Abstract
♦ Background: Encapsulating peritoneal sclerosis (EPS) is the most serious complication of peritoneal dialysis, having high morbidity and mortality. To improve outcomes, early diagnosis is needed to direct treatment during the early inflammatory phase. However, in the early inflammatory phase, clinical features are nonspecific, and no reliable diagnostic criteria have been established. Because bacterial peritonitis and termination of dialysis are two important risk factors triggering the progression of EPS, patients with refractory bacterial peritonitis necessitating dialysis catheter removal are at particularly high risk of developing EPS. Many of these patients might indeed experience non-resolving sterile peritonitis (probably the inflammatory phase of EPS) before progression to full-blown disease (that is, the presence of intestinal obstruction). We undertook a retrospective study to compare, in this particular situation, the clinical characteristics of patients with or without sterile peritoneal inflammation, assessing their clinical outcomes in terms of short-term mortality and the chance of developing full-blown EPS.
♦ Methods: Our retrospective review included 62 patients whose dialysis catheter was removed because of refractory peritonitis between January 2005 and December 2010.
♦ Results: Of the 62 patients identified, 39 (63%) had persistent sterile peritoneal inflammation (“high-risk” group, n = 39), and 23 (37%) had resolution of inflammation without significant intra-abdominal collection after catheter withdrawal (“control” group, n = 23). Compared with the control group, the high-risk group had a significantly longer PD duration (71.6 ± 43.3 months vs 42.3 ± 29.9 months, p = 0.003), a higher dialysate-to-plasma ratio (D/P) of creatinine (0.768 ± 0.141 vs 0.616 ± 0.091, p = 0.004), and a higher computed tomography score for EPS (7.69 ± 2.98 vs 1.00 ± 1.00, p < 0.001). During the 6-month study period, the high-risk group had a higher chance of developing full-blown EPS (31% vs 0%, p = 0.002) and a higher 6-month all-cause mortality (36% vs 4.3%, p = 0.004).
♦ Conclusions: Persistent sterile peritoneal inflammation was common after dialysis catheter removal for refractory bacterial peritonitis, and the patients with such inflammation were at high risk of progression to full-blown EPS.
Keywords: Catheter removal, encapsulating peritoneal sclerosis, refractory peritonitis, mortality
Encapsulating peritoneal sclerosis (EPS) is a progressive inflammatory condition, leading to peritoneal fibrosis and intestinal encapsulation, with a terminal presentation of intestinal obstruction and malnutrition. It was first reported in a peritoneal dialysis (PD) patient in 1980 (1), and in various series since, its incidence has ranged from 0.8% to 3.3% (2-4). The high morbidity and mortality associated with EPS makes it the most serious complication of PD. Mortality up to 100% has been reported in patients receiving PD for more than 15 years (2-5).
The challenge in managing EPS lies in making an early diagnosis of the condition. In 2005, Nakamoto proposed a staging system for EPS (6), including pre-EPS and inflammatory, encapsulating, and ileus phases. However, clinical features in the early (inflammatory) phase are rather non-specific, which frequently leads to under-diagnosis (6,7). Most available studies refer to a “definite diagnosis” of EPS when intestinal obstruction has already occurred, which is indeed a very late feature. At that point, fibrosis has invariably set in, and medical treatment has become relatively ineffective. Identifying patients at the early inflammatory phase when they are at risk of progression to full-blown EPS (that is, the encapsulating and ileus stages), and following up with timely medical treatment, is therefore of paramount importance to stop disease progression and improve outcomes (6,7).
According to the “two-hit” theory put forward by Honda and Oda (8), withdrawal of PD and bacterial peritonitis (the second “hit”) are important triggers for the onset of EPS, especially in patients with existing peritoneal deterioration because of a long history of PD (the first “hit”). Their theory is supported by the fact that 63% of EPS cases develop within 1 year after PD withdrawal (2), and many of the patients experience non-resolving peritoneal inflammation, with persistent elevation of C-reactive protein (CRP) and the presence of ascites after peritoneal catheter removal for refractory peritonitis (3,9,10). Patients who have undergone catheter removal for refractory bacterial peritonitis are therefore in a unique clinical situation, with the coexistence of two putative “second hit” factors rendering these patients at an exceptional risk of developing EPS.
We undertook a retrospective study to identify patients with persistent sterile peritoneal inflammation after dialysis catheter removal for refractory bacterial peritonitis and to compare their clinical characteristics and outcomes, including short-term all-cause mortality and development of full-blown EPS, with the characteristics of patients without sterile peritoneal inflammation.
Methods
Patient Selection
This single-center retrospective study identified all PD patients who underwent dialysis catheter removal because of refractory peritonitis between January 2005 and December 2010 (n = 87). Among the 87 identified patients, 25 were excluded because of the presence of surgical pathology causing peritonitis (n = 5) or because of non-resolving intra-abdominal sepsis (as evidenced by a positive culture from an intra-abdominal collection) even after dialysis catheter removal and continuation of appropriate antibiotic therapy for a reasonable duration (n = 20). The final analysis included all 62 remaining patients.
During the study period, 605 episodes of peritonitis occurred in 669 patients, for an average peritonitis rate of 1 episode in 31.1 treatment-months. The peritonitis episodes leading to dialysis catheter removal are here called “index episodes.”
The index peritonitis episodes were diagnosed according to International Society for Peritoneal Dialysis (ISPD) diagnostic criteria, which include at least two of
abdominal pain or cloudy PD effluent,
leukocytosis in dialysate (>100/μL), and
positive Gram stain or culture of dialysate.
