Abstract
Shochu wastewater (SW; alcoholic distillery wastewater) contains large amounts of organic compounds (25,000 – 60,000 COD mg/L), nitrogen (1,000 – 6,000 T-N mg/L), and phosphorus (500 – 1,000 T-P mg/L). Despite its high nutrient content, SW is highly perishable, which limits its utilization for animal feed and fertilizer. Therefore, SW is mainly treated by methane fermentation. On the other hand, a feed yeast, Candida utilis, can utilize various organic compounds and be utilized as a yeast extract source and animal feed. We previously bred a mutant, C. utilis UNA1, that accumulates a large amount of nitrogen. Here, we investigated the use of C. utilis UNA1 to treat highly concentrated SW. With fed-batch cultivation using a 5-L jar fermenter, controlling pH at 5.0 with H2SO4, 62.9% of DOC, 38.4% of DTN, and 44.5% of DTP were stably removed from non-diluted barley shochu wastewater (BSW), and about 16.7 kg of freeze-dried yeast biomass was obtained. The yeast sludge biomass generated from BSW contains about 60% crude protein. Furthermore, using H2SO4 to control pH increased the sulfur content of wastewater, which increased the methionine composition of yeast sludge biomass.
Keywords: Candida utilis, Shochu wastewater, Treatment, Biomass production, Amino acid composition
Introduction
Shochu is a traditional Japanese distilled liquor made from barley, sweet-potato, rice, and other crops. In the south Kyushu region, the annual discharge of shochu waste is about 689,000 ton/year. Although shochu waste contains large amounts of nutrients (Ikeda et al. 2012), its high perishability limits its use for animal feed and fertilizer (Tsuyumu et al. 2003). Shochu waste has high concentrations of suspended solids (30,000 – 65,000 SS mg/L), organic compounds (25,000 - 60,000 COD mg/L), nitrogen (1,000 – 6,000 T-N mg/L), and phosphorus (500 – 1,000 T-P mg/L). This makes it too expensive to treat by the conventional activated sludge method. The main treatment method of shochu waste is solid–liquid separation, and the liquid part (shochu wastewater; SW) is treated by combining a methane fermentation process, a physical phosphorus removing process, and a conventional activated sludge process.
Previously, the National Research Institute of Brewing (NRIB) of Japan developed an aerobic wastewater treatment method using a combination of yeasts and activated sludge (Yoshizawa 1978). This system removes large amounts of organic compounds, requires little space, and discharges little waste sludge. This method is useful for treating food and beverage industry wastewater (Yoshizawa 1978) and has also been used to treat SW in laboratory experiments for over thirty years (Saito et al. 1983; Suzuki et al. 1991; Watanabe et al. 2009; Yoshii et al. 2001).
The wastewater treatment abilities of yeast strains can be improved by non-recombinant techniques. We found that phenotypes of the PHO regulatory system of Saccharomyces cerevisiae (Oshima 1997) are useful for improving the phosphorus accumulation capacity (Watanabe et al. 2008). We also found that positive selection of methylamine-resistant mutants to isolate URE2 mutants (Kamerud & Roon 1986) was useful for finding strains with improved nitrogen accumulation ability (Watanabe et al. 2013b). We also confirmed this method can apply to a wastewater treatment yeast Hansenula anomala (Watanabe et al. 2013c).
However, it is necessary to keep these yeasts as the dominant microorganism in the yeast treatment tank by adding chemicals, such as HClO and HCl (Yoshizawa et al. 1980), and by replacing the yeast sludge with fresh seed yeast sludge at regular intervals (Watanabe et al. 2009). On the other hand, treating wastewater with yeast is also considered an attractive way to produce yeast biomass resource. For example, cheese whey was used to produce a food yeast, Kluyveromyces fragilis (Paul et al. 2002). Yeast biomass productivities of Debaryomyces hansenii, Kluyveromyces marxianus, and Pichia stipitis were investigated using brewery’s spent grains hemicellulosic hydrolyzates (Duartc et al. 2008). A feed yeast Candida utilis was used to produce biomass from salad oil manufacturing wastewater (Zheng et al. 2005). Furthermore, the effect of C. utilis on the degradation of forages was investigated (Ando et al. 2006).
