Abstract
The use of static indicator species, in which species are expected to have a similar sensitivity or tolerance to either natural or human-induced stressors, does not account for possible shifts in tolerance along natural environmental gradients and between biogeographic regions. Their indicative value may therefore be considered at least questionable. In this paper we demonstrate how species responses (i.e. abundance) to changes in sediment grain size and organic matter (OM) alter along a salinity gradient and conclude with a plea for prudency when interpreting static indicator-based quality indices. Six model species (three polychaetes, one amphipod and two bivalves) from the North Sea, Baltic Sea and the Mediterranean Sea region were selected. Our study demonstrated that there were no generic relationships between environment and biota and half of the studied species showed different responses in different seas. Consequently, the following points have to be carefully considered when applying static indicator-based quality indices: (1) species tolerances and preferences may change along environmental gradients and between different biogeographic regions, (2) as environment modifies species autecology, there is a need to adjust indicator species lists along major environmental gradients and (3) there is a risk of including sibling or cryptic species in calculating the index value of a species.
Introduction
“Bioindicators” and “indicator species” are terms commonly used in ecological assessment and generally denote the use of observations on the status of organisms and communities to draw conclusions on environmental quality [1-7]. The knowledge of the environmental preferences of an organism or species can be applied in both temporal and spatial comparisons to detect changes in abiotic conditions or to assess the status of the habitat. The usefulness of species for the detection of both naturally and anthropogenically induced changes renders them an indicator status. Although applied in the assessment of marine systems for more than 30-40 years [8,9], bioindicators gained importance in two periods, during the 1960’s and even earlier [2-7] due to the emergent awareness of environmental pollution. Also the beginning of this century there was an increasing demand for the assessment of the ecological status of coastal and marine waters. This is exemplified by the EU Water Framework Directive (WFD; 2000/60/EC) and the Marine Strategy Framework Directive (MSFD; 2008/56/EC), in which the sensitivity/tolerance classification of species play an essential role [10].
Indicator species can signal a change in the biological condition of a particular ecosystem, and thus may be used to diagnose the health of an ecosystem. Ecological condition in aquatic environments is often evaluated by studying the sedimentary habitat and associated benthic communities. This means looking for species which, due to their autecological requirements, are constrained by narrow environmental conditions and are therefore typical and/or indicative for these conditions [3,11]. Such species seem to have a high indicator power. However, the concept of "indicator species" is slightly different from what we might call the "degree of sensitivity" of a species. In the first case a species is used because its presence, absence, or abundance indicates a precise suite of environmental conditions, for which it has tolerance or preference. Few species are used in this kind of assessment. The second case is derived from the “community composition” approach, where all the species potentially present in an environment are used to formulate the assessment. Species are listed and a score, used for the computation of a biotic index, is assigned to each species. The indicator value is then given by the proportion of the different species in the community composition [12-14]. It is essential however that the response of a particular indicator species to changes in an environmental variable is understood. This allows the level of the environmental pressure to be estimated.
Benthic indicator species not only provide information on benthic environmental conditions, but also on the perturbations affecting a community when grouped into categories such as sentinel, tolerant or opportunistic species [3,12-15]. Species can consequently be classified into ecological groups defined by their sensitivity or tolerance to given disturbances or stresses, caused by e.g., organic enrichment, industrial waste, thermal pollution but also by natural factors (e.g., salinity gradients displayed in transitional water bodies). Thus, we expect that such generic bioindication-based assessment schemes are not likely to work or are highly biased when applied over broad spatial extents. This is also supported by specific examples. Brackish or diluted water bodies like the Baltic Sea were often labelled “bad” by certain indices due either to the absence of “good” indicator species or the indices were not adjusted for these conditions [16,17]. In these naturally stressed ecosystems, where natural variability occurs on different spatial (e.g. along gradients) and temporal (regular and coincidental) scales, it is hard to differentiate natural and anthropogenic stresses [18]. Similarly another example of biased classification has been raised in the Mediterranean lagoons that could be related to the inherently reduced number of species occurring in these highly variable and eutrophic systems [18-20].
The use of sensitivity/tolerance lists of benthic faunal species to define the ecological status of waters is generally accepted and globally applied [21-26] although the majority of these lists are built one on another and based on best professional judgement [20]. These lists are useful tools and have been updated in the last decade by the completion of the worldwide species list and an improved understanding of the autecology of many species. Reference species lists associated with static “indicator values” have been used more often than other methods [24]. Caution is required however, because each species is categorised into a single ecological category. Both from terrestrial and aquatic studies we know that many species can change their life history strategy or autecology requirements along environmental gradients [27-29]. It is generally accepted that sexes and age classes may differ in ecologically significant ways. However, phenotypic variation among individuals can generate variation in ecological attributes even within a sex and age class, including, for example abiotic tolerances, resource use, or competitive ability (see [29] for references).
If aspects of the preferences and/or tolerances of a species change over typically encountered environmental gradients, this can affect the species’ sensitivity, and therefore, its indicative value for other factors such as pollution [17,30]. Further, the ability of a species to serve as an indicator may depend on its position along an environmental gradient. Moreover species may be intrinsically different in their ability to discriminate particular environmental conditions.
These problems have been faced since the initiation of benthic bioindication during the first half of the last century [15,31-35].
Following this reasoning and examples in this paper, the use of fixed reference lists needs to be reconsidered, especially in areas with strong salinity gradients, like estuaries or the Baltic Sea [16], or eutrophic systems like Mediterranean lagoons [19]. The interactions between biotic and environmental factors and the adaptive behaviour of species have to be considered in a species sensitivity/tolerance reference list, where the diagnostic power of a species would be linked to a specific ecological zone/environment and to its discriminating power. In this paper we tested the hypothesis that alterations in species’ environmental preferences and tolerances change along environmental gradients, using a large macrobenthic dataset spanning different biogeographical regions and different environmental conditions. We hypothesise that (1) relationships between organic enrichment and sediment grain size and biota change along major environmental gradients and (2) such relationships also vary among the European seas. This study is a step forward in disentangling co-varying effects, using salinity as the response variable for selected ‘test-species’, and organic matter content (OM) and grain size composition as a proxy of substrate and habitat characteristics (e.g. organic enrichment, sheltered vs. exposed waters). Based on the results, some recommendations are given to apply when using indicator species lists for ecological quality assessments.
Materials and Methods
Research strategy
The tolerance/sensitivity values of species have been developed based on life history features using a variety of techniques. Several studies have applied different methods to classify the sensitivity or tolerance of benthic organisms to various degrees of disturbance. These were usually based on best professional judgement (including literature review and experimental research) [24,36]. Another approach was the quantitative (or mathematical) determination of the tolerance values of benthic species to environmental disturbance [17,26,37]. These values were commonly combined or used within a biotic index to allow the assessment of gradients of environmental conditions [38,39]. However, whether these gradients were of anthropogenic or natural origin was generally not considered in these investigations. Salinity gradients predominantly control the distribution of organisms on the coast, in estuaries and lagoons as well as in the Baltic Sea [40,41]. Salinity is a key environmental factor, which defines structural and functional characteristics of aquatic biota [42]. Moreover, salinity is a common confounding factor in nearly all coastal environments in which human impacts are being assessed [43]. It is acknowledge that species' tolerances/preferences change along the multitude of natural and human-induced environmental variables, the quantification of the species tolerances/preferences along strong environmental gradients will allow the use of static lists to be put in context. In this study, salinity gradients were selected as the primary environmental gradient against which the preferences and tolerances of a set of species were quantified. The secondary variables, grain size distribution and organic matter content were selected as the main substrate characteristics that would influence the distribution of macrozoobenthic species [19,44].
Data availability
This paper presents collaborative research based on a collation of existing data. Table 1 provides further details on the data sources (i.e. contact person, availability). Data are compiled into appendices (Appendix S1-S4).
Table 1. Number of samples, sampling period and availability of data used in the present study.
