Abstract
Although techniques and instruments for endoscopic submucosal dissection (ESD) have improved, bleeding is still the most common complication. Minimizing the occurrence of bleeding is important because blood can interfere with subsequent procedures. Generally, ESD-related bleeding can be divided into intraprocedural and postprocedural bleedings. Postprocedural bleeding can be further classified into early post-ESD bleeding which occurs within 48 hours after ESD and late post-ESD bleeding which occurs later than 48 hours after ESD. A basic principle for avoiding intraprocedural bleeding is to watch for vessels and coagulate them before cutting. Several countertraction devices have been designed to minimize intraprocedural bleeding. Methods for reducing postprocedural bleeding include administration of proton-pump inhibitors or prophylactic coagulation after ESD. Medical adhesive spray such as n-butyl-2-cyanoacrylate is also an option for preventing postprocedural bleeding. Various endoscopic treatment modalities are used for both intraprocedural and postprocedural bleeding. However, hemoclipping is infrequently used during ESD because the clips interfere with subsequent resection. Bleeding that occurs as a result of ESD can usually be managed easily. Nonetheless, more effective ways to prevent bleeding, including reliable ESD techniques, must be developed.
Keywords: Endoscopic submucosal dissection, Hemorrhage, Prevention and control, Hemostasis
INTRODUCTION
Endoscopic submucosal dissection (ESD) was developed for en bloc resections for all tumor sizes and locations.1 ESD is an advanced technique with a long procedure time compared to endoscopic mucosal resection (EMR).2,3 In addition, intraoperative bleeding is more frequent during ESD than during EMR.4 Although ESD techniques and instruments have improved, bleeding is still the most common complication.5,6 Minimizing bleeding is important because blood interferes with subsequent endoscopic procedures. In this review, we discuss how to avoid and control ESD-related bleeding.
CLASSIFICATION OF ESD-RELATED BLEEDINGS
ESD-related bleedings are generally divided into intraprocedural and postprocedural bleedings. Postprocedural bleedings are further classified into early post-ESD bleeding within 48 hours of ESD and late post-ESD bleeding later than 48 hours after ESD.7
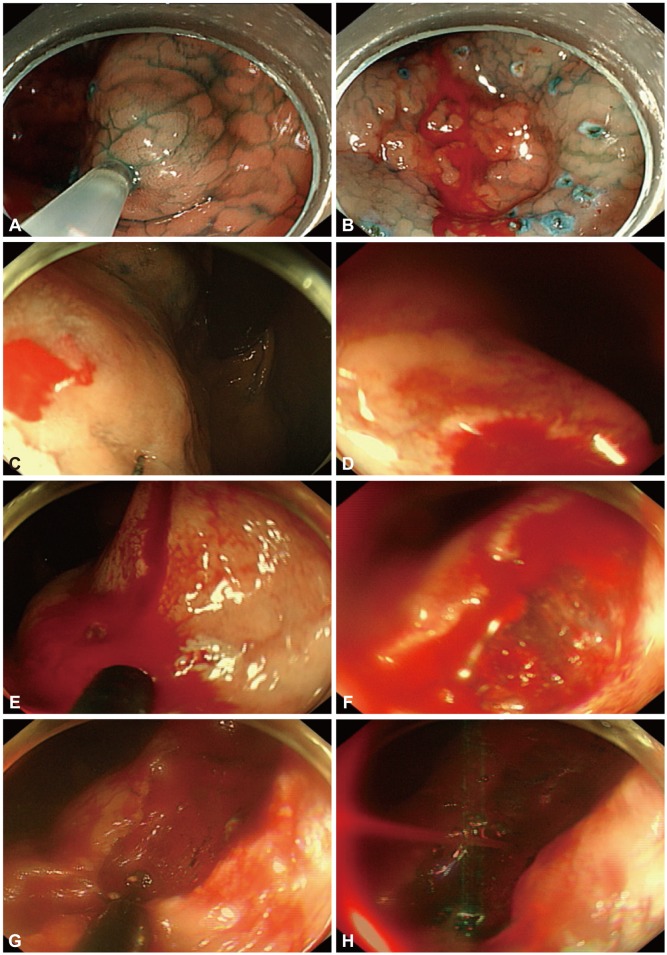
Intraprocedural bleeding can develop during ESD including during submucosal injection, incision, or dissection. Fig. 1 shows the various types of intraprocedural bleeding. Submucosal vasculature is abundant in the stomach and precisely predicting the distribution of submucosal vessel is difficult. Therefore, intraprocedural bleeding is hard to avoid and occurs in almost all ESDs. However, reported intraprocedural bleeding rates vary from 22.6% to 90.6%.4,8,9 This discrepancy in rates is due to different intraprocedural bleeding definitions, which range from inconsequential bleeding that stops spontaneously to massive bleeding that requires transfusion or termination of the ESD.
