Abstract
Biliary stents are widely used not only for palliative treatment of malignant biliary obstruction but also for benign biliary diseases. Each plastic stent or self-expandable metal stent (SEMS) has its own advantages, and a proper stent should be selected carefully for individual condition. To compensate and overcome several drawbacks of SEMS, functional self-expandable metal stent (FSEMS) has been developed with much progress so far. This article looks into the outcomes and defects of each stent type for benign biliary stricture and describes newly introduced FSEMSs according to their functional categories.
Keywords: Bile ducts, extrahepatic; Biliary tract; Drug-eluting stents; Stents
INTRODUCTION
Over the last 20 years since using biliary stents for palliative treatment of malignant biliary obstruction, the indication of biliary stents has expanded continuously to include various benign biliary diseases. Endoscopic therapies and radiologic interventional therapies have developed rapidly and nonsurgical managements using advanced stent technologies, combined with high-tech, are replacing surgical operations for biliary diseases.
The most preferential treatments of benign biliary stricture are balloon dilatation and endoscopic retrograde biliary drainage (ERBD). Plastic stents used for ERBD are easy to apply and cheap but have insufficient diameter and, therefore, short stent patency, necessitating replacement every 3 months, and often cannot resolve stricture sufficiently. Thus, a widely used alternative is a multiple large-bore plastic stents insertion method, where multiple plastic stents are inserted to the biliary tract at some time intervals. This is a very effective method, but again, with short stent patency, multiple endoscopic procedures are required. Self-expandable metal stent (SEMS) can achieve larger lumen, providing longer stent patency, and thus, has been widely used traditionally for palliation of malignant biliary obstruction. Recently, SEMS is being used for various benign biliary strictures and biliary spillage. Covered self-expendable metal stents (CSEMSs), which are easy to remove, are used mostly among others. CSEMS for benign biliary stricture requires less endoscopic procedures, technically easier than inserting multiple large-bore plastic stents, and is reported to have 80% to 90% long-term success rate after stent removal.1 SEMS has several early and late complications, however. SEMS may cause early complications, such as biliary infection, pancreatitis, bleeding, perforation, early stent migration and renal failure, or late complications, mostly symptoms associated with stent dysfunction, or mucosal hyperplasia due to chronic inflammatory reaction, biliary obstruction and cholecystitis due to biliary sludge formation or food reflux, and stent migration.2
In order to compensate and overcome such drawbacks of SEMS in malignant or benign biliary stricture, functional self-expandable metal stent (FSEMS) has been developed, focusing on the design and shape modification, and much advances have been made by frontier investigators and stent companies. This article looks into the outcomes and defects of each stent type for benign biliary stricture and describes newly introduced FSEMSs according to their functional categories.
ENDOSCOPIC MANAGEMENT OF BENIGN BILIARY STRICTURE
Benign biliary stricture is associated with various kinds of diseases, including chronic pancreatitis, anastomotic stricture after liver transplantation, inflammatory diseases, and biliary stone. Biliary stricture should not be neglected and needs to be treated immediately in order to avoid secondary biliary cirrhosis and end-stage liver failure. Endoscopic treatment of benign biliary stricture has similar efficacy to surgical operation and is preferentially recommended as a less invasive treatment. The most common endoscopic treatment is multiple stent insertion method, where one or multiple plastic stents are inserted based on the theory that gradual and continuous dilatation of stricture area may induce tissue remodeling. For maximum effect, insertion or replacement of large-bore plastic stents (10 to 11.5 Fr) at a certain interval (every 3 to 6 months) throughout 1 year is recommended.3-5
