Abstract
Endoscopic forceps biopsy is essential before planning an endoscopic resection of upper gastrointestinal epithelial tumors. However, forceps biopsy is limited by its superficiality and frequency of sampling errors. Histologic discrepancies between endoscopic forceps biopsies and resected specimens are frequent. Factors associated with such histologic discrepancies are tumor size, macroscopic type, surface color, and the type of medical facility. Precise targeting of biopsies is recommended to achieve an accurate diagnosis, curative endoscopic resection, and a satisfactory oncologic outcome. Multiple deep forceps biopsies can induce mucosal ulceration in early gastric cancer. Endoscopic resection for early gastric cancer with ulcerative findings is associated with piecemeal resection, incomplete resection, and a risk for procedure-related complications such as bleeding and perforation. Such active ulcers caused by forceps biopsy and following submucosal fibrosis might also be mistaken as an indication for more aggressive procedures, such as gastrectomy with D2 lymph node dissection. Proton pump inhibitors might be prescribed to facilitate the healing of biopsy-induced ulcers if an active ulcer is predicted after deep biopsy. It is unknown which time interval from biopsy to endoscopic resection is appropriate for a safe procedure and a good oncologic outcome. Further investigations are needed to conclude the appropriate time interval.
Keywords: Forceps biopsy, Endoscopic resection, Histologic discrepancy, Ulcer
INTRODUCTION
Endoscopic forceps biopsy is essential before planning an endoscopic resection of gastric epithelial tumors. However, histological discrepancies between endoscopic forceps biopsies and resected specimens are frequent. Biopsy-induced ulcers and subsequent fibrosis might disrupt the treatment of gastric epithelial tumors. This review presents the current problems and unsolved issues of tissue acquisition of gastric epithelial tumors expected to be suitable for endoscopic resection.
HISTOLOGICAL DISCREPANCY
Forceps biopsy is limited by its superficiality and frequency of sampling errors. Furthermore, when an adenoma is identified by forceps biopsy, the absence of cancer foci within the entire lesion cannot be guaranteed. Recent studies report high discrepancy rates between the initial endoscopic forceps biopsy and the resected specimen.1-4 For instance, in a retrospective study of 236 low-grade gastric adenomas diagnosed by forceps biopsy, the agreement rate was 63% (148/236) between the histological diagnoses based on forceps biopsy and the postendoscopic resection results.1 An upgrade of the diagnosis to high-grade adenoma or carcinoma was found in 34% (80/236) of the specimens. In another study, an upgrade of the diagnosis to carcinoma after endoscopic resection was found in 37% (23/74) of cases with low-grade dysplasia and in 90% (36/40) of cases with high-grade dysplasia.5 Lee et al.2 also reported a 45% histological discrepancy rate between endoscopic forceps biopsy and the endoscopic resection specimen if the discrepancy included histological changes from high-grade to low-grade adenoma or from moderately-differentiated to well-differentiated histology.
Factors that have been suggested as associated with such histological discrepancies include surface color (erythema), tumor size (>1 to 2 cm), morphology (depressed), and the type of medical facility (local clinic) (Table 1).1,2,6-8 Odds ratios of erythematous change and large size were 2.5 to 11.1 and 1.9 to 2.4. Those of depressed morphology were 2.8 to 7.3. Although high-grade adenoma was the significant risk factor for upgrade to carcinoma, this information can be obtained after forceps biopsy is performed. Cho et al.1 showed that the odds ratio increased to 47.6 when the three risk factors (size ≥1 cm, depressed, and erythema) were all positive. Endoscopic photos of biopsy-proven low-grade adenoma according to risk factors indicating a finding of upgrade to high-grade adenoma or invasive carcinoma with postendoscopic resection specimen were represented in Fig. 1.
Table 1.
