Abstract
Background
Precise effects of albuminuria and low estimated glomerular filtration rate (eGFR) on cardiovascular mortality, all-cause mortality, and renal events in diabetic patients are uncertain.
Materials and Methods
A systematic review was conducted of the literature through MEDLINE, EMBASE, and CINHAL from 1950 to December 2010. Cohort studies of diabetic patients providing adjusted relative risk (RR) of albuminuria and eGFR for risks of cardiovascular mortality, all-cause mortality, and renal events were selected. Two reviewers screened abstracts and full papers of each study using standardized protocol.
Results
We identified 31 studies fulfilling the criteria from 6546 abstracts. With regard to the risk of cardiovascular mortality, microalbuminuria (RR 1.76, 95%CI 1.38–2.25) and macroalbuminuria (RR 2.96 95%CI 2.44–3.60) were significant risk factors compared to normoalbuminuria. The same trends were seen in microalbuminuria (RR 1.60, 95%CI 1.42–1.81), and macroalbuminuria (RR 2.64, 95%CI 2.13–3.27) for the risk of all-cause mortality, and also in microalbuminuria (RR 3.21, 95%CI 2.05–5.02) and macroalbuminuria (RR 11.63, 95%CI 5.68–23.83) for the risk of renal events. The magnitudes of relative risks associated with low eGFR along with albuminuria were almost equal to multiplying each risk rate of low eGFR and albuminuria. No significant factors were found by investigating potential sources of heterogeneity using subgroup analysis.
Conclusions
High albuminuria and low eGFR are relevant risk factors in diabetic patients. Albuminuria and low eGFR may be independent of each other. To evaluate the effects of low eGFR, intervention, or race, appropriately designed studies are needed.
Introduction
The prevalence of diabetes is increasing globally, and management of diabetic complications is particularly important. [1], [2], [3] Diabetic nephropathy, resulting in end-stage renal events requiring renal replacement therapy, is one of the most common complications. Furthermore, in the course of diabetic nephropathy, patients have higher rates of mortality from cardiovascular disease. [4] Albuminuria is an early marker of diabetic nephropathy, and previous reports described the association between albuminuria and risks of adverse cardiovascular and kidney events. [5], [6] Albuminuria is often used as a surrogate marker for the risk of fatal and non-fatal events in clinical trials of antihyperglycemic medications or in antihypertensive therapy. [7], [8], [9] Similarly, low eGFR, which is a common manifestation of progressed diabetic nephropathy, has also been demonstrated to be an independent risk factor for cardiovascular events and death. [10], [11] Recent evidence suggests that both high albuminuria and low eGFR are independent risk factors for progressive kidney failure and cardiovascular disease. [10] In addition, the magnitudes of risk for progressive kidney failure, cardiovascular disease, and all-cause mortality were different between studies, and the unevenness may have been due to differences in study design or characteristics of participants. It is important to clarify these problems to apply this evidence to individuals.
To manage diabetic nephropathy, it is necessary to clarify the precise magnitude of the risks for cardiovascular mortality, all-cause mortality, and renal events according to the status of the patient. These observations may be useful for the screening of high-risk patients or considering interventions. Therefore, we conducted a systematic review and meta-analysis of published studies on diabetic nephropathy to provide an accurate estimation of the influence of albuminuria and low eGFR.
Methods
Data Sources and Searches
We conducted a systematic review of disease prognosis. A systematic review of the available literature according to MOOSE (meta-analysis of observational studies on epidemiology) guidelines was conducted. MEDLINE (http://ovidsp.ovid.com/), EMBASE (http://www.embase.com/), and CINHAL (http://www.ebscohost.com/cinahl/) from 1950 until December 2010 were searched, and the related literature were identified. Search strategies consisted of medical subject headings and text words, including all spellings of proteinuria, albuminuria, microalbuminuria, macroalbuminuria, and glomerular filtration rate combined with cardiovascular diseases, mortality, renal events (Table 1), and limited to cohort studies of diabetic patients. References from identified studies were also screened manually.
Table 1. Search Strategies.
| 1: diabetes mellitus AND (proteinuria OR albuminuria OR microalbuminuria OR macroalbuminuria) |
| 2: (diabetic nephropathy) |
| 3: (kidney failure, chronic) OR (glomerular filtration rate) |
| 4: (cardiovascular diseases) OR (cerebrovascular disorders) |
| 5: mortality OR death |
| 6: (cohort studies) OR (case-control studies) |
| (1 or 2) and (3 or 4 or 5) and 6 |
terms associated with Medical Subject Headings.
Study Selection
Studies were included if they were cohort studies on diabetic patients that estimated the relative risk (RR) and 95% confidence intervals (CIs) of albuminuria or low eGFR on cardiovascular mortality, all-cause mortality, or renal events, and the estimates were derived from Cox proportional hazard models. The definitions of albuminuria were pre-specified (Table 2). Studies were included if they met the definitions of albuminuria in Table 2. Cardiovascular mortality was defined as death from coronary events and/or stroke, which may be on the basis of International Classification of Diseases codes. Renal events were defined as renal replacement therapy, renal transplantation, or loss of renal function. Loss of renal function is defined as sustained eGFR or creatinine clearance below 60 ml/min/1.73 m2 or less, halving of eGFR, or doubling of serum creatinine.
Table 2. Definitions of Albuminuria.
| Measurement Method | Microalbuminuria | Macroalbuminuria | Any level of albuminuria |
| 24 hour urine collection | 30–300 mg/day or 20–200 µg/min | >300 mg/day or >200 µg/min | >30 mg/day or >20 µg/min |
| (proteinuria) | N/A | >0.3–0.5 g/day | N/A |
| Spot urine albumin creatinine ratio | 30–300 mg/g or 3.4–34 mg/mmol | >300 mg/g or >34 mg/mmol | >30 mg/g or >3.4 mg/mmol |
| (proteinuria) | N/A | >0.3–0.5 g/g | N/A |
| Spot urine albumin concentration | 3–30 mg/dl | >30 mg/dl | >3 mg/dl |
| (proteinuria) | N/A | >0.3–0.5 g/l | N/A |
| Spot urine dipstick | Specific microalbuminuria dipstick positive | N/A | N/A |
Abbreviation: N/A, not available.