Initial antibiotic protocols in our unit were devised according to the prevailing ISPD peritonitis guidelines. During the study period, our prevailing initial antibiotic regime was cefazolin plus gentamicin or cefepime alone intraperitoneally. Choice and duration of antibiotics were adjusted according to culture and sensitivity test results once they became available. It was intended that dialysis catheters be removed on day 5 of any peritonitis that failed to respond to appropriate antibiotic therapy, but the actual timing was subject to the clinical condition of individual patients and the availability of a surgical suite. Computed tomography (CT) imaging of abdomen and pelvis were arranged for all patients who underwent dialysis catheter removal for refractory peritonitis to assess for any persistent intra-abdominal collection. The imaging was usually performed at 4 weeks after catheter removal—or earlier depending on the clinical needs of individual patients. For patients who had significant intra-abdominal collections, image-guided abdominal tapping (both diagnostic and therapeutic) was routinely performed whenever possible to rule out ongoing intra-abdominal sepsis. Ongoing inflammation was defined as the presence of an otherwise unexplained abnormal CRP level at about 3 weeks after dialysis catheter removal in the presence of appropriate antibiotic treatment.
During the study period, 39 patients had persistently abnormal CRP levels and significant sterile intra-abdominal collections on CT imaging (digital or printed films) after dialysis catheter removal for refractory bacterial peritonitis, despite at least 3 weeks of appropriate antibiotic treatment after catheter removal (“high-risk” group, n = 39). In 23 patients, CRP rapidly normalized and no intra-abdominal collections were noted after dialysis catheter removal (“control” group, n = 23).
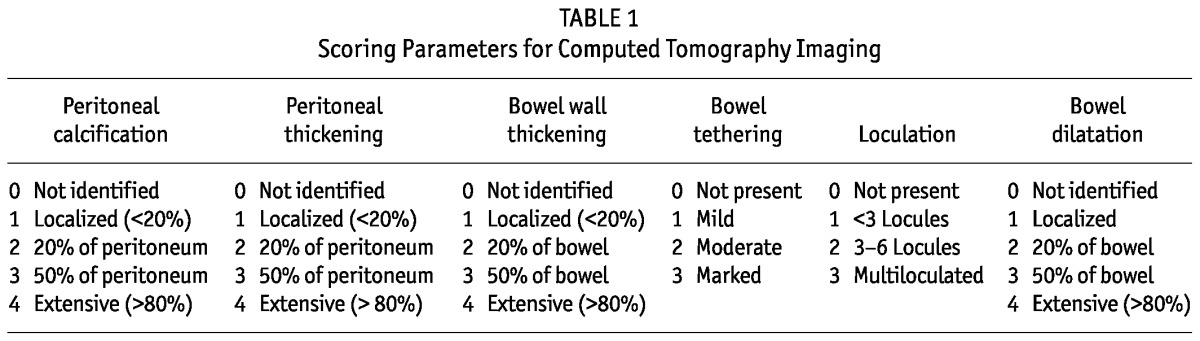
The CT images were scored according to the 6-point scoring system (Table 1) devised by Tarzi et al. (11). However, to eliminate the potential for bias contributed by the quality difference between printed films and digital images, only the digital CT images available since January 2008 were analyzed. All the digital images were reviewed and scored by an independent radiologist, who was blinded to the clinical information of the patients.
TABLE 1.
Scoring Parameters for Computed Tomography Imaging
For the study patients, variables were collected and their clinical course was followed for 6 months after the onset of peritonitis. Baseline demographic and clinical data, including age, sex, underlying renal disease, mode of PD, duration of PD, and total number of peritonitis episodes were recorded. In addition, the peritoneal equilibration test (PET) results closest in time before the index peritonitis episode and any history of ultrafiltration failure (defined as an ultrafiltration volume less than 100 mL with instillation of 2.5% dialysis fluid over 4 hours) were assessed. With respect to the index peritonitis episodes, the initial antibiotic regimen, the causative organism or organisms, and the total duration of antibiotic administration were reviewed. Levels of CRP at about 3 weeks after dialysis catheter removal were recorded. The CT imaging and Charlson comorbidity index scores were also calculated. Outcome measures— including the occurrence of full-blown EPS (defined as the presence of intestinal obstruction with CT features of bowel encapsulation) during the study period and all-cause mortality at 6 months from the time of diagnosis of peritonitis—were assessed.
Statistical Analysis
The statistical analysis was performed using the SPSS software application (version 15.0: SPSS, Chicago, IL, USA). Continuous data are expressed as mean ± standard deviation unless otherwise specified, and categorical data are expressed as numbers and percentages. For comparisons, a chi-square test or Fisher exact test was used for categorical data and a t-test for continuous variables. A p value of less than 0.05, two-tailed, was considered statistically significant.
Results
Among the 62 patients identified, 39 (63%) with persistent sterile peritoneal inflammation were classified into the high-risk group according to our inclusion criteria. These patients accounted for 44.8% of all our cases involving catheter removal and 63% of those with no underlying surgical condition or persistent intra-abdominal infection.