Suggested Possible Factors Associated with Upgraded Histology after Endoscopic Resection
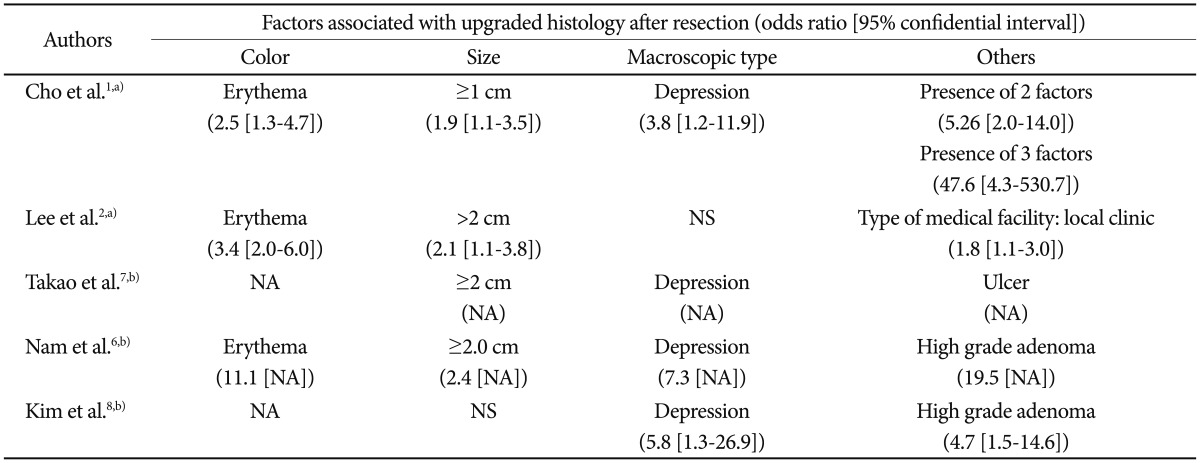
NS, not significant; NA, not applicable.
a)Multivariate analysis; b)Univariate analysis.
Fig. 1.
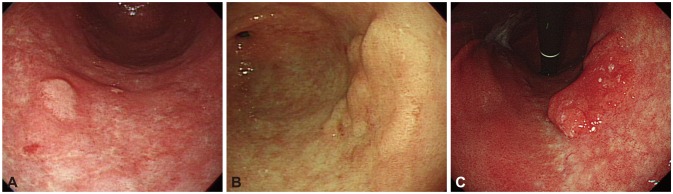
Endoscopic photos of biopsy-proven low-grade adenoma according to risk factors indicating a finding of upgrade to high-grade adenoma or invasive carcinoma with postendoscopic resection pathologic results. (A) A 0.7-cm-sized, nonerythematous, elevated lesion was classified as low-grade adenoma after endoscopic resection. (B) A 2.2-cm-sized, whitish, flat, elevated lesion was proven and classified as high-grade adenoma after endoscopic resection. (C) A 3.5-cm-sized, erythematous, flat, elevated lesion was classified as invasive carcinoma after endoscopic resection.
Importantly, an inaccurate histological diagnosis could lead to a poor clinical outcome, suggesting that a meticulous preoperative evaluation prior to treatment for cancer patients is warranted. As such, biopsy specimens should be carefully collected from large, depressed, and erythematous adenomas. The patients with these factors should be also noted that adenoma can be upgraded to cancer after endoscopic resection.
Carcinoma based on forceps biopsy could be downgraded to adenoma or nonneoplastic lesion after endoscopic resection. The rates of downgrade were 1% to 2% in a retrospective single center study.2 Adenoma based on forceps biopsy could be downgraded from adenoma to nonneoplastic lesion with rates of 3% to 11%.1,2
In Korean studies, the discrepancy rates for the histology of gastric polyps between the initial endoscopic forceps biopsy and the resected polyp were 27.1% and 39.2%.3,9 The main causes of such discrepancy rates were changes between nonneoplastic polyps (inflammatory, hyperplastic, and fundic gland) or between low- and high-grade adenoma. In these studies adenocarcinomas were confirmed after polypectomy in 4.9% (2/41) or 12.0% (11/92) of adenomas and in 1.0% (1/97) of hyperplastic polyps. There was no relationship between the size of the polyp and concordance rates in these studies.
