Abstract
Tumoral obstructions in almost the entire gastrointestinal tract can be resolved with interventional digestive endoscopy techniques. Self-expanding metal stent (SEMS) insertion in the obstructed colon is a minimally invasive and relatively simple procedure providing an effective first-line treatment for relief of acute malignant obstruction symptoms and serving either as a preoperative or “bridge to surgery” procedure or as palliative definitive care. This technique was introduced in the early 1990s. Although there is still debate about its real value, a lot of reports have been published since then and the procedure is advocated by many surgical groups as the method of choice for the initial treatment of left-sided tumoral colonic obstruction. Before the procedure, colonic obstruction has to be diagnosed by abdominal radiographs, water contrast enema and/or a computed tomography scan. The greatest information is provided by the latter and it is perhaps the method of choice prior to stenting. Skills and training are mandatory, as in all interventional procedures. The key step for success is to cross the malignant stricture with a guidewire. Care must be taken not to over insufflate an obstructed colon during the procedure. SEMS slide over the guidewire through the endoscope working channel or in parallel, outside the endoscope. An average 7% perforation rate has been reported during the procedure and other minor complications can appear in the follow up. However, as a whole, this technique seems to compare favorably with surgery.
Keywords: Self-expanding metal stent, Malignant colorectal obstruction, Emergency surgery, Interventional endoscopy
INTRODUCTION
Patients with malignant colorectal obstruction (MCRO) usually present at the emergency room (ER) because of abdominal pain, vomiting and distension. After a physical examination, abdominal radiographs show typical signs of large bowel obstruction with air-fluid levels. First therapeutic measures include fluid resuscitation with electrolyte correction. Further diagnostic procedures have to be undertaken to confirm both the colonic obstruction and the exact anatomical location. According to individual hospital policies, the colon can be cleansed with enemas and a colonoscopy can be performed. Care has to be taken not to over insufflate in order to avoid perforation. Water instead of air should be employed to allow colonoscope advancement.
However, in patients with acute abdominal pain in whom perforation is suspected, a computed tomography (CT) scan is a preferable diagnostic modality after clinical and plain abdominal radiograph evaluation. If a tumoral obstruction in the left-side colon is diagnosed, insertion of a self-expanding metal stent (SEMS) as first treatment can be considered[1].
COLONIC OBTRUCTION RELIEF WITH SEMS
As in the esophagus, duodenum or biliary tree, MCRO can be also treated in the large bowel by means of SEMS.
Dohmoto et al[2] reported the treatment of a rectal tumoral obstruction by means of a SEMS for the first time in 1990. From that time, a large number of works dealing with this topic have been annually published. Initially, they were single or a few case reports[3]. Afterwards, large series were reported[4], in addition to review articles[5] and randomized studies comparing this new modality with the classical surgical approach[6].
Figure 1 shows the increase of publications on SEMS for MCRO when the words “colon AND stent” are searched for in PubMed.
Figure 1.
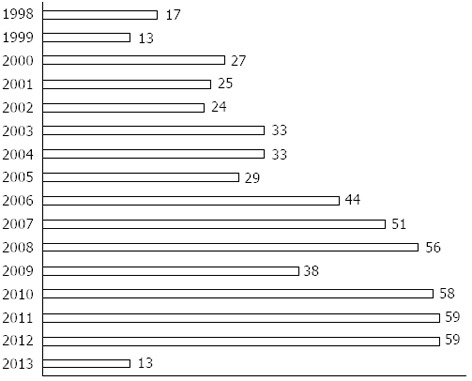
Number of scientific papers published in the last years about stents in tumoral colonic obstructions. Search was done with the terms “colon and stent” in PubMed. Year 2013 ends in the month of March.
The most valuable benefit provided by this relatively new interventional technique is to relieve obstruction by means of a minimally invasive procedure, avoiding an operation in an unstable patient. The colon can be cleansed properly and patients can undergo a scheduled surgical procedure. This kind of MCRO decompression is also called a bridge to surgery (BTS). The classical surgical approach involved a primary colostomy and a second or third operation for tumor removal and colostomy closure.
Right colon obstructions do not necessarily need bowel cleansing before surgery; therefore, the major impact of SEMS in MCRO are in the left colon[7]. In addition, non-operable patients (i.e., multiple metastases) can have the stent as a palliative measure to avoid a colostomy.
Bowel perforation is the main contraindication for stenting. In addition, in cases of multiple strictures or short life expectancy (hours or few days), other options instead of stent insertion must be undertaken.
