Abstract
HIV infection and HIV neurocognitive impairment are major global health problems. The prevalence of HIV associated neurocognitive disorders (HAND) is increasing as people with HIV are living longer due to the success of antiretroviral therapies. Our laboratory identified PrPc, the cellular non-pathogenic isoform of the prion protein, as a biomarker of HAND. In this review we discuss the published data addressing PrPc biology in normal conditions and pathologies, as well as the mechanisms of PrPc shedding and secretion. Lastly, we discuss our studies that demonstrated that PrPc is a biomarker of neurocognitive impairment in the HIV infected population.
Introduction
Human immunodeficiency virus-1, HIV, affects approximately 34 million people worldwide and continues to be a global health crisis (UNAIDS: Report on the Global AIDS Epidemic 2011, Geneva, World Health Organization). Despite successful antiretroviral therapy (ART), it is estimated that greater than 50 % of HIV infected people have HIV associated neurocognitive disorders (HAND) (Antinori et al., 2007; Heaton et al., 2010; Heaton et al., 2011). HAND is becoming more prevalent, with milder types of impairments being most frequent, as HIV infected people are living longer with the use of antiretrovirals (Nath et al., 2008; Cysique et al., 2009).
HIV enters the central nervous system (CNS) early during primary infection, predominantly transmitted by HIV-infected monocytes that constitutively cross the blood-brain barrier (BBB) (Koenig et al., 1986; Valcour et al., 2011). The transmigration of HIV-infected CD14+ CD16+ monocytes introduces virus into the CNS and causes the release of neuroinflammatory mediators including the chemokine CCL2/monocyte chemoattractant protein-1. CCL2, which is elevated in the CNS and cerebrospinal fluid (CSF) of individuals with HAND (Conant et al., 1998) and is an important mediator of HIV-associated CNS inflammation and neuropathology, induces transmigration of additional monocytes across the BBB (Williams et al., 2012b). HIV infected monocytes that cross the BBB differentiate into perivascular macrophages and become viral reservoirs, infecting resident macrophages, microglia, and to lesser extent astrocytes (Eugenin and Berman, 2003; Buckner et al., 2006; Eugenin et al., 2006a; Williams et al., 2012a). Infected and activated phagocytes and glia produce additional inflammatory and cytotoxic factors that cause BBB dysfunction and neuronal injury (Letendre, 2011).
HIV-associated neurocognitive impairment impacts the functional capacity and quality of life of an individual (Albert et al., 1995; Heaton et al., 2010; Scott et al., 2011). Predicting which individuals will develop HAND and identifying those with subtle impairment is difficult and presents a therapeutic challenge. Neurocognitive testing may not accurately reflect the dynamic nature of the disorder, as individuals with HAND frequently have fluctuating neurocognitive deficits. Furthermore, education, cultural background, local norms, and other environmental factors may affect the results of neurocognitive testing (Ances and Clifford, 2008). Thus, identification of a biomarker that is specific for HIV-associated CNS disease will facilitate the identification of individuals at risk of developing neurocognitive impairment. Biomarkers may also guide therapy and provide direction for the initiation of ART.
Our laboratory demonstrated that the soluble form of the cellular prion protein (sPrPc) is a potential biomarker of HAND. PrPc is the nonpathogenic cellular isoform of the human prion protein and is constitutively expressed in the CNS by neurons, astrocytes, brain microvascular endothelial cells (BMVEC), monocytes, macrophages, and microglia (Fournier et al., 2000; Brown, 2004; Viegas et al., 2006; Roberts et al., 2010b). We found that PrPc is increased in brain tissue of macaques with SIV (simian immunodeficiency virus) encephalitis and in humans with HAND, but not in HIV-infected individuals without neurocognitive impairment. Soluble PrPc levels in the CSF of those with HAND were also significantly elevated when compared with sPrPc levels in the CSF of HIV-infected people without cognitive impairment (Roberts et al., 2010b). These results suggest that CSF sPrPc may predict HIV-mediated CNS dysfunction and HAND.
PrP can be found in two isoforms. One is the normal non-pathogenic cellular isoform discussed above and that is the subject of this review, and the other is the scrapie isoform (PrPsc). PrPsc has the same amino acid sequence as PrPc, but a higher content of β-sheet structures that makes it resistant to proteases. This infectious form of prion protein mediates the pathogenesis of neurodegenerative diseases termed transmissible spongiform encephalopathies (TSEs) (Prusiner, 1998). In our studies, it is important to note that we are not examining PrPsc.
Structure of Cellular Prion protein
Cellular prion protein is a GPI-anchored glycoprotein located on the plasma membrane in specialized lipid raft microdomains (Brügger et al., 2004). While most PrPc is found at the plasma membrane, the protein can also localize to the cytoplasm after clathrin-mediated endocytosis (Shyng et al., 1993; Shyng et al., 1994; Sarnataro et al., 2009) and can be secreted into the extracellular environment (Roberts et al., 2010b). This 27–30 kDa protein is encoded by the PRPN gene on human chromosome 20 (Liao et al., 1986) and contains two exons with an open reading frame found in the second exon (Puckett et al., 1991).
