Abstract
Purpose
Stress has a deteriorating effect on hippocampal function. It also contributes to symptom exacerbation in many disease states, including overactive bladder and interstitial cystitis/bladder pain syndrome. We investigated the effects of various types of stresses (restraint, noise, and cold) on short-term memory and apoptosis in relation with corticotropin-releasing factor (CRF) expression.
Methods
Rats in the restraint stress group were restrained in a transparent Plexiglas cylinder for 60 minutes twice daily. Rats in the noise stress group were exposed to the 120 dB supersonic machine sound for 60 minutes twice daily. Rats in the cold stress group were placed in a cold chamber at 4℃ for 60 minutes twice daily. Each stress was applied for 10 days. A step-down avoidance test for short-term memory, immunohistochemistry for caspase-3 expression, and western blot analysis for Bax and Bcl-2 expressions were conducted.
Results
Latency time was decreased and CRF expression in the hippocampal dentate gyrus and hypothalamic paraventricular nucleus were increased in all of the stress groups. The number of caspase-3-positive cells in the hippocampal dentate gyrus was increased and the expressions of Bax and Bcl2 in the hippocampus were decreased in all of the stress groups.
Conclusions
All of the stress groups experienced short-term memory impairment induced by apoptosis in the hippocampus. The present results suggest the possibility that these stresses affecting the impairment of short-term memory may also induce functional lower urinary tract disorders.
Keywords: Restrain stress, Noise stress, Cold stress, Short-term memory, Apoptosis
INTRODUCTION
Stress exerts a deteriorating effect on hippocampal function [1], and stress-induced structural changes and neuronal damage in the hippocampus impair learning ability and memory function [2,3]. Stress during pregnancy resulted in anxiety-like behaviors in the delivered offspring [4]. Stress also contributes to symptom exacerbation in many disease states, including overactive bladder and interstitial cystitis/bladder pain syndrome [5-7]. Stress induces the release of corticotropin-releasing factor (CRF) and then activates neurons, including hippocampal pyramidal cells, and modulates brain neurotransmitter system activity [8]. CRF is a 41-amino-acid peptide that is synthesized in the hypothalamic paraventricular nucleus (PVN) in response to stress [9].
Apoptosis, programmed cell death, is a form of cell death that serves to eliminate dying cells in proliferating or differentiating cell populations; thus, it plays a crucial role in normal development and tissue homeostasis. Nevertheless, inappropriate or excessive apoptosis has been implicated in several neuropsychiatric disorders [10,11]. Two important groups of proteins involved in apoptotic cell death are the members of the Bcl-2 family [12] and a class of cysteine proteases known as caspases [13]. The Bcl-2 family is classified into two functionally distinct groups: antiapoptotic proteins and proapoptotic proteins [12,14]. Bcl-2, an antiapoptotic protein, is known to regulate the apoptotic pathways and protect against cell death. Bax, a proapoptotic protein in this family, is expressed during apoptosis and promotes cell death. Caspase-3, one of the most widely studied members of the caspase family, is one of the key executors of apoptosis [15].
Neuronal apoptosis in the cortex and hippocampus disturbs learning ability and memory function, and excessive neuronal apoptosis in the hippocampus contributes to memory dysfunction [16,17]. In particular, stress is thought to induce neuronal death, possibly through apoptosis [18,19].
However, the effects of various types of stresses on short-term memory and apoptosis have not been well examined. In the present study, we investigated the effects of various types of stresses (restraint, noise, and cold) on short-term memory and apoptosis in relation to CRF expression. For this study, a step-down avoidance test for short-term memory, immunohistochemistry for caspase-3 expression, and western blot analysis for Bax and Bcl-2 expressions were conducted.
MATERIALS AND METHODS
Animals
The experimental procedures were performed in accordance with the guidelines of the National Institutes of Health and the Korean Academy of Medical Sciences. Male Sprague-Dawley rats weighing 200±10 g each were used for the experiment. Food and water were made available ad libitum. The rats were randomly divided into four groups (n=8 in each group): control, restraint stress, noise stress, and cold stress. The animals were sacrificed immediately after completion of the step-down avoidance test.
Stress Exposure
The rats in the restraint stress group were restrained in a transparent Plexiglas cylinder (length, 21 cm; diameter, 6 cm) for 60 minutes twice daily. The rats in the noise stress group were exposed to a 120 dB supersonic machine sound for 60 minutes twice daily. The rats in the cold stress group were placed in a cold chamber at 4℃ for 60 minutes twice daily. The rats in the control group were left undisturbed. Each stress was applied for 10 days.
