Abstract
Stress pathology is associated with hypothalamic-pituitary-adrenal (HPA) axis dysregulation and aberrant glucocorticoid responses. Recent studies indicate increases in prefrontal cortical ionized calcium-binding adapter molecule 1 (Iba-1) staining following repeated restraint, reflecting increased microglial densities. Our experiments tested expression of Iba-1 staining in the prelimbic cortex (PL), infralimbic cortex (IL) and the hypothalamic paraventricular nucleus (PVN) following two-week exposure to repeated restraint (RR) and chronic variable stress (CVS), representing homotypic and heterotypic regimens, respectively. Unstressed animals served as controls. We specifically examined Iba-1 immunofluorescence in layers 2 and 3 versus layers 5 and 6 of the PL and IL, using both cell number and field staining density. Iba-1 field staining density was increased in both the PL and IL following RR in comparison to controls. This effect was not observed following CVS. Furthermore, PVN Iba-1 immunoreactivity was not affected by either stress regimen. Cell number did not vary within any brain areas or across stress exposures. Changes in microglial field density did not reflect changes in vascular density. Increases in PL and IL microglial density indicate selective microglial activation during RR, perhaps due to mild stress in the context of limited elevations in anti-inflammatory glucocorticoid actions. Supported by NIH grants [MH049698 and MH069860].
Keywords: Microglia, prefrontal cortex, chronic stress, Iba-1, glucocorticoids
1. Introduction
Chronic stress plays a prominent role in triggering or enhancing many psychiatric disorders, including major depressive disorder and schizophrenia [1, 2, 3]. Stress-responsive brain regions and limbic circuitry are receiving wide attention as potential mediators of chronic stress pathology. The medial prefrontal cortex (mPFC) is highly stress reactive and undergoes significant changes in morphology and function following chronic stress [3, 4]. These changes align with behavioral phenotypes in rats that are consistent with depression symptoms in humans [4, 5, 7].
Many of the symptoms associated with depression are accompanied by immune activation. Depressed mood, anhedonia, weight change, and fatigue are characteristics of depression, but are also related to sickness behavior [6]. Chronic restraint stress induces microglial hypertrophy in the PFC, hippocampus, and nucleus accumbens [7], suggestive of inflammatory reactions in the brain. Morphological changes in PFC microglia following chronic restraint stress are correlated with increases in neuronal ΔFosB and deficits in the T-maze; these effects are ameliorated by the microglial inhibitor minocycline [5].
Microglia have a dynamic morphology that is indicative of their function in response to various stimuli. Most microglia in the healthy brain are in a resting or ramified state, characterized by small cell bodies and thin processes [8]. However, ramified microglia are constantly surveying the environment and undergo rapid morphological changes to exert a variety of functions [8]. Microglia react to signals of neuronal damage and maintain tissue homeostasis. Resting microglia transition to a hyper-ramified state after mild stimulation, characterized by a thickening and branching of secondary processes. Physical tissue injury induces microglia to withdraw their processes to become reactive and then phagocytic [9]. Chronic stress stimulates microglia into hyper-ramification, which may represent a novel response to stress and unique microglial phenotype with mechanisms not yet fully understood. Hyper-ramification of microglia during stress may be associated with local inflammation, which can contribute to neurodegeneration, demyelination, and synaptic dysfunction [10]. For example, microglia have been implicated in actively pruning hippocampal synapses in the developing brain [11, 12].
The glucocorticoid stress response is initiated by the hypothalamic-pituitary adrenocortical (HPA) axis. The paraventricular nucleus (PVN) of the hypothalamus initiates a hormonal cascade that stimulates adrenal synthesis and release of glucocorticoids (GCs). Peripherally, GCs are potently immunosuppressant. Centrally, GCs can either activate or inhibit microglial activity in various contexts, ranging from in vitro, in vivo, acute, chronic, in combination with LPS, etc [13, 14, 15]. The effects of chronic stress-induced microglial activation are not well understood, despite the GC receptor (GR) being among the most abundant microglial steroid receptors [13]. To identify the role of GCs and microglia in chronic stress, the current study examined microglial responses to two separate stress regimens that induce distinct GC profiles. Tynan et al. demonstrated microglial hyper-ramification following repeat restraint stress, while measuring sucrose preference, body weight, and body temperature but not corticosterone [7]. Repeated restraint stress causes significant HPA axis habituation over the course of several exposures [16], and basal corticosterone secretion, adrenal hypertrophy, and thymic atrophy are generally less than those observed in non-habituating regimens [17]. These data raise questions regarding the possible role of microglia in adaptive vs. pathological consequences of chronic stress. The current study provides a side-by-side test of the impact of habituating vs. non-habituating stress protocols as a means of determining what type of stressor exposure causes microglial activation.
