Abstract
Percutaneous transhepatic cholangioscopy (PTCS) is the most widely used modality for diagnosis and treatment of biliary disease. Although many other novel technologies have been developed based on recent advances in endoscopy, PTCS has its own role. In diagnostics, PTCS is used for evaluation of indeterminate biliary strictures, bile duct tumors, and postoperative biliary strictures that cannot be reached by a peroral approach. In therapeutics, the removal of bile duct stones, dilatation of bile duct strictures including postoperative anastomosis site strictures, and local tumor therapy are indications of PTCS. Especially in a therapeutic role, PTCS has the advantage of maneuverability due to a shorter endoscopic length compared to other cholangioscopic modalities. Hence, PTCS has its own indispensable diagnostic and therapeutic roles.
Keywords: Percutaneous transhepatic cholangioscopy, Diagnostic role, Therapeutic role
INTRODUCTION
Direct visualization of the biliary system has advantages for the identification and treatment of intraductal lesions compared to indirect imaging of endoscopic retrograde cholangiopancreatography (ERCP). Intraductal endoscopy for the biliary system has been used since the initial practice of biliary surgery. The first optical choledochoscopy equipped with a lens, a distal light source, and a rinsing device was described in 1941 by McIver,1 and intraoperative cholangioscopy was successfully utilized in the 1960s by several groups. After then, modern concepts of peroral cholangioscopy (POCS) and percutaneous transhepatic cholangioscopy (PTCS) were introduced in the mid-1970s. PTCS has been the most widely used modality for diagnosis and treatment of biliary disease since it was first described by Takada et al.2 in 1974. Although it has limitations, such as the complexity of preparation before the procedure, the invasiveness of the procedure itself, and the need for a well-trained team of endoscopist, radiologist, and technician, PTCS has its own diagnostic and therapeutic roles (Table 1).3 In this review, we will discuss the role of PTCS for management of various biliary diseases.
Table 1.
The Diagnostic and Therapeutic Roles of Percutaneous Transhepatic Cholangioscopy3

a)Performed with percutaneous cholecystoscopy.
DIAGNOSTIC ROLES OF PTCS
PTCS is usually used for patients who are not able to tolerate a peroral endoscopic procedure due to cardiopulmonary problems and have altered gastrointestinal anatomy due to a previous operation. In addition to structural considerations, PTCS can provide high quality images of the biliary system and help to make a more accurate diagnosis in some cases.
Biliary stricture
Accurate diagnosis of a biliary stricture is essential, as the choice of an appropriate treatment modality depends on whether the stricture is benign or malignant. Especially in Far East Asia where there is a high incidence of hepatolithiasis and benign biliary stricture, a clinical decision for differentiating benign and malignant biliary stricture is frequently encountered. A mainstay of diagnosing biliary strictures is tissue sampling with ERCP. Conventional tissue sampling methods with ERCP include brush cytology and fluoroscopically guided endobiliary biopsy. Although these methods are simple and safe,4 the problem is low diagnostic yield. The sensitivity of brush cytology for malignant biliary stricture ranges from only 30% to 57%4-6 and that of endobiliary biopsy from 43% to 65%.6,7 For overcoming this limitation, several studies have tried and shown that combining several techniques for obtaining tissue samples from a biliary stricture at ERCP improved the diagnostic yield of biliary malignancy. Jailwala et al.6 reported that combinations of tissue sampling techniques had a higher diagnostic yield than that of any single modality for evaluating patients with suspected malignant biliary strictures. In their series of 133 patients with biliary strictures, the cumulative sensitivity of triple-tissue sampling-the combination of brush cytology, endoluminal fine needle aspiration, and endobiliary biopsy-improved to 77% compared to that of single tissue sampling. However, their low negative predictive value of 44% for malignant biliary strictures remains a limitation of conventional tissue sampling with ERCP, and this means that cytopathologic results obtained in one session of ERCP are unreliable. This problem would be inevitable without using direct visual inspection for lesions.
PTCS has an important role in differentiating benign and malignant biliary strictures. Direct visual inspection of the bile duct and precise target biopsy for a suspected lesion can overcome low diagnostic accuracy of other modalities in cases of undifferentiated strictures, and numerous studies have evaluated the role of cholangioscopy in undifferentiated biliary strictures. Typical cholangioscopic features of benign biliary stricture include smooth mucosal surface, tapered luminal narrowing, shorter length of stricture segment, multiple stricture sites, frequent presence of stones and cholangitis, and absence of definite neovascularization-the tumor vessel.8 A tumor vessel is an abnormally proliferating and tortuous vascular structure on the bile duct mucosa and is one representative feature of biliary malignancy (Fig. 1). Kim et al.9 reported that PTCS-guided biopsies combined with visual diagnosis of a tumor vessel significantly improved sensitivity and negative predictive value to 96% and 91%, respectively. In their series of 63 patients with biliary strictures, six of eight patients with negative PTCS-guided biopsies but a final diagnosis of malignancy, showed a tumor vessel. Direct visual inspection can help to make an accurate diagnosis especially in infiltrative cholangiocarcinoma, because obtaining an adequate biopsy specimen for infiltrative cholangiocarcinoma is sometimes difficult, even with PTCS, due to the striking fibrotic and inflammatory stromal responses around the cancer.