NONFLUOROSCOPIC INSERTION OF AN “OVER-THE-WIRE” STENT IN A RECTOSIGMOID MCRO
Once MCRO has been diagnosed and surgical consultation made, if the obstruction is below 25 cm from the anus (up to mid-sigmoid), a possibility is to bridge the stricture in the endoscopy office without fluoroscopy. The majority of such strictures can be traversed by means of ultrathin endoscopes (six or less millimeters in diameter). The endoscope is negotiated through the narrowed tumoral lumen until healthy colon is found. The endoscope is advanced as far as possible. A metallic Savary or a similar stiff guidewire is inserted through the working channel of the endoscope and placed beyond the malignant stenosis. The endoscope is withdrawn, leaving the guidewire in place. Important figures to record are tumoral length and the distance from the anus.
Afterwards, the endoscope is reinserted beside the guidewire and placed at the level of the stricture. A folded stent that cannot be inserted through the working channel of the endoscope because it is greater than 3.7-4.2 mm, as shown in Figure 2, is slid over the guidewire. These SEMS are called over-the-wire (OTW) to differentiate from through-the-scope (TTS) stents that have a folded diameter that allows it to be inserted undeployed through the working channel of a therapeutic endoscope (Figure 3A).
Figure 2.
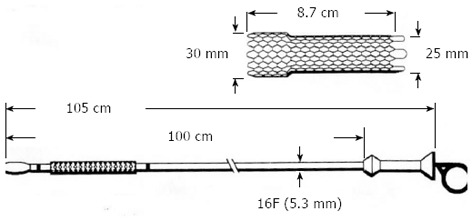
Ultraflex Precision stent from Boston Scientific. This self-expanding metal stent is called over the wire because it cannot be inserted through the working channel of a therapeutic endoscope. Many other stent manufacturers have similar stents.
Figure 3.
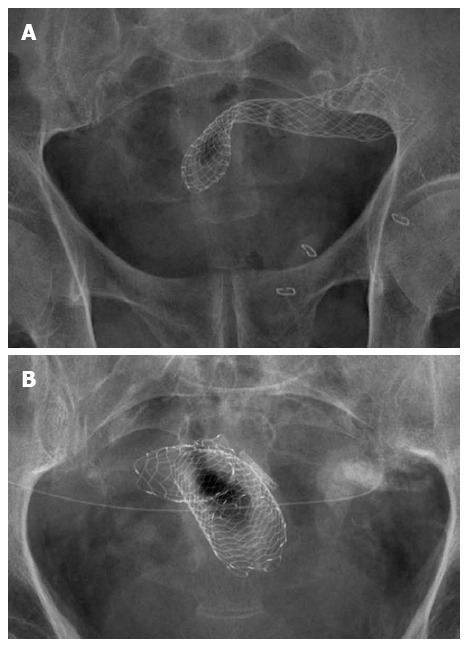
Wallflex (A) and Ultraflex (B) stents from Boston Scientific. A: This self-expanding metal stent (SEMS) is called a through the scope (TTS) stent because it can be inserted in the folded way through the working channel of a therapeutic endoscope. Many other stent manufacturers have similar stents; B: Ultraflex Precision inserted in a tumoral stricture in the sigmoid, a hemostatic clip was placed to mark the lower part of the stricture. Despite the strange configuration due to sigmoid bends, the stent was in correct position; the patient had an abdominal catheter for hydrocephaly decompression.
The endoscope gives stiffness to the system stent guidewire and prevents it from bending. The advancement of the stent through the stricture is also monitored with the endoscope. The stent is released under endoscopic vision.
This insertion technique has been used for a long time[8,9] and it has been successful in the majority of occasions, allowing the MCRO to be resolved in the endoscopy suite. Nevertheless, several points have to be underlined.
First of all, the procedure tends to always be more difficult than anticipated. Despite bowel cleansing, there are always liquid or semisolid feces in the colon that impedes good vision. The placing of a hemostatic clip in the lowest stricture margin is helpful to clearly mark where the stent has to be placed in the endoscopic view.
The endoscopist has to have skills in interventional endoscopy. A recent paper[10] pointed out that at least 30 procedures of SEMS insertion in left MCRO are the initial learning curve for mastering the technique.
With the nonfluoroscopic technique, stent deployment events beyond the stricture are not seen so they have to be “supposed”. In some OTW SEMS, like the Ultraflex Precision (Figure 2), deployment begins in the closest part to the endoscopic view, that is, in the distal tumoral end or downstream. Once the stent has been partially opened, it can be pushed if it is far from the stricture but it cannot be pulled because the open mesh can damage the colon.
After the procedure, pelvic or abdominal radiographs have to be taken to confirm proper stent deployment. When the stricture has been completely bridged, the SEMS takes an hourglass-like configuration with both ends open. Nevertheless, due to sigmoid bends, sometimes Rx images are not clear. As can be seen in Figure 3B, foreshortening occurs in the image but the SEMS was in correct position and the obstruction was resolved. In this figure, a hemostatic clip marking the lowest tumor margin is also seen. In addition, the patient had an abdominal catheter for hydrocephaly decompression.
