Abstract
Epidemiological studies suggest that prenatal exposure to different types of viral or bacterial infections may be associated with similar outcomes; i.e., an increased risk of mental illness disorders in the offspring. Infections arising from various causes have similar debilitating effects in later life, suggesting that the exact pathogen may not be the critical factor in determining the neurological and cognitive outcome in the offspring. Instead, it is thought that response of the innate immune system, specifically the increased production of inflammatory cytokines, may be the critical mediator in altering fetal brain development pre-disposing the offspring to mental illness disorders later in life. Inflammatory cytokines are essential for normal brain development. Factors such as the site of cytokine production, a change in balance between anti- and pro- inflammatory cytokines, placental transfer of cytokines, the effects of cytokines on glial cells, and the effects of glucocorticoids are important when evaluating the impact of maternal infection on fetal brain development. Although it is clear that cytokines are altered in the fetal brain following maternal infection, further evidence is required to determine if cytokines are the critical factor that alters the trajectory of brain development, subsequently leading to postnatal behavioral and neurological abnormalities.
Keywords: cytokines, mental illness, infection, pregnancy, stress, glial cells
Introduction
Genetic background and environmental factors can predispose an individual to neuropsychiatric disorders, although the prevalence of conditions such as schizophrenia and autism cannot be accounted for by these factors alone (Williams et al., 2006; Tandon et al., 2008). The exact aetiology and pathophysiology of schizophrenia and autism remain unknown, but immunological factors are thought to play a role. Evidence of immune abnormalities including dysregulation of immune-related genes, changes in the activation of glial cells, and expression of inflammatory mediators have been observed in the brains of patients with these disorders (Pardo et al., 2005; Vargas et al., 2005; Arion et al., 2007; Chez et al., 2007; Morgan et al., 2010). It has therefore been suggested that immune-induced inflammation is a candidate event in creating a vulnerability in the brain that predisposes an individual, perhaps already at risk due to genetic and environmental influences, to develop the neuropathology and behavioral and cognitive impairments observed in major disorders such as schizophrenia and autism (Rehn and Rees, 2005; Meyer et al., 2011). Due to the debilitating nature of these conditions, their relatively high incidence in the community, and the great public health burden of these disorders, it is important to identify the causative factors in order to develop interventions and treatments for them, which might then become therapies that ameliorate or correct these disorders.
Neurodevelopmental basis of mental illness
In the 1960s and 1970s, several studies showed an increase in the incidence of mental retardation in children born to mothers who were pregnant during the 1964 rubella epidemic (Stern et al., 1969; Chess, 1971; Chess et al., 1978). More recent studies, based on serological testing and clinical examination to determine rubella exposure during pregnancy have provided further convincing support for association between maternal infection during pregnancy and the development of mental retardation in the offspring (Brown et al., 2000a, 2001). The “1968 Hong Kong” influenza epidemic is another example of an exposure that, for women pregnant at the time, increased the risk of their children developing mental illness in later life—schizophrenia especially—with second trimester exposure being of particular significance (Mednick et al., 1988; Kendell and Kemp, 1989; Machón et al., 2002). In contrast, other studies concluded that prenatal exposure to influenza epidemics was unrelated to the development of mental illness in postnatal life (Crow and Done, 1992; Selten and Slaets, 1994; Morgan et al., 1997; Selten et al., 2009), but many of these were conducted retrospectively with influenza exposure based on timing of epidemics and a reliance on mothers to recall infection exposure after the pregnancy. These limitations weaken the validity of these studies and may be the reason an association between maternal influenza and an increased incidence of mental illness disorders was not found. In contrast, a study with convincing serological evidence to determine prenatal influenza exposure did find that influenza contracted during pregnancy increased the risk of schizophrenia in later life (Brown et al., 2004a).
Exposure to other infections in pregnancy have also been linked with the development of mental illness disorders, with an increased risk in children born to mothers where herpes simplex virus type 2 (Buka et al., 2001b) and toxoplasmosis (Brown et al., 2005; Mortensen et al., 2007) were detected during pregnancy. Epidemiological studies have also found that prenatal exposure to poliovirus (Fuller Torrey et al., 1988; Suvisaari et al., 1999), measles (Fuller Torrey et al., 1988), varicella-zoster (Fuller Torrey et al., 1988) and maternal genital and reproductive infections (Babulas et al., 2006) are all associated with an increased incidence of mental illness in the child in later life.
