Abstract
The incidence of rectal carcinoids is rising because of the widespread use of screening colonoscopy. Rectal carcinoids detected incidentally are usually in earlier stages at diagnosis. Rectal carcinoids estimated endoscopically as < 10 mm in diameter without atypical features and confined to the submucosal layer can be removed endoscopically. Here, we review the efficacy and safety of various endoscopic treatments for small rectal carcinoid tumors, including conventional polypectomy, endoscopic mucosal resection (EMR), cap-assisted EMR (or aspiration lumpectomy), endoscopic submucosal resection with ligating device, endoscopic submucosal dissection, and transanal endoscopic microsurgery. It is necessary to carefully choose an effective and safe primary resection method for complete histological resection.
Keywords: Carcinoid tumor, Rectum, Polypectomy, Endoscopic mucosal resection, Endoscopic submucosal dissection
Core tip: Rectal carcinoids less than 10 mm in diameter can be resected by various endoscopic techniques, such as conventional polypectomy, endoscopic mucosal resection (EMR), cap-assisted EMR (EMR-C), endoscopic submucosal dissection (ESD), or transanal endoscopic microsurgery (TEM). There are currently limited comparative data to recommend a specific endoscopic treatment. Therefore, the choice of treatment modalities for small rectal carcinoids depends on the degree of endoscopic or surgical expertise at a given facility. Furthermore, any one of the above treatment methods could have a favorable clinical outcome if performed by gastroenterologists or surgeons with special techniques. EMR-C and TEM can be used as a salvage treatment after incomplete resection by endoscopic polypectomy. The efficacy of endoscopic submucosal resection with ligating device and ESD for salvage treatment requires further investigation.
INTRODUCTION
Carcinoids, also termed well-differentiated neuroendocrine tumors (NETs), are the most common neuroendocrine tumor of the gastrointestinal tract[1]. The incidence and prevalence of carcinoid tumors have increased quickly and steadily worldwide over the past few decades[2]. Rectal carcinoids are typically small, localized, nonfunctioning tumors that rarely metastasize[2]. The Surveillance, Epidemiology, and End Results registry database of the National Cancer Institute showed that the age-adjusted incidence of rectal carcinoids has increased from approximately 0.2 per 100000 in 1973 to 0.86 per 100000 in 2004[2,3]. The increased incidence can be partially explained by widespread colorectal cancer screening, heightened awareness, and improved diagnostic modalities. Rectal carcinoids comprise 12.6% of all carcinoid tumors and represent the third largest group of the gastrointestinal carcinoids in Western countries[1]. The frequency of rectal carcinoids is higher in studies from South Korea (48%) and Taiwan (25%) compared to Western countries[4,5]. The causes of racial/ethnic differences in NETs by site are unclear and require further investigation.
The treatment of rectal carcinoids depends on the tumor size (Figure 1). Recent consensus guidelines on the management of rectal carcinoids suggests that small tumors (< 1-2 cm) confined to the mucosa or submucosa can be managed with endoscopic resection due to their low risk of metastatic spread[6]. Rectal carcinoids estimated endoscopically as < 10 mm in diameter without atypical features and confined to the submucosal layer without lymphovascular invasion rarely metastasize. Therefore, these tumors are considered good candidates for local excision, including endoscopic resection. A variety of endoscopic techniques are used to treat rectal carcinoids. Those techniques include conventional polypectomy, endoscopic mucosal resection (EMR), cap-assisted EMR (EMR-C or aspiration lumpectomy), endoscopic submucosal resection with ligating device (ESMR-L), endoscopic submucosal dissection (ESD), and transanal endoscopic microsurgery (TEM). Due to a lack of controlled prospective studies, the management of small rectal carcinoid tumors has been a matter of debate. In this Technical Advances article, we review the efficacy and safety of various endoscopic treatments for small rectal carcinoid tumors.
Figure 1.
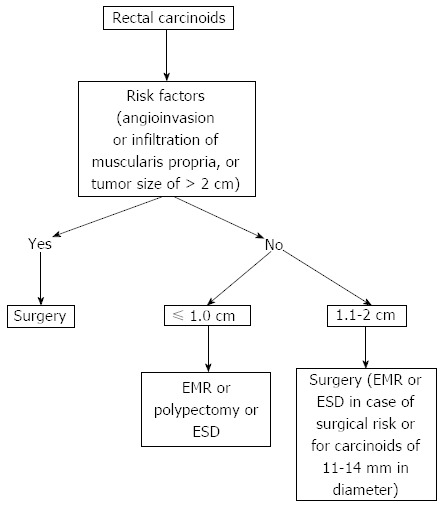
Treatment of rectal carcinoids. EMR: Endoscopic mucosal resection; ESD: Endoscopic submucosal dissection.