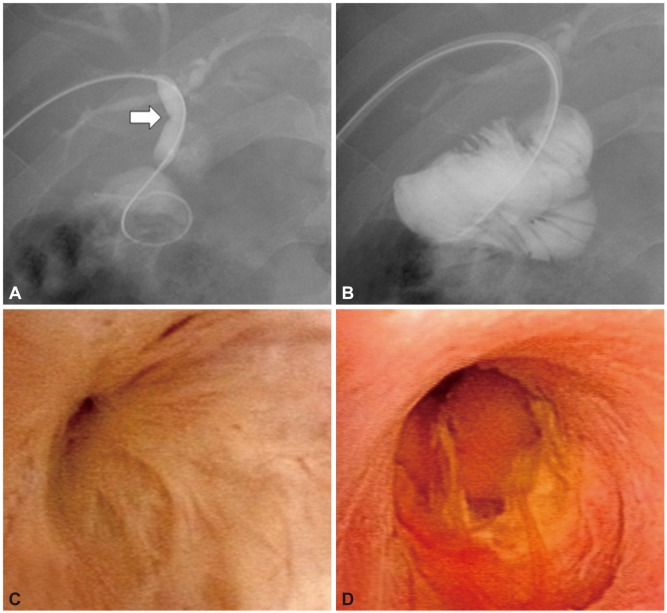
Fig. 1.
Tumor vessel-abnormally proliferating and tortuous vascular structure on the bile duct mucosa. (A, B) Two cases of indeterminate far distal bile duct lesions that could not be diagnosed with conventional endoscopic retrograde cholangiopancreatography.
Some stricture locations cannot be approached in a retrograde manner, such as used with ERCP or POCS. PTCS can be used as a valuable diagnostic modality for strictures in the far distal common bile duct stricture, hepatic hilum, and focal strictures of the intrahepatic duct (IHD) unrelated to stone. Nimura et al.10 performed 428 cases of PTCS in biliary strictures and reported that PTCS was a useful diagnostic procedure for biliary tract strictures in the distal bile duct and hepatic hilum. There are some conditions in a distal common bile duct stricture that make tissue sampling difficult using ERCP or POCS even in normal gastrointestinal anatomy. Evaluation by POCS is technically difficult particularly in the far distal common bile duct because it is hard to secure enough margins between the tip of endoscopy and the lesion for biopsy if the lesion is too close to the orifice of ampulla of Vater. Kim et al.11 evaluated the sensitivity and specificity of PTCS in 95 patients whose stricture diagnosis of benign or malignant was inconclusive, and compared these results with those of other modalities. In their study, the sensitivity and specificity for distal common bile duct stricture using a combination of PTCS-guided biopsy and tumor vessel identification were 96% and 100%, respectively-results that were superior to computed tomography (CT), magnetic resonance cholangiopancreatography (MRCP), and direct cholangiography.
Several reports have documented the usefulness of PTCS with respect to hilar strictures.10,12,13 In a study by Jung and colleagues,12 14 (7.9%) of 177 patients whose hilar strictures had been diagnosed as suspicious for malignant lesions on CT or MRCP were confirmed as benign with PTCS; diagnostic accuracy of PTCS was 88.4% by combined findings of tumor vessels and histological examination. IHD strictures that are common in Far East Asia are usually associated with an IHD stone or parasitic disease, which causes repeated episodes of cholangitis. However, focal or segmental strictures of the IHD without any evidence of an IHD stone or parasitic disease often pose diagnostic problems, and it is usually impossible to obtain a retrograde forceps biopsy specimen because of the proximal location of the stricture in these kinds of strictures. Seo et al.14 performed PTCS in 17 patients with focal strictures of the IHD without evidence of stones and successfully diagnosed 12 malignant strictures and five benign strictures. They concluded that PTCS in this situation was useful for diagnosis, including the detection of early bile duct cancer.
The more recent narrow band imaging (NBI) technique improves the diagnostic ability of PTCS for indeterminate biliary strictures. More accurate evaluation of bile duct mucosa is possible with NBI for detecting fine surface mucosal strictures and microvasculatures (Fig. 2). Itoi et al.15 reported that identification of the surface structures and vessels of the bile duct lesions by NBI observation was significantly better than with conventional observation by white light imaging. Their study was performed using peroral video cholangioscopy in development, but the NBI system can be easily applied to PTCS and used more widely than POCS because most PTCS uses video endoscopy and ensures high-quality images.