However, these studies did not investigate the stability of continuous cultivation and the wastewater treatment abilities. Because wastewater is discharged continuously over long periods, the treatment method must be highly stable. In order to continuously treat SW and produce yeast biomass at the same time, we previously bred a mutant C. utilis UNA1 with high nitrogen-accumulating ability (Watanabe et al. 2013a). In that study, we also demonstrated repeated-batch cultivation using barley shochu wastewater (BSW) (two times diluted) and obtained about 10 g dry yeast sludge biomass per liter of wastewater. Because dilution of SW requires large scale reactors and high operational costs, further works to efficiently treat undiluted SW are needed for practical application.
Here, we investigated the use of C. utilis UNA1 to treat undiluted SW. We found that we could do this by controlling the pH and continuously feeding into the reactor. We also estimated the components of yeast sludge biomass.
Materials and methods
Strains and culture conditions
Candida utilis IFO1086 strain and its highly-nitrogen-accumulating mutant UNA1 (Watanabe et al. 2013a) were used in this study. These strains were maintained on YM agar plates (0.3% yeast extract, 0.3% malt extract, 0.5% peptone, and 1% glucose, and 2% agar) at 4°C.
YM medium (0.3% yeast extract, 0.3% malt extract, 0.5% peptone, and 1% glucose) was used for pre-cultivation. Barley shochu and sweet potato shochu waste were centrifuged at 7,000 rpm for 10 min to obtain the supernatants. These shochu wastes were discharged from pot distillers. Each supernatant was used as SW for jar fermenter cultivation experiments. The physico-chemical characteristics of each SW used in this study are shown in Table 1.
Table 1.
Physico-chemical characteristics of shochu wastewater used in this study
| Barley | Sweet-potato | |
|---|---|---|
| pH (−) | 3.96 | 4.05 |
| CODMn (mg/l) | 47,000 | 17,400 |
| DOC (mg/l) | 42,000 | 16,000 |
| Total sugar (mg/l) | 1,700 | 3,500 |
| Glycerol (mg/l) | 2,200 | 1,500 |
| Organic acids (mg/l) | 26,400 | 3,500 |
| DTN (mg/l) | 6,100 | 1,100 |
| DTP (mg/l) | 800 | 290 |
| K (mg/l) | 420 | 1,800 |
| Ca (mg/l) | 100 | 300 |
| Mg (mg/l) | 100 | 120 |
Analytical methods
The absorbance at 660 nm (OD660) was measured in order to determine cell density using a spectrometer (Ultrospec 2000, Pharmacia Biotech Co. Ltd., Cambridge, England). Dissolved organic carbon (DOC) was analyzed using a TOC analyzer (TOC-5000A, Shimazu Co. Ltd., Kyoto, Japan). Nitrate (NO3-N), Dissolved total nitrogen (DTN), inorganic phosphate (PO4-P), and total phosphorus were analyzed according standard methods (APHA, AWWA, WEF 1998). pH was measured using a pH meter (HM-30S, TOA DKK Co. Ltd., Tokyo, Japan).
Repeated-batch cultivation using a 1.5-L jar fermenter
The yeast strains were pre-incubated in a test tube containing 5 mL of YM medium with shaking at 30°C for 24 h. The pre-culture was inoculated into 1 L of two-times-diluted sweet-potato shochu wastewater (SSW) in a 1.5-L jar fermenter. The cultivation conditions were agitating at 400 rpm, aerating at 0.7 LPM, and incubating at 30°C. After 24 h cultivation, 0.5 liters of culture was replaced with new 0.5 liters of SSW every 12 h. The replacing cycles were repeated 30 times for C. utilis UNA1 and 12 times for C. utilis IFO1086.
Thus, thy hydraulic retention time (HRT) and the sludge retention time (SRT) were 24 h. The DOC-volume loading of SSW was about 16 kg/m3/d.
pH controlled-batch cultivation using a 5-L jar fermenter
C. utilis UNA1 was pre-incubated in 50 mL YM medium using a 300-mL flask with shaking at 120 rpm, at 30°C for 24 h. The pre-culture was inoculated into 3 L of 5-times-diluted BSW. The cultivation conditions were agitating at 400 rpm, aerating at 0.5 LPM, and incubating at 30°C. When pH exceeded 5.0, H2SO4 solution (0.49%) was automatically added to maintain pH value at 5.0.