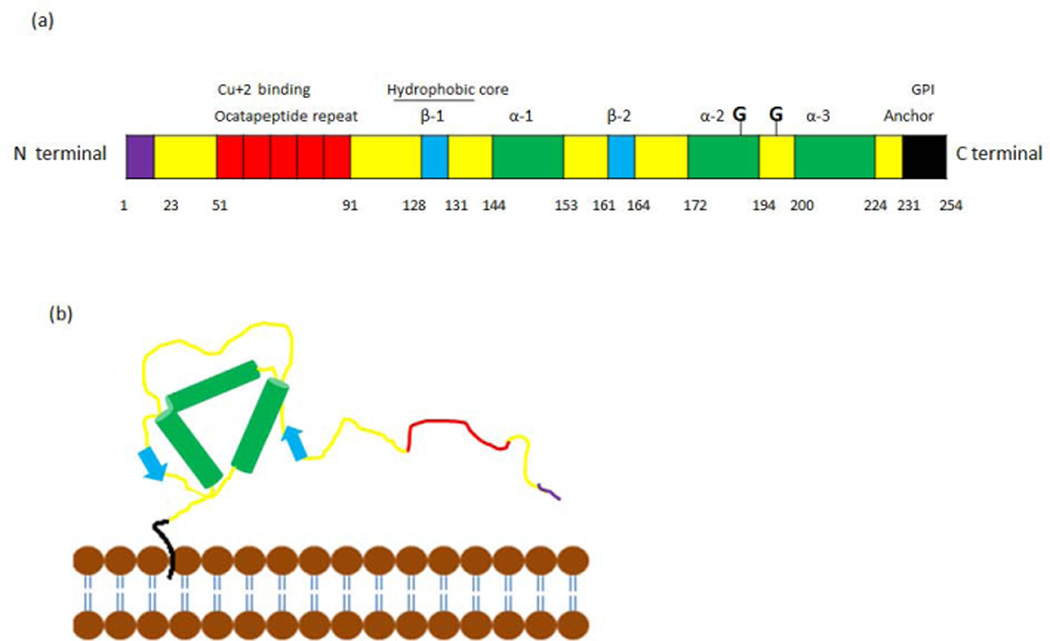
The three dimensional structure of PrPc (Figure 1a) consists of a flexible N-terminal region containing a copper-binding octapeptide repeat (residues 51–90) (Stöckel et al., 1998), a neurotoxic region, and a hydrophobic region (residues 112–130) (Figure 1b). The C-terminal region of the protein (Figure 1b) is a globular structure composed of three α-helices (amino acids 144–153, 172–94 and 200–224), two β strands (amino acids 128–131 and 161–164), and a GPI anchor that attaches the protein to the plasma membrane (Riek et al., 1997; Zahn et al., 2000). During the biosynthesis of PrPc in the endoplasmic reticulum, the N-terminal signal sequence (residue 1–22) is removed, the GPI anchor is attached to the C-terminus, and N-linked oligosaccharides are added (Stahl et al., 1987; Borchelt et al., 1993). Human PrPc is found unglycosylated, monoglycosylated, or diglycosylated at amino acids Asn 181 and Asn 197, and may have 60 different sugars attached to it, enabling binding to a broad range of partners (Haraguchi et al., 1989). PrPc can also homodimerize, which facilitates the trafficking of PrPc to the plasma membrane and confers protective properties to PrPc during stress (Béland et al., 2012).
Figure 1.
Schematic representation of the structure of PrPc a) linear representation of the primary sequence of prion protein. b) PrPc is attached in the plasma membrane through the GPI anchor.
Physiological Functions of PrPc
In the CNS, PrPc has diverse biological functions that include cellular adhesion, cytoprotection, and neuroinflammation. However, most studies of PrPc are in animal models and there are fewer reports examining the role of PrPc in human cells or within the human CNS. Studies in zebra fish embryonic cell cultures showed that PrPc mediates homophilic cell adhesion and that down regulation of PrPc results in decreased morphogenetic movement which eventually leads to developmental arrest. It was also shown that PrPc modulates cell adhesion by recruiting E-cadherin to the plasma membrane (Málaga-Trillo et al., 2009). The homophilic interactions of PrPc on monocytes and on endothelial cells were shown to be essential for the migration of monocytes across an endothelial monolayer (Viegas et al., 2006). PrPc recruits neural cell adhesion molecule (NCAM) into lipid rafts and interacts with it, promoting cell adhesion and neurite outgrowth by activating Fyn kinase (Schmitt-Ulms et al., 2001; Santuccione et al., 2005).
Glutamate induced calcium flux, which is the result of aberrant activation of the NMDA receptor, causes neuronal damage and death by excitotoxicity (Gillessen et al., 2000). PrPc interacts directly with NMDA receptors to reduce glutamate mediated excitotoxicity. This was demonstrated in neurons from PrPc null mice that had a greater cytotoxic response to NMDA receptor stimulation as compared to wild type neurons (Khosravani et al., 2008). In another study, spontaneous current activity and excitotoxicity caused by a mutated form of prion protein with the central region (amino acids 105–125) deleted was suppressed by over expression of WT PrPc (Biasini et al., 2013). In astrocytes, PrPc increased glutamate uptake that diminished excitotoxic neuronal death (Brown and Mohn, 1999; Brown, 2004). Another study demonstrated that PrPc increased uptake of synaptic zinc in neurons through AMPA receptors. Reduced zinc uptake by neurons has been implicated in neurodegenerative diseases and the involvement of PrPc in this process demonstrates the neuroprotective function of the protein (Watt et al., 2012). In addition, overexpression of PrPc protected from apoptotic stimuli such as serum deprivation (Kim et al., 2004), or Bax dependent apoptotic death (Bounhar et al., 2001; Roucou et al., 2003). A recent study also showed that stress inducible protein-1(STI1) and Laminin-γ1 induced increases in axonogenesis in wild type neurons, while PrPc null neurons showed no change, demonstrating that PrPc is involved in axonal differentiation and growth (Santos et al., 2013).
The shed form of PrPc, soluble PrPc (sPrPc), also has many biological functions including increasing the survival of wild type and PrPc null neurons (Lima et al., 2007). Through its interaction with the secreted cytoplasmic co-chaperone molecule, STI1 and neural cell adhesion molecule (NCAM), sPrPc propagates survival signals increasing axonal differentiation, neurite outgrowth and synaptic development (Kanaani et al., 2005; Santuccione et al., 2005).
Although PrPc is neuroprotective, it is also involved in neuroinflammatory processes. A study using blocking PrPc antibodies showed that it is essential for monocyte migration across an endothelial monolayer (Viegas et al., 2006). PrPc is also involved in macrophage phagocytosis, cellular taxis (de Almeida et al., 2005) and microglia activation (Brown et al., 1998). These processes are dysregulated during HIV infection and microglia activation, taxis and aggregation are important features of HIV infection of the CNS (Visentin et al., 2001). In addition, our laboratory showed that sPrPc induces production of the inflammatory mediators CCL2 and IL-6 from human astrocytes (Roberts et al., 2010b).
We previously demonstrated that CCL2 is neuroprotective against NMDA or HIV-tat-induced neuronal apoptosis (Eugenin et al., 2003; Eugenin et al., 2007). Thus, molecules considered inflammatory and/or pathogenic depending upon their expression pattern can also be neuroprotective (Eugenin et al., 2003; Eugenin et al., 2007). We propose that PrPc has a similar dual role in the pathogenesis of HIV infection, one in neuronal/glial survival and another that results in CNS inflammation and compromise.