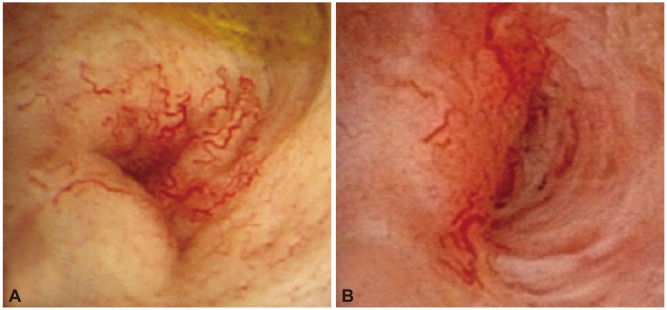
Fig. 2.
Comparison of conventional white light imaging and narrow band imaging in the case of recurred gallbladder cancer after cholecystectomy. (A) The white light imaging shows tumor vessels and a polypoid mucosal lesion. (B) The narrow band imaging shows papillary structures of mucosal surface (white arrow) and microvasculatures (black arrow) more definitely than white light imaging.
Biliary tumor
As mentioned above, one of the key roles of PTCS is the diagnosis of cholangiocarcinoma. The precise evaluation of longitudinal tumor extension is very important because this determines resectability and choice of treatment modality. Bile duct adenocarcinomas are classified into three different types according to morphologic pattern: nodular, papillary, and infiltrative.8 Although a nodular or stenotic bile duct tumor can be diagnosed without additional cholangioscopic findings, it is hard to make a definite diagnosis and preoperative staging in cases of papillary or infiltrative cholangiocarcinoma, which spreads superficially along the bile duct mucosa, without direct visualization of bile duct lesions. PTCS-guided mapping biopsy for these types of cholangiocarcinoma is helpful for defining the proximal and distal extension of the tumor and designing a type of resection.13 Lee et al.16 evaluated the discrepancy in diagnosis of hilar cholangiocarcinoma between PTCS and MRCP. In their series of 99 patients, there was relatively good overall agreement between these two modalities on the longitudinal extension of the tumor for the stenotic type of hilar cholangiocarcinoma. For polypoid or diffuse sclerosing types, however, the accuracy of MRCP was significantly lower compared with that of the stenotic type. Tamada et al.17 evaluated the accuracy of cholangiography in assessing the longitudinal extent of cholangiocarcinoma and found only 63% accuracy compared to 83% for PTCS-guided mapping biopsies. The findings of their studies demonstrate that PTCS is necessary for accurate preoperative assessment of longitudinal tumor extension. Compared to POCS, PTCS is a more invasive diagnostic procedure, but it has the advantage of precise preoperative diagnosis in the case of more complex cholangiocarcinoma. Because the proximal extension of the tumor cannot be examined by POCS in common types of cholangiocarcinoma obstructing the bile duct, PTCS should be performed for preoperative staging of this type of cholangiocarcinoma.
PTCS is also essential for diagnosis of intraductal papillary mucinous neoplasm of the bile duct (IPMN-B). Because this type of tumor is usually flat, small, or masked by thick mucin produced by the tumor and has a tendency of superficial spreading similar to the infiltrative cholangiocarcinoma, the lesion can be missed or underdiagnosed with cholangiography or other radiologic modalities.18-21 Lee et al.20 evaluated 58 cases of IPMN-B and concluded that direct visual inspection of IPMN-B was important to determine the classification and prognosis of disease. They reported that prominent papillary tumors on PTCS findings were correlated with papillary adenocarcinoma, whereas tiny, villous, coral reef-like projections and fish egg-like mucosal changes were correlated with benign papillary adenoma. In addition, saline irrigation was necessary for detection on the wall of the bile duct because various papillary mucosal lesions were covered with thick mucin. Although this disease is rare, PTCS is a useful method in assessing the diagnosis and preoperative staging of IPMN-B.
Bilioenteric anastomosis site
When tumor recurrence or benign stricture is suspected at a bilioenteric anastomosis site, PTCS is the most reliable choice for diagnosis. A peroral approach to the bilioenteric anastomosis site is usually difficult when the anatomy has been altered by surgery. PTCS can also be used as a therapeutic modality in such benign stricture cases. This topic will be discussed later.
THERAPEUTIC ROLES OF PTCS
PTCS is an effective and safe method for treating complicated choledocholithiasis, benign biliary or bilioenteric strictures, and bile duct tumor using local therapy such as argon plasma coagulation (APC) ablation, laser therapy, and photodynamic therapy (PDT). The maneuverability of a shorter cholangioscope compared to POCS enables a more precise and less cumbersome procedure. Thus, the value of PTCS in therapeutics has not changed despite recent advances in other diagnostic modalities.