Currently, early gastric cancer with poor differentiation is not included as an indication for endoscopic resection as these lesions require gastrectomy with lymph node dissection. The differentiation of the carcinoma can also change after endoscopic resection, and this discrepancy may lead to inadequate treatment (endoscopic resection) for undifferentiated tumors or unnecessary surgery with lymph node dissection for differentiated tumors. Lee et al.2 reported that six of 75 well-moderately differentiated carcinomas were found to be poorly differentiated after endoscopic resection. In another large Japanese study, differentiated types of cancer sampled by forceps biopsy showed a 97% (1,253/1,291) concordance with the final diagnosis. However, for lesions with a forceps biopsy diagnosis of undifferentiated cancer, 17% (12/69) and 27% (2/11) of undifferentiated cancers had a discrepant final diagnosis of differentiated cancer.2,7 The significant factors related to this discrepancy were the color of the lesion (normal to reddish) and the presence of mixed histology of differentiated and undifferentiated types within the lesion.7
In essence, some patients with a final diagnosis of undifferentiated cancer need additional surgery (gastrectomy with lymph node dissection) after endoscopic resection, whereas some patients with a final diagnosis of differentiated cancer may receive unnecessary surgery instead of endoscopic resection. As such, detailed explanations and counseling are recommended while planning treatments for patients with early gastric cancer.
PITFALLS OF MULTIPLE DEEP FORCEPS BIOPSY
Multiple biopsies increase diagnostic yield, and obtaining four to six samples is recommended for the diagnosis of gastric cancer.10,11 However, multiple deep biopsies can induce mucosal ulceration in early gastric cancer. In several studies, ulcerative early gastric cancer was associated with piecemeal and incomplete resection.12,13 Active ulcers and ulcer scars are also associated with a higher risk of procedure-related complications such as bleeding and perforation.12
If a biopsy was performed at a local clinic, it may be difficult to discriminate between iatrogenic ulcers (caused by forceps biopsy) and ulcers associated with malignancy in early gastric cancer. This is important as ulcer is not included in classic indication of endoscopic resection for early gastric cancer. Indeed, the classic indication for endoscopic resection of early gastric cancer is for well-differentiated intramucosal lesions <2 cm in diameter without ulceration.14 Of concern is that an ulcer caused by forceps biopsy and following submucosal fibrosis might be mistaken as an indication for an inappropriately aggressive treatment such as gastrectomy with D2 lymph node dissection.
Mucosal cancer with biopsy-induced ulcer scars can also be mistaken for a submucosal invasive cancer (another contraindication of classic indication) when endoscopic resection is performed. In this setting, there may be a nonlifting sign, defined as when the lesion is not lifted by saline solution injected into the submucosal layer of the tumor. Lesions involving massive submucosal invasion are not lifted by a submucosal saline solution injection because of the dense fibrosis associated with invasive carcinoma that prevents fluid infiltration through the submucosal connective tissue.15,16 An ulcer scar without submucosal invasion can also show a nonlifting sign. In a retrospective study of colorectal cancer, some mucosal cancers with a history of biopsy showed non-lifting signs and received unnecessary surgery.15,17 Reviewing the initial endoscopic photographs taken before performing forceps biopsy is recommended if an iatrogenic ulcer caused by forceps biopsy is suspected.
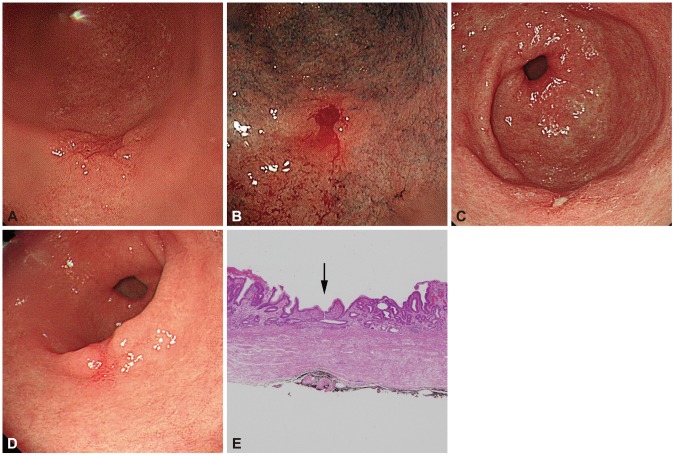
Proton pump inhibitors (PPIs) are the most potent inhibitors of gastric acid secretion available. PPIs facilitate healing of ulcerated mucosa,18 and though there is scant evidence on their efficacy after biopsy, they might be prescribed to facilitate the healing of biopsy-induced ulcers when an active ulcer is suspected (Fig. 2).
