Abstract
Recent studies have demonstrated the utility of using systems approaches to identify molecular signatures that can be used to predict vaccine immunity in humans. Such approaches are now being used extensively in vaccinology, and are beginning to yield novel insights about the molecular networks driving vaccine immunity. In this review, we present a broad review of the methodologies involved in these studies, and discuss the promise and challenges involved in this emerging field of “systems vaccinology.”
1. Introduction
Vaccines are among the greatest successes of modern medicine [1, 2]. The abiding threat of global infectious diseases poses a pressing need to develop new and effective vaccines. Despite the common origins of vaccinology and immunology in the pioneering work of scientists such as Pasteur and Jenner, the disciplines have occupied parallel universes. Thus the development of most vaccines has occurred largely through a process of trial and error, with little attention being devoted to the immunological mechanisms by which the vaccine confers protective immunity. Many vaccines, such as the live attenuated vaccines, work by mimicking pathogens, to stimulate lasting and protective immunity in the host. However, this strategy has not worked for difficult cases like HIV, malaria and tuberculosis. There is a sentiment in the scientific community that all “easy” vaccines have been discovered, and a detailed understanding of cellular and molecular mechanisms will be necessary to optimize or develop new vaccines [3, 4]. However, much of the fundamentals of modern immunology have been discerned from the use of model antigens such as ovalbumin in mice, and immunologists have paid scant attention to vaccine biology in humans.
Much progress has been made in the past two decades, in understanding how the innate immune system recognizes microbial stimuli and regulates adaptive immunity, and has been recognized by the Nobel prize in Physiology or Medicine in 2011 (to Bruce Beutler, Jules Hoffmann and Ralph Steinman). The general model of vaccination is that vaccine components program antigen presenting cells through pattern recognition receptors (e.g. Toll-like receptors, TLRs) to stimulate antigen specific T cells to proliferate and differentiate into diverse effector cells and long-lived memory T cells. T cells can also help regulate B cell activation, differentiation, antibody secretion and the development of memory B cells. This results in the synthesis of neutralizing or opsonizing antibodies and/or cell-mediated mechanisms, which lead to protection. The mechanisms of protection differ between different vaccines and the molecular pathways linking innate immunity and adaptive immunity are less well understood, especially in humans [5, 6].
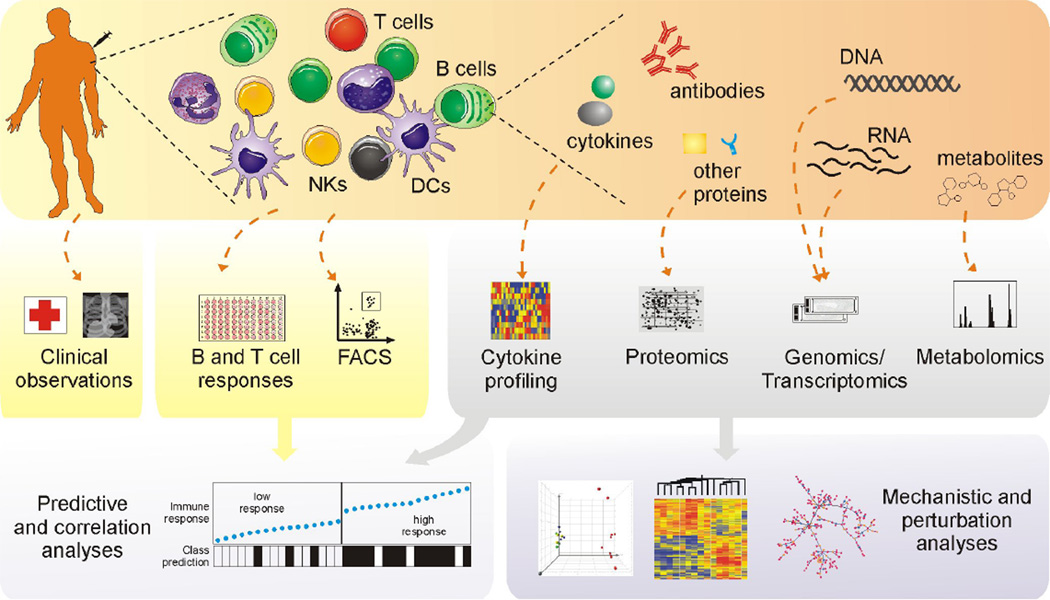
Systems vaccinology (Figure 1) is an effort to apply tools from systems biology to vaccine studies, and has started to bear fruits in the past few years [7–10]. The tools of systems biology consist of a number of high-throughput technologies (often dubbed as “omics”), including DNA microarrays, protein arrays, deep sequencing and mass spectrometry (please see Germain et al [11] for a comprehensive review). They enable system-wide unbiased molecular measurements, which can then be used to reconstruct the events in an immune response. For example, about a week after the administration of influenza vaccines, antibody producing plasmablasts increase in blood circulation and their gene expression pattern will appear in the transcriptomic profile measured from peripheral blood [12].
Figure 1. Overview of the problems and methodologies of systems vaccinology.
An immune response involves many players at the levels of organs, tissues, cells, macromolecules to metabolites. The cells are at the scale of 10−5 meter and metabolites 10−10. High-throughput technologies are employed to collect molecular data, which are used in combination with conventional immunological assays for predicting vaccine immunogenicity and for unraveling mechanisms of vaccination.
Thus, the combination of omics and conventional immunlological parameters, with the help of computational modeling, will advance vaccinology in several fronts [8, 13]. Firstly, system-wide data give offers insights into the understanding of vaccine response mechanisms, which could be used to optimize immunogenicity for better and more durable protection. Secondly, identification of early predictors of vaccine efficacy can be a powerful tool for vaccine development. In many cases, correlates of protection are not well defined, or are not equally applicable to all populations (e.g. correlates of efficacy in healthy young adults may not necessarily reflect correlates of protection in the elderly); molecular predictors may offer novel correlates of protection. Thirdly, clinical trials can be greatly sped up if molecular signatures detected in the first few days or week informs the design of the trials [8]. For instance, a number of systems biology based small studies can run in parallel in a few weeks, and the priority of a large-scale trial can be given to a vaccine that shows a desirable molecular signature. Such a gain of time will also be important during an emergence response. This is not limited to new trials, as archived samples can be retrospectively studied to understand the successes and failures, as discussed elsewhere [8].
It is becoming clear that vaccinology benefits from the methods of systems biology. Yet, this infusion will also advance systems biology because of a number of unique opportunities and challenges. For instance, vaccination is the most common, ethical way to perturb the immune system of human subjects, and thus offers a unique opportunity to learn human immunology. In this review, first, we will demonstrate the utility of “systems vaccinology” through a few successful cases. Then, we will review some of the technical aspects of systems vaccinology, concerning data management and analytical approaches. Finally, we consider approaches that go beyond the transcriptomic approaches in studying immune responses to vaccines.
2. Learning from successful vaccines
The notion that new and fundamental insights about the workings of the immune system can be discerned through the detailed study of innate and adaptive immune responses to vaccination in humans, has forged a new line of study in recent years [7, 8, 14–16]. The highly effective yellow fever vaccine YF-17D is a live attenuated virus. A single shot can confer protection for up to four decades. YF-17D is known to induce neutralizing antibodies, cytotoxic T cells and Th1/Th2 cells, and signal via Toll-like receptors 2, 7, 8 and 9 on subsets of dendritic cells [7]. Querec et al [15] and Gaucher et al [16] were the first to test systems biological approaches in evaluating immune responses to vaccination. The results from these two independent studies revealed that genes of the antiviral type I interferon pathway, complement pathway and inflammasome were induced 3–7 days after vaccination, concomitant with the development of the adaptive immune response. A broad spectrum of gene regulations for innate sensing, including TLR7, RIG-I, MDA5 and LGP2, and for innate signaling, including JUN, STAT1, IRF7 and RNF36, was also observed. Querec et al [15] further explored whether measures of adaptive immunity, such as the number of CD8+ T cells and neutralizing antibody titers, could be predicted by early gene expression signatures. In this study, computational predictive models were developed from gene expression data from one trial and tested on a second independent trial. These yielded 90% accuracy in predicting the magnitude of the CD8+ T cell response and 80% precision in the antibody response. Several of the sets of predictive genes, called “classifiers”, contain EIF2AK4, which phosphorylates eIF2a and induces cellular stress granules [17, 18]. Both the phosphorylation of eIF2a and stress granule formation were indeed observed in human PBMC and baby hamster kidney cells infected with YF-17D [15]. Furthermore, EIF2AK4 knockout mice have impaired CD8+ T cell responses when infected with YF-17D (unpublished data).