Based on Sarnak et al. [12].
Data Extraction and Quality Assessment
The literature search and screening were performed by two of the authors (TT and MS). Authors independently judged the contents of abstracts and full papers in duplicate using standardized data collection form. Additional data were not collected from authors of literature. To eliminate the potential influences of specific disease, studies were excluded if their cohorts included patients with specific complications. Studies were also excluded if they reported estimates of influences without any information about standard error, and if they did not yield an estimate that was not adjusted at least by age.
Data Synthesis and Analysis
Random-effects model were used to obtain summary estimates of RR and 95% CI. Summary estimates were obtained separately according to the level of albuminuria (microalbuminuria, macroalbuminuria, any level of albuminuria). If only subgroups of the estimate were reported (e.g., by gender), these were pooled by fixed-effects model as a within-study summary estimate. We also investigated studies providing RR associated with low eGFR according to the level of albuminuria. If the study population was representative of a particular level of eGFR (e.g., eGFR >60), it was handled as stratified. To evaluate the influences of albuminuria and low eGFR, compare the relative risks pooled by fixed-effects model according to stratified category of albuminuria (micro- and macroalbuminuria), low eGFR (< 60 mL/min/1.73 m2) and normal eGFR (≥ 60 mL/min/1.73 m2) regardless of the reference category of eGFR. Heterogeneity between studies was assessed using Cochran Q test and I2 value. Potential sources of heterogeneity were examined by subgroup analysis comparing summary estimates from subset of studies categorized by characters of participants or study design. Univariate meta-regression was used to compare the subgroups. Begg’s test [13] and Egger’s test [14] were used to evaluate possible publication bias (where P<0.05 was taken to indicate statistical significance). To evaluate an influence of a single study, sensitivity analysis is performed to examine the exclusion of any single study altered the magnitude of relative risk or test for heterogeneity. All analyses were performed using Stata (release 11.2; Stata Corporation, College Station, TX). For all tests, a two-sided p-value below 0.05 was considered significant.
Results
Literature Search and Characteristics of Studies
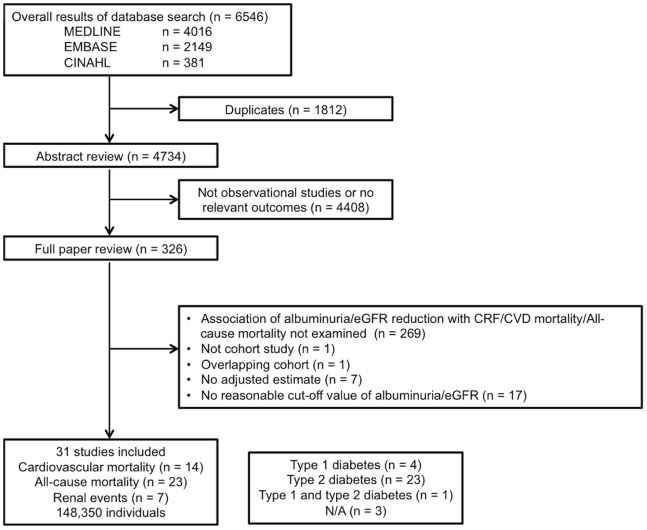
The systematic database search yielded 6546 studies, of which 326 papers were reviewed in full (Figure 1). Finally, 31 studies that fulfilled the criteria were included in the analysis, including information for 148350 participants. The crude incidence rates were 19.1 deaths from cardiovascular disease, 35.7 deaths, and 11.7 renal events (per 1000 person-years, respectively). The process of study identification is shown in the flow chart, and the study characteristics are listed in Table 3 and Table 4. Studies consisted of four studies of type 1 diabetic patients, 23 studies of type 2 diabetic patients, one study of type 1 and type 2 diabetic patients, and 3 studies of unknown type of diabetic patients. The study size was in the range of 146 to 94934, and the average follow-up period was in the range of 3 to 19 years. Regarding cardiovascular mortality, Asian population study was not included according to the criteria. We pooled the risk of two studies [15], [16] reporting only subgroups of the estimate.
Figure 1. Process for identification of eligible studies Abbreviation: N/A, not available.
Table 3. Characteristic of Studies Reporting on the Association between Albuminuria or low eGFR and Subsequent Risk of Adverse Outcomes.
| Author | Year | Country | Study size | %male | %white | Endpointsa | No. of CV mortality | No. of all-cause mortality | No. of renal events | ||
| Jager[15] | 2010 | Netherlands | 173 | 48.0 | 100.0 | CV mortality | 16 | ||||
| O'Hare[16] | 2010 | US | 94,934 | 98.0 | 87.0 | All-cause mortality | 25481 | ||||
| Grauslund[17] | 2010 | Denmark | 389 | 55.0 | N/A | CV mortality | All-cause mortality | N/A | 117 | ||
| Molitch[18] | 2010 | US | 1,439 | 52.5 | N/A | Renal events | 89 | ||||
| Ninomiya[10] | 2009 | Multicountries | 10,640 | 57.0 | N/A | CV mortality | All-cause mortality | Renal events | 432 | 817 | 107 |
| Groop[19] | 2009 | Finland | 4,201 | 51.8 | N/A | All-cause mortality | 291 | ||||
| de Boer[20] | 2009 | US | 691 | 42.1 | 80.6 | CV mortality | All-cause mortality | 169 | 378 | ||
| Vlek[6] | 2008 | Netherlands | 759 | 76.5 | N/A | CV mortality | All-cause mortality | 49 | 82 | ||
| Luk[21] | 2008 | China | 5,829 | 49.8 | N/A | Renal events | 741 | ||||
| Tong[22] | 2007 | China | 4,416 | 42.9 | N/A | All-cause mortality | Renal events | 110 | 221 | ||
| Bruno[23] | 2007 | Italy | 1,538 | 43.4 | N/A | CV mortality | All-cause mortality | 331 | 670 | ||
| Roy[24] | 2006 | US | 725 | 41.7 | 0.0 | All-cause mortality | 131 | ||||
| So[25] | 2006 | Hong Kong | 4,421 | 43.2 | N/A | Renal events | 212 | ||||
| Retnakaran[26] | 2006 | UK | 5,032 | 59.0 | 81.0 | Renal events | 584 | ||||