Repeated-fed-batch cultivation using a 5-L jar fermenter
C. utilis UNA1 was pre-incubated in 50 mL YM medium using a 300-mL flask with shaking at 120 rpm, at 30°C for 24 h. The pre-culture was inoculated into 2 L of 5-times-diluted BSW. The cultivation conditions were agitating at 400 rpm, aerating at 0.5 LPM, incubating at 30°C, and pH value was maintained at 5.0 using H2SO4 solution (0.49%). After 24 h cultivation, BSW was continuously feeding at 2 L/24 h ratio, and 2 L of culture was removed at every 24 h. After 4 times repeated this cycle, the feeding wastewater was changed to 1:1 mixture of BSW and SSW (mixed shochu wastewater; MSW). Feeding ratio and removing time were also changed to 2 L/20 h and 20 h, respectively. This cycle was also repeated 4 times.
Thus, the hydraulic retention time (HRT) and the sludge retention time (SRT) of BSW and MSW 48 h and 40 h, respectively. The DOC-volume loadings of BSW and MSW were 20.9 kg/m3/d and 18.9 kg/m3/d, respectively.
Component analysis of yeast sludge biomass
Culture from the jar fermenter cultivation was centrifuged at 7,000 rpm for 10 min to obtain yeast sludge biomass. The yeast sludge biomass was freeze-dried using a BenchTop 4 K Freeze dryers (model 4KBTZL, SP Industries, NY, USA). The freeze-dried yeast sludge biomass was analyzed for protein, fat, fiber, soluble non-nitrogen, and ash according to the manual of Association of Official Analytical Chemists (AOAC 1980). Amino acid analysis was performed by hydrolyzing the freeze-dried yeast sludge biomass with 6 N HCl at 110°C for 22 h and analyzing the hydrolysate with an amino acid auto-analyzer.
Results and discussion
Repeated-batch cultivation of sweet-potato shochu wastewater
BSW contains more organics and total nitrogen than other kinds of SW. Its discharge volume per year is also the largest. We previously confirmed that C. utilis UNA1 could efficiently and stably treat BSW by repeated-batch cultivation using a 1.5-L jar fermenter (Watanabe et al. 2013a). The discharge volume per year of SSW is almost the same as that of BSW. We first investigated the efficiency of SSW using a 1.5-L jar fermenter.
With 30-cycles repeated-batch cultivation, C. utilis UNA1 stably utilized and removed DOC, DTN and DTP from SSW (Table 2). The amounts of DOC, DTN, and DTP removed by C. utilis UNA1 were higher than those removed by C. utilis IFO1086 (Table 2). These results were similar to previous results using BSW (Table 2, (Watanabe et al. 2013a)), indicating that organic compounds of SSW are also good substrates for C. utilis biomass production. On the other hand, relatively low DOC of SSW causes relatively low biomass (OD660) production (Table 2). Thus, SSW would have to be concentrated or mixed with thick substrate to increase biomass productivity.
Table 2.
Summary performances of repeated-batch cultivation by C. utilis UNA1 and C. utilis IFO1086
| Sweet-potato shochu wastewater | Barley shochu wastewatera | ||||
|---|---|---|---|---|---|
| UNA1 | IFO1086 | UNA1 | IFO1086 | ||
| Periods | Cycles | 30 | 12 | 50 | 12 |
| DOC | Influent (mg/l) | 15877 ± 740 | 15621 ± 1201 | 19731 ± 311 | 18444 ± 557 |
| Effluent (mg/l) | 7691 ± 545 | 8179 ± 927 | 8075 ± 314 | 8217 ± 495 | |
| Removal ratio (%) | 51.6 ± 3.4 | 47.6 ± 5.9 | 59.1 ± 1.6 | 55.5 ± 2.7 | |
| DTN | Influent (mg/l) | 1013.3 ± 46.7 | 985.2 ± 71.5 | 2736.5 ± 67.0 | 2674.6 ± 59.7 |
| Effluent (mg/L) | 477.9 ± 18.8 | 489.6 ± 38.3 | 1748.4 ± 44.4 | 1792.9 ± 87.9 | |
| Removal ratio (%) | 52.8 ± 1.9 | 50.3 ± 3.9 | 36.1 ± 1.6 | 33.0 ± 3.3 | |
| DTP | Influent (mg/l) | 289.2 ± 13.9 | 264.7 ± 17.5 | 383.1 ± 7.4 | 378.7 ± 6.3 |
| Effluent (mg/l) | 116.3 ± 10.4 | 131.5 ± 16.4 | 195.4 ± 10.9 | 228.4 ± 20.6 | |
| Removal ratio (%) | 59.8 ± 3.6 | 50.3 ± 6.2 | 49.0 ± 2.8 | 39.7 ± 5.4 | |
| pH | Influent (−) | 4.08 ± 0.01 | 3.98 ± 0.01 | 4.07 ± 0.03 | 4.18 ± 0.00 |
| Effluent (−) | 7.28 ± 0.27 | 6.17 ± 0.65 | 8.00 ± 0.15 | 8.49 ± 0.07 | |
| OD660 | Effluent (−) | 32.7 ± 2.1 | 31.4 ± 3.4 | 34.9 ± 1.2 | 36.2 ±1.5 |
aThe results of barely shochu wastewater (diluted twice) previously reported (12) were shown for reference.
