Abstract
We have previously reported the high levels of glutamic acid decarboxylase 65 autoantibodies (GAD65A) in patients with type 1 diabetes and autoimmune thyroid disease. Here we describe a 32-year-old Japanese female with a thirteen-year history of type 1 diabetes whose levels of GAD65A were elevated just after the emergence of anti-thyroid autoimmunity. At 19 years of age, she developed diabetic ketoacidosis and was diagnosed with type 1 diabetes. She had GAD65A, insulinoma-associated antigen-2 autoantibodies (IA-2A), and zinc transporter-8 autoantibodies (ZnT8A), but was negative for antibodies to thyroid peroxidase (TPOAb) and thyroglobulin (TGAb) at disease onset. ZnT8A and IA-2A turned negative 2-3 years after the onset, whereas GAD65A were persistently positive at lower level (approximately 40 U/mL). However, just after the emergence of TGAb at disease duration of 12.5 years, GAD65A levels were reelevated up to 5717 U/mL in the absence of ZnT8A and IA-2A. Her thyroid function was normal and TPOAb were consistently negative. She has a HLA-DRB1*03:01/*04:01-DQB1*02:01/*03:02 genotype. Persistent positivity for GAD65A might be associated with increased risk to develop anti-thyroid autoimmunity.
Keywords: Autoimmune thyroid disease, Case report, Glutamic acid decarboxylase autoantibodies, Type 1 diabetes
Core tip: This paper describes a case of type 1 diabetes whose levels of glutamic acid decarboxylase 65 autoantibodies (GAD65A) were reelevated just after the emergence of anti-thyroid autoimmunity at disease duration of 12.5 years without any clinical signs of thyroid dysfunction. This case report suggests that persistent positivity for GAD65A is associated with increased risk to develop anti-thyroid autoimmunity.
INTRODUCTION
Type 1 diabetes is an autoimmune disease against pancreatic islet β cells and is often complicated with other autoimmune diseases, of which autoimmune thyroid disease (AITD) is the most frequent[1]. It has been reported that the prevalence of autoantibodies to thyroid peroxidase (TPOAb) and/or thyroglobulin (TGAb) are 15%-30% in patients with type 1 diabetes at the time of diagnosis of the diabetes[1-3]. Furthermore, there is heterogeneity in the natural history of AITD in patients with type 1 diabetes: AITD may be diagnosed either at diabetes onset or during the follow-up, and the measurement of TPOAb and TGAb is useful to predict the development of future AITD. We and others have previously reported the association between anti-thyroid autoimmunity and anti-islet autoantibodies, especially glutamic acid decarboxylase 65 autoantibodies (GAD65A) and zinc transporter 8 autoantibodies (ZnT8A)[4-7]. The levels and the prevalence of GAD65A are higher and more persistent in type 1 diabetic patients with AITD compared to type 1 diabetes alone[4]. Here we report a 32-year-old Japanese female with a thirteen-year history of type 1 diabetes whose levels of GAD65A were reelevated just after the emergence of anti-thyroid autoimmunity.
CASE REPORT
A 32-year-old Japanese female had developed diabetic ketoacidosis at 19 years of age and diagnosed as type 1 diabetes. She was immediately started the intensive insulin therapy and referred to our hospital for glycemic control after one month of diabetes onset. Her past medical history was unremarkable and her family history was negative for diabetes or autoimmune diseases. Her body mass index was 19.4 kg/m2 and blood pressure was 108/72 mmHg. Neither exophthalmos nor diabetic retinopathy was observed. Thyroid gland was slightly enlarged. There were no abnormal findings of the heart, lungs, or abdomen. Neurological examination was also normal. As shown in Table 1, endogenous insulin secretory capacity was decreased (Urine C-peptide 5.5 μg/d) and GAD65A (4342 U/mL; normal range < 1.5 U/mL), IA-2A (index 0.769; normal range < 0.018) and ZnT8A (index 0.055; normal range < 0.007) were all positive. However, both TPOAb and TGAb were negative. She had HLA-DRB1*03:01/*04:01-DQB1*02:01/*03:02 genotype. She was treated by insulin aspart before each meal (30 U) and glargine at lunch and dinner (10 U) and her hemoglobin A1c (HbA1c) levels were maintained between 6.2% and 7.5%.
Table 1.
