Abstract
♦ Background: The optimal approach to monitoring blood pressure (BP) in the peritoneal dialysis (PD) population is unclear. Ambulatory BP monitoring reliably predicts prognosis, but can be inconvenient. The accuracy of home BP monitoring in this population is unproven. The automated BpTRU device (BpTRU Medical Devices, Coquitlam, BC, Canada), which provides an average of up to 6 successive in-office BP measurements, has not been studied in this patient group.
♦ Methods: We studied 17 patients (average age: 54 ± 12 years; 12 men, 5 women; 94% on automated PD) attending a single center. All patients underwent office, home, BpTRU, and ambulatory BP measurement. The reference standard for analysis was daytime ambulatory BP. Correlation between the referent method and each comparator method was determined (Pearson correlation coefficient), and Bland-Altman scatter plots depicting the differences in the BP measurements were constructed.
♦ Results: Mean office BP (126.4 ± 16.9/78.8 ± 11.6 mmHg) and BpTRU BP (123.8 ± 13.7/80.7 ± 11.1 mmHg) closely approximated mean daytime ambulatory BP (129.3 ± 14.8/78.2 ± 7.9 mmHg). Mean home BP (143.8 ± 15.0/89.9 ± 28.1 mmHg) significantly overestimated mean daytime systolic BP by 14.2 mmHg (95% confidence interval: 4.3 mmHg to 24.1 mmHg; p = 0.008). Bland-Altman plots demonstrated poorest agreement between home BP and daytime ambulatory BP. No patient had “white-coat hypertension,” and only 1 patient had false-resistant hypertension. Most patients showed abnormal nocturnal dipping patterns (non-dipping: n = 11; reverse-dipping: n = 5; normal dipping: n = 1).
♦ Conclusions: We report a novel finding that BP measurement using the BpTRU device is more accurate than home BP measurement in a PD population. Potential explanations for this observation include poor home BP measurement technique, use of poorly validated home BP measurement devices, or a reduced prevalence of white-coat effect among PD patients. Our study also confirms that, in the PD population, BP measurements vary considerably with patient location, time of day, and measurement technique.
Keywords: Hypertension, blood pressure, measurement, ambulatory, home, BpTRU
Hypertension is prevalent (1,2), poorly controlled (1,2), and associated with adverse outcomes (2-4) in the peritoneal dialysis (PD) population. Excessively low blood pressure (BP) is also undesirable in this patient group (5). Given these competing risks, it is clear that accurate and reliable BP measurement is fundamental to the achievement of adequate BP control and the optimization of PD patient outcome.
The ideal approach to monitoring BP in a PD population is unknown. Compared with office BP, ambulatory BP correlates more closely with end-organ damage in this patient group (2,3). Ambulatory BP monitoring can also provide valuable information about the nocturnal BP load, allowing adjustments to be made in the timing of antihypertensive administration (“chronotherapy”) (6). In the long-term, however, repeated ambulatory BP monitoring can be inconvenient for the patient and demanding on hospital resources. Home BP measurement, another out-of-office approach to BP monitoring, is a valid alternative to ambulatory BP monitoring in patients with and without chronic kidney disease, including those receiving maintenance hemodialysis (7-9). However, for as yet unexplained reasons, the home technique appears be less accurate than standard office BP measurement in the PD population (3). The automated BpTRU device (BpTRU Medical Devices, Coquitlam, BC, Canada), which measures BP multiple times at pre-set intervals in the absence of a health professional, can negate “white-coat” effect and improve the accuracy of in-office BP measurement in patients with and without chronic kidney disease (10,11). However, the BpTRU device has not been formally studied in a PD population, and all four BP measurement techniques (office, ambulatory, home, BpTRU) have never been directly compared in any patient population.
We therefore recorded office, ambulatory, home, and BpTRU BP in a group of PD patients. We aimed to identify the relationships between daytime ambulatory BP (reference standard) and BP as measured by each of the three comparator techniques. We also wanted to explore the circadian and situational BP variations evident in this patient population.
Methods
Study Design
This cross-sectional observational study was performed in a single tertiary referral center. The study protocol was approved by the institution’s ethics review board before study commencement. All patients provided written informed consent to take part in the study.
Study Population
All patients receiving PD care at our center and scheduled to undergo a medical review during the recruitment phase of the study (1 January 2011 to 31 March 2011) were considered for study inclusion. Exclusion criteria included age less than 18 years, PD treatment duration less than 3 months, change in antihypertensive medications or PD prescription within the preceding 2 weeks, peritonitis or severe acute illness within the preceding month, presence of an arrhythmia precluding the use of an oscillometric device for measurement of BP, and inability or refusal of consent to take part in the study.