Data are means ± standard deviation of samples of each cycle.
Effect of pH control on barley shochu wastewater treatment
When SSW and BSW were treated by C. utilis UNA1, the pH increased from about 4.0 to 7.28 – 8.0 (Table 2). The preferred pH of C. utilis cultures is around 4.5 to 6.0. Previously it was shown that the consumption of organic acids by fungi and yeasts caused the pH to change from mildly acidic to mildly alkaline (Tsuyumu et al. 2003; Watanabe et al. 2009). We also thought that the remaining cations (K+ and Ca2+) and ammonium ion generation by deaminization would raise the pH to mildly alkaline. To improve the treatment efficiency and cell growth, we investigated the effect of lowering the pH to the value preferred by C. utilis by acid addition.
During 8 to 16 h cultivation with 5-times diluted BSW, maintaining the pH at 5.0 with H2SO4 raised the cell growth (OD660) compared to no pH control (Figure 1), and increased the removal of DOC (Figure 1). On the other hand, after 24 h cultivation, cell growth was lower with pH control than without it (Figure 1). Unexpectedly, the amounts of DTN and DTP that were removed were not affected by pH control (Table 3). However, the increased removal of DOC by pH control indicates that process performance can be kept high when more concentrated SW is treated.
Figure 1.
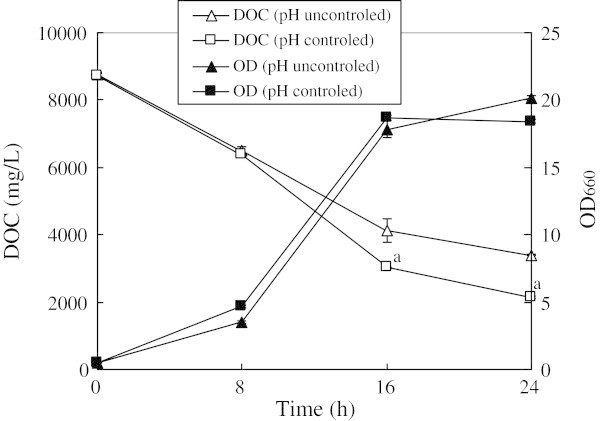
Effect of pH control on cell growth and DOC removal treating 5-times diluted barley shochu wastewater. The time course of the DOC concentration of pH uncontrolled (open triangle) and pH controlled (open square), and the cell growth (OD660) of pH uncontrolled (closed triangle) and pH controlled (closed square). The results were shown as average of three different experiments. The error bars shows standard divisions a Significantly different from pH uncontrolled (p < 0.05) by t-test.
Table 3.
The effect of pH control on process performance of batch cultivation with C. utilis UNA1 using 5-times diluted barley shochu wastewater
| pH uncontrolled | pH controlled | |
|---|---|---|
| OD660 (−) | 20.1 ± 0.2 | 18.4 ± 0.1 |
| pH (−) | 7.58 ± 0.53 | 5.00 ± 0.05 |
| DOC removal amount (mg/l) | 5337 ± 4 | 6597 ± 49 |
| DOC removal ratio (%) | 61.1 ± 0.0 | 75.5a ± 0.6 |
| DTN removal amount (mg/l) | 596.5 ± 2.9 | 563.0 ± 3.5 |
| DTN removal ratio (%) | 44.2 ± 0.2 | 41.4 ± 0.3 |
| DTP removal amount (mg/l) | 137.9 ± 1.3 | 142.9 ± 0.9 |
| DTP removal ratio (%) | 75.1 ± 0.7 | 75.8 ± 0.5 |
Data are means ± standard deviation of three independent experiments.
aSignificantly different from pH uncontrolled (p < 0.05) by t-test.
