SUMMARY
Dormancy is a crucial adaptation allowing insects to withstand harsh environmental conditions. The pre-programmed developmental arrest of diapause is a form of dormancy that is distinct from quiescence, in which development arrests in immediate response to hardship. Much progress has been made in understanding the environmental and hormonal controls of diapause. However, studies identifying transcriptional changes unique to diapause, rather than quiescence, are lacking, making it difficult to disentangle the transcriptional profiles of diapause from dormancy in general. The Asian tiger mosquito, Aedes albopictus, presents an ideal model for such a study, as diapausing and quiescent eggs can be staged and collected for global gene expression profiling using a newly developed transcriptome. Here, we use RNA-Seq to contrast gene expression during diapause with quiescence to identify transcriptional changes specific to the diapause response. We identify global trends in gene expression that show gradual convergence of diapause gene expression upon gene expression during quiescence. Functionally, early diapause A. albopictus show strong expression differences of genes involved in metabolism, which diminish over time. Of these, only expression of lipid metabolism genes remained distinct in late diapause. We identify several genes putatively related to hormonal control of development that are persistently differentially expressed throughout diapause, suggesting these might be involved in the maintenance of diapause. Our results identify key biological differences between diapausing and quiescent pharate larvae, and suggest candidate pathways for studying metabolism and the hormonal control of development during diapause in other species.
KEY WORDS: RNA-Seq, diapause, invasive species, metabolism, quiescence, Aedes albopictus
INTRODUCTION
Seasonal transitions require insects to respond to harsh environmental changes in order to survive. Diapause is an alternative developmental program that is initiated in response to a token stimulus, often photoperiod, that occurs well in advance of physiologically limiting environmental factors. Physiological changes during diapause result in developmental arrest, metabolic restructuring and stress tolerance, which allows insects to withstand seasonally occurring environmental insults, such as the harsh conditions of winter (Tauber and Tauber, 1976). For many insect species, developmental suppression continues after the physiological limitation has been lifted, until specific environmental changes or endogenous processes lead to diapause termination (Koštál, 2006).
Because of the paramount adaptive importance of diapause for insect survival during seasonal change, there has been a sustained interest in diapause physiology, which has led to the discovery of many of its environmental and hormonal controls (Denlinger, 2002; Denlinger et al., 2005). However, knowledge of the molecular regulation of diapause is only beginning to be thoroughly explored, in part because the stage in which diapause is expressed varies among insects, complicating efforts to identify common mechanisms of regulation (Denlinger, 2002). Additionally, the traditional model insect of choice, Drosophila melanogaster, has only a weak diapause phenotype (Emerson et al., 2009; Schmidt et al., 2005; but see Williams et al., 2010), minimizing the utility of this classical system for research on the molecular basis of diapause. Global gene expression profiling using microarrays or RNA sequencing (RNA-Seq) is increasingly being applied to ‘non-model’ insects – that are nonetheless excellent experimental systems for diapause – and has enabled substantial progress in documenting important transcriptional changes throughout diapause (Bao and Xu, 2011; Emerson et al., 2010; Poelchau et al., 2013b; Ragland et al., 2010; Ragland et al., 2011).
In contrast to diapause, quiescence is an alternative form of insect dormancy, in which physiological processes halt in immediate response to the reduction of an environmental, physiologically limiting factor (Hand and Podrabsky, 2000; Koštál, 2006). Once the environmental factor returns to non-limiting levels, normal activity is resumed. Both diapause and quiescence present important adaptations to avoid environmental exigencies, but there are important distinctions between these two forms of dormancy. While quiescence occurs in immediate response to an unpredictable environmental change, diapause is induced in advance of seasonally recurring changes, has an extended preparatory period, often over more than a generation, and the developmental arrest of diapause cannot be broken by an external stimulus until diapause has terminated. Metabolism is depressed in both types of dormancy, but the mode of depression can differ (Hand and Podrabsky, 2000). Diapausing and quiescent individuals can also have other important phenotypic differences that likely relate to different strategies of energy metabolism, such as differences in lipid content, the time interval required for achieving metabolic depression, and desiccation resistance (e.g. Hand and Podrabsky, 2000; Reynolds et al., 2012; Urbanski et al., 2010).
Gene expression profiling studies that have sought to identify transcriptional distinctions of diapause have usually characterized transitions between diapause stages (i.e. pre-diapause versus diapause versus post-diapause) (Bao and Xu, 2011; Emerson et al., 2010; Ragland et al., 2010; Ragland et al., 2011; but see Reynolds and Hand, 2009b). These studies have revealed important insights into the degree and mode of metabolic depression and developmental arrest, and accompanying physiological changes, in diapausing insects. However, an equally important question is how diapause is transcriptionally distinct from quiescence, and how this distinction changes throughout the dynamic progression of diapause (i.e. from early to late stages of developmental arrest). This comparison may yield alternative insights into the molecular distinctions of diapause that could be missed in other experimental designs, because two states of dormancy are being contrasted.
The Asian tiger mosquito, Aedes albopictus (Skuse), presents an excellent model system to identify molecular components of diapause. Aedes albopictus is a highly invasive vector species (Benedict et al., 2007) that enters diapause as a pharate larva within the chorion of the egg (Mori et al., 1981; Wang, 1966). Temperate populations of A. albopictus undergo a photoperiodic diapause in which a ‘short-day’ photoperiod experienced during the maternal pupal and adult stages stimulates the production of offspring destined for diapause. How diapause terminates in A. albopictus is not clear, but a certain period of time must elapse before diapause is broken [up to several months (Pumpuni, 1989), and in our laboratory, ca. 60 days], and this period can be influenced by temperature and photoperiod (Pumpuni, 1989). Like many other insect species (Hodek, 1996; Tauber et al., 1986), in A. albopictus diapause termination is followed by a period of post-diapause quiescence, which ends when environmental conditions that are favorable for direct development (i.e. immersion in water and high temperatures) stimulate direct development (i.e. hatching of the pharate larva from the egg). In contrast, maternal mosquitoes that experience a ‘long-day’ photoperiod oviposit eggs capable of quiescence: once embryonic development is complete, in the absence of a hatch stimulus, fully developed pharate larvae remain dormant within the eggs. This state of dormancy is distinct from diapause, because quiescent larvae will immediately hatch once the appropriate stimulus is received [e.g. flooding (reviewed in Hawley, 1988)]. Because the two types of dormancy are easily induced in A. albopictus, staged quiescent and diapause eggs can be easily matched and gene expression compared (Fig. 1). Additionally, there are substantial genomic resources available for expression studies in A. albopictus: while as yet there is no genome sequence available, the genome and accompanying annotations of the closely related Aedes aegypti (Nene et al., 2007) provide a powerful resource for global gene expression studies of A. albopictus (Poelchau et al., 2013a; Poelchau et al., 2013b; Poelchau et al., 2011).
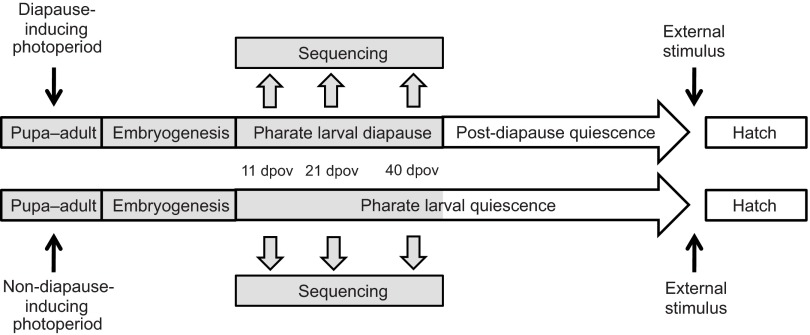
Fig. 1.
Illustration of the experimental design in the context of Aedes albopictus diapause development. Areas shaded in gray refer to the stages used in the experimental design. 11, 21 and 40 dpov refer to the embryo collection dates of 11, 21 and 40 days post-oviposition.