| Xu[27] | 2005 | USA | 1,953 | 37.6 | N/Ae | CV mortality | All-cause mortality | 223 | 627 | ||
| Yuyun[28] | 2003 | UK | 427 | 62.1 | N/A | All-cause mortality | 56 | ||||
| Bruno[29] | 2003 | Italy | 1,408 | 43.6 | N/A | Renal events | 82 | ||||
| Jude[30] | 2002 | UK | 340 | 66.5 | 66.8 | CV mortality | All-cause mortality | 44 | 63 | ||
| Ostgren[31] | 2002 | Sweden | 400 | 50.5 | N/A | All-cause mortality | 131 | ||||
| Stehouwer[32] | 2002 | Netherlands | 328 | 61.6 | N/A | All-cause mortality | 113 | ||||
| Gerstein[33] | 2001 | North and South America and Europe | 3,498 | 62.9 | N/A | All-cause mortality | 431 | ||||
| de Grauw[34] | 2001 | Netherlands | 262 | 39.0 | N/A | All-cause mortality | 57 | ||||
| Florkowski[35] | 2001 | New Zealand | 447 | 46.5 | N/A | All-cause mortality | 187 | ||||
| Casiglia[36] | 2000 | Italy | 683 | 50.2 | N/A | CV mortality | 68 | ||||
| Valmadrid[37] | 2000 | US | 840 | 45.0 | N/A | CV mortality | All-cause mortality | 364 | 529 | ||
| Hänninen[38] | 1999 | Finland | 252 | 53.2 | N/A | All-cause mortality | 21 | ||||
| Mattock[39] | 1998 | U.K. | 146 | 56.2 | 100.0 | CV mortality | All-cause mortality | 20 | 36 | ||
| Beilin[40] | 1996 | Australia | 666 | 47.1 | N/A | CV mortality | All-cause mortality | 80 | 167 | ||
| Rossing[41] | 1996 | Denmark | 939 | 52.5 | N/A | CV mortality | All-cause mortality | 74 | 207 | ||
| Gall[42] | 1995 | Denmark | 328 | 61.5 | N/A | CV mortality | 29 | ||||
| Neil[43] | 1993 | U.K. | 246 | 50.8 | N/A | All-cause mortality | 93 |
Endpoints: CV mortality, cardiovascular mortality.
Type of DM: N/A, type of DM is not documented; T1DM, population with type 1 DM; T2DM, population with type 2 DM.
Study type: Obs, based on the cohort of observational study; Trial, based on the cohort of clinical trial.
Level of Adjustment: ACE, angiotensin converting enzyme; Apo, apolipoprotein; BMI, body mass index; CVD, cardiovascular disease; dBP, diastolic blood pressure; DM, diabetes mellitus; ECG, electrocardiogram; HbA1c, glycosylated hemoglobin A1c; HDL, high-density lipoproteins; HT, hypertension; IHD, ischemic heart disease; LDL, low-density lipoproteins; PVD, peripheral vascular disease; RAAS, Renin-Angiotensin-Aldosterone System; sBP, systolic blood pressure; sCr, serum creatinine TCHO, total cholesterol; TG, triglycerides;
Cohort of American Indians.
Other abbreviations: N/A, not available; CV mortality, cardiovascular mortality; sBP, systolic blood pressure; dBP, diastolic blood pressure.
Table 4. Definitions of Albuminuria, eGFR categories and Outcomes.
| Author | Urine measurement methoda | Definition of microalbuminuria | Definition of macroalbuminuria | Definition of any level of albuminuria | eGFR categories | Criteria of renal failure | Criteria of CV mortality | Definition of CV diseaseb |
| Jager [15] | ACR | >2.0 mg/mmol | ICD code 390–459 | Heart/Brain | ||||
| O’Hare [16] | ACR | 30–299 mg/gCr | ≥300 mg/gCr | |||||
| Grauslund [17] | spot | 30–299 mg/L | ≥300 mg/L | ICD-9 codes 430.0–438.9ICD-10 codes I20.0–I25.9, I60.0–I60.9 | Heart/Brain | |||
| Molitch [18] | AER | 30–300 mg/24 h | >300 mg/24 h | sustained eGFR<60 | ||||
| Ninomiya [10] | ACR | 30–300 mg/gCr | >300 mg/gCr | >90, 60–89, <60 | death as a result of kidney disease, requirement for dialysis or transplantation, or doubling of serum creatinine to >200 µmol/L | death as a result of coronary heart disease or cerebrovascular disease | Heart/Brain | |
| Groop [19] | AER | 20–200 µg/min | >200 µg/min | |||||
| de Boer [20] | ACR | ≥30 mg/gCr | ≥60, <60 | death from coronary heart disease, myocardial infarction, sudden cardiac death, or stroke | Heart/Brain | |||
| Vlek [6] | ACR | >3 mg/mmol | >60, ≤60 | Vascular death, Stroke, Myocardial infarction | Heart/Brain | |||
| Luk [21] | ACR | 2.5–30 mg/mmol (women) 3.5–30 mg/mmol (men) | >30 mg/mmol | ICD-9 code 250.4, 585, 586 ICD-9 procedure code 39.95 (hemodialysis), 54.98 (peritoneal dialysis) | ||||
| Tong [22] | ACR | 3.5–25 mg/mmol | ≥25 mg/mmol | eGFR halving, eGFR <15 ml/min/1.73 m2, death as a result of renal causes or need for dialysis | ||||
| Bruno [23] | AER | 20–200 µg/min | >200 µg/min | ≥60, <60 | ICD code 390–459 | Heart/Brain | ||
| Roy [24] | AER | 20–200 µg/min | >200 µg/min | |||||
| So [25] | ACR | 3.5–25 mg/mmol | ≥25 mg/mmol | >3.5 mg/mmol | >90, 60–89, 30–59, 15–29 | Reduction in eGFR by 50% or progression to eGFR 15 ml/min/1.73 m2 (stage 5) or renal dialysis or death secondary to renal causes | ||
| Retnakaran [26] | spot | 50–299 mg/L | ≥300 mg/L | Creatinine clearance ≤60 ml/min per 1.73 m2 | ||||
| Xu [27] | ACR | ≥30, <300 mg/gCr | ≥300 mg/gCr | definite fatal MI, definite sudden death due to CHD, definite or possible fatal CHD, definite or possible fatal stroke, definite or possible fatal CHF, and other fatal CVD | Heart/Brain | |||
| Yuyun [28] | AER | 30–300 mg/24 h | >300 mg/24 h | |||||
| Bruno [29] | AER | 20–200 ug/min | >200 ug/min | ESRD (need for dialysis) or chronic renal failure | ||||
| Jude [30] | PER | Urine protein ≥0.5 g/24 h | from death certificates | Heart/Brain | ||||
| Ostgren [31] | qualitative | Specific microalbumiuria dipstick positive | ||||||