That similar outcomes can arise from quite varied infection in pregnancy suggests that responses in the maternal, placental or fetal compartments common to this type of immune challenge can result in altered development of the fetal brain. Importantly, whatever it is in the intra-uterine environment, or in placental and fetal tissues that is affected by maternal infection, they appear to induce long-lasting changes that, despite the much-discussed “plasticity” of the brain throughout life, nevertheless lead to a predisposition for the development of mental disorders in postnatal life, an outcome with debilitating consequences not only for the individual concerned, but also for their immediate family and society at a large.
Are cytokines the critical mediator?
The numerous epidemiological studies showing an association between prenatal viral infections and an increased risk of mental illness in later life do not identify the critical factors in the disruption of fetal brain development. It is possible that factors common to the maternal immune system, and induced by any pathogen, viral or bacterial, may be affecting fetal brain development and leading to these postnatal behavioral disorders. Research has suggested that maternal induction or a change in the balance of pro- and anti-inflammatory cytokines may be a key mechanism involved in altering the normal course of fetal brain development (Gilmore and Jarskog, 1997; Ashdown et al., 2006; Meyer et al., 2009; Watanabe et al., 2010). It is important to note that other possible mechanisms by which a maternal infection could disrupt fetal development, include maternal and fetal malnutrition, hyperthermia, fetal growth restriction, and obstetric complications such as preeclampsia and preterm birth. As these factors do not consistently arise and are not common to all maternal infections, they are not discussed in depth in this review.
To eliminate microorganisms, such as bacteria and viruses, the innate immune system triggers the activation of Toll-like receptors (TLRs) (Takeda and Akira, 2005; Takeuchi and Akira, 2007). TLR activation triggers a cascade of signal transduction events causing the transcription of genes encoding cytokines, interferons and other immunological mediators (Takeda and Akira, 2005; Takeuchi and Akira, 2007). In the adult, cytokines, a family of soluble polypeptides, have been shown to be activated by these actions of the innate immune system, in response to both bacterial and viral infections (Imanishi, 2000; Borish and Steinke, 2003). It is thought that inflammatory cytokines not only play a key role in the resolution of disease, but in contrast, can also contribute to the development of inflammation (Hanada and Yoshimura, 2002).
Serological evidence further supports the concept that inflammatory mediators, produced in response to an infectious illness, may be involved in the development of mental disorders in postnatal life. Brown et al. (2004b) identified higher levels of serum interleukin (IL)-8 during pregnancy in mothers, particularly during the second trimester, whose child eventually developed schizophrenia. Increased serum levels of tumor necrosis factor (TNF)-α at birth have also been found in mothers whose child subsequently developed schizophrenia or other psychotic disorders (Buka et al., 2001a). Abnormalities in the constitutive expression of immunological factors also appear to be involved, in that chronically increased levels of IL-1β, IL-6, IL-8 and TNF-α are positively associated with the severity of the schizophrenia or autism that eventually develops (Maes et al., 2002; Chez et al., 2007; Potvin et al., 2008; Ashwood et al., 2011).
The role of cytokines in normal brain development
Numerous studies have identified changes in cytokine levels throughout normal human pregnancy in maternal serum and amniotic fluid (Austgulen et al., 1994; Kruse et al., 2000; Heikkinen et al., 2001; Chow et al., 2008; Curry et al., 2008), but fewer have investigated cytokine levels in the human fetal brain through development, most likely due to difficulties in obtaining samples. A study conducted more than a decade ago, used in situ hybridization to find cytokine and chemokine expression in forebrain cells of human fetuses from 5 weeks of gestation (Mousa et al., 1999), suggesting a role for cytokines in normal brain development due to the presence of these factors from the first trimester. Animal studies have also demonstrated that cytokines are present in the fetal brain from early gestation (Meyer et al., 2006b, 2009) and have correlated increases in specific cytokines, such as TNF-α and IL-1β, with important development events in the brain (Dziegielewska et al., 2000). In terms of the human, the role of cytokines in normal brain development requires further research, particularly as this may be somewhat different between species. Differences may also exist in the function of specific cytokines in the prenatal and postnatal brain. For example, the cytokine IL-1β, is highly expressed in the fetal brain of rat and is thought to be an important astroglial growth factor during development (Giulian et al., 1988), but is expressed at a low level in the adult brain, although rapidly up-regulated as a host defence response to injury (Rothwell and Luheshi, 2000).