| Great Britain | North Sea 1 | North Sea 2 | Wester-schelde | Zee-schelde | Belgian Beaches | Belgium Offshore | German Baltic | Estonia | Cabras Lagoon | Venice Lagoon | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of samples | 126 | 30 | 84 | 2089 | 104 | 343 | 131 | 1012 | 1549 | 76 | 285 |
| Sampling period | 2001-2011 | 2000-2004 | 2008-2011 | 2000-2008 | 1990-2011 | 1994-1995 | 2005-2010 | 2001-2011 | 2003-2009 | 2001-2004 | 1991-2002 |
| Availability | On request | On request | On request | On request | On request | On request | On request | On request | On request | On request | On request |
| Responsible author | CG | HR | MLZ | TY | JS | SD | GVH | MLZ | JK | PM | DT |
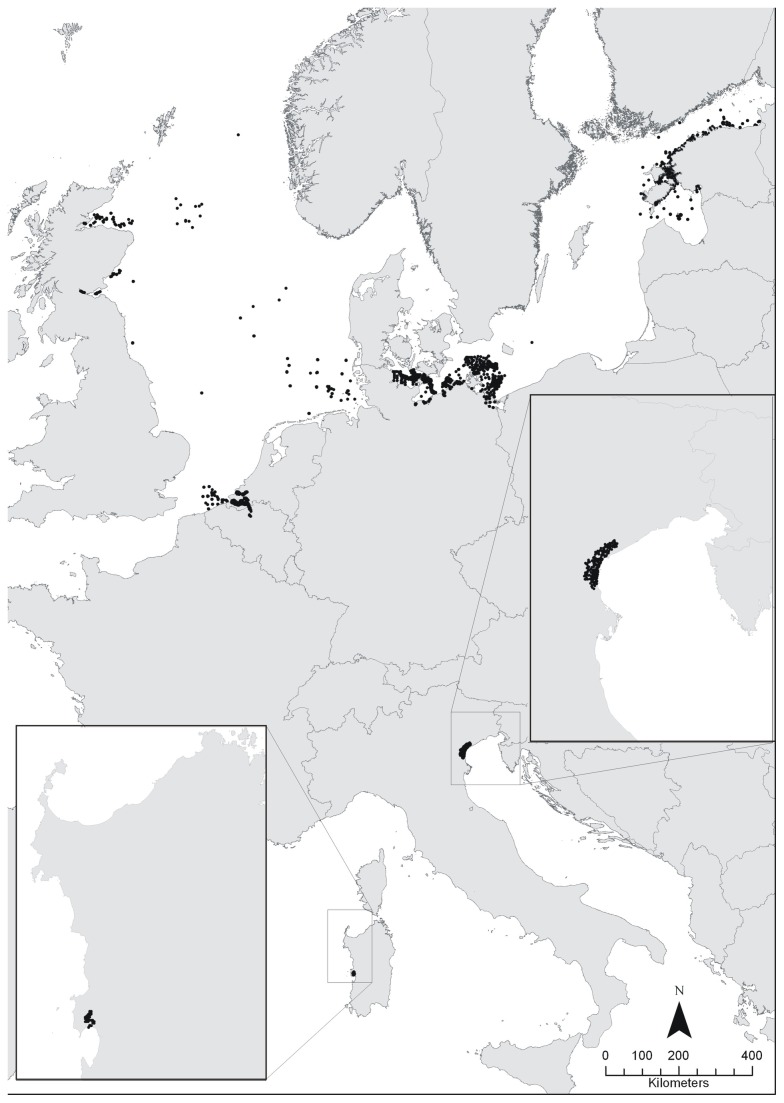
Only sample data that contained information on salinity, substrate (sediment grain size, organic matter content) and the abundance of selected species (including zero-values indicating the absence of the species at an individual station) were retained for analysis. Overall, 5468 records from the North Sea and Baltic Sea and 361 records from the Mediterranean were available for this study (Tables 2 and 3). The data originate from nine case-studies and monitoring programs from around the North Sea and Baltic Sea, covering coastal and offshore waters of the UK, Belgium, the Netherlands, Germany and Estonia (Figure 1). In the Mediterranean Sea, data were available from two Italian lagoons, namely the Venice Lagoon and the Cabras Lagoon (Isle of Sardinia). These datasets, covering a wide range in salinity, were combined into two datasets (two geographical regions), i.e. a North Sea – Baltic Sea and a Mediterranean Sea database.
Table 2. Data availability (loss on ignition (LOI) and median grain size) in the North and Baltic Sea.
|
North & Baltic Sea
|
Great Britain
|
North Sea 1
|
North Sea 2
|
Westerschelde
|
Zeeschelde
|
Belgian Beaches
|
Belgium Offshore
|
German Baltic
|
Estonia
|
Overall
|
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| LOI | oligo | 72 | 55 | 127 | |||||||
| β-meso | 362 | 1467 | 1829 | ||||||||
| α-meso | 209 | 209 | |||||||||
| β -poly | 278 | 278 | |||||||||
| α -poly | 4 | 3 | 11 | 9 | 75 | 102 | |||||
| euhalin | 113 | 27 | 81 | 332 | 91 | 644 | |||||
| grain size | oligo | 11 | 72 | 46 | 129 | ||||||
| β-meso | 88 | 93 | 269 | 1247 | 1697 | ||||||
| α-meso | 523 | 184 | 707 | ||||||||
| β -poly | 591 | 225 | 816 | ||||||||
| α -poly | 4 | 3 | 739 | 11 | 11 | 75 | 843 | ||||
| euhalin | 60 | 30 | 81 | 87 | 332 | 120 | 710 | ||||
| overall | oligo | 11 | 72 | 55 | 138 | ||||||
| β-meso | 88 | 93 | 362 | 1494 | 2037 | ||||||
| α-meso | 523 | 225 | 748 | ||||||||
| β -poly | 591 | 278 | 869 | ||||||||
| α -poly | 4 | 3 | 739 | 11 | 11 | 75 | 843 | ||||
| euhalin | 122 | 30 | 81 | 87 | 332 | 120 | 772 | ||||
Table 3. Data availability (loss on ignition (LOI) and median grain size) in the Mediterranean Sea.
|
Mediterranean
|
Cabras
|
Venice
|
Overall
|
|
|---|---|---|---|---|
| LOI | meso | 38 | 22 | 60 |
| poly | 24 | 103 | 127 | |
| eu | 14 | 160 | 174 | |
| grain size | meso | 38 | 22 | 60 |
| poly | 24 | 103 | 127 | |
| eu | 14 | 160 | 174 | |
| overall | meso | 38 | 22 | 60 |
| poly | 24 | 103 | 127 | |
| eu | 14 | 160 | 174 | |
Figure 1. Map of sample locations included in this study.
The salinity at each sampling location was either measured directly or taken from modelled data (Schelde estuary). At intertidal locations, salinity was measured from the receding water immediately after the sampling location became exposed. In this study, a reduced six-level Venice salinity system was used: euhaline zone >34 to 30, α-polyhaline zone 25 to 30, β-polyhaline zone 18 to 25, α-mesohaline zone 18 to 10, β-mesohaline zone 10 to 5 and oligohaline zone 5 to 0.5 (see [45] for details). However, due to the comparably small data-set, Mediterranean data were subdivided only into three salinity classes (poly-, meso- and oligohaline, Table 3).
Grain size distribution was taken into account as median grain size (d50 in µm) (Tables 2 and 3). Within the North/Baltic Sea dataset, stations with d50 > 2000 µm were excluded, as these were scarce and available only for a single salinity class. Overall, 4951 records were used. Median grain size was available for all Mediterranean stations. Maximum median grain size was 964 µm.
For most stations, sediment organic matter content (OM) was measured as loss on ignition (LOI in % of dry weight). For some stations in Belgium offshore waters (n=61) and the UK (n=126), LOI was calculated from the organic matter content (for Belgium the “dichromate method” for TOC [46]), LOI = 2.9324*organic matter content + 0.2979 (R2 = 0.85, p<0.01). The relationship was calculated from parallel measurements, available for Belgian waters (n = 39). Overall, 3559 data points with information on organic content of the sediment were included in the dataset. Previously proposed OM ranges were used as an indication of the level (low, moderate, high) of OM enrichment [19,44,47]. This is because OM in sediments can be a stressor when it is in excess, while at low to moderate levels (ca. up to 8-10%) it is an important food source for benthic animals [44,47].
Species selection
Species selected had to comply with the following criteria: a) wide distribution range (occurring in all study areas), b) present in at least three salinity classes and c) the species should be used in current indices and (at least partially) be classified differently within the relevant indicators/metrics. Examples of such metrics include MarBIT, M-AMBI, ES(50)0.05-values in BQI and MarLIN, all commonly used in the study areas [10,16,18,24-26,30,37,39,48]. Consequently, six model/test species were selected: 3 polychaetes (Ampharete baltica Eliason, 1955, Hediste diversicolor (O.F. Müller, 1776), and Travisia forbesii Johnston, 1840), 1 crustacean (Bathyporeia pilosa Lindström, 1855) and 2 molluscs (Cerastoderma glaucum (Bruguière, 1789) and Corbula gibba (Olivi, 1792)) (Table 4).
Table 4. Sensitivity classification of selected species in European assessment systems and sensitivity against nutrient load (MarLIN-database) referring to the following references [17,24,36,48].
| species | MARBIT (German WFD-tool) | AMBI/M-AMBI | BQI (adaptation southern Baltic) - range E(S50)0.05 | MarLIN |
|---|---|---|---|---|
| Ampharete baltica | indifferent (N) | indifferent (II) | tolerant to sensitive (6.6-10.5) | not included |
| Bathyporeia pilosa | very sensitive (O) | very sensitive (I) | tolerant (4.5-6.9) | not included |
| Cerastoderma glaucum | very sensitive (O) | tolerant (III) | tolerant (5.7-6.6) | nutrient load: intermediate intolerance, low recoverability, high sensitivity |
| Corbula gibba | indifferent (N) | very tolerant (IV) | tolerant to sensitive (5.9-9.4) | C. gibba are indicative of unstable environments such as ones with low oxygen levels and areas of eutrophication within the Mediterranean [87] |
| Hediste diversicolor | tolerant (T) | very tolerant (IV) | tolerant (5.2-6.9) | high tolerance against changes in nutrient level, may benefit |
| Travisia forbesii | very sensitive (O) | very sensitive (I) | tolerant (6.3-6.9) | not included |
The ecological requirements of these species are outlined below. The presence of muddy sediments seemed to be obligatory for the polychaete A. baltica, as small grain sizes are used for tube construction. No other sediment preferences or tolerances are known [49]. The polychaete T. forbesii is considered to be sediment-specific (regarding grain size and organic content) and therefore sensitive to changes in sediment composition [50]; sediments with especially high organic load are avoided. In contrast, H. diversicolor is known to be euryoecious, tolerating a wide range of salinity, temperature, and water quality [51]. Bathyporeia pilosa avoids very coarse sediments and prefers fine, somewhat muddy sand with at least 70% of the substrate finer than 210 µm [52]. It is more tolerant of fine particles in the sediment than other species of the same genus and is found in high densities on sediments with up to 25% silt. However, B. pilosa is often classified as “sensitive” to organic load and changes in sediment characteristics (see [52] for references). The bivalve C. gibba is widely distributed in European seas, from the Norwegian Sea southward to the Mediterranean and Black Seas (see [53] for references). Corbula gibba populations are distributed from low intertidal zones down to considerable depths. Corbula gibba, an infaunal species with a sedentary mode of life, inhabits soft bottom sediments very often mixed with molluscan shell fragments or thick muddy sand with admixed gravel and small stones [54]. Due to its high tolerance to a wide range of abiotic environmental disturbances, C. gibba is considered an indicator of environmental instability caused by pollution, low oxygen content, and/or increased turbidity [53]. The lagoon cockle C. glaucum is a euryhaline bivalve with a wide distribution along European coasts, ranging from the northern Baltic Sea to the Black Sea, the Caspian Sea and even Lake Aral, with salinities from 4 to 100 PSU [55]. In the Atlantic and the Mediterranean, C. glaucum typically inhabits closed brackish lagoons and estuaries.