Patients were requested to maintain their usual daily activities, PD prescription, and antihypertensive medications during the study period. Demographic, laboratory, clinical, and dialysis schedule details were obtained through patient interview and by manual review of medical and computer records. Mean daily ultrafiltration volumes were calculated by averaging the values recorded by patients in their diaries for the 7 days preceding the study visit. The most recent available 24-hour urinary volume was recorded. Weekly Kt/V urea was calculated using the PD Adequest software application (Baxter Healthcare, Deerfield, IL, USA) from the most recent available blood, urine, and effluent urea measurements.
BpTRU BP Measurement
One of two PD nurses used the BpTRU device to take each patient’s BP just before the patient’s medical consultation. The BpTRU device was previously validated according to British Hypertension Society protocol (12). After observing the first measurement and confirming that the device was functioning, the nurse left the examination room, and 5 additional measurements were recorded at 2-minute intervals by the device. The device automatically calculated an average value for those 5 final measurements, although a minimum of 4 measurements was deemed acceptable for calculation of an average BpTRU BP value.
Office BP Measurement
Office BP was recorded by the same PD nurse using a validated oscillometric device (Vital Signs Monitor 300 Series: Welch Allyn, Beaverton, OR, USA) during the patient’s medical consultation. The patient was allowed to rest for at least 5 minutes before the recording and was seated with his or her arm at the level of the heart during the recording. If more than one office BP was measured, an average value was calculated.
Home BP Monitoring
All patients attending our PD center routinely carry out home BP monitoring using their own personal BP measurement devices. Once they commence PD, patients are advised to purchase validated BP devices and to confirm the accuracy of their device soon after purchase by comparing it to a calibrated in-office device. Patients also receive education in correct BP measurement technique, according to European Society of Hypertension protocol (13). We asked patients to use their usual device to measure their BP twice daily (morning and evening) for the week preceding their scheduled study visit. An average BP value was manually calculated from the values recorded on the final 6 days (12 measurements), although a minimum of 10 measurements was deemed acceptable for calculation of an average home BP value. Device manufacturer and model were recorded for each patient.
Ambulatory BP Monitoring
All patients underwent 24-hour ambulatory BP measurement once within the 2-week period preceding their scheduled study visit. The same validated oscillometric ambulatory BP monitor (Spacelab 90207 monitor: SpaceLabs Medical, Redmond, WA, USA) was used for each patient. Patients attended the PD unit on a weekday morning to have the device fitted. From 0600 h to 2300 h, BP was measured every 20 minutes, and from 2300 h to 0600 h, it was measured every 30 minutes. Average values for 24-hour BP, daytime BP, and nighttime BP were calculated by the SpaceLabs software. In cases of missing data, 14 daytime measurements and 7 nighttime measurements were deemed the minimum acceptable for data analysis.
BP Variation Patterns
Patients were classified according to their office and daytime ambulatory BP, and the presence or absence of antihypertensive medications, as follows:
Normotension (office BP < 140/90 mmHg and daytime ambulatory BP < 135/85 mmHg, without treatment) or controlled hypertension (office BP < 140/90 mmHg and daytime ambulatory BP < 135/85 mmHg, with treatment)
White-coat hypertension (office BP > 140/90 mmHg and daytime ambulatory BP < 135/85 mmHg, without treatment) or false-resistant hypertension (office BP > 140/90 mmHg and daytime ambulatory BP < 135/85 mmHg, with treatment)
Masked hypertension (office BP < 140/90 mmHg and daytime ambulatory BP > 135/85 mmHg, without treatment) or residual masked hypertension (office BP < 140/90 mmHg and daytime ambulatory BP > 135/85 mmHg, with treatment)
Untreated hypertension (office BP > 140/90 mmHg and daytime ambulatory BP > 135/85 mmHg, without treatment) or residual hypertension (office BP > 140/90 mmHg and daytime ambulatory BP > 135/85 mmHg, with treatment)
Nocturnal dipping patterns were classified according to daytime and nighttime ambulatory BP, as follows:
Normal dipping: BP decreased by more than 10% from daytime to nighttime
Non-dipping: BP decreased by 0% - 10% from daytime to nighttime
Reverse-dipping: BP increased from daytime to nighttime
Statistical Analysis
All data were analyzed using the SPSS software application (version 16.0: SPSS, Chicago, IL, USA). Categorical variables are expressed as numbers and percentages and were compared using the chi-square or Fisher exact test, as appropriate. Continuous variables are expressed as a mean ± standard deviation and were compared using the Student t-test (all data normally distributed). The correlation between mean BP by the reference method (daytime ambulatory BP) and by each comparator method was determined using the Pearson correlation coefficient. Bland-Altman scatterplots (14) demonstrating the level of agreement between the comparator methods (office, home, and BpTRU BP) and the reference method (daytime ambulatory BP) were created. A two-sided p value less than 0.05 was considered statistically significant.