Effect of fed-batch cultivation on shochu wastewater treatment
C. utilis UNA1 (and C. utilis IFO1086) cannot grow in non-diluted BSW in batch cultivation, and thus dilution with water by 4 – 5 times is needed. However, dilution of BSW requires that the treatment tank be larger, which increases the operational costs. On the other hand, the culture could be kept stable and efficiently treated by repeated-batch cultivation by replacing half the volume of treated wastewater with new 2-times-diluted BSW (Watanabe et al. 2013a). Therefore, we investigated the effect of continuous feeding into large amounts of culture on SW treatment by C. utilis UNA1.
Treatment of barley shochu wastewater
By using feeding cultivation and maintaining the pH at 5.0 with H2SO4, 62.3% of DOC was stably removed from non-diluted BSW (Table 4). This value was higher than that of repeated-batch cultivation with two-times diluted BSW (Table 2). Furthermore, the cell growth (OD660) of feeding cultivation with non-diluted BSW (79.1) was twice that of repeated-batch cultivation with two-times diluted BSW (Tables 2, 4). About 16.7 kg of freeze-dried yeast biomass was obtained from 1 ton of non-diluted BSW cultivation.
Table 4.
Summary performances of fed-batch cultivation by C. utilis UNA1
| Barley | Mixed | ||
|---|---|---|---|
| DOC | Influent (mg/l) | 41969 ± 91 | 31598 ± 359 |
| Effluent (mg/l) | 15573 ± 1315 | 10830 ± 617 | |
| Removal ratio (%) | 62.9 ± 3.1 | 65.7 ± 2.0 | |
| DTN | Influent (mg/l) | 6113.8 ± 83.5 | 3660.1 ± 84.8 |
| Effluent (mg/l) | 3764.7 ± 224.7 | 1894.1 ± 19.7 | |
| Removal ratio (%) | 38.4 ± 3.7 | 48.3 ± 0.5 | |
| DTP | Influent (mg/l) | 787.0 ± 6.2 | 542.0 ± 2.2 |
| Effluent (mg/l) | 436.7 ± 34.7 | 229.2 ± 2.6 | |
| Removal ratio (%) | 44.5 ± 4.4 | 57.7 ± 0.5 | |
| OD660 | Effluent (−) | 79.1 ± 1.5 | 68.4 ± 0.7 |
pH value was controlled at 5.0 with H2SO4.
Data are means ± standard deviation of three independent experiments.
Treatment of mixed shochu wastewater
In order to concentrate SSW and to dilute BSW, we treated a 1:1 mixture of SSW and BSW (MSW). With repeated-batch cultivation, cell growth in MSW was about twice that in non-diluted SSW (Tables 2, 4). About 14.4 kg freeze-dried yeast biomass was obtained from 1 ton of MSW cultivation. Furthermore, removal ratios of DOC, DTN, and DTP of MSW treatment were higher than those of BSW (Table 4). Therefore, these results indicate that feeding cultivation of MSW with controlled pH is useful for both wastewater treatment and cell biomass production.
A marine thraustochytrid, Schizochytrium sp. strain KH105, was used to produce polyunsaturated fatty acids and xanthophylls from SW (v2006). However, it did not utilize and remove organic compounds from SW, and thus glucose addition was needed. In contrast, C. utilis UNA1 can utilize and removed over 60% of DOC, resulting in a large amount of yeast sludge biomass (Table 4).
Regarding wastewater treatment, over 30% of DOC, DTN, and DTP were still remained in yeast treated SW (Table 4). We previously confirmed that effluent of treated BSW was efficiently treated by a combination of nitrification/denitrification cycle treatment and activated sludge process (Watanabe et al. 2013a; Watanabe et al. 2009). In a laboratory-scale demonstration, 50 cycles (25 days) removed 98.9% of DOC, 95.7% of DTN, and 94.1% of DTP from BSW (Watanabe et al. 2013a). Thus, the remaining DOC, DTN, and DTP of yeast treated SW could be easily removed by conventional treatment methods.