Laboratory findings at the first visit
| Laboratory findings | |
| Urinalysis | |
| Sugar | (-) |
| Protein | (-) |
| Ketone | (-) |
| Hematological data | |
| WBC (/μL) | 5.0 × 103 |
| RBC (/μL) | 4.5 × 106 |
| Hb (g/dL) | 13.1 |
| Hct (%) | 39.9 |
| Plt (/μL) | 21.7 × 104 |
| Biochemical data | |
| TP (g/dL) | 6.6 |
| AST (IU/L) | 11 |
| ALT (IU/L) | 11 |
| γ-GTP (IU/L) | 10 |
| TC (mg/dL ) | 184 |
| TG (mg/dL ) | 50 |
| Cr (mg/dL ) | 0.5 |
| BUN (mg/dL ) | 14 |
| PG (mg/dL ) | 100 |
| HbA1c (%) | 11.90 |
| U-CPR (μg/d) | 5.5 |
| Immunological data | |
| GAD65A (U/mL) | 4342 |
| IA-2A (index) | 0.769 |
| ZnT8A (index) | 0.055 |
| TPOAb (U/mL) | (-) |
| TGAb (U/mL) | (-) |
| HLA-DRB1 | *03:01/*04:01 |
| HLA-DQB1 | *02:01/*03:02 |
HbA1c: Hemoglobin A1c; ZnT8A: Zinc transporter-8 autoantibodies; TPOAb: Thyroid peroxidase antibody; TGAb: Thyroglobulin antibody; WBC: White blood cell; RBC: Red blood cell; TP: Total protein; AST: Aspartate transaminase; ALT: Alanine aminotransferase; γ-GTP: γ-glutamyl transpeptidase; TC: Total cholesterol; TG: Triglyceride; Cr: Creatinine; BUN: Blood urea nitrogen; PG: Plasma glucose.
During the follow-up her GAD65A, anti-thyroid antibodies and thyroid function were monitored regularly. The GAD65A level gradually decreased and reached at 40 to 50 U/mL at > 10 years after diabetes onset. Her TPO/TGAbs remained negative and thyroid function was within normal range. However, GAD65A were relevated to 90 U/mL thirteen years after diabetes onset. Then we measured anti-islet autoantibodies and anti-thyroid antibodies in her stored samples (Figure 1). ZnT8A and IA-2A turned negative 2-3 years after the onset, whereas GAD65A had been persistently positive at lower level. However, TGAb emerged at disease duration of 12.5 years and GAD65A levels were relevated thereafter up to 5717 U/mL in the absence of ZnT8A and IA-2A. Ultrasound examination showed a thyroid gland of homogenous parenchyma with normal size. Furthermore, no significant elevation of TPOAb was observed and her thyroid function was normal throughout the clinical course. Her serum C-peptide level was undetectable at disease duration of 4 years and did not change after the emergence of anti-thyroid autoimmunity. Furthermore, her metabolic control and insulin requirement did not change (HbA1c 6.8%-7.1%, insulin requirement 35-41 U/d).
Figure 1.
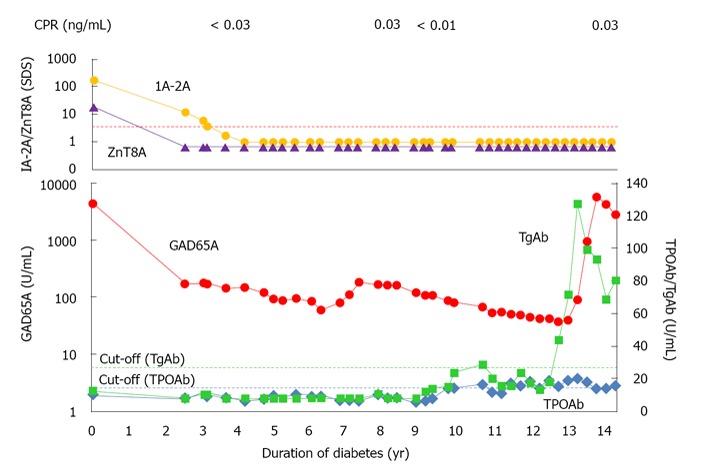
Time course of anti-islet autoantibodies and anti-thyroid antibodies. Dotted lines indicate the cut-off values of antibodies. The levels of IA-2A and ZnT8A were expressed as SDS. IA-2A: Insulinoma-associated antigen-2 autoantibodies; ZnT8A: Zinc transporter-8 autoantibodies; SDS: Standard deviation score; GAD: Glutamic acid decarboxylase.