2. Materials and Methods
2.1 Subjects
Male Sprague-Dawley rats from Harlan (Indianapolis, IN, USA), weighing 250–275 g on arrival, were housed two per cage in clear polycarbonate cages with granulated corncob bedding. Food and water were available ad libitum. The colony room was temperature and humidity controlled with a 12-hour light cycle (lights on 6:00 am; lights off 6:00 pm). Rats acclimated to the colony facility for 1 week prior to experimental manipulations. Subjects were randomly assigned to one of three groups: control unhandled (CON, n=10), repeat restraint stress (RR, n=10), and chronic variable stress (CVS, n=12). All experimental procedures were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Animals and approved by the University of Cincinnati Institutional Animal Care and Use Committee.
2.2 Stress Regimens
For RR, subjects were fixed into Plexiglas restraint tubes for 30 minutes every morning at 10:00 for 14 consecutive days. The CVS regimen lasted 14 consecutive days, with two stressors every day, and was run concurrently with the RR group. Morning stressors were applied any time between 08:00 and 11:30h, while afternoon stressors were applied any time between 13:30 and 17:00h. They included: hypoxia (30 min in 8% O2/92% N2), rotation stress (1 h at 100 rpm on a platform orbital shaker), warm swim (20 min at 31°C), cold swim (10 min at 18°C), cold room (1 h at 4°C), and overnight crowding. On the morning of the 15th day, the rats were administered an overdose of sodium pentobarbital (150mg/kg) and perfused with phosphate-buffered saline, followed by 4% paraformaldehyde. Brains were postfixed overnight in 4% paraformaldehyde and transferred to 30 % sucrose (4°C).
2.3 Immunohistochemistry
Using a sliding microtome, 30 μm sections were cut and stored at −20°C in cryoprotectant (0.1 M phosphate buffer, 30% sucrose, 1% polyvinylpyrrolidone, and 30% ethylene glycol). Sections were transferred from cryoprotectant into 50mM potassium phosphate-buffered saline (KPBS) (pH 7.2) at room temperature (RT). After rinsing off the cryoprotectant with KPBS (5×5 min), sections were incubated for 1 h at RT in blocking solution (50mM KPBS, 0.1% bovine serum albumin, 0.2% Triton X-100). Immediately thereafter, sections were incubated for 16 h at RT in primary anti-Iba-1 polyclonal rabbit antibody for visualizing microglia (1:1500; Synaptic Systems; Goettingen, Germany) and primary anti-NeuN monoclonal mouse antibody (1:200; Millipore, Temecula, CA, USA) for identifying the boundaries of the PVN and layers of the PFC. Dilutions were in blocking solution (50 mm KPBS, 0.1% bovine serum albumin, and 0.2% Triton X-100). Sections were rinsed (5×5 min) in KPBS and then incubated for 1 h at RT in both Cy 3 donkey anti-rabbit 1:500 (Jackson Immuno Research, West Grove, PA, USA) and Alexa 488 goat anti-mouse 1:500 (Molecular Probes, Eugene, OR, USA), for microglia. For blood vessels, the Alexa 488 secondary was used in conjunction with DyLight 594 labeled Lycopersicon Esculentum (Tomato) Lectin (1:400; Vector, Burlingame, CA, USA) Dilutions were with blocking solution. Sections were rinsed (4×5 min) with KPBS and mounted onto slides. Dried slides were rinsed with Nano H20 and coverslipped with Fluka mounting medium (Sigma Aldrich, St. Louis, MO, USA) to be used for imaging.
2.4 Imaging
The Zeiss Axiovision 4.6 software was used for all image quantification and anatomical landmarks were determined using features described in the Paxinos & Watson rat brain atlas (1997, [18]). The individual creating and analyzing the images was blind to treatment conditions.
For cell counts, regions of interest (ROIs) were first outlined using only the Alexa channel on 10x images. These ROIs included the PVN (AP −1.8, DV −7.7 to −8.2, ML ± 0.2 to 0.6) and PFC (AP +3.5, DV −3.0 to −5.0, ML ± 0.25 to 1.0). Using the Cy3 channel, individual microglia cells were selected manually and quantified, using the Axiovision 4.6 software.
