Abstract
The fungal genus Rhynchosporium (causative agent of leaf blotch) contains several host-specialised species, including R. commune (colonising barley and brome-grass), R. agropyri (couch-grass), R. secalis (rye and triticale) and the more distantly related R. orthosporum (cocksfoot). This study used molecular fingerprinting, multilocus DNA sequence data, conidial morphology, host range tests and scanning electron microscopy to investigate the relationship between Rhynchosporium species on ryegrasses, both economically important forage grasses and common wild grasses in many cereal growing areas, and other plant species. Two different types of Rhynchosporium were found on ryegrasses in the UK. Firstly, there were isolates of R. commune that were pathogenic to both barley and Italian ryegrass. Secondly, there were isolates of a new species, here named R. lolii, that were pathogenic only to ryegrass species. R. lolii was most closely related to R. orthosporum, but exhibited clear molecular, morphological and host range differences. The species was estimated to have diverged from R. orthosporum ca. 5735 years before the present. The colonisation strategy of all of the different Rhynchosporium species involved extensive hyphal growth in the sub-cuticular regions of the leaves. Finally, new species-specific PCR diagnostic tests were developed that could distinguish between these five closely related Rhynchosporium species.
Introduction
Leaf blotch (scald) caused by the fungal pathogen Rhynchosporium commune is an economically important disease of barley (Hordeum vulgare) crops throughout the world [1] with yield losses of 1–10% common [2]. United Kingdom (UK) survey data indicates that barley crops grown in 2005 had 0.6% area with rhynchosporium lesions on leaf two at growth stage 75 (medium milk development stage; [3]). This equates to an estimated UK national yield loss of £10.8 million per annum (at a price of £225/tonne) despite fungicide treatment [4]. The symptoms of Rhynchosporium colonisation of barley can include coalescing lesions with dark brown margins and pale green or pale brown centres [5].
Phylogeographical analyses of R. commune isolates obtained from barley across five continents led to the conclusion that the pathogen did not emerge in association with its current barley host, believed to have been first domesticated in the ‘Fertile Crescent of the Middle East about 10,000 years before the present [6]. Instead, data from phylogenetic analyses using the R. commune avirulence gene NIP1 and flanking regions [7] suggested that modern populations of R. commune originated in northern Europe approximately 2500–5000 years ago, when the pathogen switched from a wild grass species onto cultivated barley shortly after barley was introduced there, and that it subsequently spread into other barley-growing areas of the world.
The Rhynchosporium pathogen is also found on a number of other graminaceous hosts, including rye (Secale cereale), triticale (x Triticosecale), cocksfoot (Dactylis glomerata) and Italian and perennial ryegrasses (Lolium multiflorum and Lolium perenne, respectively) [8], [9], [10], [11]. Insights into the evolutionary history of the Rhynchosporium pathogen on different hosts were provided by Zaffarano et al. [12. 13], who used sequencing of several gene loci and host range testing experiments to demonstrate that the genus Rhynchosporium is comprised of at least four closely-related, but host-specialised, species. Three of the species produce conidia terminating with an oblique point, termed beak-shaped conidia [14]; (i) R. commune causing leaf blotch symptoms on barley, wall barley (Hordeum murinum), wild barley (Hordeum spontaneum), barley grass (Hordeum glaucum, Hordeum leporinum) and brome-grass (Bromus diandrus); (ii) R. agropyri on bearded couch-grass (Agropyron caninum) and couch-grass (Agropyron repens); (iii) R. secalis on rye and triticale. The remaining species R. orthosporum, which is more distantly related [13], produces cylindrically-shaped conidia [14] and is specialised on cocksfoot.
One limitation of the studies by Zaffarano et al. [12], [13] is that Rhynchosporium isolates from ryegrasses were not included. These grasses are both economically important forage grasses and commonly occurring weeds of cereal crops on most continents, and although originally native to Europe, Asia and North Africa they have now been introduced into almost all temperate countries of the world [11], [15], [16]. A previous investigation [11] had identified two types of Rhynchosporium that could cause leaf blotch on ryegrasses, with differences between them in conidial shape and host range. However, it is at present unclear how Rhynchosporium isolates from ryegrasses are related to the four described fungal species [13], none of which included ryegrass species in host range definitions Therefore, there is a need to characterise isolates from ryegrasses to determine their relatedness to the Rhynchosporium species of Zaffarano et al. [13]. Such characterisation is essential to understand any potential role for ryegrass species as a source of Rhynchosporium inoculum able to initiate leaf blotch epidemics on barley crops.
Currently, only very limited PCR (polymerase chain reaction)-based methods are available to distinguish between the four Rhynchosporium species described by Zaffarano et al. [13]. A restriction fragment length polymorphism diagnostic was developed [13] that could discriminate between isolates of R. commune and R. secalis. However, this test is time-consuming due to the requirement for an additional restriction digest step following PCR. Apart from this test, the species can be distinguished only at a molecular level on the basis of single nucleotide polymorphisms. The development of species-specific PCR diagnostic tests would provide a valuable tool to directly confirm species identity of isolates and to determine species distribution in field samples of different grass hosts.
Little is currently known about the how the four recently described Rhynchosporium species of Zaffarano et al. [13] might differ in the manner by which they colonise their plant hosts. For example, only R. commune colonisation of barley [17] and R. orthosporum colonisation of cocksfoot [10] have been the subject of detailed microscopical investigations. On both hosts, Rhynchosporium hyphae grew extracellularly (i.e. outside the plant cells) and extensive colonisation of the leaf sub-cuticular area was observed. Although Caldwell [14] made some observations about the colonisation strategy of Rhynchosporium on other grass species, no microscopic evidence is currently available to determine whether R. agropyri, R. secalis and the pathogen on ryegrasses all colonise the same sub-cuticular niche of their respective grass hosts.
This paper reports work to further investigate the different Rhynchosporium species on various grass hosts. Specifically, it uses a combination of molecular, morphological, pathological and microscopy approaches to (i) report the discovery of R. commune isolates that are pathogenic to both barley and Italian ryegrass; (ii) describe a new species that is specialised only to ryegrass species; (iii) report results of both microscopic and molecular investigations into the different host-specialised Rhynchosporium species.
Methods
Ethics Statement
Diseased plant material was collected from plots (permission obtained from IBERS-Aberystwyth University, NIAB-TAG and Rothamsted Research, all based in the UK). Diseased plant material and fungal cultures were imported into the UK under Defra plant health license number PHL174G/6192(10/2009).
Isolation of the Rhynchosporium Pathogen
Leaves of barley, couch-grass, cocksfoot, Italian ryegrass and perennial ryegrass with distinct leaf blotch lesions were collected during the period of 2009–2011 from plots, samples being collected at least ∼1 metre apart within sites. To isolate Rhynchosporium, leaf blotch lesions were cut from green leaves, rinsed first in a 70% ethanol (v/v) solution for 2 min, followed by a 10% sodium hypochlorite (v/v) solution (minimum 8% available chlorine; Fisher Scientific, UK) for 5 min, and finished by a wash in sterile distilled water for 1 min. Lesions were then dried between pieces of sterile tissue paper and incubated at 18°C on Czapek dox plates (Sigma Aldrich, UK) amended to include 0.5% mycological peptone (Oxoid, UK) and penicillin and streptomycin sulphate at a final concentration of 100 and 50 parts per million, respectively. After 3 days, mycelium growing out from lesion margins was excised and used to establish single conidial cultures on lima bean agar (LBA; Difco, UK), which were grown at 18°C for 10 days.
Fungal Isolates Obtained and Long-term Storage Procedure
A total of 44 Rhynchosporium isolates were obtained from leaves with leaf blotch lesions collected from five sites in the UK and two sites in Romania. New isolates collected included 22 isolates from Italian or perennial ryegrass in the UK, 11 isolates from couch-grass in Romania and the UK, seven isolates from cocksfoot in the UK and four isolates from barley in the UK. An additional 49 isolates, including representatives of R. commune, R. agropyri, R. secalis and R. orthosporum, obtained from international collaborators, had previously been isolated from various field sites most of which were throughout Europe. Collaborators included Dr Louise Cooke (Agri-Food and Biosciences Institute, UK), Dr James Fountaine (Scotland’s Rural College, UK), Dr Nichola Hawkins (Rothamsted Research, UK), Dr Wolfgang Knogge (Leibniz Institute of Plant Biochemistry, Germany) and Prof Bruce McDonald and Dr Tryggvi Stefansson (ETH Zürich, Switzerland). All isolates were then stored as silica stocks at −80°C [18].
DNA Extraction
All fungal isolates were revived from −80°C storage by dispensing small amounts of silica stock onto potato dextrose agar (PDA, Oxoid, UK) plates overlaid with a single cellulose disk (A.A. Packaging Ltd, UK). Plates were sealed with a double layer of parafilm (Pechiney Plastic Packaging, USA) and incubated at 18°C in the dark for 10–15 days. After this period, fungal mycelium was scraped from the surface of the overlaid cellulose disk and DNA was extracted from lyophilized tissue using a DNeasy extraction kit (Qiagen, UK), according to the manufacturer’s instructions.
RAPD-PCR and Rep-PCR Genomic Fingerprinting
Seventy-nine Rhynchosporium isolates were examined by random amplification of polymorphic DNA PCR (RAPD-PCR) fingerprinting (Table S1) as described in Murtagh et al. [19], except that a TC-512 programmable thermal controller (Techne, UK) was used for all PCR. Seven RAPD-PCR primers (Operon Technologies, UK; Table S2) were used; in preliminary testing they had been found to generate polymorphisms with a subset of Rhynchosporium isolates. No template controls were included for use with all RAPD-PCR sets and selected isolates from each of the different species were tested in duplicate to ensure that results were reproducible. PCR products (10 µl) were separated by gel electrophoresis on 1.5% agarose gels in 1×Tris-Borate-EDTA (TBE) buffer (National Diagnostics, UK) and stained with an ethidium bromide solution (200 µl of a 1 mg/ml ethidium bromide per 100 ml of 1×TBE buffer). Amplicons were viewed on a transilluminator and digital images obtained (Gene Genius Bio Imaging System, Syngene, Synoptics Ltd, UK). Unambiguous bands were chosen for scoring and their presence or absence was recorded in binary form (1 = present, 0 = absent), with data from all seven RAPD-PCR primers combined in the analyses. A neighbour joining analysis was made using Jaccard’s coefficient and a boot-strapped phylogram (based on 1,000 repeats of the tree) was produced using FreeTree software [20]. A text version of the dendrogram of the tree was exported to TreeView software [21].