Among the 39 patients in the high-risk group, 32 (82%) had undergone diagnostic abdominal paracentesis, and 9 were still on antibiotic treatment at the time of the procedure. Most of the 39 patients had one or more gastrointestinal symptoms (including vomiting, nausea, loss of appetite, abdominal distension, and diarrhea), consistent with clinical manifestations of early-phase EPS. Repeated therapeutic paracentesis was required in 20 patients for symptomatic relief. In 27 patients, the peritoneal fluid was bloodstained on aspiration. In 7 patients, small and deep-seated collections rendered paracentesis infeasible. We observed no complications related to the foregoing procedures. All specimens were negative on culture, and the subsequent clinical course of all 39 patients showed no evidence of ongoing intra-abdominal infection. All patients had persistently elevated CRP, ranging from 10 mg/L to 280 mg/L (median: 91 mg/L; interquartile range: 77 mg/L).
The 23 patients in the control group (37%) had complete resolution of inflammation without developing any intra-abdominal collection after catheter removal.
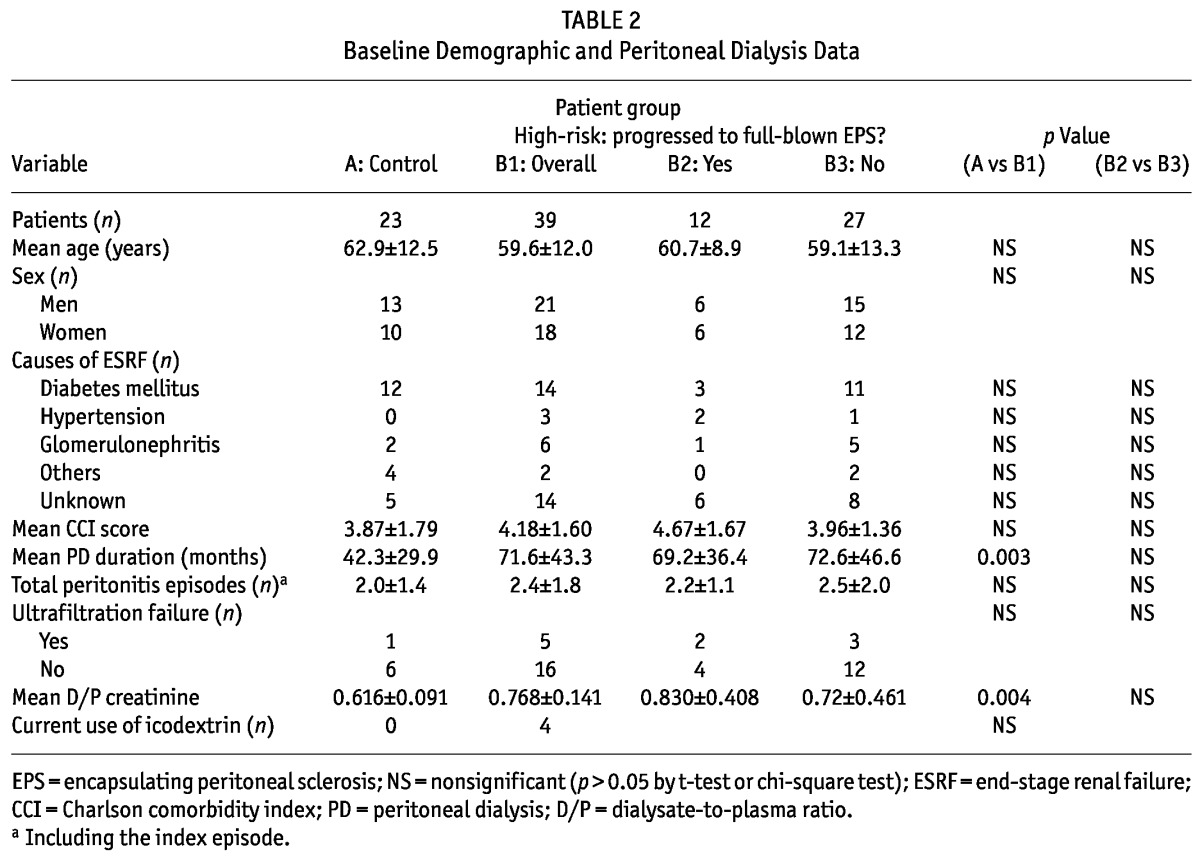
There were no significant differences in baseline characteristics between the high-risk and control groups (Table 2), except that, compared with the control group, the high-risk group had a significantly longer duration of PD (71.6 ± 43.3 months vs 42.3 ± 29.9 months, p = 0.003). Information about PET results and ultrafiltration were retrievable for only 21 (54%) and 7 (30%) of the patients in the high-risk and control groups respectively. Based on these incomplete data, the high-risk group showed a trend toward a higher transport state: that is, a higher dialysate-to-plasma ratio (D/P) of creatinine (0.768 ± 0.141 vs 0.616 ± 0.091, p = 0.004).
TABLE 2.
Baseline Demographic and Peritoneal Dialysis Data
With respect to the index peritonitis episodes, the groups showed no significant differences in terms of bacteriology (Figure 1), antibiotic regime, or timing of dialysis catheter removal. A significantly longer duration of antibiotic treatment was observed in the high-risk group (Table 3).
