Abstract
Background
Biobehavioral correlates of self-rated health in pregnancy are largely unknown.
Purpose
The goals of this study were to examine, in pregnant women, associations of self-rated health with 1) demographics, objective health status, health behaviors and psychological factors and 2) serum inflammatory markers.
Methods
In the 2nd trimester of pregnancy, 101 women provided a blood sample, completed measures of psychosocial stress, health status, and health behaviors, and received a comprehensive periodontal examination.
Results
The following independently predicted poorer self-rated health: 1) greater psychological stress, 2) greater objective health diagnoses, 3) higher body mass index, and 4) past smoking (versus never smoking). Poorer self-rated health was associated with higher serum interleukin-1β (p = .02) and marginally higher macrophage migration inhibitory factor (p = .06). These relationships were not fully accounted for by behavioral/psychological factors.
Conclusions
This study provides novel data regarding factors influencing subjective ratings of health and the association of self-rated health with serum inflammatory markers in pregnant women.
Keywords: pregnancy, inflammation, self-rated health, cytokines, subjective health, pregnant women
Introduction
A large literature links self-rated health to mortality in the general population as well as among adults with chronic health conditions (1–3). This association is surprisingly robust, remaining after accounting for physician-rated health, objective health diagnoses, disease severity, age, and health behaviors (for review see 4, 5). A notable aspect of this literature is that this predictive value is seen with single-item measures of self-rated health (for review see 4, 5). Several plausible mechanistic pathways which may explain this association have been forwarded. A key potential pathway is inflammation. Elevated serum inflammatory markers predict increased morbidity and mortality in the elderly (6–10). Moreover, inflammation may contribute to poor subjective ratings of health via “sickness behavior”; proinflammatory cytokines promote lethargy, decreased appetite, and behavioral withdrawal (11). Thus, inflammation may be a common pathway which affects subjective health as well as risk of adverse health outcomes.
In non-pregnant adults, several studies have examined associations between self-rated health and inflammation (12–19). For example, a study of 1,727 community-dwelling elderly adults found a graded association between a single-item measure of self-rated health and IL-6 (12). Among 265 adults ages 19–90 years, poorer self-rated health was associated with higher levels of inflammatory markers (IL-1β, IL-1ra, and TNF-α) among women, but not men (13). Further, self-rated health was more strongly associated with inflammation than was physician-rated patient health. Inflammatory markers have also been associated with self-rated health among women with coronary heart disease after controlling for various health confounds (15). Thus, available data indicate that self-rated health is associated with elevated serum proinflammatory proteins and that this relationship is more robust than and remains after controlling for objective indicators of health.
Inflammatory processes are of interest in the context of pregnancy. Excessive inflammation is incompatible with healthy pregnancy; elevations in proinflammatory cytokines in maternal serum and amniotic fluid are causally implicated in risk of preterm delivery in the context of infection as well as idiopathic cases (20–24). Proinflammatory cytokines can promote preterm labor by encouraging cervical ripening, weakening chorioamniotic membranes, and triggering preterm contractions (25, 26). It is not known if relationships between self-rated health and serum inflammatory markers evidenced in the general population generalize to pregnancy. Pregnancy occurs in relatively young women and elicits significant immune changes; these factors may minimize or mask the association of self-rated health with serum inflammatory markers.
In addition to assessing general health conditions and health behaviors, the current investigation also included a focus on oral health. Poor oral health is common; approximately 47% of adults in the U.S. have periodontitis (27). Rates are higher among those from lower socioeconomic backgrounds in part due to inadequate access to oral health providers as well as limited knowledge of the importance of oral health (28). Pregnancy is increasingly recognized as an ideal time for education and intervention because pregnant women have regular contact with health care providers and may be motivated to improve their health for the benefit of the fetus/child (29, 30). In addition, clinical signs of periodontal disease have been associated with elevations in serum proinflammatory markers, a focus of the current study (31). For these reasons, it was of interest to examine the extent to which oral health, in terms of both subjective symptoms and clinician-rated severity of disease, was related to global self-rated health in this population. Also of interest was the association of psychological stress with both self-rated health and inflammatory profiles. To provide a cohort with high representation of oral health conditions and significant psychological stress, women from lower socioeconomic backgrounds were targeted for recruitment. In addition, to allow for examination of potential racial differences in the association of self-rated health and variables of interest, African American and White women were relatively equally represented.
The first aim of this study was to examine, in pregnant women, the association of self-rated health with demographic factors, objective health status, health behaviors and psychological factors. The second aim was to examine the association of self-rated health with serum inflammatory markers. It was hypothesized that poorer self-rated health would be associated with poorer physical health, health behaviors, and psychological functioning. It was also predicted that poorer self-rated health would be associated with elevated serum proinflammatory cytokines, and that this relationship would not be fully accounted for by objective health indicators and psychological functioning.
Methods
Study Design
One hundred and one women were recruited from the Ohio State University Wexner Medical Center (OSUWMC) Prenatal Clinic and the general community of Columbus, Ohio. Study visits were conducted during the 2nd trimester of pregnancy. At the study visit, women provided a blood sample and completed measures of psychosocial stress, health status, and health behaviors. To define oral health status, each participant received a comprehensive periodontal examination conducted by a board certified periodontist. Birth outcomes were determined by medical record review following delivery. The study was approved by the OSU Biomedical Institutional Review Board.
Participants
In this study, effects of stress, periodontal disease, and race were of interest. In order to recruit a final cohort with high representation of psychological stress (e.g., depressive symptoms) as well as oral health conditions, we targeted women from lower socioeconomic backgrounds (annual household income <$15,000), among whom these issues are more prevalent. To allow for analyses based on race, we aimed for relatively even representation of African American and White women in the final sample. Women were recruited in-person at the Ohio State University Wexner Medical Center (OSUWMC) Prenatal Clinic and via online advertisements and postings in the community.
Women were excluded if they had chronic health conditions or medications with implications for immune function (e.g., rheumatoid arthritis, multiple sclerosis, or human immunodeficiency virus), fetal anomaly, antidepressant use, illicit drug use other than marijuana or more than two alcohol drinks per week during pregnancy (per self-report at time of phone screening or per medical record). Any woman who reported acute illness (e.g., cold or flu-like symptoms) or use of antibiotics within 10 days of a study visit was rescheduled.
Demographics
Age, race/ethnicity, marital status, education, annual family income, gravidity, and parity were collected by self-report. Pre-pregnancy body mass index (BMI; kg/m2) was calculated using self-reported pre-pregnancy weight and height measured at the study visit.
Self-Rated Health
Global self-rated health was assessed using a single-item measure from the RAND Health Survey (32). This item asked, “In general, would you say your health is: Excellent, Very Good, Good, Fair, or Poor”. This question is comparable to single-item measures of self-rated health used in prior studies demonstrating predictive validity for cardiovascular events and all-cause mortality (for review see 4, 5).
General Medical Conditions and Medication Use
The current or past occurrence of the following health diagnoses was assessed per self-report: high blood pressure (hypertension), arthritis, asthma, cancer, heart disease, diabetes, and sexually transmitted diseases [genital herpes, gonorrhea, syphilis, chlamydia, trichomoniasis, and human papilloma virus (HPV)]. The use of over-the-counter and prescription medications was also collected per self-report.
Oral Health Status
All women received a comprehensive periodontal examination by a single calibrated periodontist including assessment of probing depths and gingival recession by using UNC-15 probe. Examiner reliability was assessed in a pilot study; intra-examiner differences for probing depth and gingival recession were within 1 mm. Clinical attachment loss (formulated from probing depth and gingival recession), percentage of bleeding on probing, and plaque at six surfaces per tooth for all dentition were used to determine the extent and severity of periodontal disease. Women were asked how often they brushed their teeth, the date of their most recent dental visit, and whether they experienced painful and/or bleeding gums upon brushing their teeth.
Health Behaviors
Smoking status was defined as current, past, or never based on self-report. Exercise was operationalized as the frequency of engaging in vigorous physical activity long enough to build up a sweat with a range of “less than once per month” to “more than once per week”. Prenatal vitamin use was defined as never, some days (1–3 days per week), most days (4–6 days per week) and every day (7 days per week). Women completed the Prenatal Health Behaviors Scale (PHBS) (33, 34). In the current analyses, subscales for healthy eating (3 items), unhealthy eating (3 items), and physical strain (3 items) were utilized. For each subscale, higher scores indicate more of the given behavior.
Psychological Functioning
The Center for Epidemiological Studies Depression Scale (CES-D) is a 20-item measure of cognitive, emotional, and somatic symptoms of depression (35). The CES-D is predictive of preterm birth and immune parameters in pregnant women (36–40). The 10-item Perceived Stress Scale (PSS), is a well-validated measure which assesses a construct independent of depressive symptomatology (41). Scores have been associated with maternal neuroendocrine function (42, 43). The 6-item short form of the State-Trait Anxiety Inventory (STAI) was used to assess state anxiety (44, 45). The STAI shows strong criterion, discriminant, and predictive validity in perinatal populations (46). The Revised Prenatal Distress Questionnaire (NUPDQ) is a 17-item measure of pregnancy-specific distress including physical discomforts, financial resources to care for children, and pain during delivery (47). The Pittsburgh Sleep Quality Index (PSQI) has good diagnostic sensitivity and specificity in distinguishing good and poor sleepers (48). A score > 5 is indicative of clinically disturbed sleep.