In the adult brain, cytokine expression occurs mostly in glia, predominantly microglia and astrocytes (Meeuwsen et al., 2003; Jack et al., 2005). A similar association has been found in the fetal brain, with studies in vitro having shown that cytokines and chemokines can be produced by human fetal microglia and astrocytes (Giulian et al., 1988; Hua and Lee, 2000; Lee et al., 2002; Rezaie et al., 2002a) and that cytokine production is increased by these glial cells in response to stimulation by a viral infection (Cheeran et al., 2001; Lokensgard et al., 2001). Similar to the adult brain, glial cells in fetal brain are also thought to receive and respond to signals from inflammatory cytokines (Hanisch, 2002). Furthermore, cytokines are thought to be critically important in glial cell development, in addition to neural and synaptic maturation (Deverman and Patterson, 2009).
What is the source of the cytokines that induce fetal brain injury?
Many theories have been put forward to suggest how an infection during pregnancy could alter fetal brain development, and it is likely that cytokines play a major role. It is known that the activation of the maternal immune system by an infection during pregnancy induces the release of inflammatory cytokines (Shobokshi and Shaarawy, 2002; Zheng et al., 2012), but how these cytokines enter the fetal circulation and alter fetal brain development remains unclear.
Human and animals studies into the human influenza virus have shown that the virus or antibodies produced in response to the virus do not cross the placenta (Irving et al., 2000) and are not found in the brains of offspring where the mother had been exposed to influenza (Shi et al., 2005; Short et al., 2010; Fatemi et al., 2012). However, animal studies have shown that the activation of the maternal immune system by infections, such as influenza, alters cytokine levels in the placenta, amniotic fluid and fetal brain (Urakubo et al., 2001; Gilmore et al., 2005; Ashdown et al., 2006; Meyer et al., 2006b). These studies support the idea that it is not the virus that directly infects the fetal brain, but a substance released in either the maternal or placenta compartments that enters the fetal compartment and alters fetal brain development.
Studies have provided evidence that an increase in levels of cytokines in the maternal circulation could enter the fetal circulation, via the placenta, to influence fetal development. For example significant levels of cytokines were present in amniotic fluid and fetal tissues after the administration of specific cytokines, such as IL-2 and IL-6, to pregnant rodents (Dahlgren et al., 2006; Ponzio et al., 2007), indicating that these cytokines are able cross the placenta and enter the fetal circulation. In addition, the pro-inflammatory cytokine IL-6 was found to be capable of bi-directional transfer in a healthy term human placenta, although the same study also found that other cytokines including IL-1α and TNF-α show minimal transfer through the placenta and into the fetal circulation (Zaretsky et al., 2004).
As it still remains unclear how readily other cytokines and chemokines cross the placenta, the production of cytokines from the placenta itself is a second possibility to explain the presence of these substances in the amniotic fluid and fetal circulation. In vitro studies have shown that human trophoblast cells, from as early as the first trimester, respond to a viral mimetic that activates TLR-3 (Abrahams et al., 2006). As a result, type 1 interferons (IFN), inflammatory cytokines and other immunological mediators are inducted that may then enter both the fetal and maternal circulations.
Cytokines can be grouped according to their functions in the either inducing or suppressing inflammation. For example, IL-1β, IL-6, and TNF-α can be regarded as pro-inflammatory cytokines due to their role in early host defence against infection or disease, and in the development and progression of inflammation (Dinarello, 2000). Whereas cytokines such as IL-10 and transforming growth factor (TGF)-β can be considered as anti-inflammatory cytokines that have primarily immunomodulatory functions by limiting excess inflammatory reactions (Opal and DePalo, 2000). Researchers have hypothesized that it may not be the increased production and release of specific cytokines into the fetal compartment but instead a shift in excess pro- or anti-inflammatory cytokines during an infectious response, that may be the critical feature in disrupting normal brain development (Meyer et al., 2009; Patterson, 2009). For example, the over-expression of either the pro-inflammatory cytokine, IL-6 (Smith et al., 2007) or the anti-inflammatory cytokine, IL-10 (Meyer et al., 2008a) in pregnant rodents, causes similar cognitive and behavioral abnormalities in the offspring. As a consequence, it has been suggested that postnatal abnormalities would not arise if there were similar changes in both classes of cytokines, which therefore did not alter the balance between pro- and anti-inflammatory cytokines during fetal development (Meyer et al., 2009; Meyer, 2011). Although when a viral mimetic was administered to an IL-6 knockout mouse model or when co-administered with an IL-6 blocking antibody during pregnancy, the viral mimetic is no longer capable of producing the behavioral abnormalities and transcriptional changes in the offspring seen prior to IL-6 intervention (Smith et al., 2007). Instead this study suggests that the specificity of the cytokine response plays a key role in mediating the effects of a prenatal infection on the fetal brain.