CONVENTIONAL POLYPECTOMY OR EMR
Endoscopic resection of rectal carcinoids with conventional polypectomy or EMR is a simple procedure (Figure 2)[7-9]. However, it is difficult to achieve histologically complete resection with these techniques because 76% of rectal carcinoids extend into the submucosal layer[9,10]. In addition, crush injury of resected specimens could lead to difficulty in pathologic evaluation[7]. The histologically complete resection rate of conventional polypectomy varies from 28.6% to 100% according to previous studies[11]. Incomplete resection of the tumors often requires additional surgical intervention.
Figure 2.
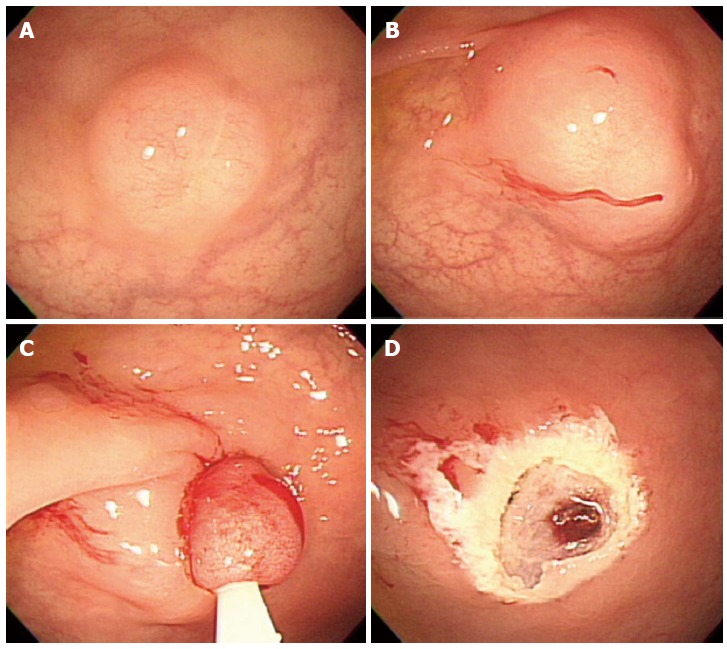
Endoscopic mucosal resection. A: An approximately 6 mm rectal carcinoid tumor; B: Injection of submucosal saline solution; C: Endoscopic mucosal resection (EMR) procedure; D: A clear, post-EMR ulcer.
POLYPECTOMY OR EMR USING TWO-CHANNEL COLONOSCOPY
Using a two-channel colonoscope, both grasping forceps and a polypectomy snare can be inserted into the gastrointestina lumen simultaneously. Therefore, rectal carcinoids can be pulled toward the center of the lumen and resected by electrocoagulation (Figure 3). Iishi et al[12] demonstrated that the complete resection rate of rectal carcinoids with a two-channel colonoscopy (9 of 10 tumors, 90%) was significantly higher than with a one-channel colonoscopy (2 of 7 tumors, 29%). In addition, there were no complications during or after endoscopic treatment. Polypectomy or EMR using the two-channel method are expected to have a deeper vertical resection margin and lead to a curative resection. However, a recent study showed a positive resection margin in 11 (26%) of 58 EMR samples collected using the two-channel method. Furthermore, the complete resection rate of this method was not different from conventional EMR[13]. Another limitation is that the mucosa can be torn before the tumor is adequately elevated with the grasping forceps[14].
Figure 3.
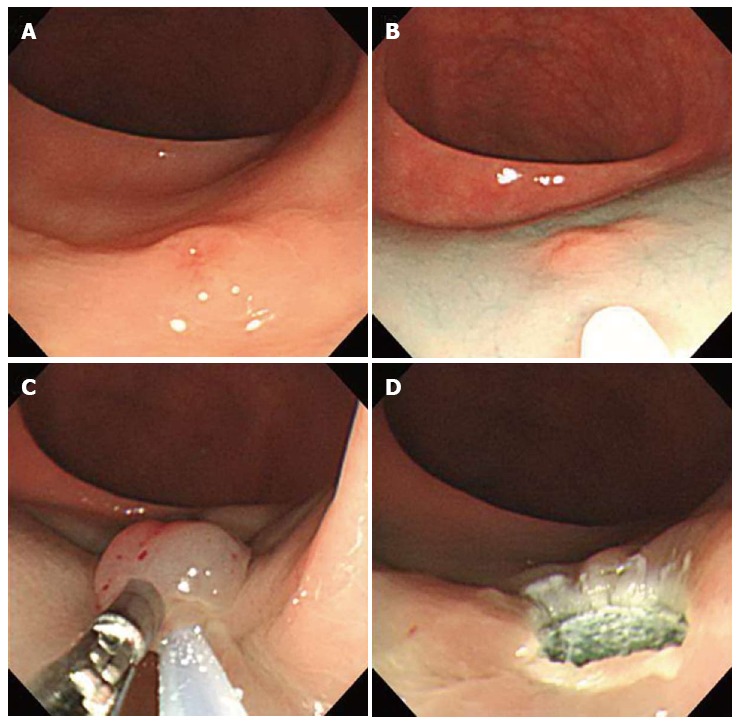
Endoscopic mucosal resection using two-channel colonoscopy. A: An approximately 5 mm rectal carcinoid tumor; B: Injection of submucosal saline solution into the base of the lesion using needle forcep; C: Pulling the lesion with grasping forcep and snare resection; D: A clear, post-endoscopic mucosal resection ulcer.