Measurement of Peripheral Blood Proinflammatory Markers
Serum interleukin (IL)-6, tumor necrosis factor (TNF)-α, and IL-1β were assayed using multiplex kits from MesoScale Discovery (MSD). Macrophage migration inhibitory factor (MIF) and C-reactive protein (CRP) were assayed using Quantikine High Sensitivity Immunoassay kits (R&D) per kit instructions, as described elsewhere (49, 50). Samples that fell out of range of the standard curve were retested, diluted 1:10 with diluent buffer included with the kit. Plates were read at a wavelength of 490nm with a correction wavelength of 690nm using a Multiscan MCC/340 plate reader (Labsystems). Sample concentrations were then extrapolated from a standard curve calculated using a four parameter logistic fit and then multiplied by the dilution factor if necessary. Blood samples were drawn between 8:00am–4:00pm. Collection times were distributed throughout the day with 28.7% collected between 8:00–10:00am, 32.7% from 10:01am–12:00pm, 19.8% from 12:01–2:00pm, and 18.8% from 2:01–4:00pm.
Statistical Analyses
Demographic and health behavior data were summarized as mean (SD) for continuous variables and frequency (%) for categorical variables. Self-rated health responses were assigned the numeric values 1=fair/poor, 2=good, 3=very good, 4=excellent; i.e. higher scores represent better self-rated health. The poor category only had one response and was therefore combined with the fair category.
It was hypothesized that 1) self-rated health would be associated with poorer physical health, health behaviors, and psychological functioning; and 2) that poorer self-rated health would be associated with elevated serum proinflammatory cytokines. These hypotheses were tested using self-rated health as a continuous variable. Pearson correlations were calculated and tested for correlations between continuous variables and self-rated health. Categorical variables were dichotomized, with the exception of periodontal disease status and smoking status, and t-tests were used to assess association with self-rated health.
To take into consideration variation in the time of day blood draws were performed, correlations between proinflammatory cytokines and time of blood draw were calculated. Also, as sensitivity analyses for the correlations of self-rated health with proinflammatory cytokines, multiple linear regression models were fit which controlled for the time of day of the blood draw.
Secondary follow-up comparisons between self-rated health categories excellent versus not excellent, fair/poor versus not fair/poor, and excellent versus fair/poor were made using chi-squared or Fisher's Exact tests. These secondary analyses were performed to explore differences between the highest and lowest health ratings and to explore non-linear relationships with self-rated health. As secondary analyses these results are not conclusive and function to further describe the observed relationships with self-rated health.
For periodontal disease status and smoking status, ANOVA was used to compare self-rated health between categories. Variables from Aim 1 identified as being significantly associated with self-rated health were included in a multivariable linear model with self-rated health as the outcome. For Aim 2, in addition to univariate correlations, multivariate linear models were fit to the self-rated health outcome in separate models for each inflammatory marker, adjusting for all variables with a p-value of 0.10 or lower in the multivariable model for Aim 1. The correlation matrix for key continuous study measures was calculated. In all analyses, Q-Q probability plots, histograms, and residuals were used to evaluate normality assumptions. For analysis of inflammatory marker outcomes, data were log-transformed (base 10) and data points more than 3 standard deviations above the next highest value were excluded.
Results
Aim 1: To examine the association of self-rated health with demographic factors, objective health status, health behaviors, and psychological factors
Self-Rated Health
Overall, 10.9% (n=11) of women rated their health as excellent, 28.7% (n=29) as very good, 44.6% (n=45) as good, 14.9% (n=15) as fair and 1% (n=1) as poor. As noted above, for analyses, fair and poor categories were merged. It was hypothesized that poorer self-rated health would be associated with poorer physical health, health behaviors, and psychological functioning.
Demographic Characteristics
Demographics are provided in Table 1. Reflecting targeted recruitment of women from disadvantaged economic backgrounds, 63.4% reported an annual household income <$15,000 and 52.5% had high school education or less. The average pre-pregnancy BMI was 27.32 (SD = 7.45; range 15.5–54.73). Overall, 4% were underweight, 42.6% were normal weight, 22.8% were overweight, and 30.7% were obese.
Table 1.
Demographic Characteristics by Self-Rated Health
| Excellent (n=11) | Very Good (n=29) | Good (n=45) | Fair/Poor (n=16) | Group Comparisons | |
|---|---|---|---|---|---|
| Race | African American vs. White; t(96)=1.13, p = .26b | ||||
| African-American (n=55)a | 10 (18.2%)* | 14 (25.5%) | 21 (38.2%) | 10 (18.2%) | |
| White (n=43) | 1 (2.3%)* | 14 (32.6%) | 22 (51.2%) | 6 (14.0%) | |
| Asian/Mixed Race (n=3) | 0 (0%) | 1 (33.3%) | 2 (66.7%) | 0 (0.0%) | |
| Income | greater vs. less than $15,000; t(99) = 0.43, p = .67 | ||||
| <$15,000 (n=64) | 9 (14.1%) | 17 (26.6%) | 27 (42.2%) | 11 (17.2%) | |
| $15,000 – 29,999 (n=30) | 2 (6.7%) | 10 (33.3%) | 14 (46.7%) | 4 (13.3%) | |
| $30,000 – 49,999 (n=7) | 0 (0.0%) | 2 (28.6%) | 4 (57.1%) | 1 (14.3%) | |
| Education | some college vs. less; t(99) = 0.6, p = .55 | ||||
| Less than high school (n=25) | 5 (20.0%) | 5 (20.0%) | 12 (48.0%) | 3 (12.0%) | |
| High school graduate (n=28) | 2 (7.1%) | 12 (42.9%) | 7 (25.0%) | 7 (25.0%) | |
| Some college or greater (n=48) | 4 (8.3%) | 12 (25.0%) | 26 (54.2%) | 6 (12.5%) | |
| Body Mass Index (BMI) | r = −.22, p = .03c | ||||
| Mean (SD) | 26.2 (8.5) | 25.9 (5.6) | 26.8 (7.1) | 32.1 (9.0) | |
| Week of Gestation | r = .13, p = .19 | ||||
| Mean (SD) | 24.6 (2.5) | 24.4 (2.6) | 23.2 (3.3) | 23.9 (2.0) | |
| Maternal Age | r= .03, p = .73 | ||||
| Mean (SD) | 25.4 (4.5) | 23.4 (3.9) | 24.2 (4.6) | 23.9 (4.2) |
includes four women endorsing both African American and White race
Fisher's Exact Test indicated a greater percentage of African-American women endorsed “excellent” health compared to Whites (p = .02)
Obese women (BMI ≥ 30) endorsed significantly lower self-rated health than non-obese women (p = .03).
Neither maternal age (r = .03, p = .73) nor week of pregnancy at the time of assessment (r = .13, p = .19) was associated with self-rated health. African Americans and Whites reported similar self-rated health on average [Mean 2.45 versus 2.23; t(96) = 1.13, p = .26]. However, a greater percentage of African Americans endorsed excellent health as compared to Whites [18.1% (10/55) vs. 2.3% (1/43); Fisher's Exact Test p = .02)]. Self-rated health was not associated with income [greater versus less than $15,000; t(99) = .43, p = .67] or education [some college versus less; t(99) = .60, p = .55]. Higher body mass index (BMI) was associated with lower self-rated health (r = −.22, p = .03).
Self-Reported Physical Health Diagnoses
Current self-reported diagnoses are presented in Table 2. Overall, 68.3% (n=69) reported no current diagnosis, 24.8% (n=25) reported one diagnosis, and 6.9% (n=7) reported two diagnoses. Compared to women with no health diagnosis, women with one or more diagnosis reported significantly poorer self-rated health [Mean 1.97 (SD = .78) versus 2.52 (SD = .87); t(99) = 3.07; p = .003].
Table 2.