Whether specific cytokines are derived in either the maternal or fetal system, or there is a change in the balance of cytokines, the stage of development of the fetal brain and/or immune system is an obvious factor in determining the consequences of a maternal infection. Epidemiological studies investigating various prenatal infections found that the risk of developing mental illness disorders can be dependent on the timing of exposure to an infection (Brown et al., 2000b, 2004a). In addition, studies investigating the administration of the viral mimetic, (Poly I:C) during gestation have provided further evidence that the time of prenatal exposure critically determines the patterns of behavioral and neurological abnormalities displayed in the offspring (Meyer et al., 2006a,b, 2008b). These studies found alterations in latent inhibition (Meyer et al., 2006a), exploratory activity (Meyer et al., 2006b) and sensorimotor gating (Meyer et al., 2008b), as well as deficits in an important neurodevelopmental market, Reelin expression (Meyer et al., 2006b) in the brain of offspring exposed to Poly I:C in early to mid gestation. Reversal learning deficits (Meyer et al., 2006b), impairments in spatial working memory (Meyer et al., 2008b) and increased apoptosis (Meyer et al., 2006b) were only demonstrated in mid-late prenatally exposed offspring. Cytokine levels in the maternal circulation and fetal brain also alter depending on the timing of Poly I:C administration, with greater levels of IL-10 and TNF-α when administered at early-mid compared to late gestation (Meyer et al., 2006a). These studies demonstrate that an immunological challenge at different times of prenatal development may have important, different neurodevelopmental consequences.
We propose two mechanisms by which an induction or a change in inflammatory cytokines during fetal life can have long term consequences on development; The effects of cytokines on 1) glial cells and 2) the hypothalamic-pituitary- adrenal axis.
The effect of cytokines on glial cells
The suggestion that an increase of particular cytokines or a change in the critical balance between classes of cytokines caused by a prenatal infection may detrimentally alter normal fetal brain development, also assumes that glial cells are likely to be involved or at least affected as these cells are shown to not only produce cytokines but respond to them in the fetal brain (Hanisch, 2002).
Glial cells, such as astrocytes and microglia, are essential for normal brain development. Astrocytes are critical for the development of neurons and synapse formation during brain development and support neuronal repair and synapse formation (Benveniste, 1998; Ullian et al., 2004). In addition, astrocytes contribute to central nervous system homeostasis by supporting neuronal repair, contributing to the metabolism of neurotransmitters and regulating metabolite levels (Dong and Benveniste, 2001). For example, astrocytes are the major cell in the brain that produces kynurenic acid and quinolinic acid, N-methyl-D-aspartate (NMDA)-receptor antagonist and agonist, respectively, that modulate excitatory glutamatergic synapses. Therefore, an increase in astrogliosis also alters kynurenic metabolism, leading to changes in NMDA receptor activity (Schwarcz et al., 2012). Changes in the concentrations of these metabolites are associated with neurodegenerative and psychiatric diseases (Schwarcz et al., 2012), and it is now known that alteration of kynurenic acid synthesis during pregnancy in rats results in abnormal behavioral outcomes postnatally (Alexander et al., 2013; Forrest et al., 2013). In addition, the enzymatic activity of indoleamine 2,3-dioxygenase (IDO), which can be enhanced or inhibited by pro- or anti- inflammatory cytokines, respectively, can change the activation of the previously described kynurenic pathway (Myint et al., 2012).