Step-Down Avoidance Test
The latency time in the step-down avoidance test was determined to evaluate short-term memory as described previously [10]. The rats were trained in a step-down avoidance test on the tenth day after each stress exposure. Two hours after training, the latency time(s) in each group was measured. The rats were placed on a 7 cm×25 cm×2.5 cm platform. The platform faced a 42 cm×25 cm grid of parallel stainless steel bars that were 0.1 cm in caliber and spaced 1 cm apart. In the training sessions, the animals received a 0.5 mA scramble foot shock for 2 seconds immediately upon stepping down. The interval of time that elapsed between the rats stepping down and placing all four paws on the grid was defined as the latency time. A latency time >300 seconds was counted as 300 seconds.
Tissue Preparation
Brain tissue preparation was performed as previously described [20]. The animals were fully anesthetized with Zoletil 50 (10 mg/kg intraperitoneally; Vibac Laboratories, Carros, France), transcardially perfused with 50 mM phosphate-buffered saline, and fixed with a freshly prepared solution consisting of 4% paraformaldehyde in 100 mM phosphate buffer (pH 7.4). The brains were then removed, postfixed in the same fixative overnight, and transferred into a 30% sucrose solution for cryoprotection. Coronal sections of 40-µm thickness were prepared using a freezing microtome (Leica, Nussloch, Germany).
CRF Immunohistochemistry
To visualize the CRF expression, CRF immunohistochemistry was conducted as previously described [21]. Sections were selected from each brain and incubated overnight with goat anti-CRF antibody (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA) followed by incubation with biotinylated goat secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA) for another hour. The secondary antibody was amplified using a Vector Elite ABC kit (1:100; Vector Laboratories). The antibody-biotin-avidin-peroxidase complexes were visualized using 0.03% 3,3'-diaminobenzidine tetrahydrochloride (DAB), and the sections were mounted onto gelatin-coated slides. The slides were air dried overnight at room temperature and the coverslips were mounted using Permount (Fisher scientific, Pitts burg, PA, USA).
Caspase-3 Immunohistochemistry
To visualize caspase-3 expression, caspase-3 immunohistochemistry was conducted as previously described [22]. Sections were selected from each brain and incubated overnight with mouse anti-caspase-3 antibody (1:500; Santa Cruz Biotechnology) followed by incubation with biotinylated mouse secondary antibody (1:200; Vector Laboratories) for another hour. The secondary antibody was amplified using the Vector Elite ABC kit (1:100; Vector Laboratories). The antibody-biotin-avidin-peroxidase complexes were visualized using 0.03% DAB, and the sections were mounted onto gelatin-coated slides. The slides were air dried overnight at room temperature and the coverslips were mounted using Permount.
Western Blot Analysis
To detect the Bax and Bcl-2 expressions, western blot was performed as previously described [22]. The hippocampal tissues were collected and then immediately frozen at -70℃. The hippocampal tissues were homogenized on ice and lysed in a lysis buffer containing 50 mM HEPES (pH 7.5), 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 1 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N'-N'-tetraacetic acid, 1.5 mM MgCl2·6H2O, 1 mM sodium orthovanadate, and 100 mM sodium flouride. The protein content was measured using a colorimetric protein assay kit (Bio-Rad Laboratories Inc., Hercules, CA, USA). Protein (30 µg) was separated on sodium dodecyl sulfate-polyacrylamide gels and transferred onto a nitrocellulose membrane. Mouse β-actin antibody (1:500; Santa Cruz Biotechnology), rabbit brain-derived neurotrophic factor (BDNF) antibody (1:1,000; Santa Cruz Biotechnology), mouse Bax antibody (1:1,000; Santa Cruz Biotechnology), and mouse Bcl-2 antibody (1:1,000; Santa Cruz Biotechnology) were used as the primary antibodies. Horseradish peroxidase (HRP)-conjugated antirabbit antibody for BDNF and TrkB (1:3,000; Vector Laboratories), and HRP-conjugated antimouse antibody for Bax and Bcl-2 (1:2,000; Vector Laboratories) were used as the secondary antibodies. The experiments were performed under normal laboratory conditions and at room temperature except those for the transferred membranes, which were performed at 4℃ with a cold pack and prechilled buffer. Band detection was performed using an enhanced chemiluminescence detection kit (Santa Cruz Biotechnology).