The proof-of-concept study was later extended to the seasonal influenza model [12]. Systems biology approaches were applied to evaluating immune responses to a live attenuated virus vaccine (LAIV), or a trivalent inactivated vaccine (TIV). The study highlighted intriguing contrasts and similarities between the two vaccines. Similar to the live attenuated YF-17D vaccine, LAIV induced the expression of several interferon-related genes, which may be common to live viral vaccines. On the other hand, the inactivated vaccine, TIV, induced a signature composed of genes highly expressed in plasma B cells. Predictive modeling of TIV data from three independent influenza seasons identified gene signatures whose activity on day 3 or 7 post-vaccination could accurately predict the antibody response of vaccinees 30 days after vaccination. The authors tested the TIV vaccine on mice that were deficient in CaMKIV, a gene identified in the predictive models, and observed an altered antibody response. In both the yellow fever and influenza vaccine studies, TNFRSF17, the receptor gene for B cell growth factor BLyS, was found to be among the top predictors of the antibody response.
YF-17D is a live virus, and the study cohorts were immunologically naïve [15, 16]. In contrast, TIV is an inactivated vaccine and the cohorts had a recall response due to previous vaccination or infection [12]. Zak et al [19] studied the systems response of Merck Ad5/HIV, a vectorized vaccine, and found patterns of cytokines and which correlate with later HIV-specific CD8+ cell response. These diverse examples together demonstrate the power of systems biology in understanding and predicting vaccine-mediated immunity. In the following, we will review the data acquisition and analysis of systems vaccinology, with a focus on transcriptomics.
3. Data handling, analysis and modeling
High throughput technologies generate large volumes of data and a critical challenge is learning how to store, analyze and model such data.
3.1 Data acquisition and management
Transcriptome analysis through DNA microarrays is now easily accessible from many service providers, while RNAseq is the rising alternative. Both approaches require RNA isolation and conversion to cDNA, but differ thereafter. For DNA microarrays (“gene chips”), cDNA is further amplified, and the resulting labeled cRNA is hybridized to a glass chamber that contain oligonucleotide probes that are designed to cover the whole genome. For RNAseq, cDNA is ligated to adaptors and amplified. The resulting fragments are then hybridized to the dense lawn of homologous adapters covalently attached to the glass support surface. Local amplification of the attached fragments results in the formation of clusters of identical sequences, covalently anchored on the surface of the flow cell. Sequence information is then derived by extending the primers complimentary to the adapter sequences one base at a time, using fluorescently labeled nucleotide derivatives. At each step, the flow cell is scanned by a laser, and the emission frequency of the incorporated fluorophore is recorded. A typical length of sequence varies from 30 to 150 nucleotides, with 50 and 100 bases options being most commonly chosen. Each cluster can be sequenced from one end or both ends, thus doubling the amount of sequence information, at the cost of additional time and reagent expenditure.
The two techniques each have their advantages. The major incentives for a system biologist to adopt the RNA-seq as a method of surveying the transcriptome lie in its independence from the probe selection, which is the basis of hybridization-based methods. This opens the possibility to detect novel transcripts, alternative splice variants of known transcripts and alternative promoter usage. In addition, the sensitivity of detection by RNA-seq is dictated entirely by the depth of sequencing (that is, the number of reads per million bases of the transcriptome). While for the pure cell population the sensitivity of detection rapidly exhausts the repertoire of the available transcripts down to the biological noise level, for mixed populations, such as PBMC, the sensitivity may become the critical factor needed to detect transcriptional responses within a minor cell subpopulation. RNA-seq ideally suits this purpose.
DNA microarrays produce intensity measurements proportionate to the abundance of each transcript, while RNAseq produces a long list of short reads. The analysis pipeline, therefore, is drastically different. After quality control, the reads from RNAseq need to be aligned to the genome, a very computational intense task, and transcripts are counted. The testing of differentially expressed genes also uses different statistical models, reflecting the data distributions [20–22].
Data management poses considerable challenges in omics studies [23, 24]. Dr Leroy Hood has long advocated that biology is becoming an information science. This is an area we need to adopt the best practices from information science, which is however no easy task for a biology lab, especially when technical and managerial issues weight on a busy work schedule. Data management should cover three principal goals: sufficient meta data, streamlined retrieval and a functional backup system. Meta data, i.e., the key parameters of data acquisition, must accompany all data so that anyone can reconstruct the experimental results in a straightforward manner. A well-known example is the MIAMI data standard for DNA microarrays [25], and other technologies followed [26–28]. Automated data retrieval and query can affect efficiency by hundreds of times, thus influence the research outcome in its own way. Data storage and backup is a matter of both functionality and security. In principle, a geographically separated backup site should be included, to safeguard against events like fires or natural disasters. Finally, everything comes down to execution. It is also well known in information science that productivity can vary a hundred times between workers. Competing with the industry for talent pool, there are real consequences when universities, as they often do, offer the lower end of prevailing wages. These factors should be considered in decisions of staffing, collaboration or delegation of services.
3.2 Significant genes, pathways and modules
The biological meaning of omics data is largely projected by the functional annotation of significant genes and pathways, which can be derived from comparing groups before/after immunization, protected/unprotected against a pathogen, and presence/absence of adverse effect. To select significant genes between categorical groups, common statistical methods for omics apply well, given proper control of false discovery rate [29]. Pathway analysis brings in specific biological context, and can be more sensitive and robust than gene level analysis [30, 31]. A substantial effort has gone into creating pathway annotations for the sets of genes, both by the non-profit community (KEGG [32], NCI-PID [33], MSigDB [34] and Reactome [35]) and commercial vendors (e.g. GeneGo, BioCarta and Ingenuity).
Immunological pathways are under-represented in current pathway databases. Efforts of manual curation of immune pathways are ongoing at InnateDB [36] and MSigDB [34]. An alternative approach is to learn immune response from existing data in the form of gene modules. Chaussabel et al clustered genes from a collection of in-house transcriptomic data, and further curated into modules that reflect immunological functions [37]. Subsequent versions and supporting software have been developed, and applied to a number of studies [38–41]. Our group has extended this approach to reconstruct gene modules from a large compendium of public data (Li et al, in preparation).
Two lines of statistical tests for pathway (gene set and module) enrichment are in mainstream practice. The first is based on the degree of overlap between member genes of a pathway and the total list of significant genes. The likelihood of over-presentation of genes in a pathway can then be calculated (hypergeometric test or Fisher's exact test). The second line is positional test, represented by Kolmogorov-Smirnov (K-S) test. The popular GSEA program implements a derivative of K-S test [34]. When one associates gene expression to phenotypes, a pattern is produced for all genes and a separate pattern is for members of a pathway. The comparison of these two patterns will tell the significance of the respective pathway. We will demonstrate this on vaccination data in the next subsection.
3.3 Correlation to immunological parameters
The premise that blood transcriptomes will (partially) capture the events of immune response can only be examined on the data, case by case. Clear validations came in the data from the yellow fever studies [15, 16], flu TIV [12] and the meningococcal conjugate vaccine, Menactra® (Li et al, in preparation) - the blood transcriptomes at day 7 faithfully mirror the expansion of plasmablast cells, which are proportional to the later antibody production.
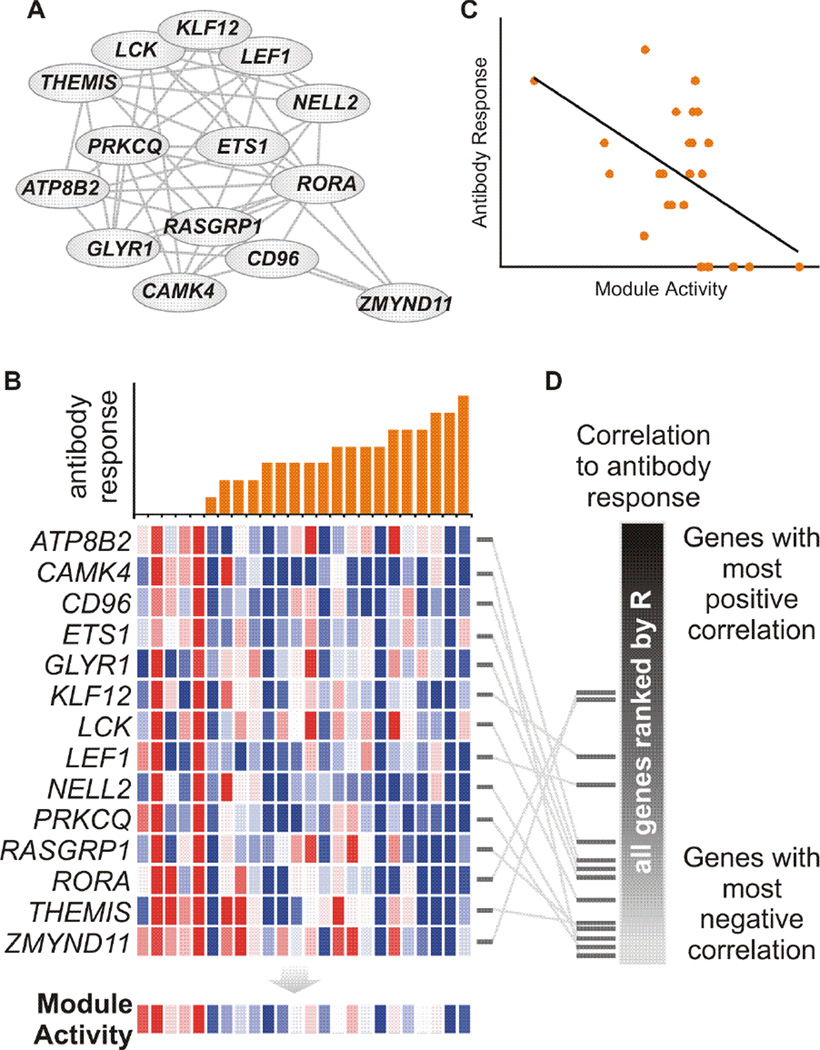
We will continue to use the antibody data for illustration as most current vaccines are thought to confer protection via antibodies [6], while the methodology in this subsection is applicable to other immunological parameters. The “curse of dimensionality” is that the number of genes is far greater than that of subjects. This creates a situation where many correlations can come from random chance. Additional information is required to further separate biology from noise. A pathway or module gains extra power from the relationship among its member genes. For the example module in Figure 2A, if the member genes do not participate in the same biological event, their expression will go in random directions so that the combined activity will not show the concerted result in Figure 2B and 2C. Such significance can be reliably assessed by computational techniques of resampling. Figure 2D shows an alternative approach, using the aforementioned K-S test. The correlation to antibody is calculated for every gene and ranked into a reference list. The positions of member genes of our example module are indicated by black bars on the side, showing significant enrichment to the negative end. The elegance of this method is that it is robust to outliers and no arbitrary cutoff is involved.