Microglia are the resident immune cells of the central nervous system, and not only play a role in phagocytosis (Tremblay et al., 2011) but also produce cytokines and chemokines (Deverman and Patterson, 2009). In the adult brain resting microglia are thought to have a ramified morphology, with long branching processes that may be sensitive to (i.e., they “monitor”) the chemical composition of the cellular environment and identify the presence of injured cells and toxins associated with infection (Rezaie and Male, 1999). More recently researchers have suggested that “resting” microglia in the postnatal brain are dynamic in nature, continually extending and retracting these branching process (Nimmerjahn et al., 2005; Tremblay et al., 2011). In either case, ramified (or resting) microglia react to insult or injury by transforming into amoeboid microglia where the ramified processes are withdrawn and the central cell body region becomes enlarged (Bilbo and Schwarz, 2009; Deverman and Patterson, 2009). Amoeboid or activated microglia are actively phagocytic, and synthesize large amounts of inflammatory cytokines such as IL-1β, IL-6 and tumor necrosis factor (TNF)-α (reviewed in Bilbo and Schwarz, 2009). More recent studies (Rezaie et al., 2002b; Monier et al., 2007), utilizing technological advances in microscopy, have provided further confirmation of earlier findings (Kershman, 1939), that in contrast to the adult brain, fetal microglia display an amoeboid appearance when first present and progressively ramify during development.
As glial cells play an important role in normal brain development, it has been suggested that an activation or change in these cells, may alter the function of these cells and have a detrimental impact on the fetal brain (Perry et al., 2010). After exposure to a prenatal infection/inflammation, the predominantly amoeboid microglia in the fetal brain can potentially remain in this state into postnatal life (Bilbo and Schwarz, 2009; Hagberg et al., 2012), possibly exposing the fetal and postnatal brain to over-expression of pro-inflammatory cytokines, such as IL-1β, IL-6 and TNF-α [reviewed in Jonakait (1997); Bilbo and Schwarz (2009)].
As further evidence of the cytokine-glial hypothesis, alterations in structure and function of astrocytes and microglia have been identified in post-mortem studies of the brains of mental illness patients. Astrogliosis has been reported in human brain studies of autistic patients (Laurence and Fatemi, 2005). For schizophrenia this association is less clear, with different studies showing decreased (Webster et al., 2005), unchanged (Falkai et al., 1999) or increased (Arnold et al., 1996) levels of astrocytes in the brains of schizophrenic patients. Increased microglial cell number (Steiner et al., 2008), microglial activation (Bayer et al., 1999), and microglial cell density (Radewicz et al., 2000) in the post-mortem brains of schizophrenic patients are more consistent findings, as also for autistic patients (Morgan et al., 2010). The localization of activated (amoeboid) microglia has also been shown to be different in the prefrontal cortex, anterior cingulate cortex and hippocampal brain regions of schizophrenic patients (Steiner et al., 2006). As previously outlined, amoeboid or activated microglia produce large amounts of inflammatory cytokines including IL-1β, IL-6 and TNF-α [reviewed in Jonakait (1997); Bilbo and Schwarz (2009)]. Therefore, it is thought that the “priming” of microglia can cause an exaggerated cytokine response by these cells to a subsequent insult, compared to “unprimed” microglia (Czeh et al., 2011; Hagberg et al., 2012), thus exacerbating brain injury.
More recently, animal studies by us and others using prenatal immune activation, to cause behavioral neuropathologies comparable to mental illnesses encountered in the human population, have provided further evidence for the importance of microglial activation in pathogenesis of mental disorders such as schizophrenia. Microglial activation was found in brains of adult offspring following treatment of the mother with lipopolysaccharide during pregnancy (Borrell et al., 2002), and also in the brains of offspring following prenatal exposure to Poly I:C (Graciarena et al., 2010; Juckel et al., 2011; Ratnayake et al., 2012; Giovanoli et al., 2013). In such studies, conducted in pregnant rats and mice, the fetal brain is very immature at the time the infection raises an immune challenge and induces the cytokine changes described above. But even in a more precocial species such as the spiny mouse, where brain development is much more advanced by the time of birth, a very low dose (0.5 mg/kg) of prenatal Poly I:C treatment also increased microglial activation in the neonatal hippocampus (Figure 2) and led to persistent behavioral impairments in juvenile offspring (Ratnayake et al., 2012), confirming that prenatal subclinical infection has profound effects on brain development.
Figure 2.