Results
Patient Characteristics
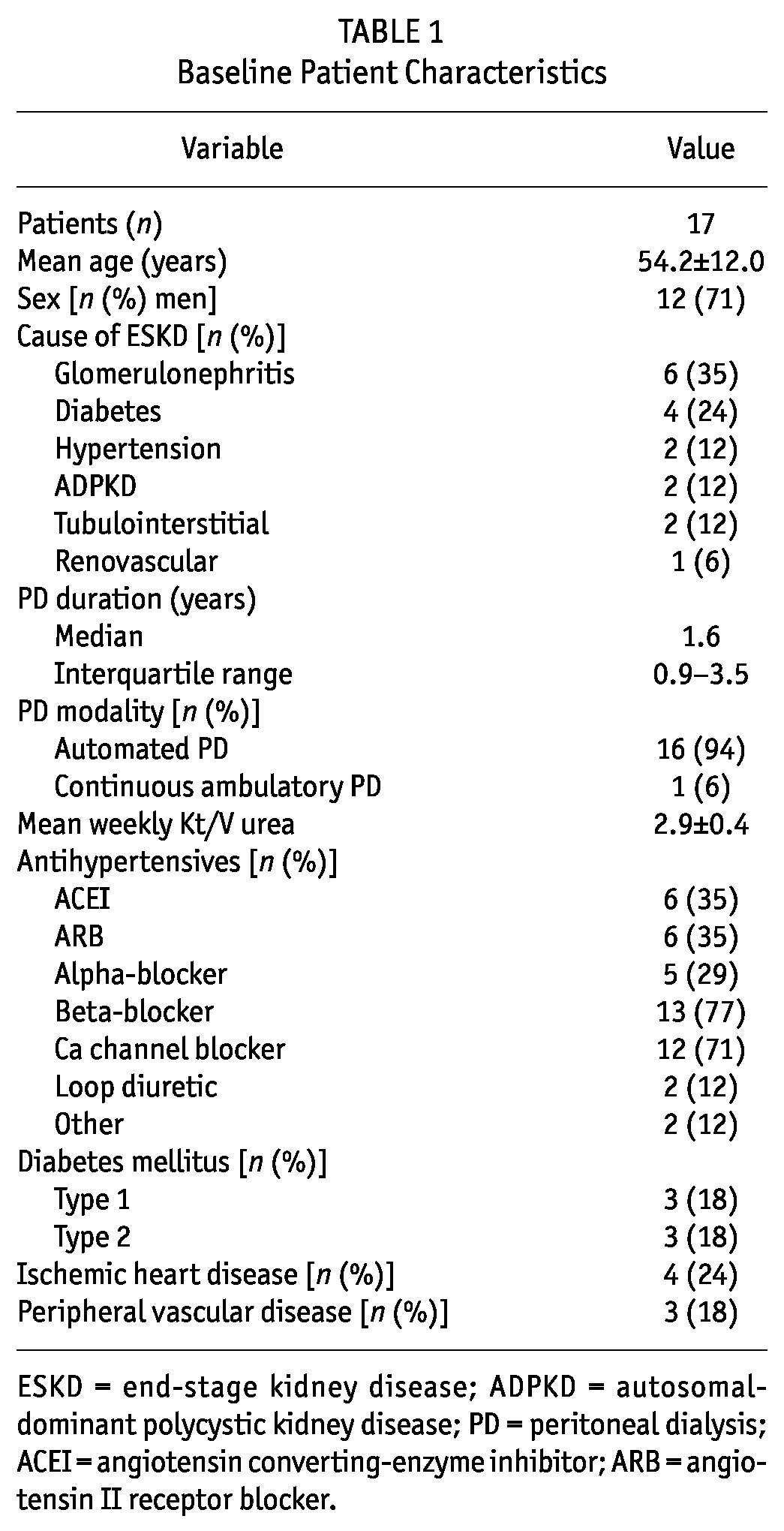
We screened 21 patients for study inclusion, and 1 patient was excluded because of being on PD for less than 3 months. Another 2 patients refused consent, and 1 patient had a failed 24-hour BP recording. The final study population therefore included 17 patients (mean age: 54 ± 12 years; 12 men, 5 women). Table 1 summarizes baseline patient characteristics. All 17 patients were receiving antihypertensive therapy: 3 were receiving 1 agent, 5 were receiving 2 agents, 3 were receiving 3 agents, and 6 were receiving 4 agents. One patient was undergoing ambulatory PD using 4 daily exchanges of Physioneal solution (Baxter Healthcare Corporation, Deerfield, IL, USA). Sixteen patients were undergoing automated PD using nocturnal Physioneal solution, with a daytime dwell of icodextrin-based Extraneal solution (Baxter Healthcare Corporation). All 17 patients had significant residual native renal function (range: 800 - 3600 mL). Only 4 patients had a mean ultrafiltration volume exceeding 1000 mL daily (range: 93 - 2277 mL).