Fig. 1.
Examples of intraprocedural bleeding. (A) A saline solution containing epinephrine (0.01 mg/mL) mixed with indigo carmine was injected into the submucosal layer using a 21-gauge needle to lift the lesion from the muscle layer. (B) Bleeding from the injected site can be controlled relatively easily with strategies including spontaneous hemostasis, saline spray with diluted epinephrine, and compression with an endoscope tip. (C) Circumferential incision in the stomach upper body. (D) Bleeding from needle knife incision. Bleeding in the upper body occurs because of abundant submucosal blood vessels with large diameters. This bleeding is more serious than bleeding from injections. Bleeding from lesions comes into the endoscope cap, which is retroflexed, obscuring the vision. (E) Bleeding from insulated tipped knife incision. (F) Bleeding could not be identified here, and therefore (G, H) additional incisions were performed in order to see the bleeding source more clearly. Coagulation of the abundant vascular networks using a knife with swift mode was performed.
Postprocedural bleeding manifests as hematemesis and/or melena and a drop in hemoglobin. Postprocedural bleeding occurs in 1.3% to 11.9% of patients who undergo ESD (Table 1).7,10-29 Although about 50% to 70% of bleeding is observed within 2 days of ESD, bleeding can develop as late as 2 weeks after the procedure.13,30 Late post-ESD bleeding that appears after patients are discharged is a concern for both endoscopists and patients, because urgent outpatient treatment is difficult.
Table 1.
Incidence of Postprocedural Bleeding
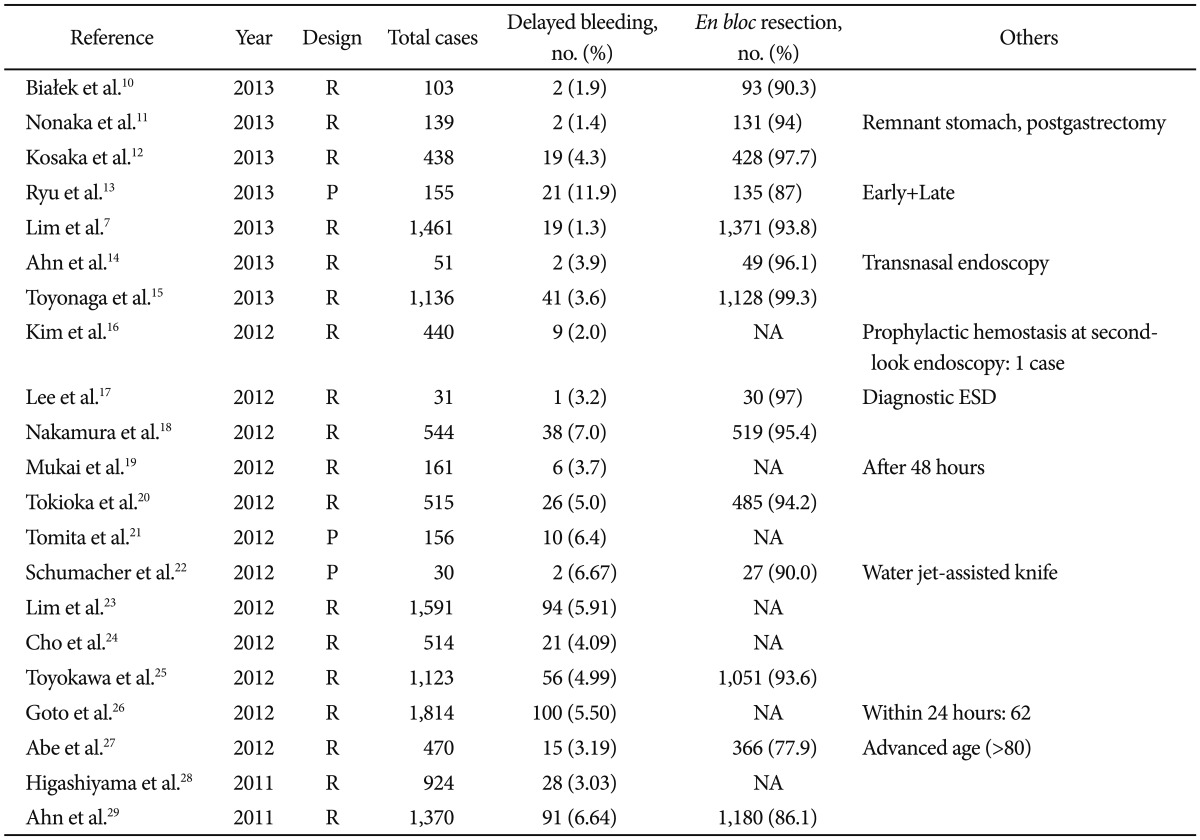
R, retrospective study; P, prospective study; NA, not analyzed; ESD, endoscopic submucosal dissection.