However, such method actually requires patients to undergo at least three endoscopic procedures a year, and even then, stricture improvement is not sufficient and there is limited report about the effect on chronic pancreatitis. The reason for requiring reprocedure every 3 months is that the maximum diameter of plastic stents capable of being inserted through working channel is limited to 12 Fr, and such relatively narrow lumen predisposes stents to be easily occluded by biliary sludge and/or bacterial biofilm formation, resulting in average 3 months of stent patency.2,6
On this basis, SEMS has been suggested as a superior treatment for being able to reduce the number of endoscopic procedures by exerting immediate expansion power (up to 30 Fr or 10 mm) of stents deployed through relatively smaller (7 to 8 Fr) delivery system. In bare self-expandable metal stent (BSEMS), however, stent itself is recognized as a foreign body, causing benign reactive tissue ingrowth and resultant narrowing of the stent lumen.7 This causes irreversible tissue hyperplasia and stent integration into the tissue, precluding stent removal, which is why BSEMS is not recommended for benign diseases and is only used for malignant biliary stricture. Covered stents has been used based on the theory that the polymeric sheath covering a stent may work as a physical barrier to prevent tissue hyperplasia. Studies using partially covered self-expandable metal stents (PCSEMSs) reported 90% success rates for treating benign stricture, although the rate was lower in patients with chronic pancreatitis. In addition, stent removal was successful in every patient, stent migration occurred in 14% of patients, and some patients developed tissue hyperplasia.8 Other studies afterwards reported approximately 50% patency rate, which appeared to be decreased with longer follow-up period.9-11 In order to overcome the limitations by tissue hyperplasia in PCSEMS, fully covered self-expandable metal stents (FCSEMSs) have been studied. FCSEMS appears to have much higher stent patency than PCSEMS but very frequent stent migration.12-14 Moreover, in both PCSEMS and FCSEMS, the covering itself may cause the occlusion of the side branches of biliary tree, leading to secondary complications, and any polymer may work as a substrate for bile salt deposition and biofilms formation, inducing stent occlusion by stone or sludge formation. What's surprising is that high rates of occlusion by tumor ingrowth have been reported even for PCSEMS and FCSEMS.15
Taken together, it appears that SEMS has not surpassed the efficacy of multiple plastic stents insertion method yet.16 Considering the merits and demerits of each stent type, studies have started efforts to enhance the functions of stents to strengthen their strengths and make up for the weaknesses, and functional development of stents is still ongoing.
STUDIES FOR FUNCTIONAL STENT
Antimigratory stent
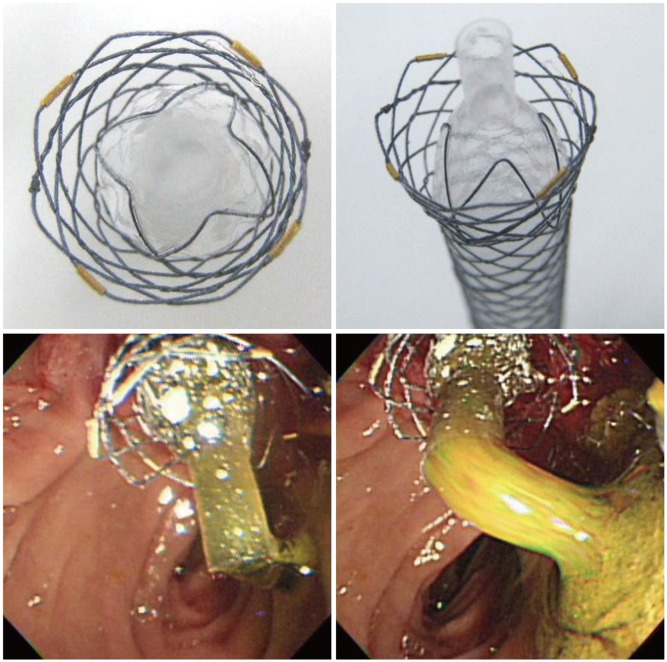
Among studies on stents with anchoring fin to prevent stent migration, Mahajan et al.17 reported a study on FCSEMS covered with Gore-tex expanded polytetrafluoroethylene (Viabil; Conmed, Utica, NY, USA) (Fig. 1). This stent has serrated anchoring pin protruding from part of the stent, contributing to potent antimigratory effect. It has been reported to achieve very high (83%) improvement in biliary stricture, but stent removal is not easy. Cholangioscopic examination, performed in half of the patients after stent removal, found biliary mucosal ulcer formation and hemorrhage by the anchoring pin.
Fig. 1.
Fully covered self-expandable metal stent attached with antimigratory anchoring fins (Viabil; Conmed).
A study using flared end FCSEMS (Niti-S; Taewoong Medical, Goyang, Korea), which has expanded shape at both ends of the stent to allow easier stent removal while maintaining the antimigratory effect at the same time, reported superior efficacy and lower migration rate than conventional FCSEMS but is limited by small sample size.18
Park et al.19 used flared end FCSEMS with anchoring flaps attached at the proximal area (M.I.Tech, Seoul, Korea) and reported promising results of 91% stricture improvement, with successful stent removal and without stent migration (Fig. 2). Although this study is limited by lack of observation on mucosal injury by the anchoring flap, further large study is expected.