To quantify the percent area occupied by Iba-1 immunoreactivity, or field density, Zeiss Apotome Deconvolution software was used to take 40x Cy3 Z-stack images. Z-stacks were taken from the medial parvocellular subdivision of the PVN (AP −1.8, DV −7.8 to −8.0, ML ± 0.2 to 0.4). In the PFC, Z-stacks were from layers 2 and 3 of the infralimbic cortex (IL), layers 2 and 3 of the prelimbic cortex (PL), layers 5 and 6 of the IL, or layers 5 and 6 of the PL. Using the LSM Image Browser, Z-stacks were merged into several projection images. Each projection image consisted of 5 consecutive 1 μm images from the Z-stack. Using Axiovision automatic program measurements, a consistent threshold level was used to select positive staining and the percent area occupied by the Iba-1 immunoreactivity above threshold was calculated. Objects comprising less than 10 pixels were removed. DyLight lectin labeling was quantified with field density in a similar way. Because the lectin lightly labeled microglial processes, objects 0–300 pixels were removed to ensure that percent area was calculated for vasculature alone. Any extraneous objects were manually removed.
Data analysis was performed using Sigma Stat (Systat Software, San Jose, CA, USA). Data are shown as mean ± SE. Outliers were removed if the values fell outside of 1.96 times the SD and 1.5 times the interquartile range [19]. For any outlier value, that subject was removed from analysis. Analysis consisted of one-way ANOVAs with Fisher’s Least Significant Difference (LSD) post hoc test using group (CON, CVS, and RR) as between-subjects factor.
3. Results
3.1 Stress regimens and adrenal size
The efficacy of the CVS protocol was confirmed by increased adrenal weights [F(2, 29) = 7.49; p = 0.002] (Table 1). Relative to controls, adrenal weight was increased in the CVS group but not in RR animals, consistent with a greater cumulative impact of the CVS regimen on adrenal growth (p < 0.05, Fisher’s LSD post hoc).
Table 1.
Average adrenal weights divided by body weights across stress groups.
| Group | Adrenal wt (mg)/Body wt (g) |
|---|---|
| CVS | 0.150 ± 0.0039* |
| RR | 0.138 ± 0.0058 |
| CON | 0.125 ± 0.0042 |
The * indicates greater than CON. (mean ± SE)
3.2 Iba-1
The analysis of Iba-1 positive cells in the PVN and PFC revealed only ramified and hyper-ramified microglia, and not reactive or phagocytic. The field density was used to determine whether stress had stimulated microglia in the PVN or PFC from the resting ramified state to a hyper-ramified state. For cell counts and field density, NeuN immunolabeling helped identify boundaries of the PVN (Fig 1) and discriminate between IL, PL, layers 2 and 3, layers 5 and 6.
Figure 1.
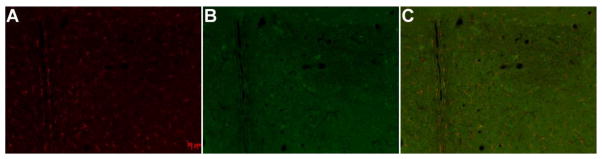
Localization of Iba-1 immunofluorescence in the PVN (a.) Iba-1 on Cy3 (b.) NeuN on Alexa 488 shows the boundaries of the PVN (c.) Iba-1 and NeuN merged
3.3 Iba-1 in the PVN
Cell counts in the PVN, analyzed by one-way ANOVA, showed no effect of stress on the number of Iba-1 positive microglia (Fig 2a). The average field densities of the medial parvocellular subdivision of the PVN also did not differ across groups (Fig 3).
Figure 2.
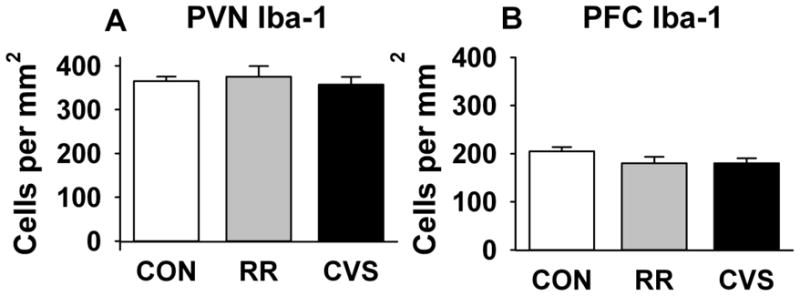
Iba-1 positive cell counts in (a.) PVN and (b.) PFC. There were no RR or CVS-related differences in the number of Iba-1 positive microglia in any region examined.
Figure 3.