Cholelithiasis: bile duct stone
Patients with complicated cholelithiasis and who have failed ERCP are good candidates for bile duct stone removal with PTCS. Several methods, including basket stone removal, electrohydraulic shock-wave lithotripsy (EHL), and laser lithotripsy, are used for the removal of bile duct stones with PTCS. Direct contact with stones by EHL or a laser lithotripsy probe is particularly useful and safe for the fragmentation of hard stones that cannot be destroyed by mechanical basket lithotripsy while avoiding mucosal injury of bile duct.
IHD stones-hepatolithiasis-are frequent in East Asia, including Taiwan, Japan, and Korea.22 Hepatolithiasis is often associated with bile duct stenoses or strictures that lead to the development of recurrent cholangitis and secondary biliary cirrhosis. These conditions may result in severe complications, such as biliary sepsis, liver abscess, portal hypertension, and cholangiocarcinoma; therefore, appropriate therapies for hepatolithiasis are essential. Radical treatment for hepatolithiasis is surgical removal of the stones via resection of the stenotic bile duct and the destroyed hepatic parenchyma.23 However, application of this method is difficult in patients with stones in more than two lobes of the liver, history of previous biliary surgery, high postoperative risk, or who refuse surgery. Although bilateral hepatectomy has been reported to be safe and effective following improvements in surgical techniques and perioperative care,24,25 surgery alone is often inadequate for the complete removal of hepatolithiasis. Hence, PTCS is a reasonable choice for treatment of hepatolithiasis with a supplement of surgery.
The initial success rate of PTCS with EHL for the treatment for hepatolithiasis is above 80%.26-30 Huang and colleagues26 reported results from up to 22 years of follow-up of 245 patients who underwent PTCS combined with EHL and documented that complete clearance of hepatolithiasis was achieved initially in 85.3% of patients and the complication rate was 4%. Using the same treatment modality, Lee et al.27 reported that complete clearance of intrahepatic stones was achieved in 80% of 92 patients and that a severe intrahepatic stricture was an important factor affecting the immediate success rate of PTCS and the recurrence rate of hepatolithiasis. A complete clearance of hepatolithiasis is important because it is related to a better long-term outcome. The incidence of recurrent cholangitis or cholangiocarcinoma was significantly higher in those with incompletely removed recurrent hepatolithiasis compared to those without coexisting hepatolithiasis.26 These results indicate that PTCS is superior to POCS for management of hepatolithiasis. Okugawa et al.31 reported that complete clearance of hepatolithiasis with POCS was achieved only in 64% of cases. Although they did not directly compare POCS to PTCS and concluded that treatment of hepatolithiasis with POCS was an effective method, lithotomy with POCS for hepatolithiasis is less efficient than PTCS because POCS is harder to handle and needs more procedure time than PTCS.
The recurrence rate of hepatolithiasis is another important consideration for treatment. Overall recurrence rate varies, ranging from 35% to 60%.26-30 One of the most important factors associated with recurrence is the combined bile duct strictures. Tsunoda et al.32 classified intrahepatic stones according to location of the stones and the presence or absence of stenotic lesions and/or localized dilation of the intrahepatic bile ducts: type I, no marked dilatation or stricture of intrahepatic bile ducts; type II, diffuse dilatation of the intrahepatic biliary tree without IHD strictures and frequently a stricture of the distal common bile duct; type III, unilateral solitary or multiple cystic intrahepatic dilatation frequently associated with stenosis of the left or right intrahepatic bile ducts; and type IV, the same attributes as type II but with bilateral involvement of the hepatic lobes. Based on this classification, the recurrence rate in patients with type III and IV hepatolithiasis increased gradually to 50% at 60 months of follow-up.27 In addition, underlying biliary disease is also a factor affecting the recurrence of hepatolithiasis. In one study, the recurrence rate in patients with advanced biliary cirrhosis was higher than in those with no or mild cirrhosis and the overall recurrence rate was 35% with a mean follow-up period of 42 months.27 PTCS should therefore be performed carefully in patients with severe biliary strictures or advanced biliary cirrhosis in view of the increased risk of recurrence, although PTCS combined with EHL is an effective and relatively safe treatment for intrahepatic stones.
Stricture dilatation
Postoperative biliary stricture at the anastomosis site is caused by benign conditions, including fibrosis and scarring after intraoperative injury,33,34 or malignant conditions such as tumor recurrence. Although relatively rare, postoperative biliary stricture is a serious complication after biliary surgery because this can lead to recurrent cholangitis, cholelithiasis, secondary biliary cirrhosis, and hepatic failure. Moreover, the proper management of postoperative biliary stricture is now more important because of increasing cases of liver transplantation.