EMR-C OR ASPIRATION LUMPECTOMY
Aspiration lumpectomy is an endoscopic approach for a tumor that can be easily resected by lifting the mucosa away from the submucosa with saline injection, followed by aspirating the lesion into a transparent cap or cylinder[15]. In 1996, Imada-Shirakat et al[16] reported that histologically complete resection was achieved in eight patients with rectal carcinoids less than 10 mm and located within the submucosal layer using this technique. There were no recurrences or distant metastasis found during the mean observation period of 13.3 mo. Nagai et al[14] demonstrated that the rate of complete resection with aspiration lumpectomy (100%) was significantly higher (P < 0.05) than with saline assisted snare resection (termed ‘strip biopsy’) in a small series of consecutive patients with rectal carcinoids. Jeon et al[17] used this technique for secondary endoscopic treatment to remove the remnant tumor after primary EMR or polypectomy, which is technically difficult due to submucosal fibrosis of residual tissue. This study demonstrated that EMR-C is a useful method for salvage treatment of a failed en bloc resection of rectal carcinoids after primary EMR or polypectomy. One of the interesting findings of this study is that all 7 patients had positive microscopic margins after primary EMR but negative endoscopic and histological findings based on a biopsy of the scarred tissue. The pathologic findings from all tissue obtained by salvage resection showed the existence of remnant tumor. This result suggests that a negative biopsy in a surveillance examination does not prove the absence of a remnant tumor and that false negative results might be due to embedding or the residual remnant tumor during tissue healing after the primary resection
ENDOSCOPIC SUBMUCOSAL RESECTION WITH LIGATING DEVICE
In 1999, Berkelhammer et al[18] first introduced the band-snare resection as a method of EMR for small rectal carcinods. This method may provide a more appropriate resection margin compared to standard polypectomy (Figure 4). A randomized controlled study comparing ESMR-L to EMR showed that the complete resection rate of ESMR-L (100%, 8/8) was significantly higher than EMR (57.1%, 4/7), and all patients were followed-up for 3 years without any recurrence[19]. In a large case series including 61 patients, the complete resection rate of ESMR-L was 95.2% (60 out of 63 lesions)[20]. The complete resection rate for lesions located in the lower rectum was 98.3%, which was significantly higher than lesions in the upper rectum and rectosigmoid colon (50%). In a large-scale study comparing ESMR-L (45 lesions) and EMR (55 lesions) including 100 cases, the overall ESMR-L complete resection rate was higher than EMR (93.3% vs 65.5%, respectively, P = 0.001)[21]. In addition, this study demonstrated that the location of the tumors had no influence on the complete resection rate when ESMR-L was performed, in contrast to the results of EMR. Recently, Moon et al[22] introduced EMR using a double ligation technique (ESMR-DL) to treat 11 patients with small rectal carcinoids. The lesion was aspirated into the ligating device, and an elastic band was placed around the base. Then, a detachable snare was used to perform a ligation below the elastic band, and the lesion was removed with snare resection above the band. After ESMR-DL, there were no immediate or delayed complications such as bleeding or perforation.
Figure 4.
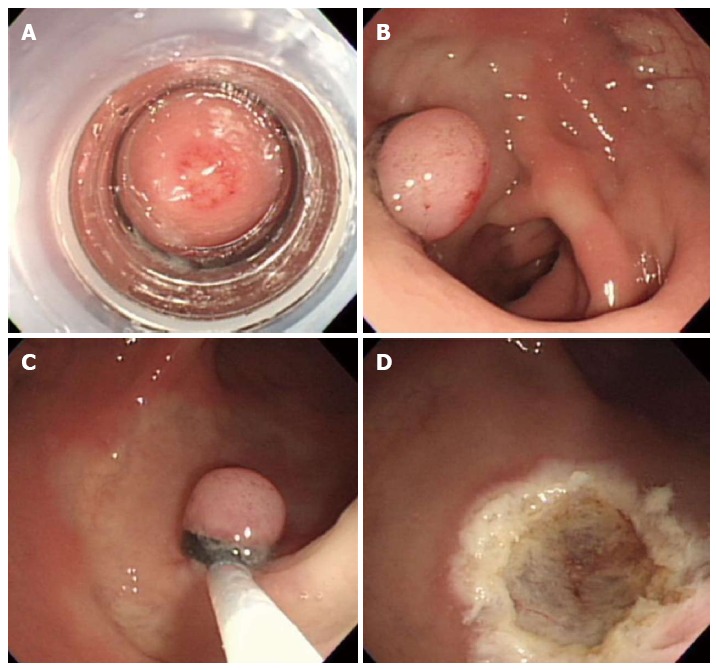
Endoscopic submucosal resection with ligating device. A: Aspiration of a carcinoid tumor into the ligating device; B: Deployed elastic band; C: Snare resection performed below the band; D: A clear, post-endoscopic submucosal resection with ligating device ulcer.