Fig. 2.

Fully covered self-expandable metal stent attached with anchoring fins (M.I.Tech). Permission for use granted by M.I.Tech.
Antireflux stent
In inoperable pancreatobiliary malignancy, SEMS inserted for bile drainage provides long-term patency rate as it can obtain larger lumen than a plastic stent. However, insertion through the duodenal ampulla may induce duodeno-biliary reflux, leading to ascending cholangitis. A study using barium found that duodeno-biliary reflux occurred in every patient.20
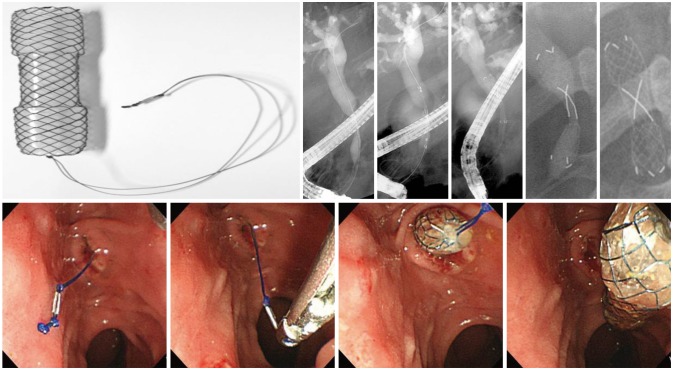
The theory of accelerated stent occlusion due to biofilm formation and food-bile mixing contents led to the development of antireflux stent, where an antireflux valve is attached to a conventional plastic stent. Despite being a plastic stent, it was found to have approximately 1.5 times longer stent patency period, but follow-up studies have not been performed yet.21 For the same purpose, studies on SEMS with antireflux valve have been conducted (Figs. 3, 4).22-24 Results of such studies were not always positive, however, because the attached antireflux valve often caused malfunction depending on its design. Antireflux valve designed to minimize the risk of malfunction led to decreased resistance for antireflux function, while that designed for greater resistance interfered with bile drainage, working against the original purpose of stent insertion. A new type of antireflux valve is currently being developed to overcome such issues.
Fig. 3.
Antireflux fully covered self-expandable metal stent (FCSEMS; M.I.Tech). FCSEMS attached with antireflux valve at distal ends to enable biliary drainage while preventing reflux of contents. Permission for use granted by M.I.Tech.
Fig. 4.
Fully covered self-expandable metal stent with ball-shaped distal ends (Bumpy stent; Taewoong Medical). Permission for use granted by Taewoong Medical and by courtesy of Prof. Jae Chul Hwang, Ajou University, Korea.
Easy removable stent or shape-modifying stent
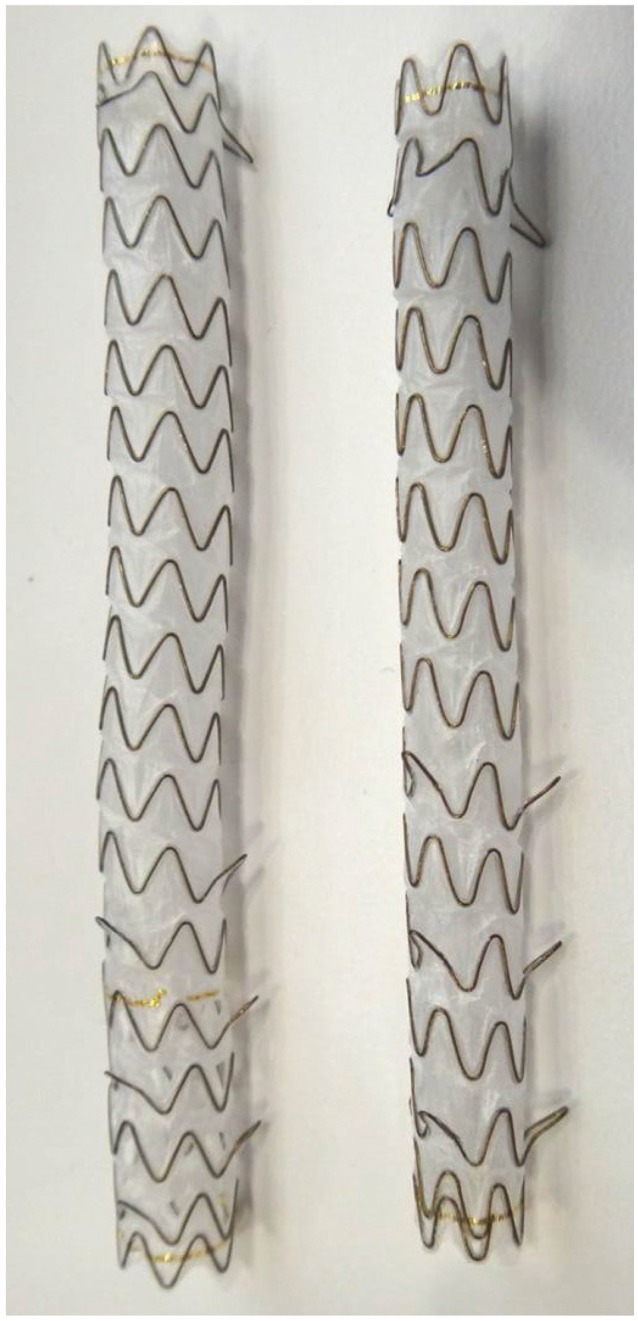
Stenotic areas are very short (0.5 to 1.0 cm) in conditions such as anastomotic stenosis after liver transplantation. Using relatively long SEMS may cause tissue necrosis and fibrosis due to pressure injuries to normal biliary tract and, on the contrary, using too short SEMS may increase the risk of stent migration. Easy removable FCSEMS was developed to make up for such flaws (Fig. 5).25-27 Such shape-modifying FCSEMS has slightly narrower waist to prevent stent migration and a long lasso for easier removal of the stent in the future.
Fig. 5.
A shape-modifying fully covered self-expandable metal stent (BONASTENT M-Intraductal; Standard Sci-Tech Inc.) and cases of stent insertion for benign biliary stricture. Lasso is placed in the duodenal lumen outside the duodenal ampulla for easier stent removal in the future. Permission for use granted by Standard Sci-Tech Inc. and courtesy of Prof. Jong Ho Moon, Soonchunhyang University, Korea.