If the cause of biliary stricture is tumor recurrence, of course, the treatment of choice is surgery if possible. However, a clinical dilemma is encountered in benign cases due to the higher morbidity and mortality rates of reoperation. Treatment with PTCS could be considered a preferred method because of its high success rate and fewer complications compared to reoperation. PTCS-guided stricture dilatation basically consists of balloon dilatation and indwelling catheter (PTCS catheter of nelaton catheter) or stent placement (Fig. 3). Kim et al.35 reported that the initial technical success rate and overall success rate of treatment with balloon dilatation and indwelling catheter using PTCS for bilioenteric anastomotic strictures were 100% and 81.0%, respectively, in their series of 21 patients during a follow-up ranging from 12 to 79 months. The overall duration of indwelling catheter maintenance was 2.4 months in the no stricture recurrence group and 1.6 months in the recurrence group, and there was no statistical difference between groups. Although there has been no documented study comparing the success rate of reoperation to that of PTCS for postoperative biliary stricture, their results showing overall success is comparable to that of surgical treatment, according to reports evaluating surgical therapy and percutaneous balloon dilatation.36,37
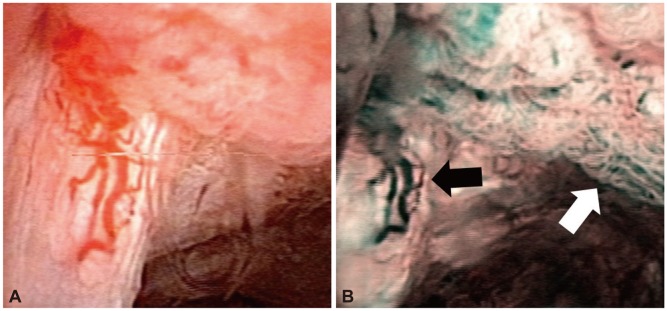
Fig. 3.
Postoperative hepaticojejunostomy site stricture dilatation with balloon dilatation and indwelling catheter placement via percutaneous transhepatic cholangioscopy (PTCS). (A) Balloon dilatation was performed on hepaticojejunostomy site stricture. White arrow indicates a waist of balloon at stricture site. (B) After balloon dilatation, 18 Fr PTCS catheter was placed through stricture site. (C) Cholangioscopic view of tight stricture at hepaticojejunostomy site. (D) Cholangioscopic view after 2 months of stricture dilatation showing widened lumen and erythematous mucosal change.
PTCS-guided stricture dilatation has advantages compared to percutaneous cholangiography-guided intervention. First, postoperative biliary stricture is often accompanied by bile duct stones proximal to the narrowed anastomosis site. If stone fragmentation with EHL or laser lithotripsy is needed, PTCS can remove stones simultaneously with stricture dilatation during the same session. Second, remnant surgical suture materials at the anastomosis site can be removed under the guidance of PTCS before or after stricture dilatation. Several case reports have indicated that the surgical clip or nonabsorbable suture used for ligation could be a nidus for bile duct stone formation38-41 as the exposed suture materials can cause bile stasis and result in the deposition of calcium bilirubinate. Removal of the suture material with forceps may reduce the recurrence of bile duct stones, and this procedure cannot be performed with cholangiographic guidance. PTCS is also helpful in differentiating benign from malignant strictures by allowing direct visualization and target biopsies, as discussed previously. Moreover, there was a case report of making new tract with PTCS in patients with complete membranous occlusion of bilioenteric anastomosis.42 Hence, PTCS-based treatment of patients with benign bilioenteric anastomotic strictures is a safe and effective method that frequently can be used as a substitute for surgery.
Local therapy for bile duct tumor
Although there has been no large-scale study, local ablation therapy with PTCS for various bile duct tumors has been tried and has shown promising results. PTCS-guided PDT has advantages over conventional cholangiography in determining the appropriate location of the PDT probe and accurately evaluating tumor extent. In addition, this method is useful for evaluating the response to PDT. Shim et al.43 used PDT in 24 patients with unresectable cholangiocarcinoma and reported successful outcomes, including decreased tumor mass and improved quality of life indices. APC for premalignant biliary lesion has also been reported.44,45 The superficial depth of ablation and ability to treat large surface areas in a short procedural time are advantages of PTCS-directed APC ablation. Brauer et al.45 reported a case of cholangioscopy-guided APC ablation for IPMN-B, despite failing to find a clinical benefit. Jazrawi et al.44 reported that a patient with biliary papillomatosis was treated successfully with APC under cholangioscopic guidance. Although surgical resection is the treatment of choice for these kinds of biliary lesions, local therapy with APC can be applied as an eligible substitution in patients who cannot tolerate surgery due to underlying medical conditions (Fig. 4). Ethanol injection to hepatocellular carcinoma (HCC) invading the bile duct under the guidance of PTCS has also been tried. Choi et al.46 conducted PTCS-guided ethanol injection to HCC in 10 patients and evaluated the safety and efficacy of the procedure. They reported that eight in 10 patients showed a response to therapy and there were no immediate complications.
Fig. 4.