Here, we identify global gene expression differences between diapausing and quiescent A. albopictus at three separate time points representing early, middle and late diapause. We find that gene expression patterns converge over time between the two states of dormancy to a quiescence expression profile. We identify key metabolic distinctions between diapause and quiescence that are important early in diapause, of which only differences in lipid metabolism remain throughout the course of diapause. Finally, we identify several genes with putative hormonal functions that are implicated throughout diapause, suggesting future avenues of investigation of the hormonal control of diapause maintenance.
MATERIALS AND METHODS
Experimental design
The experimental design (Fig. 1), insect rearing and RNA extraction have been described in a previous paper (Poelchau et al., 2013a). Tissue was generated from a laboratory F13 A. albopictus strain collected from Manassas, VA, USA. Larvae were reared at 21°C, ca. 80% relative humidity and a 16 h:8 h light:dark photoperiod until pupation (see Armbruster and Hutchinson, 2002; Armbruster and Conn, 2006). At pupation, mosquitoes were transferred to adult cages maintained under either diapause-inducing, short-day (D; 8 h:16 h light:dark) or non-diapause-inducing, long-day photoperiod treatments (ND; 16 h:8 h light:dark). We established four separate 9.5 liter adult cages (biological replicates) for each photoperiod treatment (D, ND) with ca. 100 mosquitoes per cage. Females were blood-fed on a human host 9–16 days after eclosion, and ca. 7 and 14 days thereafter. Egg collection to provide pharate larvae for RNA extraction and diapause measurements began 3 days after the first blood-feeding. Females were allowed to oviposit into a small brown jar lined with unbleached seed germination paper and half-filled with ca. 50 ml deionized water, which was placed into each cage 6–7 h after lights on. Egg papers were removed and replaced every 24 h for 26 days. Collected eggs were slowly air-dried on the papers 72 h after removal, and kept at 80% relative humidity, a 8 h:16 h light:dark cycle and 21°C until further use. We note that diapausing pharate larvae may be more metabolically active at 21°C than at lower temperatures, but this temperature is still ecologically relevant, because diapausing pharate larvae spend considerable time at higher temperatures during the fall before the onset of winter. Additionally, we are able to eliminate confounding effects of temperature by maintaining pharate larvae from all treatments at the same temperature. Eggs designated for RNA extraction were snap-frozen in liquid nitrogen and stored at −80°C at 11 (early diapause), 21 (mid-diapause) or 40 (late diapause or post-diapause quiescence) days post-oviposition (dpov; counted from the start of the oviposition period). Frozen eggs from each photoperiod, time point and replicate were ground in TRI Reagent (Sigma-Aldrich, St Louis, MO, USA), followed by RNA extraction according to the manufacturer's instructions. DNA was removed using Turbo-DNAfree (Applied Biosystems/Ambion, Austin, TX, USA). Three biological replicates from each photoperiod and development stage were chosen from the four available replicates based on RNA quality and quantity, as measured on an RNA chip (Bioanalyzer 2100, Agilent Technologies, Santa Clara, CA, USA). Only two biological replicates for 40 dpov pharate larvae reared on an ND photoperiod were chosen because of low RNA quality in the remaining replicates. Specific libraries chosen for each time period are listed in supplementary material Table S1. Incubator malfunction resulted in temperature irregularities for some 40 dpov eggs (ca. 4°C fluctuations on three consecutive days), but we discarded eggs scheduled for snap-freezing on these days. Furthermore, these temperature fluctuations should not result in systematic differences in gene expression between ND and D treatments, because ND and D eggs were stored together and experienced the same environmental conditions throughout the experiment.
For diapause incidence measurements, for each biological replicate, 14- to 28-day-old eggs were hatched, the number of hatched larvae recorded, and the egg papers with remaining, un-hatched eggs re-dried. This procedure was repeated twice, after 7 and 14 days. The remaining eggs were bleached (Trpis, 1970) to visualize and record the number of embryonated but unhatched (diapause) eggs. Diapause incidence was calculated as: (no. embryonated unhatched eggs)/(no. hatched eggs + no. embryonated unhatched eggs) (Urbanski et al., 2012). Percent embryonation was calculated as: (no. embryonated unhatched eggs + no. hatched eggs)/total no. eggs.
Sequence assembly and annotation
Sequencing, assembly and annotation are described in detail in Poelchau et al. (Poelchau et al., 2013a). Briefly, paired-end, barcoded Illumina mRNA-Seq libraries were constructed from each of the 17 RNA samples, and a proportion of each library was sequenced on three lanes on an Illumina HiSeq 2000 sequencer by the University of Maryland Genomics Institute. Cleaned reads were assembled into contigs after digital normalization (C. T. Brown, A. Howe, Q. Zhang, A. B. Pyrkosz and T. H. Brom, unpublished, arXiv:1203.4802) using Velvet (Zerbino and Birney, 2008) and Oases (Schulz et al., 2012). Contigs were then merged with two previous assemblies (Poelchau et al., 2011; Poelchau et al., 2013b) using a reference-based assembly approach outlined in Poelchau et al. (Poelchau et al., 2013a). Resulting contigs were annotated based on protein models from A. aegypti, Culex quinquefasciatus, Anopheles gambiae and D. melanogaster, and based on the A. aegypti genome sequence (Nene et al., 2007). Raw reads are available in NCBI's short read archive under accession number SRA063587, and the assembly can be downloaded at http://www.albopictusexpression.org/?q=data.
Gene expression analysis
Transcriptome assemblies without a genomic reference will generate redundant contigs for each identified gene model, in part because of allelic variation and/or alternative splicing. To account for this redundancy in our gene expression calculations, we used the program RSEM v.1.2.0 (Li and Dewey, 2011) to generate composite gene expression measures for each identified gene model. We mapped cleaned read pairs to the A. albopictus transcriptome using the program's default parameters.
Read counts were processed with the program edgeR (Robinson and Oshlack, 2010) in the R software environment (www.r-project.org). Only contigs with annotations to proteins or A. aegypti genome features based on gene set AaegL1.2 (as opposed to un-annotated A. aegypti genome sequence) were used in all subsequent analyses, as these required functional annotations. Additionally, gene models with fewer than two counts per million reads across all libraries were removed from the analysis, as these are not likely to show statistically significant differential expression (see Robinson et al., 2010). Read counts were TMM normalized (Robinson and Oshlack, 2010), which accounts for library size and expression bias, and log2 fold-change and its significance was calculated for each gene model between D and ND libraries for each time point (11, 21 and 40 dpov). We classified a gene as differentially expressed (DE) if its absolute log2 fold-change was greater than 0.5, with a Benjamini–Hochberg-corrected P<0.05. Previous RNA-Seq studies performed in our laboratory using the same A. albopictus strain, sequencing center and normalization methods show strong congruence with qRT-PCR results (Poelchau et al., 2013b), and RNA-Seq expression data have repeatedly been shown to produce accurate gene expression estimates, given proper normalization (Bullard et al., 2010; Feng et al., 2010; Fu et al., 2009; Poelchau et al., 2013b).
A distance matrix of gene expression patterns (R function dist) was summarized using multi-dimensional scaling (R function cmdscale) after transformation for linear modeling via the function voom in limma (Smyth, 2004; Smyth, 2005). Standardized expression patterns of all DE genes were also visualized as Z-scores in heat maps generated by hierarchical clustering (function hclust in R). Variability of gene expression within all D and ND DE genes, calculated as coefficients of variation (CV), was assessed via a Wilcoxon signed-rank test. All expression information is available at http://www.albopictusexpression.org/?q=data.