Health Conditions and Medication Use by Self-Rated Health
| Excellent (n=11) | Very Good (n=29) | Good (n=45) | Fair/Poor (n=16) | Group Comparisons | |
|---|---|---|---|---|---|
| Physical Health Diagnoses a | no vs. one or more diagnosis; t(99) = 3.07; p = .003 | ||||
| Asthma (n=19) | 1 (9.1%) | 5 (17.2%) | 8 (17.8%) | 5 (31.3%) | |
| HPV (n=7) | 0 (0.0%) | 1 (3.4%) | 4 (8.9%) | 2 (12.5%) | |
| Genital Herpes (n=6) | 0 (0.0%) | 2 (6.8%) | 2 (4.4%) | 2 (12.5%) | |
| Arthritis (n=4) | 0 (0.0%) | 0 (0.0%) | 2 (4.4%) | 2 (12.5%) | |
| Gestational diabetes (n=1) | 0 (0.0%) | 0 (0.0%) | 1 (2.2%) | 0 (0.0%) | |
| Genital warts (n=1) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (6.3%) | |
| Chlamydia (n=1) | 0 (0.0%) | 0 (0.0%) | 1 (2.2%) | 0 (0.0%) | |
| Oral Health Status b | F(3,97) = 1.6, p = .19 | ||||
| Gingivitis (n=33) | 3 (27.3%) | 13 (44.8%) | 13 (28.9%) | 4 (25.0%) | |
| Slight periodontitis (n= 28) | 5 (45.5%) | 7 (24.1%) | 11 (24.4%) | 5 (31.3%) | |
| Moderate periodontitis (n=27) | 0 (0.0%) | 7 (24.1%) | 14 (31.1%) | 6 (37.5%) | |
| Severe periodontitis (n=13) | 3 (27.3%) | 2 (6.9%) | 7 (15.6%) | 1 (6.3%) | |
| Painful/bleeding gums when brushing | t(97) = 2.59, p = .01 | ||||
| Yes (n=59) | 3 (27.3%) | 14 (48.3%) | 31 (68.9%) | 11 (68.8%) | |
| Medications and Supplements c | none vs. one or more; t(99) = .60, p = .55 | ||||
| Acetaminophen (n=8) | 0 (0.0%) | 4 (13.8%) | 4 (8.9%) | 2 (12.5%) | |
| Albuterol (n=5) | 1 (9.1%) | 1 (3.4%) | 1 (2.2%) | 2 (12.5%) | |
| Iron supplements (n=5) | 0 (0.0%) | 1 (3.4%) | 4 (8.9%) | 0 (0.0%) | |
| Anti-nausea medications (promethazine or ondasetron; n=7) | 0 (0.0%) | 3 (10.3%) | 2 (4.4%) | 1 (6.3%) | |
| Antihistamines (cetirizine, pseudophedrine Hcl, loratadine, or diphenhydramine; n=5) | 0 (0.0%) | 2 (6.8%) | 3 (6.6%) | 0 (0.0%) | |
| Antacids (famotidine or ranitidine Hcl; n=3) | 0 (0.0%) | 1 (3.4%) | 1 (2.2%) | 0 (0.0%) | |
| Oxycodone (n=1) | 0 (0.0%) | 0 (0.0%) | 1 (2.2%) | 0 (0.0%) | |
| Clindamycin (n=1) | 0 (0.0%) | 0 (0.0%) | 1 (2.2%) | 0 (0.0%) | |
| Methadone (n=1) | 0 (0.0%) | 0 (0.0%) | 1 (2.2%) | 0 (0.0%) | |
| Nicotine patch (n=1) | 0 (0.0%) | 0 (0.0%) | 1 (2.2%) | 0 (0.0%) |
per self-report;
per clinical evaluation;
excluding prenatal vitamins
Oral Health
Women were classified into four mutually exclusive disease categories based on comprehensive periodontal examination (Table 2). All evidenced at least gingivitis. Self-rated health did not differ significantly based on periodontal disease category [F(3,97) = 1.6, p = .19]. Endorsement of painful and/or bleeding gums upon brushing was associated with poorer self-rated health [mean SRH 2.15 versus 2.60; t(97) = 2.59, p = .01].
Medications and Supplements
Summarized in Table 2, 72.3% (n=73) reported no use of over-the-counter medications, prescription medications, or supplements (excluding prenatal vitamins), 19.8% (n=20) reported one medication/supplement, and 6.9% (n=8) reported two or three. Self-rated health did not differ among women with current medication/supplement use versus those with no use [Mean 2.25 (SD = .78) versus 2.37 (SD = .94); t(99) = .60, p = .55].
Recreational Drug Use
In terms of recreational drugs, 23% reported use of alcohol during their pregnancy occurring only before knowledge of the pregnancy and 3% reported use after knowledge of the pregnancy. At some time during the current pregnancy, 16% reported use of marijuana, 3% reported use of heroin, 1% reported use of barbiturates, and none reported use of amphetamines or cocaine.
Alcohol use at any time during pregnancy was not associated with self-rated health [t(99) = .51, p = .61]. However, as described, the majority of such occurrences were prior to knowledge of pregnancy. Reported marijuana use during pregnancy was not associated with self-rated health as a continuous measure; however, secondary analyses showed that marijuana use was more common among those endorsing fair/poor self-rated health (6/16; 37.5%) than those endorsing excellent health (10/85; 11.8%) [χ2(1) = 6.7, p = .01].
General Health Behaviors
General health behaviors are summarized in Table 3. Self-rated health was significantly higher among women who had never been smokers than in women who were past smokers [Mean 2.57 vs. 2.11; t(98) = 2.32, p = .02]. Self-rated health for current smokers (Mean 2.29) did not differ significantly from never or past smokers.
Table 3.
Health Behaviors by Self-Rated Health
| Excellent (n=11) | Very Good (n=29) | Good (n=45) | Fair/Poor (n=16) | Group Comparisons | |
|---|---|---|---|---|---|
| Smoking Status | never vs. past t(98) = 2.32, p = .02 | ||||
| Current (n=24) | 1 (4.1%) | 9 (37.5%) | 10 (41.7%) | 4 (16.7%) | |
| Past (n=35) | 2 (5.7%) | 6 (19.4%) | 21 (60.0%) | 6 (17.1%) | |
| Never (n=42) | 8 (19.1%) | 14 (33.3%) | 14 (33.3%) | 6 (14.3%) | |
| Prenatal Vitamin Use | never/some days vs. most/all days; t(99) = .65, p = .52 | ||||
| Never (n=6) | 1 (16.6%) | 2 (33.3%) | 2 (33.3%) | 1 (16.6%) | |
| Some days (1–3 days/week; n=16) | 0 (0%) | 8 (50%) | 7 (43.8%) | 1 (6.3%) | |
| Most days (4–6 days/week; n=22) | 5 (22.7%) | 4 (18.2%) | 9 (40.9%) | 4 (18.2%) | |
| Nearly every day (n=57) | 5 (8.7%) | 15 (26.3%) | 27 (47.4%) | 10 (17.5%) | |
| Exercise | once per week vs. less; t(99) = 0.81, p = .42 | ||||
| Less than once per month (n=37) | 4 (10.8%) | 12 (32.4%) | 16 (43.2%) | 5 (13.5%) | |
| Once per month (n=11) | 0 (0.0%) | 4 (36.6%) | 4 (36.6%) | 3 (27.3%) | |
| 2–3 times per month (n=14) | 1 (7.1%) | 2 (14.2%) | 9 (64.3%) | 2 (14.2%) | |
| Once per week (n=21) | 0 (0.0%) | 6 (28.6%) | 10 (47.6%) | 5 (23.8%) | |
| More than once per week (n=18) | 6 (33.3%) | 5 (27.8%) | 6 (33.3%) | 1 (5.5%) | |
| Healthy Eating | r = .23, p = .02a | ||||
| Mean (SD) | 11.18 (1.17) | 9.55 (2.49) | 8.82 (2.09) | 9.37 (2.36) | |
| Unhealthy Eating | r = −.04, p = .71 | ||||
| Mean (SD) | 6.27 (2.28) | 5.38 (2.9) | 5.93 (2.4) | 6.12 (2.73) | |
| Physical Strain | r = .05, p = .65 | ||||
| Mean (SD) | 4.00 (1.67) | 4.72 (2.56) | 4.36 (2.86) | 3.88 (3.10) |
Women rating their health as “Excellent” reported greater healthy eating behavior than the other 3 groups combined (p = .01)
Self-rated health did not differ by prenatal vitamin use [never/some days versus most days/every day, t(99) = .65, p = .52] or exercise [once per week versus less, t(99) = .81, p = .42]. Reported healthy eating behavior, as measured with the Prenatal Health Behavior Scale (PHBS), was correlated with health categorization (r = .23, p = .02); women endorsing excellent health reported greater frequency of healthy eating (e.g., consuming high fiber foods) as compared to the other three groups (p = .01). Scores on the unhealthy eating (e.g., consuming high fat foods) and physical strain subscales of the PHBS were not correlated with self-rated health categorization (ps > .05)
Psychological Factors
Summary statistics are provided in Table 4. Self-rated health was negatively correlated with depressive symptoms (r = −.27, p = .01), perceived stress (r = −.27, p = .01), and pregnancy-specific distress (r = −.22, p = .03) but not state anxiety (r = −.16, p = .12).
Table 4.
Psychological Factors by Self-Rated Health
| Excellent (n=11) | Very Good (n=29) | Good (n=45) | Fair/Poor (n=16) | Group Comparisons | |
|---|---|---|---|---|---|
| Depressive Symptoms | r = −.27, p = .01 | ||||
| Mean (SD) | 10.36 (12.07) | 12.03 (9.10) | 16.53 (11.47) | 19.63 (10.99) | |
| Clinically significant symptoms (n=42) | 2 (18.2%) | 7 (24.1%) | 22 (48.9%) | 11 (68.8%) | |
| Perceived Stress | r = −.27, p = .01 | ||||
| Mean (SD) | 13.27 (4.96) | 15.97 (8.11) | 17.42 (7.11) | 20.56 (6.24) | |
| State Anxiety | r = −.16, p = .12 | ||||
| Mean (SD) | 9.73 (4.34) | 10.55 (3.25) | 11.33 (3.64) | 11.63 (4.18) | |
| Pregnancy-Specific Distress | r = −.22, p = .03 | ||||
| Mean (SD) | 8.73 (7.10) | 8.14 (5.01) | 10.4 (6.61) | 12.56 (5.91) | |
| Sleep Quality | r = −.15, p = .14a | ||||
| Total Score | 5.36 (3.0) | 6.97 (3.0) | 6.39 (3.8) | 8.13 (3.7) | |
| Clinically disturbed sleep1 | 5 (45%) | 17 (59%) | 24 (55%) | 11 (69%) |
Sleep quality was significantly worse among women endorsing “Fair/Poor” versus “Excellent” (p = .05)
The pregnancy-specific distress questionnaire included items assessing stress specific to physical symptoms of pregnancy, specifically “being tired or having low energy during your pregnancy” and “physical symptoms of pregnancy such as vomiting, swollen feet, or backaches”. Neither of these pregnancy-specific physical health symptoms were individually associated with self-rated health (ps > .70).
As expected, African Americans reported greater frequency of racial discrimination during their lifetimes compared to Whites [t(96) = 4.14, p <.001]. Self-rated health was not significantly correlated with reported occurrence of racial discrimination among African Americans (r = −.21, p = .13) or among Whites (r = .09, p = .58).