Statistics
The response of a species to an environmental gradient is affected by the simultaneous response of the species to multiple other parameters in natural systems. This increases heterogeneity and often masks the relationship between the gradient parameter and the response variable in ecological models focussing on single parameter gradients. Due to the interlinkage of environmental variables one possibility for dealing with this problem is to look at the maximum [56] or 95th percentile [45] as the response variable. The modelling of an “outer envelope” [57] might be more appropriate than modelling the mean response as the outer envelope is less susceptible to the influence of other parameters. The advantage of the 95th percentile over the maximum value is the lower sensitivity to outliers.
Quantile regression spline (QRS) models were used to reconstruct the response of the species along the gradient of the substrate parameter for each salinity class. The chosen procedure mainly follows the description in Anderson (2008) [57]. The QRS for the 95th percentile were modelled for a set of four models per species and salinity class with a polynomial function of degree = 2, 3, 4 and 5. The quantile regression was modelled using the function ‘rq’ of the package quantreg [58] and a smoother was added using the function ‘bs’ [59] of the R statistical analysis software (R Development Core Team, 2012 [60]). Akaike’s information criterion (correction for small sample sizes AICc, [61]) was used for model selection. This automatic choice was modified if another model had a similar AICc value (within 2 units) and visually showed a better fit in the scatterplot of the data. Vertical dashed lines in the figures indicating the highest predicted density of the species in selected plots were interpreted as an estimated optimum of the species. QRS were modelled for all selected species.
Results
Neither consistent relationships in species responses to changes in sediment variables across salinity gradients, nor similar responses in different seas were found. The quantile regression spline models (quantile regression spline) did not show good fit with the data in three cases (A. baltica, T. forbesii and C. glaucum). In these three cases, simple bubble plots were used to illustrate non-significant trends.
Test species
Ampharete baltica was rare under fully marine conditions, but was found mainly in fine sandy bottoms with low to medium organic matter content in the Western Baltic (mainly polyhaline waters, Figure 2). However, in the Baltic Proper, sediment specificity seemed to become weaker as both low and high organic matter content sediments were inhabited, suggesting that the two different populations have adapted to specific conditions. It might be that the species exhibits high plasticity in its response to this environmental gradient.
Figure 2. Abundance of Ampharete baltica in relation to bottom salinity and organic sediment content in three sea areas.
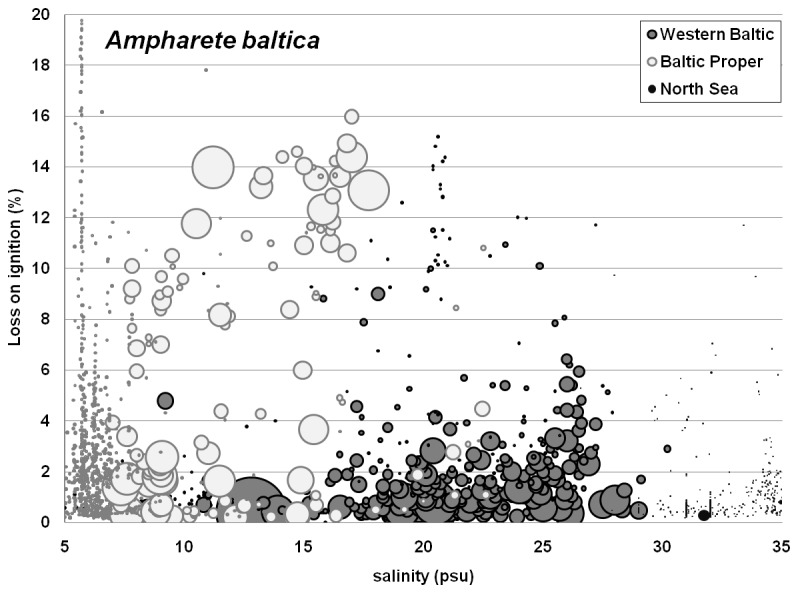
The relative bubble size indicates the density of the species with smallest dots referring to species absence and largest bubble size to ca. 1700 ind/m2. Note that the symbol shading used in Figures 2, 3 and 5 is not consistent as different issues are shown.
Travisia forbesii is restricted to a narrow range of clean medium sands (d50: 250 - 500 µm) under fully marine conditions (Figure 3). In mesohaline waters, the species was found also in clean fine sands with a lower limit of about 100 µm. No occurrence was reported from coarse sediments in mesohaline waters (which may be attributed to data deficiency).
Figure 3. Abundance of Travisia forbesii along the full salinity gradient combining the North and Baltic Sea data set and along median grain size.
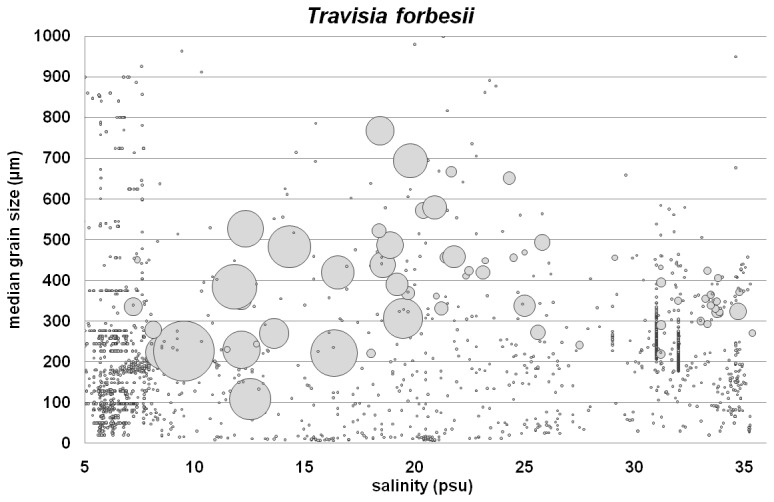
Relative bubble size: species density (absent – ca. 670 ind/m2). Note that the symbol shading used in Figures 2, 3 and 5 is not consistent as different issues are shown.
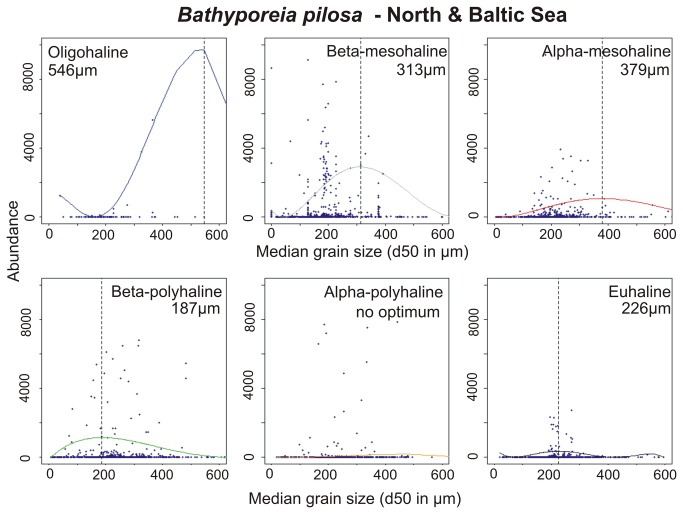
Bathyporeia pilosa was present along almost the full spectra of available substrates from very fine sand to coarse sediments. Highest abundances were found on medium sand in ß-mesohaline areas and on fine sand (d50: 100-250 µm) in β-polyhaline and euhaline areas (Figure 4). In the remaining parts of the salinity gradient, medium sands seemed to be preferred. No optimum was found for the α-polyhaline section as high abundances occurred throughout the full substrate spectrum.
Figure 4. Abundance-response curves (95th percentile QRS) of Bathyporeia pilosa along a sediment grain size gradient in six different salinity classes in the North Sea and the Baltic Sea.
The dashed line marks the modelled optimum. Note that the y-axis had to be cut in some cases due to outliers.
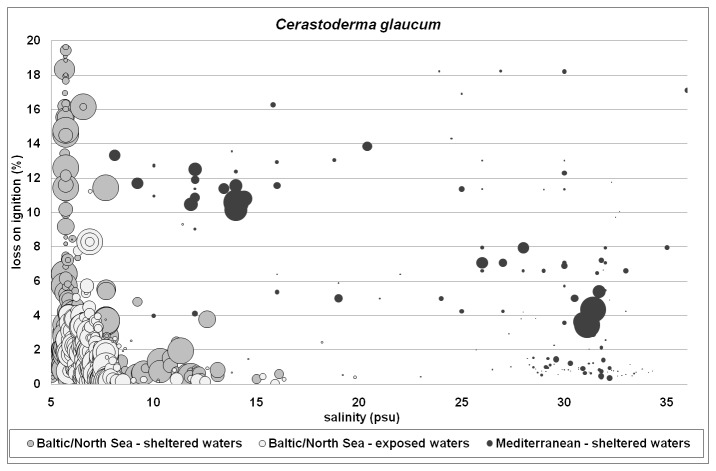
The bivalve C. glaucum was restricted to β-mesohaline and lower α-mesohaline waters in the Baltic Sea. It showed obvious differences in the ability to deal with organic enrichment between sheltered (e.g., estuaries, lagoons and inner bights) and more exposed (e.g., outer bights and offshore) waters. In exposed areas, it rarely occurred on sandy substrates with a LOI > 5% (Figure 5). In sheltered areas, it seemed to be tolerant to organic enrichment, indicated by consistently high densities throughout the range covered (0-20%). Also in the Mediterranean lagoons, it was found throughout the full organic gradient, but with highest densities in sediments with an intermediate to high organic load (LOI 3-14%). Additionally, the Mediterranean C. glaucum was present throughout the full salinity range. The tolerance of C. glaucum to OM content is apparently dependent on the OM content to which different populations are normally exposed.