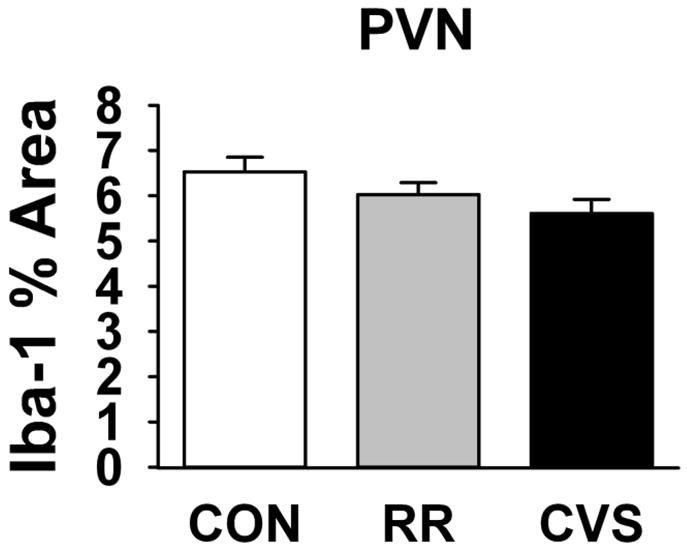
Iba-1 percent area occupied in the PVN. There was no effect of RR or CVS on PVN microglia coverage.
3.4 Iba-1 in the PFC
Iba-1 cell counts in the PFC indicated no effect of stress on the number of microglia (Fig 2b). The projection images show that the proportion of hyper-ramified microglia increases after chronic homotypic stress (RR, Fig 4b) but not after chronic heterotypic stress (CVS, Fig 4c). Microglia after CVS remained in a resting or ramified state that did not differ from controls (CON, Fig 4a).
Figure 4.
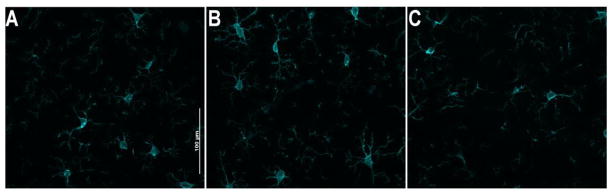
Iba-1 projection images in layers 2/3 of the PL from (a.) CON (b.) RR, showed thickening of microglial processes (c.) CVS
3.5 Iba-1 in the PL PFC
In the PL PFC, one-way ANOVA revealed an overall effect of stress on Iba-1 percent area [F(2, 25) = 3.71; p = 0.039] (Fig 5a). Only RR had greater Iba-1 field density than CON and it was also higher than the CVS group (p < 0.05, Fisher’s LSD post hoc). There was no difference between CON and CVS. There was a significant effect of stress in specifically layers 2 and 3 of the PL [F(2, 25) = 4.13, p = 0.028], RR again had greater Iba-1 coverage than both CON and CVS (p < 0.05, Fisher’s LSD post hoc) (Fig 5b). This effect failed to reach significance in layers 5 and 6 of the PL [F(2, 25) = 2.98; p = 0.069] (Fig 5c).
Figure 5.
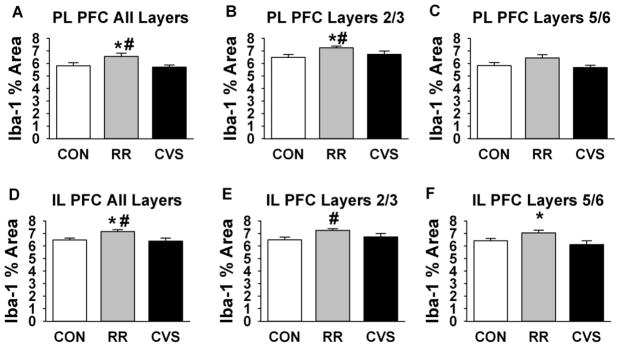
Iba-1 staining is increased in the PL and IL following RR, but not CVS. # indicates difference from CON and * indicates difference from CVS (p < 0.05) (a–c.) Iba-1 field density in the PL (a.) Averaged (b.) Layers 2/3 (c.) Layers 5/6 (d–f.) Iba-1 field density in the IL (d.) overall e.) Layers 2/3 (f.) Layers 5/6
3.6 Iba-1 in the IL PFC
An overall effect of stress on Iba-1 staining density was also observed in the IL [F(2, 25) = 5.241; p = 0.013] (Fig 5d), with RR being greater than both CON and CVS (p < 0.05, Fisher’s LSD post hoc). Again, the CVS group did not significantly differ from CON. There was a significant effect of stress on Iba-1 field density in layers 2–3 [F(2, 25) = 3.51; p = 0.047], with the RR group having significantly greater staining density than controls (p < 0.05, Fisher’s LSD post hoc) (Fig 5e). There was also a significant effect of stress on Iba-1 field density in layers 5–6 [F(2, 25) = 4.17; p = 0.029], with the RR group having significantly greater staining density than the CVS group (p < 0.05, Fisher’s LSD post hoc) (Fig 5f).