One of interesting studies on shape modification developed and used a star-shaped, winged plastic stent with small central lumen based on the theory that tubular plastic stents contribute to biofilm formation and luminal occlusion.28 It would be interesting to try various shapes of SEMS by using this concept.
Antihyperplasia stent or drug-eluting stent
Patients with gastrointestinal cancer induced obstruction often experience aggravation in their clinical status due to delayed stent occlusion, despite earlier clinical improvement after inserting a metal stent. Although covered SEMS is designed to endure tumor growth relatively longer than BSEMS, stent occlusion is inevitable over time in most cases due to the natural history of malignant tumor. This led to the development of drug eluting stent and radioactive stent, which are groundbreaking efforts to improve stent patency not just by structural modification but by adding functions such as antihyperplasia or antitumor effect.
Drug-eluting stent, which is highly used for vascular interventional treatment, has not been commercially available for the bile duct yet. Studies have been started based on the ideas of maximizing the effect of local drug-elution to relieve malignant obstruction, to delay luminal restenosis due to tumor ingrowth, or to prevent mucosal hyperplasia in benign stricture.29 Among the front runners are drug-eluting FCSEMS using paclitaxel, although the observed stent patency and patient survival have not been significantly different yet.30,31 These studies have limitations in lack of ample laboratory findings to support the results and lack of consideration on stent design to enhance patency rate. Interestingly, another study in South Korea found higher tumor growth rate in drug-eluting stent insertion group than in the control group.32
Which drug is more appropriate, or whether local control of malignant biliary obstruction is possible, have not been clarified yet. Studies using paclitaxel demonstrated its effect by ex vivo cell culture assays, but it is not effective as a systemic chemotherapy for pancreatic cancer or hepatobiliary cancer. Gemcitabine and fluorouracile has gained attention in this light, but the key is how to overcome their weakness of rapid drug emission as hydrophilic drugs. Unlike vascular interventional stents, a drug-eluting stent needs a new design to allow longer drug elution throughout broader contact surface between the stent and tumor and should be able to maintain constant release of drug, which can mix easily with the ingredients of the stent membrane. It also should be able to prevent bile-induced membrane damage to allow longer drug release. Various studies are currently underway to determine appropriate drug concentration and type and shape of stent membrane.33-35
Radioactive stent
A radioactive material can be attached to stents for a kind of brachytherapy in addition to expanding stenotic lumen. The purposes of continuous low-dose radiation inside the lumen after stent expansion is to inhibit tumor growth, prolong stent patency rate, and even to try palliative therapy of tumor itself.