Disturbed sleep was common in this sample, with 55% receiving a score ≥ 5 on the PSQI (Table 4). There was no significant correlation of self-rated health with sleep quality score (r = −.15, p = .14). However, secondary tests demonstrated that sleep quality was significantly worse among women rating their health as fair/poor compared to those rating their health as excellent [8.13 (SD = 3.7) versus 5.36 (SD= 3.0), p = .05].
Summary of Simple Associations
In sum, health factors significantly associated with poorer self-rated health as a continuous measure were greater health diagnoses, higher body mass index, smoking status (past versus never), presence of painful/bleeding gums, and less healthy eating behaviors. Greater depressive symptoms, perceived stress and pregnancy-specific distress were strong correlates of poorer self-rated health. In addition, both marijuana use and poor sleep were more common among those in the lowest versus highest self-rated health category. Self-rated health as a continuous measure did not differ by race. However, in secondary categorical analyses, African Americans were more likely to endorse excellent health as compared to Whites (p = .02). This difference was not accounted for by income, objective health indicators, or psychological factors.
Unique predictors
As expected, predictors in our model were significantly correlated with each other (Table 5). Next we examined the unique predictive value of factors significantly associated with the continuous self-rated health outcome in our initial tests of Aim 1. As measures of stress were highly correlated, a composite stress measure was created by averaging the z-scores for depressive symptoms (CES-D), perceived stress, and pregnancy-specific distress. A multivariable linear model which included the presence of health diagnoses, BMI, smoking status (current/past/never), composite stress, painful/bleeding gums, and healthy eating was fit to the self-rated health outcome (Table 6). In this multivariate model, healthy eating (p = .31) and bleeding gums (p = .13) were no longer statistically significant correlates of self-rated health. The other variables remained significantly associated with poorer self-rated health: greater composite stress (p < .001), the presence of health diagnoses (p = .001), higher BMI (p = .01), and smoking status (past versus never) (p = .003). Together, these factors accounted for 30.7% of the variance in self-rated health.
Table 5.
Correlation Matrix
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12 | 13. | 14. | 15. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Self-Rated Health | --- | .03 | .13 | −.22* | −.29** | .23* | −.27** | −.27** | −.22* | −.15 | −.23* | −.19+ | −.12 | −.13 | −.09 |
| 2. Maternal Age | --- | −.02 | .03 | −.05 | .16 | .02 | .03 | .04 | .15 | −.10 | .21* | −.04 | −.10 | −.06 | |
| 3. Weeks Gestation | --- | −.06 | −.05 | −.13 | −.01 | .06 | .05 | −.04 | −.01 | .04 | −.04 | −.16 | .12 | ||
| 4. BMI | --- | .09 | −.01 | −.06 | .01 | −.09 | −.06 | .31** | .15 | .60*** | .53*** | .22* | |||
| 5. # of Health Conditions | --- | .01 | .09 | .01 | −.07 | .09 | .03 | .12 | −.01 | .03 | .20* | ||||
| 6. Healthy Eating Behavior | --- | −.25* | −.22* | −.20* | −.20* | −.02 | .10 | −.10 | .04 | −.13 | |||||
| 7. Depressive Symptoms | --- | .63*** | .42*** | .51*** | −.20* | −.11 | .02 | −.13 | .20* | ||||||
| 8. Perceived Stress | --- | .54*** | .33*** | −.18 | .07 | −.04 | −.14 | .15 | |||||||
| 9. Pregnancy-Specific Stress | --- | .30** | −.12 | −.02 | −.03 | −.13 | −.03 | ||||||||
| 10. PSQI | --- | −.10 | −.08 | .001 | −.11 | −.03 | |||||||||
| 11. IL-1β | --- | −.02 | .17 | .38*** | .18 | ||||||||||
| 12. MIF | --- | .16 | .01 | .05 | |||||||||||
| 13. CRP | --- | .58*** | .21* | ||||||||||||
| 14. IL-6 | --- | .26* | |||||||||||||
| 15. TNF-a | --- |
p < .05;
p < .01;
p < .001;
p = .055
Table 6.
Multivariate Model for Association with Self-Rated Health
| B (SE) | β | Δ R2 | t (91) | p-value | |
|---|---|---|---|---|---|
| Health Diagnoses | −0.54 (0.16) | −0.29 | 0.08 | −3.31 | .001 |
| Body Mass Index (BMI) | −0.03 (0.01) | −0.23 | 0.05 | −2.67 | .01 |
| Smoking Status=Current | −0.27(0.19) | −0.13 | 0.09 | −1.40 | .17a |
| Smoking Status=Past | −0.61 (0.17) | −0.33 | −3.49 | .001a | |
| Composite Stress | −0.35 (0.09) | −0.33 | 0.11 | −3.88 | < .001 |
p-value for overall smoking status was .003; F(2,95) = 6.10
Note: R2 = 0.31 for model as a whole
Aim 2: To examine the association of self-rated health with serum inflammatory markers
Next, we examined the association between self-rated health and serum inflammatory markers. It was predicted that poorer self-rated health would be associated with elevated serum proinflammatory cytokines, and that this relationship would not be fully accounted for by objective health indicators and psychological functioning. Three high outliers were excluded from analyses; one each for TNF-α, MIF, and IL-6. Initial analyses examined the simple relationship between self-rated health and inflammatory markers. Subsequent analyses examined this relationship after controlling for factors which emerged as predictors of self-rated health in Aim 1.
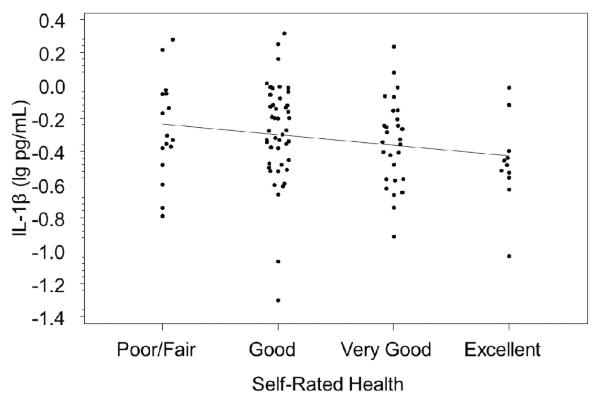
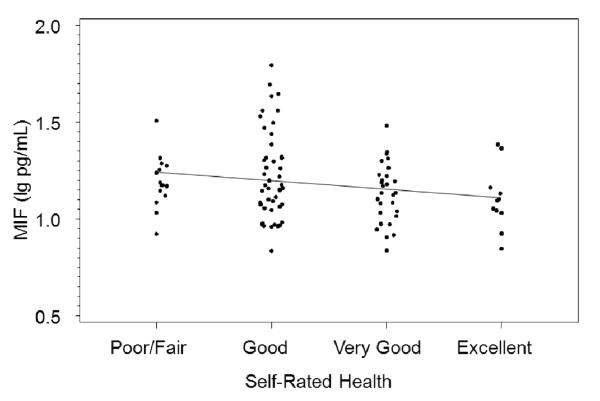
There was a significant negative correlation between IL-1β and self-rated health (r = −.23, p = .02; Figure 1) and a marginal negative correlation between macrophage migration inhibitory factor (MIF) and self-rated health (r = −.19, p=.06; Figure 2). There were no significant correlations between self-rated health and serum IL-6, TNF-α, or CRP (ps > .20). Time of day blood was drawn was not correlated with any of the proinflammatory cytokines (ps > .19; p = .63 for IL-1β and p = .26 for MIF). Adjusting for time of day did not change the correlations of IL-1β and MIF with self-rated health.
Figure 1.
Serum interleukin (IL)-1β and self-rated health (r = −.23, p = .02).
Figure 2.
Serum macrophage migration inhibitory factor (MIF) and self-rated health (r = −.19, p=.055)
Each of these correlation analyses were repeated in linear models controlling for variables with p-values of 0.1 or lower in the multivariable model for Aim 1: BMI, smoking status (never/past/current), presence of an objective health diagnosis, and composite stress. Adjusting for these factors reduced the relationship of self-rated health with IL-1β marginally (change in standardized beta from −.178 to −.194, adjusted p-value =.09) and reduced the relationship with MIF considerably (from −.194 to −.112, adjusted p-value = .21). Without control variables included, IL-1β and MIF accounted for 3.6% and 3.8% of the variation in self-rated health, respectively. With control variables, this was reduced to 1.9% for IL-1β and 2.2% for MIF.
Discussion
The first aim of this study was to examine demographic, behavioral, and psychosocial correlates of self-rated health among pregnant women. It was hypothesized that poorer self-rated health would be associated with poorer physical health, health behaviors, and psychological functioning. Multivariate modeling demonstrated that the following were independent predictors of poorer self-rated health as a continuous measure: 1) greater composite stress (i.e., depressive symptoms, perceived stress, and pregnancy-specific distress), 2) the presence of objective health diagnoses, 3) higher body mass index, and 4) a history of smoking.
It is well-established that depressive symptoms are associated with poorer self-rated health (e.g., 51, 52). Pregnant women who have lower incomes and/or lack social support are at higher risk for depressive symptoms (53). In this study, 41.6% of women scored ≥ 16 on the Center for Epidemiological Studies Depression Scale (CES-D). Rates differed significantly with self-rated health; 68.8% of women who endorsed fair/poor health scored at or above this clinical cut-off compared to 18.2% of those who endorsed excellent health. Thus, in high risk groups, inquiring as to global self-rated health at regular prenatal visits may provide an efficient means to identify women who may benefit from depression screening.