Figure 5. Abundance of Cerastoderma glaucum with relation to salinity and LOI both in the North/Baltic Sea and in the Mediterranean.
Relative bubble size: density (absent - ca. 10000 ind/m2). Note that the symbol shading used in Figures 2, 3 and 5 is not consistent as different issues are shown.
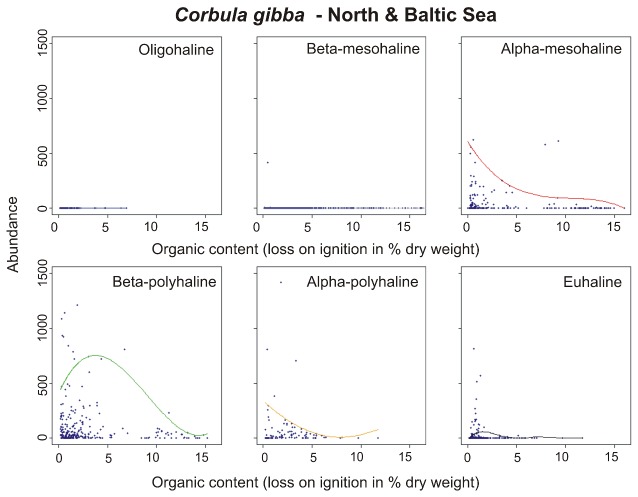
Corbula gibba was found from α-mesohaline to euhaline waters with abundances up to 1500 ind/m². This bivalve showed no clear habitat preferences, but high organic load was avoided (Figure 6). The species was not found on pure mud and seemed to prefer organic contents between 0 and 5 %.
Figure 6. Abundance-response curves (95th percentile QRS) of Corbula gibba along gradient of organic content (loss on ignition) in six different salinity classes in the North Sea and the Baltic Sea.
The dashed line marks the modelled optimum. Note that the y-axis had to be cut in some cases due to outliers.
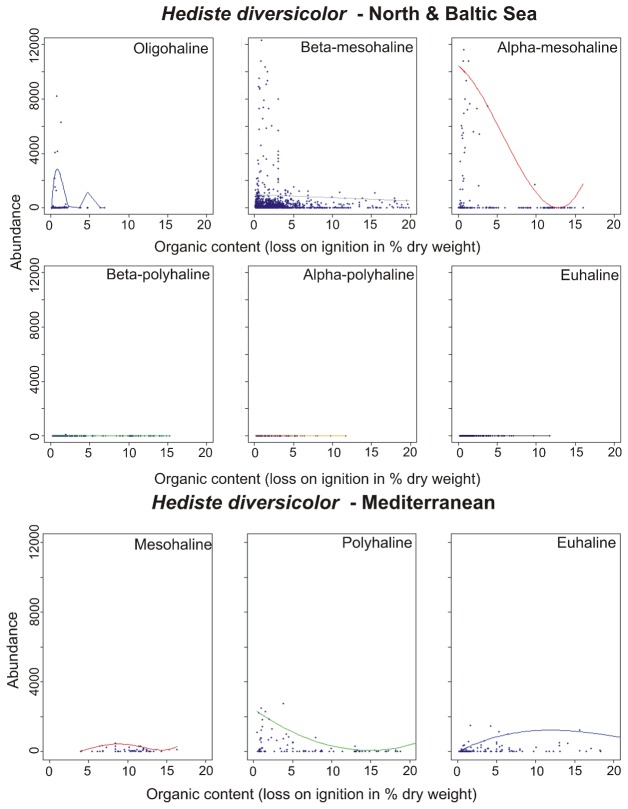
The polychaete H. diversicolor is regarded as one of the most tolerant benthic species, settling under a large variety of conditions. Nevertheless, it reached its highest abundance in Baltic mesohaline waters in substrates with low organic load (Figure 7). Similar to C. glaucum, it populated different salinity classes in the Baltic and Mediterranean Sea, respectively. In the Baltic Sea, H. diversicolor occurred from oligohaline to ß-mesohaline, and in the Mediterranean Sea it preferred mesohaline to euhaline waters.
Figure 7. Abundance-response curves (95th percentile QRS) of Hediste diversicolor along gradient of organic content (loss on ignition) in six different salinity classes in the North Sea and the Baltic Sea (top) and the Mediterranean (bottom).
The dashed line marks the modelled optimum. Note that the y-axis had to be cut in some cases due to outliers.
Discussion
The distribution patterns of species are controlled by different factors of which the relative importance varies as a function of spatial scale [62]. The relative contribution of biotic interactions is considered to be more important for the community assembly processes of macrozoobenthos at smaller spatial scales, whereas life history traits and abiotic environmental conditions become relatively more significant at larger spatial scales. Nevertheless, the investigation areas covered within this study are characterised by large spatial and temporal variability in a number of abiotic environmental variables such as salinity, temperature, oxygen, ice disturbance, etc. Consequently, even at small spatial scales the formation of communities is largely determined by changes in these abiotic variables compared to biotic interactions or community assembly processes [18,63]. In this study only sediment characteristics and salinity were used to detect shifts in the tolerances or preferences of the selected species, while other variables with potential confounding effects were not included. For example, the distribution of trace metals is largely controlled by the granulometry and organic matter content of the sediments [64,65]. Thus, potential contamination from anthropogenic sources rather than sediment characteristics might be causally linked to our observations, but considering the large spatial scale of our study this appears less likely. However, the relationships within communities with abiotic environmental gradients demonstrate that species distributions are driven by influences that are multivariate in origin. This may lead to confusing interpretations on cause-effect relationships when based on information from too narrow sections along environmental gradients. Grémare et al. [30] therefore suggest that the use of a single sensitivity/tolerance list in different geographical areas (such as in AMBI) should be interpreted with care. The use of biological indicators as objective or subjective alternatives for assessing soft-bottom communities is discussed in Dauvin et al. 2012 [39]. Discrepancies between the values of indices are due to the dominance of species characterized as sensitive in one location and tolerant in another. Therefore, natural gradients in salinity (and others such as depth or sediment characteristics) may obscure the detection of macrobenthic responses to human pollution [66]. In systems with a large salinity gradient, the relationships between environmental factors, species distributions and biological traits (specific responses to certain salinities or sediments) may change considerably, as demonstrated in this study. Many species inhabiting variable environments are opportunistic, euryplastic, or transient. If species occur in a large range of environmental parameters (e.g., salinity) the whole range has to be taken into account when interpreting their value as ecological condition indicators. Detailed biogeographic, ecological, and genomic data would be required to accurately interpret indicator species responses. Since studies and data in this respect are rare, we have tried to highlight some issues relevant to the use of static indicators using existing data from monitoring programmes. Despite being aware of the limits of our approach, we believe that the information obtained is robust enough to draw reliable conclusions and inform other ecological applications.
This study has clearly supported the hypothesis that there was no generic response of species abundances to OM content or grain size (environmental “stressors”), along a salinity gradient. The abundance data for A. baltica, C. glaucum, H. diversicolor and C. gibba showed that tolerance to OM content changed with different salinity regimes. In other species however, such as B. pilosa and T. forbesii, it was the relative abundance in different sediment types that changed with salinity regime. These apparent differential preferences could be due to several factors.
In the example of A. baltica, broader substrate preferences might be a consequence of lower interspecific competition in specific salinity regimes. Yet, also the existence of a similar and closely related, cryptic species with different sediment preferences in the Baltic Proper is possible. Ampharete baltica was considered by Jirkov (2001) [67] as a likely member of the Ampharete lindstroemi species complex. Maybe the validity of the species has to be checked [68]. Locally adapted genotypes can present an equally valid explanation for A. baltica, which would require molecular investigation. Alternatively, as a boreal or arctic species, temperature dependency might be stronger than its dependence on a certain type of sediment.
The apparent sediment specificity of T. forbesii in full marine waters might be the result of interspecific competition. Otherwise, its ability to populate fine sands in mesohaline waters could be the result of local adaptation. Cryptic speciation might be at work here as well.
The widespread occurrence of H. diversicolor in a wide variety of habitats might in fact also represent the distribution of several cryptic species in one reported taxon [69]. Potentially, one or several of those species might be regarded as being sensitive (e.g., highest abundances at low organic load). Several studies indicate that H. diversicolor, as considered in the present paper, is probably a mix of several cryptic and morphologically very similar species [70]. Consequently, H. diversicolor is most likely just as strongly differentiated into populations in the western Mediterranean as in the North Sea and Baltic Sea. However, these molecular studies have not yet been translated into an adapted taxonomy for H. diversicolor. As H. diversicolor is just one example, it is likely that the same problem exist for several other species, currently identified as sensitive or tolerant according to different sensitivity lists (e.g. C. glaucum – [71]). The autecology of sibling and cryptic species can differ completely, as they may occupy different niches [72-74]. Although there is considerable variability in the distribution of cryptic species within and between waterbodies, a distinction between such cryptic species cannot be made without genetic analysis. Phylogeographic studies may help to better distinguish and identify possible cryptic species in the future. This may help to assign appropriate indicator values where populations are likely to be genetically separated. Therefore, the investigation of the cryptic species concept in relation to the indicative value of species presents a major topic for further scientific exploration. Furthermore, such future research would shed light on the relative importance and scope for investigations into other possible explanations of the observed patterns (i.e. biotic interactions and interactions between different environmental variables, as hypothesised in this study [75]).