Data Analysis
The area in the selected region of the hippocampus was measured using Image-Pro Plus software (Media Cybernetics, Silver Spring, MD, USA). The number of CRF-positive cells in the hippocampal dentate gyrus and hypothalamic PVN was counted hemilaterally. The number of caspase-3-positive cells in the hippocampal dentate gyrus was also counted hemilaterally. To compare the relative expressions of Bax and Bcl-2, the detected bands were calculated densitometrically using Image-Pro Plus software. The statistical analysis was performed using one-way analysis of variance followed by the Duncan post hoc test. The results are presented as the mean±standard error of the mean. Values of P<0.05 were considered statistically significant.
RESULTS
Effects of Various Types of Stresses on Short-term Memory
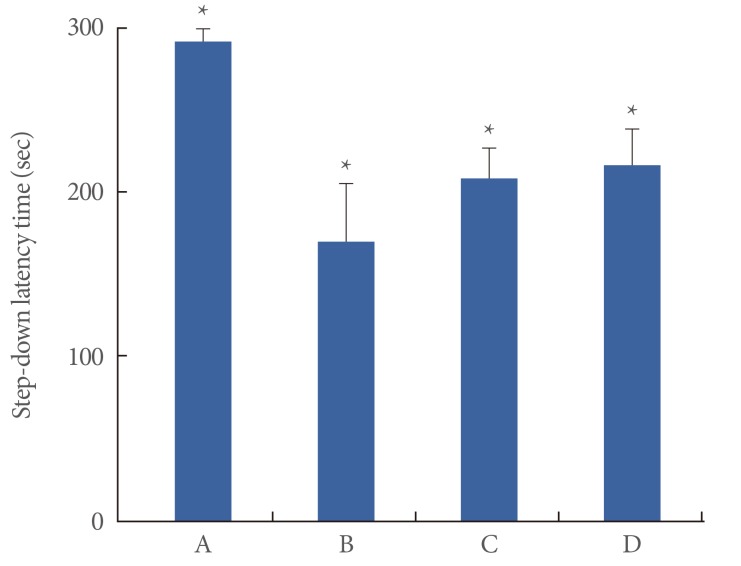
A step-down avoidance test was performed to evaluate the effects of the various stress types on short-term memory. The results of the step-down avoidance test are shown in Fig. 1. The latency time was 291.95±7.19 seconds in the control group, 170.33±34.15 seconds in the restraint stress group, 216.87±21.74 seconds in the noise stress group, and 208.75±18.16 seconds in the cold stress group. The latency time was decreased in all the stress groups compared to that in the normal group (P<0.05), suggesting that these stresses caused a deterioration in short-term memory.
Fig. 1.
The effects of various types of stresses on short-term memory. The data are presented as the mean±standard error of the mean. (A) Control group, (B) restraint stress group, (C) noise stress group, (D) cold stress group. *P<0.05 compared to the control group.
Effects of Various Types of Stresses on CRF Expression in the Hippocampal Dentate Gyrus and Hypothalamic PVN
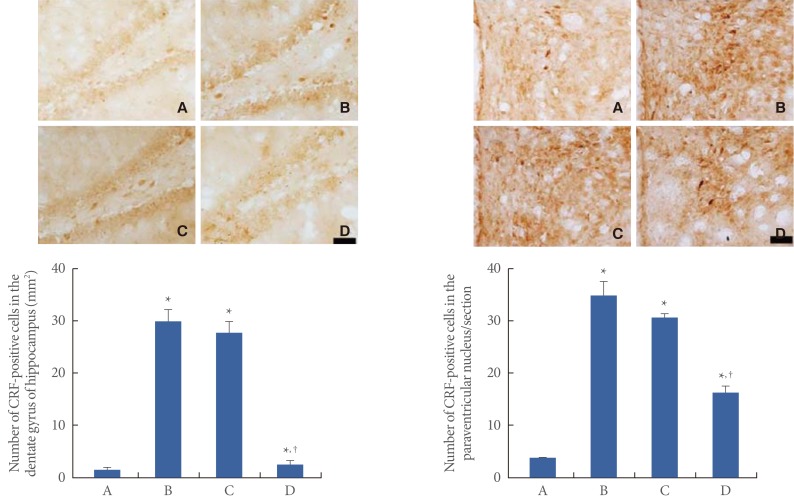
Photomicrographs of CRF-positive cells in the hippocampal dentate gyrus are presented in Fig. 2 (left). The number of CRF-positive cells was 1.15±0.64/mm2 in the control group, 29.84±2.32/mm2 in the restraint stress group, 27.65±2.23/mm2 in the noise stress group, and 2.54±0.70/mm2 in the cold stress group. Photomicrographs of CRF-positive cells in the hypothalamic PVN are presented in Fig. 2 (right). The number of CRF-positive cells was 7.02±0.47/section in the control group, 69.95±5.50/section in the restraint stress group, 61.66±1.40/section in the noise stress group, and 32.58±2.23/section in the cold stress group. Results indicated that CRF expression in the hippocampal dentate gyrus and the hypothalamic PVN increased in all the stress groups compared to that in the control group (P<0.05). However, cold stress had a lesser enhancing effect on CRF expression than restraint or noise stress (P<0.05).
