Abstract
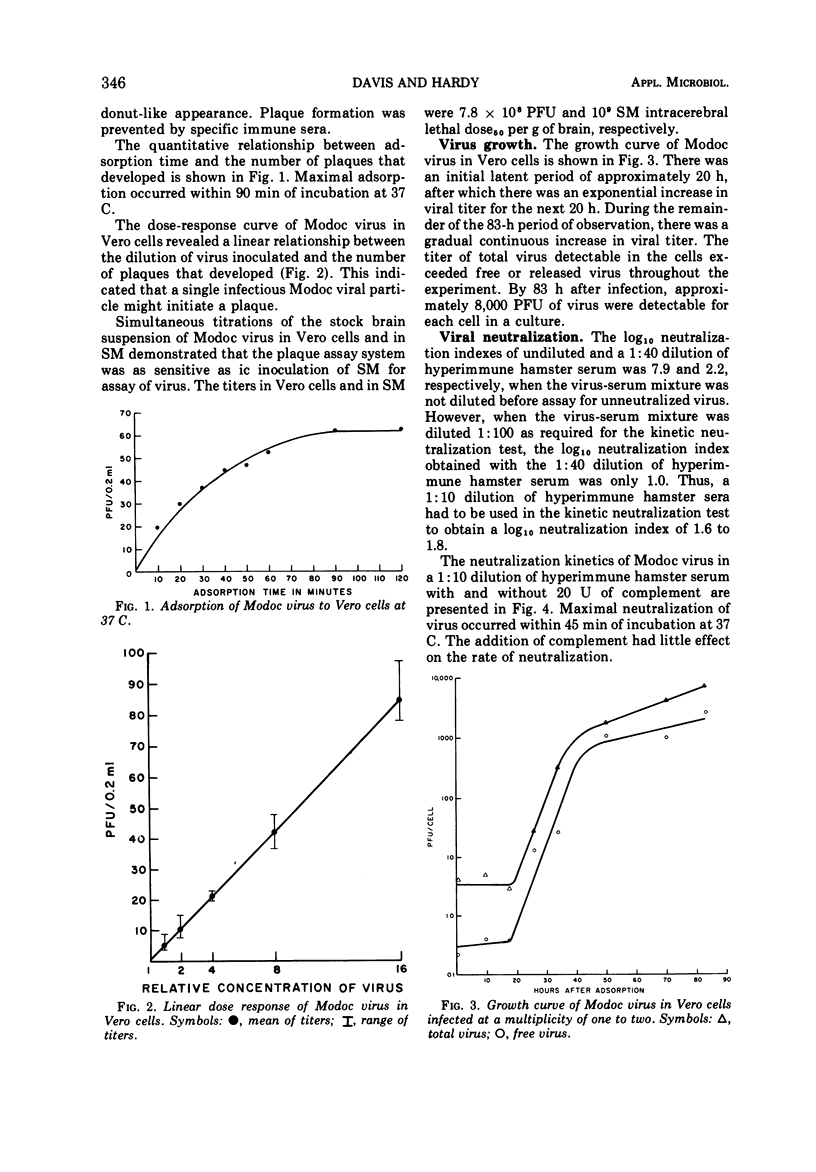
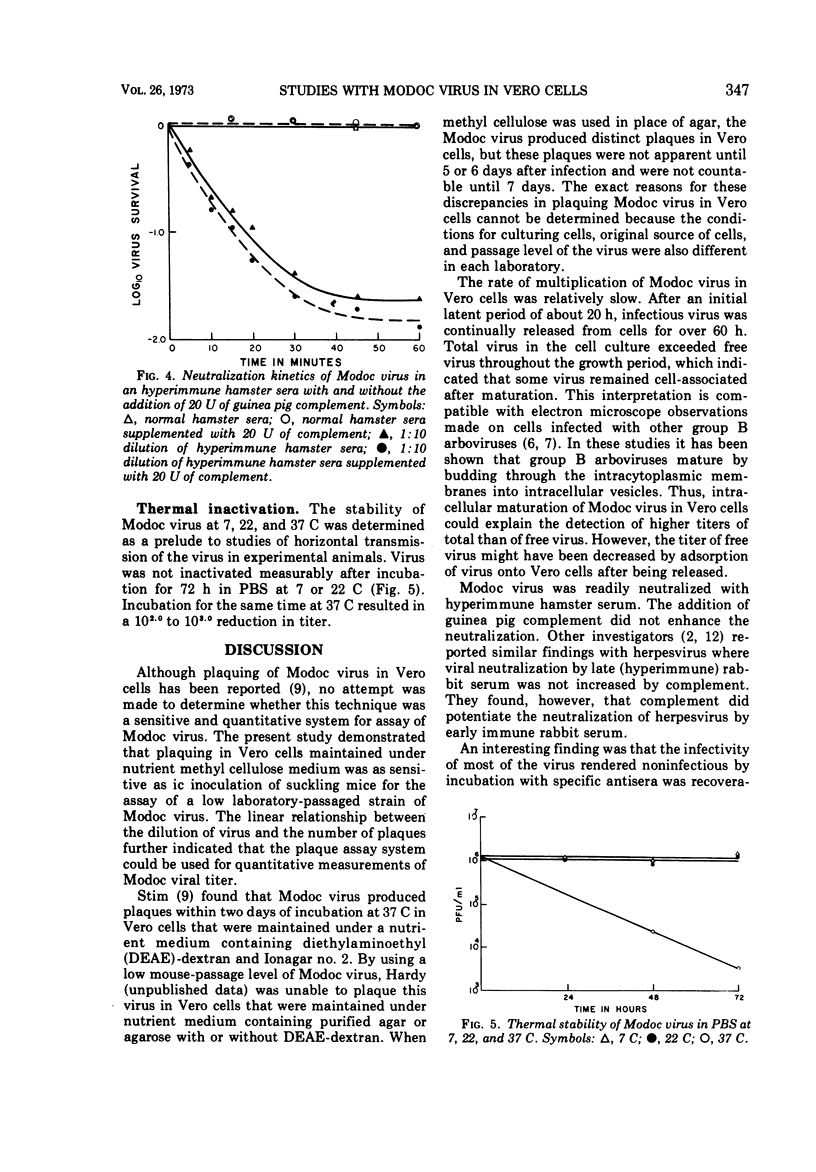
A sensitive and quantitative assay system is described for plaquing Modoc virus in Vero cells. Neutralizing antibodies to Modoc virus could be detected by using this in vitro system by their interference with viral plaque formation. Virus was readily neutralized within 30 min at 37 C by a 1:10 dilution of hyperimmune hamster serum. The rate of neutralization and the total amount of virus neutralized was not altered significantly by the addition of 20 U of guinea pig complement to the hyperimmune hamster serum. A study of the growth of Modoc virus in Vero cells is also presented. After an initial latent period of 20 h, viral titer increased exponentially for 20 h. By 83 h after infection, 8,000 plaque-forming units of virus were detected per cell. The stability of viral infectivity in phosphate-buffered saline at pH 7.4 was evaluated. No reduction in viral titer was detected after 3 days at 7 or 22 C. A continuous decrease in infectivity at 37 C was observed, however, throughout the observation period.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CASALS J. Antigenic relationship between Powassan and Russian spring-summer encephalitis viruses. Can Med Assoc J. 1960 Feb 13;82:355–358. [PMC free article] [PubMed] [Google Scholar]
- Hampar B., Notkins A. L., Mage M., Keehn M. A. Heterogeneity in the properties of 7 S and 19S rabbit-neutralizing antibodies to herpes simplex virus. J Immunol. 1968 Mar;100(3):586–593. [PubMed] [Google Scholar]