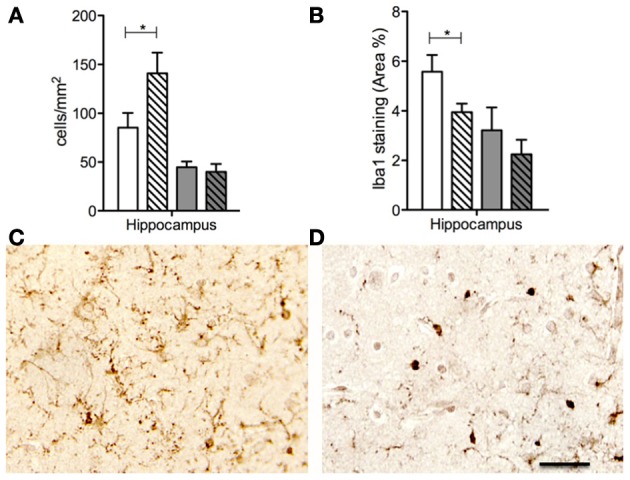
Number of Iba1 immunopositive cell bodies (A) and percentage Iba1 staining in the hippocampus in PBS (solid bars) and Poly I:C (dashed bars) prenatally exposed animals at 1d (white bars) and 100d (gray bars) postnatal age. Iba1 immunopositive staining in the hippocampus from animals prenatally exposed to PBS (C) and Poly I: (D) at 1d postnatal age. Note an increased number Iba1 immunopositive cell bodies in the Poly I:C (A,D) neonatal brains compared to the PBS (C). Although, note an increase in the percentage of Iba1 staining in PBS neonatal brains (B,C) compared to Poly I:C (D). The combination of these two findings, of more microglial cell bodies and fewer branching processes, make it possible to conclude that there are more activated, and fewer ramified, microglia in the neonatal hippocampus of animals born to mothers administered with the viral mimetic, Poly I:C. The data are shown as means ± SEM in all graphs. * refers p < 0.05. Scale bar = 100μM in (C,D). Adapted from Ratnayake et al. (2012).
The effects of cytokines on the hypothalamic-pituitary-adrenal axis
The immune system and the hypothalamic-pituitary-adrenal (HPA) axis, responsible for generalized stress responses, are inextricably linked (Franchimont et al., 2002; Chrousos and Kino, 2005; Silverman et al., 2005; Gibb et al., 2011). Cytokines can potently activate the HPA axis, to increase adrenocorticotropic hormone (ACTH), corticotropin-releasing hormone (CRH), arginine vasopressin and corticosterone levels (Besedovsky et al., 1986; Muller et al., 1999; Dunn, 2000; Schmidt et al., 2003). Therefore, cytokines, that have entered the fetal circulation due to a maternal infection, can potentially stimulate the release of CRH and arginine vasopressin from the fetal hypothalamus. The cytokine-elicited release of these hormones, shown in Figure 1, in turn, stimulates the secretion of ACTH from the anterior pituitary gland, and thus glucocorticoids from the adrenal cortex. In turn, glucocorticoids inhibit the induction of pro-inflammatory cytokines while stimulating the production of anti-inflammatory cytokines (Chrousos and Kino, 2005).
Figure 1.
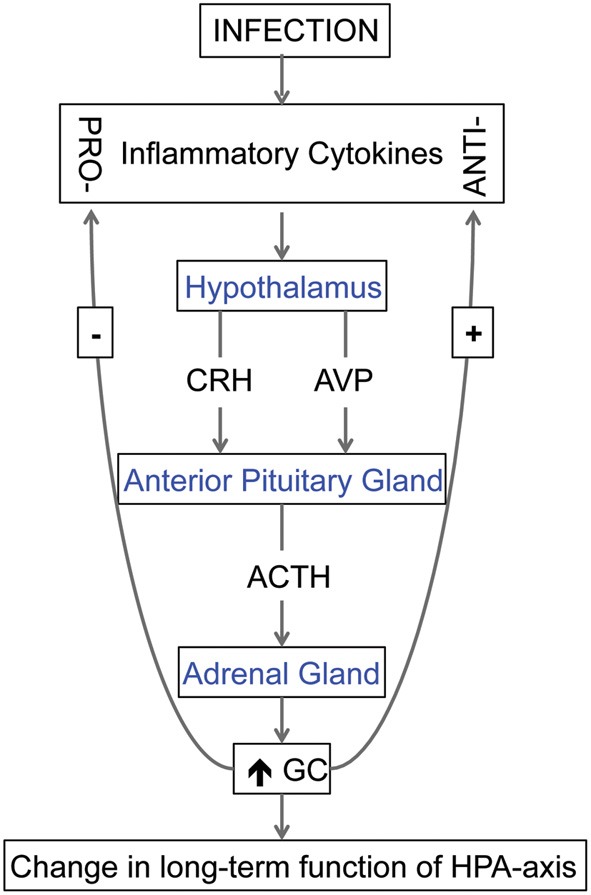
The relationship between cytokines and hypothalamic-pituitary-adrenal (HPA) axis. Cytokines, produced in response to an infection, can stimulate the release of corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) from the hypothalamus. These hormones stimulate the secretion of adrenocorticotrophic hormone (ACTH) from the anterior pituitary gland, and, in turn, the secretion of glucocorticoids (GC) from the adrenal cortex. During fetal life, excess GC can change the long-term function of the HPA-axis and if exposed to the fetal brain, GC can also modify brain development. In addition, GC can inhibit the induction of pro-inflammatory cytokines while stimulating the production of anti-inflammatory cytokines, a process that can also act to change the function of HPA-axis.