Fig. 2.
The effects of various types of stresses on corticotropin-releasing factor (CRF) expression. (Left) Hippocampal dentate gyrus. Upper: Photomicrographs of the CRF-positive cells in the hippocampal dentate gyrus. The scale bar represents 50 µm. Lower: Number of CRF-positive cells in the hippocampal dentate gyrus. (Right) Hypothalamic paraventricular nucleus (PVN). Upper: Photomicrographs of the CRF-positive cells in the dentate gyrus of the PVN. The scale bar represents 50 µm. Lower: Number of CRF-positive cells in the PVN. The data are presented as the mean±standard error of the mean. (A) Control group, (B) restraint stress group, (C) noise stress group, (D) cold stress group. *P<0.05 compared to the control group. †P<0.05 compared to the restraint stress group.
Effects of Various Stress Types on Caspase-3 Expression in the Hippocampal Dentate Gyrus
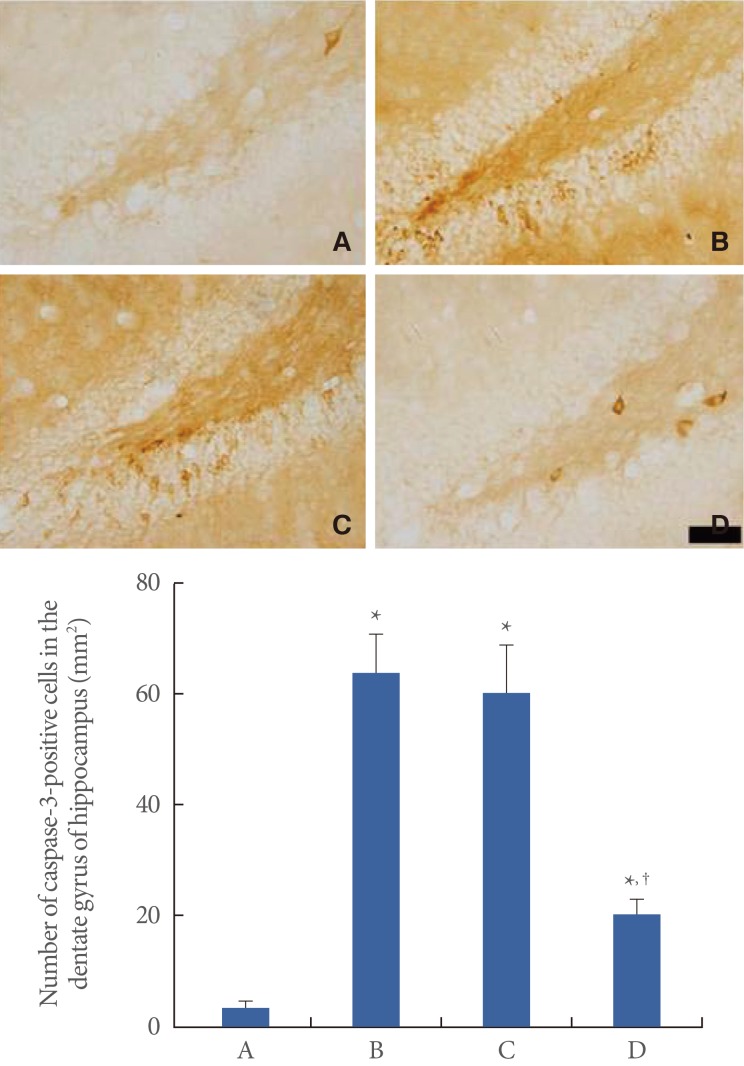
Photomicrographs of caspase-3-positive cells in the hippocampal dentate gyrus are shown in Fig. 3. The number of caspase-3-positive cells was 3.59±1.10/mm2 in the control group, 64.00±6.91/mm2 in the restraint stress group, 60.38±8.58/mm2 in the noise stress group, and 20.77±2.88/mm2 in the cold stress group. In the present results, the number of caspase-3-positive cells in the hippocampal dentate gyrus was increased in all the stress groups compared to that in the normal group (P<0.05). However, cold stress had a lesser enhancing effect on caspase-3 expression than restraint and noise stress (P<0.05).