Radioactive stent has iodine-125 seed, a radioactive material, implanted on the stent, the inside of which containing conventional metal stent to allow technically easier procedure. Since irregular arrangement of seeds may cause some sites not receiving irradiation, seeds should be arranged regularly and symmetrically at 10 to 15 mm intervals. An iodine-125 seed has 59.6 days of half-life, approximately 7.7 cGy irradiation per hour, and 20 mm of effective irradiating distance. The number of radioactive seeds should be selected based on treatment plan established for individual patient.
Most studies using radioactive stents are focused on esophageal or biliary malignant obstruction. Many of them suggested that radioactive stent were relatively safe and easy to apply. Some of them claimed longer stent patency period and survival period than conventional metal stents with statistically significant difference, although clinical application is still quite early considering their small sample sizes.36-38
Bioabsorbable stent
Bioabsorbable self-expandable stent has various advantages compared to conventional plastic stent or SEMS; larger lumen than plastic stent allows improved patency rate and reduced biofilm formation, it does not require removal of stent and it can reduce biliary mucosal hyperplasia compared to SEMS and it can also be combined with antibacterial or antitumor materials, or equipped with drug-eluting function.
Bioabsorbable stent is a braided structure of filaments made of absorbent polydioxanone or polylactide. Barium sulfate may also be included when making the filament for radiopaque property. Radial force may be enhanced by installing an elastomeric block inside the stent. Since the radial force of bioabsorbable stent is weaker than conventional metal stent, however, additional expansion of the lumen by balloon dilatation is required after stent insertion.
Bioabsorbable stent is still in laboratory stages, without clinical use so far.39-42 It is noteworthy that recently a research team in Finland has gained an unparalleled position in this field when they reported the results of a phase 1 clinical study after long and hard work in animal tests.43
Choosing from different types of functional stents
As described above, each functional stent has different advantages and disadvantages. Efficacy of each functional stent needs to be confirmed by additional prospective, large-scale clinical studies. It would be also very important, at this point, to determine which functional stent should be used for which target disease, based on the advantages and disadvantages of each stent, although such data are still very scant. For malignant biliary obstruction, radioactive or drug-eluting stents with longer stent patency might be used as an alternative to overcome tumor growth and, furthermore, to elicit antitumor effect. In addition, antimigratory, easy removal or bioabsorbable stents might be used for benign biliary stricture with the purpose of maintenance for certain period of time. With more elaborate nanotechnology and drug-eluting technology in the human body, far more ideal stents with broader indications will be available for more varied clinical diseases in the future.