NONFLUOROSCOPIC INSERTION OF A “TTS” STENT IN A LEFT COLON MCRO
Insertion of OTW stents far from the mid-sigmoid (around 25 cm from the anus) is difficult because the assembly stent guidewire tends to bend, despite the endoscope being placed side-to-side. If the MCRO has been traversed with the ultrathin endoscope, a 0.035 inch guidewire can be inserted through the working channel of the endoscope and placed as far as possible beyond the tumor (in upstream position). The ultrathin endoscope is removed, leaving the guidewire in place. This guidewire is back loaded in a therapeutic channel endoscope which is carefully advanced until the tumor. A TTS stent can be easily inserted. The endoscope gives enough stiffness to the system to advance the undeployed stent through the tumor.
Extreme care should be taken not to dislodge the guidewire placed beyond the stricture in the maneuvers of ultrathin endoscope withdrawal or therapeutic endoscope advancement.
MCRO must be never dilated before stenting because there is a great risk of tumor perforation.
ENDOSCOPIC INSERTION OF SEMS IN MCRO WITH FLUOROSCOPIC GUIDANCE
This method is considered as the ideal for many endoscopists[11]. Fluoroscopic facilities are necessary. C-arms fluoroscopic devices used sometimes for Endoscopic Retrograde Cholangiopancreatography (ERCP) are not good if they have no capacity to image the entire abdomen and if the patient table cannot be easily moved (Figure 4).
Figure 4.
C-arms fluoroscopic devices used sometimes for endoscopic retrograde cholangiopancreatography are not good for colonic stenting unless they have capacity to image the entire abdomen and if the patient table cannot be easily moved.
A therapeutic endoscope is advanced until the tumoral stricture is found. Using a gastroscope or short colonoscope with large working channel is very useful to facilitate devices exchange during the procedure.
With the endoscope in front of the stricture, an ERCP catheter loaded with a hydrophilic tip guidewire is passed through the working channel. The most important step is “cannulation” of the stricture with the guidewire. Almost all the strictures have an orifice, although sometimes it can be very difficult to find. As shown in Figure 5, gentle probing of the tumor with the guidewire leads to finally finding the path. The correct position of the guidewire beyond the stricture is given by the fluoroscopic view. If the patient is in the supine position (lying on his/her back), anatomical orientation is improved.
Figure 5.
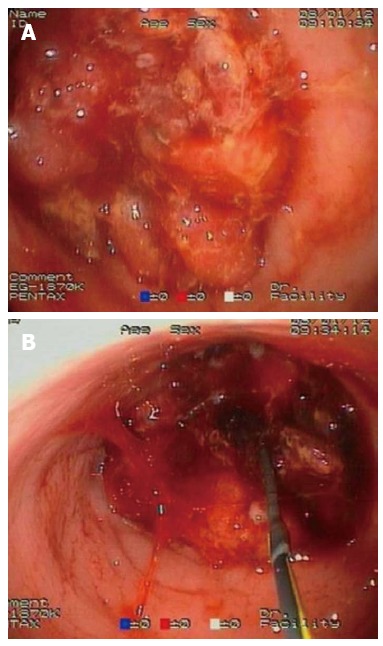
Endoscopic retrograde cholangiopancreatography catheter loaded with a hydrophilic tip guidewire. A: the obstructive tumor appears not to have any orifice that enabled stenting; B: gentle probing of the tumor with the guidewire leads to finally finding the path.
After traversing the tumor with the guidewire, the catheter is slid over it and contrast medium is injected to delineate the stricture. The catheter is removed, always leaving the guidewire tip as far as possible in the colon. A TTS stent is passed over the guidewire and deployed inside the tumor with fluoroscopic guidance of upstream maneuvers and endoscopic monitoring of downstream (in the endoscopic view) events.
SCIENTIFIC EVALUATION OF SEMS FOR MCRO
As previously said and as shown in Figure 1, a lot of papers have been published on this topic (Table 1). Nevertheless, few are randomized studies comparing the traditional surgical approach of MCRO with SEMS treatment.
Table 1.
