Abstract
Objective
To evaluate the effects of medical comorbidity on anxiety treatment outcomes.
Methods
Data were analyzed from 1,004 primary care patients enrolled in a trial of a collaborative care intervention for anxiety. Linear mixed models accounting for baseline characteristics were used to evaluate effects of overall medical comorbidity [2 or more chronic medical conditions (CMCs) vs. fewer than 2 CMCs] and specific CMCs (migraine, asthma, and gastrointestinal disease) on anxiety treatment outcomes at 6, 12, and 18 months.
Results
At baseline, patients with two or more CMCs (n = 582; 58.0%) reported more severe anxiety symptoms [10.5 (95% CI = 10.1 to 10.9) vs. 9.5 (95% CI = 9.0 to 10.0); p = .003) and anxiety-related disability [17.6 (95% CI = 17.0 to 18.2) vs. 16.0 (95% CI = 15.3 to 16.7); p = .001). However, their clinical improvement was comparable to those with one or zero CMCs (predicted Δ in anxiety symptoms = −3.9 vs. −4.1 at 6 months, −4.6 vs. − 4.4 at 12 months, −4.9 vs. −5.0 at 18 months; predicted Δ in anxiety-related disability = −6.4 vs. −6.9 at 6 months, −6.9 vs. −7.3 at 12 months, −7.3 vs. −7.5 at 18 months). The only specific CMC with a detrimental effect was migraine, which was associated less improvement in anxiety symptoms at 18 months (predicted Δ = −4.1 vs. −5.3).
Conclusions
Effectiveness of the anxiety intervention was not significantly affected by presence of multiple CMCs; however, migraine sufferers displayed less improvement at long-term follow up.
Clinical Trials Registration
www.clinicaltrials.gov Identifier: NCT00347269
Keywords: anxiety, medical illness, asthma, migraine, primary care, randomized controlled trial
Introduction
Anxiety disorders are strongly associated with many chronic medical conditions (CMCs; 1–4) Increased prevalence of anxiety disorders is observed in patients with a diverse array of CMCs, including cardiovascular disease (1, 2), gastrointestinal disease (1, 5), respiratory disease (1, 6, 7), migraine (1, 8), chronic pain (1, 9, 10), and cancer (11). Many of these associations remain significant after controlling for multiple potential confounds (e.g., demographic variables, co-occurring mental disorders; 1, 2). Overall degree of medical comorbidity demonstrates a “dose-response” relationship to prevalence of anxiety disorders, with odds of meeting criteria for an anxiety disorder increasing in a linear fashion as number of CMCs increases (1, 12).
Patients with anxiety disorders also display higher frequencies of certain CMCs than those observed in the general population [e.g., irritable bowel syndrome (13, 14), asthma (7)], and report lower levels of health-related quality of life (15, 16). Perhaps of greatest clinical significance, anxiety disorders have been shown to independently contribute to worse medical symptom severity and functional impairment in some CMCs [e.g., asthma (17), cardiovascular disease (18), diabetes (19)] and to increase risk for incidence or disease progression in others (e.g., cardiovascular disease; 20, 21).
Medical comorbidity complicates assessment and at times may lead to under-recognition of anxiety disorders (22). Overlap between symptoms of anxiety disorders and CMCs can present a diagnostic challenge even for clinicians specialized in anxiety assessment. Many of these challenging differential diagnoses involve panic disorder (PD), which is characterized by numerous somatic symptoms that could be attributable to medical illnesses (e.g., shortness of breath, dizziness). However, other anxiety disorders are also partly defined by symptoms that can also result from CMCs [e.g., hyper-arousal associated with posttraumatic stress disorder (PTSD); fatigue associated with generalized anxiety disorder (GAD)]. Treatments for certain CMCs (e.g., oral corticosteroids) also can produce symptoms that mimic anxiety disorders (e.g., restlessness).
Medical comorbidity is also thought to complicate treatment of anxiety disorders (4, 23). However, very few empirical investigations have quantified the impact of medical comorbidity on anxiety treatment outcomes. One exception is an analysis of outcomes from a randomized controlled trial (RCT) of a collaborative care intervention for PD in primary care (24). In that study, more medically ill patients had more severe anxiety at baseline, but displayed reductions in anxiety, depression, and disability that were comparable to the reductions observed in the less medically ill group. The investigators concluded that the empirically supported treatments for PD used in the study [cognitive-behavioral therapy (CBT) and pharmacotherapy) worked equally well regardless of medical comorbidity.
The current study builds on the investigation of Roy-Byrne et al. (24) by evaluating the effects of medical comorbidity on outcomes from a large (N = 1004) RCT of the Coordinated Anxiety Learning and Management (CALM) intervention for a broad range of anxiety disorders [GAD, PD, PTSD, and social anxiety disorder (SAD)] in primary care (25). The CALM intervention was shown to be superior to usual care (UC) in reducing anxiety symptoms and anxiety-related disability during 18 months of follow-up (25, 26). However, it is unknown whether co-occurring medical illness influenced treatment outcomes. The principal aim of the current study was to assess the effects of medical comorbidity on anxiety symptoms and anxiety-related disability measured over the 18 month study period. On the basis of prior results (24), we predicted that greater medical comorbidity would be associated with more severe anxiety symptoms and anxiety-related disability at baseline, but not with degree of improvement in symptoms and disability during the study follow-up period.
The secondary aim of this study was to explore whether distinct CMCs commonly associated with anxiety disorders have unique effects of anxiety treatment outcomes. To investigate this, we selected several specific CMCs that demonstrate strong associations with anxiety disorders, have widely recognized stress-related features, and were endorsed with sufficient frequency in this sample to justify separate evaluation of their potential interactions with treatment outcome. We limited these analyses to three CMCs in order to balance interest in evaluating disorder-specific effects with the risk of Type I error. The CMCs that best met our selection criteria were migraine, asthma, and gastrointestinal disease (1–8, 13, 14, 17, 27, 28). Exploratory analyses examined whether these specific CMCs had similar or divergent influences on anxiety treatment outcomes.
Method
Participants
Participants were patients enrolled in the CALM study, an RCT conducted in 17 primary care clinics in 4 U.S. regions (Seattle, WA; Los Angeles, CA; San Diego, CA; and Little Rock, AR). Patients provided informed consent to participate, and the study was approved by Institutional Review boards at all study sites. Patients were referred to the study by their primary care providers (PCPs); in some clinics, referral was facilitated by a 5-item anxiety screener (29).
