Abstract
AIM: To investigate whether discharge scoring criteria are as safe as clinical criteria for discharge decision and allow for earlier discharge.
METHODS: About 220 consecutive outpatients undergoing colonoscopy under sedation with Meperidine plus Midazolam were enrolled and assigned to 2 groups: in Control-group (110 subjects) discharge decision was based on the clinical assessment; in PADSS-group (110 subjects) discharge decision was based on the modified Post-Anaesthetic Discharge Scoring System (PADSS). Measurements of the PADDS score were taken every 20 min after colonoscopy, and patients were discharged after two consecutive PADSS scores ≥ 9. The investigator called each patient 24-48 h after discharge to administer a standardized questionnaire, to detect any delayed complications. Patients in which cecal intubation was not performed and those who were not found at follow-up phone call were excluded from the study.
RESULTS: Thirteen patients (7 in Control-group and 6 in PADSS-group) were excluded from the study. Recovery from sedation was faster in PADSS-group than in Control-group (58.75 ± 18.67 min vs 95.14 ± 10.85 min, respectively; P < 0.001). Recovery time resulted shorter than 60 min in 39 patients of PADSS-group (37.5%), and in no patient of Control-group (P < 0.001). At follow-up phone call, no patient declared any hospital re-admission because of problems related to colonoscopy and/or sedation. Mild delayed post-discharge symptoms occurred in 57 patients in Control-group (55.3%) and in 32 in PADSS-group (30.7%). The most common symptoms were drowsiness, weakness, abdominal distension, and headache. Only 3 subjects needed to take some drugs because of post-discharge symptoms.
CONCLUSION: The Post-Anaesthetic Discharge Scoring System is as safe as the clinical assessment and allows for an earlier patient discharge after colonoscopy performed under sedation.
Keywords: Colonoscopy, Conscious sedation, Patient discharge, Recovery room, Complications
Core tip: About 220 consecutive outpatients undergoing colonoscopy under sedation were enrolled to investigate whether the Post-Anaesthetic Discharge Scoring System (PADSS) is a safe clinical assessment for earlier patient discharge after colonoscopy. The patients were assigned to two groups: in Control-group (110 subjects) discharge decision was based on the clinical assessment; in PADSS-group (110 subjects) discharge decision was based on the modified PADSS. Recovery from sedation was faster in PADSS-group than in Control-group (58.75 min vs 95.14 min, P < 0.001). Recovery time resulted shorter than 60 min in 39 patients in PADSS-group (37.5%), and in no patient in Control-group (P < 0.001).
INTRODUCTION
Colonoscopy frequently causes considerable discomfort or pain to patients, and analgesia and sedation are often necessary for a successful colonoscopy. The decision to use premedication and the kind of premedication are influenced by national and cultural differences among countries[1], and by the rules regulating the drugs use. Propofol Deep Sedation is frequently used in some countries such as United States, whereas conscious sedation induced by means of a combination of a benzodiazepine and an opiate is more frequently used in other countries such as Italy[2-5], because of its excellent analgesic and sedative effects[6]. Moreover, Propofol can only be administered by anesthetists in Italy.
The annual number of colonoscopies performed on an outpatient basis is increasing, and the increase is expected to continue, because of the screening programs for the colon cancer prevention that are ongoing in many countries. Likewise, the number of examinations performed under sedation is also increasing, and this fact can cause some problems to digestive endoscopy centers, as they are often not provided with sufficiently spacious observation rooms. At the time of discharge from the digestive endoscopy center, patients should be home-ready: they should be clinically stable and able to rest at home. Although the discharge after ambulatory surgery and anesthesia can involve legal implications[7,8], there is very little information and documentation about the recovery pattern and home-readiness of the ambulatory gastrointestinal endoscopy. The Guidelines for Sedation in Digestive Endoscopy of the Italian Society of Digestive Endoscopy (SIED) do not recommend the use of discharge scoring systems to assess the home-readiness, and generically state that “the patient must be awake and well-oriented, and vital parameters must be acceptable and stable”[9,10].
Based on these observations and considering the aging population, it becomes even more important to have clear, evidence-based discharge criteria in clinical use, as patient safety must be our first priority. Several scoring systems have been devised to guide the process of discharge and home-readiness, to ensure patient safety[11]. This prospective study was planned to evaluate whether the discharge scoring criteria are as safe as clinical criteria for discharge decision and allow for earlier discharge.