Some series about self-expanding metal stents in malignant colorectal obstruction published in the last years n (%)
| Ref. | Publication year | No. of patients | Technical success | Clinical success | Perforation rate |
| García-Cano et al[4] | 2006 | 175 | 162 (92.6) | 138 (78.8) | 7 (4) |
| Ptok et al[12] | 2006 | 48 | 44 (92) | 44 (92) | 0 |
| Soto et al[13] | 2006 | 62 | 58 (93.54) | 56 (90.3) | 3 (4.8) |
| Karoui et al[14] | 2007 | 31 | 30 (97) | 27 (87) | 0 |
| Lee et al[15] | 2007 | 80 | 78 (97.5) | 77 (96.2) | 0 |
| 1Repici et al[16] | 2007 | 44 | 42 (95.4) | 41 (93.1) | 0 |
| Repici et al[17] | 2008 | 42 | 40 (95.2) | 40 (95.2) | 1 (2.38) |
| Im et al[18] | 2008 | 51 | 51 (100) | 43 (84.3) | 1 (1.9) |
| Fernández-Esparrach et al[19] | 2010 | 47 | 44 (94) | 44 (94) | 3 (7) |
| Small et al[20] | 2010 | 233 | 224 (96.1) | 222 (95.2) | 18 (7.7) |
| Park et al[21] | 2010 | 151 | 149 (98.6) | 140 (92.7) | 0 |
| Branger et al[22] | 2010 | 93 | 86 (92.5) | 80 (86) | 3 (3.2) |
| Donnellan et al[23] | 2010 | 43 | 40 (93) | 40 (93) | 2 (4.6) |
| Lee JH et al[24] | 2010 | 46 | 46 (100) | 39 (84.8) | 2 (4.3) |
| Lee HJ et al[25] | 2011 | 71 | 68 (95.8) | 68 (94) | 4 (5.6) |
| Luigiano et al[26] | 2011 | 39 | 36 (92.3) | 35 (89.7) | 2 (5.1) |
| Jiménez-Pérez et al[27] | 2011 | 182 | 177 (98) | 141 (94) | 5 (3) |
| Tominaga et al[28] | 2012 | 24 | 24 (100) | 20 (83) | 0 |
| Yoshida et al[29] | 2013 | 33 | 33 (100) | 32 (97) | 0 |
| Bonfante et al[30] | 2013 | 48 | 46 (96) | 46 (96) | 1 (2) |
In 2007, there are two articles published by Repici et al. One about the Ultraflex Precision stent in the left colon and the other on right colon stenting.
In a recent review from a surgical standpoint[31], it appears that technical and clinical success rates for stenting are lower than expected. SEMS is sometimes associated with a high incidence of clinical and silent perforation. Stenting instead of loop colostomy can be recommended only if the appropriate expertise is available in the hospital. The goal of stenting, a decrease of the stoma rate, can be advocated only if the complication rates of stenting are lower than those of stoma creation in the emergency situation. Until now, this has been not demonstrated in a prospective randomized trial.
Furthermore, when pathology surgical specimens are compared, tumors resected after stenting differed significantly in terms of ulceration at or near the tumor, perineural invasion and lymph node invasion. These findings are found less in tumors operated on without previous stenting[32].
On the contrary, many studies in clinical practice favor stenting as first-line treatment for left MCRO. Randomized trials in this setting appear to be difficult and perhaps randomization is not the only answer for structured objective evaluation of endoscopic therapy[33].
In one of the largest retrospective endoscopic series published in 2010[20], there were reported outcomes on 168 patients who underwent SEMS placement for definitive palliation and 65 patients with SEMS inserted as a BTS. Technical and immediate clinical success rates were 96% and 99% in the palliative group and 95% and 98% in the preoperative group 41/168 (24%). Patients in the palliative group had complications, including perforation (9%), occlusion (9%), migration (5%) and erosion/ulcer (2%). Mean stent patency was 145 d. The majority of patients were free of obstruction from implantation until death. Therefore, this large group of patients had their normal intestinal transit restored without having undergone an operation and without a stoma. Unfortunately, patients on oncological bevacizumab treatment triple the perforation rate.
Preoperatively placed stents remained in situ for a mean of 25.4 d and remained patent until surgery in 73.8% of patients. Complications were present in 23.1% of patients and 94% of them underwent elective colectomy. Conclusions drawn from this large cohort of patient are that colorectal SEMS placement is relatively safe and effective but has a complication rate of nearly 25%. However, only perforation (less than 10%) is a life-threatening complication. Other complications such as stent occlusion can be managed endoscopically.
Some surgical groups found SEMS treatment for MCRO in operable patients (BTS) very useful to carry on a laparoscopic procedure. Law et al[34] evaluated surgical outcomes after stent insertion for obstructing colorectal malignancy and these patients were compared with a laparoscopic and open approach. Their experience showed that after successful SEMS insertion for MCRO, elective surgical resection could be performed safely. The combined endoscopic and laparoscopic procedure provided a less invasive alternative to the multistage open operations and it was found feasible for patients with obstructing colon cancer.