The duality of the neuron damaging and neuroprotective property of PrPc is further demonstrated in CNS amyloid disease. Studies showed that PrPc binds with high affinity to Aβ oligomers which are cleavage products of amyloid precursor protein (APP) involved in Alzheimer disease, suggesting that PrPc may be a receptor mediating impairment of synaptic plasticity by the oligomers (Laurén et al., 2009). In contrast, sPrPc was shown to prevent the aggregation of amyloid β fibrils and to inhibit the formation of spherical oligomers and their toxic effect on neurons (Nieznanski et al., 2012) therefore suggesting a protective role. However, others reported that PrPc did not have a role in Aβ oligomers synaptoxocity and that the absence of PrPc did not prevent the blocking of long term potentiation (Balducci et al., 2010; Calella et al., 2010; Kessels et al., 2010), and learning and memory deficits (Cissé et al., 2011) caused by Aβ oligomers. Therefore, the function of PrPc in Alzheimer’s disease is still not well understood.
Mechanisms of PrPc release from the cell membrane
PrPc is released from cells through endoproteolytic processing, exosomal release, and cleavage from the GPI anchor. The endoprotelytic processing of prion protein occurs through α-cleavage, β-cleavage, and ectodomain shedding. Alpha cleavage, which takes place between Lys110/His111 or His111/Met112, gives rise to an 11 kDa N1-fragment and an 18 kDa C1-fragment, destroying the neurotoxic region which is associated with fibril formation (Vincent et al., 2000; Vincent et al., 2001; Mangé et al., 2004). Several proteases including members of the A disintegrin and metalloproteinase family, ADAM 10 and ADAM 17, and TNF-α converting enzyme, TACE were thought to mediate α-cleavage of PrPc (Vincent et al., 2000; Vincent et al., 2001). However, recent studies have argued against the involvement of these proteases in α-cleavage of PrPc. ADAM10 knock-out mice did not have a significant decrease in α-cleavage (Altmeppen et al., 2011) and transgenic mice with neurons that over expressed ADAM 10 did not show increased PrPc cleavage products (Endres et al., 2009). βeta cleavage of PrPc occurs in response to oxidative stress and occurs near the octapeptide region (McMahon et al., 2001).
PrPc can also be cleaved near the GPI anchor, releasing an almost full-length protein lacking any GPI modification, through protease-mediated shedding (Borchelt et al., 1993). The cleavage occurs between Gly228 and Arg229, three amino acids away from the GPI anchor (Zhao et al., 2006). Cell culture experiments identified ADAM 10 as the sheddase of PrPc (Taylor et al., 2009) and demonstrated that ADAM 9 indirectly regulates the sheddase activity of ADAM 10 (Tousseyn et al., 2009; Moss et al., 2011).
PrPc is transported from the plasma membrane to endosomes and is recycled back to the cell surface (Shyng et al., 1993; Shyng et al., 1994) or stored within intraluminal vesicles in multivesicular bodies (Laine et al., 2001; Peters et al., 2003). PrPc can be transferred from late endosomes, to lysosomes for degradation (Peters et al., 2003) or released into the extracellular space on 50–90 nm lipid vesicles known as exosomes, with the GPI anchor still attached to the membrane surface (Vella et al., 2007; Vella et al., 2008). Multiple CNS cells including neurons, macrophages, endothelial cells, and leukocytes release exosomes, and exosomes have been detected in human plasma and CSF (Caby et al., 2005; Vella et al., 2008). This suggests that exosomal microparticles are an important mechanism for PrPc shedding and likely promote sPrPc-mediated signaling during HIV infection of CNS.
PrPc as a biomarker for HAND
HIV infection of the CNS leads to cytotoxic and protective responses that mediate the dynamic processes of HIV CNS pathogenesis. Therefore, the severity of an individual’s neurocognitive impairment is likely to change during the course of the disease (Antinori et al., 2007). For this reason, indicators of neurologic dysfunction prior to the onset of clinical symptoms are important for identifying individuals who are likely to develop cognitive impairment with the goal of enabling long term monitoring of their CNS functions.
PrPc is an adhesion and signaling molecule involved in several processes including transmigration of leukocytes across the endothelium (Roberts et al., 2010a). The process of transmigration is disrupted during HIV infection of the CNS (Eugenin et al., 2006c; Eugenin et al., 2006b; Roberts et al., 2010a; Roberts et al., 2010b; Williams et al., 2012b). Thus, we proposed that dysregulation of adhesion molecules, including PrPc can result in increased monocyte influx into the CNS and damage to the BBB. We found that CSF sPrPc was elevated in HIV infected people with neurocognitive disorders as compared to uninfected people and HIV infected individuals with no cognitive impairment (Roberts et al., 2010a; Roberts et al., 2010b). The significant increase of sPrPc in individuals with HAND was independent of several factors including viral load or CD4+T cells but correlated with increased CSF CCL2. These data indicate that increased sPrPc was not a consequence of immune suppression but rather was mediated by HIV infection and CCL2, demonstrating the importance of this chemokine in PrPc release. Elevated CCL2 level in the CSF of humans is an indicator of neurocognitive impairment (Conant et al., 1998), while in pigtail macaques it is predictive of severity of encephalitis (Zink et al., 2001). Our in vitro studies examining the effect of CCL2 on BMVEC, neurons and astrocytes also showed that this chemokine induced increased PrPc release between 30 min to 24 hrs (Roberts et al., 2010b). Therefore we propose that high CCL2 levels together with HIV infection induce shedding of PrPc in the CSF as one of the pathogenic processes leading to HAND.
Our findings suggest that astrocytes, BMVEC and neurons are the main source of sPrPc in HIV infected individuals with HAND. In addition, HIV infected peripheral blood monocytes showed increased release of PrPc followed by a sudden decrease 4 days post infection which was maintained for up to 7 days, the last time point assayed (Roberts et al., 2010b). We suggest that this shed PrPc may facilitate monocyte entry into the CNS by dysregulating the normal homotypic PrPc - PrPc interactions that mediate baseline transmigration for surveillance.
To study the release of PrPc during the course of HIV CNS pathogenesis, the pigtail macaque model of NeuroAIDS, in which 90% of animals develop SIV encephalitis (SIVE) within 3 months of infection (Clements et al., 2008), was used. CSF samples from different stages of SIV infection showed that animals that developed severe encephalitis had elevated sPrPc levels during early and late stages of infection as compared to uninfected animals and animals with mild SIVE. As these stages are characterized by elevated CCL2 in the CNS, thus, supporting our proposal that CCL2 and/HIV infection regulate sPrPc release.