TABLE 1.
Baseline Patient Characteristics
BP Data
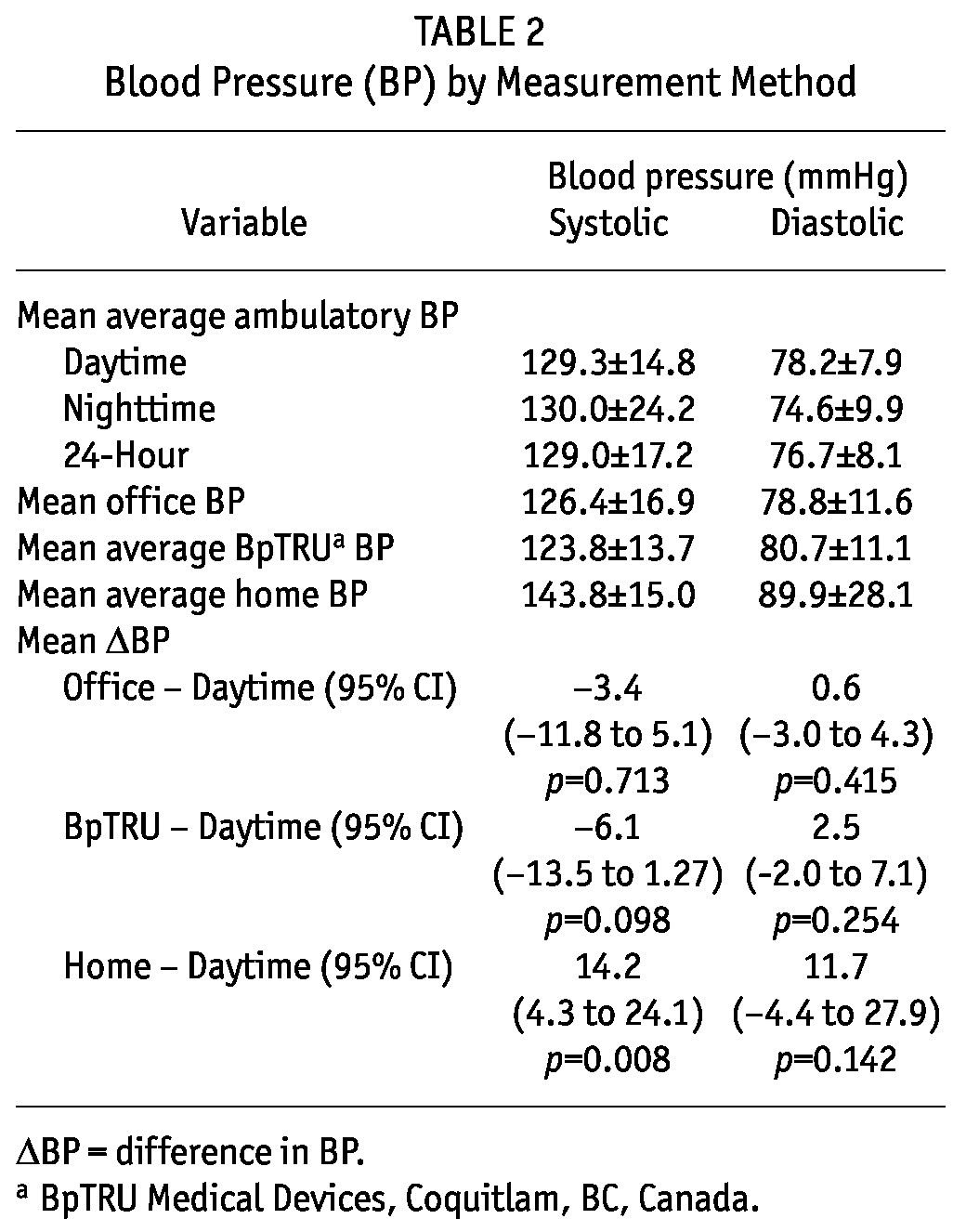
Table 2 shows the mean BP measurements according to the four BP measurement techniques. Office BP (126.4 ± 16.9/78.8 ± 11.6 mmHg) and BpTRU BP (123.8 ± 13.7/78.8 ± 11.6 mmHg) were similar to daytime ambulatory BP (129.3 ± 14.8/78.2 ± 7.9 mmHg). Home systolic BP was 14.2 mmHg higher than daytime ambulatory systolic BP [95% confidence interval (CI): 4.3 mmHg to 24.1 mmHg; p < 0.008] and home diastolic BP was 11.7 mmHg higher than daytime ambulatory diastolic BP (95% CI: -4.4 mmHg to 27.9 mmHg, p = 0.142).