Figure 2. Antibody correlation analysis of a gene module/pathway.
(A) Gene module that was learned from previous data (Li et al., in preparation). Genes are connected by significant coexpression in previous studies. This module links the CAMK4 activity to T cell activation, a possible mechanism for the observed Camk4−/− phenotype in Nakaya et al [12]. (B) Example computed from flu TIV data [12]. Each column represents a subject. Top: antibody response after a month (max fold change of hemagglutination-inhibition antibody titers or HAI) and bottom: gene expression change after three days (log2 scale). Module activity is taken as the mean value of member genes (last row of bottom). (C) Pearson correlation between module activity and antibody response. (D) The significance of antibody correlation can also be tested via positional statistics, e.g. the implementation in GSEA. All genes are ranked by their Pearson correlation to antibody, and the positions of module member genes are shown as horizontal bars.
3.4 Dissecting signals from complex mix of cells in tissues
Immune cells can migrate through the blood stream between lymph nodes, spleen and peripheral tissues. During an immune response, their trafficking pattern changes, as does gene expression in certain cells upon the encounter of antigens and cytokines. Thus, the transcriptomes measured in peripheral blood will reflect both the change of transcriptional programs within individual cells, as well as the change in the representation of cell populations. How to unravel the signals from such a complex tissue remains a complex issue to systems biology.
Several studies have modeled tissue transcriptome as a linear combination of signals from the pure individual cell types. That is, overall gene expression E = S × P, where S is the expression signal from each single cell type, P the percentage of each cell type. Lu et al started the term “expression deconvolution” in modeling yeast transcriptomes [42]. “Deconvolution” means to recover P from the above equation, given E and S. In Lu et al, S is the expression signal at each cell cycle phases [42]. Wang et al took a similar approach in studying mammary gland development and turmorigenesis [43]. Abbas et al [44] adopted this approach on immune cells. They first generated S on sorted subpopulations (18 immune cell types, including activated cells), then used it to deconvolute cell composition P from blood transcriptome (E) of SLE patients [44]. Shen-Orr et al [45] took on the problem from the other direction: given E and P, they tried to allocate S of each subpopulation and identify differential gene expression within S. In this scenario, the number of cell types, e.g. monocytes, lymphocytes, neutrophils, eosinophils and basophils, can be measured by a Coulter counter.
A less ambitious goal is to identify the most relevant cell types in the data of mixed populations. Each cell type uniquely expresses a set of signature genes. When the signal is strong, one can simply test the overlap between cell type specific signatures and differentially expressed genes (DEGs). This was demonstrated in that day 7 microarray data after influenza vaccination carried a predominately plasma B cell signature [12]. A more sophisticated method was proposed by Bolen et al [46]. They used K-S test to compute an enrichment score (Ei) for each cell type specific signatures on each patient, whose genes were ranked by expression abundance. A similar enrichment score (Ej) was computed for DEGs per patient. Across all patients, the best Ei correlated to Ej points to the most relevant cell type.
Chikina et al [47] took a machine learning approach in dissecting tissue specific expression in C. elegans, where the training data included large-scale single gene expression in single cells. Such a “gold standard” is not available for human cells, though more quality microarray data are now available for sorted cells [48–51], a potential contributor to this line of research. It should be noted that the limits of all the above methods are yet to be characterized. Finally, we should be aware that the definition of cell types by a handful of markers is an oversimplification. Each cell subpopulation is not homogeneous, but rather a distribution of cells at different states [52, 53]. We have yet to see computational formalisms to capture this kind of “continuous population”.
3.5 Predictive modeling
Computational modeling strives to capture rules from past data, so that new events can be predicted without direct measurement. When the prediction is categorical, the common set of machine learning tools are “classifiers”. We have applied DAMIP (discriminant analysis – mixed integer program [54]) classifier to our yellow fever and flu studies [12, 15], while many other classifiers have been applied or developed in the context of omics, including Linear discrimination analysis (LDA [55]), K-nearest neighbors (KNN [56]), partitioning around medoids (PAM [57]), Support Vector Machines [58] and Random Forest [59].The “rules” in some classifiers are easy to interpret, e.g. a decision tree classifier can work in the same manner as an immunologist decides that CD19+ is B cells and CD19− is something else. Many others involve more complicated mathematical and computational formulations. There are, however, standard performance metrics, e.g. a receiver operating characteristics (ROC) curve to plot true positives vs false positives.
The variables used in classifiers are often gene expression values, but there are other types of data and different data types can be mixed. Feature selection is therefore a key factor in generating a robust predictor model. A good number of genes are often required because a) omics data are often noisier than conventional biomarker tests or b) the underlying mathematical models combine the variables in a more complex manner. However, there has been intense discussions on the robustness of classifies, especially when they are applied cross studies [60]. The Microarray Quality Control consortium performed one of the most comprehensive evaluations of classification models using microarray data [61]. They found that the performance of classifiers is dependent on how well the biological endpoints are defined. This will also be the case in vaccine studies, where it is often difficult to define a good or poor response. An advantage of the DAMIP method used in Querec et al [15] and Nakaya et al [12] is that it has added tolerance to errors in class definition [54]. The MAQC report also pointed out that multiple classifiers can achieve equally good results, and good practice is often more important than the classification methods being used [61].
Either in the case of predicting disease susceptibility or in the case of predicting vaccine immunogenicity, the more relevant question is probably what goes into the classifiers. Pathways and modules can carry prior knowledge and have a filtering effect on spurious signals. To take advantage of such, people have started combining the genes in a pathway into a single variable to feed into classifiers [62–64]. The methodology can be extended further to use multiple networks and multiple classifiers together [65]. How to extract pathway and network information and incorporate that into predictive models will be an important ongoing research.
4. Beyond transcriptomics
Each of the high-throughput technologies has its own domain, and measures the complex human immune system from a specific angle. While transcriptomes are the surrogate of gene activities, proteomics and metabolomics directly measure the molecules. Phosphoproteomics may offer the best value in in vitro studies of intense sampling, due to the fast kinetics of phosphorylation events. Cell based chemical screens can also be a powerful tool to delineate cellular transcriptional and signaling networks [66].
4.1 Metabolomics and vaccine immunity
Metabolomics is the high-throughput technology measuring metabolites, small molecules usually under 2000 Dalton [67]. Even though metabolism permeates every aspect of biology [68], the metabolomics technologies have not caught up with transcriptomics and proteomics, somewhat viewed as “the last frontier of systems biology” [69]. Metabolites can be measured by mass spectrometry or nuclear magnetic resonance, while untargeted mass spectrometry gives the most desired throughput – modern instruments can profile several thousand metabolites simultaneously. Important contributions from metabolomics are now starting to emerge [70–75].
In recent literature, serum metabolomic changes during infection have been reported for Mycobacterium leprae in human [76], rhinovirus and respiratory syncytial virus in human [77], Salmonella [78] and lymphocytic choriomeningitis virus [79] in mice. Studies on the malaria parasite, Plasmodium falciparum, have been particularly noteworthy. Olszewski et al [80] used mass spectrometry based metabolomics to profile the developmental cycle of the parasite, and found that Plasmodium converts host arginine to ornithine through its arginase. As arginine is the source used to produce nitric oxide, a key component in the host immune response, the authors proposed this arginine depletion as a strategy to evade host immunity. The same group went on to show that Plasmodium has a tricarboxylic acid metabolism fundamentally different from the canonical Krebs cycle [71]. Based on the same metabolomic data, Plata et al [81] reconstructed the Plasmodium falciparum metabolic network.