Between June 2006 and April 2008, 1004 patients with GAD, PD, PTSD, and/or SAD, aged 18 to 75 years, English- or Spanish-speaking, were enrolled in the study. Most co-occurring mental disorders were permitted; active suicidal intent or plan, psychosis, Bipolar I, and substance use disorders (except alcohol and marijuana abuse) were cause for exclusion. Table 1 reports the demographic and diagnostic characteristics of this sample.
Table 1.
| All (n = 1004) | Zero or One Chronic Medical Conditions (n =422 ) | Two+ Chronic Medical Conditions (n =582) | p value | |
|---|---|---|---|---|
| Age in years, mean (SD) | 43.5 (13.4) | 37.7 (11.7) | 47.7 (13.1) | <.001 |
|
| ||||
| Gender, % Women | 71.1 (714) | 69.2 (292) | 72.5 (422) | .252 |
|
| ||||
| Education | .033 | |||
| < High school | 5.5 (55) | 3.6 (15) | 6.9 (40) | |
|
| ||||
| 12 years | 16.5 (165) | 15.0 (63) | 17.6 (102) | |
|
| ||||
| > 12 years | 78.0 (782) | 81.5 (343) | 75.6 (439) | |
|
| ||||
| Ethnicity | .048 | |||
| Hispanic | 19.5 (196) | 22.0 (93) | 17.7 (103) | |
|
| ||||
| African American | 11.6 (116) | 8.8 (37) | 13.6 (79) | |
|
| ||||
| White | 56.6 (568) | 57.8 (244) | 55.7 (324) | |
|
| ||||
| Other | 12.4 (124) | 11.4 (48) | 13.1 (76) | |
|
| ||||
| Diagnosesc | ||||
| Panic Disorder | 47.3 (475) | 48.6 (205) | 46.4 (270) | .493 |
|
| ||||
| Generalized Anxiety | 75.3 (756) | 73.2 (309) | 76.8 (447) | .194 |
|
| ||||
| Social Phobia | 40.3 (405) | 41.7 (176) | 39.4 (229) | .452 |
|
| ||||
| Posttraumatic Stress | 18.0 (181) | 12.8 (54) | 21.8 (127) | <.001 |
|
| ||||
| Major Depression | 64.5 (648) | 57.4 (242) | 69.8 (406) | <.001 |
|
| ||||
| Type of health insurancec | ||||
| Medicaid | 10.1 (101) | 5.0 (21) | 13.8 (80) | <.001 |
|
| ||||
| Medicare | 12.4 (124) | 3.6 (15) | 18.7 (109) | <.001 |
|
| ||||
| Other government insuranced | 3.5 (35) | 3.8 (16) | 3.3 (19) | .643 |
|
| ||||
| Private insurance | 74.8 (749) | 78.1 (328) | 72.3 (421) | .039 |
|
| ||||
| No insurance | 14.1 (141) | 16.4 (69) | 12.4 (72) | .069 |
|
| ||||
| Any Opiate Use | 8.6 (86) | 1.9 (8) | 13.4 (78) | <.001 |
|
| ||||
| Any Pain | 43.9 (441) | 26.3 (111) | 56.7 (330) | <.001 |
|
| ||||
| Proportion assigned to CALM | 50.1 (503) | 51.7 (218) | 49.0 (285) | .400 |
|
| ||||
| Baseline BSI-A, mean (SD) | 10.1 (5.2) | 9.5 (5.1) | 10.5 (5.3) | .003 |
|
| ||||
| Baseline SDS, mean (SD) | 17.0 (7.3) | 16.0 (7.3) | 17.6 (7.1) | .001 |
Data are reported as % (n) unless otherwise indicated.
Baseline characteristics for patients with Zero or One vs. Two or More Chronic Medical Conditions were compared using t tests and χ2 tests for continuous and categorical variables, respectively.
Because patients could have more than one, n’s may total more than 1004.
Includes Veterans’ Administration benefits, TRICARE, county programs, or other government insurance.
CALM = Coordinated Anxiety Learning and Management; BSI-A = Brief Symptom Inventory – Anxiety subscale (possible score range = 0 to 24); SDS = Sheehan Disability Scale (modified to capture anxiety-related disability; possible score range = 0 to 30)
Design of the CALM Study
The overall design of the CALM study has been described in prior reports (25, 30). Briefly, eligible participants were randomly assigned to either CALM or UC; randomization was stratified by clinic and presence/absence of major depressive disorder (MDD). Blinded telephone assessments were performed by the RAND Survey Research Group at baseline, 6, 12, and 18 months. Study retention was high and similar for the CALM and UC groups, with more than 80% of participants assessed at each follow-up evaluation point (6, 12, and 18 months).
Intervention
Patients assigned to UC received care as usual from their PCP, with no restrictions imposed (e.g., patients could receive pharmacotherapy, in-house counseling if available, or be referred out for specialty care). Patients assigned to CALM met with an Anxiety Clinical Specialist (ACS) and were given the choice of computer-assisted CBT delivered by the ACS, medication management, or both. The vast majority of patients assigned to CALM (n = 482; 95.8%) had at least one intervention contact with the ACS; of these, 166 (34.4%) had only CBT visits, 43 (8.9%) had only medication management visits, and 273 (56.6%) had both CBT and medication management visits (25).
Medication management was delivered by the PCP and supported by the ACS, who facilitated consultation with the CALM study psychiatrist as needed and encouraged medication adherence and healthy behaviors. Prior to the study enrollment period, a local study psychiatrist provided a one-time training to PCPs at participating clinics focused on pharmacotherapy for anxiety disorders. The simple pharmacotherapy algorithm focused on first-line use of selective serotonin reuptake inhibitor or serotonin norepinephrine reuptake inhibitor antidepressants, dose optimization, and side effect monitoring; followed by second and third step combinations of two antidepressants or an antidepressant and a benzodiazepine for refractory patients.
Computer-assisted CBT was provided by the ACS, and consisted of standard CBT elements such as self-monitoring, psychoeducation, breathing retraining, cognitive restructuring, exposure to feared internal and external stimuli, and relapse prevention (31). In cases where patients met criteria for more than one of the four target anxiety disorders, CBT focused on the disorder the patient judged to be most distressing or disabling.