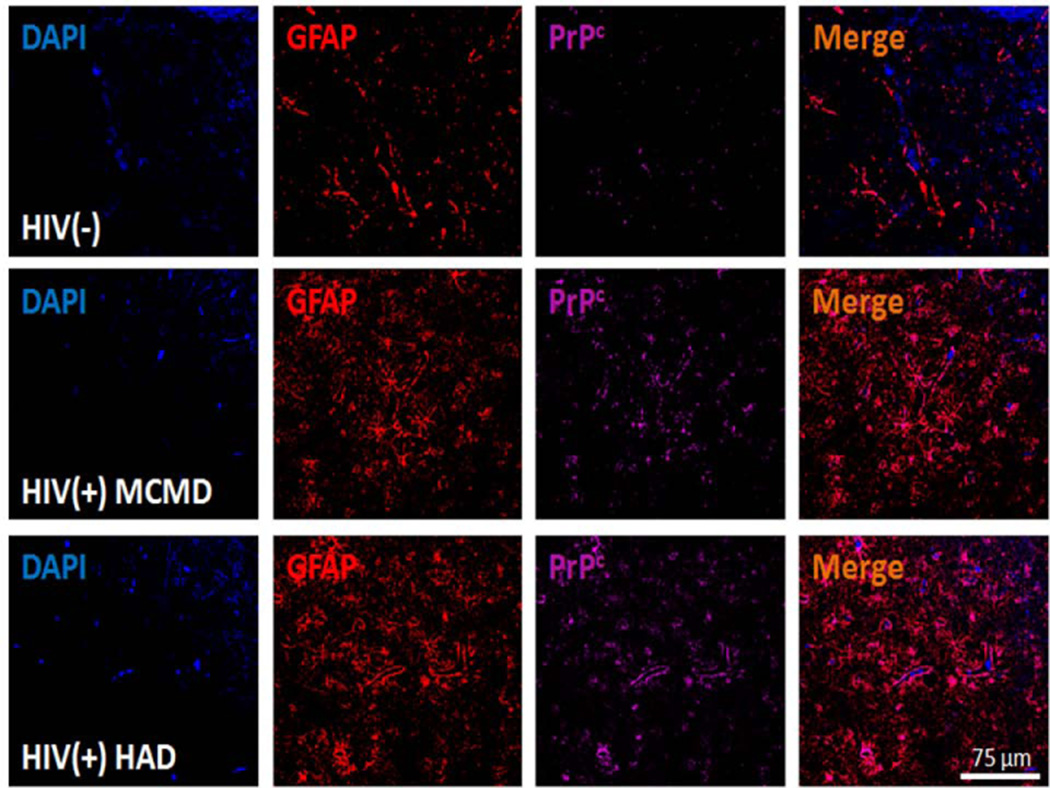
Our previously published immunostaining and confocal microscopy studies showed that PrPc was selectively increased in the brain tissue of HIV infected people with HAND as compared to uninfected people and HIV infected people with no cognitive impairment. Neuronal and astrocytic PrPc was increased in individuals with both minor motor cognitive disorders (MCMD) and HIV encephalitis (HIVE) (Roberts et al., 2010a; Roberts et al., 2010b). Astrocytes in individuals with MCMD had morphologic changes including hypertrophy, proliferation and extensive process formation along with elevated PrPc expression (Roberts et al., 2010a; Roberts et al., 2010b). An example of this increased PrPc in the brain tissue of individuals with HAND is shown in Figure 2. Astrocytes from HIV (−) people had minimal PrPc staining, while tissue from individuals with MCMD or HAD had highly increased PrPc.
Figure 2.
PrPc is increased significantly in the brain of individuals with HIV-1-associated neurocognitive impairment. Brain tissue sections from uninfected (HIV (−)), HIV infected with minor cognitive mild disorders (HIV(+) MCMD) and HIV infected with dementia (HIV (+) HAD) individuals were evaluated by immunohistochemistry using confocal microscopy. Astrocyte specific expression of PrPC is demonstrated by GFAP (Cy3-red; astrocytic marker), and PrPC (FITC-Cy5, cyan) co-localization indicates the presence of this protein in areas close to the BBB. PrPC expression was elevated in MCMD and HAD individuals with HIV infection as compared to uninfected individuals.
Our studies identified CSF sPrPc as a potential biomarker for HAND. We are expanding upon these finding using CSF samples from a large cohort of HIV infected people with and without cognitive impairment. If validated with this larger cohort, we propose that sPrPc will provide a new tool to diagnose and manage CNS disease in HIV infected individuals. CSF biomarkers are useful because the CSF reflects events occurring in the CNS. The correlation of HAND and elevated PrPc was not observed in sera samples where sPrPc levels were significantly decreased relative to uninfected individuals. In addition, in the sera samples there was no significant difference between sPrPc levels in HIV infected people with or without cognitive impairment (Roberts et al., 2010b).
Is PrPc a biomarker for other neurocognitive disorders?
Although altered PrPc levels have been described in several neurocognitive disorders, they do not appear to be a biomarker of cognitive impairment. Individuals with Alzheimer’s disease have upregulated PrPc in several regions of the brain (Voigtländer et al., 2001; Rezaie et al., 2005). However, levels of sPrPc in the CSF of people with Alzheimer’s disease were lower and did not correlate with brain PrPc expression or with cognitive impairment. In people with Lewy body dementia (Rezaie et al., 2005) and in those with Creutzfeldt-Jakob disease (CJD) (Torres et al., 2012), CSF PrPc was lower than in individuals with no neurocognitive disorders. CSF sPrPc was also decreased in people with other neurocognitive dysfunctions and neuroinflammatory diseases such as Parkinson’s disease and multiple sclerosis (Meyne et al., 2009). Although CCL2 plays a role in the pathogenesis of multiple sclerosis (Mahad and Ransohoff, 2003), there was no increase in shed PrPc in this disorder, underscoring the importance of HIV infection in concert with elevated CCL2 in mediating the shedding of PrPc. These findings indicate that increased CNS PrPc is not a general indicator of dementia and that elevation of both CNS PrPc and CSF sPrPc occurs specifically during CNS HIV infection in the presence of CCL2. A study demonstrating that PrPc expression in the brain is increased after acute stroke but not acute ischemia also supports the fact that PrPc is not correlative with general neuroinflammation and neuronal injury.