Chemical composition of C. utilis in each treatment process
The chemical compositions of yeast sludge biomass generated in each treatment process, including protein, fat, fiber, soluble non-nitrogen, ash, and minerals are shown in Table 5. With repeated-batch treatment, the crude protein contents of yeast sludge biomass generated in BSW (58.5% and 59.9%) were higher than those in SSW (35.6% and 36.0%). On the other hand, with fed-batch cultivation, the chemical compositions of C. utilis UNA1 differed little between BSW and MSW. Therefore, this indicates that MSW is useful for stabilizing yeast components. Using pH control with fed-batch cultivation had little effect on the total protein contents of C. utilis UNA1 generated in BSW.
Table 5.
General compositions of yeast sludge biomass generated in each treatment process with shochu wastewater
| Composition | Repeated-batch cultivation | Fed-batch cultivationa | ||||
|---|---|---|---|---|---|---|
| (% of dry matter) | Barleyb | Barleyb | Sweet-potato | Sweet-potato | Barley | Mixed |
| IFO1086 | UNA1 | IFO1086 | UNA1 | UNA1 | UNA1 | |
| Crude protein | 58.5c | 59.9c | 35.6c | 36.0c | 58.2 | 56.9 |
| Crude fat | 1.0 | 1.2 | 0.2 | 0.2 | 1.0 | 0.2 |
| Crude fiber | 2.3 | 3.7 | 7.2 | 6.8 | 2.1 | 4.3 |
| Soluble non-nitrogen | 28.7 | 25.4 | 42.9 | 42.6 | 28.8 | 27.9 |
| Crude ash | 9.5 | 9.8 | 14.1 | 14.4 | 9.9 | 10.7 |
| Ca | 0.15 | 0.17 | 0.08 | 0.10 | 0.07 | 0.09 |
| P | 1.67 | 1.60 | 1.69 | 1.70 | 1.58 | 1.55 |
| Mg | 0.25 | 0.22 | 0.25 | 0.25 | 0.15 | 0.16 |
| K | 1.22 | 1.28 | 2.64 | 2.67 | 1.28 | 1.67 |
These experiments were conducted in triplicate.
apH value was controlled at 5.0 with H2SO4.
bThe results of barely shochu wastewater (diluted twice) treated with repeated-batch cultivation previously reported (12) were shown for reference.
cSignificantly different barley to sweet potato (p < 0.05) by t-test.
The amino acid compositions of yeast sludge biomass made with the two types of SW were similar (Table 6). Yeast sludge biomass protein contains considerable amounts of essential amino acids. The lysine and proline contents of yeast sludge biomass generated in BSW treatment process were lower and higher than those in SSW, respectively. Using H2SO4 to control pH and fed-batch cultivation increased methionine content of C. utilis UNA1. Because methionine contains sulfur (S), the H2SO4 used to control pH would be expected to increase the synthesis of methionine by C. utilis UNA1. These results indicate that C. utilis UNA1 sludge biomass generated in SW treatment process could be a rich source of yeast extract and animal feed protein.
Table 6.
Amino acid compositions of yeast sludge biomass generated in each treatment process with shochu wastewater
| Amino acid | Repeated-batch cultivation | Fed-batch cultivationa | ||||
|---|---|---|---|---|---|---|
| (% of crude protein) | Barley | Barley | Sweet-potato | Sweet-potato | Barley | Mixed |
| IFO1086 | UNA1 | IFO1086 | UNA1 | UNA1 | UNA1 | |
| Arginineb | 4.47 | 4.32 | 4.58 | 4.06 | 4.76 | 5.19 |
| Glycine | 4.32 | 4.29 | 4.76 | 4.41 | 4.13 | 4.53 |
| Histidineb | 2.49 | 2.12 | 2.02 | 1.73 | 2.48 | 2.23 |
| Isoleucineb | 3.91 | 3.93 | 4.11 | 4.08 | 3.87 | 4.10 |
| Leucine | 3.87 | 4.07 | 5.29 | 5.84 | 3.96 | 4.64 |
| Lysineb | 0.56 | 0.89 | 3.73 | 5.21 | 0.76 | 2.21 |
| Methionineb | 0.97 | 0.77 | 1.55 | 1.37 | 2.27c | 1.99c |
| Phenylalanineb | 8.21 | 7.64 | 6.41 | 4.96 | 7.83 | 6.99 |
| Tyrosine | 4.73 | 4.21 | 2.37 | 1.96 | 4.46 | 4.01 |
| Valineb | 3.87 | 3.91 | 4.90 | 4.93 | 4.00 | 4.53 |
| Serine | 3.97 | 4.01 | 5.14 | 5.17 | 4.17 | 4.84 |
| Alanine | 1.87 | 2.34 | 2.99 | 3.73 | 2.06 | 2.47 |
| Aspartic acid | 2.02 | 2.52 | 3.47 | 4.5 | 2.15 | 2.87 |
| Glutamic acid | 3.98 | 4.66 | 5.83 | 7.09 | 4.17 | 4.95 |
| Proline | 13.04 | 12.74 | 5.60 | 3.92 | 12.50 | 7.40 |
| Threonineb | 5.12 | 5.05 | 5.76 | 5.54 | 4.93 | 5.54 |
These experiments were conducted in triplicate.