ESD
Endoscopic submucosal dissection is considered a valuable endoscopic treatment for early gastric cancer and large superficial gastric neoplasms. This technique provides a higher en bloc and histologically complete resection rate than EMR, enables accurate pathologic diagnoses, and is less invasive than surgery (Figure 5)[23]. Recently, ESD has been applied to the treatment of large colorectal neoplasms and has been reported to be more effective than either EMR or EMR-precutting[24]. However, ESD has the disadvantage of a considerably higher risk for perforation because the technique involves dissection of the submucosal tissue beneath the lesion. In addition, highly trained endoscopists are required. Thus, the safety issues associated with this technique must be solved. As a result, ESD is not yet widely accepted for the treatment of colorectal neoplasms[25].
Figure 5.
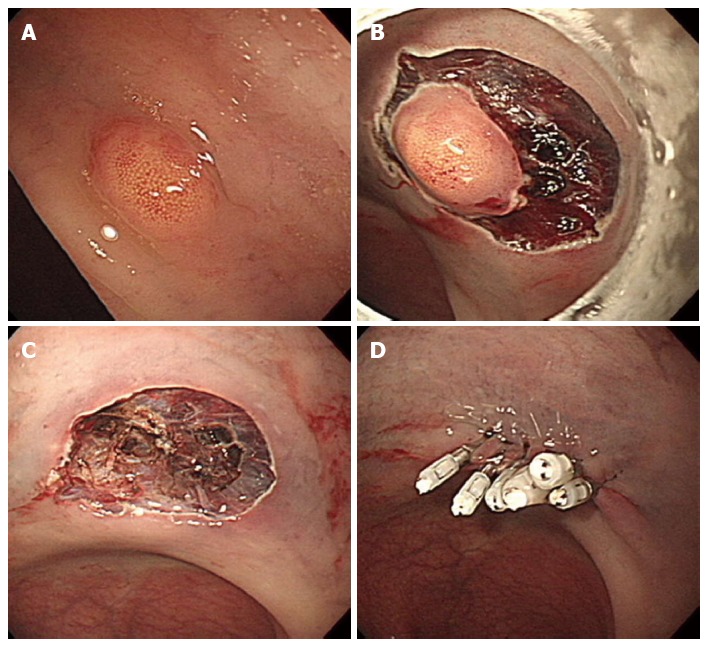
Endoscopic submucosal dissection. A: An approximately 5 mm rectal carcinoid tumor; B: Mucosal incision and submucosal dissection; C: A clear, post-endoscopic submucosal dissection ulcer; D: Endoscopic closure of the ulcer floor with endoclips.
There have been few studies reporting the efficacy and safety of ESD for the resection of rectal carcinoids. Recently, Onozato et al[26] reported that ESD was technically feasible in five cases with rectal carcinoids less than 10 mm. In addition, no complications were observed, and all lesions were completely resected histologically. In a meta-analysis including four studies[27-30], ESD was a more effective procedure for the treatment of rectal carcinoids and had a higher complete resection rate than EMR[31]. ESD was more effective than EMR in complete histological resection [odds ratio, 0.29; 95%CI: 0.14-0.58; P = 0.000]. Additionally, ESD was as safe as EMR (rate difference, -0.01; 95%CI: -0.07 - 0.05; P = 0.675). The recurrence rate did not differ significantly between the EMR and ESD groups. The duration of ESD was longer than EMR. Because the rectum is fixed in the retroperitoneum, the risk of peritonitis following perforation is lower than in other parts of the colon. One of limitations of ESD with a knife is the inability to fix the knife to the target lesion, which leads to high complications such as bleeding and perforation. New grasping type scissor forceps, which can grasp and incise the targeted tissue using an electrosurgical current, may reduce these complications[32]. More recently, there have been a few studies comparing ESD to other endoscopic treatment modalities besides EMR. Kim et al[33] reported a large retrospective analysis including 115 patients, which were classified into an EMR group (n = 33), ESMR-L group (n = 40), and ESD group (n = 44). The curative resection rate in the EMR group was 77.4%, which was significantly lower than that of the ESMR-L (95%) and ESD groups (97.7%). This result suggests that ESMR-L and ESD may be superior to conventional EMR. A recent study by Choi et al[25] comparing ESMR-L (n = 29) with ESD (n = 31) for the endoscopic treatment of rectal carcinoids showed that the complete resection rate was 80.6% in the ESD group and 82.8% in the ESMR-L group (P = 0.833). The resection time was significantly longer in the ESD group than in the ESMR-L group. The authors concluded that ESMR-L might be considered the treatment of choice for small rectal carcinoid tumors because of reduced procedure time. A small comparative study by a Japanese group[34] also showed a similar result to the above study. A retrospective analysis of 3 types of endoscopic resection technique by Zhao et al[35] demonstrated that complete resection rates using the EMR, EMR-C, and ESD were 80%, 100%, and 100%, respectively. The average procedure time was the shortest in the EMR-C group. This study concluded that EMR-C might be the best endoscopic excision method, considering the clinical efficacy, surgical time, and complication rate.