TABLE 2.
Blood Pressure (BP) by Measurement Method
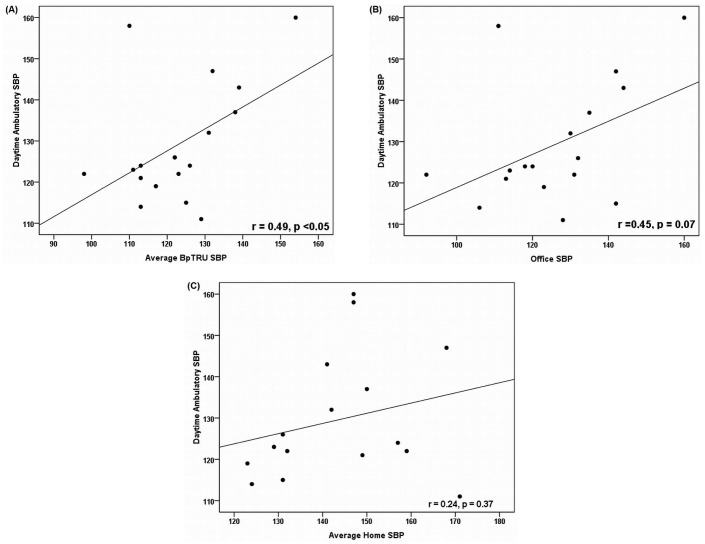
We observed a significant correlation between BpTRU systolic BP and daytime ambulatory systolic BP (r = 0.49, p < 0.05), a nonsignificant correlation between office systolic BP and daytime ambulatory systolic BP (r = 0.45, p = 0.07), and no correlation between home systolic BP and daytime ambulatory systolic BP (r = 0.24, p = 0.37, Figure 1).
Figure 1 —
Associations between daytime ambulatory systolic blood pressure (SBP) measurements and comparator measurements (in millimeters of mercury). Scatterplots show associations for comparator measurements (A) by BpTRU (BpTRU Medical Devices, Coquitlam, BC, Canada), r = 0.49, p < 0.05; (B) by office measurement, r = 0.45, p = 0.07; and (C) by home measurement, r = 0.24, p = 0.37.
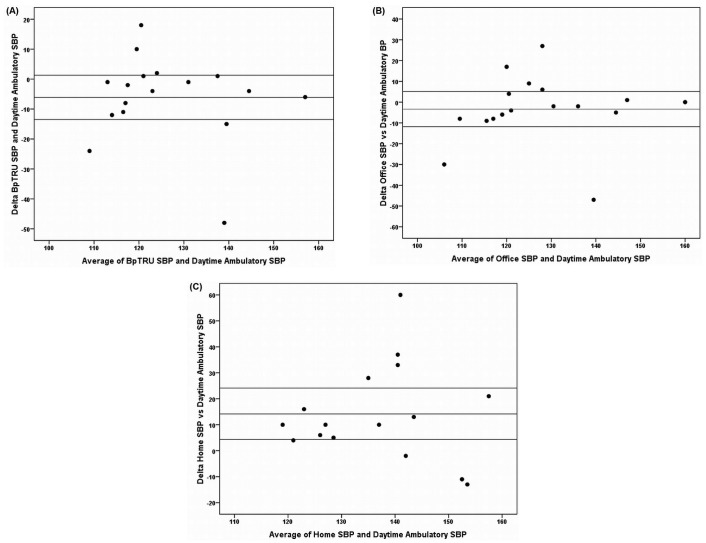
Figure 2 shows Bland-Altman scatterplots depicting the level of agreement between daytime ambulatory systolic BP (reference standard) and systolic BP by each of the comparator methods (office, home, BpTRU). Agreement was poorest between home BP and daytime ambulatory BP: that is, the limits of agreement were widest (95% CI for mean difference between methods: 4.3 mmHg to 24.1 mmHg), and a statistically significant positive bias of 14.2 mmHg was demonstrated. Individual cases of a substantial discrepancy depending on the method of BP measurement were identified. These discrepancies were greatest for home BP measurement compared with ambulatory BP measurement (differences of up to 60 mmHg, Figure 2).