Although glucocorticoids are essential for normal brain development, exposure of the fetal brain to excess glucocorticoids can also modify fetal brain development and permanently alter the function of the HPA axis in postnatal life (Matthews, 2000; Welberg and Seckl, 2001; Seckl, 2004; Kapoor et al., 2006). Pro-inflammatory cytokines, such as IL-1, IL-6 and TNF-α, can alter the actions of glucocorticoids by interfering with their intracellular signaling system (Dunn, 2000; Bornstein et al., 2004; Giovanoli et al., 2013). The use of specific cytokine or glucocorticoid receptor knock-out mice in future studies could further help distinguish the role of each of these molecules during prenatal infection, and may reveal the link between infection and the neuroendocrine system that affects brain development.
The involvement of the HPA axis on cytokine changes in the fetal brain is dependent on the ability of the fetus to produce an appropriate stress response involving the HPA axis, and therefore, the time of the infection during pregnancy can be expected to modify the impact on the fetal brain development. Particularly as HPA axis development is strongly linked to the development of specific regions of the brain (Matthews, 2002). Hormones produced by the HPA axis can be detected early in development, by 12 weeks of gestation (Ng, 2000). The hormones, such as CRH and ACTH, have important actions during fetal development on the regulation of the HPA axis and the subsequent secretion of adrenal-derived glucocorticoids. Appropriate levels of glucocorticoids, such as cortisol and DHEA, are essential in maintaining intrauterine homeostasis and neuroprotective mechanisms, where as excessive levels of these hormones can be deleterious to fetal development.
It is well known that changes during fetal development can permanently programme the HPA axis, to have a long lasting impact on neuroendocrine function in postnatal life [reviewed in Matthews (2002); Bale et al. (2010); Howerton and Bale (2012)]. As it is not within the scope of this article to extensively review this concept, briefly, previous studies have demonstrated that adult animals exposed to a prenatal stressor, show DNA methylation and expression changes in glucocorticoid receptor and CRH (Nemeroff, 1992; Kapoor et al., 2008; Mueller and Bale, 2008). DNA modification by methylation can alter long term gene function, for example by silencing gene expression, without altering sequence variation. Therefore, it possible that a prenatal stress response, caused by exposure to an infection or inflammatory event, may result in the long lasting, epigenetic reprogramming of genes involved in HPA axis function.
Conclusion
While mental illnesses such as schizophrenia and autism have been associated with prenatal infection, the variety of infections producing similar outcomes suggest that a response by the immune system common to all infections may be the important factor in the etiology of mental illness disorders. The common pathway may include induction of cytokines, triggered by activation of TLRs to assist in the resolution of disease but which then produce a pro-inflammatory environment that has downstream effects that are detrimental to fetal brain development. It is not entirely clear if significant amounts of cytokines enter the fetal circulation by placental transfer, or if placental and fetal production of cytokines is also important. However, the evidence is convincing that cytokines produced in response to infection during pregnancy have detrimental and long-lasting effects on the brain.
Inflammatory cytokines can affect the function of glia in the developing brain. Early deregulation of glial cells, such as microglia, may set in motion a cascade of events that lead to permanent alterations in these cells. There is substantial evidence for microglial activation in the post-mortem brains of patients that suffered a mental illness (Bayer et al., 1999; Radewicz et al., 2000; Steiner et al., 2006, 2008; Morgan et al., 2010), as well as in animal models of prenatal infection (Borrell et al., 2002; Graciarena et al., 2010; Juckel et al., 2011; Ratnayake et al., 2012), suggesting that permanently primed or “sensitized” microglia may be an important cellular component of the neuropathology of mental disorders that arise after birth. Another action of cytokines that may have long-term consequences is their ability to elicit a stress response via the activation of fetal HPA, which can subsequently cause long-term changes in HPA axis function in postnatal life.