TEM
Transanal endoscopic microsurgery was originally designed by Buess et al[36] in the 1980s. The procedure allows full thickness excisions as high as 20 cm from the anal verge to be performed using a 40-mm operating rectoscope. Although TEM is not superior to conventional transanal excision (TAE) for resecting lesions in the lower rectum, it has distinct advantages for removing lesions in the mid and upper rectum[37]. In addition to improved access to more proximal lesions, TEM provides several advantages over TAE, including improved visualization with better exposure, higher likelihood of achieving clear resection margins, and lower recurrence rates[38]. The application of TEM for rectal carcinoids has been described in several small case series[6]. Kinoshita et al[39] reported clinical experience including 27 patients with rectal carcinoids treated by TEM. In this study, TEM was performed as a primary excision (n = 14) or as completion surgery after incomplete resection by endoscopic polypectomy (n = 13). Negative margins were obtained in all cases. There was no additional radical surgery performed, and patients were followed-up for 70 mo without recurrence. The largest series in the United States included 24 patients over a 12-year period[40]. There were 6 (25%) primary surgical resections, and 18 (75%) resections were performed after incomplete snare excisions during colonoscopy. This study showed all negative margins, a similar zero rate of recurrence and a similarly low morbidity rate. In addition to its usefulness in primary surgical resection of rectal carcinoids especially in the mid and upper rectum, TEM can be used as a salvage treatment after incomplete resection by endoscopic polypectomy. The possible complications of TEM include bleeding and perforation. In addition, transient soiling can occur due to the large width of the rectoscope tube[37].
FUTURE PERSPECTIVES AND CONCLUSIONS
In rectal carcinoids estimated endoscopically as < 10 mm in diameter, endoscopic treatment is a feasible option. Although endoscopic resection of rectal carcinoids with conventional polypectomy or EMR is a simple procedure, it is difficult to achieve histologically complete resection. EMR-C, ESMR-L, and ESD showed similar efficacy and safety. However, there are currently limited comparative data to recommend a specific endoscopic treatment. Therefore, the choice of treatment modalities for small rectal carcinoids depends on the degree of endoscopic or surgical expertise at a given facility. Furthermore, any one of the above treatment methods could have a favorable clinical outcome if performed by gastroenterologists or surgeons with special techniques.
Endoscopic treatment for rectal carcinoid requires special techniques for a deeper resection to achieve clear margins. For this purpose, lesions are usually lifted using submucosal injection with saline solution with or without epinephrine. In addition, adequate submucosal injection is important for the reduction of thermal damage to tissue as well as the prevention of complication such as bleeding or perforation. Although electrocauterization during endoscopic resection could destroy remnant tumor, its burning or coagulation artifact may make the pathologic examination of resection margin difficult. Therefore, to separate the margin of carcinoid tumor from the underlying muscle layer adequately could provide better pathological assessment of radial margins and the depth of invasion[41].
EMR-C and TEM can be used as a salvage treatment after incomplete resection by conventional polypectomy or EMR. However, the efficacy of ESMR-L and ESD for salvage treatment requires further investigation. Endoscopic tattooing of colonic lesions helps to localize polypectomy sites that may difficult to identify with repeat endoscopy[42]. In cases with positive resection margin after endoscopic treatment of rectal carcinoids, tattooing the area of resection will help facilitate the lesion site location for further resection.
Newly developed over-the-scope clip (OTSC) has a higher compression force and the capacity to capture a larger volume of tissue than the through-the-scope clip[43]. Recent prospective study has shown that perforations occurring after full- thickness resection of gastric subepithelial tumors less than 3 cm could be managed by OTSC closure[44]. Although further prospective clinical trial is required, this study suggests that endoscopic full-thickeness resection with OTSC closure can be applied to selected patients with colonic subepithelial lesions to have malignant potential. Finally, a prospective large-scale study is warranted for the assessment of therapeutic efficacy of various endoscopic treatments and long-term outcome.