Seventy-one Rhynchosporium isolates were examined with repetitive-sequence-based PCR (rep-PCR) genomic fingerprinting (Table S1) using primer pair combinations ERIC2/BOXA1R and ERICF/BOXA1R [22], [23] (Table S2). No template controls were included for use with both primer pairs and selected isolates from each of the different species were tested in duplicate to ensure that results were reproducible. Reactions were carried out in 20 µl volumes, each containing 10 µl Jumpstart RedTaq mastermix (Sigma Aldrich, UK), 7 µl sterile distilled water, 1 µl each of both primers (10 pmol µl−1 stock) and 1 µl of template DNA (10 ng). For testing of five isolates (RS04ITA D-2.2, RS04ITA D-3.1, RS04ITA D-4.1, RS04ITA D-6.1, RS04ITA D-6.2), 10 µl of template DNA (total 10 ng) was added (achieved by increasing overall reaction volumes) because only dilute concentrations of template DNA were available. PCR was carried out using a PTC-100 programmable thermal controller (MJ Research, USA) and reaction conditions were an initial hold at 96°C for 2 min, followed by 35 cycles of 94°C for 30 sec, 52°C for 1 min and 65°C for 5 min. PCR products were then analysed as described for RAPD-PCR products.
Phylogenetic Analyses
Partial sequences of the alpha-tubulin, beta-tubulin and ITS (internal transcribed spacer) gene loci were obtained for ten isolates (2lm11, 3ar10, 6ar10 and 10ar10, 59dg09, Rs04ITA D-6.2, 4lm11, 7lm11, 13lp11 and 15lp11; isolate details and accompanying GenBank accession numbers provided in Table S3) using primers (Table S2) and reaction conditions described by Zaffarano et al. [12]. No template controls were included in reactions to amplify the three gene loci. Jumpstart high fidelity mix (Roche, Germany) was used in all reactions, with reaction components selected according to the manufacturer’s instructions with the following modifications; no DMSO was included and each reaction included 10 mM of each dNTP (Fermentas, UK) and 10–25 ng of template DNA. PCR products were visualised on a 1% agarose gel to ensure the presence of a single amplicon and purified using a MinElute kit (Qiagen, UK) according to the manufacturer’s instructions. DNA was sent to Eurofin MWG Operon for bi-directional sequencing using an ABI 3730XL machine, with the exception of the beta-tubulin PCR product for which only primer BTUB21F was used.
Individual sequences were imported into the BioEdit Sequence Alignment Editor (version 7.0.9.0; [24]) and trace sequence data of poor read quality were removed. Sequences were imported into the Geneious Pro 5.5.6 software package and the partial alpha-tubulin, beta-tubulin and ITS (partial 18S rRNA, ITS1, 5.8S rRNA, partial ITS2) sequences were edited to 1538, 542 and 492 bases in length (including gaps), respectively. For the ten isolates partially sequenced in the present study, sequence data of all three gene loci were concatenated. The CLUSTAL W algorithm contained in the Geneious software package was then used to align these sequences with the concatenated haplotype sequences of R. commune, R. agropyri, R. secalis and R. orthosporum obtained by Zaffarano et al. [12].
The relationship of isolates and haplotypes was inferred using the coalescent-based Bayesian Markov Chain Monte Carlo (MCMC) method implemented in the program BEAST version 1.4.1 [25]. To allow a direct comparison with previous studies, a strict molecular clock model was applied and the phylogenetic tree was internally calibrated by assuming a time-to-most-recent-common-ancestor (TMRCA) of 2487–4791 years (mean 3625) as inferred for the cluster R commune/R. agropyri/R. secalis [12]. The MCMC analysis was run for 107 generations, sampling every 1000th iteration after an initial burn-in of 10%. The performance of the MCMC process was checked for stationarity and large effective sample sizes in TRACER (available from http://beast.bio.ed.ac.uk/Tracer). A maximum clade credibility tree was constructed after discarding the first 10% of inferred trees. The mean and corresponding credibility intervals of the estimated TMRCAs were depicted using TRACER.
Microscopic Analysis of Conidial Morphology
Forty isolates, including isolates of R. commune, R. agropyri, R. secalis, R. orthosporum or Rhynchosporium isolates collected from ryegrasses (Table S1), were grown on LBA plates at 15°C in the dark. After 10 days of growth, sterile distilled water (2 ml) was added to each LBA plate and conidia were dislodged using an L-shaped sterile plastic spreader. Conidial suspensions were placed in sterile 2 ml microfuge tubes after filtration through two layers of sterile muslin to remove mycelial fragments. Conidial dimensions of 26 isolates were measured using fresh (within 8 hours of harvest) conidial suspensions maintained on ice (∼0°C), while conidial suspensions of 18 isolates (including four that were also measured fresh) were stored at −20°C and measured later. The shape of individual conidia of individual isolates was recorded as either beak-shaped or cylindrically-shaped [14]. Both the length and width of 25 mature (defined as having two cells clearly divided by a septum) conidia were measured with a digital CCD camera (Hamamatsu C8484 05G01) using HCimage software (Hamamatsu Photonics K.K., Japan).
Light microscopic images of representative conidia of 17 isolates were also made on a Zeiss axiophot light microscope; images were obtained using a QImaging monochrome camera equipped with a Retiga XEi liquid crystal RGB filter and operated using MetaMorph ver. 7.6 software. Before statistical analysis of data, isolates were separated into two different groups; those with beak-shaped conidia (R. commune including two isolates from Italian ryegrass, R. agropyri and R. secalis) and those with cylindrically-shaped conidia (R. orthosporum and Rhynchosporium isolates from ryegrasses). Data were analysed using ANOVA (GenStat version 14; [26]).
Host Range Testing
Twenty-two Rhynchosporium isolates (Table 1) were revived from −80°C silica stocks onto LBA plates as described previously in the text, with plates sealed with a double layer of parafilm and grown at 18°C in the dark for 10–12 days. Conidia were harvested from LBA plates as described previously in the text, and concentrations of suspensions were determined using a haemocytometer (Improved Neubauer haemocytometer, Weber Scientific International, UK). Aliquots (5–10 ml) of fungal inoculum (5×105 conidia ml−1) were prepared using sterile distilled water (containing 0.01% Tween 80; Fisher Scientific, UK) in 50 ml Falcon tubes (CellStar, USA) and frozen at −20°C until required.
Table 1. Host range experiments suggest general host-specialisation of Rhynchosporium species.
| Development of leaf blotch lesionsa, b | ||||||||
| Species | Isolate | Original host | Origin | Collected | Barleyc | Cocksfootd | Italian ryegrasse | Perennial ryegrasse |
| R. commune | 19hv09 | Barley | Hertfordshire, UK | 2009 | + + | − − | − −, − − | − −, − − |
| R. commune | 53hv09 | Barley | Hertfordshire, UK | 2009 | + + | − − | − −, − − | − −, − − |
| R. commune | 73hv09 | Barley | Hertfordshire, UK | 2009 | + + | − − | − −, − − | − −, − − |
| R. commune | D.1.1 | Wall barley | Switzerland | 2004 | + + | − − | − −, − − | − −, − − |
| R. commune | E.1.2 | Wall barley | Switzerland | 2004 | + + | − − | − −, − − | − −, − − |
| R. commune | 2lm11 | Italian ryegrass | Shropshire, UK | 2011 | + + | − − | − −,++ | − −, − − |
| R. commune | 5lm11 | Italian ryegrass | Shropshire, UK | 2011 | + + | − − | − −, − + | − −, − − |
| R. orthosporum | 57dg09 | Cocksfoot | Aberystwyth, UK | 2009 | − − | + + | − −, − − | − −, − − |
| R. orthosporum | 59dg09 | Cocksfoot | Aberystwyth, UK | 2009 | − − | − − | − −, − − | − −, − − |
| R. orthosporum | RsCH04 Bär A.1.1.3 | Cocksfoot | Switzerland | 2004 | − − | + + | − −, − − | − −, − − |
| R. orthosporum | RS04CG-BAR-A.1.1.3 | Cocksfoot | Switzerland | 2004 | − − | + + | − −, − − | − −, − − |
| R. orthosporum | RS04CG-BAR-A.1.1.4 | Cocksfoot | Switzerland | 2004 | − − | + + | − −, − − | − −, − − |
| R. lolii | 6lm11 | Italian ryegrass | Aberystwyth, UK | 2011 | − − | − − | + −, − − | − +, − − |
| R. lolii | 9lm11 | Italian ryegrass | Aberystwyth, UK | 2011 | − − | − − | − −,+− | + −, − − |
| R. lolii | 10lm11 | Italian ryegrass | Aberystwyth, UK | 2011 | − − | − − | − +,+− | + −, − − |
| R. lolii | 21lm11 | Italian ryegrass | Shropshire, UK | 2011 | − − | − − | − +,++ | − −, − − |
| R. lolii | 22lm11 | Italian ryegrass | Shropshire, UK | 2011 | − − | − − | + −, − + | − +, − − |
| R. lolii | 11lp11 | Perennial ryegrass | Aberystwyth, UK | 2011 | − − | − − | + −,+− | − +, − + |
| R. lolii | 13lp11 | Perennial ryegrass | Aberystwyth, UK | 2011 | − − | − − | − −, − − | − −,++ |
| R. lolii | 15lp11 | Perennial ryegrass | Shropshire, UK | 2011 | − − | − − | − −, − − | − +, − − |
| R. lolii | 16lp11 | Perennial ryegrass | Surrey, UK | 2011 | − − | − − | − −, − − | − −,++ |
| R. lolii | 20lp11 | Perennial ryegrass | Hertfordshire, UK | 2011 | − − | − − | − −,+− | − −, − + |
| n/a | Water control | n/af | n/a | n/a | − − | − − | − −, − − | − −, − − |
Leaf blotch disease symptoms developed at 23 days post inoculation when isolates of Rhynchosporium commune, R. orthosporum or R. lolii were cross-inoculated onto different hosts under controlled environment conditions.
Whole plants scored for presence (+) or absence (−) of leaf blotch lesions;
Scores for replicate plants are given;
Plants 14-days-old at time of inoculation;
Plants 35-days-old at time of inoculation;
Plants 35- or 40- days-old at time of inoculation (results separated by a comma);
n/a, not applicable.
All plants were grown and inoculated under controlled environment conditions, with a constant temperature of 15°C and a relative humidity of 70%. There was a 12 hour photoperiod with available light set at 700 µmol m2/sec. Seeds were sown in Rothamsted prescription mix compost (Petersfield Products, UK) at approximately 2 cm depth. Before inoculation, barley (cv. Optic) (14-day-old plants; seed supplied by the Rothamsted farm), cocksfoot (35-day-old plants) and both Italian and perennial ryegrass (35- or 40-day-old plants; seeds supplied by Herbiseed Ltd., UK) plants were grown individually in pots (4 cm×4 cm).