Fig. 3.
The effects of various stress types on caspase-3 expression in the hippocampal dentate gyrus. Upper: Photomicrographs of the caspase-3-positive cells in the hippocampal dentate gyrus. The scale bar represents 50 µm. Lower: Number of caspase-3-positive cells in the hippocampal dentate gyrus. The data are presented as the mean±standard error of the mean. (A) Normal group, (B) restraint stress group, (C) noise stress group, (D) cold stress group. *P<0.05 compared to the normal group. †P<0.05 compared to the restraint stress group.
Effects of Various Types of Stresses on Bax and Bcl-2 Expressions in the Hippocampus
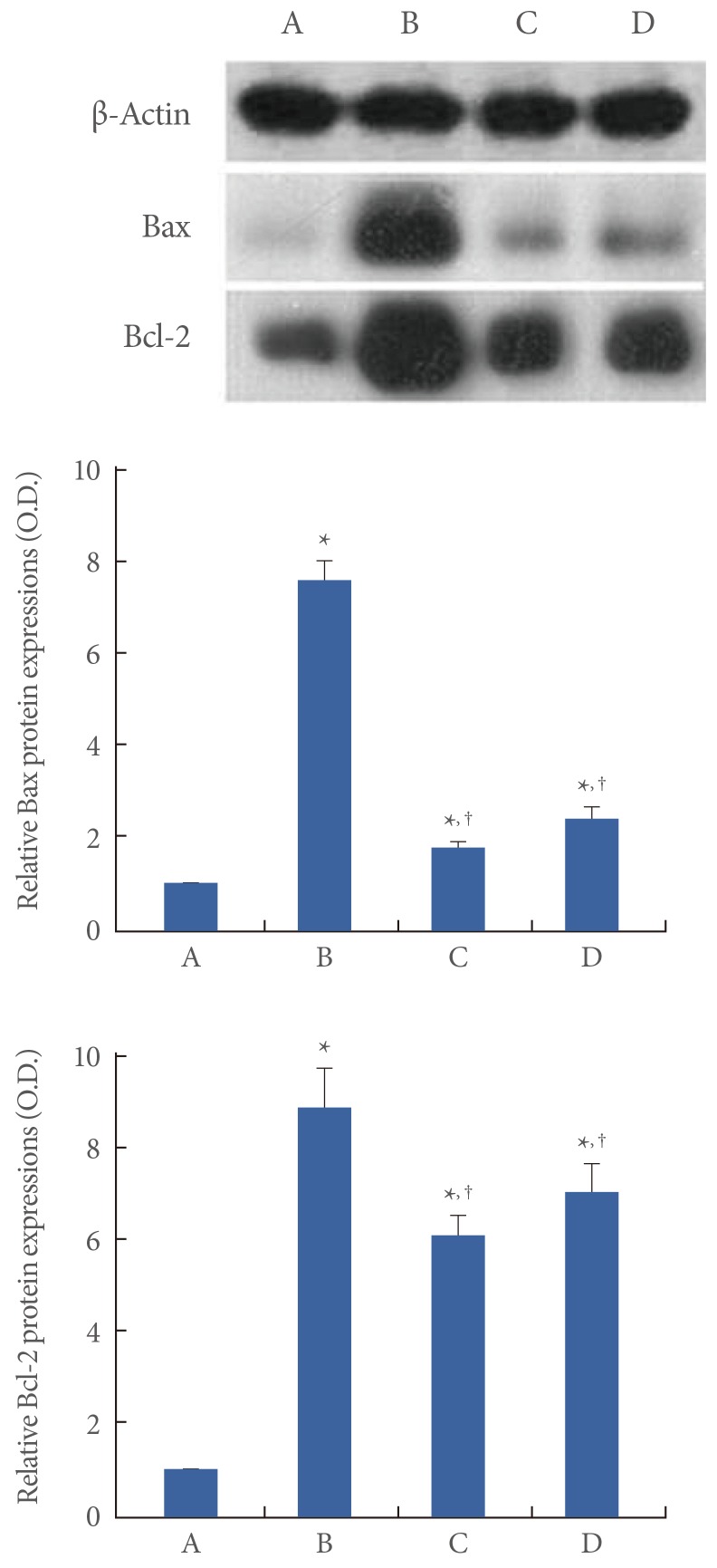
Next, we determined the relative expressions of Bax and Bcl-2. When the Bax level (24 kDa) in the control group was set to 1.00, it was 7.65±0.41 in the restraint stress group, 1.80±0.10 in the noise stress group, and 2.45±0.26 in the cold stress group (Fig. 4, left). When the Bcl-2 level (26-29 kDa) in the normal group was set at 1.00, it was 8.91±0.89 in the restraint stress group, 6.14±0.44 in the noise stress group, and 7.08±0.63 in the cold stress group (Fig. 4, right). In the present study, the expressions of Bax and Bcl-2 in the hippocampus were increased in all the stress groups compared to those in the normal group (P<0.05). However, the restraint stress exerted a more potent enhancing effect on Bax and Bcl2 expression than noise or cold stress (P<0.05).