Footnotes
Supported by Grant funded by the Catholic Cancer Center made in the program of 2010; and the National Research Foundation of Korea grant funded by the Korea government, No. 2010-0023295
P- Reviewer Königsrainer AA S- Editor Ma YJ L- Editor A E- Editor Liu XM
References
- 1.Modlin IM, Kidd M, Latich I, Zikusoka MN, Shapiro MD. Current status of gastrointestinal carcinoids. Gastroenterology. 2005;128:1717–1751. doi: 10.1053/j.gastro.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 2.Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, Caplin M, Delle Fave G, Kaltsas GA, Krenning EP, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61–72. doi: 10.1016/S1470-2045(07)70410-2. [DOI] [PubMed] [Google Scholar]
- 3.Scherübl H. Rectal carcinoids are on the rise: early detection by screening endoscopy. Endoscopy. 2009;41:162–165. doi: 10.1055/s-0028-1119456. [DOI] [PubMed] [Google Scholar]
- 4.Cho MY, Kim JM, Sohn JH, Kim MJ, Kim KM, Kim WH, Kim H, Kook MC, Park do Y, Lee JH, et al. Current Trends of the Incidence and Pathological Diagnosis of Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs) in Korea 2000-2009: Multicenter Study. Cancer Res Treat. 2012;44:157–165. doi: 10.4143/crt.2012.44.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsai HJ, Wu CC, Tsai CR, Lin SF, Chen LT, Chang JS. The epidemiology of neuroendocrine tumors in taiwan: a nation-wide cancer registry-based study. PLoS One. 2013;8:e62487. doi: 10.1371/journal.pone.0062487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anthony LB, Strosberg JR, Klimstra DS, Maples WJ, O’Dorisio TM, Warner RR, Wiseman GA, Benson AB, Pommier RF. The NANETS consensus guidelines for the diagnosis and management of gastrointestinal neuroendocrine tumors (nets): well-differentiated nets of the distal colon and rectum. Pancreas. 2010;39:767–774. doi: 10.1097/MPA.0b013e3181ec1261. [DOI] [PubMed] [Google Scholar]
- 7.Matsui K, Iwase T, Kitagawa M. Small, polypoid-appearing carcinoid tumors of the rectum: clinicopathologic study of 16 cases and effectiveness of endoscopic treatment. Am J Gastroenterol. 1993;88:1949–1953. [PubMed] [Google Scholar]
- 8.Higaki S, Nishiaki M, Mitani N, Yanai H, Tada M, Okita K. Effectiveness of local endoscopic resection of rectal carcinoid tumors. Endoscopy. 1997;29:171–175. doi: 10.1055/s-2007-1004158. [DOI] [PubMed] [Google Scholar]
- 9.Ono A, Fujii T, Saito Y, Matsuda T, Lee DT, Gotoda T, Saito D. Endoscopic submucosal resection of rectal carcinoid tumors with a ligation device. Gastrointest Endosc. 2003;57:583–587. doi: 10.1067/mge.2003.142. [DOI] [PubMed] [Google Scholar]
- 10.Soga J. Carcinoids of the rectum: an evaluation of 1271 reported cases. Surg Today. 1997;27:112–119. doi: 10.1007/BF02385898. [DOI] [PubMed] [Google Scholar]
- 11.Lee SH, Park SJ, Kim HH, Ok KS, Kim JH, Jee SR, Seol SY, Kim BM. Endoscopic resection for rectal carcinoid tumors: comparison of polypectomy and endoscopic submucosal resection with band ligation. Clin Endosc. 2012;45:89–94. doi: 10.5946/ce.2012.45.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Iishi H, Tatsuta M, Yano H, Narahara H, Iseki K, Ishiguro S. More effective endoscopic resection with a two-channel colonoscope for carcinoid tumors of the rectum. Dis Colon Rectum. 1996;39:1438–1439. doi: 10.1007/BF02054536. [DOI] [PubMed] [Google Scholar]
- 13.Sung HY, Kim SW, Kang WK, Kim SY, Jung CK, Cho YK, Park JM, Lee IS, Choi MG, Chung IS. Long-term prognosis of an endoscopically treated rectal neuroendocrine tumor: 10-year experience in a single institution. Eur J Gastroenterol Hepatol. 2012;24:978–983. doi: 10.1097/MEG.0b013e3283551e0b. [DOI] [PubMed] [Google Scholar]
- 14.Nagai T, Torishima R, Nakashima H, Ookawara H, Uchida A, Kai S, Sato R, Murakami K, Fujioka T. Saline-assisted endoscopic resection of rectal carcinoids: cap aspiration method versus simple snare resection. Endoscopy. 2004;36:202–205. doi: 10.1055/s-2004-814248. [DOI] [PubMed] [Google Scholar]
- 15.Oshitani N, Hamasaki N, Sawa Y, Hara J, Nakamura S, Matsumoto T, Kitano A, Arakawa T. Endoscopic resection of small rectal carcinoid tumours using an aspiration method with a transparent overcap. J Int Med Res. 2000;28:241–246. doi: 10.1177/147323000002800507. [DOI] [PubMed] [Google Scholar]