Relatedly, greater symptoms of sleep disturbance, as measured by the PSQI, were strongly correlated with depressive symptoms. Although a linear association between sleep and self-rated health was not evidenced, secondary analyses showed that women reporting fair/poor health had significantly poorer sleep quality than those endorsing excellent health. This is consistent with prior data showing that sleep disturbance influences subjective ratings of health (54–57). Similar to effects seen in non-pregnant adults (58) sleep disturbance in pregnancy has been associated with higher circulating proinflammatory cytokine levels (59, 60). Therefore, this aspect of self-rated health in pregnancy warrants further attention.
Smoking is a leading cause of low birth weight in the U.S. (61) and increases risk for prematurity, birth complications and sudden infant death syndrome (SIDS) (62). Psychosocial factors implicated in smoking continuation include community social norms and undervaluing the degree of risk conferred by smoking (63–65). Thus, it is notable that in the current sample, the association between self-rated health and smoking status was driven by lower self-rated health among past smokers, among whom 86.2% (25/29) reported quitting during their current pregnancy. In contrast, current smokers did not differ from women who were never smokers in self-rated health. Consistent with the health belief model (66), these data suggest that perceptions of personal susceptibility to negative health effects of smoking contribute to motivation for smoking cessation.
As seen in low income adults in general (67), poor periodontal health was highly prevalent in this sample. All women met criteria for at least gingivitis and 39.6% met criteria for moderate or severe periodontal disease. Self-rated health was not associated with periodontal disease severity; however, only one woman (with slight disease) reported that she had been previously diagnosed. Thus, this lack of association may be attributable to unawareness. Although it may result in tooth movement when chewing and eventual tooth loss, periodontal disease often does not cause pain. The most common symptom is bleeding gums. Overall, 58.4% of women reported painful and/or bleeding gums upon brushing and this symptom was associated with poorer self-rated health, although this association was no longer statistically significant in multivariate analyses.
Periodontal disease has been associated with marked increases in preterm birth risk in numerous studies, however it is not clear if this association is causal (for review see 17, 68). Although initial intervention studies reported reduced rates of preterm birth in women treated for periodontal disease during pregnancy, subsequent large randomized clinical trials (e.g.,69, 70–72) demonstrated no effect (for review see 73, 74, 75). If a causal association does exist, it is possible that treatment during pregnancy is too late to benefit birth outcomes in the current pregnancy (76). However, as poor periodontal health is associated with increased risk of chronic health conditions including cardiovascular diseases and diabetes (77, 78), recognition and treatment of oral health conditions during pregnancy may provide long-term health benefits for women. Moreover, mothers' oral health status is a strong predictor of the oral health status of their children due to vertical transmission of caries as well as maternal oversight of tooth brushing, diet, and healthcare utilization (79). Reflecting poor maternal health habits in this sample, only 53.5% reported brushing their teeth at least twice per day, as recommended by the American Dental Association, and only 40.8% had a dental visit in the past year, as compared to 71.2% of adults in Ohio overall (80). Pregnancy is a time of increased contact with healthcare providers, providing an opportunity for education, referral, and intervention.
In this sample, race was not associated with self-rated health when examined as a continuous measure. However, secondary analyses showed that African American women were significantly more likely to endorse excellent health as compared to Whites. This effect was not accounted for by any factors assessed, such as socioeconomic status, depressive symptoms, health behaviors, or presence of objective health conditions.
In older adults in the U.S., self-rated health is consistently lower among African Americans versus Whites after accounting for socioeconomic factors (81). However, this racial difference is most consistent among those at higher socioeconomic status (82) and may be less evident in younger groups. For example, a previous study of ethnically diverse pregnant women found no racial differences in self-rated health after accounting for socioeconomic status (83).
A possible explanation for the better self-rated health among African Americans versus Whites in the current study is a reference effect. Compared to Whites, African Americans experience a greater burden of serious health conditions including diabetes, hypertension, and preterm birth (84). Global self-rated health is likely influenced by comparisons to others in one's social network (85). Thus, if young African American women are consistently exposed to greater illness in their immediate social circles, they may rate their own health more positively in contrast. Regardless of the factors underlying this racial difference, the relationship between self-rated health and serum inflammatory markers examined in Aim 2 was not significantly changed by the inclusion of race as a covariate, indicating that racial differences did not drive this effect.
The second aim of this study was to examine whether self-rated health is associated with serum inflammatory markers in pregnant women. In non-pregnant adults, poorer self-rated health has been associated with elevations in serum inflammatory markers including IL-6, IL-1β, IL1-ra, and TNF-α (12–15, 17–19). These associations were not fully accounted for by behavioral and psychological factors, suggesting that more direct physiological pathways may contribute. In particular, sickness responses (e.g., fatigue, behavioral withdrawal, other depressive-like symptoms) induced by inflammatory activity may be perceived by an individual in the absence of a diagnosable disease and/or well before a specific disease is detectable by objective measures. These mild and vague symptoms may influence ratings of self-rated health. In this manner, self-rated health may provide information about physical health status above and beyond physician assessments of diagnosable conditions. Thus, based on prior studies, we hypothesized that poorer self-rated health would be associated with elevated serum proinflammatory cytokines, and that this relationship would not be fully accounted for by objective health indicators and psychological functioning.
In the current study, poorer self-rated health was significantly associated with higher serum IL-1β and marginally higher macrophage migration inhibitory factor (MIF) in simple correlations. With the addition of covariates (predictors of self-rated health identified in Aim 1), the variation in self-rated health accounted for by MIF and IL-1B was reduced by 42% and 47%, respectively. In contrast, IL-6, TNF-α, and CRP, markers that have been associated with self-rated health in older adults, showed no significant association in the current sample. Overall, these data support a modest relationship between self-rated health and serum inflammatory markers in pregnancy, which was largely but not fully explained by behavioral/psychological correlates measured. The relationship between self-rated health and inflammation may be weaker and less consistent in pregnant women due to complex and dynamic changes in immune and neuroendocrine parameters in pregnancy. Moreover, prior studies show that the relationship between self-rated health and serum inflammatory markers is stronger with increasing age (e.g., 14, 19); this relationship may be more evident in older adults due to age-related increases in inflammatory markers.
In prior studies of self-rated health and serum inflammatory markers, MIF has not been a focus. Notably, MIF has the unique ability to counteract the anti-inflammatory properties of glucocorticoids in a dose-response fashion (86, 87). Due to its broad effects and essential role in both innate and adaptive immune function, antagonism of MIF is a potential therapeutic target for many diseases with an inflammatory component (e.g., rheumatoid arthritis, vascular disease) (88–91). In pregnancy, elevated serum MIF has been associated with preeclampsia, preterm delivery, gestational diabetes, and clinical depression (92–94). Our group has reported that, among pregnant women, depressive symptoms predict more robust serum MIF responses following the immune challenge of seasonal trivalent influenza virus vaccination (37). Thus, we examined MIF in the current study due to its unique proinflammatory properties, prior associations with psychosocial factors, and emerging links with perinatal outcomes.
An important limitation of this study is that it did not permit analyses related to birth outcomes. Among the 101 women in this study, only 9 delivered preterm (< 37 weeks gestation). This rate of preterm birth is lower than among the overall population in the U.S. (~18% among African Americans versus 11% in Whites). The lower rates of preterm birth in the study cohort likely reflect exclusion of women with major health conditions and drug/alcohol abuse. An aim of future studies should be adequate statistical power to examine self-rated health and inflammation in relation to birth outcomes.
To provide high representation of women with signficant psychological stress, oral health conditions, and to permit analyses based on race, the current study targeted White and African American women from lower socioeconomic backgrounds. Generalizability to other groups is unknown. This study included assessment at only one timepoint in pregnancy. However, prior data show that, despite significant changes in depressive symptoms and subjective physical functioning, global self-rated health shows small changes from prior to conception through each trimester of pregnancy (95).
In this study, in which assessments were conducted in the 2nd trimester, there was no association between self-rated health and physical symptoms of pregnancy as measured by the prenatal distress quesitonnaire. However, the 2nd trimester of pregnancy is commonly regarded as a relatively symptom-free period. In the 1st trimester, considerable and rapid hormonal changes contribute to a high prevalence of vomiting/nausea and fatigue. During the 3rd trimester, physical growth of the fetus can contribute to backaches, difficulty sleeping, frequent urination, and shortness of breath. Therefore, physical symptoms of pregnancy may have a greater influence on self-rated health during the 1st and 3rd trimesters. Moreover, some conditions of pregnancy appear in later gestation; e.g., testing for gestational diabetes occurs at 24–28 weeks and had not yet been conducted for most women at the time of their study visit. Thus, longitudinal data inclusive of more comprehensive assessment of physical symptoms of pregnancy would be informative in future studies of inflammatory markers and self-rated health in pregnancy.
Relatedly, this study did not include a non-pregnant comparison group. Factors which influence self-rated health may differ during pregnancy versus non-pregnancy due to pregnancy-specific symptoms, as described above, and because the health of the fetus may influence a mother's perception of her overall health. Supporting the latter, examination of specific items on the prenatal distress questionniare showed that of the 17 items, only two were significantly correlated with self-rated health. These were concerns “about whether you might have an unhealthy baby” (r = −.32, p = .001) and “about the effect of ongoing health problems such as high blood pressure or diabetes on your pregnancy” (r = −.23, p = .02). In addition, concern “about whether the baby might be affected by alcohol, cigarettes, or drugs that you have taken” was marginally associated with self-rated health (r = −.19, p = .06). It is therefore of note that the association of pregnancy specific distress with self-rated heatlh appears to be driven by health-related anxieties. However, this should be interpreted with caution since these correlations were conducted in a post-hoc manner and not adjusted for multiple comparisons. Inclusion of a non-pregnant comparison group in the future would allow for examination of the extent to which specific health factors, particularly those that may affect fetal development, influence women's self-rated health during pregnancy as compared to non-pregnancy
Moreover, it is possible that pregnancy-related changes in circulating cytokines influence self-rated health. Available data suggest that compared to nonpregnancy, normal pregnancy is characterized by mild elevations in both pro- and antiinflammatory cytokine levels, with exaggerated increases seen in pregnancies affected by conditions such as preeclampsia (96–102). Comparison of non-pregnant women to women in each trimester of pregnancy would be informative for testing if the association between self-rated health and serum inflammatory markers differs meaningfully based on pregnancy status/stage.