Gene Ontology and Kegg pathway enrichment analyses
Global gene expression data sets, such as those derived from RNA-Seq experiments, can provide insights into changes involving functionally related groups of genes that underlie specific physiological processes, e.g. Gene Ontology (GO) categories (Ashburner et al., 2000) or Kegg pathways (Kanehisa and Goto, 2000; Kanehisa et al., 2012). We asked whether these functional groups were over-represented among DE genes at each time period using the program GOseq, which corrects enrichment analyses for biases arising from variable transcript lengths in RNA-Seq data sets (Young et al., 2010). We also performed the same analysis for genes that were DE throughout all three time points in order to identify functional groups of genes that were differentially expressed throughout diapause. Generic GO Slim assignments for each gene model were downloaded from EnsemblMetazoa BioMart (Haider et al., 2009), and Kegg pathway assignments from http://www.genome.jp/kegg/. In addition to the suite of GO Slim categories and Kegg pathways, we manually composed gene lists representative of pathways or physiological processes with likely relevance for diapause in A. albopictus based on gene expression studies from other organisms (following Poelchau et al., 2013b): insulin signaling, which is instrumental for insect growth and metabolism (see Ragland et al., 2010; Wu and Brown, 2006); ecdysone signaling [from ‘molting’ genes in Brody (Brody, 1999)]; and heat shock proteins (Hsps), which are a subset of the gene ontology category ‘response to stress’ (GO:0006950). Uncorrected P-values from the GOseq analysis were Benjamini–Hochberg-corrected for multiple testing using the p.adjust function in limma (Smyth, 2004; Smyth, 2005). Functional groups with corrected P-values <0.05 and five or more DE genes were considered significantly enriched.
RESULTS
Diapause incidence
Diapause incidence of each biological replicate ranged from 87.5 to 100% in the diapause-inducing photoperiod treatments, and percent hatch ranged from 77.4 to 82.9% in the non-diapause treatment (supplementary material Table S1) (Poelchau et al., 2013a). Embryonation ranged from 82.9 to 98.9% across all replicates. Diapause incidence was not 100% for all replicates, indicating that a mixture of mostly diapause, but some quiescent, pharate larvae were sequenced in the D libraries at some time points. However, this is not likely to generate spurious results, but rather makes our analysis more conservative, as fewer genes are likely to be detected as DE.
Gene expression analysis
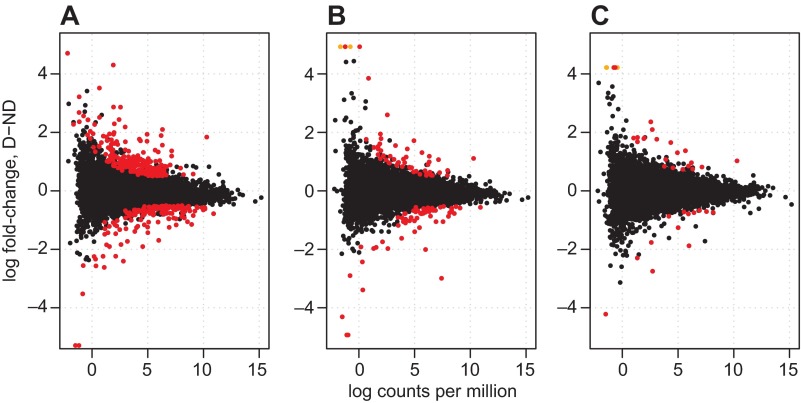
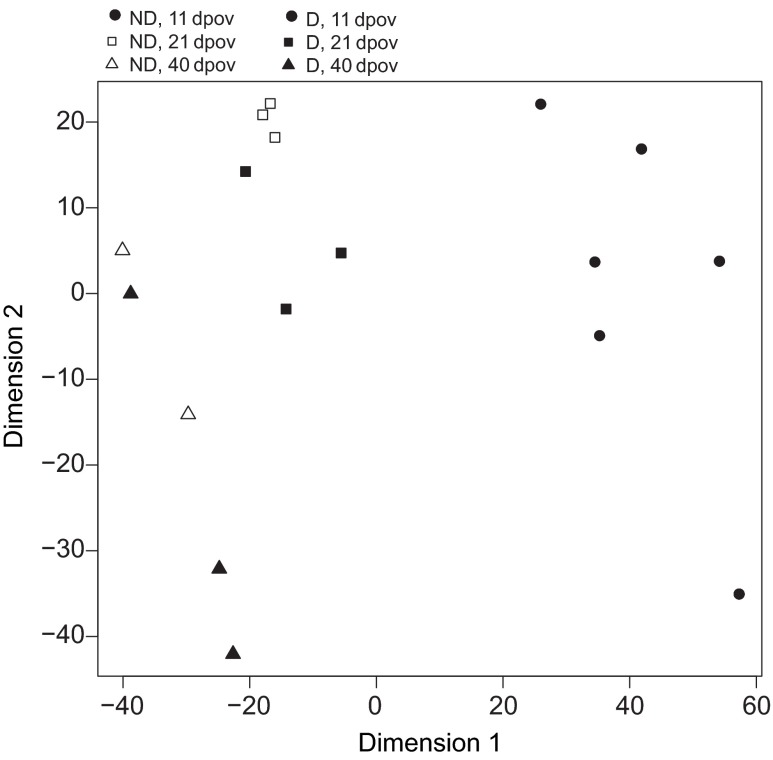
The number of genes that were DE between D and ND conditions decreased from early to middle to late diapause (Fig. 2, Table 1). Multi-dimensional scaling of the gene expression results clustered libraries generally by day post-oviposition and photoperiod treatment, although one D library at 40 dpov clustered with the two ND libraries (Fig. 3). The first MDS axis appeared to separate libraries by day post-oviposition (explaining 37.6% of the variation in the data), with the libraries clustering in chronological order, and tighter clustering occurring between the 21 and 40 dpov libraries. Also, the 11 dpov D libraries were located towards the ‘earlier’ side of the axis relative to the 11 dpov ND libraries. This is interesting, given previous observations from A. albopictus that suggest a developmental delay of embryos during diapause preparation (Poelchau et al., 2013b). The 21 and 40 dpov libraries, in contrast, show no such temporal separation between the D and ND treatments. The second axis, which explains 14.1% of the variation, roughly separated the libraries by photoperiod. Taken together, these results indicate that gene expression during A. albopictus quiescence and diapause converges over time. Normalized read counts, log-fold changes and their P-values, and descriptions of all genes in the data set are available in supplementary material Table S2.
Fig. 2.
Log fold-change expression versus log abundance of TMM-normalized gene expression at (A) 11, (B) 21 and (C) 40 days post-oviposition. Each point represents an individual gene. Genes with higher expression under diapausing (D) conditions have positive fold-change values, and genes with higher expression under non-diapausing (ND) conditions have negative fold-change values. Genes that qualified as significantly differentially expressed (corrected P<0.05; absolute log2 fold-change >0.5) are in red, and genes that are significantly differentially expressed, but are only expressed in one of the two conditions are in orange.
Table 1.
Functional categories that were significantly enriched for differentially expressed (DE) genes at 11, 21 and 40 days post-oviposition (dpov), as well as groups enriched in DE genes throughout all three time periods (‘DE throughout’)
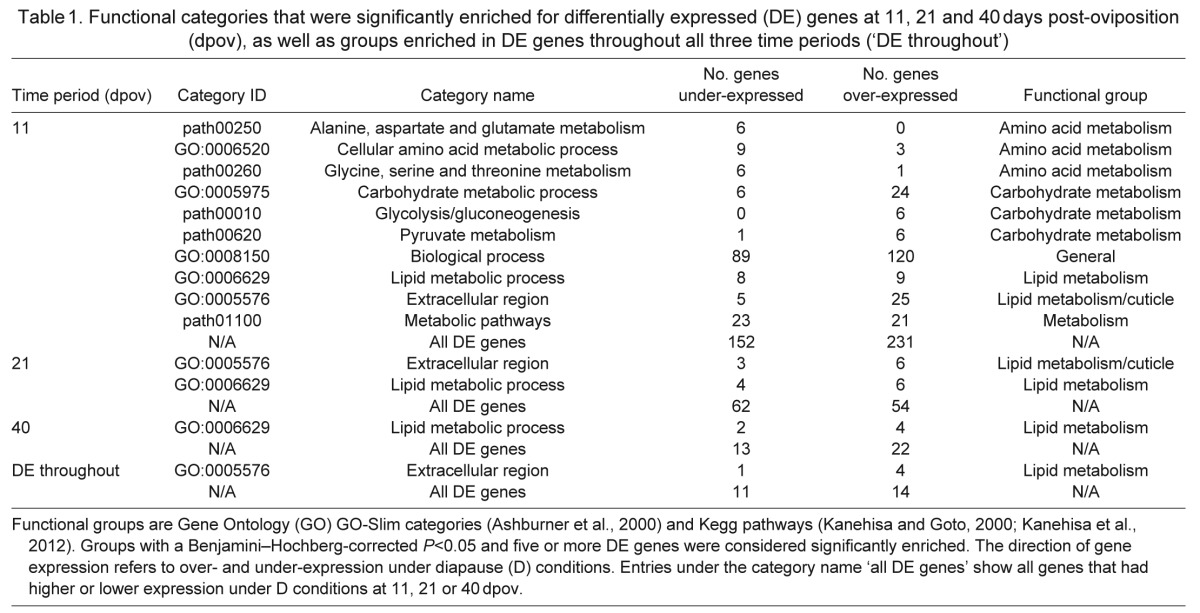
Fig. 3.