Figure 2 —
Agreement between daytime ambulatory systolic blood pressure (SBP) and comparator measurements (in millimeters of mercury). Bland-Altman scatterplots show the mean of the ambulatory and comparator SBP measurements on the x axes and the difference of those readings on the y axes. Horizontal lines mark the mean difference in SBP between the methods and the upper and lower limits of the 95% confidence interval for that difference. (A) BpTRU (BpTRU Medical Devices, Coquitlam, BC, Canada) comparator measurement. (B) Office comparator measurement. (C) Home comparator measurement. ΔSBP = (SBP by comparator method) - (SBP daytime ambulatory measurement).
In 10 patients, BP was controlled; 3 patients had residual hypertension; 1 had false-resistant hypertension (office BP 142/91 mmHg vs daytime ambulatory BP 115/74 mmHg); 1 had residual masked hypertension (office BP 111/66 mmHg vs daytime ambulatory BP 158/76 mmHg); and 2 had borderline residual masked hypertension (office BP 130/89 mmHg vs daytime ambulatory BP 132/91 mmHg, office BP 135/71 mmHg vs daytime ambulatory BP 137/70 mmHg). An abnormal nocturnal dipping pattern was evident in 16 patients (non-dipping, n = 11; reverse-dipping, n = 5; normal dipping, n = 1).
Discussion
We report a novel finding that, in a PD population, measurement of BP in the office using either a standard automated device or a BpTRU device may be more reliable than measurement of BP at home by the patient using their own BP measurement device. We suggest two potential explanations for our findings.
First, we propose that PD patients may experience less white-coat effect than other patient groups, enhancing the accuracy of in-office BP measurement techniques (that is, standard office BP measurement and BpTRU BP measurement) in this unique patient population. Patients on PD frequently encounter medical environments and health care professionals. In many centers such as ours, they routinely measure their own BP at home and are very familiar with the BP measurement procedure. Consequently, it is plausible to hypothesize that they may have a negligible alerting response to in-office BP measurement. Supporting this concept, only 1 patient in our study had false-resistant (white-coat) hypertension, despite the fact that the office BP was measured in the presence of both a PD nurse and a nephrologist. Previous studies have observed a similarly low prevalence of white-coat effect in PD populations (1,3,15). The largest of those, a multicenter study of Italian PD patients, reported that only 32 of 304 treated hypertensive patients had false-resistant hypertension, and only 6 of 66 untreated hypertensive patients had white-coat hypertension (1). The slightly closer correlation between BpTRU BP and daytime ambulatory BP than between standard office BP and daytime ambulatory BP observed in this study may relate more to the ability of the BpTRU device to correct for random error by providing an average of multiple BP readings than to an ability to eliminate what is already a negligible white-coat effect.
A second potential explanation for the superior accuracy in this study of both BpTRU and standard in-office BP measurement over home BP measurement may be an inherent flaw in the home BP measurement procedure. In the present study, we made an effort to optimize the accuracy of home BP measurements:
All patients were educated in a standardized BP measurement technique in advance of the study.
All patients were advised to purchase only validated BP measurement devices.
All devices were calibrated against an in-office device before study commencement.
Despite those precautions, 3 patients ultimately used devices that had not previously been validated according to a standardized protocol (16). Nonetheless, the discrepancies between home BP measurements and daytime ambulatory BP measurements were not significantly different for those 3 patients than for the population as a whole: for example, the difference in mean systolic BP between methods (+4.7 mmHg) was in the same direction and of a lesser magnitude than the difference seen in the entire population (+14.2 mmHg) and would therefore not be expected to significantly skew or exaggerate the study results.