Figure 1 —
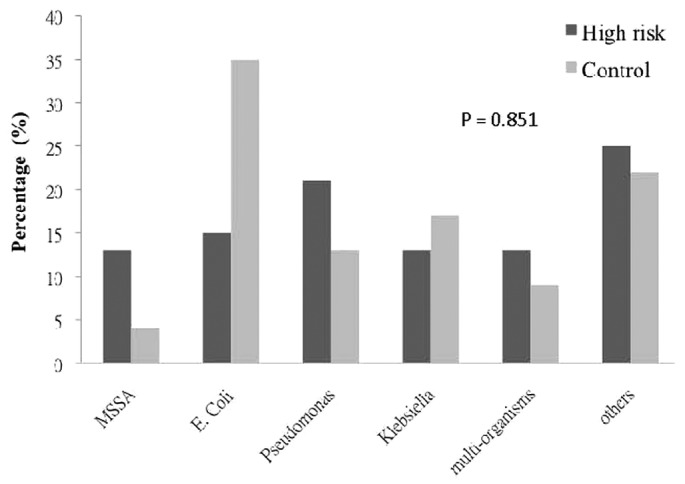
Causative organisms for the index peritonitis episode in the high-risk and control groups. p Value obtained by chi-square test. MSSA = methicillin-sensitive Staphylococcus aureus; E. coli = Escherichia coli.
TABLE 3.
Characteristics of the Index Peritonitis Episode in the High-Risk and Control Groups
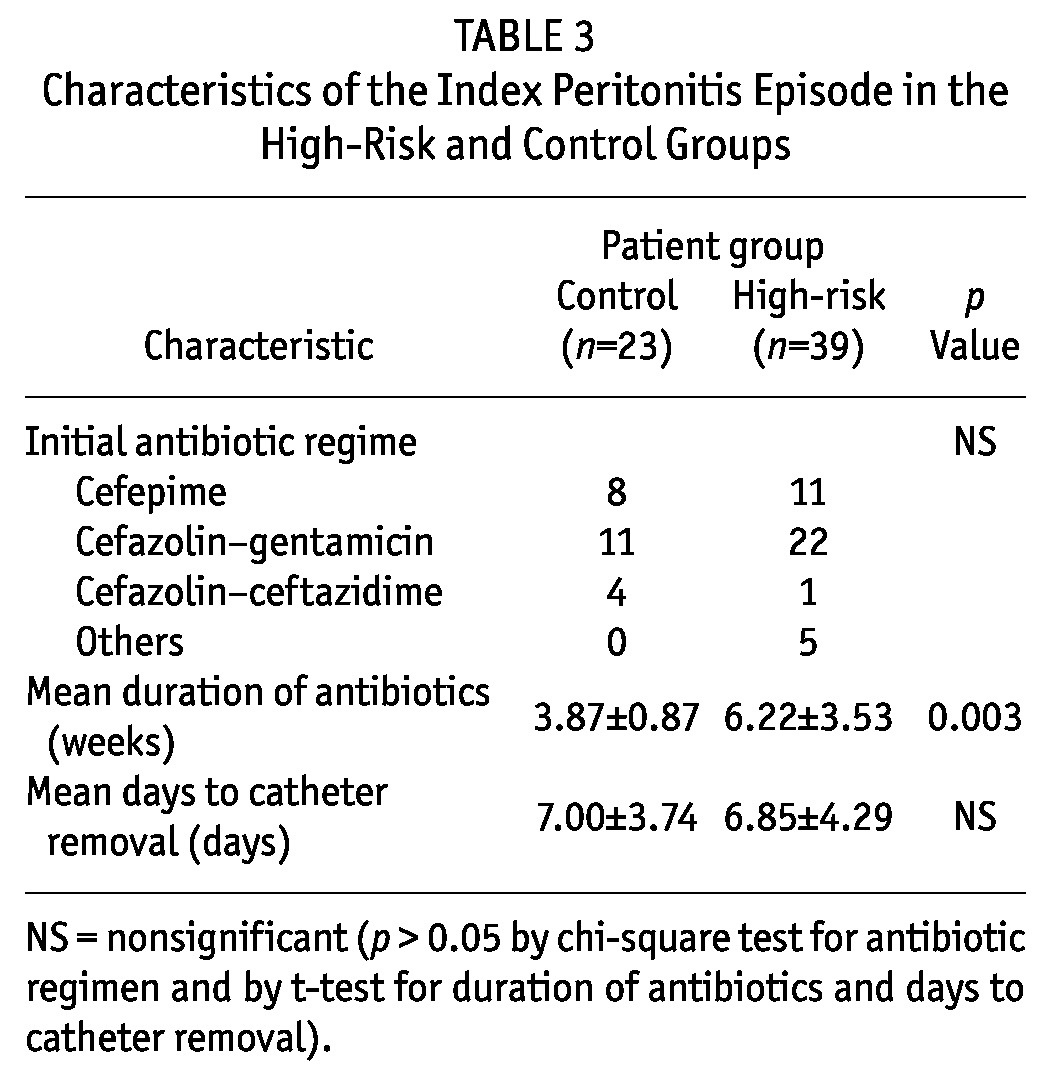
Among the study patients, 38 (61%) had their abdominal CT imaging in digital format and were reviewed by our radiologist (Table 4 and Figure 2). Scores for features of EPS were significantly higher in the high-risk group than in the control group (7.69 ± 2.98 vs 1.00 ± 1.00, p < 0.001). Apart from the presence of mild peritoneal thickening and a very small amount of loculations, all the CT features of EPS were absent in the control group.
TABLE 4.