The idea of metabolic markers for host immune status or vaccine efficacy is particularly appealing, as metabolite tests do not require polynucleotide amplification and, therefore, can be performed in the same manner as routine clinical tests of glucose and cholesterol. Many metabolites are already known to regulate immune functions, including glutamate [82, 83], tryptophan [84, 85], adenosine [86, 87], arginine [88] and eicosanoids (prostaglandins, leukotrienes, etc) [89, 90]. The signaling role of metabolites has been underestimated [91]. For instance, Al-Abed et al [92] showed that thyroxine is a potential endogenous antagonist of macrophage migration inhibitory factor (MIF), a proinflammatory cytokine. Given that more than half of the metabolites detected by metabolomics are unknown, the potential of novel discoveries is mind boggling. Finally, the understanding of metabolites can be applied to modulate immune cells, including improving vaccine efficacy [93].
Our lab has started metabolomic profiling on vaccination subjects. We have detected many of the aforementioned metabolites in our data. As part of building the computational pipeline, we developed a set of algorithms to overcome the bottleneck problem of metabolite identification (Li et al, submitted). Metabolite identification, the assignment of metabolite to spectral features, is a slow and tedious process, impeding the application of high-throughput metabolomics. Our approach leverages the collective power in the organization of metabolic pathways and networks, predicting functional activity and metabolite identity in a single step. We expect a much accelerated application of metabolomics to immunology and vaccinology.
4.2 DNA and RNA sequence variations and vaccine immunity
Several studies have evaluated the role of genetics in regulating immunity, by studying vaccine responses in monozygotic versus dizygotic twins, and demonstrated varying degrees of heritability, depending on the age of the twins, the nature of the vaccine [94–97]. Genome wide association studies (GWAS) have pinpointed a number of genetic variants that control susceptibility to infectious diseases [98], which seem to concentrate on human leukocyte antigen (HLA) regions [99–102]. Few vaccine studies have involved GWAS, other than works in smallpox vaccination [103–105].
An extremely complex host factor is the receptor repertoires of B and T lymphocytes, which can be shaped by prior antigen exposure as well as by intrinsic factors such as ageing. To what extent and under what conditions do the diversities of BCR (antibody repertoire) and TCR determine the outcome of an immune response? With the dropping of sequencing cost, it is becoming possible to address these questions. Wiley et al [106] compared different adjuvants combined with malaria vaccine PvRII through massively parallel sequencing of antibody repertoires. They found that a Toll-like receptor agonist adjuvant increased the diversity of antibodies, which led to improved antigen neutralization ability.
In transcriptomic profiling, different from DNA microarrays, RNAseq now drives the measurement to the level of splice variants of genes [19, 77]. We expect regulatory programs that specific to an immunological context to exist at the splicing level. Since the sequence variations in exons are built in RNAseq data, methods of integrating DNA variations and transcriptomes are of increasing importance.
4.3 Microbiome and vaccine immunity
In perhaps one of the most innovative ways sequence variation analyses can be applied to examine host immunity, characterization of the microbiome has emerged as a critical field of study that is revealing new insights into the symbiotic nature between the host immune system and microbiota. The microbiome comprises all microbes naturally inhabiting our bodily surfaces and they range from bacterial to viral and even fungal species. Rapidly advancing technologies in high-throughput sequencing platforms ushered in a new way of identifying and characterizing the composition of trillions of microbial species populating our bodies to a level of detail and resolution never before attainable via microbiologic techniques[107, 108]. Bacterial genomic DNA, for example, can be extracted from sampling the stool or the cecum and sequenced to determine the identity and relative abundance of specific bacterial species present in the sample. One of the many advantages of this method is the ability to characterize the overall composition of an individual’s microbiota and to identify specific patterns in dynamics or changes in microbial communities that correlate with particular experimental parameter, such as disease outcome or immune responses. Furthermore, development of softwares, such as QIIME, are enabling investigators to expedite the analysis process [109–111]. These computer programs cluster and annotate sequence data and rapidly compute levels of dissimilarity between microbial communities. Together, these tools enable compositional comparisons to be made longitudinally within and across experimental groups, and the application of these approaches in recent years are beginning to provide a better understanding of the complex interaction between the microbiota and the host immune system.
Recent studies using animal model systems have identified specific commensal species that can either promote pro-inflammatory or immunoregulatory pathways in the host [112–117]. For example, colonization of mouse gut with segmented filamentous bacteria results in enhanced levels of Th17 cells in the gut [118], while Bacteroidetes fragilis or Clostridium subspecies results in the induction IL-10 responses and regulatory CD4+ T cells [119–121]. However, critical data that led to these studies were obtained from compositional comparisons in both mouse and human populations [118, 122]. Indeed, it was the sequence analysis of bacterial communities of mice obtained from different commercial vendors that revealed the possibility of certain bacterial species contributing to development and polarization of Th17 cells [118]. Furthermore, compositional analyses of microbiota samples obtained from children living in very contrasting geographical and cultural locations revealed dramatically different composition of the host microbiota [122] with some bacterial species associated with development of inflammatory bowel disease [120]. Additional studies involving depletion of the microbiota via orally administered broad-spectrum antibiotics have also revealed critical roles of the microbiota in development and homeostasis of the immune system [112, 114, 115, 118–120, 123–127]. Together, these observations indicate that specific composition of microbiota is an important factor influencing the host immune system. Thus, it also suggests that any alterations to the gut microbiota can influence the capacity of the host immune system to mount or regulate responses against pathogens appropriately. Accordingly, some reports using mice suggest that in the absence of the microbiota, host susceptibility to infection is enhanced and the capacity to control the infection is compromised. Whether the microbiome impacts vaccine-induced immune responses is unknown. Thus relationship between microbiota and vaccine immunogenicity is a critical field to study with major implications for global public health.
4.4 Integration of multiple omics
An intuitive way of integrating DNA variations and transcriptomes is embodied in the characterization of expression quantitative trait loci (eQTL). Quantitative trait loci has been the key concept in genetic studies, where a specific genomic location is linked to a specific phenotype. If gene expression level is viewed as a phenotype, the same method can be applied to pinpoint the genetic element (eQTL) that is linked to gene expression patterns. Fairfax et al [128] sorted B cells and monocytes from 288 donors, and performed transcriptomic profiling and SNP genotyping. This study revealed a large number of transcriptional regulations that are specific to a single cell type, e.g. regulatory elements for L-selectin, DFNA5, KLF4 and LYZ genes. The authors further demonstrated preferential association of these eQTLs to autoimmune diseases.
When looking at genetic and transcriptional determinants in complex phenotypes, however, the two types of data often seem to be disconnected [129]. For instance, Chen et al [130] studied obesity in a segregating mouse population, where the candidate genes from GWAS were different from those by transcriptomics. Instead of associating individual genes to phenotypes, the authors argue for a new paradigm that molecular networks transmit the collective effects of trait genes and environmental inputs to phenotypes; such molecular networks serve as both sensors and drivers of the phenotype [131]. By combining the SNP data and transcriptomic data, a number of gene networks were constructed by Bayesian formalism [132]. Among them, a macrophage-enriched metabolic network (MEMN) was found to be predictive and causal to the disease phenotypes. New functional data from transgenic and knockout mice validated three obesity genes chosen from MEMN [130]. This methodology was further validated in a subsequent study [133] and extended to clinical human data [134].
Each high-throughput dataset by itself only captures a thin slice of the real biological complexity. The integration strategies will differ by biology and chemistry. Bayesian networks have been a favorite in modeling genetic and gene expression data [130, 135–138]. The integration of transcriptomics and metabolomics relies on metabolic network models [139–141]. Combining multiple omics and prior information is highly desirable, boosting the power of data analysis and modeling. Given the vast amount of data available both publicly and proprietary, such efforts can become a very large-scale exercise. Many examples exist in cancer research and drug development; we have yet to see more in human immunology.
5. Roads to multiscale modeling
From the methodological perspective, systems biology is roughly divided into two schools: one anchored on continuous mathematical models represented by ordinary differential equations (ODEs), and one centering on omics and statistical methods. In between is a clear gap, as omics data usually don't have the kind of resolution required by an ODE model. Interestingly, this same gap exists in the immunology field.
A large body of work has followed the kinetic modeling of viral infections [142]. Over time, investigators included host responses into the models, such as antibody opsonization and lymphocyte kinetics. For example, Lee et al [143] used differential equations to model influenza infections and predicted phenotypic outcomes. Antia et al [144] modeled CD8+ T cell proliferation and predicted that the CD8+ T cell response must have both antigen dependent and independent components. At the molecular level, mathematical/computational models were found to be necessary to understand the signaling transduction of immune cells. Altan-Bonnet and Germain [145] successfully modeled the ligand discriminating property of T cell receptor pathway through a set of differential equations, incorporating positive and negative feedback loops. Sciammas et al [146] built and tested ODE models of a molecular network in B cell differentiation.