RISK FACTORS FOR ESD-RELATED BLEEDING
To determine how to minimize ESD-related bleeding, studies have evaluated risk factors for bleeding after ESD. Intraprocedural bleeding develops more commonly with lesions in the upper third of the stomach3,8 because of abundant distribution of vessels in the submucosa.31 Technical difficulty could be another reason for frequent bleeding. In contrast to intraprocedural bleeding, postprocedural bleeding is observed more frequently at lesions in the middle or lower third of the stomach.3,32-34 Large specimen size is a well-known risk factor for postprocedural bleeding, with studies finding that postprocedural bleeding increases for lesions that are widely resected (≥40 mm).23,32,35 The possibility that antiplatelet agents are risk factors for intraprocedural or postprocedural bleeding is controversial. The 2009 American Society for Gastrointestinal Endoscopy guidelines recommended continued treatment with aspirin.36 However, the European Society of Gastrointestinal Endoscopy guidelines recommend discontinuation of aspirin for 5 days in patients with low thrombotic risk.37 The guidelines were based on observational studies, expert opinions, and best clinical practices, and rarely supported by prospective randomized studies. To draw definite conclusions, well-designed prospective studies are needed. A recent large, retrospective study showed that antithrombotic drugs are risk factors for late post-ESD bleeding.35 Since large artificial ulcers are related to a high postprocedural bleeding rate, antiplatelet agents that interfere with artificial ulcer healing could be a risk factor for postprocedural bleeding. Therefore, careful administration of antiplatelet agents could be needed, even without evidence of bleeding at the time of drug reinitiation.
PREVENTING ESD-RELATED BLEEDING
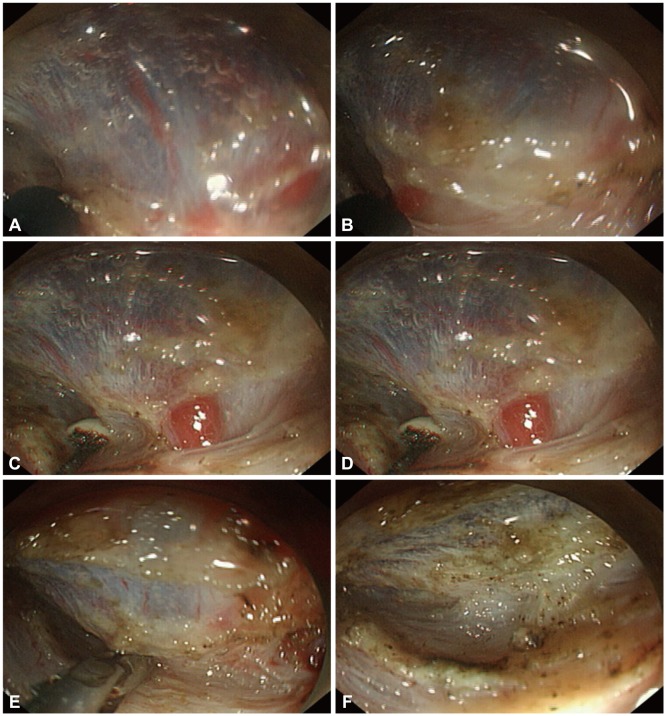
A basic principle for avoiding intraprocedural bleeding is to watch for vessels, and coagulate them before cutting (Fig. 2). In addition, understanding the anatomical characteristics of a particular area of the stomach is essential for a successful and safe ESD. The antrum has little fibrotic tissue and low density vasculature in the submucosal layer.38 The lesser curvature of the stomach body has little fibrotic tissue, similar to the antrum; however, it has large-diameter, perforating vessels. In contrast to the lesser curvature of the stomach body, the anterior and posterior wall sides have more perforating branches and fibrotic submucosa, which generally makes incision and dissection more difficult.
Fig. 2.
Watch for vessels, and coagulate them before cutting to prevent intraprocedural bleeding. Understanding stomach anatomical characteristics such as large-diameter perforating vessels in the lesser body curvature is important. Submucosal dissection in this area should be cautious compared to the antrum. (A-C) During submucosal dissection, one large vessel was identified, and dissection was stopped. (D-F) Thermocoagulation hemostasis using hemostatic forceps was performed.
