Perit Dial Int 2009; 29:489–91 [PubMed] [Google Scholar]
- 7. Korte MR, Sampimon DE, Lingsma HF, Fieren MW, Looman CW, Zietse R, et al. Risk factors associated with encapsulating peritoneal sclerosis in Dutch EPS study. Perit Dial Int 2011; 31:269–78 [DOI] [PubMed] [Google Scholar]
- 8. Martikainen TA, Teppo AM, Gronhagen-Riska C, Ekstrand AV. Glucose-free dialysis solutions: inductors of inflammation or preservers of peritoneal membrane? Perit Dial Int 2005; 25:453–60 [PubMed] [Google Scholar]
- 9. Posthuma N, Verbrugh HA, Donker AJ, van Dorp W, Dekker HA, Peers EM, et al. Peritoneal kinetics and mesothelial markers in CCPD using icodextrin for daytime dwell for two years. Perit Dial Int 2000; 20:174–80 [PubMed] [Google Scholar]
- 10. Brown EA, Van Biesen W, Finkelstein FO, Hurst H, Johnson DW, Kawanishi H, et al. Length of time on peritoneal dialysis and encapsulating peritoneal sclerosis: position paper for ISPD. Perit Dial Int 2009; 29:595–600 [PubMed] [Google Scholar]
- 11. Kawanishi H, Moriishi M. Epidemiology of encapsulating peritoneal sclerosis in Japan. Perit Dial Int 2005; 25(Suppl 4):S14–18 [PubMed] [Google Scholar]
- 12. Nakamoto H. Encapsulating peritoneal sclerosis—a clinician’s approach to diagnosis and medical treatment. Perit Dial Int 2005; 25(Suppl 4):S30–8 [PubMed] [Google Scholar]
- 13. Kawanishi H, Kawaguchi Y, Fukui H, Hara S, Imada A, Kubo H, et al. Encapsulating peritoneal sclerosis in Japan: a prospective, controlled, multicenter study. Am J Kidney Dis 2004; 44:729–37 [PubMed] [Google Scholar]
- 14. Rigby RJ, Hawley CM. Sclerosing peritonitis: the experience in Australia. Nephrol Dial Transplant 1998; 13:154–9 [DOI] [PubMed] [Google Scholar]
- 15. Nomoto Y, Kawaguchi Y, Kubo H, Hirano H, Sakai S, Kurokawa K. Sclerosing encapsulating peritonitis in patients undergoing continuous ambulatory peritoneal dialysis: a report of the Japanese Sclerosing Encapsulating Peritonitis Study Group. Am J Kidney Dis 1996; 28:420–7 [DOI] [PubMed] [Google Scholar]
- 16. Summers AM, Clancy MJ, Syed F, Harwood N, Brenchley PE, Augustine T, et al. Single-center experience of encapsulating peritoneal sclerosis in patients on peritoneal dialysis for end-stage renal failure. Kidney Int 2005; 68:2381–8 [DOI] [PubMed] [Google Scholar]
- 17. Fieren MW, Betjes MG, Korte MR, Boer WH. Posttransplant encapsulating peritoneal sclerosis: a worrying new trend? Perit Dial Int 2007; 27:619–24 [PubMed] [Google Scholar]
- 18. Summers AM, Abrahams AC, Alscher MD, Betjes M, Boeschoten EW, Braun N, et al. A collaborative approach to understanding EPS: the European perspective. Perit Dial Int 2011; 31:245–8 [DOI] [PubMed] [Google Scholar]
- 19. Braun N, Fritz P, Biegger D, Kimmel M, Reimold F, Ulmer C, et al. Difference in the expression of hormone receptors and fibrotic markers in the human peritoneum—implications for therapeutic targets to prevent encapsulating peritoneal sclerosis. Perit Dial Int 2011; 31:291–300 [DOI] [PubMed] [Google Scholar]
- 20. Bozkurt D, Sipahi S, Cetin P, Hur E, Ozdemir O, Ertilav M, et al. Does immunosuppressive treatment ameliorate morphology changes in encapsulating peritoneal sclerosis? Perit Dial Int 2009; 29(Suppl 2):S206–10 [PubMed] [Google Scholar]
- 21. Kawanishi H, Moriishi M, Tsuchiya S. Experience of 100 surgical cases of encapsulating peritoneal sclerosis: investigation of recurrent cases after surgery. Adv Perit Dial 2006; 22:60–4 [PubMed] [Google Scholar]
- 22. Kawanishi H, Moriishi M, Ide K, Dohi K. Recommendation of the surgical option for treatment of encapsulating peritoneal sclerosis. Perit Dial Int 2008; 28(Suppl 3):S205–10 [PubMed] [Google Scholar]
- 23. Kawanishi H, Watanabe H, Moriishi M, Tsuchiya S. Success ful surgical management of encapsulating peritoneal sclerosis. Perit Dial Int 2005; 25(Suppl 4):S39–47 [PubMed] [Google Scholar]
- 24. Vlijm A, Stoker J, Bipat S, Spijkerboer AM, Phoa SS, Maes R, et al. Computed tomographic findings characteristic for encapsulating peritoneal sclerosis: a case-control study. Perit Dial Int 2009; 29:517–22 [PubMed] [Google Scholar]
- 25. Honda K, Nitta K, Horita S, Tsukada M, Itabashi M, Nihei H, et al. Histologic criteria for diagnosing encapsulating peritoneal sclerosis in continuous ambulatory peritoneal dialysis patients. Adv Perit Dial 2003; 19:169–75 [PubMed] [Google Scholar]
- 26. Williams JD, Craig KJ, Topley N, Von Ruhland C, Fallon M, Newman GR, et al. Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol 2002; 13:470–9 [DOI] [PubMed] [Google Scholar]
- 27. Honda K, Nitta K, Horita S, Yumura W, Nihei H. Morphological changes in the peritoneal vasculature of patients on CAPD with ultrafiltration failure. Nephron 1996; 72:171–6 [DOI] [PubMed] [Google Scholar]
- 28. Braun N, Alscher DM, Fritz P, Edenhofer I, Kimmel M, Gaspert A, et al. Podoplanin-positive cells are a hallmark of encapsulating peritoneal sclerosis. Nephrol Dial Transplant 2011; 26:1033–41 [DOI] [PubMed] [Google Scholar]
- 29. Summers AM, Hoff CM, Topley N. How can genetic advances impact on experimental models of encapsulating peritoneal sclerosis? Perit Dial Int 2008; 28(Suppl 5):S16–20 [PubMed] [Google Scholar]
- 30. Honda K, Oda H. Pathology of encapsulating peritoneal sclerosis. Perit Dial Int 2005; 25(Suppl 4):S19–29 [PubMed] [Google Scholar]