Fig. 4.
The effects of various stress types on the expressions of Bax and Bcl-2 in the hippocampus. The data are presented as the mean±standard error of the mean. (A) Control group, (B) restraint stress group, (C) noise stress group, (D) cold stress group. *P<0.05 compared to the control group. †P<0.05 compared to the restraint stress group.
DISCUSSION
Memory impairment is closely associated with stress in humans and animal models [1-3,23]. Exposure to chronic stress induces dendrite atrophy and neuronal cell death, which causes memory impairment and behavioral abnormalities [24,25]. In the present study, a step-down avoidance test was performed to determine the effects of various types of stresses on short-term memory. All three types of stresses (restraint, noise, and cold) decreased the latency in the step-down avoidance test, showing that these stresses induced short-term memory impairment. The cold stress showed the least deteriorative effect on spatial memory among the three stress types.
The hippocampus is a brain area vulnerable to repeated stress [23]. The hippocampus and hypothalamus have been recognized as targets of glucocorticoids following stress exposure [2]. Expressions of CRF neurons in the hippocampus and hypothalamic PVN are increased in response to stress exposure [26,27]. Increased activity of CRF neurons in the hippocampus and hypothalamic PVN contributes to the release of corticotropin [28]. We determined the effects of three types of stresses on CRF expression in the hippocampal dentate gyrus and hypothalamic PVN. In the present study, all three types of stresses increased CRF expression in the hippocampus and PVN, indicating that these stresses induced the release of the stress-response hormone, CRF. Of these stresses, cold stress had the least enhancing effect on CRF expression.
The elevated glucocorticoid levels following stress exert deleterious changes on hippocampal excitability, long-term potentiation, cerebral blood flow, and spatial learning memory [29,30]. Severe and prolonged stress causes neuronal death through apoptosis [31-33]. Caspases are a family of cysteine proteases that play an important role in regulating cell apoptosis. As one of the key effectors, caspase-3 is involved in the mitochondria-mediated apoptotic pathway [22]. To confirm the involvement of caspases in stress-induced apoptosis, we examined its expression in the hippocampus. In the present study, all three types of stresses increased caspase-3 expression in the hippocampus, suggesting that these stresses enhanced apoptosis in the hippocampus. Of these stresses, cold stress had the least effect on apoptosis.
The Bcl-2 gene family plays important roles in apoptosis-regulating genes [14]. Of these genes, Bax promotes cell death, whereas Bcl-2 inhibits apoptosis and promotes cell survival [11]. Thus, we investigated the Bax and Bcl-2 expressions in the hippocampal dentate gyrus after exposure to the different stresses. In the present study, all three stress types increased the expressions of Bcl-2, the antiapoptotic protein, and Bax, the proapoptotic protein, in the hippocampus. Increased Bax expression suggests that the neuronal apoptosis in the hippocampus was facilitated by exposure to theses stresses. However, Bcl-2 expression was also increased after exposure to these stresses in the present study. These results suggest that increase in Bcl-2 expression in the hippocampus may provide compensatory protection for the neural cells following exposure to theses stresses.
Zhang et al. [34] demonstrated that occupational stress might be a contributing factor for overactive bladder and other lower urinary tract symptoms. Stress induces changes in bladder function and somatic sensitivity, resulting in increased voiding frequency [35]. In the present study, cold stress showed the least deteriorating effect on memory; meanwhile, restraint stress showed the most potent deteriorating effect on memory. We showed here that the impairment of memory function by stress is due to accelerated apoptosis in the hippocampus. The present results suggest the possibility that these stresses that impair short-term memory may also induce functional lower urinary tract disorders.