Immediately before inoculation, Falcon tubes containing Rhynchosporium inoculum were removed from storage at −20°C and allowed to defrost. Inoculum of each isolate was then transferred to a separate 20 ml plastic spray bottle. Conidial suspensions of each isolate were then sprayed onto two replicate whole plants of each graminaceous host, using a completely randomised design, until leaves were evenly coated with fine droplets. Plants were then maintained in a humid environment for 48 h by sealing them in 650–800 mm polyethylene bags (VWR International Ltd, UK). After this time, the top and bottom of each polyethylene bag were removed so that plants remained isolated but open to ambient relative humidity. In the experiments, sterile distilled water (containing 0.01% Tween 80) was used as a negative control. At 23 days post inoculation (dpi), whole plants were assessed for the presence or absence of typical leaf blotch lesions.
Barley and Italian ryegrass leaves that had been inoculated with two isolates of R. commune (2lm11 and 5lm11) that had produced typical leaf blotch lesions on both hosts (or with sterile distilled water, no lesions) were also examined in duplicate by scanning electron microscopy (SEM) (21 or 28 dpi as required, see Figure 4 legend).
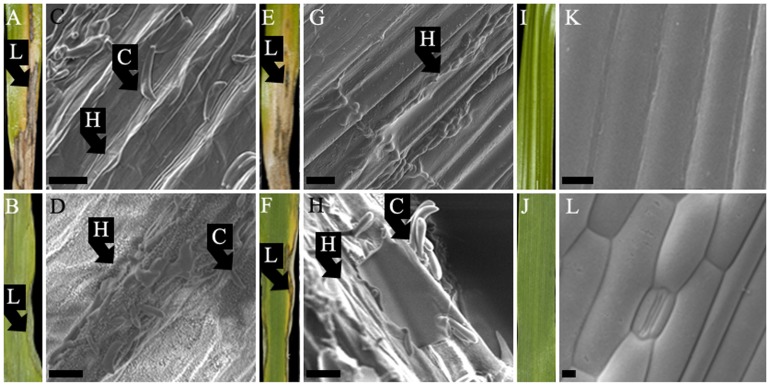
Figure 4. R. commune isolates that cause leaf blotch lesions on both Italian ryegrass and barley.
Isolate 2lm11 caused lesions (L) when inoculated onto (A) Italian ryegrass or (B) barley (cv. Optic) leaves; scanning electron microscopic (SEM) examination of these hosts (C, D) showed sub-cuticular hyphae (H) and sporulation with beak-shaped conidia (C) on both hosts. Isolate 5lm11 also caused lesions on both Italian ryegrass (E) and barley (F); SEM examination showed that the pathogen could colonise both hosts (G, H) and sporulation with beak-shaped conidia was observed on barley. Control leaves of Italian ryegrass and barley treated with water (I, J) did not develop leaf blotch symptoms and SEM examination (K, L) found no evidence for the presence of R. commune. Photographs of leaf symptoms were taken at 17 (B, F, J) and 24 (A, E, I) days post inoculation (dpi); electron micrographs were taken at 21 (D, H, L) and 28 (C, G, H) dpi. All leaf pieces were c. 4 cm long; scale bars on electron micrographs are 10 µm.
Colonisation Strategy
Plants of barley, rye, triticale, cocksfoot and Italian ryegrass were grown and inoculated under the controlled environment conditions for host range testing, except that leaves of the first three hosts were all 14-days-old at the time of inoculation and only the second fully expanded leaves were inoculated. Barley leaves (cv. Sumo) were inoculated with an isolate of R. commune (53hv09), rye leaves with an isolate of R. secalis (RS99CH1 H10B), triticale leaves with an isolate of R. secalis (I-1a), cocksfoot leaves with an isolate of R. orthosporum (RsCH04 Bär A.1.1.3) and Italian ryegrass leaves inoculated with an isolate originally collected from this host (9lm11) (see Table 2 for isolate details). Leaves of all of these graminaceous hosts with typical leaf blotch lesions were examined using SEM (14–30 dpi as required; see Figure 5 legend). In addition, couch-grass leaves colonised by R. agropyri and displaying typical leaf blotch lesions were also examined by SEM. However, it had not been possible to produce leaf blotch lesions by inoculating couch-grass with R. agropyri in controlled environments, despite the use of isolates from different geographical origins to inoculate couch grass plants of different ages. Therefore couch-grass leaf specimens colonised by R. agropyri were collected from the field in Hertfordshire (UK) in April 2010 and used for examination.
Table 2. Validation of species-specific diagnostic tests for five Rhynchosporium species.
| Species | Isolate code | Host | Geographical origin | Collected | R. commune (LinA-F/R) | R. agropyri (RA6-F/R) | R. secalis (RS25-F/R) | R. orthosporum/R. lolii (2RO-F/R) | R. lolii (ERIC2/BOXAIR) |
| R. commune | 788 | Barley | France | 1997 | + | − | − | ||
| R. commune | K1124 | Barley | UK | Unknown | + | − | − | − | |
| R. commune | QUB 12-3 | Barley | Northern Ireland, UK | Unknown | + | − | − | − | |
| R. commune | OSA 28-2-2 | Barley | Hertfordshire, UK | 2002 | + | − | − | − | |
| R. commune | FI12-63 | Barley | Finland | 1996 | + | − | − | ||
| R. commune | RS 219 | Barley | UK | 2004 | + | − | − | ||
| R. commune | QUB 9-10 | Barley | Northern Ireland, UK | Unknown | + | − | − | ||
| R. commune | R.s. 2310 4.2 | Barley | France | 2008 | + | − | − | ||
| R. commune | R.s. 2318 4.2 | Barley | France | 2008 | + | − | − | − | |
| R. commune | 19hv09 | Barley | Hertfordshire, UK | 2009 | + | − | − | − | − |
| R. commune | 53hv09 | Barley | Hertfordshire, UK | 2009 | + | − | − | − | − |
| R. commune | 73hv09 | Barley | Hertfordshire, UK | 2009 | + | − | − | − | |
| R. commune | UK7 | Barley | Aberystwyth, UK | Unknown | + | − | − | − | |
| R. commune | D.1.1 | Wall barley | Switzerland | 2004 | + | − | − | − | |
| R. commune | E.1.2 | Wall barley | Switzerland | 2004 | + | − | − | − | |
| R. commune | 2lm11 | Italian ryegrass | Shropshire, UK | 2011 | + | − | − | − | − |
| R. commune | 5lm11 | Italian ryegrass | Shropshire, UK | 2011 | + | − | − | − | − |
| R. agropyri | RS04CG-RAC-A.4.3 | Couch-grass | Switzerland | 2004 | − | + | − | − | |
| R. agropyri | RS04CG-RAC-A.5.2 | Couch-grass | Switzerland | 2004 | − | + | − | − | − |
| R. agropyri | RS04CG-RAC-A.6.1 | Couch-grass | Switzerland | 2004 | − | + | − | − | − |
| R. agropyri | 1ar10 | Couch-grass | Surrey, UK | 2010 | − | + | − | − | |
| R. agropyri | 2ar10 | Couch-grass | Surrey, UK | 2010 | − | + | − | − | − |
| R. agropyri | 3ar10 | Couch-grass | Surrey, UK | 2010 | − | + | − | − | |
| R. agropyri | 6ar10 | Couch-grass | Cluj-Napoca, Romania | 2010 | − | + | − | − | − |
| R. agropyri | 7ar10 | Couch-grass | Timisoara, Romania | 2010 | − | + | − | − | − |
| R. agropyri | 8ar10 | Couch-grass | Nottingham, UK | 2010 | − | + | − | − | − |
| R. agropyri | 9ar10 | Couch-grass | Nottingham, UK | 2010 | − | + | − | − | |
| R. agropyri | 10ar10 | Couch-grass | Nottingham, UK | 2010 | − | + | − | − | |
| R. agropyri | 11ar10 | Couch-grass | Nottingham, UK | 2010 | − | + | − | − | |
| R. secalis | RS02CH4-2a1 | Rye | Switzerland | 2002 | − | − | + | − | − |
| R. secalis | RS02CH4-4b1 | Rye | Switzerland | 2002 | − | − | + | − | − |
| R. secalis | RS02CH4-5a1 | Rye | Switzerland | 2002 | − | − | + | − | − |
| R. secalis | Rs02CH4-6a.1 | Rye | Switzerland | 2002 | − | − | + | − | |
| R. secalis | RS99CH1-H10B | Rye | Switzerland | 1999 | − | − | + | − | |
| R. secalis | RS02CH4-13a1 | Rye | Switzerland | 2002 | − | − | + | − | − |
| R. secalis | RS02CH4-14a1 | Rye | Switzerland | 2002 | − | − | + | − | |
| R. secalis | 8.4 | Rye | Russia | 2003 | − | − | + | − | |
| R. secalis | 6.2 | Rye | Russia | 2003 | − | − | + | − | − |
| R. secalis | 1E7a | Rye | Switzerland | 1999 | − | − | + | − | − |
| R. secalis | 1B8 | Rye | Switzerland | 1999 | − | − | + | − | − |
| R. secalis | 1D4a | Rye | Switzerland | 1999 | − | − | + | − | |
| R. secalis | I-1a | Triticale | Switzerland | 2002 | − | − | + | − | − |
| R. secalis | I-2a2 | Triticale | Switzerland | 2002 | − | − | + | − | − |
| R. secalis | I-3a1 | Triticale | Switzerland | 2002 | − | − | + | − | |
| R. orthosporum | 27dg09 | Cocksfoot | Aberystwyth, UK | 2009 | − | − | − | + | − |
| R. orthosporum | 51dg09 | Cocksfoot | Aberystwyth, UK | 2009 | + | − | |||
| R. orthosporum | 52dg09 | Cocksfoot | Aberystwyth, UK | 2009 | + | − | |||
| R. orthosporum | 57dg09 | Cocksfoot | Aberystwyth, UK | 2009 | − | − | − | + | |
| R. orthosporum | 59dg09 | Cocksfoot | Aberystwyth, UK | 2009 | − | − | − | + | |
| R. orthosporum | RS04CG-BAR-A.1.1.3 | Cocksfoot | Switzerland | 2004 | − | − | − | + | − |
| R. orthosporum | RS04CG-BAR-A.1.1.4 | Cocksfoot | Switzerland | 2004 | − | − | − | + | − |
| R. orthosporum | RS04ITA D-6.1 | Cocksfoot | Italy | 2004 | − | − | − | + | − |
| R. orthosporum | RS04ITA D-6.2 | Cocksfoot | Italy | 2004 | − | − | − | + | − |
| R. lolii | 1lm11 | Italian ryegrass | Shropshire, UK | 2011 | − | − | − | + | |
| R. lolii | 3lm11 | Italian ryegrass | Shropshire, UK | 2011 | − | − | − | + | + |
| R. lolii | 4lm11 | Italian ryegrass | Shropshire, UK | 2011 | − | − | − | + | + |
| R. lolii | 6lm11 | Italian ryegrass | Aberystwyth, UK | 2011 | − | − | − | + | |
| R. lolii | 7lm11 | Italian ryegrass | Aberystwyth, UK | 2011 | − | − | − | + | + |
| R. lolii | 8lm11 | Italian ryegrass | Aberystwyth, UK | 2011 | − | − | − | + | + |
| R. lolii | 9lm11 | Italian ryegrass | Aberystwyth, UK | 2011 | − | − | − | + | |