SEMS in MCRO are also inserted by interventional radiologists. In one of the first reports comparing this new method with the surgical approach[35], Martinez-Santos et al[35] found that placement of a preoperative stent in patients with left-sided malignant colon and rectal obstruction prevented 94% of unnecessary operations and a large number of colostomies after elective surgery. These results were obtained with a lower rate of severe complications as well as a shorter hospital stay. This work cannot be considered a true randomized trial because patients with MCRO received a SEMS if they presented in the ER from Monday to Friday when an interventional radiologist was present in the hospital, whereas patients were operated on if they presented on week-ends. Besides, if patients with MCRO presented out of working hours (i.e., during the night), they were stabilized with intravenous fluids, put on nil per os with a nasogastric tube and received a stent early the next morning.
Kim et al[36] found that when the colorectal obstruction had a tortuous, curved angulation of the colon or was located at or proximal to the descending colon, the endoscopic method of SEMS placement appears to be more useful than the radiological method. However, once SEMS placement was technically successful, the clinical success rate, complication rate and stent patency did not differ with the method of insertion.
In the midst of the debate between pros and cons of SEMS as the initial treatment for MCRO, a surgical group[37] reports on its experience stating that in case of colorectal obstruction, endoscopic colon stenting as a bridge to elective operation should be considered as the treatment of choice for resectable patients given the significant advantages for short and long-term outcomes. Palliative stenting is effective but associated with a high rate of long-term complications.
However, when surgery and stents are compared as a palliative measure[25], SEMS were found not only an effective and acceptable therapy for initial palliation of MCRO, but they also showed long-term efficacy comparable to that with surgery, reducing costs (i.e., hospital stay).
Some plastic tubes (such as the Dennis colorectal tube) are less expensive alternatives to clean the obstructed colon before operation. But in a recent report[38], a 4.5% perforation rate with a 1.5% mortality was reported.
Finally, the distal part of the stent should be placed at least 6 cm from the anus on the contrary patients can suffer an unpleasant tenesmus.
CONCLUSION
Despite the still ongoing scientific debate[39-43], SEMS for MCRO appears to be the modern treatment for colonic obstruction[39,44,45]. Comparison between colonic SEMS manufactured by major stent companies show no important differences between them[40]. In addition, manufacturers are continuously working on stent improvement to allow a proper obstruction decompression[46]. It is better to use bare (uncovered) stents for MCRO rather than covered ones that are more prone to have complications[41].
Endoscopically, obstructions in the entire colon can be bridged with stents[42]; however, the major impact of SEMS for MCRO are left-sided tumoral strictures. In this setting, colonic stents represent the best option when skills are available[7].
Footnotes
P- Reviewers Ibrahim M, Rodriguez DC S- Editor Qi Y L- Editor Roemmele A E- Editor Zhang DN
References
- 1.Garcia-Cano J. Endoscopic insertion of self-expanding metal stents as first step to treat malignant colorectal obstruction. Am J Gastroenterol. 2005;100:1203–1204. doi: 10.1111/j.1572-0241.2005.41837_6.x. [DOI] [PubMed] [Google Scholar]
- 2.Dohmoto M, Rupp KD, Hohlbach G. [Endoscopically-implanted prosthesis in rectal carcinoma] Dtsch Med Wochenschr. 1990;115:915. [PubMed] [Google Scholar]
- 3.Baron TH, Dean PA, Yates MR, Canon C, Koehler RE. Expandable metal stents for the treatment of colonic obstruction: techniques and outcomes. Gastrointest Endosc. 1998;47:277–286. doi: 10.1016/s0016-5107(98)70327-x. [DOI] [PubMed] [Google Scholar]