The initial treatment step (CBT, medication management, or both) was typically delivered during a 10 to 12 week period. For patients who did not respond fully to the initial treatment step, the CALM algorithm allowed for multiple treatment steps (up to 4 steps over the course of 12 months), which could include either “stepping up” (adding more of the same modality) or “stepping over” (switching to or adding the other modality). Once patients had achieved criteria for remission (25) or improved to the degree where they did not want further treatment, they entered “continued care” where they received monthly phone calls to reinforce CBT skills, medication adherence, or both, until the 12-month treatment period had elapsed. Detailed descriptions of the CALM intervention (25, 31) and the training of the ACSs are provided elsewhere (32).
Measures
Diagnostic Assessment
Diagnoses of mental disorders were established using the Mini International Neuropsychiatric Interview (MINI), version 5.0 (33). The MINI was conducted in-person by the ACS at the participant’s primary care clinic. Reliability and validity of anxiety disorder diagnoses established using the MINI are satisfactory (33).
Medical Comorbidity
Presence of medical conditions was assessed via patient self-report. Frequencies of many CMCs were high (see Table 2), and the vast majority of the sample endorsed at least one CMC (n = 801; 79.8%). The median number of CMCs endorsed was 2 (range = 0 to 11; IQR = 3); this was the case for both the CALM (median = 2; range = 0 to 11; IQR = 3) and UC (median = 2; range = 0 to 9; IQR = 2) groups. Given that medical comorbidity was the rule rather than the exception in this sample, we opted to evaluate the effect of having multiple CMCs on treatment outcome (rather than evaluating the effect of having any medical comorbidity). Patients endorsing two or more CMCs (n = 582; 58.0%) comprised the High Medical Comorbidity group, while those endorsing zero or one CMC comprised the Low Medical Comorbidity group (n = 421; 42.0%). We also evaluated the specific effects of migraine, asthma, and gastrointestinal disease on treatment outcomes. Positive status on these variables was defined as simply endorsing these items from the list in Table 2.
Table 2.
Frequencies of Chronic Medical Conditionsa
| Chronic Medical Condition | Frequency Proportion (n) | Baseline BSI-A Mean (SD) | Baseline SDS Mean (SD) |
|---|---|---|---|
| Hypertension or High Blood Pressure | 36.7 (368) | 10.5 (5.3) | 17.3 (7.5) |
| Back Problems | 33.0 (331) | 10.8 (5.3) | 17.8 (7.3) |
| Migraine Headaches | 28.6 (287) | 10.5 (5.3) | 17.7 (7.4) |
| Vision Problems (despite use of corrective lenses) | 24.3 (244) | 11.5 (5.4) | 18.7 (7.2) |
| Arthritis or Rheumatism | 24.0 (241) | 10.3 (5.2) | 17.7 (7.2) |
| Asthma | 20.6 (207) | 10.9 (5.5) | 17.8 (7.1) |
| Gastrointestinal Diseasea | 17.3 (174) | 11.0 (5.5) | 17.2 (7.6) |
| High Blood Sugar or Diabetes | 10.2 (102) | 10.8 (5.5) | 18.6 (7.1) |
| Thyroid Disease | 8.6 (86) | 10.2 (5.4) | 16.9 (7.6) |
| Heart Disease | 6.3 (63) | 11.0 (5.9) | 17.3 (7.4) |
| Chronic Bronchitis or Emphysema | 5.2 (52) | 11.0 (5.9) | 17.0 (7.4) |
| Physical Disability (birth defect; loss of limb, sight, hearing) | 4.4 (44) | 10.8 (4.7) | 19.5 (6.4) |
| Cancer diagnosed within the last 3 years | 3.5 (35) | 10.5 (5.3) | 17.2 (7.3) |
| Neurological Condition | 2.5 (25) | 13.9 (5.1) | 19.5 (7.5) |
| Stroke or Major Paralysis | 2.0 (20) | 11.9 (5.3) | 18.6 (6.3) |
| Kidney Failure | 1.1 (11) | 11.3 (5.7) | 14.1 (7.4) |
Baseline BSI-A and SDS scores are presented as descriptive data only. Differences between the baseline scores for each CMC and the baseline scores for the total sample were not formally analyzed or interpreted due to wide variation in the frequencies of specific CMCs.
This category is comprised of patients who endorsed either the item “stomach ulcer” (10.2%; n = 102) or the item “chronic inflamed bowel, enteritis, or colitis” (9.4%; n = 94). These two items were counted separately in determining whether patients belonged to the High or Low Medical Comorbidity group. The two items were collapsed into a general “Gastrointestinal Disease” category to increase power for subsequent analyses of the effect of gastrointestinal disease on treatment outcome.
BSI-A = Brief Symptom Inventory – Anxiety subscale (possible score range = 0 to 24); SDS = Sheehan Disability Scale (modified to capture anxiety-related disability; possible score range = 0 to 30).
Anxiety Symptoms
Severity of anxiety symptoms was measured using the Anxiety subscale of the well-validated Brief Symptom Inventory (BSI-A) (34). The BSI-A measures severity of psychic anxiety, which is common across all anxiety disorders targeted in the study. BSI-A scores were measured during the RAND telephone assessments at baseline, 6, 12, and 18 months.
Anxiety-related Disability
Disability was assessed using the well-validated Sheehan Disability Scale (SDS) (35), which measures the degree to which symptoms disrupt work/school, social functioning, and family/home life. For the CALM study, the instructions that precede each of the three ratings were modified to specifically target anxiety-related disability (e.g., “Anxiety, tension, and worry symptoms have disrupted your work/schoolwork…”). SDS scores were measured during the RAND telephone assessments at baseline, 6, 12, and 18 months.
Statistical Analysis/Design of the Current Study
To estimate the effect of Medical Comorbidity over time, we jointly modeled the symptom-based and functional outcomes (BSI-A and SDS) at the four assessment points by Treatment Assignment (CALM vs. UC), Time (baseline, 6, 12, and 18 months), Medical Comorbidity (High vs. Low), and the interactions of Treatment Assignment, Time, and Medical Comorbidity. In models where the 3-way (Treatment Assignment × Time × Medical Comorbidity) interaction was non-significant, we dropped the 3-way interaction and refit the model including only the 2-way interactions (Treatment Assignment × Time, Treatment Assignment × Medical Comorbidity, and Medical Comorbidity × Time). We also repeated the analyses replacing overall Medical Comorbidity with presence/absence of specific CMCs (Asthma, Migraine, and Gastrointestinal Disease). The objective of these additional analyses was to explore the potentially differential effects of specific CMCs that are commonly reported by individuals diagnosed with anxiety disorders. In all analyses, we modeled the effects of recruitment site, education level, gender, race/ethnicity, and age in order to control for potentially important demographic variables. Time was treated as a categorical variable in the analyses. To avoid restrictive assumptions, the covariance of the outcomes at the four assessment points was left unstructured.