ACKNOWLEDGEMENTS
This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MEST) (No. R11-2008-036-01001-0).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS, Magarinos AM. Stress and hippocampal plasticity: implications for the pathophysiology of affective disorders. Hum Psychopharmacol. 2001;16(S1):S7–S19. doi: 10.1002/hup.266. [DOI] [PubMed] [Google Scholar]
- 3.van der Beek EM, Wiegant VM, Schouten WG, van Eerdenburg FJ, Loijens LW, van der Plas C, et al. Neuronal number, volume, and apoptosis of the left dentate gyrus of chronically stressed pigs correlate negatively with basal saliva cortisol levels. Hippocampus. 2004;14:688–700. doi: 10.1002/hipo.10213. [DOI] [PubMed] [Google Scholar]
- 4.Seo JH, Kim TW, Kim CJ, Sung YH, Lee SJ. Treadmill exercise during pregnancy ameliorates post-traumatic stress disorder-induced anxiety-like responses in maternal rats. Mol Med Rep. 2013;7:389–395. doi: 10.3892/mmr.2012.1197. [DOI] [PubMed] [Google Scholar]
- 5.Klausner AP, Steers WD. Corticotropin releasing factor: a mediator of emotional influences on bladder function. J Urol. 2004;172(6 Pt 2):2570–2573. doi: 10.1097/01.ju.0000144142.26242.f3. [DOI] [PubMed] [Google Scholar]
- 6.Rothrock NE, Lutgendorf SK, Kreder KJ, Ratliff T, Zimmerman B. Stress and symptoms in patients with interstitial cystitis: a life stress model. Urology. 2001;57:422–427. doi: 10.1016/s0090-4295(00)00988-2. [DOI] [PubMed] [Google Scholar]
- 7.Westropp JL, Buffington CA. In vivo models of interstitial cystitis. J Urol. 2002;167(2 Pt 1):694–702. doi: 10.1016/S0022-5347(01)69129-8. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Brunson KL, Adelmann G, Bender RA, Frotscher M, Baram TZ. Hippocampal corticotropin releasing hormone: pre- and postsynaptic location and release by stress. Neuroscience. 2004;126:533–540. doi: 10.1016/j.neuroscience.2004.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonaz B, Rivest S. Effect of a chronic stress on CRF neuronal activity and expression of its type 1 receptor in the rat brain. Am J Physiol. 1998;275(5 Pt 2):R1438–R1449. doi: 10.1152/ajpregu.1998.275.5.R1438. [DOI] [PubMed] [Google Scholar]
- 10.Ko IG, Shin MS, Kim BK, Kim SE, Sung YH, Kim TS, et al. Tadalafil improves short-term memory by suppressing ischemia-induced apoptosis of hippocampal neuronal cells in gerbils. Pharmacol Biochem Behav. 2009;91:629–635. doi: 10.1016/j.pbb.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Kim SE, Ko IG, Kim BK, Shin MS, Cho S, Kim CJ, et al. Treadmill exercise prevents aging-induced failure of memory through an increase in neurogenesis and suppression of apoptosis in rat hippocampus. Exp Gerontol. 2010;45:357–365. doi: 10.1016/j.exger.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Upadhyay D, Panduri V, Ghio A, Kamp DW. Particulate matter induces alveolar epithelial cell DNA damage and apoptosis: role of free radicals and the mitochondria. Am J Respir Cell Mol Biol. 2003;29:180–187. doi: 10.1165/rcmb.2002-0269OC. [DOI] [PubMed] [Google Scholar]
- 13.Nicholson DW. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 1999;6:1028–1042. doi: 10.1038/sj.cdd.4400598. [DOI] [PubMed] [Google Scholar]
- 14.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 15.Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326(Pt 1):1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuhn HG, Biebl M, Wilhelm D, Li M, Friedlander RM, Winkler J. Increased generation of granule cells in adult Bcl-2-overexpressing mice: a role for cell death during continued hippocampal neurogenesis. Eur J Neurosci. 2005;22:1907–1915. doi: 10.1111/j.1460-9568.2005.04377.x. [DOI] [PubMed] [Google Scholar]
- 17.Sun XQ, Xu ZP, Zhang S, Cao XS, Liu TS. Simulated weightlessness aggravates hypergravity-induced impairment of learning and memory and neuronal apoptosis in rats. Behav Brain Res. 2009;199:197–202. doi: 10.1016/j.bbr.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 18.Kudryashov IE, Yakovlev AA, Kudryashova I, Gulyaeva NV. Footshock stress alters early postnatal development of electrophysiological responses and caspase-3 activity in rat hippocampus. Neurosci Lett. 2002;332:95–98. doi: 10.1016/s0304-3940(02)00937-0. [DOI] [PubMed] [Google Scholar]