A different set of tools are employed for omics data. Among a handful of examples of reconstructing immune networks from omics data, data integration and perturbation are the key to uncovering true gene regulations. Litvak et al [147] combined a gene expression time series, chromatin immunoprecipitation and simulation models, to identify a gene circuit consisting of NF-kB, Atf3 and C/EBPδ that interpret the TLR4 signals to downstream activation in macrophage. Amit et al [66] used Bayesian learning algorithms and large-scale RNAi perturbation to reconstruct the transcriptional network in dendritic cells.
It should be noted that the above examples were based on very different scales (Figure 1). Litvak et al [147] and Amit et al [66] were performed on in vitro cell cultures. ODE models operate either at the molecular level with limited players, or at the organism level with great simplifications. With the complexity captured by omics data from human subjects as many of the data in systems vaccinology, we can no longer just ignore the cell kinetics or pathway cross-talks, because they are often mixed in the same data. This poses an urgent need to integrate omic analysis with models at cell, tissue, organism and population levels [148–150]. An additional problem is that not much perturbation can be done to human subjects beyond vaccination, and in vivo time series (if any) are usually very sparse. The importance of simulation tools should be properly recognized [11, 151]. Formalisms connecting cell level data and molecular level data are expected to play a key role.
p6. Systems vaccinology on the outlook
We have used the examples of Yellow Fever virus and Influenza vaccines to illustrate some of the results and strategies in systems vaccinology. These are just a beginning. To understand the interplay between vaccines and human immunology is an overarching goal, with a whole array of possibilities and scientific questions opened up, driven by omics technologies. National Institute of Health in the United States has funded the Human Immunology Project Consortium (HIPC, immuneprofiling.org) to specifically support the study of systems biology of vaccination and infection.
Besides the immediate scientific findings, these emerging data have long term value. In the early days of a rapidly evolving field, no single lab can possibly analyze the data comprehensively and thoroughly. Sample size matters as well. Many questions, e.g. epigenetic factors in immunity or mechanisms of different vaccine types, can only be addressed when datasets are combined. Just like the model of TCGA (The Cancer Genome Atlas), a wider community of computational scientists should be welcomed to mine these data.
Many challenges come with systems vaccinology. Analyzing and modeling omics data is new to many immunologists. False discovery rate is now a commonly accepted tool in gene selection, however, testing the statistical significance of networks does not always come with a conscious effort. On the other hand, statistical significance is not equal to biological significance. In such cases, validation from additional experiments will be important. Multidisciplinary research often operates differently from the traditional R01 supported labs. How an organizational structure supports efficiency and quality, the career development of team members and fusion of cultures from different fields are among the questions every participant should be aware of.
The interaction between systems biology and vaccinology goes both ways. The unique opportunities for systems biology at large include: i) modeling molecular data in complex tissues. Immune cells are among the best characterized cell types by systems profiling; ii) multiscale modeling in humans with important medical relevance. Projects like those from HIPC present both quality data and the imperatives; iii) abundant data at the single-cell level (from flow cytometry, microfluid platforms etc.); and iv) connection of molecular structures with omics, especially for epitopes and antibodies [152, 153]. These quests will also go side by side with immunology and infectious diseases [154–158]. Together, we look forward to an exciting time of systems vaccinology.
Highlights.
Recent studies have used systems biological approaches to predict vaccine efficacy
Such studies are yielding new insights about the mechanisms underlying vaccine immunity
Data acquisition, management and modeling represent major challenges
Integration of multiple “omics” data also represent major challenges
Overcoming such challenges will enable systems approaches to guide rational vaccine design
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Plotkin S, Plotkin S. The development of vaccines: how the past led to the future. Nature reviews Microbiology. 2011;9:889. doi: 10.1038/nrmicro2668. [DOI] [PubMed] [Google Scholar]
- 2.Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nature immunology. 2011;12:509. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Germain RN. Vaccines and the future of human immunology. Immunity. 2010;33:441–450. doi: 10.1016/j.immuni.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Rappuoli R, Aderem A. A 2020 vision for vaccines against HIV, tuberculosis and malaria. Nature. 2011;473:463–469. doi: 10.1038/nature10124. [DOI] [PubMed] [Google Scholar]
- 5.Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006;124:849–863. doi: 10.1016/j.cell.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 6.Plotkin SA. Correlates of protection induced by vaccination. Clinical and Vaccine Immunology. 2010;17:1055–1065. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pulendran B. Learning immunology from the yellow fever vaccine: innate immunity to systems vaccinology. Nature Reviews Immunology. 2009;9:741–747. doi: 10.1038/nri2629. [DOI] [PubMed] [Google Scholar]
- 8.Pulendran B, Li S, Nakaya HI. Systems vaccinology. Immunity. 2010;33:516–529. doi: 10.1016/j.immuni.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oberg AL, Kennedy RB, Li P, Ovsyannikova IG, Poland GA. Systems biology approaches to new vaccine development. Current opinion in immunology. 2011;23:436–443. doi: 10.1016/j.coi.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trautmann L, Sekaly R. Solving vaccine mysteries: a systems biology perspective. Nature immunology. 2011;12:729. doi: 10.1038/ni.2078. [DOI] [PubMed] [Google Scholar]
- 11.Germain RN, Meier-Schellersheim M, Nita-Lazar A, Fraser ID. Systems Biology in Immunology–A Computational Modeling Perspective. Annual review of immunology. 2011;29:527. doi: 10.1146/annurev-immunol-030409-101317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakaya HI, Wrammert J, Lee EK, Racioppi L, Marie-Kunze S, Haining WN, et al. Systems biology of vaccination for seasonal influenza in humans. Nature immunology. 2011;12:786–795. doi: 10.1038/ni.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakaya HI, Pulendran B. Systems vaccinology: its promise and challenge for HIV vaccine development. Current Opinion in HIV and AIDS. 2012;7:24. doi: 10.1097/COH.0b013e32834dc37b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Querec T, Bennouna S, Alkan S, Laouar Y, Gorden K, Flavell R, et al. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. The Journal of experimental medicine. 2006;203:413–424. doi: 10.1084/jem.20051720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Querec TD, Akondy RS, Lee EK, Cao W, Nakaya HI, Teuwen D, et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nature immunology. 2008;10:116–125. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaucher D, Therrien R, Kettaf N, Angermann BR, Boucher G, Filali-Mouhim A, et al. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. The Journal of experimental medicine. 2008;205:3119–3131. doi: 10.1084/jem.20082292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richter JD, Sonenberg N. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature. 2005;433:477–480. doi: 10.1038/nature03205. [DOI] [PubMed] [Google Scholar]
- 18.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nature Reviews Molecular Cell Biology. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 19.Zak DE, Andersen-Nissen E, Peterson ER, Sato A, Hamilton MK, Borgerding J, et al. Merck Ad5/HIV induces broad innate immune activation that predicts CD8+ T-cell responses but is attenuated by preexisting Ad5 immunity. Proceedings of the National Academy of Sciences. 2012;109:E3503–E3512. doi: 10.1073/pnas.1208972109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, Van Baren MJ, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nature biotechnology. 2010;28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaussabel D, Ueno H, Banchereau J, Quinn C. Data management: it starts at the bench. Nature immunology. 2009;10:1225. doi: 10.1038/ni1209-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schadt EE, Linderman MD, Sorenson J, Lee L, Nolan GP. Computational solutions to large-scale data management and analysis. Nature Reviews Genetics. 2010;11:647–657. doi: 10.1038/nrg2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brazma A, Hingamp P, Quackenbush J, Sherlock G, Spellman P, Stoeckert C, et al. Minimum information about a microarray experiment (MIAME)-toward standards for microarray data. Nature genetics. 2001;29:365–372. doi: 10.1038/ng1201-365. [DOI] [PubMed] [Google Scholar]