Multi-dimensional scaling plot representing distances between expression profiles of each library across photoperiod treatments and development times (see Materials and methods for details). D and ND represent diapause-inducing photoperiods and non-diapause-inducing photoperiods, respectively; 11, 21 and 40 dpov refer to pharate larval collection at 11, 21 or 40 days post-oviposition.
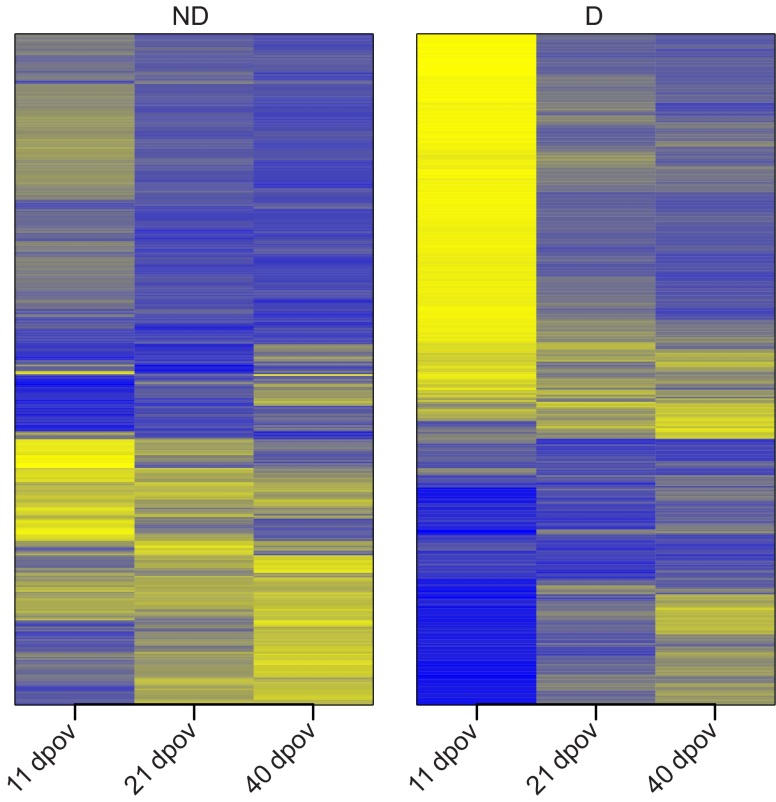
We visualized gene expression of all DE genes to identify trends in their expression convergence over time. Heat maps of standardized expression scores suggested that change in expression over time is driven by change during diapause, not quiescence (Fig. 4). To determine whether the decrease in the number of DE genes over time was driven more by change in ND or D expression, we asked whether the coefficient of variation (CV) in gene expression across time periods differed between D and ND genes. D genes had higher CVs that differed significantly from ND genes (paired Wilcoxon-rank-sum test, P<2.2e–16; ND, mean CV=0.357, D, mean CV=0.453), indicating that gene expression change over time occurs more in D rather than in ND pharate larvae.
Fig. 4.
Heat maps of A. albopictus differentially expressed genes at 11, 21 and 40 days post-oviposition (dpov) from first-instar larvae generated from females reared under diapause-inducing (D) and non-diapause-inducing (ND) photoperiods. Expression values are depicted as Z-standardized scores for each gene, where blue represents low expression and yellow represents high expression.
Gene Ontology and Kegg pathway enrichment analyses
The enrichment analyses overwhelmingly point to differential regulation of metabolic processes as a key distinction between diapause and quiescence in A. albopictus. The number of enriched processes decreased over time, reflecting the decreasing number of DE genes at each time point (Table 1). In addition, these categories converged in function: the category ‘lipid metabolism’ remained enriched across time periods, and was the only enriched category remaining at 40 dpov. We describe the categories at individual time points below.
A diversity of metabolic processes were enriched for DE genes at 11 dpov (Table 1). Several were related to amino acid metabolism, with most genes under-expressed in D conditions. In contrast, genes involved in carbohydrate metabolism, including pyruvate metabolism and glycolysis/gluconeogenesis, were primarily over-expressed. Genes involved in lipid metabolism had mostly higher expression under D conditions, although the direction of expression was mixed. The category ‘extracellular region’ was mainly comprised of lipid metabolism genes, and genes that contained chitin-binding domains. The categories ‘biological process’ and ‘metabolic pathways’ have a strong overlap in gene composition with other categories, and will not be discussed further.
At 21 dpov, the categories ‘lipid metabolism’ and ‘extracellular region’ were enriched for DE genes, where ‘extracellular region’ genes were primarily lipases and genes with chitin-binding domains. At 40 dpov, only ‘lipid metabolism’ remained enriched. Similar to 11 dpov, gene expression tended to be higher under D conditions for all of these categories at 21 and 40 dpov.
Twenty-five genes were DE throughout all three time points (Table 1), and all were expressed in the same direction continuously. The set of DE genes was enriched for genes in the category ‘extracellular region’, which contained two lipases, two triacyl-glycerol lipases and one conserved hypothetical protein containing conotoxin domains, indicating that most of the genes were involved in lipid metabolism. This is consistent with the enrichment of ‘lipid metabolism’ at all three time points (see above; Table 1). While not contributing to an enriched category, three genes related to juvenile hormone (JH) binding and metabolism were also DE: a putative JH-inducible protein, AAEL012680, under-expressed in D conditions; an ortholog of juvenile hormone esterase (JHE), AAEL005200, over-expressed; and a gene containing a JH binding protein domain, AAEL000500, over-expressed (supplementary material Table S3).
DISCUSSION
In this study, we contrast gene expression patterns throughout A. albopictus diapause and quiescence, two alternative developmental states for pharate first instar larvae, in order to identify transcriptional regulation of processes relevant to diapause. To our knowledge, this is the first study to identify global transcriptional components of diapause maintenance for pharate larval diapause, and the first RNA-Seq study to use staged comparisons of diapause versus quiescence to identify diapause-enriched transcripts. These comparisons allowed us to demonstrate on a global scale how gene expression patterns during A. albopictus diapause maintenance reflect biological distinctions of A. albopictus diapause from quiescence. Aedes albopictus eggs are refractory to hatching stimuli during diapause, yet return to a period of post-diapause quiescence. In turn, A. albopictus gene expression patterns demonstrate convergence of diapause upon quiescence over time (Figs 2, 4), rather than achieving a state entirely distinct from quiescence once diapause is broken. We note that because diapause was only measured once for each replicate between 14 and 28 dpov, the transcriptional changes between 11 and 40 dpov could be due to diapause termination leading to post-diapause quiescence in a proportion of individuals in the diapause group. Previous experiments suggest that most eggs terminate diapause between 30 and 60 dpov under the conditions utilized in this experiment (Pumpuni, 1989) (P.A.A., unpublished data). Thus, transcriptional convergence between the diapause and quiescence profiles could be driven by transcriptional change during diapause and/or by diapause loss itself. However, regardless of the mechanism, diminishing gene expression differences over time are likely to reflect a physiological convergence of diapause towards quiescence. This observation supports the model of diapause as a dynamic process, rather than a static condition (Denlinger, 2002; Koštál, 2006; Tauber et al., 1986).