Endoscopic ultrasonography (EUS) was used to evaluate submucosal vascular structure to predict intraprocedural bleeding. A retrospective study by Kikuchi et al.39 showed that the frequency of clip use was higher in lesions rich in vascular structure compared to lesions without rich vascular structure. Their subsequent prospective study revealed that EUS predicted procedure time, muscle injury incidence, and frequency of clip use. However, intraprocedural bleeding could not be predicted by preoperative EUS findings.40 Various counter-traction devices have been developed to minimize intraprocedural bleeding.41 Methods using these devices include transnasal endoscope-assisted ESD, external grasping-type forceps, yo-yo technique, and clip-band technique. All countertraction devices expose the submucosa to the endoscopist's visual field for easier and safer ESD. Some devices, however, require an additional assistant.
The best way to reduce postprocedural bleeding is administration of a proton-pump inhibitor (PPI) (Table 2).21,42-45 In several studies, PPIs were superior to histamine-2-receptor antagonists at preventing postprocedural bleeding;46 however, preoperative administration of PPIs might not offer additional benefit.47 In addition, the optimal duration of PPI administration among patients who undergo ESD is unclear. A prospective study from Japan concluded that administration of PPIs for 2 weeks for ESD-induced ulcers might be sufficient to aid healing without increasing the risk of adverse effects.48 Another study showed that the optimal duration of PPI treatment varies based on the ESD-induced ulcer size; patients with an ESD-induced ulcer larger than 40 mm should be treated with an 8-week course of PPIs.43 Another well-known method for preventing postprocedural bleeding is prophylactic coagulation of visible vessels in the resection area.33 Many endoscopists agree that prophylactic coagulation of nonbleeding visible vessels is necessary. However, aggressive, routine coagulation of all visible vessels could risk perforation, although this possibility has not been confirmed in a trial. Some investigators, therefore, have developed methods to try to identify vessels that have a low risk of bleeding. One method uses infrared imaging.49 Yoshida et al.49 classified nonbleeding visible vessels a blue or gray points using this system and water-jet pressure was applied to an artificial ulcer base to induce iatrogenic bleeding. Vessels with blood flow were dark gray from infrared absorption by hemoglobin; coagulated vessels were blue. Iatrogenic bleeding occurred only at gray and not blue points. Doppler ultrasonography (US) has also been used to distinguish low-risk vessels for bleeding. Uedo et al.50 performed prophylactic coagulation using Doppler US signals in 10 patients who underwent ESD. No postprocedural bleeding was observed. Although these were pilot studies and the methods have not been generalized, these types of efforts will improve ESD techniques and safety. Another method suggested to reduce postprocedural bleeding is secondlook endoscopy after ESD, which many endoscopists perform.26,30 However, evidence that second-look endoscopy reduces postprocedural bleeding is lacking.13,30 Recently, a new method for preventing postprocedural bleeding using medical adhesive such as n-butyl-2-cyanoacrylate was reported by a Chinese prospective study. The study revealed that medical adhesive spray is effective in preventing postprocedural bleeding (medical adhesive group vs. control group, 0% vs. 4.88%; p=0.035).51 Since medical adhesives are usually used to treat gastric varices, the results were unexpected. Medical adhesive spray could be an option for preventing postprocedural bleeding.
Table 2.
Drugs for Treating Artificial Ulcer
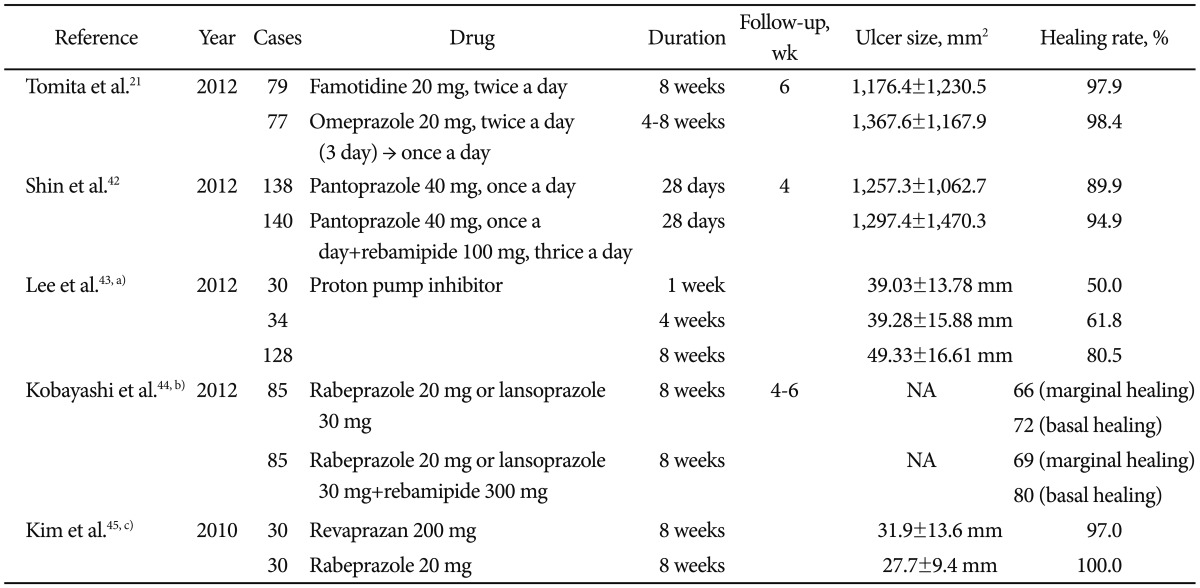
Values are presented as mean±SD.