CONCLUSIONS
When metal stent was first introduced to digestive tract 20 years, it was a groundbreaking treatment but not without certain problems. Although easier excess than operation and relatively simpler treatment of malignancy induced swallowing difficulty, defecation difficulty, or jaundice made it a considerably attractive treatment method. However, metal stent had to go through continuous structural changes to overcome issues regarding stent migration and stent occlusion due to tumor ingrowth or overgrowth. The above mentioned new functional stents still lack clinical data, but with continuous efforts to make up for their weak points, they will provide a new paradigm in stenting. Functional stents are developing rapidly in various aspects and, with the help of more elaborate nanotechnology and advanced drug-elution control, they are expected to be able to accomplish superior outcomes than currently available stents
Acknowledgments
This work was supported by a 2-year Research Grant of Pusan National University. We wish to thank Prof. Jong Ho Moon (Soonchunhyang University, Korea) and Jae Chul Hwang (Ajou University, Korea) for courtesy of excellent pictures. We also thank M.I.Tech, Taewoong Medical, and Standard Sci-Tech Inc. for permission for stents pictures.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Baron TH. Covered self-expandable metal stents for benign biliary tract diseases. Curr Opin Gastroenterol. 2011;27:262–267. doi: 10.1097/MOG.0b013e3283438a26. [DOI] [PubMed] [Google Scholar]
- 2.Kim JH. Endoscopic stent placement in the palliation of malignant biliary obstruction. Clin Endosc. 2011;44:76–86. doi: 10.5946/ce.2011.44.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costamagna G, Pandolfi M, Mutignani M, Spada C, Perri V. Long-term results of endoscopic management of postoperative bile duct strictures with increasing numbers of stents. Gastrointest Endosc. 2001;54:162–168. doi: 10.1067/mge.2001.116876. [DOI] [PubMed] [Google Scholar]
- 4.Costamagna G, Tringali A, Mutignani M, et al. Endotherapy of postoperative biliary strictures with multiple stents: results after more than 10 years of follow-up. Gastrointest Endosc. 2010;72:551–557. doi: 10.1016/j.gie.2010.04.052. [DOI] [PubMed] [Google Scholar]
- 5.Perri V, Familiari P, Tringali A, Boskoski I, Costamagna G. Plastic biliary stents for benign biliary diseases. Gastrointest Endosc Clin N Am. 2011;21:405–433. doi: 10.1016/j.giec.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Raijman I. Biliary and pancreatic stents. Gastrointest Endosc Clin N Am. 2003;13:561–592. doi: 10.1016/s1052-5157(03)00069-2. [DOI] [PubMed] [Google Scholar]
- 7.Silvis SE, Sievert CE, Jr, Vennes JA, Abeyta BK, Brennecke LH. Comparison of covered versus uncovered wire mesh stents in the canine biliary tract. Gastrointest Endosc. 1994;40:17–21. doi: 10.1016/s0016-5107(94)70004-4. [DOI] [PubMed] [Google Scholar]
- 8.Kahaleh M, Behm B, Clarke BW, et al. Temporary placement of covered self-expandable metal stents in benign biliary strictures: a new paradigm? (with video) Gastrointest Endosc. 2008;67:446–454. doi: 10.1016/j.gie.2007.06.057. [DOI] [PubMed] [Google Scholar]
- 9.Chaput U, Scatton O, Bichard P, et al. Temporary placement of partially covered self-expandable metal stents for anastomotic biliary strictures after liver transplantation: a prospective, multicenter study. Gastrointest Endosc. 2010;72:1167–1174. doi: 10.1016/j.gie.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 10.Cantù P, Hookey LC, Morales A, Le Moine O, Devière J. The treatment of patients with symptomatic common bile duct stenosis secondary to chronic pancreatitis using partially covered metal stents: a pilot study. Endoscopy. 2005;37:735–739. doi: 10.1055/s-2005-870130. [DOI] [PubMed] [Google Scholar]
- 11.Behm B, Brock A, Clarke BW, et al. Partially covered self-expandable metallic stents for benign biliary strictures due to chronic pancreatitis. Endoscopy. 2009;41:547–551. doi: 10.1055/s-0029-1214708. [DOI] [PubMed] [Google Scholar]
- 12.Traina M, Tarantino I, Barresi L, et al. Efficacy and safety of fully covered self-expandable metallic stents in biliary complications after liver transplantation: a preliminary study. Liver Transpl. 2009;15:1493–1498. doi: 10.1002/lt.21886. [DOI] [PubMed] [Google Scholar]