Conclusion
Despite the success of ART, the prevalence of HIV associated neurocognitive disorders continues to increase as HIV infected individuals live longer. Although the mechanisms that mediate HAND are not fully characterized, HIV infected CD14+ CD16+ monocytes that transmigrate across the BBB initiate CNS infection and the subsequent neuroinflammation that mediates HAND. HIV infected monocytes cross the BBB through homophilic interactions between the junctional proteins on the surface of EC and on monocytes. PrPc is one adhesion and signaling molecule that is essential for monocyte transmigration. This protein is involved not only in neuroinflammatory but also in cytoprotective processes such as neurite outgrowth, regulation of ionic current and protection against apoptosis. Studies showed that PrPc is involved in several neurocognitive disorders including Alzheimer’s disease. However, the role of PrPc in HAND had not been previously examined. Our data showed that PrPc is increased in the brain of individuals with HAND as compared to uninfected individuals or HIV infected people with no cognitive impairments. In addition, we demonstrated that in HIV infected individuals, sPrPc in the CSF is a potential biomarker. Therefore we propose that HIV infection of the CNS and its associated inflammation mediated, at least in part, by increased CCL2 altering PrPc expression and release, resulting in ongoing neuroinflammation and subsequent cognitive impairment. PrPc as a biomarker for HAND would (1) identify individuals who are at risk of developing cognitive impairment, (2) inform management decisions regarding initiation of therapy and (3) monitor the efficacy of treatment of individuals with HAND.
Acknowledgements
We thank the Fetal Tissue Repository and the NIH Center for AIDS Research grant AI-051519 at the Albert Einstein College of Medicine. This work was supported by the National Institutes of Health grants, MH090958 (BWM, JWB), MH0756799, DA025567 (JWB), MH096625 and MH076679 (EAE), and by AI071326 pre-doctoral fellowship (TKR). The authors had no financial interest. Human tissues were supplied by the Manhattan HIV Brain Bank (U01MH083501), member of the National NeuroAIDS Tissue Consortium.
References
- Albert SM, Marder K, Dooneief G, Bell K, Sano M, Todak G, Stern Y. Neuropsychologic impairment in early HIV infection: a risk factor for work disability. Archives of Neurology. 1995;52:525. doi: 10.1001/archneur.1995.00540290115027. [DOI] [PubMed] [Google Scholar]
- Altmeppen HC, Prox J, Puig B, Kluth MA, Bernreuther C, Thurm D, Jorissen E, Petrowitz B, Bartsch U, De Strooper B. Lack of a-disintegrin-and-metalloproteinase ADAM10 leads to intracellular accumulation and loss of shedding of the cellular prion protein in vivo. Mol Neurodegener. 2011;6:25. doi: 10.1186/1750-1326-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances BM, Clifford DB. HIV-associated neurocognitive disorders and the impact of combination antiretroviral therapies. Current neurology and neuroscience reports. 2008;8:455–461. doi: 10.1007/s11910-008-0073-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balducci C, Beeg M, Stravalaci M, Bastone A, Sclip A, Biasini E, Tapella L, Colombo L, Manzoni C, Borsello T. Synthetic amyloid-β oligomers impair long-term memory independently of cellular prion protein. Proceedings of the National Academy of Sciences. 2010;107:2295–2300. doi: 10.1073/pnas.0911829107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béland M, Motard J, Barbarin A, Roucou X. PrPC Homodimerization Stimulates the Production of PrPC Cleaved Fragments PrPN1 and PrPC1. The Journal of Neuroscience. 2012;32:13255–13263. doi: 10.1523/JNEUROSCI.2236-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasini E, Unterberger U, Solomon IH, Massignan T, Senatore A, Bian H, Voigtlaender T, Bowman FP, Bonetto V, Chiesa R. A Mutant Prion Protein Sensitizes Neurons to Glutamate-Induced Excitotoxicity. The Journal of Neuroscience. 2013;33:2408–2418. doi: 10.1523/JNEUROSCI.3406-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchelt DR, Rogers M, Stahl N, Telling G, Prusiner SB. Release of the cellular prion protein from cultured cells after loss of its glycoinositol phospholipid anchor. Glycobiology. 1993;3:319–329. doi: 10.1093/glycob/3.4.319. [DOI] [PubMed] [Google Scholar]
- Bounhar Y, Zhang Y, Goodyer CG, LeBlanc A. Prion protein protects human neurons against Bax-mediated apoptosis. Science Signalling. 2001;276:39145. doi: 10.1074/jbc.C100443200. [DOI] [PubMed] [Google Scholar]
- Brown DR. Role of the prion protein in copper turnover in astrocytes. Neurobiology of disease. 2004;15:534–543. doi: 10.1016/j.nbd.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Brown DR, Mohn CM. Astrocytic glutamate uptake and prion protein expression. Glia. 1999;25:282–292. doi: 10.1002/(sici)1098-1136(19990201)25:3<282::aid-glia8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Brown DR, Schmidt B, Kretzschmar HA. A prion protein fragment primes type 1 astrocytes to proliferation signals from microglia. Neurobiology of disease. 1998;4:410–422. doi: 10.1006/nbdi.1998.0169. [DOI] [PubMed] [Google Scholar]
- Brügger B, Graham C, Leibrecht I, Mombelli E, Jen A, Wieland F, Morris R. The membrane domains occupied by glycosylphosphatidylinositol-anchored prion protein and Thy-1 differ in lipid composition. Journal of Biological Chemistry. 2004;279:7530–7536. doi: 10.1074/jbc.M310207200. [DOI] [PubMed] [Google Scholar]