- 26.Lee JA, Spidlen J, Boyce K, Cai J, Crosbie N, Dalphin M, et al. MIFlowCyt: the minimum information about a Flow Cytometry Experiment. Cytometry Part A. 2008;73:926–930. doi: 10.1002/cyto.a.20623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spidlen J, Shooshtari P, Kollmann TR, Brinkman RR. Flow cytometry data standards. BMC research notes. 2011;4:50. doi: 10.1186/1756-0500-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janetzki S, Britten CM, Kalos M, Levitsky HI, Maecker HT, Melief CJ, et al. “MIATA”—Minimal Information about T Cell Assays. Immunity. 2009;31:527–528. doi: 10.1016/j.immuni.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proceedings of the National Academy of Sciences. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dinu I, Potter JD, Mueller T, Liu Q, Adewale AJ, Jhangri GS, et al. Gene-set analysis and reduction. Briefings in bioinformatics. 2009;10:24–34. doi: 10.1093/bib/bbn042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khatri P, Sirota M, Butte AJ. Ten years of pathway analysis: current approaches and outstanding challenges. PLoS computational biology. 2012;8:e1002375. doi: 10.1371/journal.pcbi.1002375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanehisa M, Goto S, Hattori M, Aoki-Kinoshita KF, Itoh M, Kawashima S, et al. From genomics to chemical genomics: new developments in KEGG. Nucleic acids research. 2006;34:D354–D357. doi: 10.1093/nar/gkj102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaefer CF, Anthony K, Krupa S, Buchoff J, Day M, Hannay T, et al. PID: the pathway interaction database. Nucleic acids research. 2009;37:D674–D679. doi: 10.1093/nar/gkn653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vastrik I, D'Eustachio P, Schmidt E, Joshi-Tope G, Gopinath G, Croft D, et al. Reactome: a knowledge base of biologic pathways and processes. Genome biology. 2007;8:R39. doi: 10.1186/gb-2007-8-3-r39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lynn DJ, Winsor GL, Chan C, Richard N, Laird MR, Barsky A, et al. InnateDB: facilitating systems-level analyses of the mammalian innate immune response. Molecular systems biology. 2008:4. doi: 10.1038/msb.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaussabel D, Quinn C, Shen J, Patel P, Glaser C, Baldwin N, et al. A modular analysis framework for blood genomics studies: application to systemic lupus erythematosus. Immunity. 2008;29:150–164. doi: 10.1016/j.immuni.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berry MP, Graham CM, McNab FW, Xu Z, Bloch SA, Oni T, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466:973–977. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaussabel D, Pascual V, Banchereau J. Assessing the human immune system through blood transcriptomics. BMC biology. 2010;8:84. doi: 10.1186/1741-7007-8-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quartier P, Allantaz F, Cimaz R, Pillet P, Messiaen C, Bardin C, et al. A multicentre, randomised, double-blind, placebo-controlled trial with the interleukin-1 receptor antagonist anakinra in patients with systemic-onset juvenile idiopathic arthritis (ANAJIS trial) Annals of the rheumatic diseases. 2011;70:747–754. doi: 10.1136/ard.2010.134254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tattermusch S, Skinner JA, Chaussabel D, Banchereau J, Berry MP, McNab FW, et al. Systems biology approaches reveal a specific interferon-inducible signature in HTLV-1 associated myelopathy. PLoS pathogens. 2012;8:e1002480. doi: 10.1371/journal.ppat.1002480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu P, Nakorchevskiy A, Marcotte EM. Expression deconvolution: a reinterpretation of DNA microarray data reveals dynamic changes in cell populations. Proceedings of the National Academy of Sciences. 2003;100:10370–10375. doi: 10.1073/pnas.1832361100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang M, Master SR, Chodosh LA. Computational expression deconvolution in a complex mammalian organ. BMC bioinformatics. 2006;7:328. doi: 10.1186/1471-2105-7-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abbas AR, Wolslegel K, Seshasayee D, Modrusan Z, Clark HF. Deconvolution of blood microarray data identifies cellular activation patterns in systemic lupus erythematosus. PloS one. 2009;4:e6098. doi: 10.1371/journal.pone.0006098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen-Orr SS, Tibshirani R, Khatri P, Bodian DL, Staedtler F, Perry NM, et al. Cell type–specific gene expression differences in complex tissues. Nature methods. 2010;7:287–289. doi: 10.1038/nmeth.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bolen CR, Uduman M, Kleinstein SH. Cell subset prediction for blood genomic studies. BMC bioinformatics. 2011;12:258. doi: 10.1186/1471-2105-12-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chikina MD, Huttenhower C, Murphy CT, Troyanskaya OG. Global prediction of tissue-specific gene expression and context-dependent gene networks in Caenorhabditis elegans. PLoS computational biology. 2009;5:e1000417. doi: 10.1371/journal.pcbi.1000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abbas A, Baldwin D, Ma Y, Ouyang W, Gurney A, Martin F, et al. Immune response in silico (IRIS): immune-specific genes identified from a compendium of microarray expression data. Genes and immunity. 2005;6:319–331. doi: 10.1038/sj.gene.6364173. [DOI] [PubMed] [Google Scholar]
- 49.Palmer C, Diehn M, Alizadeh AA, Brown PO. Cell-type specific gene expression profiles of leukocytes in human peripheral blood. BMC genomics. 2006;7:115. doi: 10.1186/1471-2164-7-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heng TS, Painter MW, Elpek K, Lukacs-Kornek V, Mauermann N, Turley SJ, et al. The Immunological Genome Project: networks of gene expression in immune cells. Nature immunology. 2008;9:1091–1094. doi: 10.1038/ni1008-1091. [DOI] [PubMed] [Google Scholar]
- 51.Novershtern N, Subramanian A, Lawton LN, Mak RH, Haining WN, McConkey ME, et al. Densely interconnected transcriptional circuits control cell states in human hematopoiesis. Cell. 2011;144:296–309. doi: 10.1016/j.cell.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bendall SC, Simonds EF, Qiu P, Amir E-aD, Krutzik PO, Finck R, et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science Signalling. 2011;332:687. doi: 10.1126/science.1198704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Newell EW, Sigal N, Bendall SC, Nolan GP, Davis MM. Cytometry by Time-of-Flight Shows Combinatorial Cytokine Expression and Virus-Specific Cell Niches within a Continuum of CD8+T Cell Phenotypes. Immunity. 2012 doi: 10.1016/j.immuni.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee E. Machine learning framework for classification in medicine and biology. Lecture Notes in Computer Science. 2009;5547:1–7. [Google Scholar]
- 55.Lee S-I, Batzoglou S. Application of independent component analysis to microarrays. Genome biology. 2003;4:76. doi: 10.1186/gb-2003-4-11-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parry R, Jones W, Stokes T, Phan J, Moffitt R, Fang H, et al. k-Nearest neighbor models for microarray gene expression analysis and clinical outcome prediction. The pharmacogenomics journal. 2010;10:292–309. doi: 10.1038/tpj.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tibshirani R, Hastie T, Narasimhan B, Chu G. Diagnosis of multiple cancer types by shrunken centroids of gene expression. Proceedings of the National Academy of Sciences. 2002;99:6567–6572. doi: 10.1073/pnas.082099299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Noble WS. What is a support vector machine? Nature biotechnology. 2006;24:1565–1567. doi: 10.1038/nbt1206-1565. [DOI] [PubMed] [Google Scholar]
- 59.Díaz-Uriarte R, De Andres SA. Gene selection and classification of microarray data using random forest. BMC bioinformatics. 2006;7:3. doi: 10.1186/1471-2105-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dupuy A, Simon RM. Critical review of published microarray studies for cancer outcome and guidelines on statistical analysis and reporting. Journal of the National Cancer Institute. 2007;99:147–157. doi: 10.1093/jnci/djk018. [DOI] [PubMed] [Google Scholar]
- 61.Shi L, Campbell G, Jones WD, Campagne F, Wen Z, Walker SJ, et al. The MicroArray Quality Control (MAQC)-II study of common practices for the development and validation of microarray-based predictive models. Nature biotechnology. 2010;28:827. doi: 10.1038/nbt.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee E, Chuang H-Y, Kim J-W, Ideker T, Lee D. Inferring pathway activity toward precise disease classification. PLoS computational biology. 2008;4:e1000217. doi: 10.1371/journal.pcbi.1000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chuang H-Y, Lee E, Liu Y-T, Lee D, Ideker T. Network-based classification of breast cancer metastasis. Molecular systems biology. 2007;3 doi: 10.1038/msb4100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haining WN, Pulendran B. Identifying gnostic predictors of the vaccine response. Current opinion in immunology. 2012 doi: 10.1016/j.coi.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pandey G, Zhang B, Chang AN, Myers CL, Zhu J, Kumar V, et al. An integrative multi-network and multi-classifier approach to predict genetic interactions. PLoS computational biology. 2010;6:e1000928. doi: 10.1371/journal.pcbi.1000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Amit I, Regev A, Hacohen N. Strategies to discover regulatory circuits of the mammalian immune system. Nature Reviews Immunology. 2011;11:873–880. doi: 10.1038/nri3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patti G, Yanes O, Siuzdak G. Metabolomics: the apogee of the omics trilogy. Nature reviews Molecular cell biology. 