MATERIALS AND METHODS
Study population
This prospective, non-randomized study was conducted on a population of 220 consecutive outpatients undergoing ambulatory elective colonoscopy in our Digestive Endoscopy Centre. Inclusion criteria were: age range 18 to 75 years, patients scheduled for elective sedated colonoscopy, and capability (evaluated by the endoscopist) of fully understanding the questionnaire. Exclusion criteria were: American Society of Anestesiology (ASA) risk class 3 or higher[12], previous colonic surgical procedure, willingness to undergo unsedated colonoscopy, inpatient status, planned endoscopic therapy, psychiatric diseases or long-term psychiatric drug addiction, concomitant neoplastic diseases, pregnancy or lactation. The first 110 subjects formed the control group (Co-group), in which discharge decision was based on clinical evaluation; the other 110 subjects formed the study group in which the discharge was based on the modified Post Anaesthetic Discharge Scoring System (PADSS-group)[13].
Oral 4-L polyethylene glycol solution was used in all patients as a preparation for colonoscopy. Conscious sedation was induced by means of an iv combination of Meperidine 40-60 mg plus Midazolam 2-5 mg according to our routine practice, in order to obtain a degree of sedation ranging from 2 to 4 of the Ramsay’s scale[14].
The study protocol was approved by the Ethical Committee of our hospital, and all patients enrolled gave their written informed consent to participate in the study.
Outcome measurement
Pre-colonoscopy and during-colonoscopy assessment: For each patient, age, gender, blood pressure (BP), blood oxygen saturation (SaO2), and heart rate (HR) were recorded. Associated medical illnesses were graded according to the American Society of Anesthesiologists’ Physical Status Classification (ASA grade)[12]. Before colonoscopy the anxiety level of the patient was evaluated on a four-point verbal scale, where 1 = no anxiety, 4 = very anxious. Pre-colonoscopy abdominal pain was assessed with the Numerical Analogue Scale (0 = no pain; 10 = unbearable pain)[15]. Heart rate, blood oxygen saturation, and blood pressure were monitored, and oxygen supplement (2 L/min) was provided throughout the duration of colonoscopy.
Post-colonoscopy assessment: Patients in which cecal intubation was not performed were excluded from the study. After colonoscopy, the patients were followed up in the recovery room, and 20 min after the end of colonoscopy they were scored using the Modified PADSS (Table 1)[13]. Afterwards, they were re-scored every 20 min, until two consecutive PADDS scores ≥ 9 were achieved.
Table 1.
Modified Post-Anaesthetic Discharge Scoring System
| Categories | Points |
| Vital signs | |
| BP and HR ± 20% of pre-endoscopy value | 2 |
| BP and HR ± 20%-40% of pre-endoscopy value | 1 |
| BP and HR ± 40% of pre-endoscopy value | 0 |
| Activity | |
| Steady gait, no dizziness or meets pre-endoscopy level | 2 |
| Requires assistance | 1 |
| Unable to ambulate | 0 |
| Nausea and vomiting | |
| No or minimal/treated with p.o. medication | 2 |
| Moderate/treated with parenteral medication | 1 |
| Severe/continues despite treatment | 0 |
| Pain | |
| Minimal or no pain (Numerical Analogue Scale = 0-3) | 2 |
| Moderate (Numerical Analogue Scale = 4-6) | 1 |
| Severe (Numerical Analogue Scale = 7-10) | 0 |
| Surgical bleeding | |
| None or Minimal (not requiring intervention) | 2 |
| Moderate (1 episode of hematemesis or rectal bleeding) | 1 |
| Severe (≥ 2 episodes of hematemesis or rectal bleeding) | 0 |
| Total score | ... |
| (Patients’ scoring ≥ 9 for two consecutive measurements are considered fit for discharge home) |
BP: Blood pressure; HR: Heart rate.
Using a 9-item questionnaire, the investigator documented each patient’s postoperative course in a follow-up phone call 24-48 h after discharge, to assess any delayed complication. Patients who were not found at follow-up phone call were excluded from the study.