| R. lolii | 10lm11 | Italian ryegrass | Aberystwyth, UK | 2011 | − | − | − | + | |
| R. lolii | 21lm11 | Italian ryegrass | Shropshire, UK | 2011 | − | − | − | + | |
| R. lolii | 22lm11 | Italian ryegrass | Shropshire, UK | 2011 | − | − | − | + | |
| R. lolii | 11lp11 | Perennial ryegrass | Aberystwyth, UK | 2011 | − | − | − | ||
| R. lolii | 12lp11 | Perennial ryegrass | Aberystwyth, UK | 2011 | − | − | − | + | + |
| R. lolii | 13lp11 | Perennial ryegrass | Aberystwyth, UK | 2011 | − | − | − | + | + |
| R. lolii | 14lp11 | Perennial ryegrass | Aberystwyth, UK | 2011 | − | − | − | + | + |
| R. lolii | 15lp11 | Perennial ryegrass | Shropshire, UK | 2011 | − | − | − | + | + |
| R. lolii | 16lp11 | Perennial ryegrass | Surrey, UK | 2011 | − | − | − | ||
| R. lolii | 17lp11 | Perennial ryegrass | Hertfordshire, UK | 2011 | − | − | − | + | + |
| R. lolii | 18lp11 | Perennial ryegrass | Hertfordshire, UK | 2011 | − | − | − | + | + |
| R. lolii | 20lp11 | Perennial ryegrass | Hertfordshire, UK | 2011 | − | − | − | + | |
| Leptosphaeria maculans | LMA1 | Oilseed rape | Unknown | Unknown | − | − | − | − | n/a |
| L. maculans | LMA5 | Oilseed rape | Unknown | Unknown | − | − | − | − | n/a |
| Pyrenopeziza brassicae | PbCRA | Oilseed rape | UK | 1988 | − | − | − | − | n/a |
| P. brassicae | PbCRB | Oilseed rape | UK | 1988 | − | − | − | − | n/a |
| Sclerotinia sclerotiorum | SSGFRII | Oilseed rape | Unknown | Unknown | − | − | − | − | n/a |
| Fusarium graminearum | 602.10 | Wheat | Unknown | Unknown | − | − | − | − | n/a |
| F. culmorum | UK 99 | Wheat | Unknown | Unknown | − | − | − | − | n/a |
| Host plant DNAa | n/ab | Barley | n/a | n/a | − | − | − | − | n/a |
| Host plant DNAa | n/a | Rye | n/a | n/a | − | − | − | − | n/a |
| Host plant DNAa | n/a | Cocksfoot | n/a | n/a | − | n/a |
All the diagnostic tests detected only the predicted Rhynchosporium species when tested against a range of fungal isolates and plant DNA. PCR using primer pair LinA-F/R produced an amplicon of 145-bp specific for R. commune isolates; RA6-F/R produced an amplicon of 461-bp specific for R. agropyri isolates; RS25-F/R produced an amplicon of 1240-bp specific for R. secalis isolates; 2RO-F/R produced an amplicon of 277-bp specific for both R. orthosporum and R. lolii isolates; rep-PCR genomic fingerprinting using primers ERIC2/BOXA1R produced an amplicon of ∼400-bp specific for isolates of R. lolii.
Barley (cv. Sumo), rye or cocksfoot DNA extracted from leaves of healthy seedlings (no leaf blotch lesions present) grown under controlled environment conditions was confirmed to be free of detectable Rhynchosporium DNA using the quantitative PCR assay of Fountaine et al. [30] (data not shown);
n/a = not applicable.
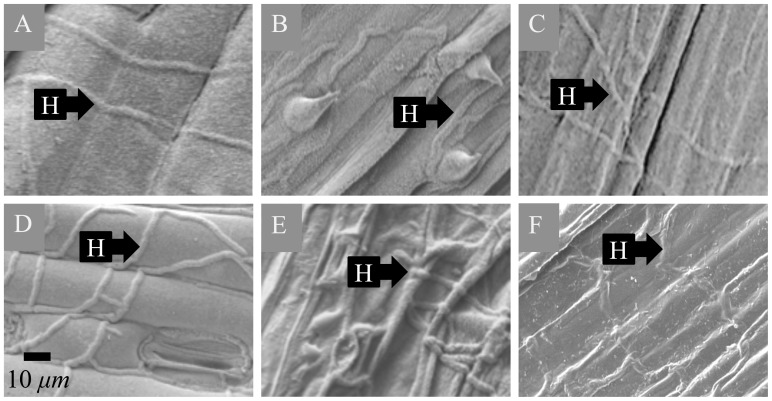
Figure 5. Five Rhynchosporium species colonise the same sub-cuticular niche in their hosts.
Sub-cuticular hyphal (H) growth of (A) R. commune (isolate 53hv09) at 28 days post inoculation (dpi) on a leaf of barley (cv. Sumo); (B) R. agropyri on a leaf of couch-grass collected from the field (Hertfordshire, UK) in April 2010; (C) R. secalis (RS99CH1 H10B) at 30 dpi on a leaf of rye; (D) R. secalis isolate (I-1a) at 28 dpi on a leaf of triticale; (E) R. orthosporum (RsCH04 Bär A.1.1.3) at 14 dpi on a leaf of cocksfoot; (F) R. lolii (9lm11) at 28 dpi on a leaf of Italian ryegrass. Scale-bars on electron micrographs are 10 µm.
For SEM, leaves were collected and placed in individual 9 cm diameter Petri dishes, which were lined with moist filter paper (Whatman No. 8) and sealed with a single layer of parafilm. Pieces of leaves, approximately 5 mm×5 mm, were cut out using a sterile razor blade. These were immediately mounted onto aluminium cryo-stubs using a smear of Tissue-Tek © O.C.T. compound (Sakura Finetek, USA) and plunged into pre-slushed liquid nitrogen (−280°C) to freeze them. The samples were then transferred under vacuum to the Alto 2100 (Gatan, UK) cryo-chamber stage, with the temperature maintained at −180°C. Sublimation of any contaminating ice and gold coating (10 nm thickness) was done in this chamber. Samples were then transferred to the stage of the scanning electron microscope (JSM 6360 LVSEM, Jeol, UK) with the temperature maintained at −150°C for examination and imaging using the on-board software (Jeol, UK).
Species-specific PCR Diagnostic Tests
Four PCR-based diagnostic tests, relying on either sequence alignments of known genetic loci (ITS and beta-tubulin) and non-coding nuclear RFLP loci (pRS52; [27]) or using RAPD-PCR derived sequence, were developed to both detect and distinguish between isolates of (i) R. commune (including two isolates from Italian ryegrass), (ii) R. agropyri, (iii) R. secalis, (iv) R. orthosporum and all other Rhynchosporium isolates obtained from ryegrasses. Primers (for all sequences see Table S2) were designed using Vector NTI software (Invitrogen, USA). All PCR reactions were set up on ice (∼0°C) and used a PTC-100 programmable thermal controller (MJ Research, USA); all testing using these diagnostic tests included no template controls. PCR products (10 µl) were separated by gel electrophoresis in 1×TBE buffer on 1% agarose gels. Gels were either stained with ethidium bromide solution as described previously in the text or incorporated with ethidium bromide (0.5 µg/ml) during preparation. Amplicons were viewed on a transilluminator and digital images recorded (Gene Genius Bio Imaging System, Syngene, Synoptics Ltd, UK).
R. commune-specific primers (LinA-F/R) were designed based on alignments of partial sequences of the ITS region and were predicted to produce a 145-bp amplicon specific only for template DNA of this species. PCR was carried out in 20 µl reaction volumes, each containing 10 µl RedTaq ReadyMix (2×concentrate, Sigma Aldrich, UK), 2 µl each of both primers (1 pmol µl−1 stock), 5 µl of sterile distilled water and 1 µl of template DNA (10 ng). Reaction conditions were as follows; 35 cycles of 95°C for 1 min, 66°C for 1 min, 72°C for 1 min before a final elongation step of 72°C for 5 min, with a final hold at 4°C.
R. agropyri-specific primers (RA6-F/R) were designed based on alignments of pRS52 sequences [27] and were predicted to produce a 461-bp amplicon specific only for template DNA of this species. PCR was carried out in 25 µl reaction volumes, each containing 12.5 µl of RedTaq ReadyMix (2×concentrate, Sigma Aldrich, UK), 1 µl each of both primers (5 pmol µl−1 stock), 9.5 µl of sterile distilled water and 1 µl of template DNA (10 ng). Reaction conditions were as follows; 35 cycles of 94°C for 1 min, 55°C for 2 min and 72°C for 2 min before a final elongation step of 72°C for 5 min, with a final hold at 4°C.
R. secalis-specific primers were developed as sequenced characterised amplified region (SCAR) markers [28]. An amplicon of ∼1240-bp was produced specifically for isolates of R. secalis in RAPD-PCR testing with primer OPW-05. This amplicon was purified from an agarose gel using a QiaQuick gel extraction kit (Qiagen, UK) and cloned using a Strataclone PCR cloning kit (Agilent Technologies, UK). Plasmids with inserts of the correct size were then purified from cultures using the Fermentas GeneJet plasmid prep kit (Fermentas, UK) and sent to Eurofins MWG Operon for bi-directional sequencing using an ABI 3730XL machine. Resulting sequence data were then used to develop SCAR-PCR primers by extending the original 10-bp RAPD primer sequence into the flanking regions. These R. secalis-specific primers (RS25-F/R) were predicted to produce a 1240-bp amplicon specific only for template DNA of this species. PCR was carried out in 25 µl reaction volumes, each containing 12.5 µl RedTaq ReadyMix (2×concentrate, Sigma Aldrich, UK), 1 µl each of both primers (1 pmol µl−1 stock), 9.5 µl of sterile distilled water and 1 µl of template DNA (10 ng). Reaction conditions were as follows; 35 cycles of 94°C for 1 min, 57°C for 2 min, 72°C for 2 min before a final elongation step of 72°C for 5 min, with a final hold at 4°C.
Primers specific for R. orthosporum and most Rhynchosporium isolates from ryegrasses (2RO-F/R) were designed using alignments of partial sequences of the beta-tubulin region and were predicted to produce a 277-bp amplicon specific for template DNA of only these two species. PCR was carried out in 25 µl reaction volumes, each containing 12.5 µl RedTaq ReadyMix (2×concentrate), 1 µl each of both primers (1 pmol µl−1 stock), 9.5 µl of sterile distilled water and 1 µl of template DNA (1 ng). Reaction conditions were as follows; 35 cycles of 94°C for 1 min, 52°C for 1 min, 72°C for 1 min before a final elongation step of 72°C for 5 min, with a final hold at 4°C.