We fitted the proposed model using a restricted maximum likelihood approach, which produces valid estimates under the missing-at-random assumption (36). This approach correctly handles the additional uncertainty arising from missing data and uses all available data to obtain unbiased estimates for model parameters (37). This is an efficient way to conduct intent-to-treat analyses as it includes all participants with a baseline assessment.
The statistical software used was SAS version 9 (SAS Institute Inc., Cary, NC). All p values were two-tailed and a conservative significance level of p < .01 was adopted to account for multiple comparisons in the analyses of study hypotheses.
Results
Baseline Characteristics Related to Medical Comorbidity
Table 1 summarizes the characteristics of participants with High and Low Medical Comorbidity. The High Medical Comorbidity group was older and more likely to be diagnosed with PTSD and MDD, to have Medicaid or Medicare, and to report significant pain and opiate use (p’s < .001). The High Medical Comorbidity group also endorsed higher anxiety symptom [10.5 (95% CI = 10.1 to 10.9) vs. 9.5 (95% CI = 9.0 to 10.0); p = .003] and anxiety-related disability [17.6 (95% CI = 17.0 to 18.2) vs. 16.0 (95% CI = 15.3 to 16.7); p = .001] at baseline. A follow-up analysis revealed small but statistically significant correlations between the number of CMCs endorsed by patients and their anxiety symptom severity (Spearman’s ρ = .14, p < .001) and anxiety-related disability (Spearman’s ρ = .12, p < .001).
Table 2 presents the frequencies with which participants endorsed specific CMCs, as well as the baseline anxiety symptom and anxiety-related disability scores endorsed by patients with each CMC.
Effects of Overall Medical Comorbidity on Anxiety Treatment Outcomes
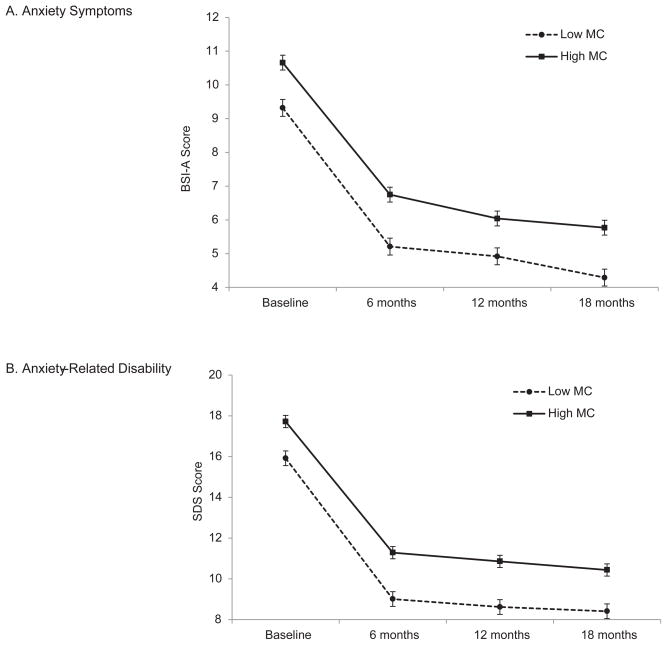
The 3-way Medical Comorbidity × Treatment Assignment × Time interaction effects on BSI-A (p = .64) and SDS (p = .44) were non-significant. We dropped the 3-way interactions and refit the models including only 2-way interactions; this also failed to reveal any significant Medical Comorbidity × Time interaction effects on BSI-A (p = .47) or SDS (p = .61).1 These results indicate that improvement in anxiety symptoms and anxiety-related disability was comparable for the High Medical Comorbidity and Low Medical Comorbidity groups (predicted Δ in BSI-A = −3.9 vs. −4.1 at 6 months, −4.6 vs. − 4.4 at 12 months, −4.9 vs. −5.0 at 18 months; predicted Δ in SDS = −6.4 vs. −6.9 at 6 months, −6.9 vs. −7.3 at 12 months, −7.3 vs. −7.5 at 18 months).
As expected based on the group differences on baseline measures, there were significant main effects of Medical Comorbidity on BSI-A [F (1,988) = 22.44, p < .001] and SDS [F (1,988) = 22.03, p < .001), with the High Medical Comorbidity group displaying higher anxiety symptom and anxiety-related disability scores at all of the assessment points (see Figure 1).
Figure 1.
Predicted scores on the (A) Brief Symptom Inventory – Anxiety subscale and (B) Sheehan Disability Scale for High and Low Medical Comorbidity groups. There are significant main effects of Medical Comorbidity on anxiety symptoms and disability, but no Medical Comorbidity × Time interaction effects. Results are not broken down by Treatment Assignment because there were no significant interaction effects involving Treatment Assignment and Medical Comorbidity. Error bars represent the standard error of the predicted means.
Effects of Specific Chronic Medical Conditions on Anxiety Treatment Outcomes
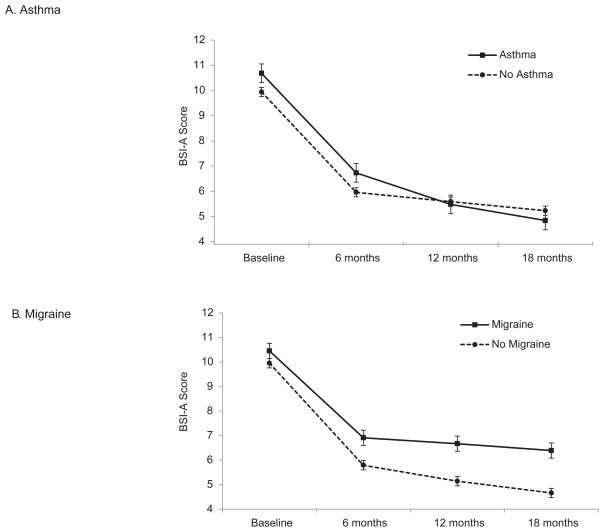
The 3-way Asthma × Treatment Assignment × Time interaction effects on BSI-A (p = .64) and SDS (p = .60) were non-significant. We dropped the 3-way interactions and refit the models including only 2-way interactions, which revealed a significant Asthma × Time effect on BSI-A (p = .004). Regardless of Treatment Assignment, asthma sufferers showed greater improvement at 18 months than those without asthma (predicted Δ in BSI-A = −5.8 vs. −4.7 at 18 months; p = .010; see Figure 2). While those with asthma started the study with slightly higher BSI-A scores, by the 18-month follow up they endorsed slightly lower BSI-A scores than participants without asthma (predicted mean BSI-A = 4.84 vs. 5.23). Participants with asthma showed only a trend toward greater improvement at 12 months (p = .048) and comparable improvement at 6 months (p = .97) relative to those without asthma. There was no main effect of asthma on BSI-A (p = .41), nor was there a significant Asthma × Time effect on SDS (p = .056), or a main effect of asthma on SDS (p = .47).