3.7 Lectin
In the medial parvocellular PVN, PL PFC, and IL PFC, vascular density assessed by DyLight lectin percent area did not show significant changes following exposure to either RR or CVS (Fig 6a,b,c). The PVN showed greater vascular density than PFC, but neither region was affected by stress.
Figure 6.
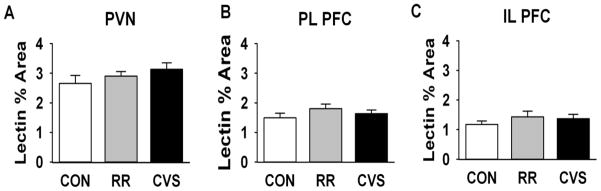
Percent area occupied by lectin immunofluorescence in the (a.) PVN (b.) PL (c.) IL. There was no effect of RR or CVS on vascular densities, as determined by lectin staining, in any region.
4. Discussion
The present study indicates a selective increase in microglial ramification in the PL and IL in response to chronic restraint, a stress exposure paradigm that results in significant habituation of the HPA axis GC response [16]. These data are consistent with previous work demonstrating microglial activation to hyper-ramification in the mPFC following repeated restraint [7]. The RR-induced changes in microglia were not accompanied by changes in vascular density, indicating that microglia changes are not secondary to stress-induced angiogenesis. Notably, this hyper-ramification was not observed after chronic variable stress exposure, a non-habituating stress regimen that causes significant HPA axis sensitization and hyperactivity [20]. Together, the data suggest that increased HPA axis drive in the context of chronic non-habituating stress may be sufficient to inhibit microglial activation induced by repeated restraint stress.
Increased microglial ramification, as demonstrated by increases in Iba-1 coverage without a change in microglial cell number, denotes an activation of these cells from a resting state to hyper-ramified state after chronic restraint stress. Iba-1 (ionized calcium-binding adaptor molecule-1) is a gene exclusive to microglia and is up-regulated during microglial activation [21, 22]. Iba-1 up-regulation is necessary for microglial membrane ruffling to confer cell motility [23]. Increases in Iba-1 coverage are consistent with increases in microglial surface area, indicating a transition from resting to hyper-ramified state. Given evidence that chronic variable stress causes greater cumulative GC exposure [17, 20, 24], it is possible that the lack of hyper-ramified microglia is due to anti-inflammatory actions of GCs. GCs are known to be anti-inflammatory in the periphery, but have a more complex role in the CNS. In the brain, GCs can reduce microglial pro-inflammatory signals [15]. Chronic variable stress induces adrenal hypertrophy, thymic involution, increased basal corticosterone and enhanced corticosterone production in response to adrenocorticotrophic hormone (ACTH) [17, 20], consistent with a hyper-activated HPA axis. It is possible that the cumulative elevations in corticosterone due to CVS exert anti-inflammatory effects that limit transformation of microglia to an activated state. Chronic restraint does not produce the same degree of GC dyshomeostasis seen in chronic variable stress [20], and thus the cumulative levels of GCs may not be sufficient to keep the microglial responses completely checked. Alternatively, chronic stress in the context of low GC exposure may enable priming of the immune system, while chronic stress with high GC exposure may inhibit immune activation.
It is important to note that GCs may also play a pro-inflammatory role in the CNS. For example, chronic high levels of GCs can enhance pro-inflammatory signals [15]. Chronic GC stimulation or stress can also cause GC insensitivity [25], which can promote inappropriately maintained inflammatory responses [10, 26]. The CVS regimen used in these studies is known to have anti-inflammatory actions in the periphery (indicated by reduced T lymphocytes percentages [27]), suggesting that this paradigm is not sufficient to induce frank GC resistance. We should note that while our studies are suggestive of a glucocorticoid mechanism, follow-up experiments are required to conclusively test links between differential glucocorticoid exposure and microglial hyper-ramification after stress.