apH value was controlled at 5.0 with H2SO4.
bEssential amino acids.
cSignificantly different from repeated-batch cultivation (p < 0.05) by t-test.
Conclusions
In the present study, we investigated the cultivation process of highly concentrated SW in order to effectively treat wastewater and to produce large amounts of yeast sludge biomass to reduce operational costs. With fed-batch cultivation using a 5-L jar fermenter, controlling pH value at 5.0 with H2SO4, 62.9% of DOC, 38.4% of DTN, and 44.5% of DTP were stably removed from non-diluted BSW, and about 16.7 kg of freeze-dry yeast biomass was obtained. Furthermore, mixed with relatively thin SSW 1:1, removal ratios of DOC, DTN, and DTP were increased, and 14.4 kg of freeze-dry yeast biomass was obtained. Yeast sludge biomass generated in BSW and MSW contains about 60% of crude protein. Thus, they are useful for yeast extract source and animal feed protein. Furthermore, using H2SO4 to control pH supplied the wastewater with sulfur (S) appeared to raise the methionine content of yeast sludge biomass.
Shohu is a Japanese traditional liquor and its method of production is similar to the methods of whisky and brandy. Whisky distillery wastewater can be treated with yeast (Yamamoto et al. 1986). Thus, yeast should have the same advantages for alcohol distillery wastewater as it has for SW.
Acknowledgments
We thank Komasa Shuzou Co. Ltd. for kindly providing shochu wastewater. This work was supported by a Research Fellowship of the Japan Society for the Promotion of Science (JSPS) for Young Scientists, and by JSPS KAKENHI Grant no. 23 · 10107.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
TW carried out the wastewater treatment and cultivation experiments and drafted the manuscript. HI and HKK interpreted the data and revised the manuscript. All authors read and approved the final manuscript.
Contributor Information
Takashi Watanabe, Email: takawata@affrc.go.jp.
Haruyuki Iefuji, Email: haiefuji@nifty.com.
Hiroko K Kitamoto, Email: kitamoto@affrc.go.jp.
References
- Ando S, Nishiguchi Y, Hayasaka K, Iefuji H, Takahashi J. Effects of Candida utilis treatment on the nutrient value of rice bran and the effect of Candida utilis on degradation of forages in vitro. Asian-Aust J Anim Sci. 2006;19:806–810. [Google Scholar]
- Official methods of analysis of AOAC. 13. Washington, DC: Association of Official Agricultual Chemists; 1980. [Google Scholar]
- Standard methods for the examination of water and wastewater. 20. Washington, DC: American Public Health Association; 1998. [Google Scholar]
- Duartc LC, Carvalheiro F, Lopes S, Neves I, Gírio FM. Yeast biomass production in brewery’s spent grains hemicellulosic hydrolyzare. Appl Biochem Biotechnol. 2008;148:119–129. doi: 10.1007/s12010-007-8046-6. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Nakano T, Fujii M, Hou D, Yoshimoto M, Kurata RA, Takamine K, Suganuma S. Physiological functions of a sweetpotato shochu distilled residue beverage with rice Koji added. J Brew Soc Japan. 2012;107:355–361. [Google Scholar]
- Kamerud JQ, Roon RJ. Asparaginase II of Saccharomyces cerevisiae: selection of four mutations that cause derepressed enzyme synthesis. J Bacteriol. 1986;165:293–296. doi: 10.1128/jb.165.1.293-296.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima Y. The phosphate system in Saccharomyces cerevisiae. Gene Genet Syst. 1997;72:323–334. doi: 10.1266/ggs.72.323. [DOI] [PubMed] [Google Scholar]