DISCUSSION
There were many reports on the association between AITD and type 1 diabetes. However, little is known on the dynamics of the humoral autoimmunity to islet autoantigens in association with anti-thyroid autoimmunity in type 1 diabetic patients who have no evidence of thyroid autoimmunity at disease onset. In patients with type 1 diabetes positive for glutamic acid decarboxylase autoantibodies (GADA), a higher prevalence of anti-thyroid antibodies was reported as compared to those without GADA[5,8]. Furthermore, we have previously reported that patients with type 1 diabetes and AITD (i.e., autoimmune polyendocrine syndrome type 3; APS3) show the higher levels of GAD65A compared to patients with type 1 diabetes alone in both cross-sectional and longitudinal observations[4]. Because high levels of GADA are observed in insulin-deficient patients as same as that of our case, production of GADA may not be associated with the residual β cell antigens. Furthermore, it has been reported that GAD is not only expressed in β cells but also in the thyroid gland[9]. Taken together it is hypothesized that the production of GADA in patients with APS3 might be attributable to a polyclonal activation of the autoimmune system in AITD and persistent positivity for GAD65A might be associated with increased risk to develop anti-thyroid autoimmunity.
It is unknown whether organ specific autoantibodies are directly involved in the pathogenesis of AITD or whether they are just secondary to tissue destruction by thyroid-infiltrating T-cells[10]. In our case, TGAb turned positive 12.5 years after the onset of type 1 diabetes. It has been questioned whether TGAb provide further diagnostic information as compared to the TPOAb in the diagnosis of AITD[3,11,12]. However, it has been reported that female gender, older (adolescence) onset, longer duration of diabetes, and positivity for GADA were risk factors for the development of thyroid disorders[3,5]. In addition, it is generally accepted that a certain type of HLA is involved in the development of type 1 diabetes and AITD[13,14]. An association between AITD and HLA-DR3[15] and DRB1*04-DQB1*03:01[16] has been reported in Caucasian population. In addition, HLA-DRB1*03:01-DQB1*02:01 and DR4-DQB1*03:02 has also been reported to contribute to both type 1 diabetes and AITD in a study of families with both diseases[17,18]. Therefore, our patient, who has HLA-DRB1*03:01-DQB1*02:01 and DRB1*04:01-DQB1*03:02 haplotypes, might be at high risk for the later development of AITD. Therefore, thyroid function test should be performed regularly in this patient for possible early diagnosis of thyroid disorders, although thyroid function is still normal.
Footnotes
P- Reviewers Markopoulos AK, Sanlioglu AD S- Editor Song XX L- Editor A E- Editor Liu XM
References
- 1.De Block CE, De Leeuw IH, Vertommen JJ, Rooman RP, Du Caju MV, Van Campenhout CM, Weyler JJ, Winnock F, Van Autreve J, Gorus FK. Beta-cell, thyroid, gastric, adrenal and coeliac autoimmunity and HLA-DQ types in type 1 diabetes. Clin Exp Immunol. 2001;126:236–241. doi: 10.1046/j.1365-2249.2001.01668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burbelo PD, Lebovitz EE, Bren KE, Bayat A, Paviol S, Wenzlau JM, Barriga KJ, Rewers M, Harlan DM, Iadarola MJ. Extrapancreatic autoantibody profiles in type I diabetes. PLoS One. 2012;7:e45216. doi: 10.1371/journal.pone.0045216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker JM, Yu J, Yu L, Wang J, Miao D, Bao F, Hoffenberg E, Nelson JC, Gottlieb PA, Rewers M, et al. Autoantibody “subspecificity” in type 1 diabetes: risk for organ-specific autoimmunity clusters in distinct groups. Diabetes Care. 2005;28:850–855. doi: 10.2337/diacare.28.4.850. [DOI] [PubMed] [Google Scholar]
- 4.Kawasaki E, Takino H, Yano M, Uotani S, Matsumoto K, Takao Y, Yamaguchi Y, Akazawa S, Nagataki S. Autoantibodies to glutamic acid decarboxylase in patients with IDDM and autoimmune thyroid disease. Diabetes. 1994;43:80–86. doi: 10.2337/diab.43.1.80. [DOI] [PubMed] [Google Scholar]