Computed Tomography (CT) Scores in the High-Risk and Control Groups
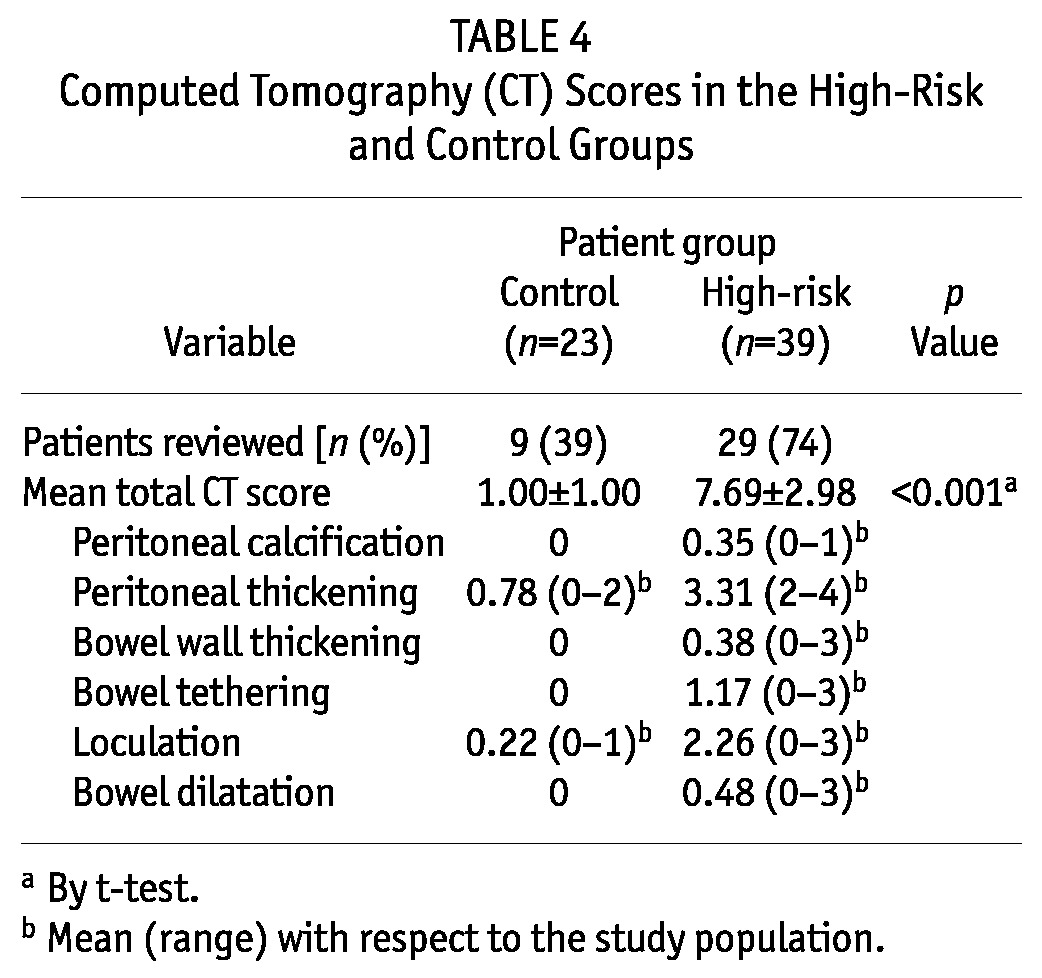
Figure 2 —
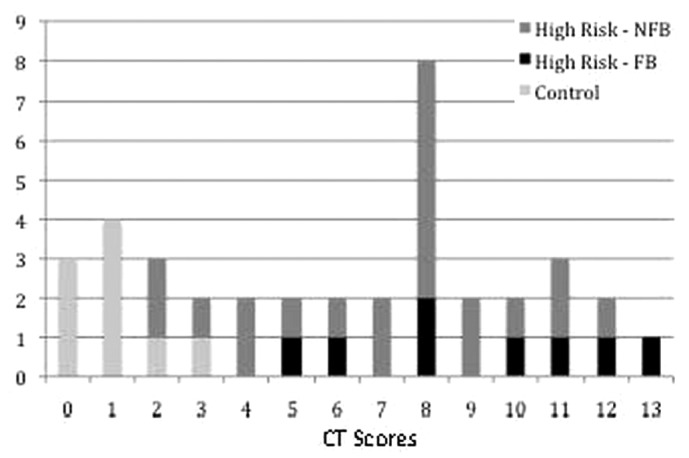
Distribution of computed tomography (CT) scores for patients in the control and high-risk groups. NFB = no full-blown encapsulating peritoneal sclerosis (EPS); FB = with full-blown EPS.
Outcome
In the high-risk group, 12 patients developed full-blown EPS during the 6-month study period; no patient in the control group developed EPS (31% vs 0%, p = 0.002). The baseline characteristics were similar for patients within the high-risk group who did and did not develop full-blown EPS (Table 2). The average time from dialysis catheter removal to the development of intestinal obstruction leading to a diagnosis of full-blown EPS was 37 ± 28.7 days (range: 17 - 119 days). These patients had persistent gastrointestinal tract malfunction requiring nasogastric tube suction and support with total parenteral nutritional. Compared with the patients who developed full-blown EPS during the follow-up period, those who did not develop EPS appeared to have better nutrition status and higher mean serum albumin at 6 months (34.1 ± 2.4 mg/dL vs 27.3 ± 10.6 mg/dL, p = 0.114).