In benthic communities, even closely related species may feed on slightly different food sources. Consequently, species are anticipated to play different role, depending on their way or in the rate at which they contribute to a distinct ecosystem feature (e.g., acting as primary producers, herbivores, predators, or detritivores) [76]. One problem is that all individuals of a given species are assumed equal, whereas characteristics such as sex and developmental stage may profoundly impact their functional roles in the ecosystem and hence the sensitivity/tolerance to stressors. However, densities and abundances are often recorded and reported with no reference to sex or developmental stage. Similarly and even more problematic, closely related species are often assumed to be equivalent (cf. expert judgement on sensitivity/tolerance), when they are clearly not [1]. The autecology of sibling species can differ completely, as they may occupy different niches. Additionally, flexible feeding strategies can vary for a species, in relation to environmental conditions [77].
Wider tolerance to salinity extremes or variability, or adaption to the lower end of the gradient is a key to surviving in low salinity waters. The horohalinicum zone (5-8 psu, sensu [78]) hosts organisms with a broad range of environmental tolerances [79] with interesting genetic implications [80]. Such waters, including inner coastal regions, are within the remit of legislative drivers such as the European Water Framework Directive. It is a paradox in estuarine quality assessment to use macroinvertebrates as biomonitors to detect pollution impacts in estuaries [3,39], since the species inventory per se is well adapted to this highly variable environment. Without suitable reference conditions in such highly dynamic habitats, it is often not possible to differentiate between the response due to anthropogenic disturbances and the natural fluctuation of environmental parameters.
Finally, the question arises whether simple presence/absence, species density or relative abundance is sufficient for the analysis and full understanding of environmental change. The main associated problem is that in distinct localities many of the purported indicator species naturally occur in relatively high densities [81] or have a high seasonal and spatial variability. There is no reliable methodology to know at which density level any indicator species would normally be represented in a community that is not affected by any kind of pollution [82]. Biomass or per capita biomass could give very valuable, additional information when evaluating environmental change.
Taking account of all of the above, defining ecosystem health is therefore currently mainly based on static reference lists of species (sensitivity/tolerance), because it is currently the most feasible way to summarize the ecology within a species community. This manner of ecosystem quality assessment is yet to be considered valuable and its outcomes should be seen as valid warning signals to the competent authorities. However, there is still significant scope for improvement here, which should be further explored in relation to current developments in, for example the European Water Framework Directive and Marine Strategy Framework Directive. From a scientific point of view, in relation to indicator species, attention should be paid to species autecology differentiating between developmental stages, and to the cryptic species issue in relation to indicator species, as elaborated above. This scientific progress is however only expected to gradually deliver valuable insights, which may come too late for the current implementation of environmental legislation. Pragmatically, best available expert knowledge, or evaluating the role of ‘’best professional judgment” in using indicators, may therefore be considered legitimately valuable compared to running data through statistical packages, and may be more routinely used [10,62,83,84]. We therefore suggest that in addition to significant scientific progress, the list of species sensitivities (scores, ecological groups) for marine and coastal environments be discussed among a wide group of experts during dedicated (periodic) meetings (e.g. Water Framework Directive indicator calibration meetings organised by the European Commission), rather than prepared by a few specialists. These discussions would have to focus especially on species with a wide ecological range of occurrence and tolerance, and with regard to their (functional) role in the local ecosystem and response to the environment, as illustrated in this study (Table 5). While useful, expert elicitation of best professional judgement can also fail [85]. There is unlikely to be any single approach that always works, and the incorporation of multiple lines of evidence in condition assessment is probably most powerful. A wide suite of tools, methods, and models would be best for these assessments rather than any one single indicator [37,86].
Table 5. Summary table of ecological requirements of selected species, given as assumptions, results and its possible explanations.
| species | Ampharete baltica | Bathyporeia pilosa | Cerastoderma glaucum | Corbula gibba | Hediste diversicolor | Travisia forbesii |
|---|---|---|---|---|---|---|
| assumption | indifferent to organic load | sensitive to changes in grain size distribution | sensitive or tolerant to organic load | tolerant to organic load | tolerant to organic load | sensitive to changes in grain size distribution |
| result | avoidance of organic load at high salinity, higher tolerance at low salinities | large differences in preferred grain size detected between different salinity classes and ecoregions | no general sensitivity to organic load detected | found on various substrates, but absent on pure mud with high organic load | found on various substrates also with very high organic load, but highest abundances were found in substrates with medium organic load | higher sediment specificity at higher salinities, widening in mesohaline waters |
| rationale | different adaptation of different populations, cryptic species | apparent substrate preferences result of strong/weak interspecific competition with grain size not being the limiting parameter | different adaptation of different populations | apparent substrate preferences result of strong/weak interspecific competition | cryptic species | apparent substrate preferences result of strong/weak interspecific competition with grain size not being the limiting parameter |
Conclusion
This paper demonstrated that static indicator species lists should be applied with caution in environmental quality assessments; the importance of interacting environmental variables, the possible existence of cryptic species and the differential sensitivity of different life stages should not be neglected when using static indicator lists. In this context we do not question the use of indicator species in general, but point out several important issues that ecologist should consider before using indicator species or indices derived from them. In this work we raise the importance of when adopting such static list approach, this is applied in a context where there is a proper prior knowledge of the system and the species that are being analysed and this in cases is context (e.g. area specific). The use of benthic indicator organisms will continue to be a mainstay of marine environmental management and impact assessment. However, instead of using generic pan-European indices or species scores, the assessment schemes would benefit if dealt with in a region- and ecosystem type-specific manner. Consequently, the following points have to be considered when assessing species indicative values:
-
1
Large variability in patterns of species life history strategies along major natural environmental gradients and between various regions and ecosystem types;
-
2
Altered species autecology (and hence indicator value) along environmental gradients (e.g. salinity);
-
3
Need for adjustment of indicator species lists to distinct salinity/organic matter ranges;
-
4
Avoid pooling of sibling or cryptic species when calculating the index value of a species and/or evaluation of the uncertainty in condition assessments due to the inclusion of sibling or cryptic species in an indicator species or condition index. In most cases there is no way out of not pooling sibling or cryptic species.
We therefore recommend progressing at both the scientific level (e.g. species autecology (life-stage dependent) and the cryptic species concept (longer term perspective)) and the application level (e.g. widely solicited expert judgement on region and habitat-specific indicator values (shorter term perspective)).
Supporting Information
Geographical coordinates of stations used for this study.
(XLSX)
Data on sediment median grain size (d50) linked with the abundance of selected species and their salinity ranges of the Baltic Sea and North Sea.
(XLSX)
Data on sediment organic content (LOI) linked with the abundance of selected species and their salinity ranges of the Baltic Sea and North Sea.
(XLSX)
Data on sediment median grain size and organic content linked with the abundance of selected species and their salinity ranges of the Mediterranean Sea.
(XLSX)
Acknowledgments
This publication was initiated and facilitated by the Benthos Ecology Working Group (BEWG), which is an expert group of the International Council for the Exploration of the Sea (ICES). The following authors are member of the BEWG: MLZ, CEP, AD, SD, CG, PM, GM, HR and GVH. We thank the Monitor taskforce (NIOZ-Yerseke) and Rijkswaterstaat for benthic sample collection of the Westerschelde data, and the Spatial Ecology Department of NIOZ-Yerseke for providing grain size data (courtesy F. Cozzoli). Data for the GB portion of the North Sea were supplied by the British Oceanographic Data Centre on behalf of the Clean Safe Seas Evidence Group (MERMAN database, http://www.bodc.ac.uk/projects/uk/merman/). The final manuscript was very much improved by the explanatory notes of three anonymous reviewers, which we appreciate as well.