NA, not analyzed.
a)Retrospective study; b)Lansoprazole 30 mg given to all patients for an initial 2 days before study medication was started; c)Matched case-control study. Intravenous pantoprazole 80 mg given 2 hours before endoscopic submucosal dissection to all patients followed by 8 mg/hr intravenous pantoprazole was given continuously.
CONTROLLING ESD-RELATED BLEEDING
For cases of intraprocedural bleeding, the hemostatic method should be chosen according to the appearance and the severity of the bleeding. Minimal bleeding can be controlled with epinephrine spray, compression by the tip of an endoscope, or subtle coagulation by electrosurgical knives such as an insulated-tipped knife. Bleeding can stop spontaneously, so waiting briefly is an option for controlling intraprocedural bleeding. Severe bleeding that cannot be easily controlled should be treated with a hemostasis device. Epinephrine mixed into a solution that acts as a submucosal cushion is useful for both peptic and ESD-induced ulcers.52 The most common method for controlling severe bleeding is thermocoagulation hemostasis using hemostatic forceps.53 These forceps have been developed especially for hemostasis and have a narrow opening angle, a small cup, and a blunt edge to ensure efficient electrocoagulation.53,54 If the exact bleeder cannot be identified, broad coagulation of the lesion or trimming the submucosal layer to expose the bleeder should be considered. However, excessive coagulation increases the resistance to electrical cutting, interfering with subsequent procedures. Hemoclipping is an alternative for hemostasis of intraprocedural bleeding; however, it is used less often during ESD because endoscopic clips interfere with subsequent resection procedures.5
To control post-ESD bleeding, various endoscopic treatment modalities can be used individually or in combination. Early in the healing period of artificial ulcer, the ulcer base is still soft with little granulation tissue, so endoscopic clips or electrocautery using hemostatic forceps can be applied to control the bleeding.5 Late in the healing period, the artificial ulcer base hardens with granulation tissue, so the injection method is preferable.5 Although almost all bleeding events can be controlled endoscopically, interventional radiologic methods for vascular embolization or surgery are also hemostasis options and should be considered when endoscopic management fails or disseminated intravascular coagulation is developed.
CONCLUSIONS
Although bleeding often occurs as a result of ESD, it can almost always be easily managed. However, more effective ways to prevent ESD-related bleeding, including reliable ESD techniques, must be developed.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Miyamoto S, Muto M, Hamamoto Y, et al. A new technique for endoscopic mucosal resection with an insulated-tip electrosurgical knife improves the completeness of resection of intramucosal gastric neoplasms. Gastrointest Endosc. 2002;55:576–581. doi: 10.1067/mge.2002.122579. [DOI] [PubMed] [Google Scholar]
- 2.Akasaka T, Nishida T, Tsutsui S, et al. Short-term outcomes of endoscopic submucosal dissection (ESD) for early gastric neoplasm: multicenter survey by osaka university ESD study group. Dig Endosc. 2011;23:73–77. doi: 10.1111/j.1443-1661.2010.01062.x. [DOI] [PubMed] [Google Scholar]
- 3.Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929–942. doi: 10.1007/s00535-006-1954-3. [DOI] [PubMed] [Google Scholar]
- 4.Oka S, Tanaka S, Kaneko I, et al. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877–883. doi: 10.1016/j.gie.2006.03.932. [DOI] [PubMed] [Google Scholar]
- 5.Oda I, Suzuki H, Nonaka S, Yoshinaga S. Complications of gastric endoscopic submucosal dissection. Dig Endosc. 2013;25(Suppl 1):71–78. doi: 10.1111/j.1443-1661.2012.01376.x. [DOI] [PubMed] [Google Scholar]
- 6.Park CH, Min JH, Yoo YC, et al. Sedation methods can determine performance of endoscopic submucosal dissection in patients with gastric neoplasia. Surg Endosc. 2013;27:2760–2767. doi: 10.1007/s00464-013-2804-z. [DOI] [PubMed] [Google Scholar]
- 7.Lim SM, Park JC, Lee H, Shin SK, Lee SK, Lee YC. Impact of cumulative time on the clinical outcomes of endoscopic submucosal dissection in gastric neoplasm. Surg Endosc. 2013;27:1397–1403. doi: 10.1007/s00464-012-2643-3. [DOI] [PubMed] [Google Scholar]