Chronic restraint stress involves a predictable and repetitive exposure regimen, and causes significant HPA axis habituation over time. In contrast, chronic variable stress is designed to be unpredictable and non-habituating, and thus the two regimens may have differential impact on neuroadaptive mechanisms. For example, our prior work indicates that these two stress regimens deviate on the populations of FosB/ΔFosB-activated neurons, indicating that they may recruit different neuronal populations [20]. Thus, it is possible that neural information signaling stressor predictability may cause differential activation of microglia. The prefrontal cortex is known to be critical for encoding of expectancy and predictability. Specifically, the mPFC is necessary for perceiving controllability of stress to prevent hyper-activation of the dorsal raphe nucleus during subsequent stress challenges [28]. Localization of microglial hyper-ramification to the mPFC may be related to expectancy or habituation to repeated stimulus exposure. Future studies are required to isolate the effect of predictability on microglial activation.
The mPFC directs working memory and attention to coordinate limbic and cognitive processes in response to pertinent emotional stimuli [4, 29]. The PFC is highly reactive to stress and modulates HPA axis activity and behavior [30]. Microglial hyper-ramification in the PFC following chronic homotypic stress is known to coincide with alterations in neuronal activity and working memory, while minocycline reduces the hyper-ramification, neuronal changes, and working memory deficits associated with repeated restraint [5]. Chronic homotypic stress does not induce microglial activation through IL-1β, MHC-II, CD86, TUNEL, or activated caspase-3 expression, but rather is linked to changes in β1-integrin [31]. These data suggest that stress-induced microglial activation differs substantially from changes that arise from neuronal injury. Because our data show microglial activation following chronic restraint stress and not after chronic variable stress, microglial activation by stress may be a necessary and beneficial adaptation to certain stressors. The connection between microglial changes and PFC function raises the intriguing possibility that the microglial response may play a role in the habituation process, a possibility warranting further investigation.
Unlike the PFC, the density and morphology of microglia in the PVN were not altered after chronic variable stress or repeated restraint stress. Iba-1 measures were unaltered in the PVN, despite the fact that both chronic homtypic and heterotypic stress regimens induce notable increases in PVN CRH mRNA [32, 33]. These data suggest that chronic stressors do not engage PVN microglia, or that GC secretion resulting from both exposure paradigms is sufficient to keep microglial activation in check.
Microglia originate from mesenchymal cells in the bone marrow that migrate to the CNS [34, 35] or from peripheral monocytes [36]. Neurodegeneration, such as that seen in Alzheimer’s disease, is linked to peripheral recruitment of immune cells from the blood [34]. Chronic stress is linked to increased sympathetic tone [37], and CNS immune activation is consistent with neurogenic hypertension [38], both of which can affect vascular integrity. Chronic social defeat stress has been shown to increase macrophage/microglial molecules implicated in trafficking from the periphery to CNS [39]. Therefore, we monitored vascular density following chronic stress to evaluate whether vascular changes may contribute to any observed increases in microglia. Stress did not alter vascular density or microglial numbers in the PFC or PVN, suggesting that chronic stress does not alter blood vessel morphology. While our data are insufficient to make conclusions as to vascular permeability, the lack of observable increases in vessels makes it unlikely that microglial changes are related to neovascularization.
Chronic variable stress and repeated restraint stress appear to have different effects on other aspects of PFC function, most notably, dendrite morphology. In the PFC, repeated restraint stress produced reductions in apical dendritic spine density and length [4]. However, Vyas et al. in 2002 found that in the hippocampus, chronic restraint stress produced reductions in CA3 pyramidal branching and shaft length, but chronic variable stress produced no such effects [40]. Microglial signals are thought to be responsible for proper synaptic pruning in the developing hippocampus and are disrupted by stress [11, 12], suggesting that there may be a connection between extent of microglial recruitment and structural changes seen in corticolimbic structures. During chronic stress, microglia may be stimulated to overprune synapses in the PFC, to contribute to pathological behavioral phenotypes. This has yet to be tested, but microglial and synaptic changes in response to stress seem to align.
The interactions of stress regimen, glucocorticoid levels, and microglial morphology are very complex. The current study suggests that microglial activation in the brain is a selective process, targeting prefrontal cortical (but not hypothalamic) circuits that are critical to stressor habituation or predictability. It is currently unknown whether microglial response specificity is controlled by differential central drive or differences in the degree to which different stress paradigms recruit anti-inflammatory processes, such as glucocorticoid secretion.
Highlights.
Chronic restraint stress produces microglial activation in the prefrontal cortex.
Chronic variable stress does not produce microglial activation.
Chronic restraint stress, but not variable stress, produces HPA axis habituation.
Microglial activation may be an adaptive process.