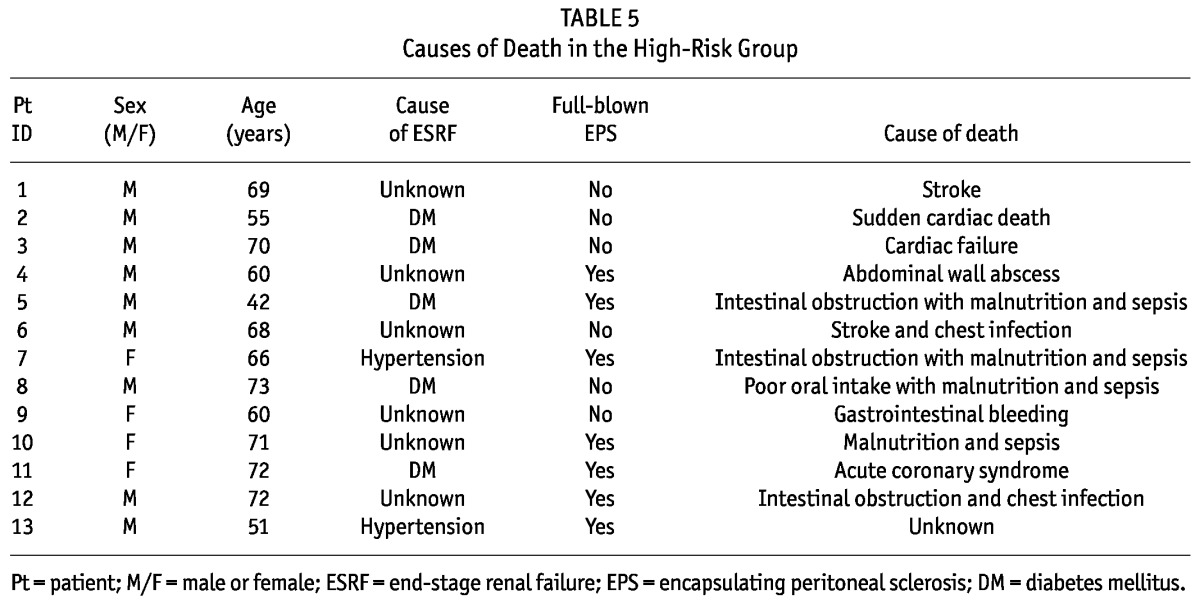
Fixed-time all-cause mortality at 6 months was calculated for the patients from the diagnosis of peritonitis. Compared with the control group, the high-risk group had a significantly higher 6-month all-cause mortality (36% vs 4.3%, p = 0.004) and a shorter survival (165 ± 37.0 days vs 178 ± 9.4 days; 95% confidence interval: -26.08 to -0.98; p = 0.035). Within the high-risk group, we observed an insignificant trend toward higher 6-month all-cause mortality for patients with full-blown EPS than for patients without full-blown EPS (50% vs 29.6%, p = 0.194). Table 5 presents the causes of death for the patients in the high-risk group.
TABLE 5.
Causes of Death in the High-Risk Group
Discussion
In the present study, we found that persistent sterile peritoneal inflammation was common (up to 63%) in patients after catheter removal for refractory bacterial peritonitis. These patients had an increased risk for developing full-blown EPS and poor short-term survival, especially those who had received PD for more than 5 years and who had a high transport membrane.
The current difficulty in managing EPS is the lack of specific diagnostic criteria in the early inflammatory phase, which is probably the only phase during which medical therapy will be effective (7). Once full-blown EPS has occurred (with the presence of encapsulation and fibrosis), mortality is high, and only surgical therapy can potentially improve the condition. Despite medical advances in proteomics (such as tests for cancer antigen 125, matrix metalloproteinase 2, or interleukin 6 either alone or in combination), its application in a diagnosis of EPS is still inconclusive (12,13). Moreover, the necessary tests might not be widely available in local PD centers, and clinical judgment is still required to select the high-risk patients that will undergo such investigations.
Using clinical criteria in a preset clinical context, we successfully identified patients at high risk of progression to full-blown EPS. According to the “two-hit” theory of Honda and Oda, patients undergoing dialysis catheter removal for refractory bacterial peritonitis have two important “second hit” factors (bacterial peritonitis and withdrawal of dialysis) which predispose them to EPS development (2,8). The accelerated fibrin production triggered by peritoneal infection, if not continuously washed out by PD (because of treatment withdrawal), will rapidly deposit and encapsulate the bowel, causing bowel dysfunction (7,8). Clinically, 38% of EPS cases were observed to develop after an episode of bacterial peritonitis (3,14), and 63% of cases occurred after PD withdrawal for various reasons (2). Although bacterial peritonitis and PD withdrawal both appear to be associated with the development of EPS, the individual roles and relative importance of these two predisposing factors remain unclear. Often, they are indeed inter-related and occur at the same time, because refractory bacterial peritonitis is a major reason for peritoneal catheter removal, leading to temporary or permanent PD withdrawal. In this regard, our study has not provided a definite answer.
In the overall study population of 62 patients who underwent peritoneal catheter removal because of refractory bacterial peritonitis, 12 (19%) developed EPS. Notwithstanding this seemingly high proportion, we were unable to identify any specific additional peritonitis-related risk factor. Hence, we still cannot provide evidence that peritonitis plays a particular role in the pathophysiology of EPS. On the other hand, persistent sterile peritoneal inflammation despite the resolution of sepsis was found to be crucial in the development of EPS under this particular circumstance. We deliberately excluded from the study patients who had bacterial peritonitis because of surgical pathology or persistent intra-abdominal sepsis because it is well known that unresolved intra-abdominal infection is associated with adhesions and fibrosis, which probably represents a different pathophysiologic mechanism and carries a different prognosis and implication for intervention than that in patients having EPS.