- Buckner CM, Luers AJ, Calderon TM, Eugenin EA, Berman JW. Neuroimmunity and the blood-brain barrier: molecular regulation of leukocyte transmigration and viral entry into the nervous system with a focus on neuroAIDS. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2006;1:160–181. doi: 10.1007/s11481-006-9017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caby M-P, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. International immunology. 2005;17:879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- Calella AM, Farinelli M, Nuvolone M, Mirante O, Moos R, Falsig J, Mansuy IM, Aguzzi A. Prion protein and Aβ-related synaptic toxicity impairment. EMBO molecular medicine. 2010;2:306–314. doi: 10.1002/emmm.201000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cissé M, Sanchez PE, Kim DH, Ho K, Yu G-Q, Mucke L. Ablation of cellular prion protein does not ameliorate abnormal neural network activity or cognitive dysfunction in the J20 line of human amyloid precursor protein transgenic mice. The Journal of Neuroscience. 2011;31:10427–10431. doi: 10.1523/JNEUROSCI.1459-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JE, Mankowski JL, Gama L, Zink MC. The accelerated simian immunodeficiency virus macaque model of human immunodeficiency virus-associated neurological disease: from mechanism to treatment. Journal of neurovirology. 2008;14:309–317. doi: 10.1080/13550280802132832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, Gallo RC, Major EO. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proceedings of the National Academy of Sciences. 1998;95:3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Vaida F, Letendre S, Gibson S, Cherner M, Woods SP, McCutchan JA, Heaton RK, Ellis RJ. Dynamics of cognitive change in impaired HIV-positive patients initiating antiretroviral therapy. Neurology. 2009;73:342–348. doi: 10.1212/WNL.0b013e3181ab2b3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida CJG, Chiarini LB, da Silva JP, e Silva PMR, Martins MA, Linden R. The cellular prion protein modulates phagocytosis and inflammatory response. Journal of leukocyte biology. 2005;77:238–246. doi: 10.1189/jlb.1103531. [DOI] [PubMed] [Google Scholar]
- Endres K, Mitteregger G, Kojro E, Kretzschmar H, Fahrenholz F. Influence of ADAM10 on prion protein processing and scrapie infectiosity <i>in vivo</i>. Neurobiology of disease. 2009;36:233–241. doi: 10.1016/j.nbd.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, Berman JW. Chemokine-dependent mechanisms of leukocyte trafficking across a model of the blood-brain barrier. Methods. 2003;29:351–361. doi: 10.1016/s1046-2023(02)00359-6. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, D'Aversa TG, Lopez L, Calderon TM, Berman JW. MCP-1 (CCL2) protects human neurons and astrocytes from NMDA or HIV-tat-induced apoptosis. Journal of neurochemistry. 2003;85:1299–1311. doi: 10.1046/j.1471-4159.2003.01775.x. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci. 2006a;26:1098–1106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006b;26:1098–1106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, King JE, Nath A, Calderon TM, Zukin RS, Bennett MV, Berman JW. HIV-tat induces formation of an LRP-PSD-95-NMDAR-nNOS complex that promotes apoptosis in neurons and astrocytes. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3438–3443. doi: 10.1073/pnas.0611699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, Gamss R, Buckner C, Buono D, Klein RS, Schoenbaum EE, Calderon TM, Berman JW. Shedding of PECAM-1 during HIV infection: a potential role for soluble PECAM-1 in the pathogenesis of NeuroAIDS. Journal of leukocyte biology. 2006c;79:444–452. doi: 10.1189/jlb.0405215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier JG, Escaig-Haye F, Grigoriev V. Ultrastructural localization of prion proteins: physiological and pathological implications. Microscopy research and technique. 2000;50:76–88. doi: 10.1002/1097-0029(20000701)50:1<76::AID-JEMT11>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Gillessen T, Budd SL, Lipton SA. Excitatory amino acid neurotoxicity. 2000 doi: 10.1007/978-1-4615-0123-7_1. [DOI] [PubMed] [Google Scholar]
- Haraguchi T, Fisher S, Olofsson S, Endo T, Groth D, Tarentino A, Borchelt DR, Teplow D, Hood L, Burlingame A. Asparagine-linked glycosylation of the scrapie and cellular prion proteins. Archives of Biochemistry and Biophysics. 1989;274:1–13. doi: 10.1016/0003-9861(89)90409-8. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. Journal of neurovirology. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaani J, Prusiner SB, Diacovo J, Baekkeskov S, Legname G. Recombinant prion protein induces rapid polarization and development of synapses in embryonic rat hippocampal neurons in vitro. Journal of neurochemistry. 2005;95:1373–1386. doi: 10.1111/j.1471-4159.2005.03469.x. [DOI] [PubMed] [Google Scholar]
- Kessels HW, Nguyen LN, Nabavi S, Malinow R. The prion protein as a receptor for amyloid-[bgr] Nature. 2010;466:E3–E4. doi: 10.1038/nature09217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravani H, Zhang Y, Tsutsui S, Hameed S, Altier C, Hamid J, Chen L, Villemaire M, Ali Z, Jirik FR. Prion protein attenuates excitotoxicity by inhibiting NMDA receptors. Science Signalling. 2008;181:551. doi: 10.1083/jcb.200711002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B-H, Lee H-G, Choi J-K, Kim J-I, Choi E-K, Carp RI, Kim Y-S. The cellular prion protein (PrP<sup>C</sup>) prevents apoptotic neuronal cell death and mitochondrial dysfunction induced by serum deprivation. Molecular Brain Research. 2004;124:40–50. doi: 10.1016/j.molbrainres.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Koenig S, Gendelman HE, Orenstein JM, Dal Canto MC, Pezeshkpour GH, Yungbluth M, Janotta F, Aksamit A, Martin MA, Fauci AS. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science (New York, NY) 1986;233:1089. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Laine J, Marc ME, Sy MS, Axelrad H. Cellular and subcellular morphological localization of normal prion protein in rodent cerebellum. European Journal of Neuroscience. 2001;14:47–56. doi: 10.1046/j.0953-816x.2001.01621.x. [DOI] [PubMed] [Google Scholar]
- Laurén J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-&bgr; oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre S. Central nervous system complications in HIV disease: HIV-associated neurocognitive disorder. Top Antivir Med. 2011;19:137–142. [PMC free article] [PubMed] [Google Scholar]