Discharge criteria: (1) Co-group: After colonoscopy, the endoscopist settled the observation time on the basis of patient’s age and clinical conditions, dosage of the administered drugs, and sedation degree. At the end of the observation time, the patient was discharged if BP, HR, and SaO2 were stable; and (2) PADSS-group: Recovery-room nurse discharged the patient after a PADSS score ≥ 9 was achieved in two consecutive measurements. The time from the end of colonoscopy to the patient discharge was recorded.
Estimation of sample size: The test power was exclusively based on the presence of two groups (Co-group and PADSS-group) resulting to be higher than 95% and suitable to reveal differences between discharges times of at least 10 min preserving a P value < 0.05.
Statistical analysis
Interval variables were analyzed using the non parametric Kruskal-Wallis test, and nominal variables were analyzed using the χ2 test, or, if necessary, the Fisher’s exact test. Results were considered statistically significant if P values were < 0.05.
RESULTS
Thirteen patients (7 in Co-group and 6 in PADSS-group) were excluded from the study, as cecal intubation was not performed or the patients were not found at follow-up phone call. Two hundred and seven patients (92 males and 115 females) could be evaluated. Their characteristics are summarized in Table 2. The two groups did not differ for age, gender, pre-colonoscopy anxiety level and ASA classification. No patient needed reversal agents.
Table 2.
Patients characteristics and main results
| Co-group (n = 103) | PADSS-group (n = 104) | |
| Age, mean ± SD, yr | 58.45 ± 11.65 | 57.21 ± 11.6 |
| Gender, M/F | 46/57 | 46/58 |
| ASA class I/II | 40/63 | 41/63 |
| Anxiety level, n | ||
| 1: none | 16 | 9 |
| 2: mild | 75 | 88 |
| 3: moderate | 10 | 6 |
| 4: severe | 2 | 1 |
| Pain before colonoscopy, mean ± SD | 1.9 ± 1.4 | 1.9 ± 0.6 |
| Recovery time, mean ± SD, minb | 95.14 ± 10.85 | 58.75 ± 18.67 |
| Recovery time < 60 min, n (%)b | 0 (0) | 39 (37.5) |
| Early or late severe complications, n | 0 | 0 |
P < 0.001, Post-Anaesthetic Discharge Scoring System (PADSS)-group vs Co-group. ASA: American Society of Anestesiology.
Recovery from sedation was faster in PADSS-group than in Co-group (58.75 ± 18.67 min and 95.14 ± 10.85 min, respectively; P < 0.001) (Table 2 and Figure 1). Recovery time resulted shorter than 60 min in 39 patients of PADSS-group (37.5%), and in no patient of Co-group (P < 0.001).
Figure 1.
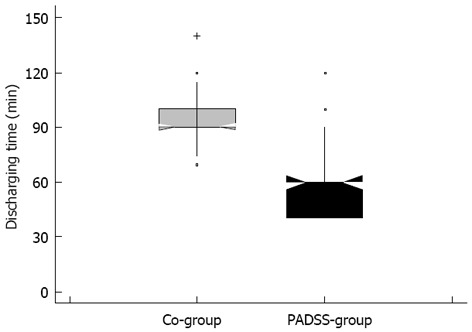
Comparison of recovery time for the two groups. PADSS: Post-Anaesthetic Discharge Scoring System.
No early complication occurred in both groups. At follow-up phone call, no patient declared any need of hospital re-admission because of problems related to colonoscopy and/or sedation. Fifty-seven patients in Co-group (55.3%) and 32 in PADSS-group (30.7%) complained of mild post-colonoscopy symptoms (Table 3), but only three of them (2 in Co-group e 1 in PADSS-group) needed to take some drugs for these symptoms. The most common symptoms were drowsiness, weakness, abdominal distension, and headache.
Table 3.
Results of post-endoscopy evaluation phone call
| Co-group(n) | PADSS-group (n) | |
| Go back to the hospital | 0 | 0 |
| Problems since discharge | 57 | 32 |
| Abdominal distension (with or without pain) | 21 | 7 |
| Fever | 1 | 2 |
| Pain at the injection site | 4 | 4 |
| Headache | 15 | 4 |
| Nausea and/or vomiting | 3 | 2 |
| Drowsiness or difficult to wake-up | 31 | 22 |
| Weakness | 20 | 19 |
| Did you take drugs for these problems? | 2 | 1 |
PADSS: Post-Anaesthetic Discharge Scoring System.