- 4.García-Cano J, González-Huix F, Juzgado D, Igea F, Pérez-Miranda M, López-Rosés L, Rodríguez A, González-Carro P, Yuguero L, Espinós J, et al. Use of self-expanding metal stents to treat malignant colorectal obstruction in general endoscopic practice (with videos) Gastrointest Endosc. 2006;64:914–920. doi: 10.1016/j.gie.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 5.Baron TH, Wong Kee Song LM, Repici A. Role of self-expandable stents for patients with colon cancer (with videos) Gastrointest Endosc. 2012;75:653–662. doi: 10.1016/j.gie.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 6.Tan CJ, Dasari BV, Gardiner K. Systematic review and meta-analysis of randomized clinical trials of self-expanding metallic stents as a bridge to surgery versus emergency surgery for malignant left-sided large bowel obstruction. Br J Surg. 2012;99:469–476. doi: 10.1002/bjs.8689. [DOI] [PubMed] [Google Scholar]
- 7.Ansaloni L, Andersson RE, Bazzoli F, Catena F, Cennamo V, Di Saverio S, Fuccio L, Jeekel H, Leppäniemi A, Moore E, et al. Guidelenines in the management of obstructing cancer of the left colon: consensus conference of the world society of emergency surgery (WSES) and peritoneum and surgery (PnS) society. World J Emerg Surg. 2010;5:29. doi: 10.1186/1749-7922-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.García-Cano J. Use of an ultrathin gastroscope to allow endoscopic insertion of enteral wallstents without fluoroscopic monitoring. Dig Dis Sci. 2006;51:1231–1235. doi: 10.1007/s10620-006-8040-9. [DOI] [PubMed] [Google Scholar]
- 9.García-Cano J, Sanchez-Manjavacas N, Viuelas M, Gomez-Ruiz C, Jimeno C, Morillas J, Redondo E, Perez-Vigara G, Perez-Garcia JI, Perez-Sola A. Use of an ultrathin endoscope to insert self-expanding metal stents in tumoral strictures of the rectosigmoid without fluoroscopy. Gastrointest Endosc. 2007;65:AB258. [Google Scholar]
- 10.Lee JH, Yoon JY, Park SJ, Hong SP, Kim TI, Kim WH, Cheon JH. The learning curve for colorectal stent insertion for the treatment of malignant colorectal obstruction. Gut Liver. 2012;6:328–333. doi: 10.5009/gnl.2012.6.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.García-Cano J. Colonic self-expandable metal stents: indications and placement techniques. In: Kozarek R, Baron T, Song HY, editors. Self-Expandable Stents in the Gastrointestinal Tract. New York: Springer; 2013. pp. 175–189. [Google Scholar]
- 12.Ptok H, Meyer F, Marusch F, Steinert R, Gastinger I, Lippert H, Meyer L. Palliative stent implantation in the treatment of malignant colorectal obstruction. Surg Endosc. 2006;20:909–914. doi: 10.1007/s00464-005-0594-7. [DOI] [PubMed] [Google Scholar]
- 13.Soto S, López-Rosés L, González-Ramírez A, Lancho A, Santos A, Olivencia P. Endoscopic treatment of acute colorectal obstruction with self-expandable metallic stents: experience in a community hospital. Surg Endosc. 2006;20:1072–1076. doi: 10.1007/s00464-005-0345-9. [DOI] [PubMed] [Google Scholar]
- 14.Karoui M, Charachon A, Delbaldo C, Loriau J, Laurent A, Sobhani I, Tran Van Nhieu J, Delchier JC, Fagniez PL, Piedbois P, et al. Stents for palliation of obstructive metastatic colon cancer: impact on management and chemotherapy administration. Arch Surg. 2007;142:619–23; discussion 623. doi: 10.1001/archsurg.142.7.619. [DOI] [PubMed] [Google Scholar]
- 15.Lee KM, Shin SJ, Hwang JC, Cheong JY, Yoo BM, Lee KJ, Hahm KB, Kim JH, Cho SW. Comparison of uncovered stent with covered stent for treatment of malignant colorectal obstruction. Gastrointest Endosc. 2007;66:931–936. doi: 10.1016/j.gie.2007.02.064. [DOI] [PubMed] [Google Scholar]
- 16.Repici A, Fregonese D, Costamagna G, Dumas R, Kähler G, Meisner S, Giovannini M, Freeman J, Petruziello L, Hervoso C, et al. Ultraflex precision colonic stent placement for palliation of malignant colonic obstruction: a prospective multicenter study. Gastrointest Endosc. 2007;66:920–927. doi: 10.1016/j.gie.2007.03.1042. [DOI] [PubMed] [Google Scholar]
- 17.Repici A, Adler DG, Gibbs CM, Malesci A, Preatoni P, Baron TH. Stenting of the proximal colon in patients with malignant large bowel obstruction: techniques and outcomes. Gastrointest Endosc. 2007;66:940–944. doi: 10.1016/j.gie.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 18.Im JP, Kim SG, Kang HW, Kim JS, Jung HC, Song IS. Clinical outcomes and patency of self-expanding metal stents in patients with malignant colorectal obstruction: a prospective single center study. Int J Colorectal Dis. 2008;23:789–794. doi: 10.1007/s00384-008-0477-1. [DOI] [PubMed] [Google Scholar]