- 19.Lucassen PJ, Vollmann-Honsdorf GK, Gleisberg M, Czeh B, De Kloet ER, Fuchs E. Chronic psychosocial stress differentially affects apoptosis in hippocampal subregions and cortex of the adult tree shrew. Eur J Neurosci. 2001;14:161–166. doi: 10.1046/j.0953-816x.2001.01629.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim SE, Shin MS, Kim CJ, Park JH, Chung KJ, Jung H, et al. Effects of tamsulosin on urinary bladder function and neuronal activity in the voiding centers of rats with cyclophosphamide-induced overactive bladder. Int Neurourol J. 2012;16:13–22. doi: 10.5213/inj.2012.16.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi C, Ohata H, Shibasaki T. Corticotropin-releasing factor (CRF) receptor subtypes in mediating neuronal activation of brain areas involved in responses to intracerebroventricular CRF and stress in rats. Peptides. 2011;32:2384–2393. doi: 10.1016/j.peptides.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 22.Hwang L, Choi IY, Kim SE, Ko IG, Shin MS, Kim CJ, et al. Dexmedetomidine ameliorates intracerebral hemorrhage-induced memory impairment by inhibiting apoptosis and enhancing brain-derived neurotrophic factor expression in the rat hippocampus. Int J Mol Med. 2013;31:1047–1056. doi: 10.3892/ijmm.2013.1301. [DOI] [PubMed] [Google Scholar]
- 23.McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 24.Lucassen PJ, Meerlo P, Naylor AS, van Dam AM, Dayer AG, Fuchs E, et al. Regulation of adult neurogenesis by stress, sleep disruption, exercise and inflammation: implications for depression and antidepressant action. Eur Neuropsychopharmacol. 2010;20:1–17. doi: 10.1016/j.euroneuro.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Givalois L, Arancibia S, Tapia-Arancibia L. Concomitant changes in CRH mRNA levels in rat hippocampus and hypothalamus following immobilization stress. Brain Res Mol Brain Res. 2000;75:166–171. doi: 10.1016/s0169-328x(99)00290-9. [DOI] [PubMed] [Google Scholar]
- 27.Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- 28.Viau V, Sawchenko PE. Hypophysiotropic neurons of the paraventricular nucleus respond in spatially, temporally, and phenotypically differentiated manners to acute vs. repeated restraint stress: rapid publication. J Comp Neurol. 2002;445:293–307. doi: 10.1002/cne.10178. [DOI] [PubMed] [Google Scholar]
- 29.Endo Y, Nishimura JI, Kobayashi S, Kimura F. Chronic stress exposure influences local cerebral blood flow in the rat hippocampus. Neuroscience. 1999;93:551–555. doi: 10.1016/s0306-4522(99)00176-1. [DOI] [PubMed] [Google Scholar]
- 30.Joëls M, de Kloet ER. Control of neuronal excitability by corticosteroid hormones. Trends Neurosci. 1992;15:25–30. doi: 10.1016/0166-2236(92)90345-9. [DOI] [PubMed] [Google Scholar]
- 31.Raff M. Cell suicide for beginners. Nature. 1998;396:119–122. doi: 10.1038/24055. [DOI] [PubMed] [Google Scholar]
- 32.Reagan LP, McEwen BS. Controversies surrounding glucocorticoid-mediated cell death in the hippocampus. J Chem Neuroanat. 1997;13:149–167. doi: 10.1016/s0891-0618(97)00031-8. [DOI] [PubMed] [Google Scholar]
- 33.Sapolsky RM. Stress, glucocorticoids, and damage to the nervous system: the current state of confusion. Stress. 1996;1:1–19. doi: 10.3109/10253899609001092. [DOI] [PubMed] [Google Scholar]
- 34.Zhang C, Hai T, Yu L, Liu S, Li Q, Zhang X, et al. Association between occupational stress and risk of overactive bladder and other lower urinary tract symptoms: a cross-sectional study of female nurses in China. Neurourol Urodyn. 2013;32:254–260. doi: 10.1002/nau.22290. [DOI] [PubMed] [Google Scholar]
- 35.Merrill L, Malley S, Vizzard MA. Repeated variate stress in male rats induces increased voiding frequency, somatic sensitivity, and urinary bladder nerve growth factor expression. Am J Physiol Regul Integr Comp Physiol. 2013;305:R147–R156. doi: 10.1152/ajpregu.00089.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]