Metabolic gene expression is the main transcriptional distinction between early diapause and quiescence (Table 1). This general result corroborates findings from many other gene expression studies of insect diapause, which have documented profound transcriptional changes related to metabolism in diapausing larvae, pupae and adult insects (e.g. Emerson et al., 2010; Ragland et al., 2010; Ragland et al., 2011; Reynolds et al., 2012), and many other physiological studies that have outlined various mechanisms of metabolic restructuring during diapause (reviewed in Hahn and Denlinger, 2007; Hahn and Denlinger, 2011; Hand et al., 2011). In general, diapausing animals survive extended periods of developmental arrest by increasing nutrient stores during diapause preparation, and reducing metabolism during developmental arrest. How these metabolic changes are achieved differs among species: insects vary in the degree that metabolism is suppressed during diapause (Chaplin and Wells, 1982; Denlinger et al., 1972; Reynolds and Hand, 2009a), and in the specific nutrient compositions that are stored and later utilized, although energy stores in form of triacylglycerides (Danks, 1987; Hahn and Denlinger, 2007), glycogen (Danks, 1987; Zhou and Miesfeld, 2009) and specialized storage proteins (Burmester, 1999; Denlinger et al., 2005) are common. Below, we discuss gene expression changes underlying the metabolism of different types of nutrient stores during diapause.
Carbohydrate metabolism
Glycolysis is the first step in generating metabolic energy from glucose. Gluconeogenesis reverses the glycolytic process, generating glucose from pyruvate, and uses many of the steps of glycolysis in reverse. Upregulation of the gluconeogenetic process has been implicated in previous studies of diapause gene expression (Baker and Russell, 2009; Emerson et al., 2010; Ragland et al., 2010; Ragland et al., 2011), and in a diapause context is considered consistent with reliance on anerobic metabolism (Hahn and Denlinger, 2011). At 11 dpov, both the glycolysis/gluconeogenesis pathway and the pyruvate metabolism pathway were enriched for DE genes, most of which had higher expression in diapause (Table 1). Pepck (phosphoenolpyruvate carboxykinase; AAEL000006, AAEL000080), which encodes a rate-limiting enzyme in gluconeogenesis, and a gapdh homolog (glyceraldehyde 3 phosphate dehydrogenase; AAEL016984) had higher expression under diapausing conditions. These results are consistent with upregulation of gluconeogenesis, which suggests a shift towards anerobic metabolism. In contrast, pyk (pyruvate kinase; AAEL012576, AAEL014913) also had higher expression, which should indicate reliance on glycolysis, because PyK converts phosphoenolpyruvate to the end-product of the glycolysis pathway, pyruvate. However, these results are not necessarily contradictory, as PyK can be inhibited via post-translational modification under fasting conditions (Feliú et al., 1976; Llorente et al., 1970).
In addition to A. albopictus, a diverse range of other organisms show upregulation of pepck during diapause in gene expression scans, such as Sarcophaga crassipalpis, Rhagoletis pomonella, Wyeomia smithii and Caenorhabditis elegans (Emerson et al., 2010; McElwee et al., 2006; Ragland et al., 2010; Ragland et al., 2011), suggesting that this enzyme may have a ubiquitous role in the metabolic restructuring of diapausing animals. In A. albopictus, pepck expression is high throughout diapause induction (Poelchau et al., 2011), preparation (Poelchau et al., 2013b) and early diapause. Collectively, our results on pepck suggest a reliance on anerobic metabolism in preparation for diapause and during early diapause that exceeds that of quiescent pharate larvae.
Amino acid metabolism
Amino acid metabolic pathways synthesize proteins, hormones and enzymes; they can also degrade amino acids to generate metabolic intermediates of glucose to be used in the citric acid cycle (Klowden, 2007). Amino acids are thought to mediate cold and desiccation resistance during diapause (e.g. Michaud and Denlinger, 2007) or to play a role in nutrient storage (Morgan and Chippendale, 1983). Several amino acid metabolic pathways were enriched at 11 dpov, mostly for genes with lower expression under D conditions (Table 1, supplementary material Table S4). Many of these genes are involved in glutamine, glycine and serine metabolism (supplementary material Table S4). These results suggest a downregulation of these pathways, which could result in (1) lower provisioning of the citric acid cycle with metabolic intermediates, consistent with a shift towards anaerobic metabolism, and (2) higher concentrations of amino acids because of decreased degradation. Consistent with this interpretation, preliminary metabolomics data from A. albopictus show higher levels of amino acids in diapausing versus non-diapausing eggs (leucine, serine, threonine, tyrosine, lysine and proline; data not shown). In general, these data point towards a key role of amino acids in early A. albopictus diapause that is consistent with increased anaerobic metabolism and increased cold and desiccation resistance.
Lipid metabolism
Lipids can serve as a fundamental energy source for diapausing insects, and are the primary fuel for embryonic development (Arrese and Soulages, 2010; Van Handel, 1993). Because of their high caloric content and water yield, they store energy more efficiently than carbohydrate-based sources (Hahn and Denlinger, 2011). Diapausing animals, which often do not feed and thus must rely on stored nutrients for survival, can be provisioned with higher lipid reserves, in particular triacylglycerides, than their non-diapause counterparts (Danks, 1987; Hahn and Denlinger, 2007; McElwee et al., 2006; Tauber et al., 1986). These stores can then be metabolized during diapause via lipases, which catalyze the hydrolysis of triacylglycerides, to generate energy. Diapausing 11 dpov pharate larvae were enriched for lipid metabolism genes (Table 1), especially genes involved in lipid store mobilization, such as lipases and hydrolases (supplementary material Table S4). Expression patterns of these genes were mixed; however, the majority of lipases, in particular the triacylglycerol lipases, were upregulated rather than downregulated, suggesting that diapausing A. albopictus pharate larvae metabolize lipid stores as an energy source at this stage. Reliance on lipid stores as an energy source during diapause is consistent with a previous physiological study of A. albopictus: 10- to 14-day-old A. albopictus eggs contained ~30% more total lipid than quiescent eggs, and pre-diapause embryos showed expression evidence of lipid storage relative to non-diapause embryos (Reynolds et al., 2012).
Lipid metabolism persisted as a distinct feature of all sampled diapause stages: lipid metabolism genes were enriched throughout all sampled time periods (Table 1), and remained primarily over-expressed under diapause conditions. This points to a consistent role of lipids as an energy store provisioning pharate larvae throughout diapause, relative to quiescence. Other studies in diapausing insects have indicated different temporal profiles of lipid metabolism across diapause: for example, in the adult diapause of the mosquito Culex pipiens, lipase expression is low in early diapause, then increases in late diapause (Sim and Denlinger, 2009). The cotton bollworm Helicoverpa armigera also downregulates lipase expression in early diapause, presumably to promote lipid storage for use as an energy source later in diapause (Bao and Xu, 2011). Our data indicate that at 11 dpov, lipids are already an important source of energy for diapausing A. albopictus, suggesting that the relative importance of this energy source is higher than for other species.
Hormone action during diapause maintenance
Insect hormones play a fundamental role in the control of development (Fraenkel, 1935; Riddiford, 1994; Wigglesworth, 1934) and diapause (Denlinger, 2002). The relative abundance of two major hormones, ecdysone and JH, during development dictates the developmental progression of the insect (Klowden, 2007; Riddiford, 1994). Changes in the relative and absolute abundance of JH or ecdysone are known to be important during the initiation, maintenance and termination of diapause in many insect species, but the nature of these changes will depend on the life-cycle stage of diapause developmental arrest (Denlinger, 2002). For example, ecdysteroids play a regulatory role in the pharate larval diapause of the gypsy moth Lymantria dispar (Lee and Denlinger, 1997; Lee et al., 1997), whereas JH mediates the hormonal control of adult diapause in C. pipiens (Readio et al., 1999; Spielman, 1974).