- 8.Jeon SW, Jung MK, Cho CM, et al. Predictors of immediate bleeding during endoscopic submucosal dissection in gastric lesions. Surg Endosc. 2009;23:1974–1979. doi: 10.1007/s00464-008-9988-7. [DOI] [PubMed] [Google Scholar]
- 9.Jang JS, Choi SR, Graham DY, et al. Risk factors for immediate and delayed bleeding associated with endoscopic submucosal dissection of gastric neoplastic lesions. Scand J Gastroenterol. 2009;44:1370–1376. doi: 10.3109/00365520903194609. [DOI] [PubMed] [Google Scholar]
- 10.Białek A, Wiechowska-Kozłowska A, Pertkiewicz J, et al. Endoscopic submucosal dissection for the treatment of neoplastic lesions in the gastrointestinal tract. World J Gastroenterol. 2013;19:1953–1961. doi: 10.3748/wjg.v19.i12.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nonaka S, Oda I, Makazu M, et al. Endoscopic submucosal dissection for early gastric cancer in the remnant stomach after gastrectomy. Gastrointest Endosc. 2013;78:63–72. doi: 10.1016/j.gie.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Kosaka T, Endo M, Toya Y, et al. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a single-center retrospective study. Dig Endosc. doi: 10.1111/den.12099. Epub 2013 Apr 7. DOI: 10.1111/den.12099. [DOI] [PubMed] [Google Scholar]
- 13.Ryu HY, Kim JW, Kim HS, et al. Second-look endoscopy is not associated with better clinical outcomes after gastric endoscopic submucosal dissection: a prospective, randomized, clinical trial analyzed on an astreated basis. Gastrointest Endosc. doi: 10.1016/j.gie.2013.02.008. Epub 2013 Mar 23. DOI: 10.1016/j.gie.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Ahn JY, Choi KD, Lee JH, et al. Is transnasal endoscope-assisted endoscopic submucosal dissection for gastric neoplasm useful in training beginners? A prospective randomized trial. Surg Endosc. 2013;27:1158–1165. doi: 10.1007/s00464-012-2567-y. [DOI] [PubMed] [Google Scholar]
- 15.Toyonaga T, Man-i M, East JE, et al. 1,635 Endoscopic submucosal dissection cases in the esophagus, stomach, and colorectum: complication rates and long-term outcomes. Surg Endosc. 2013;27:1000–1008. doi: 10.1007/s00464-012-2555-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim HH, Park SJ, Park MI, Moon W. Clinical impact of second-look endoscopy after endoscopic submucosal dissection of gastric neoplasms. Gut Liver. 2012;6:316–320. doi: 10.5009/gnl.2012.6.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee H, Kim H, Shin SK, et al. The diagnostic role of endoscopic submucosal dissection for gastric lesions with indefinite pathology. Scand J Gastroenterol. 2012;47:1101–1107. doi: 10.3109/00365521.2012.704939. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura M, Nishikawa J, Hamabe K, et al. Risk factors for delayed bleeding from endoscopic submucosal dissection of gastric neoplasms. Scand J Gastroenterol. 2012;47:1108–1114. doi: 10.3109/00365521.2012.699550. [DOI] [PubMed] [Google Scholar]
- 19.Mukai S, Cho S, Kotachi T, et al. Analysis of delayed bleeding after endoscopic submucosal dissection for gastric epithelial neoplasms. Gastroenterol Res Pract. 2012;2012:875323. doi: 10.1155/2012/875323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tokioka S, Umegaki E, Murano M, et al. Utility and problems of endoscopic submucosal dissection for early gastric cancer in elderly patients. J Gastroenterol Hepatol. 2012;27(Suppl 3):63–69. doi: 10.1111/j.1440-1746.2012.07075.x. [DOI] [PubMed] [Google Scholar]
- 21.Tomita T, Kim Y, Yamasaki T, et al. Prospective randomized controlled trial to compare the effects of omeprazole and famotidine in preventing delayed bleeding and promoting ulcer healing after endoscopic submucosal dissection. J Gastroenterol Hepatol. 2012;27:1441–1446. doi: 10.1111/j.1440-1746.2012.07144.x. [DOI] [PubMed] [Google Scholar]