- Liao Y-CJ, Lebo RV, Clawson GA, Smuckler EA. Human prion protein cDNA: molecular cloning, chromosomal mapping, and biological implications. Science. 1986 doi: 10.1126/science.3014653. [DOI] [PubMed] [Google Scholar]
- Lima FRS, Arantes CP, Muras AG, Nomizo R, Brentani RR, Martins VR. Cellular prion protein expression in astrocytes modulates neuronal survival and differentiation. Journal of neurochemistry. 2007;103:2164–2176. doi: 10.1111/j.1471-4159.2007.04904.x. [DOI] [PubMed] [Google Scholar]
- Mahad DJ, Ransohoff RM. Seminars in immunology. 1 Edition. Elsevier; 2003. The role of MCP-1 (CCL2) and CCR2 in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE) pp. 23–32. [DOI] [PubMed] [Google Scholar]
- Málaga-Trillo E, Solis GP, Schrock Y, Geiss C, Luncz L, Thomanetz V, Stuermer CAO. Regulation of embryonic cell adhesion by the prion protein. PLoS biology. 2009;7:e1000055. doi: 10.1371/journal.pbio.1000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangé A, Béranger F, Peoc’h K, Onodera T, Frobert Y, Lehmann S. Alpha-and beta-cleavages of the amino-terminus of the cellular prion protein. Biology of the Cell. 2004;96:125–132. doi: 10.1016/j.biolcel.2003.11.007. [DOI] [PubMed] [Google Scholar]
- McMahon HEM, Mangé A, Nishida N, Créminon C, Casanova D, Lehmann S. Cleavage of the amino terminus of the prion protein by reactive oxygen species. Journal of Biological Chemistry. 2001;276:2286–2291. doi: 10.1074/jbc.M007243200. [DOI] [PubMed] [Google Scholar]
- Meyne F, Gloeckner SF, Ciesielczyk B, Heinemann U, Krasnianski A, Meissner B, Zerr I. Total prion protein levels in the cerebrospinal fluid are reduced in patients with various neurological disorders. Journal of Alzheimer's Disease. 2009;17:863–873. doi: 10.3233/JAD-2009-1110. [DOI] [PubMed] [Google Scholar]
- Moss ML, Powell G, Miller MA, Edwards L, Qi B, Sang Q-XA, De Strooper B, Tesseur I, Lichtenthaler SF, Taverna M. ADAM9 Inhibition Increases Membrane Activity of ADAM10 and Controls α-Secretase Processing of Amyloid Precursor Protein. Journal of Biological Chemistry. 2011;286:40443–40451. doi: 10.1074/jbc.M111.280495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Schiess N, Venkatesan A, Rumbaugh J, Sacktor N, McArthur J. Evolution of HIV dementia with HIV infection. International Review of Psychiatry. 2008;20:25–31. doi: 10.1080/09540260701861930. [DOI] [PubMed] [Google Scholar]
- Nieznanski K, Choi J-K, Chen S, Surewicz K, Surewicz WK. Soluble Prion Protein Inhibits Amyloid β (Aβ) Fibrillization and Toxicity. Journal of Biological Chemistry. 2012 doi: 10.1074/jbc.C112.400614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters PJ, Mironov A, Jr, Peretz D, Van Donselaar E, Leclerc E, Erpel S, DeArmond SJ, Burton DR, Williamson RA, Vey M. Trafficking of prion proteins through a caveolaemediated endosomal pathway. The Journal of cell biology. 2003;162:703–717. doi: 10.1083/jcb.200304140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner SB. Prions. Proceedings of the National Academy of Sciences. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puckett C, Concannon P, Casey C, Hood L. Genomic structure of the human prion protein gene. American Journal of Human Genetics. 1991;49:320. [PMC free article] [PubMed] [Google Scholar]
- Rezaie P, Pontikis CC, Hudson L, Cairns NJ, Lanto PL. Expression of cellular prion protein in the frontal and occipital lobe in Alzheimer's disease, diffuse Lewy body disease, and in normal brain: an immunohistochemical study. Journal of Histochemistry & Cytochemistry. 2005;53:929–940. doi: 10.1369/jhc.4A6551.2005. [DOI] [PubMed] [Google Scholar]
- Riek R, Hornemann S, Wider G, Glockshuber R, Wüthrich K. NMR characterization of the full-length recombinant murine prion protein, mPrP (23-231) FEBS letters. 1997;413:282–288. doi: 10.1016/s0014-5793(97)00920-4. [DOI] [PubMed] [Google Scholar]
- Roberts TK, Buckner CM, Berman JW. Leukocyte transmigration across the blood-brain barrier: perspectives on neuroAIDS. Frontiers in bioscience : a journal and virtual library. 2010a;15:478–536. doi: 10.2741/3631. [DOI] [PubMed] [Google Scholar]
- Roberts TK, Eugenin EA, Morgello S, Clements JE, Zink MC, Berman JW. PrP<sup>C</sup>the Cellular Isoform of the Human Prion Protein, Is a Novel Biomarker of HIV-Associated Neurocognitive Impairment and Mediates Neuroinflammation. The American journal of pathology. 2010b;177:1848–1860. doi: 10.2353/ajpath.2010.091006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roucou X, Guo Q, Zhang Y, Goodyer CG, LeBlanc AC. Cytosolic prion protein is not toxic and protects against Bax-mediated cell death in human primary neurons. Journal of Biological Chemistry. 2003;278:40877–40881. doi: 10.1074/jbc.M306177200. [DOI] [PubMed] [Google Scholar]
- Santos TG, Beraldo FH, Hajj GNM, Lopes MH, Roffe M, Lupinacci F, Ostapchenko VG, Prado VF, Prado MAM, Martins VR. Laminin-γ1 chain and stress inducible protein 1 synergistically mediate PrPC-dependent axonal growth via Ca2 mobilization in dorsal root ganglia neurons. Journal of neurochemistry. 2013;124:210–223. doi: 10.1111/jnc.12091. [DOI] [PubMed] [Google Scholar]
- Santuccione A, Sytnyk V, Leshchyns' ka I, Schachner M. Prion protein recruits its neuronal receptor NCAM to lipid rafts to activate p59fyn and to enhance neurite outgrowth. Science Signalling. 2005;169:341. doi: 10.1083/jcb.200409127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnataro D, Caputo A, Casanova P, Puri C, Paladino S, Tivodar SS, Campana V, Tacchetti C, Zurzolo C. Lipid rafts and clathrin cooperate in the internalization of PrPC in epithelial FRT cells. PloS one. 2009;4:e5829. doi: 10.1371/journal.pone.0005829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt-Ulms G, Legname G, Baldwin MA, Ball HL, Bradon N, Bosque PJ, Crossin KL, Edelman GM, DeArmond SJ, Cohen FE. Binding of neural cell adhesion molecules (N-CAMs) to the cellular prion protein. Journal of Molecular Biology. 2001;314:1209–1225. doi: 10.1006/jmbi.2000.5183. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Vigil O, Heaton RK, Grant I, Ellis RJ, Marcotte TD. Script generation of activities of daily living in HIV-associated neurocognitive disorders. Journal of the International Neuropsychological Society : JINS. 2011;17:740–745. doi: 10.1017/S135561771100052X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyng S-L, Huber MT, Harris DA. A prion protein cycles between the cell surface and an endocytic compartment in cultured neuroblastoma cells. Journal of Biological Chemistry. 1993;268:15922–15928. [PubMed] [Google Scholar]