2012;13:263. doi: 10.1038/nrm3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.DeBerardinis RJ, Thompson CB. Cellular metabolism and disease: what do metabolic outliers teach us? Cell. 2012;148:1132–1144. doi: 10.1016/j.cell.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Veenstra TD. Metabolomics: the final frontier? Genome Medicine. 2012;4:40. doi: 10.1186/gm339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Munger J, Bennett BD, Parikh A, Feng X-J, McArdle J, Rabitz HA, et al. Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nature biotechnology. 2008;26:1179–1186. doi: 10.1038/nbt.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Olszewski KL, Mather MW, Morrisey JM, Garcia BA, Vaidya AB, Rabinowitz JD, et al. Branched tricarboxylic acid metabolism in Plasmodium falciparum. Nature. 2010;466:774–778. doi: 10.1038/nature09301. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 72.Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457:910–914. doi: 10.1038/nature07762. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 74.Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proceedings of the National Academy of Sciences. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yanes O, Clark J, Wong DM, Patti GJ, Sánchez-Ruiz A, Benton HP, et al. Metabolic oxidation regulates embryonic stem cell differentiation. Nature chemical biology. 2010;6:411–417. doi: 10.1038/nchembio.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Al-Mubarak R, Vander Heiden J, Broeckling CD, Balagon M, Brennan PJ, Vissa VD. Serum metabolomics reveals higher levels of polyunsaturated Fatty acids in lepromatous leprosy: potential markers for susceptibility and pathogenesis. PLoS Neglected Tropical Diseases. 2011;5:e1303. doi: 10.1371/journal.pntd.0001303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen R, Mias GI, Li-Pook-Than J, Jiang L, Lam HY, Chen R, et al. Personal omics profiling reveals dynamic molecular and medical phenotypes. Cell. 2012;148:1293–1307. doi: 10.1016/j.cell.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Antunes LCM, Arena ET, Menendez A, Han J, Ferreira RB, Buckner MM, et al. Impact of Salmonella infection on host hormone metabolism revealed by metabolomics. Infection and immunity. 2011;79:1759–1769. doi: 10.1128/IAI.01373-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wikoff WR, Kalisak E, Trauger S, Manchester M, Siuzdak G. Response and recovery in the plasma metabolome tracks the acute LCMV-induced immune response. Journal of proteome research. 2009;8:3578–3587. doi: 10.1021/pr900275p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Olszewski KL, Morrisey JM, Wilinski D, Burns JM, Vaidya AB, Rabinowitz JD, et al. Host-Parasite Interactions Revealed by< i>Plasmodium falciparum</i>Metabolomics. Cell host & microbe. 2009;5:191–199. doi: 10.1016/j.chom.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Plata G, Hsiao T-L, Olszewski KL, Llinás M, Vitkup D. Reconstruction and flux-balance analysis of the Plasmodium falciparum metabolic network. Molecular systems biology. 2010;6 doi: 10.1038/msb.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pacheco R, Oliva H, Martinez-Navío JM, Climent N, Ciruela F, Gatell JM, et al. Glutamate released by dendritic cells as a novel modulator of T cell activation. The Journal of Immunology. 2006;177:6695–6704. doi: 10.4049/jimmunol.177.10.6695. [DOI] [PubMed] [Google Scholar]
- 83.Hansen AM, Caspi RR. Glutamate joins the ranks of immunomodulators. Nature medicine. 2010;16:856–858. doi: 10.1038/nm0810-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moffett JR, Namboodiri MA. Tryptophan and the immune response. Immunology and cell biology. 2003;81:247–265. doi: 10.1046/j.1440-1711.2003.t01-1-01177.x. [DOI] [PubMed] [Google Scholar]
- 85.Gottfried E, Kreutz M, Mackensen A. Tumor metabolism as modulator of immune response and tumor progression. Seminars in Cancer Biology: Elsevier. 2012 doi: 10.1016/j.semcancer.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 86.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. The Journal of experimental medicine. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Haskó G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nature Reviews Drug Discovery. 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nature Reviews Immunology. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 89.Fritsche K. Fatty acids as modulators of the immune response. Annu Rev Nutr. 2006;26:45–73. doi: 10.1146/annurev.nutr.25.050304.092610. [DOI] [PubMed] [Google Scholar]
- 90.O'Donnell VB, Murphy RC. New families of bioactive oxidized phospholipids generated by immune cells: identification and signaling actions. Blood. 2012;120:1985–1992. doi: 10.1182/blood-2012-04-402826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wellen KE, Thompson CB. A two-way street: reciprocal regulation of metabolism and signalling. Nature Reviews Molecular Cell Biology. 2012 doi: 10.1038/nrm3305. [DOI] [PubMed] [Google Scholar]
- 92.Al-Abed Y, Metz CN, Cheng KF, Aljabari B, VanPatten S, Blau S, et al. Thyroxine is a potential endogenous antagonist of macrophage migration inhibitory factor (MIF) activity. Proceedings of the National Academy of Sciences. 2011;108:8224–8227. doi: 10.1073/pnas.1017624108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang L-S, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kohler PF, Rivera VJ, Eckert E, Bouchard TJ, Jr, Heston LL. Genetic regulation of immunoglobulin and specific antibody levels in twins reared apart. Journal of Clinical Investigation. 1985;75:883. doi: 10.1172/JCI111787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tan P-L, Jacobson RM, Poland GA, Jacobsen SJ, Pankratz VS. Twin studies of immunogenicity—determining the genetic contribution to vaccine failure. Vaccine. 2001;19:2434–2439. doi: 10.1016/s0264-410x(00)00468-0. [DOI] [PubMed] [Google Scholar]
- 96.Lee Y, Newport M, Goetghebuer T, Siegrist C, Weiss H, Pollard A, et al. Influence of genetic and environmental factors on the immunogenicity of Hib vaccine in Gambian twins. Vaccine. 2006;24:5335–5340. doi: 10.1016/j.vaccine.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 97.Poland GA, Ovsyannikova IG, Jacobson RM. Application of pharmacogenomics to vaccines. Pharmacogenomics. 2009;10:837–852. doi: 10.2217/PGS.09.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.de Bakker PI, Telenti A. Infectious diseases not immune to genome-wide association. Nature genetics. 2010;42:731. doi: 10.1038/ng0910-731. [DOI] [PubMed] [Google Scholar]
- 99.Fellay J, Ge D, Shianna KV, Colombo S, Ledergerber B, Cirulli ET, et al. Common genetic variation and the control of HIV-1 in humans. PLoS genetics. 2009;5:e1000791. doi: 10.1371/journal.pgen.1000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kamatani Y, Wattanapokayakit S, Ochi H, Kawaguchi T, Takahashi A, Hosono N, et al. A genome-wide association study identifies variants in the HLA-DP locus associated with chronic hepatitis B in Asians. Nature genetics. 2009;41:591–595. doi: 10.1038/ng.348. [DOI] [PubMed] [Google Scholar]
- 101.Zhang F-R, Huang W, Chen S-M, Sun L-D, Liu H, Li Y, et al. Genomewide association study of leprosy. New England Journal of Medicine. 2009;361:2609–2618. doi: 10.1056/NEJMoa0903753. [DOI] [PubMed] [Google Scholar]
- 102.Pereyra F, Jia X, McLaren PJ, Telenti A, de Bakker PI, Walker BD, et al. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science. 2010;330:1551. doi: 10.1126/science.1195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ovsyannikova IG, Kennedy RB, O’Byrne M, Jacobson RM, Pankratz VS, Poland GA. Genome-wide association study of antibody response to smallpox vaccine. Vaccine. 2012 doi: 10.1016/j.vaccine.2012.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kennedy RB, Ovsyannikova IG, Shane Pankratz V, Haralambieva IH, Vierkant RA, Poland GA. Genome-wide analysis of polymorphisms associated with cytokine responses in smallpox vaccine recipients. Human Genetics. 2012:1–19. doi: 10.1007/s00439-012-1174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Davis N, Crowe J, Pajewski N, McKinney B. Surfing a genetic association interaction network to identify modulators of antibody response to smallpox vaccine. Genes and immunity. 2010;11:630–636. doi: 10.1038/gene.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wiley SR, Raman VS, Desbien A, Bailor HR, Bhardwaj R, Shakri AR, et al. Targeting TLRs expands the antibody repertoire in response to a malaria vaccine. Science Translational Medicine. 2011;3:93ra69–93ra69. doi: 10.1126/scitranslmed.3002135. [DOI] [PubMed] [Google Scholar]
- 107.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huttenhower C, Gevers D, Knight R, Abubucker S, Badger JH, Chinwalla AT, et al. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Caporaso JG, Bittinger K, Bushman FD, DeSantis TZ, Andersen GL, Knight R. PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics. 2010;26:266–267. doi: 10.1093/bioinformatics/btp636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hamady M, Walker JJ, Harris JK, Gold NJ, Knight R. Error-correcting barcoded primers for pyrosequencing hundreds of samples in multiplex. Nature methods. 2008;5:235–237. doi: 10.1038/nmeth.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Carvalho FA, Nalbantoglu I, Ortega-Fernandez S, Aitken JD, Su Y, Koren O, et al. Interleukin-1β (IL-1β) promotes susceptibility of Toll-like receptor 5 (TLR5) deficient mice to colitis. Gut. 2012;61:373–384. doi: 10.1136/gut.2011.240556. [DOI] [PubMed] [Google Scholar]