The mechanisms of hormonal control of pharate larval diapause in A. albopictus are unknown. Previous transcriptome analyses of A. albopictus suggested a role for ecdysteroid signaling during the preparatory stage of diapause (Poelchau et al., 2013b; Poelchau et al., 2011). In our analysis of A. albopictus developmental arrest, we identified three genes (out of a total of 25) with putative functions related to JH action that had consistent differential expression across all time points (supplementary material Table S3). This conspicuous pattern would suggest that JH, or its absence, has a role in the maintenance of A. albopictus diapause. Endogenous JH production begins in late embryonic development, and its presence is thought to be important for dorsal closure, first-instar larval cuticle formation, and differentiation of the midgut (reviewed in Riddiford, 1994). JH continues to be present during larval feeding, inter-molt and molting phases, and its titer rises before the molt to the next larval instar. JH levels can be influenced by the environment: for example, starvation can increase JH titers (Truman et al., 2006). The JH titer is a function of JH synthesis and degradation. The enzyme that degrades JH is JHE (Klowden, 2007). A JHE homolog (AAEL005200), which has been verified experimentally in A. aegypti (Bai et al., 2007), was over-expressed under diapausing conditions, which would suggest it functions to keep JH levels low throughout diapause. Consistent with this pattern, a putative JH-inducible protein (AAEL012680), which should increase in expression under higher JH levels, was under-expressed, suggesting lower JH levels in diapausing pharate larvae. In contrast, a gene containing multiple JH binding domains (AAEL000500), which generally function to transport JH and protect it from degradation by JHEs, was over-expressed. Therefore, it is difficult to deduce a mode of JH action in the maintenance of diapause in A. albopictus. Accordingly, an experiment using adult females reared under short day-lengths did not show conclusive effects of JH topical application on subsequent hatching rates (Pumpuni, 1989). However, our data from the pharate larval stage strongly suggest that further research into JH as a regulatory hormone of diapause maintenance is worthwhile.
An inspection of the ‘biological process’ enriched category revealed a list of 15 members of the cytochrome P450 family (supplementary material Table S4). One of the diverse functions of the cytochrome P450 family is steroid hormone biosynthesis (Miller, 1988). Four of the cytochrome P450s were DE at both 11 and 21 dpov; and one of these, cyp18a1, encodes an enzyme that inactivates steroid hormones in D. melanogaster; loss-of-function mutations in D. melanogaster cause an extended final larval instar and lethality during metamorphosis (Guittard et al., 2011). Drosophila melanogaster cyp18a1 is also homologous to C. elegans daf-9, which regulates dauer, larval growth and longevity (Gerisch et al., 2001). Cyp18a1 had lower expression in diapausing A. albopictus pharate larvae, which is intriguing, given that daf-9 loss-of-function mutants form constitutive dauer larvae (Gerisch et al., 2001; Jia et al., 2002). Because of its conspicuous expression pattern – lower expression during early and mid-diapause, and lack of differential expression late in diapause – and because of the documented function of related genes, this gene represents a promising candidate for future studies into the hormonal regulation of diapause maintenance in A. albopictus.
Stress resistance
Diapausing insects use various mechanisms to tolerate adverse environmental conditions, such as extreme cold, aridity and hypoxia (Denlinger, 2002; MacRae, 2010). Hsps, in particular Hsp70, are often, but not always, upregulated in diapausing insects as protection against cold injury (Hayward et al., 2005; Rinehart et al., 2007). For example, one of the few studies contrasting gene expression during diapause versus post-diapause quiescence found that Hsp70 and Hsp23 were upregulated during Sarcophaga crassipalpis diapause (Hayward et al., 2005). Interestingly, this study also found close parallels between diapause and post-diapause quiescence: expression of these genes continued at a high level after diapause was broken, but before adult development resumed. We did not find conspicuous evidence for diapause upregulation of Hsps in our analysis of A. albopictus. This result aligns with findings from other insect species (Rinehart et al., 2007), such as C. pipiens, where Hsp70 was not upregulated in diapausing adults, despite the fact that diapausing individuals were more cold tolerant (Rinehart et al., 2006; Rinehart et al., 2007). These results suggest that other protective measures against low temperatures, such as amino acid provisioning (see ‘Amino acid metabolism’, above) or synthesis of classic cryoprotectants, distinguish A. albopictus diapause from quiescence. Finally, several immune-related genes – for example, homologs of a putative cecropin anti-microbial peptide and of Gram-negative binding proteins 3 and 4 – were over-expressed at 11 dpov (supplementary material Table S4), suggesting that pathogen defense may be particularly important in early diapausing pharate larvae. Similarly, several immune-responsive genes had higher expression in diapausing S. crassipalpis (Ragland et al., 2010). This suggests that higher investment in pathogen defense may be a common strategy during insect diapause.
Conclusions
In this study, we use gene expression profiling to gain fundamental insights into the molecular and physiological distinctions between diapause and quiescence in A. albopictus. Very little is known about the molecular mechanisms of diapause maintenance in pharate larval diapause. Because of the increasing importance of A. albopictus as a disease vector (Benedict et al., 2007), understanding the molecular regulation of this crucial life history trait could potentially provide a platform for novel vector control strategies based on the genetic or chemical disruption of diapause. We find gradual convergence of global diapause gene expression patterns towards quiescence. Metabolic differences, which are the primary distinguishing factor between early diapause and quiescent gene expression, decline over time to only include small differences in lipid metabolism, likely the main source of energy for diapausing pharate larvae. The data also suggest a role for juvenile hormone, and a member of the cytochrome P450 family, in facilitating diapause maintenance. With our experimental design, we can effectively characterize diapause as a physiological state distinct from quiescence, and therefore identify more subtle and likely important components of diapause that would be missed if compared with actively developing first-instar larvae.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the Armbruster, Elsik and Denlinger labs for helpful comments and suggestions on this work.
LIST OF SYMBOLS AND ABBREVIATIONS
- D
diapause-inducing photoperiod
- DE
differentially expressed
- dpov
days post-oviposition
- Hsp
heat shock protein
- JH
juvenile hormone
- JHE
juvenile hormone esterase
- ND
non-diapause-inducing photoperiod
- RNA-Seq
RNA sequencing
FOOTNOTES
Supplementary material available online at http://jeb.biologists.org/cgi/content/full/216/21/4082/DC1
COMPETING INTERESTS
No competing interests declared.
FUNDING
This work was supported by the National Institutes of Health [grant no. 5R21AI081041-02 to P.A.A., C.G.E. and D.L.D.] and Georgetown University. Deposited in PMC for release after 12 months.