Funding Statement
Data on C. glaucum and H. diversicolor from the Venice lagoon are from the Ministry of Infrastructures and Transport - Magistrato alle Acque di Venezia (Water Authority of Venice) through the “Consorzio Venezia Nuova”. The collection and analyses of Estonian data was supported by institutional research funding IUT02-20 of the Estonian Research Council. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Gray JS, Pearson TH (1982) Objective selection of sensitive species indicative of pollution-induced change in benthic communities. I. Comparative methodology. Mar Ecol Prog Ser 9: 111-119. doi: 10.3354/meps009111. [DOI] [Google Scholar]
- 2. Pearson TH, Gray JS, Johannessen PJ (1983) Objective selection of sensitive species indicative of pollution-induced change in benthic communities. 2. Data analyses. Mar Ecol Prog Ser 12: 237-225. doi: 10.3354/meps012237. [DOI] [Google Scholar]
- 3. Dauvin J-C, Bellan G, Bellan-Santini D (2010) Benthic indicators: From subjectivity to objectivity – Where is the line? Mar Pollut Bull 60: 947-953. doi: 10.1016/j.marpolbul.2010.03.028. PubMed: 20413132. [DOI] [PubMed] [Google Scholar]
- 4. Zettler ML, Darr A, Labrenz M, Sagert S, Selig U et al. (2013) The indicator value of organisms. In: Snoeijs P, Schubert H, Radziejewska T. Biological Oceanography of the Baltic Sea. Springer Verlag. In press [Google Scholar]
- 5. Reish DJ (1957) The relationship of the polychaetous annelid Capitella capitata to waste discharge of biological origin. In: Tarzwell CM. Biological Problems in Water Pollution. Cincinnati, OH: US: Public Health Service; pp. 195-200. [Google Scholar]
- 6. Word JQ, Myers BL, Mearns AJ (1977) Animals that are indicators of marine pollution. Annu Rep, Coastal Water Research Project, El Segundo, Calif. pp. 199-206. [Google Scholar]
- 7. Word JQ (1979) The infaunal trophic index. Annu Rep, 1978: Coastal Water Research Project, El Segundo, Calif. pp. 19-39. [Google Scholar]
- 8. Marchand M, Vas D, Duursma EK (1976) Levels of PCBs and DDTs in mussels from the NW Mediterranean. Mar Pollut Bull 7: 65-69. doi: 10.1016/0025-326X(76)90014-X. [DOI] [Google Scholar]
- 9. Carignan V, Villard M-A (2002) Selecting indicator species to monitor ecological integrity: A review. Environ Monit Assess 78: 45-61. doi: 10.1023/A:1016136723584. PubMed: 12197640. [DOI] [PubMed] [Google Scholar]
- 10. Van Hoey G, Borja A, Birchenough S, Degraer S, Fleischer D et al. (2010) The use of benthic indicators in Europe: from the Water Framework Directive to the Marine Strategy Framework Directive. Mar Pollut Bull 60: 2187-2196. doi: 10.1016/j.marpolbul.2010.09.015. PubMed: 21051051. [DOI] [PubMed] [Google Scholar]
- 11. Heink U, Kowarik I (2010) What are indicators? On the definition of indicators in ecology and environmental planning. Ecol Indic 10: 584-593. doi: 10.1016/j.ecolind.2009.09.009. [DOI] [Google Scholar]
- 12. Bellan G, Bellan-Santini D (1972) Influence de la pollution sur les peuplements marins de la région de Marseille. In: Ruivo M. Marine Pollution and Sea Life. London, UK: FAO, Fishing News (Books) Ltd. pp. 396-401. [Google Scholar]
- 13. Pérès JM, Bellan G (1972) Aperçu sur l’influence des pollutions sur les peuplements benthiques. Ruivo M. Marine Pollution and Sea Life. London, UK: FAO, Fishing News (Books) Ltd. pp. 375-386. [Google Scholar]
- 14. Leppäkoski E (1975) Assessment of degree of pollution on the basis of macrozoobenthos in marine brackish-water environments. Acta Acad Abo Ser B 35: 1-96. [Google Scholar]
- 15. Pantle R, Buck H (1955) Die biologische Überwachung der Gewässer und die Darstellung der Ergebnisse. Gas Und Wasserfach Wasser Abwasser 96: 604-607 (in German). [Google Scholar]
- 16. Zettler ML, Schiedek D, Bobertz B (2007) Benthic biodiversity indices versus salinity gradient in the southern Baltic Sea. Mar Pollut Bull 55: 258-270. doi: 10.1016/j.marpolbul.2006.08.024. PubMed: 17010998. [DOI] [PubMed] [Google Scholar]
- 17. Fleischer D, Zettler ML (2009) An adjustment of benthic ecological quality assessment to effects of salinity. Mar Pollut Bull 58: 351-357. doi: 10.1016/j.marpolbul.2008.10.016. PubMed: 19084875. [DOI] [PubMed] [Google Scholar]
- 18. Barbone E, Rosati I, Reizopoulou S, Basset A (2012) Linking classification boundaries to sources of natural variability in transitional waters: A case study of benthic macroinvertebrates. Ecol Indic 12: 105-122. doi: 10.1016/j.ecolind.2011.04.014. [DOI] [Google Scholar]
- 19. Magni P, Tagliapietra D, Lardicci C, Balthis L, Castelli A et al. (2009) Animal-sediment relationships: Evaluating the ‘Pearson-Rosenberg paradigm’ in Mediterranean coastal lagoons. Mar Pollut Bull 58: 478-486. doi: 10.1016/j.marpolbul.2008.12.009. PubMed: 19162282. [DOI] [PubMed] [Google Scholar]
- 20. Tagliapietra D, Sigovini M, Magni P (2012) Saprobity: A unified view of benthic succession models for coastal lagoons. Hydrobiologia 686: 15-28. doi: 10.1007/s10750-012-1001-8. [DOI] [Google Scholar]
- 21. Majeed SA (1987) Organic matter and biotic indices on the beaches of North Brittany. Mar Pollut Bull 18: 490-495. doi: 10.1016/0025-326X(87)90360-2. [DOI] [Google Scholar]
- 22. Grall J, Glémarec M (1997) Using biotic indices to estimate macrobenthic community perturbations in the Bay of Brest. Estuar Coast Shelf Sci 44: 43–53. doi: 10.1016/S0272-7714(97)80006-6. [DOI] [Google Scholar]
- 23. Weisberg SB, Ranasighe JA, Schaffner LC, Diaz RJ, Dauer DM et al. (1997) An estuarine index of biological integrity (B-IBI) for Chesapeake Bay. Estuaries 20: 149-158. doi: 10.2307/1352728. [DOI] [Google Scholar]
- 24. Borja A, Franco J, Pérez V (2000) A marine biotic index to establish the ecological quality of soft-bottom benthos within European estuarine and coastal environments. Mar Pollut Bull 40: 1100-1114. doi: 10.1016/S0025-326X(00)00061-8. [DOI] [Google Scholar]
- 25. Simboura N, Zenetos A (2002) Benthic indicators to use in Ecological Quality classification of Mediterranean soft bottom marine ecosystems, including a new Biotic Index. Medit Mar Sci 3: 77-111. [Google Scholar]
- 26. Rosenberg R, Blomqvist M, Nilsson HC, Cederwall H, Dimming A (2004) Marine quality assessment by use of benthic species-abundance distributions: a proposed new protocol within the European Union Water Framework Directive. Mar Pollut Bull 49: 728-739. doi: 10.1016/j.marpolbul.2004.05.013. PubMed: 15530516. [DOI] [PubMed] [Google Scholar]
- 27. Meng TT, Ni J, Harrison SP (2009) Plant morphometric traits and climate gradients in northern China: a meta-analysis using quadrat and flora data. Ann Bot 104: 1217-1229. doi: 10.1093/aob/mcp230. PubMed: 19805404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dowdall JRT (2012) Intra- and interspecific variation along environmental gradients: Adaption, plasticity, and range limits [PhD Dissertation]. Colorado State University. p. 162. [Google Scholar]
- 29. Bolnick DI, Amarasekare P, Araújo MS, Bürger R, Levine JM et al. (2012) Why intraspecific trait variation matters in community ecology. Trends Ecol Evol 26: 183-192. PubMed: 21367482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grémare A, Labrune C, Vanden Berghe E, Amouroux JM, Bachelet G et al. (2009) Comparison of the performances of two biotic indices based on the MacroBen database. Mar Ecol Prog Ser 382: 297-311. doi: 10.3354/meps08030. [DOI] [Google Scholar]
- 31. Kolkwitz R, Marsson M (1902) Grundsätze für die biologische Beurteilung des Wassers nach seiner Flora und Fauna. Mittheilungen Königlichen Versuchs Und Prüfungsanstalt Wasserversorgung Abwässerbeseitigung Berl 1: 33-72 (in German). [Google Scholar]
- 32. Kolkwitz R, Marsson M (1908) Ökologie der pflanzlichen Saprobien. Ber Deutschen Botanischen Gesellschaft 26a: 505-519 (in German) [Google Scholar]
- 33. Kolkwitz R, Marsson M (1909) Ökologie der tierischen Saprobien. Int Rev Gesamten Hydrobiol 2: 126-152 (in German). doi: 10.1002/iroh.19090020108. [DOI] [Google Scholar]
- 34. Zelinka M, Marvan P (1961) Zur Präzisierung der biologischen Klassifikation der Reinheit fließender Gewässer. Arch Hydrobiol 57: 389-407 (in German). [Google Scholar]
- 35. Wilhelmi J (1916) Übersicht über die biologische Beurteilung des Wassers. Sber Ges Naturf Fr Bln. pp. 297-306 (in German). [Google Scholar]
- 36. Meyer T, Berg T, Fürhaupter K (2008) Ostsee Makrozoobenthos Klassifizierungssystem für die Wasserrahmenrichtlinie. Referenz-Artenlisten, Bewertungsmodell und Monitoring. MARILIM Gewässeruntersuchung. p. 131. Available: http://www.marilim.de/marbit/download/files/MarBIT.pdf. [Accessed 20 May 2013].