| Stehouwer [32] | AER | 30–299 mg/24 h | ≥300 mg/24 h | |||||
| Gerstein [33] | ACR | >2.0 mg/mmol exclude dipstick–positive proteinuria | ||||||
| de Grauw [34] | spot | 20–200 mg/L | >200 mg/L | |||||
| Florkowski [35] | spot | ≥50 mg/l | ||||||
| Casiglia [36] | AER | 30–300 mg/24 h | >300 mg/24 h | >60, ≤60 | from the hospital of physicians’ files | Heart/Brain | ||
| Valmadrid [37] | qualitative | Agglutination inhibition assay positive,and reagent strip negative | Urine protein ≥0.3 g/L | ICD9 codes 402, 404, 410–414, 428, 430–438 | Heart/Brain | |||
| Hänninen [38] | AER | ≥20 µg/min | ||||||
| Mattock [39] | AER | 20–200 µg/min | UAER >200 µg/min | from death certificates | Heart | |||
| Beilin [40] | spot | 30–300 mg/L | ≥300 mg/L | ICD9 codes 390 to 458, 410 to 414 | Heart/Brain | |||
| Rossing [41] | AER | 31–299 mg/24 h | ≥300 mg/24 h | from death certificate | Heart/Brain | |||
| Gall [42] | AER | 30–299 mg/24 h | AER ≥300 mg/24 h | from death certificates | Heart/Brain | |||
| Neil [43] | spot | 40–200 mg/L | UAC >200 mg/L |
Urine measurement method: ACR, albumin creatinine ratio; AER, albumin excretion rate; PER, protein excretion rate; spot, spot urinary albumin concentration; qualitative, qualitative detection of albumin in urine.
Definition of CV disease: Heart, ischemic heart disease; Brain, cerebrovascular disease.
Micro- and macroalbuminuria were defined as risk factors in 25 studies. Any level of albuminuria (i.e., micro- or macroalbuminuria) was defined as a risk factor in 7 studies. In these studies, various means of expression of albuminuria were adopted. The magnitude of microalbuminuria was expressed as urinary albumin excretion rate (n?12), urinary albumin-creatinine ratio on spot urine samples (n?10), spot urinary albumin concentration (n?6), qualitative test of albuminuria (n?2), or urinary protein excretion rate (n?1). Almost all of the estimates were adjusted for multiple risk factors including age. In one study [17], the estimate was not adjusted for age because age was not a statistically significant risk.
Association of Albuminuria with Risk of Cardiovascular Mortality
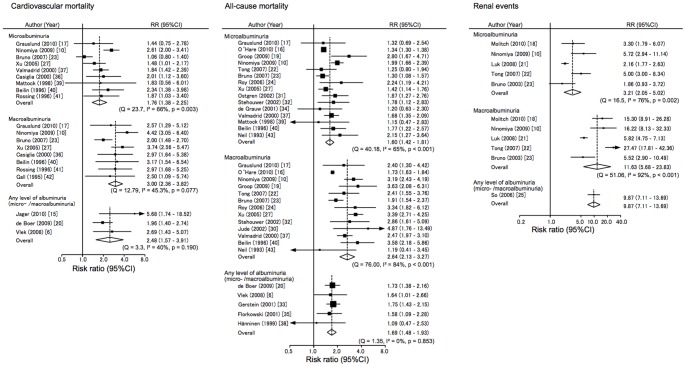
Microalbuminuria was associated with 1.76 (95% confidence interval [CI] 1.38–2.25) times greater risk of cardiovascular mortality as compared with normoalbuminuria (Figure 2), with strong heterogeneity among studies (I2 = 66%, p = 0.003 for heterogeneity). We found no significant evidence of publication bias. Subgroup analysis did not determine the suspected source of heterogeneity (Figure S1). Age stratified analysis showed no trends neither micro- nor macroalbuminuria (Figure S2). Macroalbuminuria was associated with about 2.96 (95%CI 2.44–3.60) times greater risk of cardiovascular mortality compared with normoalbuminuria, and there was no significant evidence of heterogeneity among studies. These findings suggest that there is a dose-dependent association between albuminuria and the risk of cardiovascular mortality: the influence of macroalbuminuria was significantly higher than that of microalbuminuria (p = 0.026). In the three studies for which information was available, any level of albuminuria was associated with about 2.48 times (95%CI 1.57–3.91) greater risk of cardiovascular mortality compared with normoalbuminuria, without any evidence of heterogeneity in the association.
Figure 2. Risk ratio for the association between albuminuria and cardiovascular mortality, all-cause mortality, and renal events compared with normoalbuminuria.
Abbreviations: CI, confidence interval; RR, risk ratio.
Association of Albuminuria with Risk of All-cause Mortality
Summary estimates of the influences of microalbuminuria and macroalbuminuria on all-cause mortality were 1.60 (95%CI 1.42–1.81) and 2.64 (95%CI 2.13–3.27), respectively (Figure 2): the associations were heterogeneous among studies for both (I2 = 65% and 84%, both p<0.001 for heterogeneity). There was some evidence of publication bias in microalbuminuria and macroalbuminuria (Egger’s test P?0.014 and P?0.015, respectively), which may have overestimated the strength of the association. Subgroup analysis did not determine the suspected source of heterogeneity. As to the racial difference, relative risks were not significantly different between Asians and non-Asians. A study in veterans (O’Hare et al.) [18] yielded a lower risk of all-cause mortality (HR 1.34 [95%CI 1.30–1.38] for microalbuminuria, HR 1.73 [95%CI 1.63–1.84] for macroalbuminuria), but the source of heterogeneity was not apparent (Figure S1). In age-stratified analysis, there was no significant difference between younger and older age (Figure S2). Sensitivity analysis excluding this study [18], with the highest weight in this meta-analysis, showed a similar relative risk in microalbuminuria (HR 1.65 [95% CI 1.46 – 1.87]) and macroalbuminuria (HR 2.77 [95% CI 2.34 – 3.27]); the test for heterogeneity was insignificant in microalbuminuria (I2?41.0%, P?0.06), and was still significant for macroalbuminuria (I2?51.1%, P?0.02). The summary estimate of the influence of any level of albuminuria for the risk of all-cause mortality was 1.69 (95%CI 1.48–1.93).