Figure 2.
Predicted scores on the Brief Symptom Inventory – Anxiety subscale for (A) patients with and without asthma, and (B) patients with and without migraine. Patients with asthma displayed greater improvement in anxiety symptoms at 18 months than patients without asthma; whereas patients with migraine displayed less improvement in anxiety symptoms at 18 months compared to patients without migraine. Results are not broken down by Treatment Assignment because there were no significant interaction effects involving Treatment Assignment and Asthma or Treatment Assignment and Migraine. Error bars represent the standard error of the predicted means.
The 3-way Migraine × Treatment Assignment × Time interaction effects on BSI-A (p = .031) and SDS (p = .14) were non-significant. We therefore dropped the 3-way interactions and refit the models including only 2-way interactions. This also failed to reveal any Migraine × Time effects on BSI-A (p = .018) or on SDS (p = .073) that met our a priori criterion for statistical significance. However, the Migraine × Time effect approached significance for the BSI-A [F (3,991) = 3.39, p = .018] because those with migraine showed significantly less improvement in anxiety symptoms at 18 months (predicted Δ in BSI-A = −4.1 vs. −5.3; p = .003) and tended to show less improvement at 12 months (predicted Δ in BSI-A = −3.8 vs. −4.8; p = .014), regardless of Treatment Assignment (see Figure 2). We also observed main effects of Migraine Status on BSI-A (p < .001) and SDS (p < .001), with migraine sufferers displaying more severe anxiety symptoms and anxiety-related disability at all three follow-up points (p’s = .001 to .010) but not at baseline (p’s = .17 and .079).
There were no significant effects of gastrointestinal disease on improvement in anxiety symptoms or anxiety-related disability. All 3-way and 2-way interactions involving Gastrointestinal Disease and Time were non-significant (p’s > .10).
Discussion
The past decade has witnessed growing interest in the delivery of interventions for anxiety disorders in primary care. Collaborative care interventions that incorporate elements of empirically supported treatments have been shown to improve outcomes for primary care patients with anxiety disorders (25, 38, 39); however, questions remain about factors that influence treatment outcome. The results of the current study provide information pertaining specifically to the effects of medical comorbidity on anxiety treatment outcome, and more generally to the phenomenology of anxiety disorders in the context of medical illness.
First, descriptive analyses confirmed that frequencies of major chronic medical conditions (CMCs) are high in patients who seek treatment of anxiety disorders in primary care settings. The majority of the CALM study sample endorsed two or more CMCs. Approximately one-third of participants endorsed hypertension and back problems; one-fourth endorsed migraine, vision problems despite use of corrective lenses, and arthritis; and one-fifth endorsed asthma and gastrointestinal disease. Patients with multiple CMCs were older and more likely to be diagnosed with PTSD and MDD, with nearly 70% of those with two or more CMCs meeting criteria for MDD. Those with more medical comorbidity also endorsed more severe anxiety symptoms and anxiety-related disability at baseline. Taken together, the descriptive findings point toward a relatively complicated “typical” anxiety disorder presentation in primary care (multiple co-occurring CMCs, more severe anxiety, high likelihood of depression). These results highlight the need for continuing study of methods for optimizing assessment of anxiety and other mental health problems in primary care settings, as well as ways to facilitate treatment planning, delivery of interventions, and monitoring of outcomes.
Second, consistent with our hypotheses and previous findings in primary care patients with PD (24), overall medical comorbidity did not moderate the effects of the CALM intervention on anxiety symptoms or anxiety-related disability. This suggests that the advantages of CALM over UC are robust to differences in level of medical comorbidity, broadly defined. Additionally, reductions in anxiety symptoms and anxiety-related disability were comparable for the high and low medical comorbidity groups when considered irrespective of treatment assignment. Considered in conjunction with prior results (24), these findings suggest that improvements of similar magnitude can be expected from interventions such as CALM and UC in patients with varying levels of overall medical comorbidity.
Although degree of improvement was similar in patients with high and low medical comorbidity, absolute levels of anxiety symptoms and anxiety-related disability were higher at all assessment points for patients with two or more CMCs. Given the lack of significant interaction effects, these higher absolute scores appear attributable to baseline elevations in symptoms and disability (which carried forward to subsequent assessments). Higher baseline anxiety severity in patients with more medical comorbidity could be due to a range of biopsychosocial factors. The stress of managing multiple CMCs could exacerbate anxiety disorder symptoms, while the need to address multiple CMCs could make anxiety disorder symptoms a lower treatment priority for both clinicians and patients. Symptoms of CMCs also could restrict patients’ engagement in non-treatment activities that could assist in ameliorating anxiety symptom severity (e.g., exercise, activities that provide social support). It is also possible that in some cases of anxiety-CMC comorbidity, there could be shared biological substrates associated with increased severity of both types of conditions or increased susceptibility to medical conditions in patients with more severe anxiety (4). Finally, in the absence of objective confirmation of medical diagnoses, we cannot rule out the possibility that patients with more severe anxiety were more biased toward endorsing medical conditions as a result of hypersensitivity to physical symptoms and/or health-focused anxiety.
Although the CALM intervention produced similar degrees of clinical improvement regardless of medical comorbidity level, Figure 1 shows that there is room for further improvement in the absolute levels of anxiety symptoms and anxiety-related disability endorsed by patients with more medical comorbidity. Modification of standard interventions may be needed to accomplish further reductions in anxiety severity in these patients. This could include more tailoring of CBT to address possible interactions of anxiety and medical symptoms, or augmentation with other empirically-supported strategies (e.g., acceptance-based techniques; 40) that may aid patients in coping with symptoms of medical illness as well as anxiety.