We therefore felt that the inaccuracy of home BP monitoring observed in this study related more to a failure of the patients to rigorously adhere to standardized BP measurement protocol (for example, incorrectly measuring BP after physical exertion, after intake of caffeine, or without the arm at the level of the heart) when taking their measurements unsupervised at home than to an excessive use of non-validated BP measurement devices. Indeed, our study is not the first to demonstrate that home BP measurement may be less accurate than standard office BP measurement in a PD population. A study comparing office, home, and ambulatory BP measurement (without BpTRU BP measurement) in a PD population demonstrated that standard office BP was more closely related than home BP to ambulatory BP (3). That finding held true regardless of whether systolic, diastolic, or mean BP was being assessed or whether office and home BP were being compared to 24-hour or daytime ambulatory BP.
Although most patients in the present study had an overall BP that was controlled, comparing favorably with reports from other centers (1,2), only 1 patient had a normal nocturnal dipping pattern. Nocturnal non-dipping has previously been reported to affect up to two thirds of PD patients (1-3,17-19) and has been associated with adverse outcomes in this population. Interestingly, a previous study demonstrated a higher prevalence of nocturnal non-dipping and a higher mean left ventricular mass index in patients on automated PD than in those on continuous ambulatory PD (3). Although speculative, that difference suggests that the process of carrying out automated dialysis throughout the night, combined with a relative absence of ultrafiltration during the day, might somehow (for example, by nocturnal sympathetic stimulation or increased volume overload) contribute to nocturnal non-dipping, which might in turn lead to the development of left ventricular hypertrophy. Our study population was composed almost entirely of patients undergoing automated PD, and they had a particularly high prevalence of abnormal nocturnal dipping (94%), adding strength to the latter hypothesis. However, it should also be noted that a larger, more recent study, while not specifically looking at nocturnal dipping patterns, did not confirm any association between PD modality (automated PD, continuous ambulatory PD) and inferior overall BP control or patient survival (20). Furthermore, the single patient undergoing continuous ambulatory PD in our study did not exhibit a normal nocturnal dipping pattern, at least for systolic BP (average daytime BP: 111/74 mmHg; average nighttime BP: 114/68 mmHg), but did show excellent overall BP control.
Our study has some limitations. No inferences from this cross-sectional study can be made regarding the relative ability of each individual BP measurement method to stratify cardiovascular risk or predict prognosis. Neither can the effect that the incorporation of these methods into clinical practice might have on patient outcomes be determined. Small patient numbers limited our ability to detect more subtle, yet potentially clinically significant, differences in BP between methods. The study was not powered to perform subgroup analyses. For example, the impact of timing of administration and overall number of antihypertensive agents or the degree of BP control (for example, controlled BP vs residual hypertension) on the reliability of BP measurement by each modality or on patterns of BP variability could not be determined. The results of this single-center study— which enrolled entirely white patients, most of whom were receiving automated PD—cannot necessarily be extrapolated to populations of patients with dissimilar baseline clinical characteristics.
Conclusions
In summary, our study found that, in a PD population, standard office BP measurement and BpTRU BP measurement were both more accurate than home BP measurement with respect to daytime ambulatory BP measurement. We also demonstrated that white-coat effect was rare, but that nocturnal non-dipping was highly prevalent in this patient population. Although ambulatory BP monitoring should remain the “gold standard” for measuring BP in a PD population in view of its proven ability to predict prognosis, we propose that BpTRU BP measurement might reliably and preferentially replace home BP measurement as a first-line means of validating standard in-office BP measurement in situations in which ambulatory BP monitoring is unavailable or will unduly delay treatment decisions.
Disclosures
The authors declare that no financial conflict of interest exists.
Acknowledgments
The authors thank Gemma Prendergast and Sharon Fahy for assistance with patient enrolment and data collection.