In conclusion, this study provides novel information regarding predictors of self-rated health and the association of self-rated health with inflammatory biomarkers in pregnant women. From a clinical standpoint, these data contribute to understanding of factors that are valued and possibly undervalued in women's subjective evaluations of their own health during pregnancy. In addition, these data provide evidence that, as seen in non-pregnant adults, self-rated health may be associated with inflammatory status in pregnant women and that this effect may not be fully accounted for by objective health factors. Continued research is needed to determine the consistency, strength, and clinical relevance of this association in the context of pregnancy.
Acknowledgments
We appreciate the contributions of Clinical Research Assistants Kelly Marceau and Rebecca Long to data collection. We would like to thank our study participants and the staff at the OSU Clinical Research Center and Wexner Medical Center Prenatal Clinic.
Role of the Funding Sources
This study was supported by NICHD (HD061644, LMC and HD067670, LMC) and a seed grant from the Ohio State University College of Dentistry (BL & LMC). The project described was supported by the Ohio State University Clinical Research Center, funded by the National Center for Research Resources, Grant UL1RR025755 and is now at the National Center for Advancing Translational Sciences, Grant 8UL1TR000090-05. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflict of Interest Statement
The authors have no conflict of interest to disclose.
References
- 1.Dasbach EJ, Klein R, Klein BEK, Moss SE. Self-Rated Health and Mortality in People with Diabetes. American Journal of Public Health. 1994;84:1775–1779. doi: 10.2105/ajph.84.11.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosworth HB, Siegler IC, Brummett BH, Barefoot JC, Williams RB, Clapp-Channing NE, Mark DB. The association between self-rated health and mortality in a well-characterized sample of coronary artery disease patients. Medical Care. 1999;37:1226–1236. doi: 10.1097/00005650-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Thong MSY, Kaptein AA, Benyamini Y, Krediet RT, Boeschoten EW, Dekker FW, NECOSAD AD. Association between a self-rated health question and mortality in young and old dialysis patients: A cohort study. American Journal of Kidney Diseases. 2008;52:111–117. doi: 10.1053/j.ajkd.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Idler EL, Benyamini Y. Self-rated health and mortality: A review of twenty-seven community studies. Journal of Health and Social Behavior. 1997;38:21–37. [PubMed] [Google Scholar]
- 5.Benyamini Y, Idler EL. Community studies reporting association between self-rated health and mortality - Additional studies, 1995 to 1998. Research on Aging. 1999;21:392–401. [Google Scholar]
- 6.Cappola AR, Xue QL, Ferrucci L, Guralnik JM, Volpato S, Fried LP. Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. Journal of Clinical Endocrinology and Metabolism. 2003;88:2019–2025. doi: 10.1210/jc.2002-021694. [DOI] [PubMed] [Google Scholar]
- 7.Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr, Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. American Journal of Medicine. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 8.Roubenoff R, Parise H, Payette HA, Abad LW, D'Agostino R, Jacques PF, Wilson PWF, Dinarello CA, Harris TB. Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: The Framingham Heart Study. American Journal of Medicine. 2003;115:429–435. doi: 10.1016/j.amjmed.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Bruunsgaard H, Andersen-Ranberg K, Hjelmborg JVB, Pedersen BK, Jeune B. Elevated levels of tumor necrosis factor alpha and mortality in centenarians. American Journal of Medicine. 2003;115:278–283. doi: 10.1016/s0002-9343(03)00329-2. [DOI] [PubMed] [Google Scholar]
- 10.Maggio M, Guralnik JM, Longo DL, Ferrucci L. Interleukin-6 in aging and chronic disease: A magnificent pathway. Journals of Gerontology Series a-Biological Sciences and Medical Sciences. 2006;61:575–584. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain, Behavior, and Immunity. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen HJ, Pieper CF, Harris T, Rao KM, Currie MS. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 1997;52:M201–208. doi: 10.1093/gerona/52a.4.m201. [DOI] [PubMed] [Google Scholar]
- 13.Lekander M, Elofsson S, Neve I-M, Hansson L-O, Unden A-L. Self-rated Health Is Related to Levels of Circulating Cytokines. Psychosomatic Medicine. 2004;66:559–563. doi: 10.1097/01.psy.0000130491.95823.94. [DOI] [PubMed] [Google Scholar]
- 14.Unden AL, Andreasson A, Elofsson S, Brismar K, Mathsson L, Ronnelid J, Lekander M. Inflammatory cytokines, behaviour and age as determinants of self-rated health in women. Clin Sci (Lond) 2007;112:363–373. doi: 10.1042/CS20060128. [DOI] [PubMed] [Google Scholar]
- 15.Janszky I, Lekander M, Blom M, Georgiades A, Ahnve S. Self-rated health and vital exhaustion, but not depression, is related to inflammation in women with coronary heart disease. Brain Behavior and Immunity. 2005;19:555–563. doi: 10.1016/j.bbi.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Dowd JB, Zajacova A. Does self-rated health mean the same thing across socioeconomic groups? Evidence from biomarker data. Annals of Epidemiology. 2010;20:743–749. doi: 10.1016/j.annepidem.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christian LM, Glaser R, Porter K, Malarkey WB, Beversdorf D, Kiecolt-Glaser JK. Poorer self-rated health is associated with elevated inflammatory markers among older adults. Psychoneuroendocrinology. 2011;36:1495–1504. doi: 10.1016/j.psyneuen.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanno K, Ohsawa M, Onoda T, Itai K, Sakata K, Tanaka F, Makita S, Nakamura M, Omama S, Ogasawara K, Ogawa A, Ishibashi Y, Kuribayashi T, Koyama T, Okayama A. Poor self-rated health is significantly associated with elevated C-reactive protein levels in women, but not in men, in the Japanese general population. Journal of Psychosomatic Research. 2012;73:225–231. doi: 10.1016/j.jpsychores.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Andreasson AN, Szulkin R, Unden AL, von Essen J, Nilsson LG, Lekander M. Inflammation and positive affect are associated with subjective health in women of the general population. J Health Psychol. 2012 doi: 10.1177/1359105311435428. [DOI] [PubMed] [Google Scholar]
- 20.Dizon-Townson DS. Preterm labour and delivery: a genetic predisposition. Paediatric and Perinatal Epidemiology. 2001;15:57–62. doi: 10.1046/j.1365-3016.2001.00008.x. [DOI] [PubMed] [Google Scholar]
- 21.Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin-6 in pre-term labor: association with infection. Journal of Clinical Investigation. 1990;85:1392–1400. doi: 10.1172/JCI114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romero R, Yoon BH, Mazor M, Gomez R, Diamond MP, Kenney JS, et al. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and Gram stain in patients with preterm labor and intact membranes. American Journal of Obstetrics and Gynecology. 1993;169:805–816. doi: 10.1016/0002-9378(93)90009-8. [DOI] [PubMed] [Google Scholar]
- 23.Romero R, Yoon BH, Mazor M, Gomez R, Gonzales R, Diamond MP. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and Gram stainn in the detection of microbial invasion in patients with preterm premature rupture of membranes. American Journal of Obstetrics and Gynecology. 1993;169:839–851. doi: 10.1016/0002-9378(93)90014-a. [DOI] [PubMed] [Google Scholar]
- 24.Murtha AP, Sinclair T, Hauser ER, Swamy GK, Herbert WN, Heine RP. Maternal serum cytokines in preterm premature rupture of membranes. Obstetrics and Gynecology. 2007;109:121–127. doi: 10.1097/01.AOG.0000250474.35369.12. [DOI] [PubMed] [Google Scholar]
- 25.Hagberg H, Mallard C, Jacobsson B. Role of cytokines in preterm labour and brain injury. British Journal of Obstetrics and Gynaecology. 2005;112:16–18. doi: 10.1111/j.1471-0528.2005.00578.x. [DOI] [PubMed] [Google Scholar]
- 26.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Sem. Fetal Neonatal Med. 2006;11:317–326. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. Prevalence of periodontitis in adults in the United States: 2009 and 2010. Journal of Dental Research. 2012;91:914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- 28.Boggess KA, Urlaub M, Moos MK, Polinkovsky M, El-Khorazaty J, Lorenz C. Knowledge and beliefs regarding oral health among pregnant women. Journal of the American Dental Association. 2011;142:1275–1282. doi: 10.14219/jada.archive.2011.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bates SB, Riedy CA. Changing knowledge and beliefs through an oral health pregnancy message. Jour al of Public Health Dentistry. 2012;72:104–111. doi: 10.1111/j.1752-7325.2011.00289.x. [DOI] [PubMed] [Google Scholar]
- 30.Anderson C, Harris MS, Kovarik R, Skelton J. Discovering expectant mothers' beliefs about oral health: an application of the Centering Pregnancy Smiles program. Int Q Community Health Educ. 2009;30:115–140. doi: 10.2190/IQ.30.2.c. [DOI] [PubMed] [Google Scholar]