- 16.Imada-Shirakata Y, Sakai M, Kajiyama T, Kin G, Inoue K, Torii A, Kishimoto H, Ueda S, Okuma M. Endoscopic resection of rectal carcinoid tumors using aspiration lumpectomy. Endoscopy. 1997;29:34–38. doi: 10.1055/s-2007-1024058. [DOI] [PubMed] [Google Scholar]
- 17.Jeon SM, Lee JH, Hong SP, Kim TI, Kim WH, Cheon JH. Feasibility of salvage endoscopic mucosal resection by using a cap for remnant rectal carcinoids after primary EMR. Gastrointest Endosc. 2011;73:1009–1014. doi: 10.1016/j.gie.2010.12.029. [DOI] [PubMed] [Google Scholar]
- 18.Berkelhammer C, Jasper I, Kirvaitis E, Schreiber S, Hamilton J, Walloch J. “Band-snare” resection of small rectal carcinoid tumors. Gastrointest Endosc. 1999;50:582–585. doi: 10.1016/s0016-5107(99)70092-1. [DOI] [PubMed] [Google Scholar]
- 19.Sakata H, Iwakiri R, Ootani A, Tsunada S, Ogata S, Ootani H, Shimoda R, Yamaguchi K, Sakata Y, Amemori S, et al. A pilot randomized control study to evaluate endoscopic resection using a ligation device for rectal carcinoid tumors. World J Gastroenterol. 2006;12:4026–4028. doi: 10.3748/wjg.v12.i25.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mashimo Y, Matsuda T, Uraoka T, Saito Y, Sano Y, Fu K, Kozu T, Ono A, Fujii T, Saito D. Endoscopic submucosal resection with a ligation device is an effective and safe treatment for carcinoid tumors in the lower rectum. J Gastroenterol Hepatol. 2008;23:218–221. doi: 10.1111/j.1440-1746.2008.05313.x. [DOI] [PubMed] [Google Scholar]
- 21.Kim HH, Park SJ, Lee SH, Park HU, Song CS, Park MI, Moon W. Efficacy of endoscopic submucosal resection with a ligation device for removing small rectal carcinoid tumor compared with endoscopic mucosal resection: analysis of 100 cases. Dig Endosc. 2012;24:159–163. doi: 10.1111/j.1443-1661.2011.01190.x. [DOI] [PubMed] [Google Scholar]
- 22.Moon JH, Kim JH, Park CH, Jung JO, Shin WG, Kim JP, Kim KO, Hahn T, Yoo KS, Park SH, et al. Endoscopic submucosal resection with double ligation technique for treatment of small rectal carcinoid tumors. Endoscopy. 2006;38:511–514. doi: 10.1055/s-2006-925074. [DOI] [PubMed] [Google Scholar]
- 23.Onozato Y, Ishihara H, Iizuka H, Sohara N, Kakizaki S, Okamura S, Mori M. Endoscopic submucosal dissection for early gastric cancers and large flat adenomas. Endoscopy. 2006;38:980–986. doi: 10.1055/s-2006-944809. [DOI] [PubMed] [Google Scholar]
- 24.Lee EJ, Lee JB, Lee SH, Youk EG. Endoscopic treatment of large colorectal tumors: comparison of endoscopic mucosal resection, endoscopic mucosal resection-precutting, and endoscopic submucosal dissection. Surg Endosc. 2012;26:2220–2230. doi: 10.1007/s00464-012-2164-0. [DOI] [PubMed] [Google Scholar]
- 25.Choi CW, Kang DH, Kim HW, Park SB, Jo WS, Song GA, Cho M. Comparison of endoscopic resection therapies for rectal carcinoid tumor: endoscopic submucosal dissection versus endoscopic mucosal resection using band ligation. J Clin Gastroenterol. 2013;47:432–436. doi: 10.1097/MCG.0b013e31826faf2b. [DOI] [PubMed] [Google Scholar]
- 26.Onozato Y, Kakizaki S, Ishihara H, Iizuka H, Sohara N, Okamura S, Mori M, Itoh H. Endoscopic submucosal dissection for rectal tumors. Endoscopy. 2007;39:423–427. doi: 10.1055/s-2007-966237. [DOI] [PubMed] [Google Scholar]
- 27.Lee DS, Jeon SW, Park SY, Jung MK, Cho CM, Tak WY, Kweon YO, Kim SK. The feasibility of endoscopic submucosal dissection for rectal carcinoid tumors: comparison with endoscopic mucosal resection. Endoscopy. 2010;42:647–651. doi: 10.1055/s-0030-1255591. [DOI] [PubMed] [Google Scholar]
- 28.Park HW, Byeon JS, Park YS, Yang DH, Yoon SM, Kim KJ, Ye BD, Myung SJ, Yang SK, Kim JH. Endoscopic submucosal dissection for treatment of rectal carcinoid tumors. Gastrointest Endosc. 2010;72:143–149. doi: 10.1016/j.gie.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 29.Zhou PH, Yao LQ, Qin XY, Xu MD, Zhong YS, Chen WF, Ma LL, Zhang YQ, Qin WZ, Cai MY, et al. Advantages of endoscopic submucosal dissection with needle-knife over endoscopic mucosal resection for small rectal carcinoid tumors: a retrospective study. Surg Endosc. 2010;24:2607–2612. doi: 10.1007/s00464-010-1016-z. [DOI] [PubMed] [Google Scholar]