Because different comorbid CMCs may influence anxiety treatment outcomes in distinct ways, we undertook exploratory analyses to examine treatment outcomes for patients who endorsed asthma, migraine, and gastrointestinal disease. We found preliminary evidence that these conditions have divergent effects on anxiety-related treatment outcomes. We did not observe any effects of gastrointestinal disease on improvement of anxiety symptoms or anxiety-related disability during the 18 month study period. Additionally, asthma did not appear detrimental to treatment efficacy; in fact, asthma sufferers showed more improvement than patients without asthma at the 18-month follow-up. While asthma sufferers started the study with slightly more severe anxiety symptoms, by the end of the study they had “caught up” with patients without asthma and reported comparably low anxiety symptoms. These results are encouraging; particularly in light of evidence suggesting that anxiety disorders and asthma can potentiate one another (27). While symptom severity and functional impairment due to anxiety and asthma may be interrelated, it appears that anxiety disorders can be just as successfully treated in asthma sufferers as in other primary care patients. Further, treatment of anxiety should be a priority in this subgroup, as reduction in anxiety symptoms could conceivably improve asthma-related outcomes as well (27).
Migraine sufferers, on the other hand, displayed some evidence of poorer response to treatment. They tended to show less improvement in anxiety symptoms over time, and their anxiety symptoms were significantly less improved at the 18-month follow-up. Main effects also indicated that migraine sufferers endorsed significantly higher absolute levels of anxiety symptoms and anxiety-related disability at all follow-up assessments. These effects were unlikely to be strictly due to baseline elevations in anxiety-related symptoms and disability, as patients with and without migraines did not differ significantly on these measures at baseline.
Several studies have found particularly strong associations between migraine headaches and anxiety disorders, with some reporting that migraine had the strongest association of all assessed CMCs (1, 16). In addition, having an anxiety or mood disorder diagnosis predicts worse outcome of migraine treatment (41). Our preliminary results suggest that anxiety-migraine comorbidity also may complicate the treatment of anxiety, with the negative impact observed most clearly in long-term follow-up. Several explanations for this finding are possible. To the extent that migraine headaches cause a restriction of activities, they may prevent the corrective learning experiences (and anxiety reduction) that occur with regular exposure to anxiety-provoking stimuli and situations. Such an effect could be expected to appear after treatment withdrawal (in this case at 18 months) because at this point patients no longer have the instruction, direct support, and accountability for exposure practice that results from regular contact with a clinician. However, an argument against this interpretation is that the other CMCs evaluated (particularly gastrointestinal disease) also can lead to a restriction of activities and therefore it is unclear why this detrimental effect would only be observed in relation to migraine. Alternatively, migraine may be a causal factor in certain types of anxiety (e.g., anxiety related to pain or the anticipation of pain2) that are less responsive to standard CBT or pharmacotherapy; or patients with anxiety-migraine comorbidity may have higher levels of neurobiological or temperamental diatheses (e.g., neuroticism, anxiety sensitivity) that predispose them to both conditions and make their anxiety symptoms more difficult to treat.
Limitations
This study evaluated the effects of medical comorbidity on outcomes from an RCT of a multi-faceted intervention for a range of anxiety disorders. Due to statistical power considerations, the study was not designed to evaluate higher level (4-way) interactions involving principal anxiety diagnosis (GAD, PD, PTSD, or SAD). In addition, patients were not randomized to receive specific treatment components (i.e., CBT, pharmacotherapy) and thus we were unable to evaluate possible moderation effects of CMCs on patients’ response to these components. The results reported here cannot be assumed to apply uniformly to each individual anxiety disorder or to CBT versus pharmacotherapy.
Statistical power considerations also limited our ability to examine effects of specific CMCs on anxiety treatment outcomes; however, we undertook exploratory analyses of the effects of three high frequency CMCs in this sample that were of conceptual interest, and which taken together represent a broad range of medical comorbidity commonly found in patients with anxiety disorders. The divergent outcomes for asthma, migraine, and gastrointestinal disease sufferers illustrate the limitations of examining medical comorbidity in the aggregate. Additional investigation is needed to further elucidate the effects of specific CMCs on treatments for anxiety disorders.
Measurement of medical comorbidity was based entirely on patients’ self-report. Group membership (High versus Low Medical Comorbidity) therefore depended on the accuracy of their answers to the survey questions focused on medical illnesses. It would have been ideal to corroborate diagnoses via examination of medical records; however, these data were not available to investigators. In addition, if more detailed assessment of medical conditions had been incorporated into the baseline assessment, we could have included variables related to the severity of medical illness. Future research should go beyond assessing the presence/absence of CMCs, with the aim of incorporating information about the severity of co-occurring medical illness into models of anxiety treatment response.
Finally, the results of this investigation may not generalize to groups of patients who were not well-represented in the study sample (e.g., patients from underrepresented ethnic minority groups; patients with low levels of education). Future investigations should attempt to evaluate the effects of medical comorbidity on anxiety treatment outcome in more diverse samples.
Conclusions
Co-occurring CMCs are common in primary care patients with anxiety disorders, and are associated with more severe baseline anxiety and higher frequencies of co-occurring MDD and PTSD. Nevertheless, patients with multiple CMCs achieve similar degrees of improvement in anxiety symptoms and anxiety-related disability as patients with one or zero CMCs. Different CMCs may have divergent effects on anxiety treatment outcomes. This study suggested that migraine was associated with poorer long-term improvement in anxiety symptoms. Future studies are needed to corroborate this finding and to further evaluate the effects of specific CMCs on anxiety treatment outcomes.
While this study makes important contributions to the literature on anxiety disorders and medical comorbidity (especially in demonstrating that patients with multiple CMCs can benefit from anxiety treatment as much as those with low medical comorbidity), it also highlights the need for further study of interactions between medical conditions and the etiology, phenomenology, and treatment of anxiety disorders. The high prevalence of anxiety disorder/CMC comorbidity, the commonplace occurrence of complicated presentations (multiple CMCs, more severe anxiety, co-occurring depression), and the apparently divergent effects of distinct CMCs all indicate that better understanding of these relationships is crucial to maximizing the impact of primary care-based interventions for anxiety disorders.
Acknowledgments
This work was supported by grants U01 MH057858 (Dr. Roy-Byrne), U01 MH058915 (Dr. Craske), U01 MH 070022 (Dr. Sullivan), U01 MH070018 (Dr. Sherbourne), U01 MH057835 and K24 MH64122 (Dr. Stein), and K01 MH072952 (Dr. Chavira) from the National Institute of Mental Health, Bethesda, MD, USA.