The specificity of all four diagnostic tests was evaluated by screening them against template DNA from a collection of Rhynchosporium isolates (Table 2). Specificity of the four tests was further confirmed by screening against template DNA of other crop pathogens, including the closely related Pyrenopeziza brassicae [29], and different plant hosts (e.g. barley) by PCR with the addition of the appropriate DNA template (in all cases, 10 ng template DNA was added; Table 2). In addition, the sensitivities of the four diagnostic tests were evaluated by screening against a total of 25 ng of mixed template DNA, with different amounts of DNA of the respective Rhynchosporium species (10 ng, 5 ng, 1 ng, 100 pg, 1 pg, 0 pg) used in a background of corresponding healthy host plant DNA (15 ng, 20 ng, 24 ng, 24.9 ng, 24.99 ng, 25 ng, respectively; confirmed to be free of detectable Rhynchosporium DNA using the quantitative PCR assay of Fountaine et al. [30] (data not shown). However, in the 20 or 25 µl reaction volume, as appropriate, 2 µl of template DNA was included by reducing by 1 µl the volume of sterile distilled water. The R. commune test was evaluated using isolate UK7 [31] in a background of healthy barley (cv. Sumo) plant template DNA, the R. agropyri test evaluated using isolate 7ar10 in a background of healthy barley (cv. Sumo) plant template DNA, the R. secalis test evaluated using isolate I-1a in a background of healthy rye plant template DNA and the R. orthosporum/most isolates from ryegrasses test evaluated using isolate 57dg09 in a background of healthy cocksfoot plant template DNA.
Finally, a fifth PCR-based test, based on data obtained from the rep-PCR genomic fingerprinting, was developed to distinguish between confirmed (using primer pair 2RO-F/R) isolates of R. orthosporum and most isolates from ryegrasses. Rep-PCR genomic fingerprinting using primer pair ERIC2/BOXA1R against a collection of 71 Rhynchosporium isolates was carried out as described previously, with these primers predicted to produce an ∼400-bp amplicon for most isolates from ryegrasses but not for isolates of R. orthosporum.
Nomenclature
The electronic version of this article in Portable Document Format (PDF) is a work with an ISSN or ISBN number that will represent a published work according to the International Code of Nomenclature for algae, fungi, and plants and hence the new names contained in the electronic publication of a PLOS ONE article are effectively published under that Code from the electronic edition alone, so there is no longer any need to provide printed copies.
In addition, the new name contained in this work has been submitted to MycoBank (MB 803876) from where it will be made available to the Global Names Index. The unique MycoBank number can be resolved and the associated information viewed through any standard web browser by appending the MycoBank number contained in this publication to the prefix http://www.mycobank.org/mb/. The online version of this work is archived and available from the following digital repositories: PubMed Central, LOCKSS.
Results
DNA Fingerprint Analysis
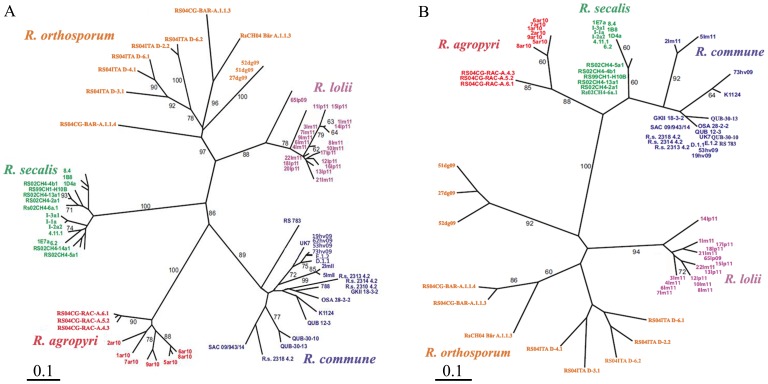
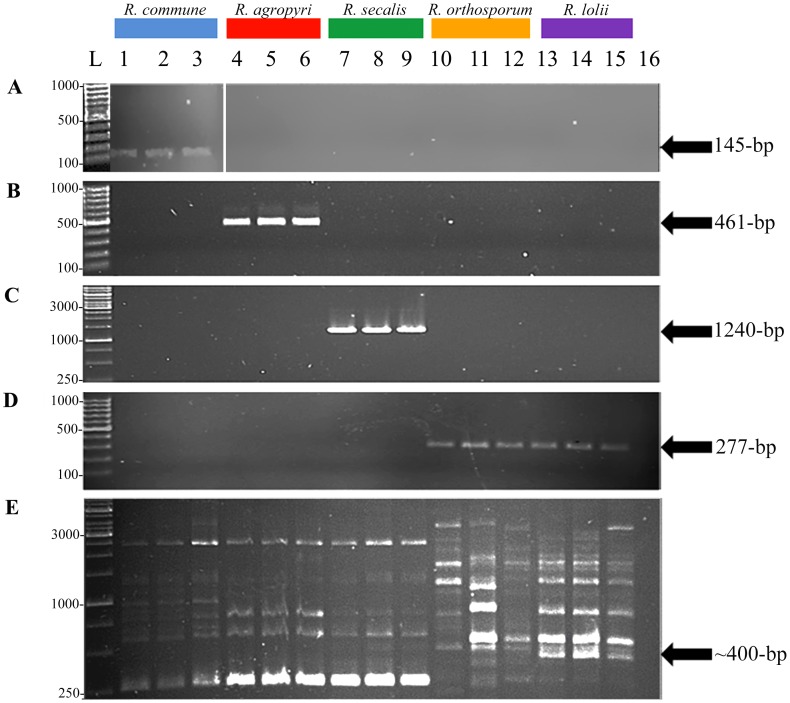
Both RAPD-PCR (Fig. 1A) and rep-PCR (Fig. 1B) methodologies could discriminate between isolates of R. commune, R. agropyri, R. secalis and R. orthosporum obtained from proximate geographical origins. Furthermore, in both analyses two distinct types of Rhynchosporium isolates were found on ryegrasses. Two isolates (2lm11 and 5lm11) collected from Italian ryegrass clustered within the main R. commune grouping, forming a terminal clade. However, all other isolates collected from both Italian and perennial ryegrasses formed a separate grouping with strong bootstrap support values (>85% for both DNA fingerprinting methods) distinct from all the other previously described species of Rhynchosporium (Figs 1A and 1B), consistent with the presence of a novel species. This grouping was tentatively named Rhynchosporium lolii, subject to further evidence of speciation that would allow a formal description (see below). R. orthosporum was the closest sister species to R. lolii, the latter of which showed an intermixing of isolates from both Italian and perennial ryegrasses.
Figure 1. DNA fingerprinting methods distinguish between five Rhynchosporium species.
(A) RAPD-PCR fingerprinting of 79 isolates using combined data from seven RAPD-PCR primers; (B) rep-PCR genomic fingerprinting of 71 isolates using combined data from two primer pairs (ERIC2/BOXA1R and ERICF/BOXA1R). Both unrooted trees were constructed by neighbour-joining analyses with branch lengths drawn to show genetic distance derived from Jaccard’s coefficient of band matching (scale bar: 0.1 = 10% genetic difference). Numbers at nodes indicate the percentage bootstrap support (based on 1000 re-samplings) for the groupings; only values (A) >60% and (B) >70% are shown, for clarity. Both fingerprinting methods discriminated between isolates of R. commune (blue), R. agropyri (red), R. secalis (green), R. orthosporum (yellow) and R. lolii (purple). Note that two isolates of R. commune (2lm11 and 5lm11) were collected from Italian ryegrass.
DNA Phylogeny and Times to the Most Recent Common Ancestor (TMRCA)
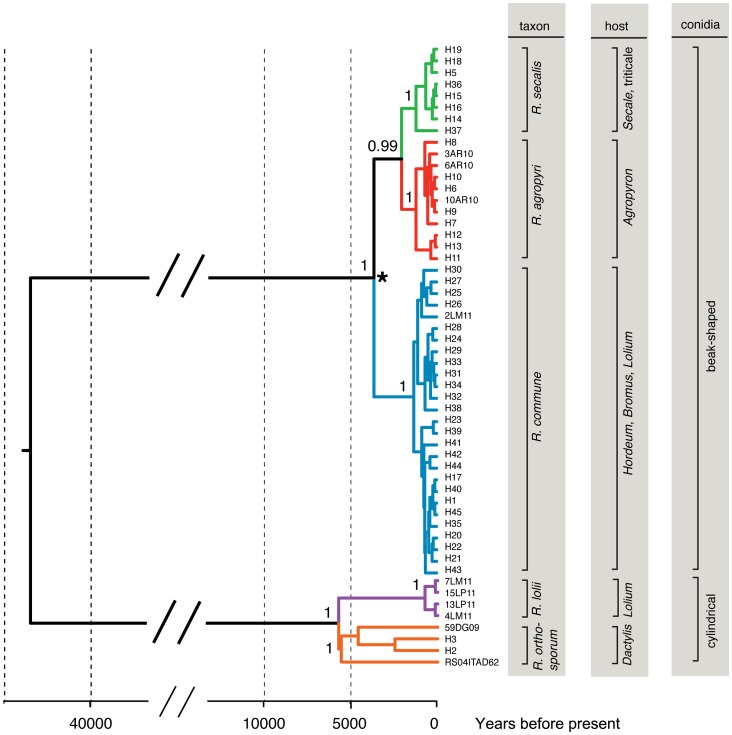
Bayesian phylogenetic analysis resulted in a tree topology with strongly supported clusters for all of the previously described Rhynchosporium species (Fig. 2). The phylogenetic analysis confirmed that two distinct Rhynchosporium species had been isolated from ryegrasses. Isolate 2lm11 was identified as R. commune, while isolates 4lm11, 7lm11, 13lp11 and 15lp11 grouped distinctly as the new R. lolii species. As with the previous DNA fingerprint analysis, R. orthosporum and R. lolii clustered as sister species, clearly separated from the three other species by a deep phylogenetic split. Estimates of TMRCA suggested a mean age of 736 years (160–1464 highest posterior density, HPD) for R. lolii and that it diverged from R. orthosporum ca. 5735 ybp (4335–7241). This TMRCA estimate for R. lolii overlaps largely with previous estimates for R. secalis (566–1922 ybp), R. agropyri (491–2023 ybp) and R. commune (775–1952 ybp) by Zaffarano et al. [12] (Figure S1).
Figure 2. Multilocus phylogeny to determine the evolutionary relationships between five Rhynchosporium species.