- 30.Onozato Y, Kakizaki S, Iizuka H, Sohara N, Mori M, Itoh H. Endoscopic treatment of rectal carcinoid tumors. Dis Colon Rectum. 2010;53:169–176. doi: 10.1007/DCR.0b013e3181b9db7b. [DOI] [PubMed] [Google Scholar]
- 31.Zhong DD, Shao LM, Cai JT. Endoscopic mucosal resection vs endoscopic submucosal dissection for rectal carcinoid tumours: a systematic review and meta-analysis. Colorectal Dis. 2013;15:283–291. doi: 10.1111/codi.12069. [DOI] [PubMed] [Google Scholar]
- 32.Akahoshi K, Motomura Y, Kubokawa M, Matsui N, Oda M, Okamoto R, Endo S, Higuchi N, Kashiwabara Y, Oya M, et al. Endoscopic submucosal dissection of a rectal carcinoid tumor using grasping type scissors forceps. World J Gastroenterol. 2009;15:2162–2165. doi: 10.3748/wjg.15.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim KM, Eo SJ, Shim SG, Choi JH, Min BH, Lee JH, Chang DK, Kim YH, Rhee PL, Kim JJ, et al. Treatment outcomes according to endoscopic treatment modalities for rectal carcinoid tumors. Clin Res Hepatol Gastroenterol. 2013;37:275–282. doi: 10.1016/j.clinre.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Niimi K, Goto O, Fujishiro M, Kodashima S, Ono S, Mochizuki S, Asada-Hirayama I, Konno-Shimizu M, Mikami-Matsuda R, Minatsuki C, et al. Endoscopic mucosal resection with a ligation device or endoscopic submucosal dissection for rectal carcinoid tumors: an analysis of 24 consecutive cases. Dig Endosc. 2012;24:443–447. doi: 10.1111/j.1443-1661.2012.01303.x. [DOI] [PubMed] [Google Scholar]
- 35.Zhao ZF, Zhang N, Ma SR, Yang Z, Han X, Zhao YF, Gao F, Gong ZJ, Yang L. A comparative study on endoscopy treatment in rectal carcinoid tumors. Surg Laparosc Endosc Percutan Tech. 2012;22:260–263. doi: 10.1097/SLE.0b013e3182512e0f. [DOI] [PubMed] [Google Scholar]
- 36.Buess G, Theiss R, Günther M, Hutterer F, Pichlmaier H. Endoscopic surgery in the rectum. Endoscopy. 1985;17:31–35. doi: 10.1055/s-2007-1018451. [DOI] [PubMed] [Google Scholar]
- 37.Saclarides TJ. TEM/local excision: indications, techniques, outcomes, and the future. J Surg Oncol. 2007;96:644–650. doi: 10.1002/jso.20922. [DOI] [PubMed] [Google Scholar]
- 38.Tsai BM, Finne CO, Nordenstam JF, Christoforidis D, Madoff RD, Mellgren A. Transanal endoscopic microsurgery resection of rectal tumors: outcomes and recommendations. Dis Colon Rectum. 2010;53:16–23. doi: 10.1007/DCR.0b013e3181bbd6ee. [DOI] [PubMed] [Google Scholar]
- 39.Kinoshita T, Kanehira E, Omura K, Tomori T, Yamada H. Transanal endoscopic microsurgery in the treatment of rectal carcinoid tumor. Surg Endosc. 2007;21:970–974. doi: 10.1007/s00464-006-9155-y. [DOI] [PubMed] [Google Scholar]
- 40.Kumar AS, Sidani SM, Kolli K, Stahl TJ, Ayscue JM, Fitzgerald JF, Smith LE. Transanal endoscopic microsurgery for rectal carcinoids: the largest reported United States experience. Colorectal Dis. 2012;14:562–566. doi: 10.1111/j.1463-1318.2011.02726.x. [DOI] [PubMed] [Google Scholar]
- 41.Cipolletta L, Rotondano G, Bianco MA, Garofano ML, Meucci C, Prisco A, Cipolletta F, Piscopo R. Self-assembled hydro-jet system for submucosal elevation before endoscopic resection of nonpolypoid colorectal lesions (with video) Gastrointest Endosc. 2009;70:1018–1022. doi: 10.1016/j.gie.2009.04.041. [DOI] [PubMed] [Google Scholar]
- 42.McArthur CS, Roayaie S, Waye JD. Safety of preoperation endoscopic tattoo with india ink for identification of colonic lesions. Surg Endosc. 1999;13:397–400. doi: 10.1007/s004649900997. [DOI] [PubMed] [Google Scholar]
- 43.Baron TH, Wong Kee Song LM, Zielinski MD, Emura F, Fotoohi M, Kozarek RA. A comprehensive approach to the management of acute endoscopic perforations (with videos) Gastrointest Endosc. 2012;76:838–859. doi: 10.1016/j.gie.2012.04.476. [DOI] [PubMed] [Google Scholar]
- 44.Schlag C, Wilhelm D, von Delius S, Feussner H, Meining A. EndoResect study: endoscopic full-thickness resection of gastric subepithelial tumors. Endoscopy. 2013;45:4–11. doi: 10.1055/s-0032-1325760. [DOI] [PubMed] [Google Scholar]