References
- 1. Cocchi R, Degli Esposti E, Fabbri A, Lucatello A, Sturani A, Quarello F, et al. Prevalence of hypertension in patients on peritoneal dialysis: results of an Italian multicentre study. Nephrol Dial Transplant 1999; 14:1536–40 [DOI] [PubMed] [Google Scholar]
- 2. Konings CJ, Kooman JP, Schonck M, Dammers R, Cheriex E, Palmans Meulemans AP, et al. Fluid status, blood pressure, and cardiovascular abnormalities in patients on peritoneal dialysis. Perit Dial Int 2002; 22:477–87 [PubMed] [Google Scholar]
- 3. Wang MC, Tseng CC, Tsai WC, Huang JJ. Blood pressure and left ventricular hypertrophy in patients on different peritoneal dialysis regimens. Perit Dial Int 2001; 21:36–42 [PubMed] [Google Scholar]
- 4. Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barré PE. Impact of hypertension on cardiomyopathy, morbidity and mortality in end-stage kidney disease. Kidney Int 1996; 49:1379–85 [DOI] [PubMed] [Google Scholar]
- 5. Goldfarb-Rumyantzev A, Baird B, Leypoldt J, Cheung A. The association between BP and mortality in patients on chronic peritoneal dialysis. Nephrol Dial Transplant 2005; 20:1693–1701 [DOI] [PubMed] [Google Scholar]
- 6. Hermida R, Ayala D, Mojon A, Fernandez J. Bedtime dosing of antihypertensive medications reduces cardiovascular risk in CKD. J Am Soc Nephrol 2011; 22:2213–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Agarwal R, Anderson MJ. Prognostic importance of clinic and home blood pressure recordings in patients with chronic kidney disease. Kidney Int 2006; 69:406–11 [DOI] [PubMed] [Google Scholar]
- 8. Agarwal R, Satyan S, Alborzi P, Light RP, Tegegne GG, Mazengia HS, et al. Home blood pressure measurements for managing hypertension in hemodialysis patients. Am J Nephrol 2009; 30:126–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ohkubo T, Imai Y, Tsuji I, Nagai K, Kato J, Kikuchi N, et al. Home blood pressure measurement has a stronger predictive power for mortality than does screening blood pressure measurement: a population-based observation in Ohasama, Japan. J Hypertens 1998; 16:971–5 [DOI] [PubMed] [Google Scholar]
- 10. O’Shaughnessy MM, Newman CA, Kinsella SM, Reddan DN, Lappin DW. In-office assessment of blood pressure in chronic kidney disease: usual measurement versus automated BpTRU measurement. Blood Press Monit 2011; 16:124–8 [DOI] [PubMed] [Google Scholar]
- 11. Myers M, Godwin M, Dawes M, Kiss A, Tobe S, Grant F, et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomised parallel design controlled trial. BMJ 2011; 342:d286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mattu GS, Heran BS, Wright JM. Overall accuracy of the BpTRU—an automated electronic blood pressure device. Blood Press Monit 2004; 9:47–52 [DOI] [PubMed] [Google Scholar]
- 13. Parati G, Stergiou GS, Asmar R, Bilo G, de Leeuw P, Imai Y, et al. European Society of Hypertension guidelines for blood pressure monitoring at home: a summary report of the Second International Consensus Conference on Home Blood Pressure Monitoring. J Hypertens 2008; 26:1505–26 [DOI] [PubMed] [Google Scholar]
- 14. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measure ment. Lancet 1986; 1:307–10 [PubMed] [Google Scholar]
- 15. Koc M, Toprak A, Tezcan H, Bihorac A, Akoglu E, Ozener IC. Uncontrolled hypertension due to volume overload contributes to higher left ventricular mass index in CAPD patients. Nephrol Dial Transplant 2002; 17:1661–6 [DOI] [PubMed] [Google Scholar]
- 16. dabl Educational Trust. Devices > Sphygmomanometers for Clinical Use > Automated Devices for Clinical Use [Web page]. Blackrock, Ireland: dabl Educational Trust; 2013. [Available online at: http://www.dableducational.org/sphygmomanometers/devices_1_clinical.html#ClinTable; accessed 1 February 2012] [Google Scholar]
- 17. Luik AJ, Struijk DG, Gladziwa U, von Olden RW, von Hooff JP, de Leeuw PW, et al. Diurnal blood-pressure variations in haemodialysis and CAPD patients. Nephrol Dial Transplant 1994; 9:1616–21 [PubMed] [Google Scholar]
- 18. Cheigh JS, Serur D, Paguirigan M, Stenzel KH, Rubin A. How well is hypertension controlled in CAPD patients? Adv Perit Dial 1994; 10:55–8 [PubMed] [Google Scholar]
- 19. Tonbul Z, Altintepe L, Sözlü C, Yeksan M, Yildiz A, Türk S. Ambulatory blood pressure monitoring in haemodialysis and continuous ambulatory peritoneal dialysis (CAPD) patients. J Hum Hypertens 2002; 16:585–9 [DOI] [PubMed] [Google Scholar]
- 20. Cnossen TT, Usvyat L, Kotanko P, van der Sande FM, Kooman JP, Carter M, et al. Comparison of outcomes on continuous ambulatory peritoneal dialysis versus automated peritoneal dialysis: results from a USA database. Perit Dial Int 2011; 31:679–84 [DOI] [PubMed] [Google Scholar]