DISCUSSION
The increasing number of digestive endoscopic examinations performed under sedation has highlighted the problem of the space and personnel required to recover the patients, and the need to identify criteria that can be used to determine when they can safely go home under the care of a friend or relative. Most centers still rely on clinical criteria for practical discharge decision after colonoscopy. Efforts to shorten recovery time by using sedative agents with shorter half life are gaining increasing popularity. The European Guidelines concerning Non-Anaesthesiologist Administered Propofol (NAAP) for Gastrointestinal Endoscopy was published in 2010[16], but 21 national societies of anaesthesiology in Europe signed a Consensus Statement to declare their disagreement with the NAAP guidelines[17]. Moreover, because of the well-known risks of Propofol administration, the manufacturers of the drug have added the following restriction: “For general anesthesia or monitored anesthesia care (MAC) sedation, DIPRIVAN Injectable Emulsion should be administered only by persons trained in the administration of general anesthesia and not involved in the conduct of the surgical/diagnostic procedure”. For these reasons, drugs with a very short duration of action, such as Propofol and Remifentanil, are only administered by anesthetists in Italy, and their use under the direction of a gastroenterologist can have medico-legal implications[18]. Therefore, sedation is generally obtained by means of Meperidine and Midazolam. However, Meperidine is an opioid analgesic with long duration of action (2-4 h)[19], and the duration of the impairment after sedation and post-colonoscopy observation time are unavoidably long.
Several cognitive and psychomotor tests are available to assess the impairment after sedation, but most of them are toilsome and poorly suitable for clinical practice[20-22]. The clinical scoring systems are based on clear, concise and standardized discharge criteria that can be used to determine when patients can safely go home under the care of a relative. The Aldrete scoring system and the PADSS have received widespread acceptance in assessing postanesthetic recovery[23], and are currently used to assess home-readiness after ambulatory surgery. Conversely, to date there is very little information about their use in ambulatory gastrointestinal endoscopy.
In our study, the PADSS resulted as safe as clinical assessment and allowed for earlier patient discharge after colonoscopy performed under sedation. No patient had to be re-admitted because of complications, and just three patients (2 in Co-group and 1 in PADSS-group) taken some drugs for mild and transient symptoms (Table 3). Our data are comparable to those reported by a previous prospective study, in which the patients undergoing endoscopic procedures under sedation were assessed with the PADSS and were discharged within two hours[24]. Furthermore, in our study 37.5% of patients in PADSS-group could be discharged within 60 min from the end of colonoscopy. This observation is quite interesting, as the patients were only discharged after two consecutive measurements achieving a PADSS score ≥ 9. Since the measurements were taken every 20 min, the theoretical shortest time for patient discharge would be 40 min. We prudentially planned to discharge the patients after two measurements of PADSS score, as there are very few studies dealing with its use in digestive endoscopy, and no specific information is provided in literature on potential discharge problems. However, the discharge time could probably be even shorter, as prior reports suggested that patients can be discharged without problems after just one PADSS score ≥ 9[23].
The patient’s readiness for discharge needs to be addressed in a simple, clear and reproducible manner, to replace subjective clinical impression by assigning numeric values to parameters. Our trial was conducted in a large busy hospital, and its results show that well-defined discharge scoring criteria offer measurable advantage in decreasing total procedure time by shortening recovery time, and can represent a useful tool for all digestive endoscopy centers in which Meperidine is routinely used for sedation. The use of a standardized discharge scoring system can increase the flow of patients through the recovery process and allows for safe discharge without increasing post-discharge complications and without using any additional resources. The shorter mean recovery time achieved in the PADDS-group in comparison with the Co-group (about 37 min) entails a shorter time spent by the nurse in the recovery room. However, it would be quite hard to quantify such a time saving in terms of cost saving, as several patients are contemporaneously followed up by the recovery-room nurse. Nonetheless, the use of a standardized discharge scoring system represents a more cost-efficient manner while still maintaining quality of care, and becomes essential if discharge decision is entrusted to the nursing staff, which needs to evaluate the post-endoscopy course of the patient in a systematic way, applying to physician for consultation only when necessary.