REFERENCES
- Armbruster P. A., Conn J. E. (2006). Geographic variation of larval growth in North American Aedes albopictus (Diptera: Culicidae). Ann. Entomol. Soc. Am. 99, 1234-1243 [Google Scholar]
- Armbruster P. A., Hutchinson R. A. (2002). Pupal mass and wing length as indicators of fecundity in Aedes albopictus and Aedes geniculatus (Diptera: Culicidae). J. Med. Entomol. 39, 699-704 [DOI] [PubMed] [Google Scholar]
- Arrese E. L., Soulages J. L. (2010). Insect fat body: energy, metabolism, and regulation. Annu. Rev. Entomol. 55, 207-225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M., Ball C. A., Blake J. A., Botstein D., Butler H., Cherry J. M., Davis A. P., Dolinski K., Dwight S. S., Eppig J. T., et al. (2000). Gene Ontology: tool for the unification of biology. Nat. Genet. 25, 25-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai H., Ramaseshadri P., Palli S. R. (2007). Identification and characterization of juvenile hormone esterase gene from the yellow fever mosquito, Aedes aegypti. Insect Biochem. Mol. Biol. 37, 829-837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D. A., Russell S. (2009). Gene expression during Drosophila melanogaster egg development before and after reproductive diapause. BMC Genomics 10, 242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao B., Xu W. H. (2011). Identification of gene expression changes associated with the initiation of diapause in the brain of the cotton bollworm, Helicoverpa armigera. BMC Genomics 12, 224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict M. Q., Levine R. S., Hawley W. A., Lounibos L. P. (2007). Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis. 7, 76-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody T. (1999). The Interactive Fly: gene networks, development and the Internet. Trends Genet. 15, 333-334 [DOI] [PubMed] [Google Scholar]
- Bullard J. H., Purdom E., Hansen K. D., Dudoit S. (2010). Evaluation of statistical methods for normalization and differential expression in mRNA-Seq experiments. BMC Bioinformatics 11, 94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmester T. (1999). Evolution and function of the insect hexamerins. Eur. J. Entomol. 96, 213-225 [Google Scholar]
- Chaplin S. B., Wells P. H. (1982). Energy reserves and metabolic expenditures of monarch butterflies overwintering in Southern California. Ecol. Entomol. 7, 249-256 [Google Scholar]
- Danks H. V. (1987). Insect Dormancy: An Ecological Perspective. Ottowa, ON: Biological Survey of Canada (Terrestrial Arthropods) [Google Scholar]
- Denlinger D. L. (2002). Regulation of diapause. Annu. Rev. Entomol. 47, 93-122 [DOI] [PubMed] [Google Scholar]
- Denlinger D. L., Wilis J. H., Fraenkel G. (1972). Rates and cycles of oxygen consumption during pupal diapause in Sarcophaga flesh flies. J. Insect Physiol. 18, 871-882 [DOI] [PubMed] [Google Scholar]
- Denlinger D. L., Yocum G. D., Rinehart J. L. (2005). Hormonal control of diapause. In Comprehensive Molecular Insect Science (ed. Gilbert L., Iatrou K., Gill S.), pp. 615-650 Amsterdam: Elsevier Press; [Google Scholar]
- Emerson K. J., Uyemura A. M., McDaniel K. L., Schmidt P. S., Bradshaw W. E., Holzapfel C. M. (2009). Environmental control of ovarian dormancy in natural populations of Drosophila melanogaster. J. Comp. Physiol. A 195, 825-829 [DOI] [PubMed] [Google Scholar]
- Emerson K. J., Bradshaw W. E., Holzapfel C. M. (2010). Microarrays reveal early transcriptional events during the termination of larval diapause in natural populations of the mosquito, Wyeomyia smithii. PLoS ONE 5, e9574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliú J. E., Hue L., Hers H. G. (1976). Hormonal control of pyruvate kinase activity and of gluconeogenesis in isolated hepatocytes. Proc. Natl. Acad. Sci. USA 73, 2762-2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L., Liu H., Liu Y., Lu Z., Guo G., Guo S., Zheng H., Gao Y., Cheng S., Wang J., et al. (2010). Power of deep sequencing and agilent microarray for gene expression profiling study. Mol. Biotechnol. 45, 101-110 [DOI] [PubMed] [Google Scholar]
- Fraenkel G. S. (1935). A hormone causing pupation in the blowfly Calliphora erythrocephala. Proc. R. Soc. B 118, 1-12 [Google Scholar]
- Fu X., Fu N., Guo S., Yan Z., Xu Y., Hu H., Menzel C., Chen W., Li Y., Zeng R., et al. (2009). Estimating accuracy of RNA-Seq and microarrays with proteomics. BMC Genomics 10, 161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerisch B., Weitzel C., Kober-Eisermann C., Rottiers V., Antebi A. (2001). A hormonal signaling pathway influencing C. elegans metabolism, reproductive development, and life span. Dev. Cell 1, 841-851 [DOI] [PubMed] [Google Scholar]
- Guittard E., Blais C., Maria A., Parvy J. P., Pasricha S., Lumb C., Lafont R., Daborn P. J., Dauphin-Villemant C. (2011). CYP18A1, a key enzyme of Drosophila steroid hormone inactivation, is essential for metamorphosis. Dev. Biol. 349, 35-45 [DOI] [PubMed] [Google Scholar]
- Hahn D. A., Denlinger D. L. (2007). Meeting the energetic demands of insect diapause: nutrient storage and utilization. J. Insect Physiol. 53, 760-773 [DOI] [PubMed] [Google Scholar]
- Hahn D. A., Denlinger D. L. (2011). Energetics of insect diapause. Annu. Rev. Entomol. 56, 103-121 [DOI] [PubMed] [Google Scholar]
- Haider S., Ballester B., Smedley D., Zhang J. J., Rice P., Kasprzyk A. (2009). BioMart Central Portal – unified access to biological data. Nucleic Acids Res. 37, W23-W27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand S. C., Podrabsky J. E. (2000). Bioenergetics of diapause and quiescence in aquatic animals. Thermochim. Acta 349, 31-42 [Google Scholar]
- Hand S. C., Menze M. A., Borcar A., Patil Y., Covi J. A., Reynolds J. A., Toner M. (2011). Metabolic restructuring during energy-limited states: insights from Artemia franciscana embryos and other animals. J. Insect Physiol. 57, 584-594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley W. A. (1988). The biology of Aedes albopictus. J. Am. Mosq. Control Assoc. 1, Suppl., 1-39 [PubMed] [Google Scholar]
- Hayward S. A. L., Pavlides S. C., Tammariello S. P., Rinehart J. P., Denlinger D. L. (2005). Temporal expression patterns of diapause-associated genes in flesh fly pupae from the onset of diapause through post-diapause quiescence. J. Insect Physiol. 51, 631-640 [DOI] [PubMed] [Google Scholar]
- Hodek I. (1996). Diapause development, diapause termination and the end of diapause. Eur. J. Entomol. 93, 475-487 [Google Scholar]
- Jia K., Albert P. S., Riddle D. L. (2002). DAF-9, a cytochrome P450 regulating C. elegans larval development and adult longevity. Development 129, 221-231 [DOI] [PubMed] [Google Scholar]
- Kanehisa M., Goto S. (2000). KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M., Goto S., Sato Y., Furumichi M., Tanabe M. (2012). KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 40, D109-D114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klowden M. J. (2007). Physiological Systems in Insects. Burlington, MA: Academic Press; [Google Scholar]
- Koštál V. (2006). Eco-physiological phases of insect diapause. J. Insect Physiol. 52, 113-127 [DOI] [PubMed] [Google Scholar]
- Lee K., Denlinger D. L. (1997). A role for ecdysteroids in the induction and maintenance of the pharate first instar diapause of the gypsy moth, Lymantria dispar. J. Insect Physiol. 43, 289-296 [DOI] [PubMed] [Google Scholar]
- Lee K., Valaitis A. P., Denlinger D. L. (1997). Further evidence that diapause in the gypsy moth, Lymantria dispar, is regulated by ecdysteroids: a comparison of diapause and nondiapause strains. J. Insect Physiol. 43, 897-903 [DOI] [PubMed] [Google Scholar]
- Li B., Dewey C. N. (2011). RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12, 323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente P., Marco R., Sols A. (1970). Regulation of liver pyruvate kinase and the phosphoenolpyruvate crossroads. Eur. J. Biochem. 13, 45-54 [DOI] [PubMed] [Google Scholar]
- MacRae T. H. (2010). Gene expression, metabolic regulation and stress tolerance during diapause. Cell. Mol. Life Sci. 67, 2405-2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElwee J. J., Schuster E., Blanc E., Thornton J., Gems D. (2006). Diapause-associated metabolic traits reiterated in long-lived daf-2 mutants in the nematode Caenorhabditis elegans. Mech. Ageing Dev. 127, 458-472 [DOI] [PubMed] [Google Scholar]