- 37. Keely NB, Macleod CK, Forrest BM (2012) Combining best professional judgement and quantile regression splines to improve characterisation of macrofaunal responses to enrichment. Ecol Indic 12: 154-166. doi: 10.1016/j.ecolind.2011.03.022. [DOI] [Google Scholar]
- 38. Pelletier MC, Gold AJ, Heltshe JF, Buffum HW (2010) A method to identify estuarine macroinvertebrate pollution indicator species in the Virginian Biogeographic Province. Ecol Indic 10: 1037-1048. doi: 10.1016/j.ecolind.2010.03.005. [DOI] [Google Scholar]
- 39. Dauvin JC, Alizier S, Rolet C, Bakalem A, Bellan G et al. (2012) Response of different benthic indices to diverse human pressures. Ecol Indic 12: 143-153. doi: 10.1016/j.ecolind.2011.03.019. [DOI] [Google Scholar]
- 40. Ysebaert T, Herman PMJ, Meire P, Craeymeersch J, Verbeek H et al. (2003) Large-scale spatial patterns in estuaries: estuarine macrobenthic communities in the Schelde estuary, NW Europe. Estuar Coast Shelf Sci 57: 335-355. doi: 10.1016/S0272-7714(02)00359-1. [DOI] [Google Scholar]
- 41. Zettler ML, Schiedek D, Glockzin M (2008) Zoobenthos. In: Feistel R, Nausch G, Wasmund N. State and Evolution of the Baltic Sea, 1952 - 2005. A Detailed 50-Year Survey of Meteorology and Climate, Physics, Chemistry, Biology, and Marine Environment. Hoboken: John Wiley & Sons; pp. 517-541. [Google Scholar]
- 42. Telesh IV, Khlebovich VV (2010) Principal processes within the estuarine salinity gradient: A review. Mar Pollut Bull 61: 149-155. doi: 10.1016/j.marpolbul.2010.02.008. PubMed: 20304437. [DOI] [PubMed] [Google Scholar]
- 43. Ganoulis J (2009) Risk analysis of water pollution (2nd ed.). Weinheim: Wiley VCH Verlag. [Google Scholar]
- 44. Hyland J, Balthis LW, Karakassis I, Magni P, Petrov A et al. (2005) Organic carbon content of sediments as an indicator of stress in the marine benthos. Mar Ecol Prog Ser 295: 91-103. doi: 10.3354/meps295091. [DOI] [Google Scholar]
- 45. Reusser DA, Lee H (2011) Evolution of natural history information in the 21st century – developing an integrated framework for biological and geographical data. J Biogeogr 38: 1225-1239. doi: 10.1111/j.1365-2699.2011.02515.x. [DOI] [Google Scholar]
- 46. Mebius LJ (1960) A rapid method for the determination of organic carbon in soil. Anal Chim Acta 22: 120-124. doi: 10.1016/S0003-2670(00)88254-9. [DOI] [Google Scholar]
- 47. Magni P, De Falco G, Como S, Casu D, Floris A et al. (2008) Distribution and ecological relevance of fine sediments in organic-enriched lagoons: the case study of the Cabras lagoon (Sardinia, Italy). Mar Pollut Bull 56: 549-564. doi: 10.1016/j.marpolbul.2007.12.004. PubMed: 18234238. [DOI] [PubMed] [Google Scholar]
- 48. MarLIN (The Marine Life Information Network). Available: http://www.marlin.ac.uk/. [Accessed 20 May 2013].
- 49. Hartmann-Schröder G (1996) Annelida, Borstenwürmer, Polychaeta. (2. Aufl.) In: Dahl F, Die Tierwelt Deutschlands und der angrenzenden Meeresteile nach ihren Merkmalen und ihrer Lebensweise. Teil 58. Jena: Gustav Fischer Verlag; p. 648 (in German). [Google Scholar]
- 50. Krause JC (2002) he effects of marine sediment extraction on sensitive macrozoobenthic populations in the southern Baltic Sea [PhD Thesis]. University of Rostock. [Google Scholar]
- 51. Scaps P (2002) A review of the biology, ecology and potential use of the common ragworm Hediste diversicolor (OF Müller) (Annelida: Polychaeta). Hydrobiologia 470: 203-218. doi: 10.1023/A:1015681605656. [DOI] [Google Scholar]
- 52. d’Udekem d'Acoz C (2004) The genus Bathyporeia Lindström, 1855, in western Europe (Crustacea: Amphipoda: Pontoporeiidae). Zool Verhand 348: 1-164. [Google Scholar]
- 53. Hrs-Brenko M (2006) The basket shell, Corbula gibba Olivi, 1792 (Bivalve Mollusks) as a species resistant to environmental disturbances: A review. Acta Adriat 47: 49–64. [Google Scholar]
- 54. Yonge CM (1946) On the habits and adaptations of Aloidis (Corbula) gibba . J Mar Biol Assoc UK 26: 358-376. doi: 10.1017/S0025315400012182. PubMed: 20992402. [DOI] [PubMed] [Google Scholar]
- 55. Russell PJ, Petersen GH (1973) The use of ecological data in the elucidation of some shallow water European Cardium species. Malacologia 14: 223-232. [Google Scholar]
- 56. Thrush SF, Hewitt JE, Herman PMJ, Ysebaert T (2005) Multi-scale analysis of species-environment relationships. Mar Ecol Prog Ser 302: 13-26. doi: 10.3354/meps302013. [DOI] [Google Scholar]
- 57. Anderson MJ (2008) Animal-sediment relationships re-visited: Characterising species' distributions along an environmental gradient using canonical analysis and quantile regression splines. J Exp Mar Biol Ecol 366: 16-27. doi: 10.1016/j.jembe.2008.07.006. [DOI] [Google Scholar]
- 58. Koenker R (2012) quantreg: Quantile Regression. R package version 4.79. Available: http://CRAN.R-project.org/package=quantreg.
- 59. Hastie TJ (1992) Generalized additive models. Chapter 7 of Statistical Models in S. In: Chambers JM, Hastie TJ. Wadsworth Publishing House & Brooks/Cole. [Google Scholar]
- 60. R Development Core Team (2012) R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; Available: http://www.R-project.org/. [Google Scholar]
- 61. Burnham KP, Anderson DR (2002) Model selection and multi-model inference: a practical information-theoretic approach, 2nd edition. New York: Springer Verlag. [Google Scholar]
- 62. Gray S, Elliott M (2009) Ecology of Marine Sediments: From Science to Management, second edition. New York: Oxford University Press. [Google Scholar]
- 63. Herkül K, Kotta J, Kotta I, Orav-Kotta H (2006) Effects of physical disturbance, isolation and key macrozoobenthic species on community development, recolonisation and sedimentation processes. Oceanologia 48 (S): 267-282 [Google Scholar]
- 64. Cai MH, Lin J, Hong QQ, Wang Y, Cai MG (2011) Content and distribution of trace metals in surface sediments from the northern Bering Sea, Chukchi Sea and adjacent Arctic areas. Mar Pollut Bull 63: 523-527. doi: 10.1016/j.marpolbul.2011.02.007. PubMed: 21377172. [DOI] [PubMed] [Google Scholar]
- 65. Rubio B, Nombela MA, Vilas F (2000) Geochemistry of major and trace elements in sediments of the Ria de Vigo (NW Spain): an assessment of metal pollution. Mar Pollut Bull 40: 968-980. doi: 10.1016/S0025-326X(00)00039-4. [DOI] [Google Scholar]
- 66. Rakocinski CF, Brown SS, Gaston GR, Heard RW, Walker WW et al. (1997) Macrobenthic responses to natural and contaminant related gradients in northern Gulf of Mexico estuaries. Ecol Appl 7: 1278-1298. doi: 10.1890/1051-0761(1997)007[1278:MRTNAC]2.0.CO;2. [DOI] [Google Scholar]
- 67. Jirkov IA (2001) Polychaeta of the Arctic Ocean. Moscow: Yanus-K; (in Russian). [Google Scholar]
- 68. Parapar J, Helgason GV, Jirkov I, Moreira J (2012) Polychaetes of the genus Ampharete (Polychaeta: Ampharetidae) collected in Icelandic waters during the BIOICE project. Helgol Mar Res 66: 331-344. [Google Scholar]
- 69. Virgilio M, Fauvelot C, Costantini F, Abbiati M, Backeljau T (2009) Phylogeography of the common ragworm Hediste diversicolor (Polychaeta: Nereididae) reveals cryptic diversity and multiple colonization events across its distribution. Mol Ecol 18: 1980-1994. doi: 10.1111/j.1365-294X.2009.04170.x. PubMed: 19344353. [DOI] [PubMed] [Google Scholar]
- 70. Röhner M, Bastrop R, Jürss K (1997) Genetic differentiation in Hediste diversicolor (Polychaeta: Nereididae) for the North Sea and the Baltic Sea. Mar Biol 130: 171-180. doi: 10.1007/s002270050236. [DOI] [Google Scholar]
- 71. Nikula R, Väinölä R (2003) Phylogeography of Cerastoderma glaucum (Bivalvia: Cardiidae) across Europe: a major break in the Eastern Mediterranean. Mar Biol 143: 339-350. doi: 10.1007/s00227-003-1088-6. [DOI] [Google Scholar]
- 72. Kesäniemi JE, Rawson PD, Lindsay SM, Knott KE (2012) Phylogenetic analysis of cryptic speciation in the polychaete Pygospio elegans . Ecol Evol 2: 994-1007. doi: 10.1002/ece3.226. PubMed: 22837844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gamenick I, Vismann B, Grieshaber MK, Giere O (1998) Ecophysiological differentiation of Capitella capitata (Polychaeta). Sibling species from different sulfidic habitats. Mar Ecol Prog Ser 175: 155-166. doi: 10.3354/meps175155. [DOI] [Google Scholar]
- 74. Luttikhuizen PC, Bol A, Cardoso JFMF, Dekker R (2011) Overlapping distributions of cryptic Scoloplos cf. armiger species in the western Wadden Sea. J Sea Res 66: 231-237. doi: 10.1016/j.seares.2011.07.002. [DOI] [Google Scholar]
- 75. Baird HP, Miller KJ, Stark JS (2012) Genetic Population Structure in the Antarctic Benthos: Insights from the Widespread Amphipod, Orchomenella franklini . PLOS ONE 7: e34363. doi: 10.1371/journal.pone.0034363. PubMed: 22479613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Covich AP, Palmer MA, Crowl TA (1999) The role of benthic invertebrate species in freshwater ecosystems. Zoobenthic species influence energy flows and nutrient cycling. BioScience 49: 119-127. [Google Scholar]