Phylogenetic analysis (maximum clade credibility tree) of combined partial sequences of the alpha-tubulin, beta-tubulin and internal transcribed spacer loci show consistent differences between R. commune, R. agropyri, R. secalis, R. orthosporum and R. lolii. Concatenated haplotype (H) sequences sourced from Zaffarano et al. [12] were combined with sequence data obtained from individual isolates in the present study. Posterior probabilities are indicated for major speciation nodes. The asterisk (*) indicates the calibration point used to infer absolute times (ybp; years before present) to the most recent common ancestor (TMRCA) for these Rhynchosporium species (see also Figure S1).
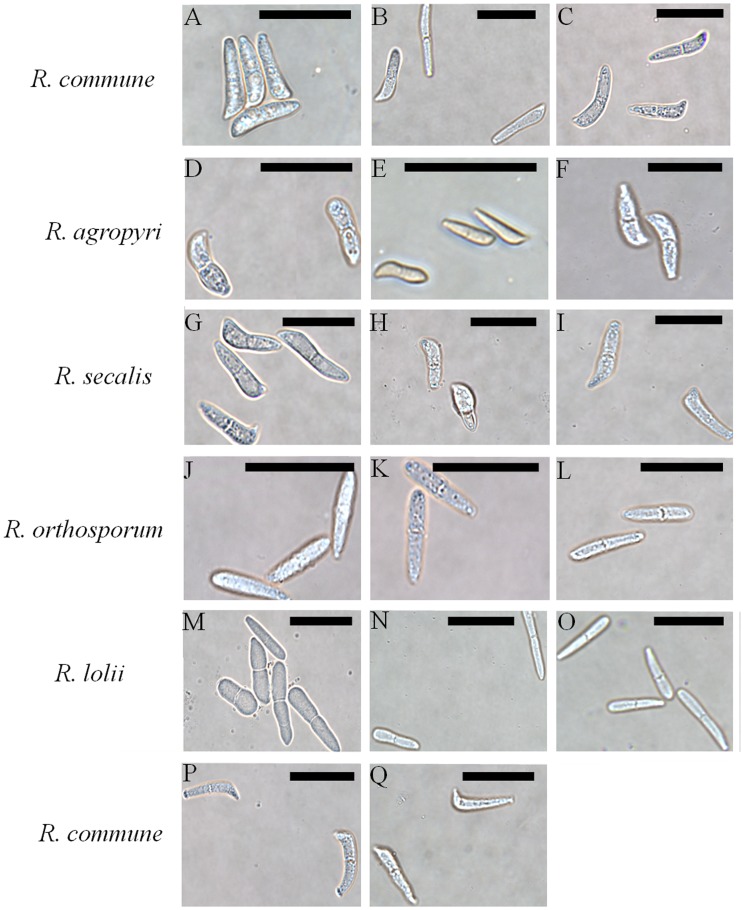
Conidial Morphology
Isolates could be divided into two distinct groups on the basis of conidial shape. Isolates of R. commune (including isolates 2lm11 and 5lm11 from Italian ryegrass), R. agropyri and R. secalis had beak-shaped conidia, while isolates of R. orthosporum and the new R. lolii species group from ryegrass had cylindrically-shaped conidia (Fig. 3). No statistically significant differences in conidial length were observed between isolates of R. commune (mean = 16.80 µm, standard error of the mean = 0.611), R. agropyri (15.41 µm, 0.576) or R. secalis (15.64 µm, 0.773) (F 2,35 = 1.49, P = 0.239). In addition, no significant differences in conidial width were observed between isolates of R. commune (2.95 µm, 0.152), R. agropyri (3.30 µm, 0.143) or R. secalis (3.19 µm, 0.192) (F 2,35 = 1.48, P = 0.241). In contrast, statistically significant differences in conidial length were observed between isolates of R. orthosporum (16.41 µm, 0.611) and R. lolii (19.36 µm, 0.546); (F 1,35 = 12.09, P<0.01). However, no significant differences in conidial width were observed between isolates of R. orthosporum (2.92 µm, 0.152) and R. lolii (2.77 µm, 0.136) (F 1,35 = 0.53, P = 0.473). This differentiation in conidial morphology between the two groups, i.e. cylindrical vs. beak-shaped, was consistent with a deep phylogenetic split (see also Fig. 2).
Figure 3. Five Rhynchosporium species divided into two groups by conidial shape.
Isolates of R. commune, R. agropyri and R. secalis have beak-shaped conidia, while isolates of R. orthosporum and R. lolii have cylindrically-shaped conidia. Isolates shown are R. commune (collected from barley/wall barley) (A-C), R. agropyri (D-F), R. secalis (G-I), R. orthosporum (J-L), R. lolii (M-O) and R. commune (from Italian ryegrass) (P,Q). Isolates shown are (A-Q): 19hv09, D.1.1, E.1.2, 4ar10, 8ar10, Rs04CH Rac A.6.1, 1D4a, Rs02CH4-6a.1, I-3a1, 27dg09, 57dg09, RsCH04 Bär A.1.1.3, 12lp11, 20lp11, 22lm11, 2lm11, 5lm11. Scale bars are 20 µm.
Host Range Testing
At 23 dpi under controlled environment conditions, five isolates of R. commune produced leaf blotch symptoms on barley (cv. Optic) but not on cocksfoot or ryegrasses. Likewise, four out of five isolates of R. orthosporum produced leaf blotch lesions only on cocksfoot but not on barley or ryegrasses (Table 1).
However, isolates collected from ryegrasses showed two distinct host range profiles (Table 1). Firstly, two isolates of R. commune collected from Italian ryegrass caused leaf blotch symptoms (Fig. 4A, B, E, F) on both barley (cv. Optic) and Italian ryegrass (although no disease symptoms developed on perennial ryegrass); by 23 dpi, both hosts showed extensive leaf blotch symptoms and total cell collapse was observed (Table 1). When examined with scanning electron microscopy, leaves of both barley (21 dpi) and Italian ryegrass (28 dpi) were extensively colonised by sub-cuticular hyphae (Fig. 4C, D, G, H). Moreover, beak-shaped conidia were observed (Fig. 4C, D, H) erupting through the leaf cuticle of both hosts (although no sporulation was observed on Italian ryegrass leaves inoculated with isolate 5lm11; Fig. 4G).
Secondly, ten isolates of R. lolii obtained from either Italian or perennial ryegrass produced leaf blotch lesions only on ryegrasses and not on barley or cocksfoot (Table 1). Some isolates of R. lolii produced leaf blotch symptoms on both Italian and perennial ryegrass; therefore, there was no evidence for specialisation between these hosts.
Colonisation Strategy
Scanning electron microscopy results showed that isolates of R. commune on barley (Fig. 5A), R. agropyri on couch-grass (Fig. 5B), R. secalis on rye (Fig. 5C), R. secalis on triticale (Fig. 5D), R. orthosporum on cocksfoot (Fig. 5E) and R. lolii on Italian ryegrass (Fig. 5F) all colonised their respective hosts in a similar manner. In all of these pathosystems, clear evidence was found for extracellular growth of the pathogen, with extensive, branching hyphae colonising the sub-cuticular area of the host leaf tissue. In addition, sub-cuticular hyphal growth was also observed on most of these hosts on areas of leaf tissue with no visible leaf blotch symptoms, i.e. there was evidence of asymptomatic colonisation surrounding lesions (data not shown).
Species-specific PCR Diagnostic Tests
Species-specific endpoint PCR diagnostic tests were developed to detect and distinguish between isolates of (i) R. commune, (ii) R. agropyri, (iii) R. secalis and (iv) R. orthosporum/R. lolii. Multiple alignments of known genetic loci were made (or RAPD-PCR derived sequence used) and a series of different primer pairs were designed with the theoretical ability to discriminate between species due to sequence divergence at the 3′ end and/or within the primers. Many of these primer pairs failed to discriminate sufficiently between species, with false positives produced in some reactions (data not shown). However, a subset of these primer pairs consistently discriminated between the Rhynchosporium species. These diagnostic primers were evaluated by screening against a set of Rhynchosporium isolates representing the different species from proximate geographical origins (Table 2). The R. commune diagnostic test (primer set LinA-F/R) unambiguously produced the predicted amplicon of 145-bp (Fig. 6A) only with 10 ng template DNA from R. commune isolates. However, it is noted that an extremely faint and ambiguous PCR signal was produced for a single isolate of R. agropyri (RS04CG-RAC-A.4.3); no signal was detected when the PCR was repeated with only 34 cycles (data not shown). The R. agropyri test (RA6-F/R) produced the predicted 461-bp (Fig. 6B) amplicon only with 10 ng template DNA from R. agropyri isolates, while the R. secalis test (RS25-F/R) produced the predicted 1240-bp (Fig. 6C) amplicon only with 10 ng template DNA from R. secalis isolates. The R. orthosporum/R. lolii test (2RO-F/R) produced the predicted 277-bp (Fig. 6D) amplicon only with 1 ng template DNA from isolates of these two species; in addition, no amplicons were produced when this test was further screened against 10 ng template DNA from representative isolates of R. commune, R. agropyri and R. secalis (data not shown). None of the four species-specific diagnostic tests detected 10 ng template DNA of either plant host or other fungal pathogens. These included five other pathogens of crops (Table 2), including the closely related P. brassicae [29].
Figure 6. PCR-based diagnostic tests to distinguish between five Rhynchosporium species.
Five primer pairs (LinA-F/R; RA6-F/R; RS25-F/R; 2RO-F/R; ERIC2/BOXA1R) were tested with representative isolates of R. commune (lanes 1–3; K1124, QUB 12-3, OSA 28-2-2), R. agropyri (lanes 4–6; RS04CG-RAC-A.6.1, 6ar10, 10ar10), R. secalis (lanes 7–9; RS99CH1 H10B, E7a, I-1a), R. orthosporum (lanes 10–13; 27dg09, RS04CG-BAR-A.1.1.4, RS04ITA D-4.1) or R. lolii (lanes 13–15; 1lm11, 9lm11 and 18lp11). (A) LinA-F/R produced a 145-base pair (bp) amplicon specific for R. commune isolates; (B) RA6-F/R produced a 461-bp amplicon specific for R. agropyri isolates; (C) RS25-F/R produced a 1240-bp amplicon specific for R. secalis isolates; (D) 2ROR-F/R produced a 277-bp amplicon specific for both R. orthosporum and R. lolii isolates; use of rep-PCR genomic fingerprinting primers ERIC2/BOXA1R produced a ∼400-bp amplicon specific only for isolates of R. lolii. Different isolates are displayed in (B, C) lanes 1–3: FI12-63, QUB 9-10, 2lm11; (D) lanes 1–15∶19hv09, UK7, 2lm11, 10ar10, 6ar10, RS04CG-RAC-A.6.1, RS02CH4-4b1, RS02CH4-14a1, 6.2, 27dg09, RS04CG-BAR-A.1.1.4, RS04ITA D-4.1, 9lm11, 14lp11 and 18lp11; (E) lanes 3, 6 and 14∶2lm11, 1ar10 and 8lm11, respectively. Note that in (A) lanes 1–3 have been inserted from a different gel image. Lane labelled ‘L’ contains a 100-bp ladder (A, B, D) or a 1-kilobase ladder (C, E) (both Fermentas, UK); lane 16 is a no template control.