In various studies, elevated CRP and the presence of intra-abdominal collections have commonly been observed in patients with technique failure (9,10,15). In a Korean study by Moon et al. (9), persistent elevation of CRP at 72 hours was found to be a significant predictor for the development of EPS in patients after dialysis catheter removal for refractory peritonitis. Another local study by Szeto et al. (10) also found that 14% of their patients in the same clinical context experienced persistent symptomatic intra-abdominal collections, and 67% and 70% of the patients also had elevated inflammatory markers and bowel obstruction respectively. Those features are likely the clinical manifestations of the inflammatory phase of EPS. However, in that study, imaging (either by ultrasonography or CT) was arranged only for those who experienced persistent abdominal symptoms or features of ongoing infection, possibly leading to underdetection of subclinical collections. In contrast, our center routinely performed CT imaging for all patients after dialysis catheter removal, allowing for the detection of subclinical yet clinically significant intra-abdominal collections. These examinations explain our higher rate of intra-abdominal collection after dialysis catheter removal (63% vs 14% in the other study), which is indeed a more accurate reflection of the scale of the problem. Thus, patients who undergo dialysis catheter removal for refractory peritonitis are particularly “at risk” for the development of EPS and should be actively monitored for features of peritoneal inflammation, including persistently elevated CRP and the presence of intra-abdominal collections.
In our study, a significantly longer duration of PD and higher membrane transport were observed in the high-risk group, findings that have consistently been identified as risk factors for EPS (2-5,16). Prolonged exposure to bioincompatible hypertonic glucose solution leads to the development of a high-transport state (17), which is indirect evidence of peritoneal deterioration (that is, the “first hit”), subsequently leading to development of EPS upon a “second hit.” Regular PETs should be arranged in all PD patients for the purpose of peritoneal function monitoring. However, use of icodextrin was low in our study. That lower use might be partly attributed to cost concerns in our locality, but in addition, icodextrin dialysate was not available for patients using Fresenius connection systems. Those patients had to rely on high-tonicity dialysate to tackle the ultrafiltration problem.
Characteristic CT features for EPS and higher CT scores were observed for patients in the high-risk group in the current study (18-20). On CT, features such as peritoneal calcification, bowel wall thickening, bowel tethering, and bowel dilatation were found only in the high-risk group. A previous study reported that individual CT changes are not specific for EPS, because they can also be found in long-term PD patients, but the combination of features is still useful in making a diagnosis of EPS (18). Our study also demonstrated that CT changes are already evident in the early inflammatory phase of EPS. Taken together, CT changes might indeed provide important clues for the early identification of patients at risk of developing EPS. Early CT imaging should therefore probably be advocated for patients with high-risk characteristics after catheter removal for refractory peritonitis. In this context, a detailed case-by-case discussion with the radiologists to look for the specific features would be particularly helpful. However, screening CT imaging for EPS outside the setting of the present study has not been found to be useful (11,20).
Limitations
The present study has a few limitations. First, data collection was incomplete because of the retrospective study design. Data concerning transport status was available for only 45% of the study population. A considerable number of patients failed to undergo PET studies because of fragility or other personal reasons, which might have had a significant effect on the comparisons of transport status and the incidence of ultrafiltration failure in the two patient groups. There are also inadequacies in the assessment of CT score. The fact that only 61% of the CT scans (39% in the control group, 74% in high-risk group) were used for scoring and that only 1 independent radiologist was responsible for the scoring (instead of 2) might potentially create bias and affect the reliability of the results. Furthermore, in the present study, we reported only those patients who developed EPS early after an episode of peritonitis. Given the findings that many patients who did not develop full-blown EPS indeed had high CT scores, it is possible that more patients in the high-risk group would have developed full-blown EPS after a longer duration of follow-up. On the other hand, other patients in our center developed EPS after renal transplantation, late (that is, months to years) after an episode of peritonitis, after conversion to hemodialysis because of technique failure, or while still on PD without a preceding peritonitis. In the absence of reliable screening tools or monitoring protocols, these patients were typically diagnosed in the late stage of EPS when they presented with severe gastrointestinal symptoms from intestinal obstruction. The findings in the present study might therefore not be applicable to the groups of patients who develop EPS without a preceding episode of refractory peritonitis requiring catheter removal. Furthermore, although a high CT score was found to be associated with a higher risk for EPS, we observed no cut-off value that would help to clearly diagnose the condition. Finally, this is just a single-center study involving a small number of patients of Chinese ethnicity, and therefore the results need to be confirmed by large-scale study and might not be generalizable to other ethnic groups. A multicenter prospective study with a standard screening and follow-up protocol would be desirable to clarify the issue.
Conclusions
In the present study, we observed that persistent sterile peritoneal inflammation was common after dialysis catheter removal for refractory bacterial peritonitis and that it might predispose the patients to develop EPS and to progress to full-blown disease. Close observation through serial monitoring of inflammatory markers and abdominal CT imaging after catheter removal might help with early identification of the high-risk group of patients. Indeed, CT imaging might be a useful adjunct in the identification of the early inflammatory phase of EPS, which might possibly be more amendable to therapeutic intervention. After all, high clinical suspicion is the key to early diagnosis. Hopefully, with early identification and treatment, the outcome of this serious condition might be improved. Nevertheless, further large-scale prospective studies are needed to confirm our findings.
Disclosures
The authors have no financial conflicts of interest to declare.