Association of Albuminuria with Risk of Renal Events
Summary estimates of the influences of microalbuminuria and macroalbuminuria on renal events were 3.21 (95%CI 2.05–5.02) and 11.63 (95%CI 5.68–23.83), respectively (Figure 2): the risk estimates of micro- and macroalbuminuria were diverse across studies (I2 = 76% and 92%, p = 0.02 and p<0.001 for heterogeneity). We found no significant evidence of publication bias. Subgroup analysis did not show any significant differences between characteristics of participants or study design (Figure S1). Asians have almost the same risk for renal events as non-Asians in both micro- and macroalbuminuria. Age stratified analysis showed no trends in microalbuminuric or macroalbuminuric patients (Figure S2). One study evaluating the influences of any level of albuminuria showed the same trend.
Combined Impacts of Low eGFR on Albuminuria
A few studies [6], [10], [19], [20] evaluated the combined influence of low eGFR on albuminuria in terms of the risk for the outcomes. As compared to those with normoalbuminuria, the risk of cardiovascular mortality tended to increase by 1.70-fold (95%CI 0.83–3.49) in subjects with normoalbuminuria and eGFR of <60 mL/min/1.73 m2 (Figure 3). Similarly, the presence of albuminuria was significantly associated with 2.46-fold (95%CI 1.96–3.07) increased risk of cardiovascular mortality. Furthermore, subjects with both albuminuria and eGFR <60 mL/min/1.73 m2 were at 4.20 times (95%CI 3.11–5.68) higher risk of cardiovascular mortality compared to those with neither of these risk factors.
Figure 3. Risk ratio for the association of low eGFR with the risk of each outcome according to the presence of albuminuria, compared with normal eGFR and normoalbuminuria.
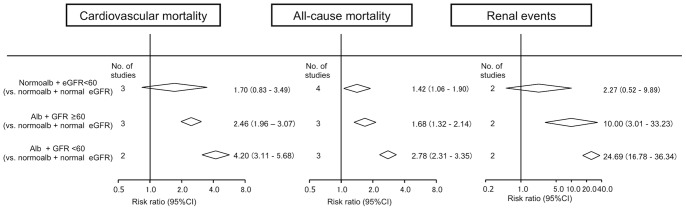
Albuminuria was defined as any level of albuminuria or pooled estimate of microalbuminuria and macroalbuminuria. Abbreviations: normoalb, normoalbuminuria; alb, albuminuria.
Discussion
This study explored the influences of albuminuria and low eGFR on cardiovascular mortality, all-cause mortality, and renal events in diabetic patients using meta-analysis methods with 148350 cases. Microalbuminuria and macroalbuminuria are significant risk factors for each outcome. Similar to the influences of albuminuria, low eGFR also increased the risk of each adverse outcome.
This meta-analysis suggested that low eGFR and albuminuria may be independent risk factors for cardiovascular mortality, all-cause mortality, and renal events. Recent published new CKD staging from Kidney Disease: Improving Global Outcomes (KDIGO) was defined by these two factors, eGFR and albuminuria. [44], [45] However, conventional staging of diabetic nephropathy was classified only by degree of albuminuria. [46] Many reports and meta-analysis indicated albuminuria as one of the main risk factors for cardiovascular mortality and all-cause mortality in diabetic patients. [47] Although the number of reports was limited, some indicated the influences of low eGFR on the risk of each outcome in diabetic nephropathy. [10], [19], [20] However, other reports concluded that low eGFR was not always a significant risk factor for these outcomes. [6], [25] Thus, the influences of albuminuria and low eGFR are not consistent among studies adjusted for each other. Further large prospective studies are needed to clarify the independent influences of albuminuria and low eGFR on the three outcomes in diabetic nephropathy.
The interaction between eGFR and albuminuria may be important in considering the possibility of albuminuria and low eGFR as independent risk factors for the three outcomes. Previous meta-analyses of general and high-risk cohorts indicated no interaction between eGFR and albuminuria on the risks of cardiovascular mortality, all-cause mortality, and renal events. [48], [49] Similarly, in our results of diabetic nephropathy consisting of 4 data or less, stratified analysis demonstrated that the magnitudes of relative risks of these events with low eGFR and albuminuria were almost equivalent to those obtained by multiplying each risk rate of low eGFR and albuminuria. These results suggested that there is no interaction between eGFR and albuminuria in each adverse outcome. In our meta-analysis, only two studies evaluated the interaction between eGFR and albuminuria. [10], [25] One of these studies that included stratified analysis indicated that increasing risk of cardiovascular mortality and all-cause mortality in low eGFR were significantly higher in patients with macroalbuminuria but not those with normoalbuminuria. [25] Moreover, in a previous meta-analysis, one of eight general and high-risk cohorts showed significant interaction between eGFR and albuminuria for the risk of ESRD. [49] Based on these studies, the significance of the interaction between eGFR and albuminuria is still variable. Detailed analysis of cohort studies, including an unusual case of diabetic nephropathy, such as low eGFR with normoalbuminuria and high GFR with macroalbuminuria, are needed to resolve the precise interaction of them.
There was heterogeneity among studies for cardiovascular mortality, all-cause mortality, and renal events in the presence of microalbuminuria or macroalbuminuria. There are some possible causes of the heterogeneity in this study. One of the possible reasons is a large cohort with different results from the others. Another possible reason is the diversity of study design. A large study with an exceptional setting [18] may lead to heterogeneity of the outcome. The report by O’Hare et al. had the highest weight in this meta-analysis, and its relative risk was even lower than the pooled risk of all-cause mortality. [18] Therefore, this large cohort study of veterans should have some different setting from other studies. The multiplicity of study design is an unavoidable limitation of meta-analyses, which is another possible reason of heterogeneity. The entry criteria, treatment, or adjustment for confounders were different between studies, and the different settings may affect results to uneven extents. Although some other factors, such as blood pressure control or use of ACE inhibitors for renal events, are possible factors for heterogeneity, these factors were not fully evaluated in the studies included in this analysis. [50], [51] Based on these results, standardization of study design is needed, including treatment strategy or adjustment of confounders.