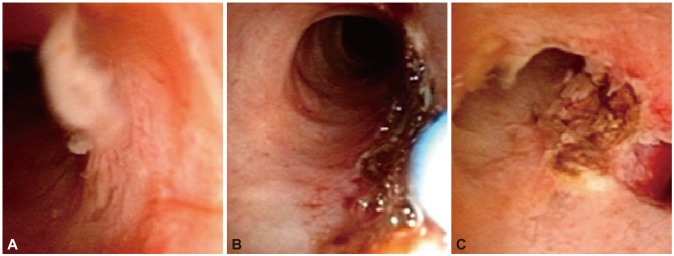
(A-C) Argon plasma coagulation ablation to intraductal papillary adenoma.
CONCLUSIONS
Although PTCS is a time-consuming, cumbersome procedure and has a risk of complications associated with cutaneobiliary fistula (e.g., hemobilia, infection, tumor seeding on sinus tract),47 PTCS has its own indispensable diagnostic and therapeutic roles.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.McIver MA. An instrument for visualizing the interior of the common duct at operation. Surgery. 1941;9:112–114. [Google Scholar]
- 2.Takada T, Kobayashi S, Yamada A, Uchida Y, Hayashi N. A new technique for the diagnosis and therapy of cholangitic hepatic abscesses; percutaneous transhepatic cholangial drainage (auther's transl) Nihon Shokakibyo Gakkai Zasshi. 1974;71:657–665. [PubMed] [Google Scholar]
- 3.Judah JR, Draganov PV. Intraductal biliary and pancreatic endoscopy: an expanding scope of possibility. World J Gastroenterol. 2008;14:3129–3136. doi: 10.3748/wjg.14.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ponchon T, Gagnon P, Berger F, et al. Value of endobiliary brush cytology and biopsies for the diagnosis of malignant bile duct stenosis: results of a prospective study. Gastrointest Endosc. 1995;42:565–572. doi: 10.1016/s0016-5107(95)70012-9. [DOI] [PubMed] [Google Scholar]
- 5.Macken E, Drijkoningen M, Van Aken E, Van Steenbergen W. Brush cytology of ductal strictures during ERCP. Acta Gastroenterol Belg. 2000;63:254–259. [PubMed] [Google Scholar]
- 6.Jailwala J, Fogel EL, Sherman S, et al. Triple-tissue sampling at ERCP in malignant biliary obstruction. Gastrointest Endosc. 2000;51(4 Pt 1):383–390. doi: 10.1016/s0016-5107(00)70435-4. [DOI] [PubMed] [Google Scholar]
- 7.Schoefl R, Haefner M, Wrba F, et al. Forceps biopsy and brush cytology during endoscopic retrograde cholangiopancreatography for the diagnosis of biliary stenoses. Scand J Gastroenterol. 1997;32:363–368. doi: 10.3109/00365529709007685. [DOI] [PubMed] [Google Scholar]
- 8.Seo DW, Lee SK, Yoo KS, et al. Cholangioscopic findings in bile duct tumors. Gastrointest Endosc. 2000;52:630–634. doi: 10.1067/mge.2000.108667. [DOI] [PubMed] [Google Scholar]
- 9.Kim HJ, Kim MH, Lee SK, Yoo KS, Seo DW, Min YI. Tumor vessel: a valuable cholangioscopic clue of malignant biliary stricture. Gastrointest Endosc. 2000;52:635–638. doi: 10.1067/mge.2000.108969. [DOI] [PubMed] [Google Scholar]
- 10.Nimura Y, Kamiya J, Hayakawa N, Shionoya S. Cholangioscopic differentiation of biliary strictures and polyps. Endoscopy. 1989;21(Suppl 1):351–356. doi: 10.1055/s-2007-1012989. [DOI] [PubMed] [Google Scholar]
- 11.Kim EH, Kim HJ, Oh HC, et al. The usefulness of percutaneous transhepatic cholangioscopy for identifying malignancies in distal common [corrected] bile duct strictures. J Korean Med Sci. 2008;23:579–585. doi: 10.3346/jkms.2008.23.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung JY, Lee SK, Oh HC, et al. The role of percutaneous transhepatic cholangioscopy in patients with hilar strictures. Gut Liver. 2007;1:56–62. doi: 10.5009/gnl.2007.1.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nimura Y. Staging cholangiocarcinoma by cholangioscopy. HPB (Oxford) 2008;10:113–115. doi: 10.1080/13651820801992658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seo DW, Kim MH, Lee SK, et al. Usefulness of cholangioscopy in patients with focal stricture of the intrahepatic duct unrelated to intrahepatic stones. Gastrointest Endosc. 1999;49:204–209. doi: 10.1016/s0016-5107(99)70487-6. [DOI] [PubMed] [Google Scholar]