- 19.Fernández-Esparrach G, Bordas JM, Giráldez MD, Ginès A, Pellisé M, Sendino O, Martínez-Pallí G, Castells A, Llach J. Severe complications limit long-term clinical success of self-expanding metal stents in patients with obstructive colorectal cancer. Am J Gastroenterol. 2010;105:1087–1093. doi: 10.1038/ajg.2009.660. [DOI] [PubMed] [Google Scholar]
- 20.Small AJ, Coelho-Prabhu N, Baron TH. Endoscopic placement of self-expandable metal stents for malignant colonic obstruction: long-term outcomes and complication factors. Gastrointest Endosc. 2010;71:560–572. doi: 10.1016/j.gie.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Park S, Cheon JH, Park JJ, Moon CM, Hong SP, Lee SK, Kim TI, Kim WH. Comparison of efficacies between stents for malignant colorectal obstruction: a randomized, prospective study. Gastrointest Endosc. 2010;72:304–310. doi: 10.1016/j.gie.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 22.Branger F, Thibaudeau E, Mucci-Hennekinne S, Métivier-Cesbron E, Vychnevskaia K, Hamy A, Arnaud JP. Management of acute malignant large-bowel obstruction with self-expanding metal stent. Int J Colorectal Dis. 2010;25:1481–1485. doi: 10.1007/s00384-010-1003-9. [DOI] [PubMed] [Google Scholar]
- 23.Donnellan F, Cullen G, Cagney D, O’Halloran P, Harewood GC, Murray FE, Patchett SE. Efficacy and safety of colonic stenting for malignant disease in the elderly. Int J Colorectal Dis. 2010;25:747–750. doi: 10.1007/s00384-010-0917-6. [DOI] [PubMed] [Google Scholar]
- 24.Lee JH, Ross WA, Davila R, Chang G, Lin E, Dekovich A, Davila M. Self-expandable metal stents (SEMS) can serve as a bridge to surgery or as a definitive therapy in patients with an advanced stage of cancer: clinical experience of a tertiary cancer center. Dig Dis Sci. 2010;55:3530–3536. doi: 10.1007/s10620-010-1370-7. [DOI] [PubMed] [Google Scholar]
- 25.Lee HJ, Hong SP, Cheon JH, Kim TI, Min BS, Kim NK, Kim WH. Long-term outcome of palliative therapy for malignant colorectal obstruction in patients with unresectable metastatic colorectal cancers: endoscopic stenting versus surgery. Gastrointest Endosc. 2011;73:535–542. doi: 10.1016/j.gie.2010.10.052. [DOI] [PubMed] [Google Scholar]
- 26.Luigiano C, Ferrara F, Fabbri C, Ghersi S, Bassi M, Billi P, Polifemo AM, Landi P, Cennamo V, Consolo P, et al. Through-the-scope large diameter self-expanding metal stent placement as a safe and effective technique for palliation of malignant colorectal obstruction: a single center experience with a long-term follow-up. Scand J Gastroenterol. 2011;46:591–596. doi: 10.3109/00365521.2011.551886. [DOI] [PubMed] [Google Scholar]
- 27.Jiménez-Pérez J, Casellas J, García-Cano J, Vandervoort J, García-Escribano OR, Barcenilla J, Delgado AA, Goldberg P, Gonzalez-Huix F, Vázquez-Astray E, et al. Colonic stenting as a bridge to surgery in malignant large-bowel obstruction: a report from two large multinational registries. Am J Gastroenterol. 2011;106:2174–2180. doi: 10.1038/ajg.2011.360. [DOI] [PubMed] [Google Scholar]
- 28.Tominaga K, Maetani I, Sato K, Shigoka H, Omuta S, Ito S, Saigusa Y. Favorable long-term clinical outcome of uncovered D-weave stent placement as definitive palliative treatment for malignant colorectal obstruction. Dis Colon Rectum. 2012;55:983–989. doi: 10.1097/DCR.0b013e31825c484d. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida S, Watabe H, Isayama H, Kogure H, Nakai Y, Yamamoto N, Sasaki T, Kawakubo K, Hamada T, Ito Y, et al. Feasibility of a new self-expandable metallic stent for patients with malignant colorectal obstruction. Dig Endosc. 2013;25:160–166. doi: 10.1111/j.1443-1661.2012.01353.x. [DOI] [PubMed] [Google Scholar]
- 30.Bonfante P, D’Ambra L, Berti S, Falco E, Cristoni MV, Briglia R. Managing acute colorectal obstruction by “bridge stenting” to laparoscopic surgery: Our experience. World J Gastrointest Surg. 2012;4:289–295. doi: 10.4240/wjgs.v4.i12.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grundmann RT. Primary colon resection or Hartmann’s procedure in malignant left-sided large bowel obstruction? The use of stents as a bridge to surgery. World J Gastrointest Surg. 2013;5:1–4. doi: 10.4240/wjgs.v5.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sabbagh C, Chatelain D, Trouillet N, Mauvais F, Bendjaballah S, Browet F, Regimbeau JM. Does use of a metallic colon stent as a bridge to surgery modify the pathology data in patients with colonic obstruction? A case-matched study. Surg Endosc. 2013;27:3622–3631. doi: 10.1007/s00464-013-2934-3. [DOI] [PubMed] [Google Scholar]