- 13.García-Pajares F, Sánchez-Antolín G, Pelayo SL, et al. Covered metal stents for the treatment of biliary complications after orthotopic liver transplantation. Transplant Proc. 2010;42:2966–2969. doi: 10.1016/j.transproceed.2010.07.084. [DOI] [PubMed] [Google Scholar]
- 14.Cahen DL, Rauws EA, Gouma DJ, Fockens P, Bruno MJ. Removable fully covered self-expandable metal stents in the treatment of common bile duct strictures due to chronic pancreatitis: a case series. Endoscopy. 2008;40:697–700. doi: 10.1055/s-2008-1077353. [DOI] [PubMed] [Google Scholar]
- 15.Shim CS, Cho YD, Kim YS, et al. Long term follow up results of membrane covered self expanding metal stent in patients with malignant biliary obstruction. Gastrointest Endosc. 1999;49(4 Suppl):AB131. [Google Scholar]
- 16.van Boeckel PG, Vleggaar FP, Siersema PD. Plastic or metal stents for benign extrahepatic biliary strictures: a systematic review. BMC Gastroenterol. 2009;9:96. doi: 10.1186/1471-230X-9-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahajan A, Ho H, Sauer B, et al. Temporary placement of fully covered self-expandable metal stents in benign biliary strictures: midterm evaluation (with video) Gastrointest Endosc. 2009;70:303–309. doi: 10.1016/j.gie.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 18.Tringali A, Familiari P, Mutignani M, Perri V, Costamagna G. Self-expandable, removable, fully covered metal stents to dilate common bile duct strictures secondary to chronic pancreatitis: preliminary results. Gastrointest Endosc. 2010;71:AB169–AB170. [Google Scholar]
- 19.Park DH, Lee SS, Lee TH, et al. Anchoring flap versus flared end, fully covered self-expandable metal stents to prevent migration in patients with benign biliary strictures: a multicenter, prospective, comparative pilot study (with videos) Gastrointest Endosc. 2011;73:64–70. doi: 10.1016/j.gie.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 20.Misra SP, Dwivedi M. Reflux of duodenal contents and cholangitis in patients undergoing self-expanding metal stent placement. Gastrointest Endosc. 2009;70:317–321. doi: 10.1016/j.gie.2008.12.054. [DOI] [PubMed] [Google Scholar]
- 21.Dua KS, Reddy ND, Rao VG, Banerjee R, Medda B, Lang I. Impact of reducing duodenobiliary reflux on biliary stent patency: an in vitro evaluation and a prospective randomized clinical trial that used a biliary stent with an antireflux valve. Gastrointest Endosc. 2007;65:819–828. doi: 10.1016/j.gie.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Hu B, Wang TT, Shi ZM, et al. A novel antireflux metal stent for the palliation of biliary malignancies: a pilot feasibility study (with video) Gastrointest Endosc. 2011;73:143–148. doi: 10.1016/j.gie.2010.08.048. [DOI] [PubMed] [Google Scholar]
- 23.Kim DU, Kwon CI, Kang DH, Ko KH, Hong SP. New antireflux self-expandable metal stent for malignant lower biliary obstruction: in vitro and in vivo preliminary study. Dig Endosc. 2013;25:60–66. doi: 10.1111/j.1443-1661.2012.01324.x. [DOI] [PubMed] [Google Scholar]
- 24.Hwang JC, Kim JH, Yoo BM, et al. Temporary placement of a newly designed, fully covered, self-expandable metal stent for refractory bile leaks. Gut Liver. 2011;5:96–99. doi: 10.5009/gnl.2011.5.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tee HP, James MW, Kaffes AJ. Placement of removable metal biliary stent in post-orthotopic liver transplantation anastomotic stricture. World J Gastroenterol. 2010;16:3597–3600. doi: 10.3748/wjg.v16.i28.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moon JH, Choi HJ, Koo HC, et al. Feasibility of placing a modified fully covered self-expandable metal stent above the papilla to minimize stent-induced bile duct injury in patients with refractory benign biliary strictures (with videos) Gastrointest Endosc. 2012;75:1080–1085. doi: 10.1016/j.gie.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 27.Hu B, Gao DJ, Yu FH, Wang TT, Pan YM, Yang XM. Endoscopic stenting for post-transplant biliary stricture: usefulness of a novel removable covered metal stent. J Hepatobiliary Pancreat Sci. 2011;18:640–645. doi: 10.1007/s00534-011-0408-3. [DOI] [PubMed] [Google Scholar]