- 15.Itoi T, Sofuni A, Itokawa F, et al. Peroral cholangioscopic diagnosis of biliary-tract diseases by using narrow-band imaging (with videos) Gastrointest Endosc. 2007;66:730–736. doi: 10.1016/j.gie.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 16.Lee SS, Kim MH, Lee SK, et al. MR cholangiography versus cholangioscopy for evaluation of longitudinal extension of hilar cholangiocarcinoma. Gastrointest Endosc. 2002;56:25–32. doi: 10.1067/mge.2002.125363. [DOI] [PubMed] [Google Scholar]
- 17.Tamada K, Yasuda Y, Nagai H, et al. Limitation of cholangiography in assessing longitudinal spread of extrahepatic bile duct carcinoma to the hepatic side. J Gastroenterol Hepatol. 1999;14:691–698. doi: 10.1046/j.1440-1746.1999.01894.x. [DOI] [PubMed] [Google Scholar]
- 18.Sakamoto E, Hayakawa N, Kamiya J, et al. Treatment strategy for mucin-producing intrahepatic cholangiocarcinoma: value of percutaneous transhepatic biliary drainage and cholangioscopy. World J Surg. 1999;23:1038–1043. doi: 10.1007/s002689900620. [DOI] [PubMed] [Google Scholar]
- 19.Kim YS, Myung SJ, Lee SK, Kim MH. Role of percutaneous transhepatic cholangioscopy in biliary papillomatosis: can it change treatment modality? Gastrointest Endosc. 1998;47:563–564. doi: 10.1016/s0016-5107(98)70274-3. [DOI] [PubMed] [Google Scholar]
- 20.Lee SS, Kim MH, Lee SK, et al. Clinicopathologic review of 58 patients with biliary papillomatosis. Cancer. 2004;100:783–793. doi: 10.1002/cncr.20031. [DOI] [PubMed] [Google Scholar]
- 21.Seo DW, Lee SK, Kim MH. Biliary papillomatosis. Gastrointest Endosc. 2000;51:67. doi: 10.1016/s0016-5107(00)70140-4. [DOI] [PubMed] [Google Scholar]
- 22.Kim MH, Sekijima J, Lee SP. Primary intrahepatic stones. Am J Gastroenterol. 1995;90:540–548. [PubMed] [Google Scholar]
- 23.Chen DW, Tung-Ping Poon R, Liu CL, Fan ST, Wong J. Immediate and long-term outcomes of hepatectomy for hepatolithiasis. Surgery. 2004;135:386–393. doi: 10.1016/j.surg.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Yang T, Lau WY, Lai EC, et al. Hepatectomy for bilateral primary hepatolithiasis: a cohort study. Ann Surg. 2010;251:84–90. doi: 10.1097/SLA.0b013e3181b2f374. [DOI] [PubMed] [Google Scholar]
- 25.Lin CC, Lin PY, Ko CJ, Chen YL, Chen ST, Kuo SJ. Hepatic resection for bilateral hepatolithiasis: a 20-year experience. ANZ J Surg. Epub 2012 Sep 26; doi: 10.1111/j.1445-2197.2012.06283.x. DOI: 10.1111/j.1445-2197.2012.06283.x. [DOI] [PubMed] [Google Scholar]
- 26.Huang MH, Chen CH, Yang JC, et al. Long-term outcome of percutaneous transhepatic cholangioscopic lithotomy for hepatolithiasis. Am J Gastroenterol. 2003;98:2655–2662. doi: 10.1111/j.1572-0241.2003.08770.x. [DOI] [PubMed] [Google Scholar]
- 27.Lee SK, Seo DW, Myung SJ, et al. Percutaneous transhepatic cholangioscopic treatment for hepatolithiasis: an evaluation of long-term results and risk factors for recurrence. Gastrointest Endosc. 2001;53:318–323. doi: 10.1016/s0016-5107(01)70405-1. [DOI] [PubMed] [Google Scholar]
- 28.Yeh YH, Huang MH, Yang JC, Mo LR, Lin J, Yueh SK. Percutaneous trans-hepatic cholangioscopy and lithotripsy in the treatment of intrahepatic stones: a study with 5 year follow-up. Gastrointest Endosc. 1995;42:13–18. doi: 10.1016/s0016-5107(95)70236-9. [DOI] [PubMed] [Google Scholar]
- 29.Jan YY, Chen MF. Percutaneous trans-hepatic cholangioscopic lithotomy for hepatolithiasis: long-term results. Gastrointest Endosc. 1995;42:1–5. doi: 10.1016/s0016-5107(95)70234-2. [DOI] [PubMed] [Google Scholar]
- 30.Chen C, Huang M, Yang J, et al. Reappraisal of percutaneous transhepatic cholangioscopic lithotomy for primary hepatolithiasis. Surg Endosc. 2005;19:505–509. doi: 10.1007/s00464-004-8125-5. [DOI] [PubMed] [Google Scholar]
- 31.Okugawa T, Tsuyuguchi T, Sudhamshu KC, et al. Peroral cholangioscopic treatment of hepatolithiasis: long-term results. Gastrointest Endosc. 2002;56:366–371. doi: 10.1016/s0016-5107(02)70040-0. [DOI] [PubMed] [Google Scholar]