Fig. 2.
(A) Endoscopic finding which was obtained at initial endoscopy at a local clinic. No definite ulcer was found. (B) Endoscopic finding just after performing forceps biopsy at a local clinic. Biopsy was performed at the center of the tumor surface. (C) Endoscopic finding at the tertiary referral center. An ulcer was shown on the tumor surface. (D) Endoscopic finding at endoscopic resection. The previously noted ulcer was healed after treating with proton pump inhibitors for 15 days. (E) Microscopic findings of resected specimen (H&E stain, ×40). The center of the specimen (arrow) shows a healed ulcer with submucosal fibrosis.
Rebiopsies are frequently performed at tertiary referral centers when initial biopsies and diagnosis were performed at a local clinic. There are several disadvantages to repeated biopsy, such as patient discomfort, costs, and increased risks following the invasive procedure. Biopsy-induced ulcers and subsequent fibrosis are also unwanted side effects observed prior to endoscopic resection. However, repeated biopsy might be recommended if the medical record from the local clinic is confusing or incomplete, or if adenomas are suspected to have undergone carcinomatous changes.
INTERVAL FROM FORCEPS BIOPSY TO ENDOSCOPIC RESECTION
It is unknown which time interval from biopsy to endoscopic resection is appropriate for a safe procedure and satisfactory oncologic outcome. A retrospective study has suggested performing an endoscopic resection within 3 weeks after biopsy because prolongation past this interval may be associated with nonlifting signs in endoscopically resectable colorectal cancer.15 Considering that active ulcers may interfere with successful submucosal dissection, performing the endoscopic resection when a biopsy-induced ulcer has just healed would be the most appropriate timing if an active ulcer was predicted when the biopsy was performed. Further investigations are needed to conclude the appropriate time interval from biopsy to resection.
CONCLUSIONS
Histological discrepancies between endoscopic forceps biopsies and resected specimens are a frequently encountered problem. It is recommended to collect biopsy samples carefully from gastric epithelial lesions with factors suggestive of carcinomatous changes, such as those that are large, erythematous, or depressed. Furthermore, biopsy can induce mucosal ulceration, which might interfere with curative submucosal dissection and increase procedure-related risk. Ulcerative changes can also be mistaken as a contraindication of classic indication to endoscopic resection. As such, careful review of the initial endoscopic findings prior to forceps biopsy in addition to detailed explanations and counseling are recommended while planning treatments for early gastric cancer. The optimal time interval from biopsy to endoscopic resection for a safe procedure and good oncologic outcome remains undefined, and further investigations are warranted.
Acknowledgments
This work was supported by grant 1210270 from the National Cancer Center, Korea.
Footnotes
The author has no financial conflicts of interest.