- 28.Raju GS, Sud R, Elfert AA, Enaba M, Kalloo A, Pasricha PJ. Biliary drainage by using stents without a central lumen: a pilot study. Gastrointest Endosc. 2006;63:317–320. doi: 10.1016/j.gie.2005.07.053. [DOI] [PubMed] [Google Scholar]
- 29.Lee DK. Drug-eluting stent in malignant biliary obstruction. J Hepatobiliary Pancreat Surg. 2009;16:628–632. doi: 10.1007/s00534-009-0135-1. [DOI] [PubMed] [Google Scholar]
- 30.Suk KT, Kim JW, Kim HS, et al. Human application of a metallic stent covered with a paclitaxel-incorporated membrane for malignant biliary obstruction: multicenter pilot study. Gastrointest Endosc. 2007;66:798–803. doi: 10.1016/j.gie.2007.05.037. [DOI] [PubMed] [Google Scholar]
- 31.Song TJ, Lee SS, Yun SC, et al. Paclitaxel-eluting covered metal stents versus covered metal stents for distal malignant biliary obstruction: a prospective comparative pilot study. Gastrointest Endosc. 2011;73:727–733. doi: 10.1016/j.gie.2010.11.048. [DOI] [PubMed] [Google Scholar]
- 32.Jang SI, Kim JH, You JW, et al. Efficacy of a metallic stent covered with a paclitaxel-incorporated membrane versus a covered metal stent for malignant biliary obstruction: a prospective comparative study. Dig Dis Sci. 2013;58:865–871. doi: 10.1007/s10620-012-2472-1. [DOI] [PubMed] [Google Scholar]
- 33.Jang SI, Kim JH, Kim M, et al. Porcine feasibility and safety study of a new paclitaxel-eluting biliary stent with a Pluronic-containing membrane. Endoscopy. 2012;44:825–831. doi: 10.1055/s-0032-1309881. [DOI] [PubMed] [Google Scholar]
- 34.Chung MJ, Kim H, Kim KS, Park S, Chung JB, Park SW. Safety evaluation of self-expanding metallic biliary stents eluting gemcitabine in a porcine model. J Gastroenterol Hepatol. 2012;27:261–267. doi: 10.1111/j.1440-1746.2011.06866.x. [DOI] [PubMed] [Google Scholar]
- 35.Gwon DI, Lee SS, Kim EY. Cefotaxime-eluting covered self-expandable stents in a canine biliary model: scanning electron microscopic study of biofilm formation. Acta Radiol. 2012;53:1127–1132. doi: 10.1258/ar.2012.120220. [DOI] [PubMed] [Google Scholar]
- 36.Zhongmin W, Xunbo H, Jun C, et al. Intraluminal radioactive stent compared with covered stent alone for the treatment of malignant esophageal stricture. Cardiovasc Intervent Radiol. 2012;35:351–358. doi: 10.1007/s00270-011-0146-6. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Liu JL, Cai ZZ, et al. A novel approach for treatment of unresectable extrahepatic bile duct carcinoma: design of radioactive stents and an experimental trial in healthy pigs. Gastrointest Endosc. 2009;69(3 Pt 1):517–524. doi: 10.1016/j.gie.2008.05.069. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, Liu JL, Cai ZZ, et al. A novel approach for treatment of unresectable pancreatic cancer: design of radioactive stents and trial studies on normal pigs. Clin Cancer Res. 2007;13:3326–3332. doi: 10.1158/1078-0432.CCR-07-0154. [DOI] [PubMed] [Google Scholar]
- 39.Ginsberg G, Cope C, Shah J, et al. In vivo evaluation of a new bioabsorbable self-expanding biliary stent. Gastrointest Endosc. 2003;58:777–784. doi: 10.1016/s0016-5107(03)02016-9. [DOI] [PubMed] [Google Scholar]
- 40.Meng B, Wang J, Zhu N, Meng QY, Cui FZ, Xu YX. Study of biodegradable and self-expandable PLLA helical biliary stent in vivo and in vitro. J Mater Sci Mater Med. 2006;17:611–617. doi: 10.1007/s10856-006-9223-9. [DOI] [PubMed] [Google Scholar]
- 41.Itoi T, Kasuya K, Abe Y, Isayama H. Endoscopic placement of a new short-term biodegradable pancreatic and biliary stent in an animal model: a preliminary feasibility study (with videos) J Hepatobiliary Pancreat Sci. 2011;18:463–467. doi: 10.1007/s00534-010-0364-3. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto K, Yoshioka T, Furuichi K, et al. Experimental study of poly-L-lactic acid biodegradable stents in normal canine bile ducts. Cardiovasc Intervent Radiol. 2011;34:601–608. doi: 10.1007/s00270-010-0045-2. [DOI] [PubMed] [Google Scholar]
- 43.Nordback I, Räty S, Laukkarinen J, et al. A novel radiopaque biodegradable stent for pancreatobiliary applications--the first human phase I trial in the pancreas. Pancreatology. 2012;12:264–271. doi: 10.1016/j.pan.2012.02.016. [DOI] [PubMed] [Google Scholar]