Our study has some limits. First, it is a single centre study. Second, it is not a randomized trial. Moreover, although the scoring criterion is a reliable tool, it can not replace the critical thinking or professional judgment, as it does not allow to identify all the possible problems (for instance, a hypoglicemic crisis). Calculating scores of PADSS entails that post-endoscopy vital sign parameters should be compared with pre-endoscopy values, to ensure the patient’s return to homeostasis. However, if some pre-endoscopy values were abnormally elevated because of anxiety or pain, expecting the post-endoscopy values to be within ± 20% range may not be appropriate.
In conclusion, having well-defined discharge scoring criteria is imperative in order to ensure a quick and safe discharge. Our study suggests that almost all patients undergoing sedation with Meperidine and Midazolam can be discharged within 2 h of colonoscopy, using the modified PADSS score. However, further and wider randomized trials are needed to confirm our observation.
COMMENTS
Background
The number of colonoscopies performed under sedation on an outpatient basis is increasing as the screening programs for the colon cancer prevention are ongoing in many countries. This fact can cause some problem to digestive endoscopy centres, as they are often not provided with sufficiently spacious recovery rooms.
Research frontiers
At the time of discharge after colonoscopy, patients should be home-ready, and this issue can involve legal implications. Nevertheless, there is very little information and documentation about the recovery patterns and home-readiness after colonoscopy, and many guidelines do not include the use of any standardized discharge scoring system.
Innovations and breakthroughs
In this prospective study, recovery from sedation resulted faster in Post-Anaesthetic Discharge Scoring System (PADSS)-group than in Control-group (58.75 min vs 95.14 min, P < 0.001), and no patient had to be re-admitted because of complications.
Applications
This study demonstrated that the use of PADSS is safe and allows for an earlier patient discharge after colonoscopy performed under sedation.
Terminology
PADSS is a clinical scoring system based on clear, concise and standardized discharge criteria, and is currently used to assess home-readiness after ambulatory surgery.
Peer review
This is an interesting study, which has important clinical applications.
Footnotes
P- Reviewers de Silva AP, Gaya DR, Kemik O, Lee JFY S- Editor Wen LL L- Editor A E- Editor Liu XM
References
- 1.Ladas SD, Satake Y, Mostafa I, Morse J. Sedation practices for gastrointestinal endoscopy in Europe, North America, Asia, Africa and Australia. Digestion. 2010;82:74–76. doi: 10.1159/000285248. [DOI] [PubMed] [Google Scholar]
- 2.Froehlich F, Harris JK, Wietlisbach V, Burnand B, Vader JP, Gonvers JJ. Current sedation and monitoring practice for colonoscopy: an International Observational Study (EPAGE) Endoscopy. 2006;38:461–469. doi: 10.1055/s-2006-925368. [DOI] [PubMed] [Google Scholar]
- 3.Lee H, Kim JH. Superiority of split dose midazolam as conscious sedation for outpatient colonoscopy. World J Gastroenterol. 2009;15:3783–3787. doi: 10.3748/wjg.15.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radaelli F, Meucci G, Minoli G. Colonoscopy practice in Italy: a prospective survey on behalf of the Italian Association of Hospital Gastroenterologists. Dig Liver Dis. 2008;40:897–904. doi: 10.1016/j.dld.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 5.Waring JP, Baron TH, Hirota WK, Goldstein JL, Jacobson BC, Leighton JA, Mallery JS, Faigel DO. Guidelines for conscious sedation and monitoring during gastrointestinal endoscopy. Gastrointest Endosc. 2003;58:317–322. doi: 10.1067/s0016-5107(03)00001-4. [DOI] [PubMed] [Google Scholar]
- 6.Wong RC. The menu of endoscopic sedation: all-you-can-eat, combination set, á la carte, alternative cuisine, or go hungry. Gastrointest Endosc. 2001;54:122–126. doi: 10.1067/mge.2001.116115. [DOI] [PubMed] [Google Scholar]
- 7.Forceville X, Oxeda C, Leloup E, Bouju P, Amiot JF, Dupouey B, Arnaud F. Is it possible to avoid a penal offence in carrying out ambulatory anesthesia? Cah Anesthesiol. 1991;39:427–433. [PubMed] [Google Scholar]
- 8.Vayre P, Jost JL. Medico-legal implications of ambulatory surgery. Chirurgie 1993- 1994;119:137–140; discussion 140-141. [PubMed] [Google Scholar]
- 9.SIED , SIAARTI , ANOTE Linee Guida per la Sedazione in Endoscopia Digestiva. Giorn Ital End Dig. 2000;23(Suppl):29–39. [Google Scholar]
- 10.Conigliaro R, Battistini A, De Masi E, Fanti L, Ficano L, Rossi A. Linee-Guida per la Sedazione in Endoscopia Digestiva. Revisione Febbraio 2006. Available from: http: //www.sied.it. [Google Scholar]
- 11.Awad IT, Chung F. Factors affecting recovery and discharge following ambulatory surgery. Can J Anaesth. 2006;53:858–872. doi: 10.1007/BF03022828. [DOI] [PubMed] [Google Scholar]
- 12.American Society of Anesthesiologists. ASA physical status classification system. Available from: http://www.asahq.org/clinical/physicalstatus.htm.