As diabetes is a common disease with high risk of macrovascular and microvascular complications, we focused on diabetic patients. In this sense, we excluded patients without diabetes from this study. Due to this restriction of subjects, our study precisely compared the outcomes of the studies of diabetic cohorts. On the other hand, out study was not able to describe the risk of patients with diabetes compared to those without diabetes.
The strength of this study is the listing of all studies allowing readers to see the inconsistency across cohorts. The limitations of this study should also be noted. First, the numbers of studies regarding the associations between low eGFR and cardiovascular mortality, all-cause mortality, and renal events were small. Although low eGFR was considered as a risk factor for cardiovascular events according to the guidelines developed by KDIGO in 2002, there were few studies from this viewpoint prior to this time. [44] Second, each study had its own definition of normal eGFR as the reference category for multivariate analysis. Some studies [10], [19] defined normal eGFR as >90 mL/min/1.73 m2, while others [6], [20] used a definition of >60 mL/min/1.73 m2. The difference in definition may have affected the magnitude of pooled risk ratio for each outcome. Third, there were differences in measurement and expression of albuminuria, such as daily excretion of albumin, or the ratio of urinary albumin to creatinine. Moreover, measurement of urinary albumin was still not standardized. [52], [53], [54] A standardized method for measurement of albuminuria is essential for comparing data across studies. Furthermore, collection of urine was also not standardized. Spot urine sample collection in the morning or daily collection of urine would lead to different magnitudes of risk ratio., [55] With regard to expression of urinary albumin, some guidelines [56], [57], [58] use albumin/creatinine ratio. However, other expressions were also used in different studies, such as 24-h excretion or concentration of urinary albumin. Fourth, there may be problems associated with reporting bias, especially for renal events. Some studies measuring serum creatinine at baseline did not report renal outcome. The outcome reporting bias may have increased the influence of renal outcome, which is a very large risk ratio compared with cardiovascular or all-cause mortality. Fifth, the numbers of studies reporting the influence of low eGFR were small. Our search strategy limited objects as “diabetes with albuminuria/proteinuria” or “diabetic nephropathy.” Therefore, studies of diabetic patients with low eGFR may not have been included in our systematic review due to our search strategy. Sixth, making the best use of information about study design or baseline characteristics, the threshold of study size was not used as a limitation in study selection. These selection criteria resulted in more than half of the selected studies consisted of less than 1000 participants.
With regard to the effects of albuminuria and eGFR in diabetic patients, the Chronic Kidney Disease Prognosis Consortium (CKDPC) reported a precise estimate of risk [59]. In addition, our study provided further information showing the inconsistency of study design or subgroup analysis, and presented pooled risk ratio by category of albuminuria and low eGFR for use in clinical care. Moreover, information about intervention or race (except Caucasian) is limited in both the report of CKDPC and this systematic review.
In summary, we conducted a systematic review and meta-analysis, including 148350 cases, and described the impacts of albuminuria and low eGFR on the risks of cardiovascular mortality, all-cause mortality, and renal events. Micro- and macroalbuminuria were significant risk factors for all three outcomes, and low eGFR and albuminuria may be independent risk factors. There was less evidence exploring the influences of low eGFR as independent risk factor on the outcomes. To evaluate the effects of low eGFR, intervention, or race, including Asian subjects, individual patient data meta-analysis or long-term prospective studies based on individual patient data are needed.
Supporting Information
Subgroup analysis for examination of potential sources of heterogeneity in the association between micro- or macroalbuminuria and cardiovascular mortality, all-cause mortality or renal events.
(TIFF)
Age stratified analysis for the association between albuminuria and cardiovascular mortality, all-cause mortality, and renal events compared with normoalbuminuria.
(TIFF)
Funding Statement
This study was supported in part by a Grant-in-Aid for Diabetic Nephropathy Research, from the Ministry of Health, Labor and Welfare of Japan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
References
- 1. Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, et al. (2011) National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet 378: 31–40. [DOI] [PubMed] [Google Scholar]
- 2.Yang SH, Dou KF, Song WJ (2010) Prevalence of diabetes among men and women in China. The New England journal of medicine 362: 2425–6; author reply 2426. [DOI] [PubMed]
- 3. Soriguer F, Goday A, Bosch-Comas A, Bordiú E, Calle-Pascual A, et al. (2012) Prevalence of diabetes mellitus and impaired glucose regulation in Spain: the Di@bet.es Study. Diabetologia. 55: 88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, et al. (2003) Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney international 63: 225–232. [DOI] [PubMed] [Google Scholar]
- 5. Wada T, Shimizu M, Toyama T, Hara A, Kaneko S, et al. (2012) Clinical impact of albuminuria in diabetic nephropathy. Clinical and experimental nephrology 16: 96–101. [DOI] [PubMed] [Google Scholar]
- 6. Vlek ALM, Van der Graaf Y, Spiering W, Algra A, Visseren FLJ (2008) Cardiovascular events and all-cause mortality by albuminuria and decreased glomerular filtration rate in patients with vascular disease. Journal of internal medicine 264: 351–360. [DOI] [PubMed] [Google Scholar]
- 7. The Diabetes Control and Complications Trial Research Group (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The New England journal of medicine 329: 977–986. [DOI] [PubMed] [Google Scholar]
- 8. Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, et al. (1995) Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes research and clinical practice 28: 103–117. [DOI] [PubMed] [Google Scholar]