- 77. Maurer D, Nguyen H, Robertson G, Gerlinger T (1999) The infaunal trophic index (ITI): its suitability for marine environmental monitoring. Ecol Appl 9: 699-713. doi: 10.1890/1051-0761(1999)009[0699:TITIII]2.0.CO;2. [DOI] [Google Scholar]
- 78. Kinne O (1971) Salinity: animals-invertebrates, In Kinne O. Marine Ecology, Vol. 1, Environmental Factors, Part 2. New York: John Wiley and Sons; pp. 821-996. [Google Scholar]
- 79. Deaton LE, Greenberg MJ (1986) There is no horohalinicum. Estuaries 9: 20-30. doi: 10.2307/1352189. [DOI] [Google Scholar]
- 80. Khlebovich VV, Abramova EN (2000) Some problems of crustacean taxonomy related to the phenomenon of Horohalinicum. Hydrobiologia 417: 109-111. doi: 10.1023/A:1003863623267. [DOI] [Google Scholar]
- 81. Warwick RM (1993) Environmental impact studies on marine communities: Pragmatical considerations. Aust J Ecol 18: 63-80. doi: 10.1111/j.1442-9993.1993.tb00435.x. [DOI] [Google Scholar]
- 82. Marques JC, Salas F, Patrício J, Teixeira H, Neto JM (2009) Ecological indicators for coastal and estuarine environmental assessment. A user guide. Southampton: WIT-Press. [Google Scholar]
- 83. Weisberg SB, Thompson B, Ranasinghe JA, Montagne DE, Cadien DB et al. (2008) The level of agreement among experts applying best professional judgment to assess the condition of benthic infaunal communities. Ecol Indic 8: 389-394. doi: 10.1016/j.ecolind.2007.04.001. [DOI] [Google Scholar]
- 84. Teixeira H, Borja Á, Weisberg SB, Ranasinghe JA, Cadien DB et al. (2010) Assessing coastal benthic macrofauna community condition using best professional judgement – Developing consensus across North America and Europe. Mar Pollut Bull 60: 589-600. doi: 10.1016/j.marpolbul.2009.11.005. PubMed: 19969316. [DOI] [PubMed] [Google Scholar]
- 85. Thompson B, Weisberg SB, Melwani A, Lowe S, Ranasinghe JA et al. (2012) Low levels of agreement among experts using best professional judgment to assess benthic condition in the San Francisco Estuary and Delta. Ecol Indic 12: 167-173. doi: 10.1016/j.ecolind.2011.03.020. [DOI] [Google Scholar]
- 86. Magni P, Hyland J, Manzella G, Rumohr H, Viaroli P et al. , editors (2005) Proceedings of the Workshop "Indicators of Stress in the Marine Benthos", Torregrande-Oristano, (Italy): IOC Workshop Reports, 195. Available: http://unesdoc.unesco.org/images/0013/001397/139719e.pdf. [Accessed 2013 May 20].
- 87. Crema R, Castelli A, Prevedelli D (1991) Long term eutrophication effects on macrofaunal communities in Northern Adriatic Sea. Mar Pollut Bull 22: 503-508. doi: 10.1016/0025-326X(91)90405-H. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Geographical coordinates of stations used for this study.
(XLSX)
Data on sediment median grain size (d50) linked with the abundance of selected species and their salinity ranges of the Baltic Sea and North Sea.
(XLSX)
Data on sediment organic content (LOI) linked with the abundance of selected species and their salinity ranges of the Baltic Sea and North Sea.
(XLSX)
Data on sediment median grain size and organic content linked with the abundance of selected species and their salinity ranges of the Mediterranean Sea.
(XLSX)
Data Availability Statement
This paper presents collaborative research based on a collation of existing data. Table 1 provides further details on the data sources (i.e. contact person, availability). Data are compiled into appendices (Appendix S1-S4).
Table 1. Number of samples, sampling period and availability of data used in the present study.
| Great Britain | North Sea 1 | North Sea 2 | Wester-schelde | Zee-schelde | Belgian Beaches | Belgium Offshore | German Baltic | Estonia | Cabras Lagoon | Venice Lagoon | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of samples | 126 | 30 | 84 | 2089 | 104 | 343 | 131 | 1012 | 1549 | 76 | 285 |
| Sampling period | 2001-2011 | 2000-2004 | 2008-2011 | 2000-2008 | 1990-2011 | 1994-1995 | 2005-2010 | 2001-2011 | 2003-2009 | 2001-2004 | 1991-2002 |
| Availability | On request | On request | On request | On request | On request | On request | On request | On request | On request | On request | On request |
| Responsible author | CG | HR | MLZ | TY | JS | SD | GVH | MLZ | JK | PM | DT |
Only sample data that contained information on salinity, substrate (sediment grain size, organic matter content) and the abundance of selected species (including zero-values indicating the absence of the species at an individual station) were retained for analysis. Overall, 5468 records from the North Sea and Baltic Sea and 361 records from the Mediterranean were available for this study (Tables 2 and 3). The data originate from nine case-studies and monitoring programs from around the North Sea and Baltic Sea, covering coastal and offshore waters of the UK, Belgium, the Netherlands, Germany and Estonia (Figure 1). In the Mediterranean Sea, data were available from two Italian lagoons, namely the Venice Lagoon and the Cabras Lagoon (Isle of Sardinia). These datasets, covering a wide range in salinity, were combined into two datasets (two geographical regions), i.e. a North Sea – Baltic Sea and a Mediterranean Sea database.
Table 2. Data availability (loss on ignition (LOI) and median grain size) in the North and Baltic Sea.
|
North & Baltic Sea
|
Great Britain
|
North Sea 1
|
North Sea 2
|
Westerschelde
|
Zeeschelde
|
Belgian Beaches
|
Belgium Offshore
|
German Baltic
|
Estonia
|
Overall
|
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| LOI | oligo | 72 | 55 | 127 | |||||||
| β-meso | 362 | 1467 | 1829 | ||||||||
| α-meso | 209 | 209 | |||||||||
| β -poly | 278 | 278 | |||||||||
| α -poly | 4 | 3 | 11 | 9 | 75 | 102 | |||||
| euhalin | 113 | 27 | 81 | 332 | 91 | 644 | |||||
| grain size | oligo | 11 | 72 | 46 | 129 | ||||||
| β-meso | 88 | 93 | 269 | 1247 | 1697 | ||||||
| α-meso | 523 | 184 | 707 | ||||||||
| β -poly | 591 | 225 | 816 | ||||||||
| α -poly | 4 | 3 | 739 | 11 | 11 | 75 | 843 | ||||
| euhalin | 60 | 30 | 81 | 87 | 332 | 120 | 710 | ||||
| overall | oligo | 11 | 72 | 55 | 138 | ||||||
| β-meso | 88 | 93 | 362 | 1494 | 2037 | ||||||
| α-meso | 523 | 225 | 748 | ||||||||
| β -poly | 591 | 278 | 869 | ||||||||
| α -poly | 4 | 3 | 739 | 11 | 11 | 75 | 843 | ||||
| euhalin | 122 | 30 | 81 | 87 | 332 | 120 | 772 | ||||
Table 3. Data availability (loss on ignition (LOI) and median grain size) in the Mediterranean Sea.
|
Mediterranean
|
Cabras
|
Venice
|
Overall
|
|
|---|---|---|---|---|
| LOI | meso | 38 | 22 | 60 |
| poly | 24 | 103 | 127 | |
| eu | 14 | 160 | 174 | |
| grain size | meso | 38 | 22 | 60 |
| poly | 24 | 103 | 127 | |
| eu | 14 | 160 | 174 | |
| overall | meso | 38 | 22 | 60 |
| poly | 24 | 103 | 127 | |
| eu | 14 | 160 | 174 | |
Figure 1. Map of sample locations included in this study.
The salinity at each sampling location was either measured directly or taken from modelled data (Schelde estuary). At intertidal locations, salinity was measured from the receding water immediately after the sampling location became exposed. In this study, a reduced six-level Venice salinity system was used: euhaline zone >34 to 30, α-polyhaline zone 25 to 30, β-polyhaline zone 18 to 25, α-mesohaline zone 18 to 10, β-mesohaline zone 10 to 5 and oligohaline zone 5 to 0.5 (see [45] for details). However, due to the comparably small data-set, Mediterranean data were subdivided only into three salinity classes (poly-, meso- and oligohaline, Table 3).
Grain size distribution was taken into account as median grain size (d50 in µm) (Tables 2 and 3). Within the North/Baltic Sea dataset, stations with d50 > 2000 µm were excluded, as these were scarce and available only for a single salinity class. Overall, 4951 records were used. Median grain size was available for all Mediterranean stations. Maximum median grain size was 964 µm.
For most stations, sediment organic matter content (OM) was measured as loss on ignition (LOI in % of dry weight). For some stations in Belgium offshore waters (n=61) and the UK (n=126), LOI was calculated from the organic matter content (for Belgium the “dichromate method” for TOC [46]), LOI = 2.9324*organic matter content + 0.2979 (R2 = 0.85, p<0.01). The relationship was calculated from parallel measurements, available for Belgian waters (n = 39). Overall, 3559 data points with information on organic content of the sediment were included in the dataset. Previously proposed OM ranges were used as an indication of the level (low, moderate, high) of OM enrichment [19,44,47]. This is because OM in sediments can be a stressor when it is in excess, while at low to moderate levels (ca. up to 8-10%) it is an important food source for benthic animals [44,47].