Sensitivity testing, using mixed amounts of fungal/host plant DNA (25 ng template DNA in total), demonstrated that each of these four diagnostic tests were specific to their respective Rhynchosporium species in a background of host plant DNA, and that the sensitivity of each diagnostic test to the target species template DNA differed as follows; the R. commune test was by far the least sensitive test and required ∼2.5 ng, the R. orthosporum/R. lolii test required 1 ng and both the R. agropyri and R. secalis tests required 1 pg (data not shown).
Finally, the fifth rep-PCR genomic fingerprinting based diagnostic (using primer pair ERIC2/BOXA1R) was also developed; this produced an amplicon of ∼400-bp for isolates of R. lolii but not for isolates of R. orthosporum (Table 2; Fig. 6E). Therefore, when this test was used in combination with the R. orthosporum/R. lolii endpoint diagnostic test (Table 2; Fig 6D), these two species could readily be distinguished from each other.
Taxonomy
Results described above provided clear evidence for the presence of a new species of Rhynchosporium, based on combined molecular, morphological and host specialisation data. Therefore, the new species, Rhynchosporium lolii, is now formally described:
Rhynchosporium lolii King, West, Brunner, Dyer and Fitt sp. nov. [urn:lsid:mycobank.org:names:803876] Etymology: Referring to the host genus, i.e. Lolium.
Type: UK: Shropshire: Newport, isolated from Lolium perenne leaves, May 2011, Collector: Kevin M. King; 15lp11 (IMI 502640– holotype (dried culture); CBS 135745 and IMI 502640– ex-holotype cultures).
Rhynchosporium lolii is genetically most closely related to Rhynchosporium orthosporum, and both species have conidia that are erect, cylindrically shaped and medianly septate. Conidia of R. lolii have a mean length of 19.36 µm and width of 2.77 µm; they are statistically significantly longer than those of R. orthosporum. The two species can be distinguished by the following fixed nucleotide differences between R. lolii and R. orthosporum (presented as the gene and the nucleotide characters fixed in R. lolii in parenthesis, based on numbering of the partial sequences deposited in GenBank in the present study); alpha-tubulin positions 85 (G), 100 (T), 499 (G); beta-tubulin position 309 (A). PCR amplification with primer pair 2RO-F/R produces an amplicon of 277 base pairs for both R. lolii and R. orthosporum, but amplification with rep-PCR genomic fingerprinting primer pair ERIC2/BOXA1R produces an amplicon of approximately 400 base pairs specifically for R. lolii. R. lolii causes leaf blotch lesions on ryegrass species but not on cocksfoot or barley.
Discussion
This work provides the first conclusive evidence for the occurrence of both R. commune and a new species, R. lolii, on ryegrasses in the UK. Two Rhynchosporium isolates from Italian ryegrass (2lm11 and 5lm11) clustered within the main R. commune species group according to both DNA fingerprinting and sequence data. However, in both RAPD-PCR and rep-PCR genomic fingerprinting the two isolates formed a terminal clade with strong bootstrap support. It is unclear whether this was an artefact because these isolates originated from the same geographic location, or whether there was some genuine difference between R. commune isolates occurring on ryegrasses and those on barley.
In addition, the confirmed R. commune isolates from Italian ryegrass both had beak-shaped conidia and could colonise, cause leaf blotch symptoms and sporulate on both Italian ryegrass and barley (cv. Optic). These data support previous work by Wilkins [11], where some Rhynchosporium isolates were collected from Italian ryegrass that could cause disease symptoms on both this host and barley. However, it is noted that data in both the present and previous study [11] suggest that isolates of R. commune collected from barley do not appear to cause leaf blotch symptoms on Italian ryegrass. Nevertheless, we propose that the species description for R. commune [13] be amended to include Italian ryegrass as an additional host species.
The second species, R. lolii, occurred widely on ryegrasses in England and Wales. This new species was most closely related to R. orthosporum and these two sister species had cylindrically-shaped conidia, unlike other species of Rhynchosporium. However, all of the molecular approaches used revealed consistent differences between R. lolii and R. orthosporum. There was therefore sufficient phylogenetic evidence for the identification and description of the new species R. lolii. Such molecular phylogenetic evidence is now being used routinely to define new species of filamentous fungi and yeasts [32], [13], [33], [34], [35] because strongly supported molecular divergence between taxa, especially when based on multiple gene genealogies, indicates a lack of gene flow consistent with speciation events [36], [37], [38].
The new species R. lolii could also be distinguished on the basis of morphological differences, since it had cylindrically-shaped conidia that were typically longer than those of R. orthosporum. Furthermore, host range testing demonstrated that isolates of R. lolii caused leaf blotch symptoms only on ryegrass species whereas isolates of R. orthosporum caused leaf blotch symptoms only on cocksfoot. The occurrence of these host-specialised Rhynchosporium species is similar to that for other plant pathogen genera, for example Zymoseptoria and Colletotrichum that both include several species that are specialised to different wild and cultivated (cereal) grasses [34], [39].
Isolates of R. orthosporum and R. lolii from proximate geographical origins could also be distinguished by the new PCR-based diagnostic tests. The divergence of the two species was supported by results from a molecular clock model implemented in BEAST [25], which suggested that R. lolii diverged from R. orthosporum ca. 5735 ybp (range 4335–7241 ybp). Moreover, the TMRCA estimate of a mean age of 736 years (160–1464 HPD) for R. lolii largely overlaps with previous estimates for other Rhynchosporium species, suggesting independent speciation events during the same period [12].
This study has therefore clarified the identity of Rhynchosporium species occurring on ryegrasses, which are both economically important forage grasses and commonly occurring weeds of cereal crops throughout the world [11], [40], [15], [16]. These findings will be of practical use to both farmers and breeders, for example when considering the use of cocksfoot and ryegrass species as forage grasses [11]. Furthermore, these data suggest that ryegrasses could potentially be a reservoir of R. commune inoculum able to initiate barley leaf blotch epidemics. Similar roles for wild grasses as potential sources of inoculum have been suggested previously for the wheat pathogens Pyrenophora tritici-repentis (cause of tan spot), Oculimacula yallundae (synonym Tapesia yallundae, cause of eyespot) and Colletotrichum species (cause of anthracnose disease) [41], [42], [39]. Further work is now required to investigate the worldwide frequency and distribution of R. commune on ryegrass species.
This work has also demonstrated that all five Rhynchosporium species colonise their respective hosts in a very similar manner morphologically, with extensive hyphal growth observed in the sub-cuticular region of the leaves of all host species. It provides the first SEM evidence of morphological events relating to the colonisation strategy of R. agropyri on couch-grass, R. secalis on rye and triticale and R. lolii on Italian ryegrass. Few plant pathogenic fungi are known to grow in the sub-cuticular region, although other pathogens known to exploit this niche include Diplocarpon rosae (cause of blackspot) on roses (Rosa spp.) [43] and P. brassicae (cause of light leaf spot) on oilseed rape (Brassica napus) [44], [45]. Increased opportunities for horizontal gene transfer between these species due to occupation of the same sub-cuticular niche could have impacts on both disease emergence and metabolic capabilities [46].
Finally, the present study has developed the first endpoint PCR diagnostic tests that can directly detect and distinguish between isolates of R. commune, R. agropyri, R. secalis and R. orthosporum [14], [13]. However, the test developed for R. orthosporum also detected isolates of R. lolii; therefore an additional rep-PCR genomic fingerprinting based test was developed that distinguished these two sister species from each other. Rapid, simple and cheap diagnostic tests to distinguish between all these host-specialised Rhynchosporium species will benefit breeders, farmers and researchers because leaf blotch is a serious disease of barley crops across the world, especially in areas with cool temperate climates [1]. Moreover, leaf blotch is also an important disease of rye, triticale, cocksfoot and ryegrasses [8], [9], [10], [11]. These diagnostic tests may be of use in the detection of asymptomatic colonisation by these Rhynchosporium species on their respective hosts, such as has been observed on barley [47] and other grass hosts (present study). In addition, they will also complement PCR-based tests for other pathogens, such as Ramularia collo-cygni [48], an emerging pathogen of barley crops in northern Europe and New Zealand [49].
Supporting Information
Estimates of time to most recent common ancestor (TMRCA) for the five Rhynchosporium species. Estimates were inferred from the phylogenetic reconstruction shown in Fig. 2. Indicated are mean (○) and 95% credibility intervals (vertical bars, i.e. highest posterior density, HPD).
(TIFF)
Rhynchosporium isolates used for DNA fingerprint testing and spore morphology measurements.
(DOCX)
Primers used for DNA fingerprinting, amplification of gene loci or for discrimination between five Rhynchosporium species.
(DOCX)
GenBank accession numbers for sequences obtained in the present study.
(DOCX)
Acknowledgments
The authors would like to thank the following people from Rothamsted Research, UK: Dr Simon Atkins for initially designing the sequences of primer pair LinA-F/R, Mrs Lynda Castle for help preparing the figures, Mrs Jean Devonshire for guidance in scanning electron microscopy, Mrs Sandra Harvey for technical support, Dr Nichola Hawkins and Professor John Lucas for helpful advice, Mr Graham Shephard for photography, and Mr Rodger White for guidance in experimental design and statistical analysis. The authors also thank Mr Mohammed Rafi (University of Hertfordshire) and Miss Cassie King for help in preparing the figures and our collaborators (mentioned in body of text) for their generous provision of some of the isolates used in this study. Finally, the authors thank Dr Pete Wilkins (IBERS-Aberystwyth University) and Dr Phillip Brook (NIAB–TAG) for hosting visits to collect samples from experimental field plots.
Funding Statement
This work was supported by funding from the Perry Foundation (http://www.perryfoundation.co.uk) and the University of Hertfordshire (http://www.herts.ac.uk). Rothamsted Research (http://www.rothamsted.ac.uk) receives strategic funding from the Biotechnology and Biological Sciences Research Council (http://www.bbsrc.ac.uk). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Zhan J, Fitt BDL, Pinnschmidt HO, Oxley SJP, Newton AC (2008) Resistance, epidemiology and sustainable management of Rhynchosporium secalis populations on barley. Plant Pathol 57: 1–14. [Google Scholar]
- 2. Shipton WA, Boyd JR, Ali SM (1974) Scald of barley. Rev. Plant Pathol. 53: 839–861. [Google Scholar]
- 3.HGCA website. Available: http://www.hgca.com/publications/documents/cropresearch/barley_growth_guide.pdf. Accessed: 22 Jul 2013.