- Johnson H. N. Ecological implications of antigenically related mammalian viruses for which arthropod vectors are unknown and avian associated soft tick viruses. Jpn J Med Sci Biol. 1967 Dec;20 (Suppl):160–166. [PubMed] [Google Scholar]
- Johnson H. N. Long-term persistence of Modoc virus in hamster-kidney cells. In vivo and in vitro demonstration. Am J Trop Med Hyg. 1970 May;19(3):537–539. doi: 10.4269/ajtmh.1970.19.537. [DOI] [PubMed] [Google Scholar]
- Karabatsos N., Buckley S. M. Susceptibility of the baby-hamster kidney-cell line (BHK-21) to infection with arboviruses. Am J Trop Med Hyg. 1967 Jan;16(1):99–105. doi: 10.4269/ajtmh.1967.16.99. [DOI] [PubMed] [Google Scholar]
- Matsumura T., Stollar V., Schlesinger R. W. Studies on the nature of dengue viruses. V. Structure and development of dengue virus in Vero cells. Virology. 1971 Nov;46(2):344–355. doi: 10.1016/0042-6822(71)90036-5. [DOI] [PubMed] [Google Scholar]
- Murphy F. A., Harrison A. K., Gary G. W., Jr, Whitfield S. G., Forrester F. T. St. Louis encephalitis virus infection in mice. Electron microscopic studies of central nervous system. Lab Invest. 1968 Dec;19(6):652–662. [PubMed] [Google Scholar]
- YOSHINO K., TANIGUCHI S. STUDIES ON THE NEUTRALIZATION OF HERPES SIMPLEX VIRUS. I. APPEARANCE OF NEUTRALIZING ANTIBODIES HAVING DIFFERENT GRADES OF COMPLEMENT REQUIREMENT. Virology. 1965 May;26:44–53. doi: 10.1016/0042-6822(65)90024-3. [DOI] [PubMed] [Google Scholar]