- 32.Tsunoda T, Tsuchiya R, Harada N, et al. Long-term results of surgical treatment for intrahepatic stones. Jpn J Surg. 1985;15:455–462. doi: 10.1007/BF02470091. [DOI] [PubMed] [Google Scholar]
- 33.Pitt HA, Miyamoto T, Parapatis SK, Tompkins RK, Longmire WP., Jr Factors influencing outcome in patients with postoperative biliary strictures. Am J Surg. 1982;144:14–21. doi: 10.1016/0002-9610(82)90595-5. [DOI] [PubMed] [Google Scholar]
- 34.Collins PG, Gorey TF. Iatrogenic biliary stricture: presentation and management. Br J Surg. 1984;71:980–982. doi: 10.1002/bjs.1800711224. [DOI] [PubMed] [Google Scholar]
- 35.Kim JH, Lee SK, Kim MH, et al. Percutaneous transhepatic cholangioscopic treatment of patients with benign bilio-enteric anastomotic strictures. Gastrointest Endosc. 2003;58:733–738. doi: 10.1016/s0016-5107(03)02144-8. [DOI] [PubMed] [Google Scholar]
- 36.Kocher M, Cerna M, Havlik R, Kral V, Gryga A, Duda M. Percutaneous treatment of benign bile duct strictures. Eur J Radiol. 2007;62:170–174. doi: 10.1016/j.ejrad.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 37.Vos PM, van Beek EJ, Smits NJ, Rauws EA, Gouma DJ, Reeders JW. Percutaneous balloon dilatation for benign hepaticojejunostomy strictures. Abdom Imaging. 2000;25:134–138. doi: 10.1007/s002619910032. [DOI] [PubMed] [Google Scholar]
- 38.Bak B, Ornsholt J. Non-absorbable suture material as a nidus for the formation of common bile duct stones. Ann Chir Gynaecol. 1985;74:146–147. [PubMed] [Google Scholar]
- 39.Herline AJ, Fisk JM, Debelak JP, Shull HJ, Jr, Chapman WC. Surgical clips: a cause of late recurrent gallstones. Am Surg. 1998;64:845–848. [PubMed] [Google Scholar]
- 40.Martinez J, Combs W, Brady PG. Surgical clips as a nidus for biliary stone formation: diagnosis and therapy. Am J Gastroenterol. 1995;90:1521–1524. [PubMed] [Google Scholar]
- 41.Kinoshita H, Sajima S, Hashino K, et al. A case of intrahepatic gallstone formation around nylon suture for hepatectomy. Kurume Med J. 2000;47:235–237. doi: 10.2739/kurumemedj.47.235. [DOI] [PubMed] [Google Scholar]
- 42.Yang DH, Lee SK, Moon SH, et al. Percutaneous transhepatic cholangioscopic intervention in the management of complete membranous occlusion of bilioenteric anastomosis: report of two cases. Gut Liver. 2009;3:352–355. doi: 10.5009/gnl.2009.3.4.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shim CS, Cheon YK, Cha SW, et al. Prospective study of the effectiveness of percutaneous transhepatic photodynamic therapy for advanced bile duct cancer and the role of intraductal ultrasonography in response assessment. Endoscopy. 2005;37:425–433. doi: 10.1055/s-2005-861294. [DOI] [PubMed] [Google Scholar]
- 44.Jazrawi SF, Nguyen D, Barnett C, Tang SJ. Novel application of intraductal argon plasma coagulation in biliary papillomatosis (with video) Gastrointest Endosc. 2009;69:372–374. doi: 10.1016/j.gie.2008.03.1095. [DOI] [PubMed] [Google Scholar]
- 45.Brauer BC, Fukami N, Chen YK. Direct cholangioscopy with narrowband imaging, chromoendoscopy, and argon plasma coagulation of intraductal papillary mucinous neoplasm of the bile duct (with videos) Gastrointest Endosc. 2008;67:574–576. doi: 10.1016/j.gie.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 46.Choi JM, Lee SK, Lee SS, et al. Efficacy of percutaneous cholangioscopic ethanol injection in hepatocellular carcinoma invading the bile duct. Korean J Gastrointest Endosc. 2005;30:305–311. [Google Scholar]
- 47.Oh HC, Lee SK, Lee TY, et al. Analysis of percutaneous transhepatic cholangioscopy-related complications and the risk factors for those complications. Endoscopy. 2007;39:731–736. doi: 10.1055/s-2007-966577. [DOI] [PubMed] [Google Scholar]