References
- 1. Bradley JA, McWhinnie DL, Hamilton DN, Starnes F, Macpherson SG, Seywright M, et al. Sclerosing obstructive peritonitis after continuous ambulatory peritoneal dialysis. Lancet 1983; 2:113–14 [DOI] [PubMed] [Google Scholar]
- 2. Brown MC, Simpson K, Kerssens JJ, Mactier RA. on behalf of the Scottish Renal Registry. Encapsulating peritoneal sclerosis in the new millennium: a national cohort study. Clin J Am Soc Nephrol 2009; 4:1222–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kawanishi H, Kawaguchi Y, Fukui H, Hara S, Imada A, Kubo H, et al. Encapsulating peritoneal sclerosis in Japan: a prospective, controlled, multicenter study. Am J Kidney Dis 2004; 44:729–37 [PubMed] [Google Scholar]
- 4. Summers AM, Clancy MJ, Syed F, Harwood N, Brenchley PE, Augustine T, et al. Single-center experience of encapsulating peritoneal sclerosis in patients on peritoneal dialysis for end-stage renal failure. Kidney Int 2005; 68:2381–8 [DOI] [PubMed] [Google Scholar]
- 5. Balasubramaniam G, Brown EA, Davenport A, Cairns H, Cooper B, Fan SL, et al. The Pan-Thames EPS study: treatment and outcomes of encapsulating peritoneal sclerosis. Nephrol Dial Transplant 2009; 24:3209–15 [DOI] [PubMed] [Google Scholar]
- 6. Nakamoto H. Encapsulating peritoneal sclerosis—a clinician’s approach to diagnosis and medical treatment. Perit Dial Int 2005; 25(Suppl 4):S30–8 [PubMed] [Google Scholar]
- 7. Brown EA, Van Biesen W, Finkelstein FO, Hurst H, Johnson DW, Kawanishi H, et al. on behalf of the ISPD Working Party. Length of time on peritoneal dialysis and encapsulating peritoneal sclerosis: position paper for ISPD. Perit Dial Int 2009; 29:595–600 [PubMed] [Google Scholar]
- 8. Honda K, Oda H. Pathology of encapsulating peritoneal sclerosis. Perit Dial Int 2005; 25(Suppl 4):S19–29 [PubMed] [Google Scholar]
- 9. Moon SJ, Han SH, Kim DK, Lee JE, Kim BS, Kang SW, et al. Risk factors for adverse outcomes after peritonitis-related technique failure. Perit Dial Int 2008; 28:352–60 [PubMed] [Google Scholar]
- 10. Szeto CC, Kwan BC, Chow KM, Pang WF, Kwong VW, Leung CB, et al. Persistent symptomatic intra-abdominal collection after catheter removal for PD-related peritonitis. Perit Dial Int 2011; 31:34–8 [DOI] [PubMed] [Google Scholar]
- 11. Tarzi RM, Lim A, Moser S, Ahmad S, George A, Balasubramaniam G, et al. Assessing the validity of an abdominal CT scoring system in the diagnosis of encapsulating peritoneal sclerosis. Clin J Am Soc Nephrol 2008; 3:1702–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sampimon DE, Korte MR, Barreto DL, Vlijm A, de Waart R, Struijk DG, et al. Early diagnostic markers for encapsulating peritoneal sclerosis: a case-control study. Perit Dial Int 2010; 30:163–9 [DOI] [PubMed] [Google Scholar]
- 13. Hirahara I, Inoue M, Okuda K, Ando Y, Muto S, Kusano E. The potential of matrix metalloproteinase-2 as a marker of peritoneal injury, increased solute transport, or progression to encapsulating peritoneal sclerosis during peritoneal dialysis—a multicentre study in Japan. Nephrol Dial Transplant 2007; 22:560–7 [DOI] [PubMed] [Google Scholar]
- 14. Rigby RJ, Hawley CM. Sclerosing peritonitis: the experience in Australia. Nephrol Dial Transplant 1998; 13:154–9 [DOI] [PubMed] [Google Scholar]
- 15. Bilgic A, Sezer S, Ozdemir FN, Akgul A, Arat Z, Haberal M. Clinical outcome after transfer from peritoneal dialysis to hemodialysis. Adv Perit Dial 2006; 22:94–8 [PubMed] [Google Scholar]
- 16. Korte MR, Sampimon DE, Lingsma HF, Fieren MW, Looman CW, Zietse R, et al. on behalf of the Dutch Multicenter EPS Study. Risk factors associated with encapsulating peritoneal sclerosis in Dutch EPS study. Perit Dial Int 2011; 31:269–78 [DOI] [PubMed] [Google Scholar]
- 17. Brimble KS, Walker M, Margetts PJ, Kundhal KK, Rabbat CG. Meta-analysis: peritoneal membrane transport, mortality, and technique failure in peritoneal dialysis. J Am Soc Nephrol 2006; 17:2591–8 [DOI] [PubMed] [Google Scholar]
- 18. Vlijm A, Stoker J, Bipat S, Spijkerboer AM, Phoa SS, Maes R, et al. Computed tomographic findings characteristic for encapsulating peritoneal sclerosis: a case-control study. Perit Dial Int 2009; 29:517–22 [PubMed] [Google Scholar]
- 19. George C, Al-Zwae K, Nair S, Cast JE. Computed tomography appearances of sclerosing encapsulating peritonitis. Clin Radiology 2007; 62:732–7 [DOI] [PubMed] [Google Scholar]
- 20. Goodlad C, Tarzi R, Gedroyc W, Lim A, Moser S, Brown EA. Screening for encapsulating peritoneal sclerosis in patients on peritoneal dialysis: role of CT scanning. Nephrol Dial Transplant 2011; 26:1374–9 [DOI] [PubMed] [Google Scholar]