- 4.HGCA website. Available: http://publications.hgca.com/publications/documents/cropresearch/G44_-_HGCA_Barley_Disease_Management_Guide_2011_(Complete_version).pdf. Accessed: 22 Jul 2013.
- 5. Avrova A, Knogge W (2012) Rhynchosporium commune: a persistent threat to barley cultivation. Mol Plant Pathol 13: 986–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Badr A, Müller K, Schäfer-Pregl R, El Rabey H, Effgen S, et al. (2000) On the origin and domestication history of barley (Hordeum vulgare). Mol Biol Evol 17: 499–510. [DOI] [PubMed] [Google Scholar]
- 7. Brunner PC, Schürch S, McDonald BA (2007) The origin and colonization history of the barley scald pathogen Rhynchosporium secalis . J Evol Biol 20: 1311–1321. [DOI] [PubMed] [Google Scholar]
- 8. Brooks FT (1928) Observations on Rhynchosporium secalis (Oud.) Davis, leaf blotch of barley and rye. New Phytol 11: 215–219. [Google Scholar]
- 9. Welty RE, Metzger RJ (1996) First report of scald of triticale caused by Rhynchosporium secalis in North America. Plant Dis 80: 1220–1223. [Google Scholar]
- 10. Fernandez JP, Welty RE (1991) Histopathology of orchardgrass infected by Rhynchosporium orthosporum . Mycologia 83: 774–778. [Google Scholar]
- 11. Wilkins P (1973) Infection of Lolium multiflorum with Rhynchosporium species. Plant Pathol 22: 107–111. [Google Scholar]
- 12. Zaffarano PL, McDonald BA, Linde CC (2008) Rapid speciation following recent host shifts in the plant pathogenic fungus Rhynchosporium . Evolution 62: 1418–1436. [DOI] [PubMed] [Google Scholar]
- 13. Zaffarano PL, McDonald BA, Linde CC (2011) Two new species of Rhynchosporium . Mycologia 103: 195–202. [DOI] [PubMed] [Google Scholar]
- 14. Caldwell RM (1937) Rhynchosporium scald of barley, rye and other grasses. J Agric Res 55: 175–198. [Google Scholar]
- 15. Charmet G, Balfourier F, Chatard V (1996) Taxonomic relationships and interspecific hybridization in the genus Lolium (grasses). Genet Resour Crop Evol 43: 319–327. [Google Scholar]
- 16. Preston C, Wakelin AM, Dolman FC, Bostamam Y, Boutsalis P (2009) A decade of glyphosate-resistant Lolium around the world: mechanisms, genes, fitness, and agronomic management. Weed Sci 57: 435–441. [Google Scholar]
- 17. Howlett SG, Cooke BM (1987) Scanning electron microscopy of sporulation in Rhynchosporium secalis . Trans Br Mycol Soc 88: 547–557. [Google Scholar]
- 18. Lange BJ, Boyd WJR (1968) Preservation of fungal spores by drying on porcelain beads. Phytopathology 58: 1711–1712. [Google Scholar]
- 19. Murtagh GJ, Dyer PS, McClure PC, Crittenden PD (1999) Use of randomly amplified polymorphic DNA markers as a tool to study variation in lichen-forming fungi. Lichenologist 31: 257–267. [Google Scholar]
- 20. Hampl V, Pavlícek A, Flegr J (2001) Construction and bootstrap analysis of DNA fingerprinting-based phylogenetic trees with a freeware program FreeTree: Application to trichomonad parasites. Int J Syst Evol Microbiol 51: 731–735. [DOI] [PubMed] [Google Scholar]
- 21. Page RDM (1996) TREEVIEW: An application to display phylogenetic trees on personal computers. Comput Appl Biosci 12: 357–358. [DOI] [PubMed] [Google Scholar]
- 22. Versalovic J, Koeuth T, Lupski JR (1991) Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res 19: 6823–6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Versalovic J, Schneider M, de Bruijn FJ, Lupski JR (1994) Genomic fingerprinting of bacteria using repetitive sequence based polymerase chain reaction. Methods Mol Cell Biol 5: 25–40. [Google Scholar]
- 24. Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41: 95–98. [Google Scholar]
- 25. Drummond AJ, Rambaut A (2007) BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol 7: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Payne RW, Murray DA, Harding SA, Baird DB, Soutar DM (2009) GenStat for Windows (12th Edition) Introduction. VSN International, Hemel Hempstead.
- 27. Zaffarano PL, McDonald BA, Linde CC (2009) Phylogeographical analyses reveal global migration patterns of the barley scald pathogen Rhynchosporium secalis . Mol Ecol 18: 279–293. [DOI] [PubMed] [Google Scholar]
- 28. Nicholson P, Rezanoor HN, Simpson DR, Joyce D (1997) Differentiation and quantification of the cereal eyespot fungi Tapesia yallundae and Tapesia acuformis using a PCR assay. Plant Pathol 46: 842–856. [Google Scholar]
- 29. Goodwin SB (2002) The barley scald pathogen Rhynchosporium secalis is closely related to the discomycetes Tapesia and Pyrenopeziza . Mycol Res 106: 645–654. [Google Scholar]
- 30. Fountaine JM, Shaw MW, Napier B, Ward E, Fraaije BA (2007) Application of real-time and multiplex polymerase chain reaction assays to study leaf blotch epidemics in barley. Phytopathology 97: 297–303. [DOI] [PubMed] [Google Scholar]
- 31.Lehnackers H, Knogge W (1990) Cytological studies on the infection of barley cultivars with known resistance genotypes by Rhynchosporium secalis. Canadian Journal of Botany, 68, 1953–1961.
- 32. Fisher MC, Koenig GL, White TJ, Taylor JW (2002) Molecular and phenotypic description of Coccidioides posadasii sp. nov, previously recognized as the non-Californian population of Coccidioides immitis . Mycologia 94: 73–84. [PubMed] [Google Scholar]
- 33. Holland SL, Dyer PS, Bond CJ, James SA, Roberts IN, et al. (2011) Candida argentea sp. nov., a copper and silver resistant yeast species. Fungal Biol 115: 909–918. [DOI] [PubMed] [Google Scholar]
- 34. Stukenbrock EH, Quaedvlieg W, Javan-Nikhah M, Zala M, Crous PW, et al. (2012) Zymoseptoria ardabiliae and Z. pseudotritici, two progenitor species of the septoria tritici leaf blotch fungus Z. tritici (synonym: Mycosphaerella graminicola). Mycologia 104: 1397–1407. [DOI] [PubMed] [Google Scholar]
- 35. Barrs VR, van Doorn TM, Houbraken J, Kidd SE, Martin P, et al. (2013) Aspergillus felis sp. nov., an emerging agent of invasive aspergillosis in humans, cats and dogs. PLoS One 8: e64871 doi:10.1371/journal.pone.0064871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Taylor JW, Jacobson DJ, Kroken S, Kasuga T, Geiser DW, et al. (2000) Phylogenetic species recognition and species concepts in fungi. Fungal Genet Biol 31: 21–31. [DOI] [PubMed] [Google Scholar]
- 37. Dettman JR, Jacobson DJ, Turner E, Pringle A, Taylor JW (2003) Reproductive isolation and phylogenetic divergence in Neurospora – comparing methods of species recognition in a model eukaryote. Evolution 57: 2721–2741. [DOI] [PubMed] [Google Scholar]
- 38.Taylor JW, Turner E, Pringle A, Dettman J, Johannesson H (2006). Fungal species: thoughts on their recognition, maintenance and selection. In: Fungi in the Environment (Eds GM Gadd, SC Watkinson & PS Dyer), 313–339. Cambridge University Press, Cambridge.
- 39. Crouch JA, Tredway LP, Clarke BB, Hillman BI (2009) Phylogenetic and population genetic divergence correspond with habitat for the pathogen Colletotrichum cereale and allied taxa across diverse grass communities. Mol Ecol 18: 123–135. [DOI] [PubMed] [Google Scholar]
- 40. Lutman PJW, Storkey J, Martin H, Holland J (2009) Abundance of weeds in arable fields in southern England in 2007/08. Asp Appl Biol 91: 163–168. [Google Scholar]
- 41. Kastelein P, Köhl J, Gerlagh M, Goossen-van de Geijn HM (2002) Inoculum sources of the tan spot fungus Pyrenophora tritici-repentis in The Netherlands. Meded Rijksuniv Gent Fak Landbouwkd Toegep Biol Wet 67: 257–267. [PubMed] [Google Scholar]
- 42. Dyer PS, Bradshaw RE (2002) First report of apothecia of Tapesia yallundae occurring on the wild grass Holcus lanatus (Yorkshire fog) in New Zealand. Plant Pathol 51: 806. [Google Scholar]
- 43. Gachomo EW, Dehne H-W, Steiner U (2009) Efficacy of triazoles and strobilurins in controlling black spot disease of roses caused by Diplocarpon rosae . Ann Appl Biol 154: 259–267. [Google Scholar]
- 44. Boys EF, Roques SE, Ashby AM, Evans N, Latunde-Dada AO, et al. (2007) Resistance to infection by stealth: Brassica napus (winter oilseed rape) and Pyrenopeziza brassicae (light leaf spot). Eur J Plant Pathol 118: 307–321. [Google Scholar]
- 45. Boys EF, Roques SE, West JS, Werner CP, King GJ, et al. (2012) Effects of R gene-mediated resistance in Brassica napus (oilseed rape) on asexual and sexual sporulation of Pyrenopeziza brassicae (light leaf spot). Plant Pathol 61: 543–554. [Google Scholar]
- 46. Fitzpatrick DA (2012) Horizontal gene transfer in fungi. FEMS Microbiol Lett 329: 1–8. [DOI] [PubMed] [Google Scholar]
- 47. Davis H, Fitt BDL (1990) Symptomless infection of Rhynchosporium secalis on leaves of winter barley. Mycol Res 94: 557–560. [Google Scholar]
- 48. Taylor JMG, Paterson LJ, Havis ND (2010) A quantitative real-time PCR assay for the detection of Ramularia collo-cygni from barley (Hordeum vulgare) . Lett Appl Microbiol 50: 493–499. [DOI] [PubMed] [Google Scholar]
- 49. Walters DR, Havis ND, Oxley SJ (2008) Ramularia collo-cygni: the biology of an emerging pathogen of barley. FEMS Microbiol Lett 279: 1–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Estimates of time to most recent common ancestor (TMRCA) for the five Rhynchosporium species. Estimates were inferred from the phylogenetic reconstruction shown in Fig. 2. Indicated are mean (○) and 95% credibility intervals (vertical bars, i.e. highest posterior density, HPD).
(TIFF)
Rhynchosporium isolates used for DNA fingerprint testing and spore morphology measurements.
(DOCX)
Primers used for DNA fingerprinting, amplification of gene loci or for discrimination between five Rhynchosporium species.
(DOCX)
GenBank accession numbers for sequences obtained in the present study.
(DOCX)