- Paul D, Mukhopadhyay R, Chatterjee BP, Guha AK. Nutritional profile of food yeast kluyveromyces fragilis biomass grown on whey. Appl Biochem Biotechnol. 2002;97:209–218. doi: 10.1385/ABAB:97:3:209. [DOI] [PubMed] [Google Scholar]
- Saito K, Hasuo T, Terada S, Fujikawa S, Tadenuma M. Treatment of waste water discharged from muscovado-spirits (kokuto-shochu) production using yeast. J Brew Soc Japan. 1983;78:954–958. doi: 10.6013/jbrewsocjapan1915.78.954. [DOI] [Google Scholar]
- Suzuki O, Sato S, Iefuji H, Shimoi H, Tadenuma M, Yoshizawa K. Treatment of waste water from shochu distillery with a flocculent yeast hansenula anomala J-224. J Brew Soc Japan. 1991;85:137–141. [Google Scholar]
- Tsuyumu A, Iefuji H, Iwashita K, Ozaki N, Fukushima T. Solid–liquid separation technique for shochu wastewater with high organic concentration using filamentous fungi. J Jpn Soc Water Environ. 2003;26:295–300. doi: 10.2965/jswe.26.295. [DOI] [Google Scholar]
- Watanabe T, Ozaki N, Iwashita K, Fujii T, Iefuji H. Breeding of wastewater treatment yeasts that accumulate high concentrates of phosphorus. Appl Microbiol Biotechnol. 2008;80:331–338. doi: 10.1007/s00253-008-1529-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Masaki K, Iwashita K, Fujii T, Iefuji H. Treatment and phosphorus removal from high-concentration organic wastewater by the yeast hansenula anomala J224 PAWA. Bioresour Technol. 2009;100:1781–1785. doi: 10.1016/j.biortech.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Fujii T, Iefuji H, Kitamoto HK. Treatment and reutilization of shochu wastewater using yeasts. (Review) Yousui to Haisui. 2013;55:605–612. [Google Scholar]
- Watanabe T, Iefuji H, Kitamoto HK. Genome-wide screening to study breeding methods to improve the nitrogen accumulation ability of yeast without gene recombinat techniques. Biosci Biotehcnol Biochem. 2013;77:917–922. doi: 10.1271/bbb.120730. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Iefuji H, Kitamoto HK. J Brew Soc Japan. 2013. Breeding of wastewater treatment yeasts having high nitrogen removal ability. [Google Scholar]
- Yamamoto N, Hashimoto K, Sato S, Nakao T, Shimoi H, Saito K, Tadenuma M. Dynamic state of yeasts and yeast-lysing microorganisms in the treatment of waste water of pot-still from whisky distillery. J Brew Soc Japan. 1986;81:633–634. doi: 10.6013/jbrewsocjapan1915.81.633. [DOI] [Google Scholar]
- Yamazaki T, Aki T, Shinozaki M, Taguchi M, Kawamoto S, Ono K. Utilization of shochu distillery wastewater for production of polyunsaturated fatty acids and xanthophylls using thraustochytrid. J Biosci Bioeng. 2006;102:323–327. doi: 10.1263/jbb.102.323. [DOI] [PubMed] [Google Scholar]
- Yoshii H, Furuta T, Ikeda M, Ito T, Iefuji H, Linko P. Characterization of the cellulose-binding ability of geotrichum sp. M111 Cells and its application to dehydration of the distilled waste of sweet potato shochu. Biosci Biotechnol Biochem. 2001;65:2187–2192. doi: 10.1271/bbb.65.2187. [DOI] [PubMed] [Google Scholar]
- Yoshizawa K. Treatment of waste-water discharged from sake brewery using yeast. J Ferment Technol. 1978;56:389–395. [Google Scholar]
- Yoshizawa K, Momose H, Hasuno T, Suzuki O, Takano K. Disinfection of microorganism in a yeast tank using sterilizers during wastewater treatment. Hakkokogakukaishi. 1980;58:139–144. [Google Scholar]
- Zheng S, Yang M, Yang Z. Biomass production of yeast isolate from salad oil manufacturing wastewater. Bioresour Technol. 2005;96:1183–1187. doi: 10.1016/j.biortech.2004.09.022. [DOI] [PubMed] [Google Scholar]