Acknowledgments
Grants from the National Institute of Health [MH049698 and MH069860] supported this research. We would also like to thank Rylon Hofacer for assistance with the project, Ben Packard and Mark Dolgas for their assistance with the project, and Stuart Tobet for advice on vascular staining protocols.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brown GW, Harris T. Life events and illness. New York: Guilford Press; 1989. [Google Scholar]
- 2.Ventura J, Nuechterlein KH, Lukoff D, Hardesty JP. A prospective study of stressful life events and schizophrenic relapse. Journal of Abnormal Psychology. 1989;98(4):407–411. doi: 10.1037//0021-843x.98.4.407. [DOI] [PubMed] [Google Scholar]
- 3.Wilber AA, Walker AG, Southwood CJ, Farrell MR, Lin GL, Rebec GV, Wellman CL. Chronic stress alters neural activity in medial prefrontal cortex during retrieval of extinction. Neuroscience. 2011;174:115–131. doi: 10.1016/j.neuroscience.2010.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radley JJ, Rocher AB, Miller M, Janssen WGM, Liston C, Hof PR, McEwen BS, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal. cortex. 2006;16(3):313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- 5.Hinwood M, Morandini J, Day TA, Walker FR. Evidence that microglia mediate the neurobiological effects of chronic psychological stress on the medial prefrontal cortex. Cerebral Cortex. 2011;22(6):1442–1454. doi: 10.1093/cercor/bhr229. [DOI] [PubMed] [Google Scholar]
- 6.Maes M, Berk M, Goehler L, Song C, Anderson G, Galecki P, Leonard B. Depression and sickness behavior are janus-faced responses to shared inflammatory pathways. BioMed Central Medicine. 2012;10:66. doi: 10.1186/1741-7015-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tynan RJ, Naicker S, Hinwood M, Nalivaiko E, Buller KM, Pow DV, Day TA, Walker FR. Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain, Behavior, and Immunity. 2010;24(7):1058–1068. doi: 10.1016/j.bbi.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annual Review of Immunology. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 9.Streit WJ, Walter SA, Pennell NA. Reactive microgliosis. Progress in Neurobiology. 1999;57(6):563–581. doi: 10.1016/s0301-0082(98)00069-0. [DOI] [PubMed] [Google Scholar]
- 10.Glezer I, Rivest S. Glucocorticoids: protectors of the brain during innate immune responses. Neuroscientist. 2004;10(6):538–552. doi: 10.1177/1073858404263494. [DOI] [PubMed] [Google Scholar]
- 11.Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333(6048):1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 12.Wei L, Simen A, Mane S, Kaffman A. Early life stress inhibits expression of a novel innate immune pathway in the developing hippocampus. Neuropsychopharmacology. 2012;37(2):567–580. doi: 10.1038/npp.2011.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sierra A, Gottfried-Blackmore A, Milner TA, McEwen B, Bulloch K. Steroid hormone receptor expression and function in microglia. Glia. 2008;56(6):659–674. doi: 10.1002/glia.20644. [DOI] [PubMed] [Google Scholar]
- 14.Chang JY, Liu LZ. Inhibition of microglial nitric oxide production by hydrocortisone and glucocorticoid precursors. Neurochemical Research. 2000;25(7):903–908. doi: 10.1023/a:1007511221666. [DOI] [PubMed] [Google Scholar]
- 15.Sorrells SF, Sapolsky RM. An inflammatory review of glucocorticoid actions in the CNS. Brain, Behavior, and Immunity. 2007;21(3):259–272. doi: 10.1016/j.bbi.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girotti M, Pace TWW, Gaylord RI, Rubin BA, Herman JP, Spencer RL. Habituation to repeated restraint stress is associated with lack of stress-induced c-fos expression in primary sensory processing areas of the rat brain. Neuroscience. 2006;138 (4):1067–1081. doi: 10.1016/j.neuroscience.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi D, Engeland WC, Herman JP. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. American Journal of Physiology, Endocrinology and Metabolism. 2006;291(5):E965–E973. doi: 10.1152/ajpendo.00070.2006. [DOI] [PubMed] [Google Scholar]
- 18.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Sydney, Australia: Academic Press; 1997. [Google Scholar]
- 19.McClave J, Dietrich F. Statistics. 6. Englewood Cliffs, NJ: Macmillan College Publishing Company, Inc; 1994. [Google Scholar]
- 20.Flak JN, Solomon MB, Jankord R, Krause EG, Herman JP. Identification of chronic stress-activated regions reveals a potential recruited circuit in rat brain. European Journal of Neuroscience. 2012;36 (4):2547–2555. doi: 10.1111/j.1460-9568.2012.08161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imai Y, Ibata I, Ito D, Ohsawa K, Kohsaka S. A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochemical and Biophysical Research Communications. 1996;224:855–862. doi: 10.1006/bbrc.1996.1112. [DOI] [PubMed] [Google Scholar]