- 22.Schumacher B, Charton JP, Nordmann T, Vieth M, Enderle M, Neuhaus H. Endoscopic submucosal dissection of early gastric neoplasia with a water jet-assisted knife: a Western, single-center experience. Gastrointest Endosc. 2012;75:1166–1174. doi: 10.1016/j.gie.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 23.Lim JH, Kim SG, Kim JW, et al. Do antiplatelets increase the risk of bleeding after endoscopic submucosal dissection of gastric neoplasms? Gastrointest Endosc. 2012;75:719–727. doi: 10.1016/j.gie.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 24.Cho SJ, Choi IJ, Kim CG, et al. Aspirin use and bleeding risk after endoscopic submucosal dissection in patients with gastric neoplasms. Endoscopy. 2012;44:114–121. doi: 10.1055/s-0031-1291459. [DOI] [PubMed] [Google Scholar]
- 25.Toyokawa T, Inaba T, Omote S, et al. Risk factors for perforation and delayed bleeding associated with endoscopic submucosal dissection for early gastric neoplasms: analysis of 1123 lesions. J Gastroenterol Hepatol. 2012;27:907–912. doi: 10.1111/j.1440-1746.2011.07039.x. [DOI] [PubMed] [Google Scholar]
- 26.Goto O, Fujishiro M, Oda I, et al. A multicenter survey of the management after gastric endoscopic submucosal dissection related to postoperative bleeding. Dig Dis Sci. 2012;57:435–439. doi: 10.1007/s10620-011-1886-5. [DOI] [PubMed] [Google Scholar]
- 27.Abe N, Gotoda T, Hirasawa T, et al. Multicenter study of the long-term outcomes of endoscopic submucosal dissection for early gastric cancer in patients 80 years of age or older. Gastric Cancer. 2012;15:70–75. doi: 10.1007/s10120-011-0067-8. [DOI] [PubMed] [Google Scholar]
- 28.Higashiyama M, Oka S, Tanaka S, et al. Risk factors for bleeding after endoscopic submucosal dissection of gastric epithelial neoplasm. Dig Endosc. 2011;23:290–295. doi: 10.1111/j.1443-1661.2011.01151.x. [DOI] [PubMed] [Google Scholar]
- 29.Ahn JY, Jung HY, Choi KD, et al. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc. 2011;74:485–493. doi: 10.1016/j.gie.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 30.Goto O, Fujishiro M, Kodashima S, et al. A second-look endoscopy after endoscopic submucosal dissection for gastric epithelial neoplasm may be unnecessary: a retrospective analysis of postendoscopic submucosal dissection bleeding. Gastrointest Endosc. 2010;71:241–248. doi: 10.1016/j.gie.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 31.Muraki Y, Enomoto S, Iguchi M, Fujishiro M, Yahagi N, Ichinose M. Management of bleeding and artificial gastric ulcers associated with endoscopic submucosal dissection. World J Gastrointest Endosc. 2012;4:1–8. doi: 10.4253/wjge.v4.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okada K, Yamamoto Y, Kasuga A, et al. Risk factors for delayed bleeding after endoscopic submucosal dissection for gastric neoplasm. Surg Endosc. 2011;25:98–107. doi: 10.1007/s00464-010-1137-4. [DOI] [PubMed] [Google Scholar]
- 33.Takizawa K, Oda I, Gotoda T, et al. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection: an analysis of risk factors. Endoscopy. 2008;40:179–183. doi: 10.1055/s-2007-995530. [DOI] [PubMed] [Google Scholar]
- 34.Tsuji Y, Ohata K, Ito T, et al. Risk factors for bleeding after endoscopic submucosal dissection for gastric lesions. World J Gastroenterol. 2010;16:2913–2917. doi: 10.3748/wjg.v16.i23.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koh R, Hirasawa K, Yahara S, et al. Antithrombotic drugs are risk factors for delayed postoperative bleeding after endoscopic submucosal dissection for gastric neoplasms. Gastrointest Endosc. doi: 10.1016/j.gie.2013.03.008. Epub 2013 Apr 24. DOI: 10.1016/j.gie.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 36.ASGE Standards of Practice Committee. Anderson MA, Ben-Menachem T, et al. Management of antithrombotic agents for endoscopic procedures. Gastrointest Endosc. 2009;70:1060–1070. doi: 10.1016/j.gie.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 37.Boustière C, Veitch A, Vanbiervliet G, et al. Endoscopy and antiplatelet agents. European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2011;43:445–461. doi: 10.1055/s-0030-1256317. [DOI] [PubMed] [Google Scholar]
- 38.Toyonaga T. ESD ATLAS. Seoul: Hanguguihak; 2007. [Google Scholar]
- 39.Kikuchi D, Iizuka T, Hoteya S, et al. Usefulness of endoscopic ultrasound for the prediction of intraoperative bleeding of endoscopic submucosal dissection for gastric neoplasms. J Gastroenterol Hepatol. 2011;26:68–72. doi: 10.1111/j.1440-1746.2010.06412.x. [DOI] [PubMed] [Google Scholar]