- 33.Cotton PB. Randomization is not the (only) answer: a plea for structured objective evaluation of endoscopic therapy. Endoscopy. 2000;32:402–405. doi: 10.1055/s-2000-642. [DOI] [PubMed] [Google Scholar]
- 34.Law WL, Poon JT, Fan JK, Lo OS. Colorectal resection after stent insertion for obstructing cancer: comparison between open and laparoscopic approaches. Surg Laparosc Endosc Percutan Tech. 2013;23:29–32. doi: 10.1097/SLE.0b013e318275743b. [DOI] [PubMed] [Google Scholar]
- 35.Martinez-Santos C, Lobato RF, Fradejas JM, Pinto I, Ortega-Deballón P, Moreno-Azcoita M. Self-expandable stent before elective surgery vs. emergency surgery for the treatment of malignant colorectal obstructions: comparison of primary anastomosis and morbidity rates. Dis Colon Rectum. 2002;45:401–406. doi: 10.1007/s10350-004-6190-4. [DOI] [PubMed] [Google Scholar]
- 36.Kim JW, Jeong JB, Lee KL, Kim BG, Jung YJ, Kim W, Kim HY, Ahn DW, Koh SJ, Lee JK. Comparison of clinical outcomes between endoscopic and radiologic placement of self-expandable metal stent in patients with malignant colorectal obstruction. Korean J Gastroenterol. 2013;61:22–29. [PubMed] [Google Scholar]
- 37.Gianotti L, Tamini N, Nespoli L, Rota M, Bolzonaro E, Frego R, Redaelli A, Antolini L, Ardito A, Nespoli A, et al. A prospective evaluation of short-term and long-term results from colonic stenting for palliation or as a bridge to elective operation versus immediate surgery for large-bowel obstruction. Surg Endosc. 2013;27:832–842. doi: 10.1007/s00464-012-2520-0. [DOI] [PubMed] [Google Scholar]
- 38.Yamada T, Shimura T, Sakamoto E, Kurumiya Y, Komatsu S, Iwasaki H, Nomura S, Kanie H, Hasegawa H, Orito E, et al. Preoperative drainage using a transanal tube enables elective laparoscopic colectomy for obstructive distal colorectal cancer. Endoscopy. 2013;45:265–271. doi: 10.1055/s-0032-1326030. [DOI] [PubMed] [Google Scholar]
- 39.Feo L, Schaffzin DM. Colonic stents: the modern treatment of colonic obstruction. Adv Ther. 2011;28:73–86. doi: 10.1007/s12325-010-0094-6. [DOI] [PubMed] [Google Scholar]
- 40.Cheung DY, Kim JY, Hong SP, Jung MK, Ye BD, Kim SG, Kim JH, Lee KM, Kim KH, Baik GH, et al. Outcome and safety of self-expandable metallic stents for malignant colon obstruction: a Korean multicenter randomized prospective study. Surg Endosc. 2012;26:3106–3113. doi: 10.1007/s00464-012-2300-x. [DOI] [PubMed] [Google Scholar]
- 41.Choi JH, Lee YJ, Kim ES, Choi JH, Cho KB, Park KS, Jang BK, Chung WJ, Hwang JS. Covered self-expandable metal stents are more associated with complications in the management of malignant colorectal obstruction. Surg Endosc. 2013;27:3220–3227. doi: 10.1007/s00464-013-2897-4. [DOI] [PubMed] [Google Scholar]
- 42.Yao LQ, Zhong YS, Xu MD, Xu JM, Zhou PH, Cai XL. Self-expanding metallic stents drainage for acute proximal colon obstruction. World J Gastroenterol. 2011;17:3342–3346. doi: 10.3748/wjg.v17.i28.3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghazal AH, El-Shazly WG, Bessa SS, El-Riwini MT, Hussein AM. Colonic endolumenal stenting devices and elective surgery versus emergency subtotal/total colectomy in the management of malignant obstructed left colon carcinoma. J Gastrointest Surg. 2013;17:1123–1129. doi: 10.1007/s11605-013-2152-2. [DOI] [PubMed] [Google Scholar]
- 44.Tirosh D, Perry Z, Walfisch S, Rozental A, Fich A, Krugliak P, Mizrahi S, Kirshtein B. Endoscopic self-expanding metal stents for acute colonic obstruction. Am Surg. 2013;79:30–34. [PubMed] [Google Scholar]
- 45.Iversen LH. Aspects of survival from colorectal cancer in Denmark. Dan Med J. 2012;59:B4428. [PubMed] [Google Scholar]
- 46.Puértolas S, Bajador E, Puértolas JA, López E, Ibarz E, Gracia L, Herrera A. Study of the behavior of a bell-shaped colonic self-expandable NiTi stent under peristaltic movements. Biomed Res Int. 2013;2013:370582. doi: 10.1155/2013/370582. [DOI] [PMC free article] [PubMed] [Google Scholar]