- 9. Haller H, Ito S, Izzo JL, Januszewicz A, Katayama S, et al. (2011) Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. The New England journal of medicine 364: 907–917. [DOI] [PubMed] [Google Scholar]
- 10. Ninomiya T, Perkovic V, De Galan BE, Zoungas S, Pillai A, et al. (2009) Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. Journal of the American Society of Nephrology?: JASN 20: 1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yokoyama H, Oishi M, Kawai K, Sone H (2008) Reduced GFR and microalbuminuria are independently associated with prevalent cardiovascular disease in Type 2 diabetes: JDDM study 16. Diabetic medicine: a journal of the British Diabetic Association 25: 1426–1432. [DOI] [PubMed] [Google Scholar]
- 12. Sarnak MJ, Levey AS (2003) Schoolwerth AC, Coresh J, Culleton B, et al (2003) Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108: 2154–2169. [DOI] [PubMed] [Google Scholar]
- 13. Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50: 1088–1101. [PubMed] [Google Scholar]
- 14. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed) 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Florkowski CM, Scott RS, Coope PA, Moir CL (2001) Predictors of mortality from type 2 diabetes mellitus in Canterbury, New Zealand; a ten-year cohort study. Diabetes research and clinical practice 53: 113–120. [DOI] [PubMed] [Google Scholar]
- 16. Tong PCY, Kong AP, So WY, Yang X, Ng MCY, et al. (2007) Interactive effect of retinopathy and macroalbuminuria on all-cause mortality, cardiovascular and renal end points in Chinese patients with Type 2 diabetes mellitus. Diabetic medicine?: a journal of the British Diabetic Association 24: 741–746. [DOI] [PubMed] [Google Scholar]
- 17. Molitch ME, Steffes M, Sun W, Rutledge B, Cleary P, et al. (2010) Development and progression of renal insufficiency with and without albuminuria in adults with type 1 diabetes in the diabetes control and complications trial and the epidemiology of diabetes interventions and complications study. Diabetes care 33: 1536–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O’Hare AM, Hailpern SM, Pavkov ME, Rios-Burrows N, Gupta I, et al. (2010) Prognostic implications of the urinary albumin to creatinine ratio in veterans of different ages with diabetes. Archives of internal medicine 170: 930–936. [DOI] [PubMed] [Google Scholar]
- 19. So WY, Kong APS, Ma RCW, Ozaki R, Szeto CC, et al. (2006) Glomerular filtration rate, cardiorenal end points, and all-cause mortality in type 2 diabetic patients. Diabetes care 29: 2046–2052. [DOI] [PubMed] [Google Scholar]
- 20. De Boer IH, Katz R, Cao JJ, Fried LF, Kestenbaum B, et al. (2009) Cystatin C, albuminuria, and mortality among older adults with diabetes. Diabetes care 32: 1833–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jager A, Kostense PJ, Ruhé HG, Heine RJ, Nijpels G, et al. (1999) Microalbuminuria and peripheral arterial disease are independent predictors of cardiovascular and all-cause mortality, especially among hypertensive subjects: five-year follow-up of the Hoorn Study. Arteriosclerosis, thrombosis, and vascular biology 19: 617–624. [DOI] [PubMed] [Google Scholar]
- 22. Grauslund J, Jørgensen TMM, Nybo M, Green A, Rasmussen LM, et al. (2010) Risk factors for mortality and ischemic heart disease in patients with long-term type 1 diabetes. Journal of diabetes and its complications 24: 223–228. [DOI] [PubMed] [Google Scholar]
- 23. Groop P, Thomas MC, Moran JL, Wadèn J, Thorn LM, et al. (2009) The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes 58: 1651–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luk AOY, So W-Y, Ma RCW, Kong APS, Ozaki R, et al. (2008) Metabolic syndrome predicts new onset of chronic kidney disease in 5,829 patients with type 2 diabetes: a 5-year prospective analysis of the Hong Kong Diabetes Registry. Diabetes care 31: 2357–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bruno G, Merletti F, Bargero G, Novelli G, Melis D, et al. (2007) Estimated glomerular filtration rate, albuminuria and mortality in type 2 diabetes: the Casale Monferrato study. Diabetologia 50: 941–948. [DOI] [PubMed] [Google Scholar]
- 26. Roy M, Rendas-Baum R, Skurnick J (2006) Mortality in African-Americans with Type 1 diabetes: The New Jersey 725. Diabetic medicine: a journal of the British Diabetic Association 23: 698–706. [DOI] [PubMed] [Google Scholar]
- 27. Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR (2006) Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes 55: 1832–1839. [DOI] [PubMed] [Google Scholar]
- 28. Xu J, Lee ET, Best LG, Begum M, Knowler WC, et al. (2005) Association of albuminuria with all-cause and cardiovascular disease mortality in diabetes: the Strong Heart Study. The British Journal of Diabetes & Vascular Disease 5: 334–340. [Google Scholar]
- 29. Yuyun MF, Dinneen SF, Edwards OM, Wood E, Wareham NJ (2003) Absolute level and rate of change of albuminuria over 1 year independently predict mortality and cardiovascular events in patients with diabetic nephropathy. Diabetic medicine?: a journal of the British Diabetic Association 20: 277–282. [DOI] [PubMed] [Google Scholar]
- 30. Bruno G, Biggeri A, Merletti F, Bargero G, Ferrero S, et al. (2003) Low incidence of end-stage renal disease and chronic renal failure in type 2 diabetes: a 10-year prospective study. Diabetes care 26: 2353–2358. [DOI] [PubMed] [Google Scholar]
- 31. Jude EB, Anderson SG, Cruickshank JK, Srivatsa A, Tentolouris N, et al. (2002) Natural history and prognostic factors of diabetic nephropathy in type 2 diabetes. QJM: monthly journal of the Association of Physicians 95: 371–377. [DOI] [PubMed] [Google Scholar]
- 32. Ostgren CJ, Lindblad U, Melander A, Råstam L (2002) Survival in patients with type 2 diabetes in a Swedish community: skaraborg hypertension and diabetes project. Diabetes care 25: 1297–1302. [DOI] [PubMed] [Google Scholar]
- 33. Stehouwer CDA, Gall M, Twisk JWR, Knudsen E, Emeis JJ, et al. (2002) Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in type 2 diabetes: progressive, interrelated, and independently associated with risk of death. Diabetes 51: 1157–1165. [DOI] [PubMed] [Google Scholar]
- 34. Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, et al. (2001) Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA: the journal of the American Medical Association 286: 421–426. [DOI] [PubMed] [Google Scholar]