References
- 1.Cho SJ, Choi IJ, Kim CG, et al. Risk of high-grade dysplasia or carcinoma in gastric biopsy-proven low-grade dysplasia: an analysis using the Vienna classification. Endoscopy. 2011;43:465–471. doi: 10.1055/s-0030-1256236. [DOI] [PubMed] [Google Scholar]
- 2.Lee CK, Chung IK, Lee SH, et al. Is endoscopic forceps biopsy enough for a definitive diagnosis of gastric epithelial neoplasia? J Gastroenterol Hepatol. 2010;25:1507–1513. doi: 10.1111/j.1440-1746.2010.006367.x. [DOI] [PubMed] [Google Scholar]
- 3.Sung HY, Cheung DY, Cho SH, et al. Polyps in the gastrointestinal tract: discrepancy between endoscopic forceps biopsies and resected specimens. Eur J Gastroenterol Hepatol. 2009;21:190–195. doi: 10.1097/MEG.0b013e3283140ebd. [DOI] [PubMed] [Google Scholar]
- 4.Lee SB, Kang HY, Kim KI, Ahn DH. The diagnostic accuracy of endoscopic biopsy for gastric dysplasia. J Gastric Cancer. 2010;10:175–181. doi: 10.5230/jgc.2010.10.4.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jung MK, Jeon SW, Park SY, et al. Endoscopic characteristics of gastric adenomas suggesting carcinomatous transformation. Surg Endosc. 2008;22:2705–2711. doi: 10.1007/s00464-008-9875-2. [DOI] [PubMed] [Google Scholar]
- 6.Nam KW, Song KS, Lee HY, et al. Spectrum of final pathological diagnosis of gastric adenoma after endoscopic resection. World J Gastroenterol. 2011;17:5177–5183. doi: 10.3748/wjg.v17.i47.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takao M, Kakushima N, Takizawa K, et al. Discrepancies in histologic diagnoses of early gastric cancer between biopsy and endoscopic mucosal resection specimens. Gastric Cancer. 2012;15:91–96. doi: 10.1007/s10120-011-0075-8. [DOI] [PubMed] [Google Scholar]
- 8.Kim JH, Kim SH, Park WH, et al. Predictable factors of histologic discrepancy of gastric cancer between the endoscopic forceps biopsy and endoscopic treatment specimen. Korean J Gastroenterol. 2012;59:354–359. doi: 10.4166/kjg.2012.59.5.354. [DOI] [PubMed] [Google Scholar]
- 9.Yoon WJ, Lee DH, Jung YJ, et al. Histologic characteristics of gastric polyps in Korea: emphasis on discrepancy between endoscopic forceps biopsy and endoscopic mucosal resection specimen. World J Gastroenterol. 2006;12:4029–4032. doi: 10.3748/wjg.v12.i25.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham DY, Schwartz JT, Cain GD, Gyorkey F. Prospective evaluation of biopsy number in the diagnosis of esophageal and gastric carcinoma. Gastroenterology. 1982;82:228–231. [PubMed] [Google Scholar]
- 11.Faigel DO, Eisen GM, Baron TH, et al. Tissue sampling and analysis. Gastrointest Endosc. 2003;57:811–816. doi: 10.1016/s0016-5107(03)70047-9. [DOI] [PubMed] [Google Scholar]
- 12.Ohnita K, Isomoto H, Yamaguchi N, et al. Factors related to the curability of early gastric cancer with endoscopic submucosal dissection. Surg Endosc. 2009;23:2713–2719. doi: 10.1007/s00464-009-0473-8. [DOI] [PubMed] [Google Scholar]
- 13.Oka S, Tanaka S, Kaneko I, et al. Endoscopic submucosal dissection for residual/local recurrence of early gastric cancer after endoscopic mucosal resection. Endoscopy. 2006;38:996–1000. doi: 10.1055/s-2006-944780. [DOI] [PubMed] [Google Scholar]
- 14.Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219–225. doi: 10.1007/pl00011720. [DOI] [PubMed] [Google Scholar]
- 15.Han KS, Sohn DK, Choi DH, et al. Prolongation of the period between biopsy and EMR can influence the nonlifting sign in endoscopically resectable colorectal cancers. Gastrointest Endosc. 2008;67:97–102. doi: 10.1016/j.gie.2007.05.057. [DOI] [PubMed] [Google Scholar]
- 16.Uno Y, Munakata A. The non-lifting sign of invasive colon cancer. Gastrointest Endosc. 1994;40:485–489. doi: 10.1016/s0016-5107(94)70216-0. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi N, Saito Y, Matsuda T, Fu KI. False-positive nonlifting sign. Gastrointest Endosc. 2008;68:408. doi: 10.1016/j.gie.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 18.Sheen E, Triadafilopoulos G. Adverse effects of long-term proton pump inhibitor therapy. Dig Dis Sci. 2011;56:931–950. doi: 10.1007/s10620-010-1560-3. [DOI] [PubMed] [Google Scholar]