Acronyms
- CMC
chronic medical condition
- CI
confidence interval
Footnotes
Results do not change substantially when “Number of CMCs” is used instead of High vs. Low Medical Comorbidity groups. The Number of CMCs x Treatment Assignment x Time and Number of CMCs x Time effects on BSI-A and SDS scores were non-significant (all p’s > .25).
A post-hoc analysis evaluated whether other pain-related CMCs had similar effects on anxiety outcomes; however, neither back problems nor arthritis showed any significant effects on improvement of anxiety symptoms or anxiety-related disability.
Disclosures: Dr. Roy-Byrne is a consultant to Valant Medical Solutions (EMR Company), and is Editor-in-Chief of Journal Watch Psychiatry, Depression and Anxiety, and UpToDate Psychiatry. Dr. Stein is paid for his editorial work on the journal Depression and Anxiety and the evidence-based medical information provider, UpToDate Psychiatry. The other authors have no disclosures.
References
- 1.El-Gabalawy R, Mackenzie CS, Shooshtari S, Sareen J. Comorbid physical health conditions and anxiety disorders: a population-based exploration of prevalence and health outcomes among older adults. Gen Hosp Psychiatry. 2011;33:556–64. doi: 10.1016/j.genhosppsych.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Ormel J, Demler O, Stang PE. Comorbid mental disorders account for the role impairment of commonly occurring chronic physical disorders: results from the National Comorbidity Survey. J Occup Environ Med. 2003;45:1257–66. doi: 10.1097/01.jom.0000100000.70011.bb. [DOI] [PubMed] [Google Scholar]
- 3.Sareen J, Cox BJ, Clara I, Asmundson GJ. The relationship between anxiety disorders and physical disorders in the U.S. National Comorbidity Survey. Depress Anxiety. 2005;21:193–202. doi: 10.1002/da.20072. [DOI] [PubMed] [Google Scholar]
- 4.Roy-Byrne PP, Davidson KW, Kessler RC, Asmundson GJ, Goodwin RD, Kubzansky L, Lydiard RB, Massie MJ, Katon W, Laden SK, Stein MB. Anxiety disorders and comorbid medical illness. Gen Hosp Psychiatry. 2008;30:208–25. doi: 10.1016/j.genhosppsych.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Blanchard EB, Keefer L, Lackner JM, Galovski TE, Krasner S, Sykes MA. The role of childhood abuse in Axis I and Axis II psychiatric disorders and medical disorders of unknown origin among irritable bowel syndrome patients. J Psychosom Res. 2004;56:431–6. doi: 10.1016/S0022-3999(03)00627-5. [DOI] [PubMed] [Google Scholar]
- 6.Katon W, Lozano P, Russo J, McCauley E, Richardson L, Bush T. The prevalence of DSM-IV anxiety and depressive disorders in youth with asthma compared with controls. J Adolesc Health. 2007;41(5):455–63. doi: 10.1016/j.jadohealth.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodwin RD, Jacobi F, Thefeld W. Mental disorders and asthma in the community. Arch Gen Psychiatry. 2003;60:1125–30. doi: 10.1001/archpsyc.60.11.1125. [DOI] [PubMed] [Google Scholar]
- 8.Stewart WF, Linet MS, Celentano DD. Migraine headaches and panic attacks. Psychosom Med. 1989;51:559–69. doi: 10.1097/00006842-198909000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Arnold LM, Hudson JI, Keck PE, Auchenbach MB, Javaras KN, Hess EV. Comorbidity of fibromyalgia and psychiatric disorders. J Clin Psychiatry. 2006;67:1219–25. doi: 10.4088/jcp.v67n0807. [DOI] [PubMed] [Google Scholar]
- 10.McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain. 2003;106:127–33. doi: 10.1016/s0304-3959(03)00301-4. [DOI] [PubMed] [Google Scholar]
- 11.Kangas M, Henry JL, Bryant RA. The course of psychological disorders in the 1st year after cancer diagnosis. J Consult Clin Psychol. 2005;73:763–8. doi: 10.1037/0022-006X.73.4.763. [DOI] [PubMed] [Google Scholar]
- 12.van Balkom AJ, Beekman AT, de Beurs E, Deeg DJ, van Dyck R, van Tilburg W. Comorbidity of the anxiety disorders in a community-based older population in The Netherlands. Acta Psychiatr Scand. 2000;101:37–45. doi: 10.1034/j.1600-0447.2000.101001037.x. [DOI] [PubMed] [Google Scholar]
- 13.Lydiard RB. Increased prevalence of functional gastrointestinal disorders in panic disorder: clinical and theoretical implications. CNS Spectr. 2005;10:899–908. doi: 10.1017/s1092852900019878. [DOI] [PubMed] [Google Scholar]
- 14.Lydiard RB, Greenwald S, Weissman MM, Johnson J, Drossman DA, Ballenger JC. Panic disorder and gastrointestinal symptoms: findings from the NIMH Epidemiologic Catchment Area project. Am J Psychiatry. 1994;151:64–70. doi: 10.1176/ajp.151.1.64. [DOI] [PubMed] [Google Scholar]
- 15.Cass AR, Volk RJ, Nease DE., Jr Health-related quality of life in primary care patients with recognized and unrecognized mood and anxiety disorders. Int J Psychiatry Med. 1999;29:293–309. doi: 10.2190/CPYJ-2HBF-RGCN-64V2. [DOI] [PubMed] [Google Scholar]
- 16.Sareen J, Jacobi F, Cox BJ, Belik SL, Clara I, Stein MB. Disability and poor quality of life associated with comorbid anxiety disorders and physical conditions. Arch Intern Med. 2006;166:2109–16. doi: 10.1001/archinte.166.19.2109. [DOI] [PubMed] [Google Scholar]
- 17.Richardson LP, Lozano P, Russo J, McCauley E, Bush T, Katon W. Asthma symptom burden: relationship to asthma severity and anxiety and depression symptoms. Pediatrics. 2006;118:1042–51. doi: 10.1542/peds.2006-0249. [DOI] [PubMed] [Google Scholar]
- 18.Katon W, Lin EH, Kroenke K. The association of depression and anxiety with medical symptom burden in patients with chronic medical illness. Gen Hosp Psychiatry. 2007;29:147–55. doi: 10.1016/j.genhosppsych.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Ludman E, Katon W, Russo J, Simon G, Von Korff M, Lin E, Ciechanowski P, Kinder L. Panic episodes among patients with diabetes. Gen Hosp Psychiatry. 2006;28:475–81. doi: 10.1016/j.genhosppsych.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Kubzansky LD, Cole SR, Kawachi I, Vokonas P, Sparrow D. Shared and unique contributions of anger, anxiety, and depression to coronary heart disease: a prospective study in the normative aging study. Ann Behav Med. 2006;31:21–9. doi: 10.1207/s15324796abm3101_5. [DOI] [PubMed] [Google Scholar]