- Shyng S-L, Heuser JE, Harris DA. A glycolipid-anchored prion protein is endocytosed via clathrin-coated pits. The Journal of cell biology. 1994;125:1239–1250. doi: 10.1083/jcb.125.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl N, Borchelt DR, Hsiao K, Prusiner SB. Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell. 1987;51:229. doi: 10.1016/0092-8674(87)90150-4. [DOI] [PubMed] [Google Scholar]
- Stöckel J, Safar J, Wallace AC, Cohen FE, Prusiner SB. Prion protein selectively binds copper (II) ions. Biochemistry. 1998;37:7185–7193. doi: 10.1021/bi972827k. [DOI] [PubMed] [Google Scholar]
- Taylor DR, Parkin ET, Cocklin SL, Ault JR, Ashcroft AE, Turner AJ, Hooper NM. Role of ADAMs in the ectodomain shedding and conformational conversion of the prion protein. Journal of Biological Chemistry. 2009;284:22590–22600. doi: 10.1074/jbc.M109.032599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres M, Cartier L, Matamala JM, Hernández N, Woehlbier U, Hetz C. Altered Prion Protein Expression Pattern in CSF as a Biomarker for Creutzfeldt-Jakob Disease. PloS one. 2012;7:e36159. doi: 10.1371/journal.pone.0036159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tousseyn T, Thathiah A, Jorissen E, Raemaekers T, Konietzko U, Reiss K, Maes E, Snellinx A, Serneels L, Nyabi O. ADAM10, the rate-limiting protease of regulated intramembrane proteolysis of Notch and other proteins, is processed by ADAMS-9, ADAMS-15, and the γ-secretase. Journal of Biological Chemistry. 2009;284:11738–11747. doi: 10.1074/jbc.M805894200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Sithinamsuwan P, Letendre S, Ances B. Pathogenesis of HIV in the central nervous system. Current HIV/AIDS Reports. 2011;8:54–61. doi: 10.1007/s11904-010-0070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella LJ, Greenwood DLV, Cappai R, Scheerlinck J-PY, Hill AF. Enrichment of prion protein in exosomes derived from ovine cerebral spinal fluid. Veterinary immunology and immunopathology. 2008;124:385–393. doi: 10.1016/j.vetimm.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Vella LJ, Sharples RA, Lawson VA, Masters CL, Cappai R, Hill AF. Packaging of prions into exosomes is associated with a novel pathway of PrP processing. The Journal of pathology. 2007;211:582–590. doi: 10.1002/path.2145. [DOI] [PubMed] [Google Scholar]
- Viegas P, Chaverot N, Enslen H, Perrière N, Couraud P-O, Cazaubon S. Junctional expression of the prion protein PrPC by brain endothelial cells: a role in trans-endothelial migration of human monocytes. Journal of cell science. 2006;119:4634–4643. doi: 10.1242/jcs.03222. [DOI] [PubMed] [Google Scholar]
- Vincent B, Paitel E, Frobert Y, Lehmann S, Grassi J, Checler F. Phorbol ester-regulated cleavage of normal prion protein in HEK293 human cells and murine neurons. Journal of Biological Chemistry. 2000;275:35612–35616. doi: 10.1074/jbc.M004628200. [DOI] [PubMed] [Google Scholar]
- Vincent B, Paitel E, Saftig P, Frobert Y, Hartmann D, De Strooper B, Grassi J, Lopez-Perez E, Checler F. The disintegrins ADAM10 and TACE contribute to the constitutive and phorbol ester-regulated normal cleavage of the cellular prion protein. Journal of Biological Chemistry. 2001;276:37743–37746. doi: 10.1074/jbc.M105677200. [DOI] [PubMed] [Google Scholar]
- Visentin S, Renzi M, Levi G. Altered outward-rectifying K current reveals microglial activation induced by HIV-1 Tat protein. Glia. 2001;33:181–190. doi: 10.1002/1098-1136(200103)33:3<181::aid-glia1017>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Voigtländer T, Klöppel S, Birner P, Jarius C, Flicker H, Verghese-Nikolakaki S, Sklaviadis T, Guentchev M, Budka H. Marked increase of neuronal prion protein immunoreactivity in Alzheimer's disease and human prion diseases. Acta Neuropathologica. 2001;101:417–423. doi: 10.1007/s004010100405. [DOI] [PubMed] [Google Scholar]
- Watt NT, Taylor DR, Kerrigan TL, Griffiths HH, Rushworth JV, Whitehouse IJ, Hooper NM. Prion protein facilitates uptake of zinc into neuronal cells. Nature Communications. 2012;3:1134. doi: 10.1038/ncomms2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DW, Eugenin EA, Calderon TM, Berman JW. Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis. J Leukoc Biol. 2012a;91:401–415. doi: 10.1189/jlb.0811394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DW, Eugenin EA, Calderon TM, Berman JW. Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis. Journal of leukocyte biology. 2012b;91:401–415. doi: 10.1189/jlb.0811394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R, Liu A, Lührs T, Riek R, Von Schroetter C, García FL, Billeter M, Calzolai L, Wider G, Wüthrich K. NMR solution structure of the human prion protein. Proceedings of the National Academy of Sciences. 2000;97:145–150. doi: 10.1073/pnas.97.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Klingeborn M, Simonsson M, Linné T. Proteolytic cleavage and shedding of the bovine prion protein in two cell culture systems. Virus research. 2006;115:43–55. doi: 10.1016/j.virusres.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Zink MC, Coleman GD, Mankowski JL, Adams RJ, Tarwater PM, Fox K, Clements JE. Increased macrophage chemoattractant protein-1 in cerebrospinal fluid precedes and predicts simian immunodeficiency virus encephalitis. Journal of Infectious Diseases. 2001;184:1015–1021. doi: 10.1086/323478. [DOI] [PubMed] [Google Scholar]