- 31.Beck JD, Offenbacher S. Relationships among clinical measures of periodontal disease and their associations with systemic markers. Annals of Periodontology. 2002;7:79–89. doi: 10.1902/annals.2002.7.1.79. [DOI] [PubMed] [Google Scholar]
- 32.Ware JE, Sherbourne CD. The MOS 36-Item-Short-Form Health Survey (SF-36): I. Conceptual framework and item selection. Medical Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 33.Lobel M. The Revised Prenatal Life Events Scale (PLES) New York Stony Brook University; Stony Brook: 1996. [Google Scholar]
- 34.Lobel M, Cannella DL, Graham JE, DeVincent C, Schneider J, Meyer BA. Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health Psychology. 2008;27:604–615. doi: 10.1037/a0013242. [DOI] [PubMed] [Google Scholar]
- 35.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 36.Christian LM, Franco A, Glaser R, Iams J. Depressive symptoms are associated with elevated serum proinflammatory cytokines among pregnant women. Brain, Behavior, and Immunity. 2009;23:750–754. doi: 10.1016/j.bbi.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christian LM, Franco A, Iams JD, Sheridan J, Glaser R. Depressive symptoms predict exaggerated inflammatory response to in vivo immune challenge during human pregnancy. Brain, Behavior, and Immunity. 2010;24:49–53. doi: 10.1016/j.bbi.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li D, Liu L, Odouli R. Presence of depressive symptoms during early pregnancy and the risk of preterm delivery: a prospective cohort study. Human Reproduction. 2009;24:146–153. doi: 10.1093/humrep/den342. [DOI] [PubMed] [Google Scholar]
- 39.Phillips GS, Wise LA, Rich-Edwards JW, Stampfer MJ, Rosenberg L. Prepregnancy depressive symptoms and preterm birth in the Black Women's Health Study. Annals of Epidemiology. 2010;20:8–15. doi: 10.1016/j.annepidem.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orr ST, James SA. Blackmore Prince C: Maternal prenatal depressive symptoms and spontaneous preterm births among African-American women in Baltimore, Maryland. American Journal of Epidemiology. 2002;156:797–802. doi: 10.1093/aje/kwf131. [DOI] [PubMed] [Google Scholar]
- 41.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- 42.Wadhwa PD, Dunkel-Schetter C, Chicz-DeMet A, Porto M, Sandman CA. Prenatal psychosocial factors and the neuroendocrine axis in human pregnancy. Psychosomatic Medicine. 1996;58:432–446. doi: 10.1097/00006842-199609000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Hobel CJ, Dunkel-Schetter C, Roesch SC, Castro LC, Arora CP. Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks' gestation in pregnancies ending in preterm delivery. American Journal of Obstetrics and Gynecology. 1999;180:S257–263. doi: 10.1016/s0002-9378(99)70712-x. [DOI] [PubMed] [Google Scholar]
- 44.Spielberger CD. State-trait anxiety inventory : a comprehensive bibliography. 2nd Ed Consulting Psychologists Press; Palo Alto, CA (577 College Ave., Palo Alto 94306): 1989. [Google Scholar]
- 45.Marteau TM, Bekker H. The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI) British Journal of Clinical Psychology. 1992;31(Pt 3):301–306. doi: 10.1111/j.2044-8260.1992.tb00997.x. [DOI] [PubMed] [Google Scholar]
- 46.Meades R, Ayers S. Anxiety measures validated in perinatal populations: A systematic review. Journal of Affective Disorders. 2011;133:1–15. doi: 10.1016/j.jad.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 47.Lobel M. The revised prenatal distress questionnaire (NUPDQ) New York: State University of New York; Stony Brook: 1996. [Google Scholar]
- 48.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 49.Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc. Natl. Acad. Sci. USA. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glaser R, Robles T, Sheridan J, Malarkey WB, Kiecolt-Glaser JK. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses following influenza vaccination in older adults. Archives of General Psychiatry. 2003;60:1009–1014. doi: 10.1001/archpsyc.60.10.1009. [DOI] [PubMed] [Google Scholar]
- 51.Han B. Depressive symptoms and self-rated health in community-dwelling older adults: A longitudinal study. Journal of the American Geriatrics Society. 2002;50:1549–1556. doi: 10.1046/j.1532-5415.2002.50411.x. [DOI] [PubMed] [Google Scholar]
- 52.Mulsant BH, Ganguli M, Seaberg EC. The relationship between self-rated health and depressive symptoms in an epidemiological sample of community-dwelling older adults. Journal of the American Geriatrics Society. 1997;45:954–958. doi: 10.1111/j.1532-5415.1997.tb02966.x. [DOI] [PubMed] [Google Scholar]
- 53.Lancaster CA, Gold KJ, Flynn HA, Yoo H, Marcus SM, Davis MM. Risk factors for depressive symptoms during pregnancy: a systematic review (vol 202, pg 5, 2010) American Journal of Obstetrics and Gynecology. 2011;205:236–236. doi: 10.1016/j.ajog.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hale L, Hill TD, Burdette AM. Does sleep quality mediate the association between neighborhood disorder and self-rated physical health? Preventive Medicine. 2010;51:275–278. doi: 10.1016/j.ypmed.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 55.Magee CA, Caputi P, Iverson DC. Relationships between self-rated health, quality of life and sleep duration in middle aged and elderly Australians. Sleep Med. 2011;12:346–350. doi: 10.1016/j.sleep.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 56.Geiger SD, Sabanayagam C, Shankar A. The relationship between insufficient sleep and self-rated health in a nationally representative sample. J Environ Public Health. 2012;2012:518263. doi: 10.1155/2012/518263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shankar A, Charumathi S, Kalidindi S. Sleep duration and self-rated health: the national health interview survey 2008. Sleep. 2011;34:1173–1177. doi: 10.5665/SLEEP.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jain S, Bower J, Irwin MR. Psychoneuroimmunology of Fatigue and Sleep Disturbance: The Role of Pro-inflammatory Cytokines. The Oxford Handbook of Psychoneuroimmunology. 2012:321. [Google Scholar]
- 59.Okun ML, Hall M, Coussons-Read ME. Sleep disturbances increase interleukin-6 production during pregnancy: implications for pregnancy complications. Reprod Sci. 2007;14:560–567. doi: 10.1177/1933719107307647. [DOI] [PubMed] [Google Scholar]
- 60.Okun ML, Coussons-Read ME. Sleep disruption during pregnancy: how does it influence serum cytokines? Journal of Reproductive Immunology. 2007;73:158–165. doi: 10.1016/j.jri.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 61.Committee on Understanding Premature Birth and Assuring Healthy Outcomes . Preterm birth : causes, consequences, and prevention. National Academies Press; Washington, D.C.: 2007. [Google Scholar]
- 62.Monga M. Maternal Cardiovascular, Respiratory, and Renal Adapation to Pregnancy. In: Creasy RRRK, Iams JD, editors. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 6th ed Saunders Elsevier; Philadelphia, PA: 2009. [Google Scholar]
- 63.Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain, Behavior, and Immunity. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 64.Weatherall M. Prevention of falls and fall-related fractures in community-dwelling older adults: a meta-analysis of estimates of effectiveness based on recent guidelines. Intern Med J. 2004;34:102–108. doi: 10.1111/j.1444-0903.2004.t01-15-.x. [DOI] [PubMed] [Google Scholar]
- 65.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Executive summary: heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 66.Janz NK, Becker MH. The Health Belief Model: a decade later. Health Education Quarterly. 1984;11:1–47. doi: 10.1177/109019818401100101. [DOI] [PubMed] [Google Scholar]
- 67.Borrell LN, Crawford ND. Social disparities in periodontitis among United States adults 1999–2004. Community Dentistry and Oral Epidemiology. 2008;36:383–391. doi: 10.1111/j.1600-0528.2007.00406.x. [DOI] [PubMed] [Google Scholar]
- 68.Khader YS, Ta'ani Q. Periodontal diseases and the risk of preterm birth and low birth weight: A meta-analysis. Journal of Periodontology. 2005;76:161–165. doi: 10.1902/jop.2005.76.2.161. [DOI] [PubMed] [Google Scholar]
- 69.Offenbacher S, Beck JD, Jared HL, Mauriello SM, Mendoza LC, Couper DJ, Stewart DD, Murtha AP, Cochran DL, Dudley DJ, Reddy MS, Geurs NC, Hauth JC. Effects of periodontal therapy on rate of preterm delivery: a randomized controlled trial. Obstetrics and Gynecology. 2009;114:551–559. doi: 10.1097/AOG.0b013e3181b1341f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Michalowicz BS, Hodges JS, DiAngelis AJ, Lupo VR, Novak MJ, Ferguson JE, Buchanan W, Bofill J, Papapanou PN, Mitchell DA, Matseoane S, Tschida PA, Study O. Treatment of periodontal disease and the risk of preterm birth. New England Journal of Medicine. 2006;355:1885–1894. doi: 10.1056/NEJMoa062249. [DOI] [PubMed] [Google Scholar]