- 22.Imai Y, Kohsaka S. Intracellular signaling in M-CSF-induced microglia activation: role of Iba1. Glia. 2002;40(2):164–174. doi: 10.1002/glia.10149. [DOI] [PubMed] [Google Scholar]
- 23.Kanazawa H, Ohsawa K, Sasaki Y, Kohsaka S, Imai Y. Macrophage/microglia-specific protein Iba-1 enhances membrane ruffling and Rac activation via phospholipase-C-γ-dependent pathway. Journal of Biol Chemistry. 2002;277:20026–20032. doi: 10.1074/jbc.M109218200. [DOI] [PubMed] [Google Scholar]
- 24.Marin MT, Cruz FC, Planeta CS. Chronic restraint or variable stresses differently affect the behavior, corticosterone secretion and body weight in rats. Physiology and Behavior. 2007;90 (1):29–35. doi: 10.1016/j.physbeh.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 25.Reber SO. Stress and animal models of inflammatory bowel disease-an update on the role of the hypothalamo-pituitary-adrenal axis. Psychoneuroendocrinology. 2012;37 (1):1–19. doi: 10.1016/j.psyneuen.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Miller GE, Chen E, Sze J, Marin T, Arevalo JMG, Doll R, Ma R, Cole SW. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-κB signaling. Stress and Synaptic Plasticity. 2007;64(4):266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basso AM, Depiante-Depaoli M, Cancela L, Molina V. Seven-day variable-stress regime alters cortical-adrenoceptor binding and immunologic responses: reversal by imipramine. Pharmacology Biochemistry and Behavior. 1993;45:665–672. doi: 10.1016/0091-3057(93)90522-u. [DOI] [PubMed] [Google Scholar]
- 28.Amat J, Paul E, Zarza C, Watkins LR, Maier SF. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. The Journal of Neuroscience. 2006;26(51):13264–13272. doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simpson JR, Jr, Snyder A, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: I. during cognitive task performance. PNAS. 2001;98(2):683–687. doi: 10.1073/pnas.98.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaferi A, Bhatnagar S. Corticotropin-releasing hormone receptors in the medial prefrontal cortex regulat hypothalamic-pituitary-adrenal activity and anxiety-related behavior regardless of prior stress experience. Brain Research. 2007;1186:212–223. doi: 10.1016/j.brainres.2007.07.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hinwood M, Tynan RJ, Charnley JL, Beynon SB, Day TA, Walker FR. Chronic stress induced remodeling of the prefrontal cortex: structural re-organization of microglia and the inhibitory effect of minocycline. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhs151. [DOI] [PubMed] [Google Scholar]
- 32.Imaki T, Nahan JL, Rivier C, Sawchenko PE, Vale W. Differential regulation of corticotropin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. The Journal of Neuroscience. 1991;11(3) doi: 10.1523/JNEUROSCI.11-03-00585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herman JP, Adams D, Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995;61(2):180–190. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- 34.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–20. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Bechmann I, Galea I, Perry VH. What is the blood-brain barrier (not)? Trends in Immunology. 2007;28(1):5–11. doi: 10.1016/j.it.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Bechmann I, Goldmann J, Kovac AD, Kwidzinski E, Simburger E, Naftolin F, Dirnagl U, Nitsch R, Priller J. Circulating monocytic cells infiltrate layers of anterograde axonal degeneration where they transform into microglia. FASEB J. 2005;19:647–649. doi: 10.1096/fj.04-2599fje. [DOI] [PubMed] [Google Scholar]
- 37.Grippo AJ, Beltz TG, Johnson AK. Behavior and cardiovascular changes in the chronic mild stress model of depression. Physiology and Behavior. 2003;78(4–5):703–710. doi: 10.1016/s0031-9384(03)00050-7. [DOI] [PubMed] [Google Scholar]
- 38.Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, Sriramula S, Francis J, Sumners C, Raizada MK. Brain microglial cytokines in neurogenic hypertension. AHA Hypertension. 2010;56:297–303. doi: 10.1161/HYPERTENSIONAHA.110.150409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wohleb E, Hanke M, Corona A, Powell N, Stiner LM, Bailey MT, Nelson RJ, Godbout JP, Sheridan JF. β-adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. Journal of Neuroscience. 2011;31(17):6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vyas A, Mitra R, Rao BSS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. The Journal of Neuroscience. 2002;22(15):6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