- Michaud M. R., Denlinger D. L. (2007). Shifts in the carbohydrate, polyol, and amino acid pools during rapid cold-hardening and diapause-associated cold-hardening in flesh flies (Sarcophaga crassipalpis): a metabolomic comparison. J. Comp. Physiol. B 177, 753-763 [DOI] [PubMed] [Google Scholar]
- Miller W. L. (1988). Molecular biology of steroid hormone synthesis. Endocr. Rev. 9, 295-318 [DOI] [PubMed] [Google Scholar]
- Morgan T. D., Chippendale G. M. (1983). Free amino acids of the hemolymph of the Southwestern corn borer and the European corn borer in relation to their diapause. J. Insect Physiol. 29, 735-740 [Google Scholar]
- Mori A., Oda T., Wada Y. (1981). Studies on the egg diapause and overwintering of Aedes albopictus in Nagasaki. Tropical Medicine 23, 79-90 [Google Scholar]
- Nene V., Wortman J. R., Lawson D., Haas B., Kodira C., Tu Z. J., Loftus B., Xi Z., Megy K., Grabherr M., et al. (2007). Genome sequence of Aedes aegypti, a major arbovirus vector. Science 316, 1718-1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelchau M. F., Reynolds J. A., Denlinger D. L., Elsik C. G., Armbruster P. A. (2011). A de novo transcriptome of the Asian tiger mosquito, Aedes albopictus, to identify candidate transcripts for diapause preparation. BMC Genomics 12, 619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelchau M. F., Reynolds J. A., Elsik C. G., Denlinger D. L., Armbruster P. A. (2013a). Transcriptome sequencing as a platform to elucidate molecular components of the diapause response in the Asian tiger mosquito, Aedes albopictus. Physiol. Entomol. 38, 173-181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelchau M. F., Reynolds J. A., Elsik C. G., Denlinger D. L., Armbruster P. A. (2013b). Deep sequencing reveals complex mechanisms of diapause preparation in the invasive mosquito, Aedes albopictus. Proc. Biol. Sci. 280, 20130143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumpuni C. B. (1989). Factors influencing photoperiodic control of egg diapause in Aedes albopictus (Skuse). PhD thesis, Department of Biological Sciences, University of Notre Dame, IN, USA: [Google Scholar]
- Ragland G. J., Denlinger D. L., Hahn D. A. (2010). Mechanisms of suspended animation are revealed by transcript profiling of diapause in the flesh fly. Proc. Natl. Acad. Sci. USA 107, 14909-14914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland G. J., Egan S. P., Feder J. L., Berlocher S. H., Hahn D. A. (2011). Developmental trajectories of gene expression reveal candidates for diapause termination: a key life-history transition in the apple maggot fly Rhagoletis pomonella. J. Exp. Biol. 214, 3948-3959 [DOI] [PubMed] [Google Scholar]
- Readio J., Chen M. H., Meola R. (1999). Juvenile hormone biosynthesis in diapausing and nondiapausing Culex pipiens (Diptera: Culicidae). J. Med. Entomol. 36, 355-360 [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Hand S. C. (2009a). Decoupling development and energy flow during embryonic diapause in the cricket, Allonemobius socius. J. Exp. Biol. 212, 2065-2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J. A., Hand S. C. (2009b). Embryonic diapause highlighted by differential expression of mRNAs for ecdysteroidogenesis, transcription and lipid sparing in the cricket Allonemobius socius. J. Exp. Biol. 212, 2075-2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J. A., Poelchau M. F., Rahman Z., Armbruster P. A., Denlinger D. L. (2012). Transcript profiling reveals mechanisms for lipid conservation during diapause in the mosquito, Aedes albopictus. J. Insect Physiol. 58, 966-973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddiford L. M. (1994). Cellular and molecular actions of juvenile hormone. I. General considerations and premetamorphic actions. In Advances in Insect Physiology (ed. Evans P. D.), pp. 213-274 San Diego, CA: Academic Press; [Google Scholar]
- Rinehart J. P., Robich R. M., Denlinger D. L. (2006). Enhanced cold and desiccation tolerance in diapausing adults of Culex pipiens, and a role for Hsp70 in response to cold shock but not as a component of the diapause program. J. Med. Entomol. 43, 713-722 [DOI] [PubMed] [Google Scholar]
- Rinehart J. P., Li A., Yocum G. D., Robich R. M., Hayward S. A., Denlinger D. L. (2007). Up-regulation of heat shock proteins is essential for cold survival during insect diapause. Proc. Natl. Acad. Sci. USA 104, 11130-11137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M. D., Oshlack A. (2010). A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 11, R25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M. D., McCarthy D. J., Smyth G. K. (2010). edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139-140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt P. S., Matzkin L., Ippolito M., Eanes W. F. (2005). Geographic variation in diapause incidence, life-history traits, and climatic adaptation in Drosophila melanogaster. Evolution 59, 1721-1732 [PubMed] [Google Scholar]
- Schulz M. H., Zerbino D. R., Vingron M., Birney E. (2012). Oases: robust de novo RNA-seq assembly across the dynamic range of expression levels. Bioinformatics 28, 1086-1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim C., Denlinger D. L. (2009). Transcription profiling and regulation of fat metabolism genes in diapausing adults of the mosquito Culex pipiens. Physiol. Genomics 39, 202-209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth G. K. (2004). Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3, e3 [DOI] [PubMed] [Google Scholar]
- Smyth G. K. (2005). Limma: linear models for microarray data. In Bioinformatics and Computational Biology Solutions Using R and Bioconductor (ed. Gentleman R., Carey V., Dudoit S., Irizarry R., Huber W.), pp. 397-420 New York, NY: Springer; [Google Scholar]
- Spielman A. (1974). Effect of synthetic juvenile hormone on ovarian diapause of Culex pipiens mosquitoes. J. Med. Entomol. 11, 223-225 [DOI] [PubMed] [Google Scholar]
- Tauber M. J., Tauber C. A. (1976). Insect seasonality: diapause maintenance, termination, and postdiapause development. Annu. Rev. Entomol. 21, 81-107 [Google Scholar]
- Tauber M. J., Tauber C. A., Masaki S. (1986). Seasonal Adaptations of Insects. New York, NY: Oxford University Press; [Google Scholar]
- Trpis M. (1970). A new bleaching and decalcifying method for general use in zoology. Can. J. Zool. 48, 892-893 [Google Scholar]
- Truman J. W., Hiruma K., Allee J. P., Macwhinnie S. G., Champlin D. T., Riddiford L. M. (2006). Juvenile hormone is required to couple imaginal disc formation with nutrition in insects. Science 312, 1385-1388 [DOI] [PubMed] [Google Scholar]
- Urbanski J. M., Benoit J. B., Michaud M. R., Denlinger D. L., Armbruster P. A. (2010). The molecular physiology of increased egg desiccation resistance during diapause in the invasive mosquito, Aedes albopictus. Proc. Biol. Sci. 277, 2683-2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanski J. M., Mogi M., O'Donnell D. L., DeCotiis M., Toma T., Armbruster P. A. (2012). Rapid adaptive evolution of photoperiodic response during invasion and range expansion across a climatic gradient. Am. Nat. 179, 490-500 [DOI] [PubMed] [Google Scholar]
- Van Handel E. (1993). Fuel metabolism of the mosquito Culex quinquefasciatus embryo. J. Insect Physiol. 39, 831-833 [Google Scholar]
- Wang R. L. (1966). Observations on the influence of photoperiod on egg diapause in Aedes albopictus Skuse. Acta Entomologica Sinica 15, 75-77 [Google Scholar]
- Wigglesworth V. B. (1934). The physiology of ecdysis in Rhodnius prolixus. II. Factors controlling moulting and metamorphosis. Q. J. Microsc. Sci. 77, 191-222 [Google Scholar]
- Williams K. D., Schmidt P. S., Sokolowski M. B. (2010). Photoperiodism in insects: molecular basis and consequences of diapause. In Photoperiodism: The Biological Calendar (ed. Nelson R. J., Denlinger D. L., Somers D. E.), pp. 287-317 New York, NY: Oxford University Press; [Google Scholar]
- Wu Q., Brown M. R. (2006). Signaling and function of insulin-like peptides in insects. Annu. Rev. Entomol. 51, 1-24 [DOI] [PubMed] [Google Scholar]
- Young M. D., Wakefield M. J., Smyth G. K., Oshlack A. (2010). Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol. 11, R14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbino D. R., Birney E. (2008). Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 18, 821-829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G., Miesfeld R. L. (2009). Energy metabolism during diapause in Culex pipiens mosquitoes. J. Insect Physiol. 55, 40-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.