- 21.Smoller JW, Pollack MH, Wassertheil-Smoller S, Jackson RD, Oberman A, Wong ND, Sheps D. Panic attacks and risk of incident cardiovascular events among postmenopausal women in the Women’s Health Initiative Observational Study. Arch General Psychiatry. 2007;64:1153–60. doi: 10.1001/archpsyc.64.10.1153. [DOI] [PubMed] [Google Scholar]
- 22.Katon WJ, Richardson L, Russo J, Lozano P, McCauley E. Quality of mental health care for youth with asthma and comorbid anxiety and depression. Med Care. 2006;44:1064–72. doi: 10.1097/01.mlr.0000237421.17555.8f. [DOI] [PubMed] [Google Scholar]
- 23.American Psychiatric Association Work Group on Panic Disorder. Practice guideline for the treatment of patients with panic disorder. Am J Psychiatry. 2009;166(2 Suppl):1–34. [PubMed] [Google Scholar]
- 24.Roy-Byrne P, Stein MB, Russo J, Craske M, Katon W, Sullivan G, Sherbourne CD. Medical illness and response to treatment in primary care panic disorder. Gen Hosp Psychiatry. 2005;27:237–43. doi: 10.1016/j.genhosppsych.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Roy-Byrne P, Craske MG, Sullivan G, Rose RD, Edlund MJ, Lang AJ, Bystritsky A, Welch SS, Chavira DA, Golinelli D, Campbell-Sills L, Sherbourne CD, Stein MB. Delivery of evidence-based treatment for multiple anxiety disorders in primary care: a randomized controlled trial. JAMA. 2010;303:1921–8. doi: 10.1001/jama.2010.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Craske MG, Stein MB, Sullivan G, Sherbourne C, Bystritsky A, Rose RD, Lang AJ, Welch S, Campbell-Sills L, Golinelli D, Roy-Byrne P. Disorder-specific impact of coordinated anxiety learning and management treatment for anxiety disorders in primary care. Arch Gen Psychiatry. 2011;68:378–88. doi: 10.1001/archgenpsychiatry.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lehrer P, Feldman J, Giardino N, Song HS, Schmaling K. Psychological aspects of asthma. J Consult Clin Psychol. 2002;70:691–711. doi: 10.1037//0022-006x.70.3.691. [DOI] [PubMed] [Google Scholar]
- 28.Haque DB, Rahman DK, Hoque DA, Hasan DA, Chowdhury DR, Khan DS, Alam DM, Habib PM, Mohammad PQ. Precipitating and relieving factors of migraine versus tension type headache. BMC Neurol. 2012;12:82. doi: 10.1186/1471-2377-12-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Means-Christensen AJ, Sherbourne CD, Roy-Byrne PP, Craske MG, Stein MB. Using five questions to screen for five common mental disorders in primary care: diagnostic accuracy of the Anxiety and Depression Detector. Gen Hosp Psychiatry. 2006;28:108–18. doi: 10.1016/j.genhosppsych.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Sullivan G, Craske MG, Sherbourne C, Edlund MJ, Rose RD, Golinelli D, Chavira DA, Bystritsky A, Stein MB, Roy-Byrne PP. Design of the Coordinated Anxiety Learning and Management (CALM) study: innovations in collaborative care for anxiety disorders. Gen Hosp Psychiatry. 2007;29:379–87. doi: 10.1016/j.genhosppsych.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Craske MG, Rose RD, Lang A, Welch SS, Campbell-Sills L, Sullivan G, Sherbourne C, Bystritsky A, Stein MB, Roy-Byrne PP. Computer-assisted delivery of cognitive behavioral therapy for anxiety disorders in primary-care settings. Depress Anxiety. 2009;26:235–42. doi: 10.1002/da.20542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rose RD, Lang AJ, Welch SS, Campbell-Sills L, Chavira DA, Sullivan G, Sherbourne C, Bystritsky A, Stein MB, Roy-Byrne PP, Craske MG. Training primary care staff to deliver a computer-assisted cognitive-behavioral therapy program for anxiety disorders. Gen Hosp Psychiatry. 2011;33:336–42. doi: 10.1016/j.genhosppsych.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 34.Derogatis L. BSI-18: Brief Symptom Inventory 18 Administration, Scoring, and Procedures Manual. Minneapolis, MN: NCS Pearson, Inc; 2001. [Google Scholar]
- 35.Sheehan DV, Harnett-Sheehan K, Raj BA. The measurement of disability. Int Clin Psychopharmacol. 1996;11(Suppl 3):89–95. doi: 10.1097/00004850-199606003-00015. [DOI] [PubMed] [Google Scholar]
- 36.Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. Cary, NC: SAS Institute, Inc; 1996. [Google Scholar]
- 37.Little R, Rubin D. Statistical Analysis with Missing Data. New York, NY: John Wiley & Sons; 1987. [Google Scholar]
- 38.Roy-Byrne PP, Katon W, Cowley DS, Russo J. A randomized effectiveness trial of collaborative care for patients with panic disorder in primary care. Arch Gen Psychiatry. 2001;58:869–76. doi: 10.1001/archpsyc.58.9.869. [DOI] [PubMed] [Google Scholar]
- 39.Rollman BL, Belnap BH, Mazumdar S, Houck PR, Zhu F, Gardner W, Reynolds CF, Schulberg HC, Shear MK. A randomized trial to improve the quality of treatment for panic and generalized anxiety disorders in primary care. Arch Gen Psychiatry. 2005;62:1332–41. doi: 10.1001/archpsyc.62.12.1332. [DOI] [PubMed] [Google Scholar]
- 40.Kerns RD, Sellinger J, Goodin BR. Psychological treatment of chronic pain. Annu Rev Clin Psychol. 2011;7:411–34. doi: 10.1146/annurev-clinpsy-090310-120430. [DOI] [PubMed] [Google Scholar]
- 41.Seng EK, Holroyd KA. Psychiatric comorbidity and response to preventative therapy in the treatment of severe migraine trial. Cephalalgia. 2012;32:390–400. doi: 10.1177/0333102411436333. [DOI] [PubMed] [Google Scholar]