- 5.Kordonouri O, Charpentier N, Hartmann R. GADA positivity at onset of type 1 diabetes is a risk factor for the development of autoimmune thyroiditis. Pediatr Diabetes. 2011;12:31–33. doi: 10.1111/j.1399-5448.2010.00666.x. [DOI] [PubMed] [Google Scholar]
- 6.Horie I, Kawasaki E, Ando T, Kuwahara H, Abiru N, Usa T, Yamasaki H, Ejima E, Kawakami A. Clinical and genetic characteristics of autoimmune polyglandular syndrome type 3 variant in the Japanese population. J Clin Endocrinol Metab. 2012;97:E1043–E1050. doi: 10.1210/jc.2011-3109. [DOI] [PubMed] [Google Scholar]
- 7.Jonsdottir B, Andersson C, Carlsson A, Delli A, Forsander G, Ludvigsson J, Marcus C, Samuelsson U, Ortqvist E, Lernmark A, et al. Thyroid autoimmunity in relation to islet autoantibodies and HLA-DQ genotype in newly diagnosed type 1 diabetes in children and adolescents. Diabetologia. 2013;56:1735–1742. doi: 10.1007/s00125-013-2934-9. [DOI] [PubMed] [Google Scholar]
- 8.Bárová H, Perusicová J, Hill M, Sterzl I, Vondra K, Masek Z. Anti-GAD-positive patients with type 1 diabetes mellitus have higher prevalence of autoimmune thyroiditis than anti-GAD-negative patients with type 1 and type 2 diabetes mellitus. Physiol Res. 2004;53:279–286. [PubMed] [Google Scholar]
- 9.Christie MR, Brown TJ, Cassidy D. Binding of antibodies in sera from Type 1 (insulin-dependent) diabetic patients to glutamate decarboxylase from rat tissues. Evidence for antigenic and non-antigenic forms of the enzyme. Diabetologia. 1992;35:380–384. doi: 10.1007/BF00401206. [DOI] [PubMed] [Google Scholar]
- 10.McIntosh RS, Asghar MS, Weetman AP. The antibody response in human autoimmune thyroid disease. Clin Sci (Lond) 1997;92:529–541. doi: 10.1042/cs0920529. [DOI] [PubMed] [Google Scholar]
- 11.Padberg S, Heller K, Usadel KH, Schumm-Draeger PM. One-year prophylactic treatment of euthyroid Hashimoto’s thyroiditis patients with levothyroxine: is there a benefit? Thyroid. 2001;11:249–255. doi: 10.1089/105072501750159651. [DOI] [PubMed] [Google Scholar]
- 12.Kordonouri O, Klinghammer A, Lang EB, Grüters-Kieslich A, Grabert M, Holl RW. Thyroid autoimmunity in children and adolescents with type 1 diabetes: a multicenter survey. Diabetes Care. 2002;25:1346–1350. doi: 10.2337/diacare.25.8.1346. [DOI] [PubMed] [Google Scholar]
- 13.Kawasaki E, Gill RG, Eisenbarth GS. Type 1 diabetes mellitus. In: Eisenbarth GS Molecular mechanisms of endocrine and organ specific autoimmunity., editor. Austin, TX: R.G. Landes Company; 1999. pp. 149–182. [Google Scholar]
- 14.Brand OJ, Gough SC. Immunogenetic mechanisms leading to thyroid autoimmunity: recent advances in identifying susceptibility genes and regions. Curr Genomics. 2011;12:526–541. doi: 10.2174/138920211798120790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tandon N, Zhang L, Weetman AP. HLA associations with Hashimoto’s thyroiditis. Clin Endocrinol (Oxf) 1991;34:383–386. doi: 10.1111/j.1365-2265.1991.tb00309.x. [DOI] [PubMed] [Google Scholar]
- 16.Petrone A, Giorgi G, Mesturino CA, Capizzi M, Cascino I, Nistico L, Osborn J, Di Mario U, Buzzetti R. Association of DRB1*04-DQB1*0301 haplotype and lack of association of two polymorphic sites at CTLA-4 gene with Hashimoto’s thyroiditis in an Italian population. Thyroid. 2001;11:171–175. doi: 10.1089/105072501300042901. [DOI] [PubMed] [Google Scholar]
- 17.Levin L, Ban Y, Concepcion E, Davies TF, Greenberg DA, Tomer Y. Analysis of HLA genes in families with autoimmune diabetes and thyroiditis. Hum Immunol. 2004;65:640–647. doi: 10.1016/j.humimm.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 18.Golden B, Levin L, Ban Y, Concepcion E, Greenberg DA, Tomer Y. Genetic analysis of families with autoimmune diabetes and thyroiditis: evidence for common and unique genes. J Clin Endocrinol Metab. 2005;90:4904–4911. doi: 10.1210/jc.2004-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]