- 13.Chung F, Chan VW, Ong D. A post-anesthetic discharge scoring system for home readiness after ambulatory surgery. J Clin Anesth. 1995;7:500–506. doi: 10.1016/0952-8180(95)00130-a. [DOI] [PubMed] [Google Scholar]
- 14.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656–659. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Downie WW, Leatham PA, Rhind VM, Wright V, Branco JA, Anderson JA. Studies with pain rating scales. Ann Rheum Dis. 1978;37:378–381. doi: 10.1136/ard.37.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dumonceau JM, Riphaus A, Aparicio JR, Beilenhoff U, Knape JT, Ortmann M, Paspatis G, Ponsioen CY, Racz I, Schreiber F, et al. European Society of Gastrointestinal Endoscopy, European Society of Gastroenterology and Endoscopy Nurses and Associates, and the European Society of Anaesthesiology Guideline: Non-anesthesiologist administration of propofol for GI endoscopy. Endoscopy. 2010;42:960–974. doi: 10.1055/s-0030-1255728. [DOI] [PubMed] [Google Scholar]
- 17.Perel A. Non-anaesthesiologists should not be allowed to administer propofol for procedural sedation: a Consensus Statement of 21 European National Societies of Anaesthesia. Eur J Anaesthesiol. 2011;28:580–584. doi: 10.1097/EJA.0b013e328348a977. [DOI] [PubMed] [Google Scholar]
- 18.Axon AE. The use of propofol by gastroenterologists: medico-legal issues. Digestion. 2010;82:110–112. doi: 10.1159/000285570. [DOI] [PubMed] [Google Scholar]
- 19.McQuaid KR, Laine L. A systematic review and meta-analysis of randomized, controlled trials of moderate sedation for routine endoscopic procedures. Gastrointest Endosc. 2008;67:910–923. doi: 10.1016/j.gie.2007.12.046. [DOI] [PubMed] [Google Scholar]
- 20.Horiuchi A, Nakayama Y, Fujii H, Katsuyama Y, Ohmori S, Tanaka N. Psychomotor recovery and blood propofol level in colonoscopy when using propofol sedation. Gastrointest Endosc. 2012;75:506–512. doi: 10.1016/j.gie.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 21.Marshall SI, Chung F. Discharge criteria and complications after ambulatory surgery. Anesth Analg. 1999;88:508–517. doi: 10.1097/00000539-199903000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Padmanabhan U, Leslie K, Eer AS, Maruff P, Silbert BS. Early cognitive impairment after sedation for colonoscopy: the effect of adding midazolam and/or fentanyl to propofol. Anesth Analg. 2009;109:1448–1455. doi: 10.1213/ane.0b013e3181a6ad31. [DOI] [PubMed] [Google Scholar]
- 23.Ead H. From Aldrete to PADSS: Reviewing discharge criteria after ambulatory surgery. J Perianesth Nurs. 2006;21:259–267. doi: 10.1016/j.jopan.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Amornyotin S, Chalayonnavin W, Kongphlay S. Recovery pattern and home-readiness after ambulatory gastrointestinal endoscopy. J Med Assoc Thai. 2007;90:2352–2358. [PubMed] [Google Scholar]