- 40.Kikuchi D, Iizuka T, Hoteya S, et al. Prospective study about the utility of endoscopic ultrasound for predicting the safety of endoscopic submucosal dissection in early gastric cancer (T-HOPE 0801) Gastroenterol Res Pract. 2013;2013:329385. doi: 10.1155/2013/329385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakurazawa N, Kato S, Fujita I, Kanazawa Y, Onodera H, Uchida E. Supportive techniques and devices for endoscopic submucosal dissection of gastric cancer. World J Gastrointest Endosc. 2012;4:231–235. doi: 10.4253/wjge.v4.i6.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shin WG, Kim SJ, Choi MH, et al. Can rebamipide and proton pump inhibitor combination therapy promote the healing of endoscopic submucosal dissection-induced ulcers? A randomized, prospective, multicenter study. Gastrointest Endosc. 2012;75:739–747. doi: 10.1016/j.gie.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 43.Lee SH, Lee CK, Chung IK, et al. Optimal duration of proton pump inhibitor in the treatment of endoscopic submucosal dissection-induced ulcers: a retrospective analysis and prospective validation study. Dig Dis Sci. 2012;57:429–434. doi: 10.1007/s10620-011-1941-2. [DOI] [PubMed] [Google Scholar]
- 44.Kobayashi M, Takeuchi M, Hashimoto S, et al. Contributing factors to gastric ulcer healing after endoscopic submucosal dissection including the promoting effect of rebamipide. Dig Dis Sci. 2012;57:119–126. doi: 10.1007/s10620-011-1850-4. [DOI] [PubMed] [Google Scholar]
- 45.Kim YG, Jang BI, Kim TN. A matched case-control study of a novel acid-pump antagonist and proton-pump inhibitor for the treatment of iatrogenic ulcers caused by endoscopic submucosal dissection. Gut Liver. 2010;4:25–30. doi: 10.5009/gnl.2010.4.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Z, Wu Q, Liu Z, Wu K, Fan D. Proton pump inhibitors versus histamine-2-receptor antagonists for the management of iatrogenic gastric ulcer after endoscopic mucosal resection or endoscopic submucosal dissection: a meta-analysis of randomized trials. Digestion. 2011;84:315–320. doi: 10.1159/000331138. [DOI] [PubMed] [Google Scholar]
- 47.Ono S, Kato M, Ono Y, et al. Effects of preoperative administration of omeprazole on bleeding after endoscopic submucosal dissection: a prospective randomized controlled trial. Endoscopy. 2009;41:299–303. doi: 10.1055/s-0029-1214530. [DOI] [PubMed] [Google Scholar]
- 48.Niimi K, Fujishiro M, Goto O, et al. Prospective single-arm trial of two-week rabeprazole treatment for ulcer healing after gastric endoscopic submucosal dissection. Dig Endosc. 2012;24:110–116. doi: 10.1111/j.1443-1661.2011.01178.x. [DOI] [PubMed] [Google Scholar]
- 49.Yoshida Y, Matsuda K, Tamai N, et al. A pilot study using an infrared imaging system in prevention of post-endoscopic submucosal dissection ulcer bleeding. Gastric Cancer. doi: 10.1007/s10120-013-0231-4. Epub 2013 Feb 8. DOI: 10.1007/s10120-013-0231-4. [DOI] [PubMed] [Google Scholar]
- 50.Uedo N, Takeuchi Y, Ishihara R, et al. Endoscopic Doppler US for the prevention of ulcer bleeding after endoscopic submucosal dissection for early gastric cancer: a preliminary study (with video) Gastrointest Endosc. 2010;72:444–448. doi: 10.1016/j.gie.2010.03.1128. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Chen Y, Qu CY, Zhou M, Ni QW, Xu LM. Effects of medical adhesives in prevention of complications after endoscopic submucosal dissection. World J Gastroenterol. 2013;19:2704–2708. doi: 10.3748/wjg.v19.i17.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsui N, Sugi M, Bai X, et al. Effective hemostasis with hypertonic saline-epinephrine solution for uncontrolled bleeding during endoscopic submucosal dissection of the stomach. Dig Endosc. 2012;24:476. doi: 10.1111/j.1443-1661.2012.01322.x. [DOI] [PubMed] [Google Scholar]
- 53.Fujishiro M, Abe N, Endo M, et al. Current managements and outcomes of peptic and artificial ulcer bleeding in Japan. Dig Endosc. 2010;22(Suppl 1):S9–S14. doi: 10.1111/j.1443-1661.2010.00961.x. [DOI] [PubMed] [Google Scholar]
- 54.Enomoto S, Yahagi N, Fujishiro M, et al. Novel endoscopic hemostasis technique for use during endoscopic submucosal dissection. Endoscopy. 2007;39(Suppl 1):E156. doi: 10.1055/s-2006-925254. [DOI] [PubMed] [Google Scholar]