- 35. De Grauw WJ, Van de Lisdonk EH, Van Gerwen WH, Verstappen M, Van den Hoogen HJ, et al. (2001) Microalbuminuria in patients with Type 2 diabetes mellitus from general practice: course and predictive value. Diabetic medicine?: a journal of the British Diabetic Association 18: 139–143. [DOI] [PubMed] [Google Scholar]
- 36. Casiglia E, Zanette G, Mazza a, Donadon V, Donada C, et al. (2000) Cardiovascular mortality in non-insulin-dependent diabetes mellitus. A controlled study among 683 diabetics and 683 age- and sex-matched normal subjects. European journal of epidemiology 16: 677–684. [DOI] [PubMed] [Google Scholar]
- 37. Valmadrid CT, Klein R, Moss SE, Klein BE (2000) The risk of cardiovascular disease mortality associated with microalbuminuria and gross proteinuria in persons with older-onset diabetes mellitus. Archives of internal medicine 160: 1093–1100. [DOI] [PubMed] [Google Scholar]
- 38. Hänninen J, Takala J, Keinänen-Kiukaanniemi S (1999) Albuminuria and other risk factors for mortality in patients with non-insulin-dependent diabetes mellitus aged under 65 years: a population-based prospective 5-year study. Diabetes research and clinical practice 43: 121–126. [DOI] [PubMed] [Google Scholar]
- 39. Mattock MB, Barnes DJ, Viberti G, Keen H, Burt D, et al. (1998) Microalbuminuria and coronary heart disease in NIDDM: an incidence study. Diabetes 47: 1786–1792. [DOI] [PubMed] [Google Scholar]
- 40. Beilin J, Stanton KG, McCann VJ, Knuiman MW, Divitini ML (1996) Microalbuminuria in type 2 diabetes: an independent predictor of cardiovascular mortality. Australian and New Zealand journal of medicine 26: 519–525. [DOI] [PubMed] [Google Scholar]
- 41. Rossing P, Hougaard P, Borch-Johnsen K, Parving HH (1996) Predictors of mortality in insulin dependent diabetes: 10 year observational follow up study. BMJ (Clinical research ed) 313: 779–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gall MA, Borch-Johnsen K, Hougaard P, Nielsen FS, Parving HH (1995) Albuminuria and poor glycemic control predict mortality in NIDDM. Diabetes 44: 1303–1309. [DOI] [PubMed] [Google Scholar]
- 43. Neil A, Hawkins M, Potok M, Thorogood M, Cohen D, et al. (1993) A prospective population-based study of microalbuminuria as a predictor of mortality in NIDDM. Diabetes care 16: 996–1003. [DOI] [PubMed] [Google Scholar]
- 44. Inventory BD, Index CD (2002) K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. American journal of kidney diseases: the official journal of the National Kidney Foundation 39: S1–266. [PubMed] [Google Scholar]
- 45. Levey AS, De Jong PE, Coresh J, Nahas M El, Astor BC, et al. (2010) The definition, classification and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney international 80: 17–28. [DOI] [PubMed] [Google Scholar]
- 46. Gross JL, De Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, et al. (2005) Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes care 28: 164–176. [DOI] [PubMed] [Google Scholar]
- 47. Dinneen SF, Gerstein HC (1997) The association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus. A systematic overview of the literature. Archives of internal medicine 157: 1413–1418. [PubMed] [Google Scholar]
- 48. Van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, et al. (2011) Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney international 79: 1341–1352. [DOI] [PubMed] [Google Scholar]
- 49. Gansevoort RT, Matsushita K, Van der Velde M, Astor BC, Woodward M, et al. (2011) Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes in both general and high-risk populations. A collaborative meta-analysis of general and high-risk population cohorts. Kidney International 80: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pohl MA, Blumenthal S, Cordonnier DJ, De Alvaro F, Deferrari G, et al. (2005) Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the irbesartan diabetic nephropathy trial: clinical implications and limitations. Journal of the American Society of Nephrology?: JASN 16: 3027–3037. [DOI] [PubMed] [Google Scholar]
- 51. Lewis EJ, Hunsicker LG, Bain RP, Rohde RD (1993) The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. The New England journal of medicine 329: 1456–1462. [DOI] [PubMed] [Google Scholar]
- 52. Miller WG, Bruns DE, Hortin GL, Sandberg S, Aakre KM, et al. (2009) Current issues in measurement and reporting of urinary albumin excretion. Clinical chemistry 55: 24–38. [DOI] [PubMed] [Google Scholar]
- 53. Sviridov D, Meilinger B, Drake SK, Hoehn GT, Hortin GL (2006) Coelution of other proteins with albumin during size-exclusion HPLC: Implications for analysis of urinary albumin. Clinical chemistry 52: 389–397. [DOI] [PubMed] [Google Scholar]
- 54.Brinkman JW, Bakker SJL, Gansevoort RT, Hillege HL, Kema IP, et al.. (2004) Which method for quantifying urinary albumin excretion gives what outcome? A comparison of immunonephelometry with HPLC. Kidney international Supplement: S69–75. [DOI] [PubMed]
- 55. Witte EC, Lambers Heerspink HJ, De Zeeuw D, Bakker SJL, De Jong PE, et al. (2009) First morning voids are more reliable than spot urine samples to assess microalbuminuria. Journal of the American Society of Nephrology: JASN 20: 436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Diabetes VII, In C (2011) Standards of medical care in diabetes–2011. Diabetes care 34 Suppl 1S11–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. KDOQI Clinical Practice Guidelines, Clinical Practice Recommendations for Diabetes, Chronic Kidney Disease (2007) American journal of kidney diseases?: the official journal of the National Kidney Foundation. 49: S12–154. [DOI] [PubMed] [Google Scholar]
- 58. Levey AS, Eckardt K-U, Tsukamoto Y, Levin A, Coresh J, et al. (2005) Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney international 67: 2089–2100. [DOI] [PubMed] [Google Scholar]
- 59. Fox CS, Matsushita K, Woodward M, Bilo HJG, Chalmers J, et al. (2012) Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet 380: 1662–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Subgroup analysis for examination of potential sources of heterogeneity in the association between micro- or macroalbuminuria and cardiovascular mortality, all-cause mortality or renal events.
(TIFF)
Age stratified analysis for the association between albuminuria and cardiovascular mortality, all-cause mortality, and renal events compared with normoalbuminuria.
(TIFF)