- 71.Macones GA, Parry S, Nelson DB, Strauss JF, Ludmir J, Cohen AW, Stamilio DM, Appleby D, Clothier B, Sammel MD. Jeffcoat M: Treatment of localized periodontal disease in pregnancy does not reduce the occurrence of preterm birth: results from the Periodontal Infections and Prematurity Study (PIPS) American Journal of Obstetrics and Gynecology. 2010;202:e1–8. doi: 10.1016/j.ajog.2009.10.892. [DOI] [PubMed] [Google Scholar]
- 72.Newnham JP, Newnham IA, Ball CM, Wright M, Pennell CE, Swain J, Doherty DA. Treatment of periodontal disease during pregnancy: a randomized controlled trial. Obstetrics and Gynecology. 2009;114:1239–1248. doi: 10.1097/AOG.0b013e3181c15b40. [DOI] [PubMed] [Google Scholar]
- 73.Chambrone L, Pannuti CM, Guglielmetti MR, Chambrone LA. Evidence grade associating periodontitis with preterm birth and/or low birth weight: II: a systematic review of randomized trials evaluating the effects of periodontal treatment. Journal of Clinical Periodontology. 2011;38:902–914. doi: 10.1111/j.1600-051X.2011.01761.x. [DOI] [PubMed] [Google Scholar]
- 74.Kim AJ, Lo AJ, Pullin DA, Thornton-Johnson DS, Karimbux NY. Scaling and Root Planing Treatment for Periodontitis to Reduce Preterm Birth and Low Birth Weight: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Periodontology. 2012 doi: 10.1902/jop.2012.110636. [DOI] [PubMed] [Google Scholar]
- 75.Polyzos NP, Polyzos IP, Mauri D, Tzioras S, Tsappi M, Cortinovis I, Casazza G. Effect of periodontal disease treatment during pregnancy on preterm birth incidence: a metaanalysis of randomized trials. American Journal of Obstetrics and Gynecology. 2009;200:225–232. doi: 10.1016/j.ajog.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 76.Xiong X, Buekens P, Goldenberg RL, Offenbacher S, Qian X. Optimal timing of periodontal disease treatment for prevention of adverse pregnancy outcomes: before or during pregnancy? American Journal of Obstetrics and Gynecology. 2011:205. doi: 10.1016/j.ajog.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 77.James NM, Smouse PE, Carroll BJ, Haines RF. Affective illness and HLA frequencies: no compelling association. Neuropsychobiology. 1980;6:208–216. doi: 10.1159/000117754. [DOI] [PubMed] [Google Scholar]
- 78.Miller GE, Rohleder N, Stetler C, Kirschbaum C. Clinical depression and regulation of the inflammatory response during acute stress. Psychosomatic Medicine. 2005;67:679–687. doi: 10.1097/01.psy.0000174172.82428.ce. [DOI] [PubMed] [Google Scholar]
- 79.Dye BA, Vargas CM, Lee JJ, Magder L, Tinanoff N. Assessing the relationship between children's oral health status and that of their mothers. Journal of the American Dental Association. 2011;142:173–183. doi: 10.14219/jada.archive.2011.0061. [DOI] [PubMed] [Google Scholar]
- 80.Centers for Disease Control . National Oral Health Serveillance System: Dental Visits. [accessed February 12, 2013]. from http://apps.nccd.cdc.gov/nohss. [Google Scholar]
- 81.Racial/ethnic disparities in self-rated health status among adults with and without disabilities--United States, 2004–2006. MMWR. Morbidity and Mortality Weekly Report. 2008;57:1069–1073. [PubMed] [Google Scholar]
- 82.Farmer MM, Ferraro KF. Are racial disparities in health conditional on socioeconomic status? Social Science and Medicine. 2005;60:191–204. doi: 10.1016/j.socscimed.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 83.Ostrove JM, Adler NE, Kuppermann M, Washington AE. Objective and subjective assessments of socioeconomic status and their relationship to self-rated health in an ethnically diverse sample of pregnant women. Health Psychology. 2000;19:613–618. doi: 10.1037//0278-6133.19.6.613. [DOI] [PubMed] [Google Scholar]
- 84.Frieden TR, et al. CDC Health Disparities and Inequalities Report - United States, 2011. MMWR. Surveillance Summaries. 2011;60(Suppl):1–2. [PubMed] [Google Scholar]
- 85.Manderbacka K, Kareholt I, Martikainen P, Lundberg O. The effect of point of reference on the association between self-rated health and mortality. Social Science and Medicine. 2003;56:1447–1452. doi: 10.1016/s0277-9536(02)00141-7. [DOI] [PubMed] [Google Scholar]
- 86.Flaster H, Bernhagen J, Calandra T, Bucala R. The macrophage migration inhibitory factor-glucocorticoid dyad: regulation of inflammation and immunity. Molecular Endocrinology. 2007;21:1267–1280. doi: 10.1210/me.2007-0065. [DOI] [PubMed] [Google Scholar]
- 87.Calandra T, Bucala R. Macrophage migration inhibitory factor: a counter-regulator of glucocorticoid action and critical mediator of septic shock. Journal of Inflammation. 1995;47:39–51. [PubMed] [Google Scholar]
- 88.Morand EF, Bucala R, Leech M. Macrophage migration inhibitory factor - An emerging therapeutic target in rheumatoid arthritis. Arthritis and Rheumatism. 2003;48:291–299. doi: 10.1002/art.10728. [DOI] [PubMed] [Google Scholar]
- 89.Burger-Kentischer A, Gobel H, Kleemann R, Zernecke A, Bucala R, Leng L, Finkelmeier D, Geiger G, Schaefer HE, Schober A, Weber C, Brunner H, Rutten H, Ihling C, Bernhagen J. Reduction of the aortic inflammatory response in spontaneous atherosclerosis by blockade of macrophage migration inhibitory factor (MIF) Atherosclerosis. 2006;184:28–38. doi: 10.1016/j.atherosclerosis.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 90.Denkinger CM, Denkinger M, Kort JJ, Metz C, Forsthuber TG. In vivo blockade of macrophage migration inhibitory factor ameliorates acute experimental autoimmune encephalomyelitis by impairing the homing of encephalitogenic T cells to the central nervous system. Journal of Immunology. 2003;170:1274–1282. doi: 10.4049/jimmunol.170.3.1274. [DOI] [PubMed] [Google Scholar]
- 91.Pearce BD. NARSAD's 16th annual scientific symposium. New York City: 2004. The role of a unique immunohormonal molecule (MIF) in depression during pregnancy. [Google Scholar]
- 92.Todros T, Bontempo S, Piccoli E, Ietta F, Romagnoli R, Biolcati M, Castellucci M, Paulesu L. Increased levels of macrophage migration inhibitory factor (MIF) in preeclampsia. European Journal of Obstetrics Gynecology and Reproductive Biology. 2005;123:162–166. doi: 10.1016/j.ejogrb.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 93.Pearce BD, Garvin SE, Grove J, Bonney EA, Dudley DJ, Schendel DE, Thorsen P. Serum macrophage migration inhibitory factor in the prediction of preterm delivery. American Journal of Obstetrics and Gynecology. 2008;199:46, e41–46. doi: 10.1016/j.ajog.2007.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yilmaz Ö , Küçük M, Kebapçilar L, Altindag T, Yüksel A, Yuvanç HO, Dal T, Savran Y. Macrophage migration-inhibitory factor is elevated in pregnant women with gestational diabetes mellitus. Gynecological Endocrinology. 2012;28:76–79. doi: 10.3109/09513590.2011.588757. [DOI] [PubMed] [Google Scholar]
- 95.Haas JS, Jackson RA, Fuentes-Afflick E, Stewart AL, Dean ML, Brawarsky P, Escobar GJ. Changes in the health status of women during and after pregnancy. Journal of General Internal Medicine. 2005;20:45–51. doi: 10.1111/j.1525-1497.2004.40097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Curry AE, Vogel I, Skogstrand K, Drews C, Schendel DE, Flanders WD, Hougaard DM, Thorsen P. Maternal plasma cytokines in early- and mid-gestation of normal human pregnancy and their association with maternal factors. Journal of Reproductive Immunology. 2008;77:152–160. doi: 10.1016/j.jri.2007.06.051. [DOI] [PubMed] [Google Scholar]
- 97.Vassiliadis S, Ranella A, Papadimitriou L, Makrygiannakis A, Athanassakis I. Serum levels of pro- and anti-inflammatory cytokines in non-pregnant women, during pregnancy, labour and abortion. Mediators of Inflammation. 1998;7:69–72. doi: 10.1080/09629359891199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Austgulen R, Lien E, Liabakk NB, Jacobsen G, Arntzen KJ. Increased Levels of Cytokines and Cytokine Activity Modifiers in Normal-Pregnancy. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 1994;57:149–155. doi: 10.1016/0028-2243(94)90291-7. [DOI] [PubMed] [Google Scholar]
- 99.Opsjon SL, Wathen NC, Tingulstad S, Wiedswang G, Sundan A, Waage A, Austgulen R. Tumor-Necrosis-Factor, Interleukin-1, and Interleukin-6 in Normal Human-Pregnancy. American Journal of Obstetrics and Gynecology. 1993;169:397–404. doi: 10.1016/0002-9378(93)90096-2. [DOI] [PubMed] [Google Scholar]
- 100.Makhseed M, Raghupathy R, Azizieh F, Farhat R, Hassan N, Bandar A. Circulating cytokines and CD30 in normal human pregnancy and recurrent spontaneous abortions. Human Reproduction. 2000;15:2011–2017. doi: 10.1093/humrep/15.9.2011. [DOI] [PubMed] [Google Scholar]
- 101.Szarka A, Rigo J, Jr, Lazar L, Beko G, Molvarec A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol. 2010;11:59. doi: 10.1186/1471-2172-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Molvarec A, Szarka A, Walentin S, Beko G, Karádi I, Prohászka Z, Rigó J, Knez K, Zorn B, Tomazevic T. Serum leptin levels in relation to circulating cytokines, chemokines, adhesion molecules and angiogenic factors in normal pregnancy and preeclampsia. Reprod Biol Endocrinol. 2011;9:124. doi: 10.1186/1477-7827-9-124. [DOI] [PMC free article] [PubMed] [Google Scholar]