- 112.Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, et al. ATP drives lamina propria TH17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 113.Cebra JJ. Influences of microbiota on intestinal immune system development. The American journal of clinical nutrition. 1999;69:1046s–1051s. doi: 10.1093/ajcn/69.5.1046s. [DOI] [PubMed] [Google Scholar]
- 114.Hall JA, Bouladoux N, Sun CM, Wohlfert EA, Blank RB, Zhu Q, et al. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity. 2008;29:637–649. doi: 10.1016/j.immuni.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ivanov II, Frutos RdL, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell host & microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nature Reviews Immunology. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Smith K, McCoy KD, Macpherson AJ. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Seminars in immunology: Elsevier. 2007:59–69. doi: 10.1016/j.smim.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 118.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 120.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 121.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science Signalling. 2011;331:337. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proceedings of the National Academy of Sciences. 2010;107:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 124.Hill DA, Hoffmann C, Abt MC, Du Y, Kobuley D, Kirn TJ, et al. Metagenomic analyses reveal antibiotic-induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis. Mucosal immunology. 2009;3:148–158. doi: 10.1038/mi.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Abt MC, Osborne LC, Monticelli LA, Doering TA, Alenghat T, Sonnenberg GF, et al. Commensal Bacteria Calibrate the Activation Threshold of Innate Antiviral Immunity. Immunity. 2012 doi: 10.1016/j.immuni.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hill DA, Siracusa MC, Abt MC, Kim BS, Kobuley D, Kubo M, et al. Commensal bacteria-derived signals regulate basophil hematopoiesis and allergic inflammation. Nature medicine. 2012 doi: 10.1038/nm.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Science Signalling. 2011;108:5354. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fairfax BP, Makino S, Radhakrishnan J, Plant K, Leslie S, Dilthey A, et al. Genetics of gene expression in primary immune cells identifies cell type-specific master regulators and roles of HLA alleles. Nature genetics. 2012;44:502–510. doi: 10.1038/ng.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yeger-Lotem E, Riva L, Su LJ, Gitler AD, Cashikar AG, King OD, et al. Bridging high-throughput genetic and transcriptional data reveals cellular responses to alpha-synuclein toxicity. Nature genetics. 2009;41:316–323. doi: 10.1038/ng.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen Y, Zhu J, Lum PY, Yang X, Pinto S, MacNeil DJ, et al. Variations in DNA elucidate molecular networks that cause disease. Nature. 2008;452:429–435. doi: 10.1038/nature06757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schadt EE. Molecular networks as sensors and drivers of common human diseases. Nature. 2009;461:218–223. doi: 10.1038/nature08454. [DOI] [PubMed] [Google Scholar]
- 132.Lum PY, Chen Y, Zhu J, Lamb J, Melmed S, Wang S, et al. Elucidating the murine brain transcriptional network in a segregating mouse population to identify core functional modules for obesity and diabetes. Journal of neurochemistry. 2006;97:50–62. doi: 10.1111/j.1471-4159.2006.03661.x. [DOI] [PubMed] [Google Scholar]
- 133.Yang X, Deignan JL, Qi H, Zhu J, Qian S, Zhong J, et al. Validation of candidate causal genes for obesity that affect shared metabolic pathways and networks. Nature genetics. 2009;41:415–423. doi: 10.1038/ng.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Emilsson V, Thorleifsson G, Zhang B, Leonardson AS, Zink F, Zhu J, et al. Genetics of gene expression and its effect on disease. Nature. 2008;452:423–428. doi: 10.1038/nature06758. [DOI] [PubMed] [Google Scholar]
- 135.Friedman N, Linial M, Nachman I, Pe'er D. Using Bayesian networks to analyze expression data. Journal of computational biology. 2000;7:601–620. doi: 10.1089/106652700750050961. [DOI] [PubMed] [Google Scholar]
- 136.Sachs K, Perez O, Pe'er D, Lauffenburger DA, Nolan GP. Causal protein-signaling networks derived from multiparameter single-cell data. Science Signalling. 2005;308:523. doi: 10.1126/science.1105809. [DOI] [PubMed] [Google Scholar]
- 137.Yeung KY, Bumgarner RE, Raftery AE. Bayesian model averaging: development of an improved multi-class, gene selection and classification tool for microarray data. Bioinformatics. 2005;21:2394–2402. doi: 10.1093/bioinformatics/bti319. [DOI] [PubMed] [Google Scholar]
- 138.Xing H, McDonagh PD, Bienkowska J, Cashorali T, Runge K, Miller RE, et al. Causal modeling using network ensemble simulations of genetic and gene expression data predicts genes involved in rheumatoid arthritis. PLoS computational biology. 2011;7:e1001105. doi: 10.1371/journal.pcbi.1001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Joyce AR, Palsson BØ. The model organism as a system: integrating'omics' data sets. Nature Reviews Molecular Cell Biology. 2006;7:198–210. doi: 10.1038/nrm1857. [DOI] [PubMed] [Google Scholar]
- 140.Li S, Pozhitkov A, Ryan RA, Manning CS, Brown-Peterson N, Brouwer M. Constructing a fish metabolic network model. Genome biology. 2010;11:R115. doi: 10.1186/gb-2010-11-11-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Oberhardt MA, Palsson BØ, Papin JA. Applications of genome-scale metabolic reconstructions. Molecular systems biology. 2009;5 doi: 10.1038/msb.2009.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Perelson AS. Modelling viral and immune system dynamics. Nature Reviews Immunology. 2002;2:28–36. doi: 10.1038/nri700. [DOI] [PubMed] [Google Scholar]
- 143.Lee HY, Topham DJ, Park SY, Hollenbaugh J, Treanor J, Mosmann TR, et al. Simulation and prediction of the adaptive immune response to influenza A virus infection. Journal of virology. 2009;83:7151–7165. doi: 10.1128/JVI.00098-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Antia R, Ganusov VV, Ahmed R. The role of models in understanding CD8+ T-cell memory. Nature Reviews Immunology. 2005;5:101–111. doi: 10.1038/nri1550. [DOI] [PubMed] [Google Scholar]
- 145.Altan-Bonnet G, Germain RN. Modeling T cell antigen discrimination based on feedback control of digital ERK responses. PLoS biology. 2005;3:e356. doi: 10.1371/journal.pbio.0030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sciammas R, Li Y, Warmflash A, Song Y, Dinner AR, Singh H. An incoherent regulatory network architecture that orchestrates B cell diversification in response to antigen signaling. Molecular systems biology. 2011;7 doi: 10.1038/msb.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Litvak V, Ramsey SA, Rust AG, Zak DE, Kennedy KA, Lampano AE, et al. Function of C/EBPδ in a regulatory circuit that discriminates between transient and persistent TLR4-induced signals. Nature immunology. 2009;10:437–443. doi: 10.1038/ni.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kirschner DE, Chang ST, Riggs TW, Perry N, Linderman JJ. Toward a multiscale model of antigen presentation in immunity. Immunological reviews. 2007;216:93–118. doi: 10.1111/j.1600-065X.2007.00490.x. [DOI] [PubMed] [Google Scholar]
- 149.Young D, Stark J, Kirschner D. Systems biology of persistent infection: tuberculosis as a case study. Nature Reviews Microbiology. 2008;6:520–528. doi: 10.1038/nrmicro1919. [DOI] [PubMed] [Google Scholar]
- 150.Auffray C, Chen Z, Hood L. Systems medicine: the future of medical genomics and healthcare. Genome Med. 2009;1:2. doi: 10.1186/gm2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Meier-Schellersheim M, Fraser ID, Klauschen F. Multiscale modeling for biologists. Wiley Interdisciplinary Reviews: Systems Biology and Medicine. 2009;1:4–14. doi: 10.1002/wsbm.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wu X, Zhou T, Zhu J, Zhang B, Georgiev I, Wang C, et al. Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science. 2011;333:1593–1602. doi: 10.1126/science.1207532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Davies MN, Flower DR. Harnessing bioinformatics to discover new vaccines. Drug discovery today. 2007;12:389–395. doi: 10.1016/j.drudis.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 154.Zak DE, Aderem A. Systems biology of innate immunity. Immunological reviews. 2008;227:264–282. doi: 10.1111/j.1600-065X.2008.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pascual V, Chaussabel D, Banchereau J. A genomic approach to human autoimmune diseases. Annual review of immunology. 2010;28:535. doi: 10.1146/annurev-immunol-030409-101221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zaas AK, Chen M, Varkey J, Veldman T, Hero AO, Lucas J, et al. Gene expression signatures diagnose influenza and other symptomatic respiratory viral infections in humans. Cell host & microbe. 2009;6:207–217. doi: 10.1016/j.chom.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Huang Y, Zaas AK, Rao A, Dobigeon N, Woolf PJ, Veldman T, et al. Temporal dynamics of host molecular responses differentiate symptomatic and asymptomatic influenza a infection. PLoS genetics. 2011;7:e1002234. doi: 10.1371/journal.pgen.1002234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tan S-L, Ganji G, Paeper B, Proll S, Katze MG. Systems biology and the host response to viral infection. Nature biotechnology. 2007;25:1383–1389. doi: 10.1038/nbt1207-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]