It is possible to conclude that exposure to prenatal infection via the actions of cytokines can predispose or increase the susceptibility of an individual to future inflammatory events or postnatal stressors, as the function of the systems that act to resolve these insults have been permanently altered. Therefore, we believe it is important for future research to investigate two-hit models of mental illness disorders i.e., a first hit during fetal life—a prenatal viral or bacterial infection that drives an early susceptibility to mental illness via the activation of inflammatory pathways, such as the persistent activation of microglia, or changes in HPA axis function- in combination with a second hit during postnatal life—an immune activator or environmental factors such as chronic stressors or trauma. The development of these models may provide a unique period of time, prior to the “second hit,” to develop and target potential interventions or treatments to prevent the “full-blown” onset of a mental illness disorder.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Abrahams V. M., Schaefer T. M., Fahey J. V., Visintin I., Wright J. A., Aldo P. B., et al. (2006). Expression and secretion of antiviral factors by trophoblast cells following stimulation by the TLR-3 agonist, Poly (I: C). Hum. Reprod. 21, 2432 10.1093/humrep/del178 [DOI] [PubMed] [Google Scholar]
- Alexander K. S., Pocivavsek A., Wu H. Q., Pershing M. L., Schwarcz R., Bruno J. P. (2013). Early developmental elevations of brain kynurenic acid impair cognitive flexibility in adults: reversal with galantamine. Neuroscience 238, 19–28 10.1016/j.neuroscience.2013.01.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arion D., Unger T., Lewis D. A., Levitt P., Mirnics K. (2007). Molecular evidence for increased expression of genes related to immune and chaperone function in the prefrontal cortex in schizophrenia. Biol. Psychiatry 62, 711–721 10.1016/j.biopsych.2006.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold S. E., Franz B. R., Trojanowski J. Q., Moberg P. J., Gur R. E. (1996). Glial fibrillary acidic protein-immunoreactive astrocytosis in elderly patients with schizophrenia and dementia. Acta Neuropathol. 91, 269–277 10.1007/s004010050425 [DOI] [PubMed] [Google Scholar]
- Ashdown H., Dumont Y., Ng M., Poole S., Boksa P., Luheshi G. N. (2006). The role of cytokines in mediating effects of prenatal infection on the fetus: implications for schizophrenia. Mol. Psychiatry 11, 47–55 10.1038/sj.mp.4001748 [DOI] [PubMed] [Google Scholar]
- Ashwood P., Krakowiak P., Hertz-Picciotto I., Hansen R., Pessah I., Van de Water J. (2011). Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav. Immun. 25, 40–45 10.1016/j.bbi.2010.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austgulen R., Lien E., Liabakk N. B., Jacobsen G., Arntzen K. J. (1994). Increased levels of cytokines and cytokine activity modifiers in normal pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 57, 149–155 10.1016/0028-2243(94)90291-7 [DOI] [PubMed] [Google Scholar]
- Babulas V., Factor-Litvak P., Goetz R., Schaefer C. A., Brown A. S. (2006). Prenatal exposure to maternal genital and reproductive infections and adult schizophrenia. Am. J. Psychiatry 163, 927–929 10.1176/appi.ajp.163.5.927 [DOI] [PubMed] [Google Scholar]
- Bale T. L., Baram T. Z., Brown A. S., Goldstein J. M., Insel T. R., McCarthy M. M., et al. (2010). Early life programming and neurodevelopmental disorders. Biol. Psychiatry 68, 314–319 10.1016/j.biopsych.2010.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer T. A., Buslei R., Havas L., Falkai P. (1999). Evidence for activation of microglia in patients with psychiatric illnesses. Neurosci. Lett. 271, 126–128 10.1016/S0304-3940(99)00545-5 [DOI] [PubMed] [Google Scholar]
- Benveniste E. N. (1998). Cytokine actions in the central nervous system. Cytokine Growth Factor Rev. 9, 259–275 10.1016/S1359-6101(98)00015-X [DOI] [PubMed] [Google Scholar]
- Besedovsky H., del Rey A., Sorkin E., Dinarello C. A. (1986). Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science 233, 652–654 10.1126/science.3014662 [DOI] [PubMed] [Google Scholar]
- Bilbo S. D., Schwarz J. M. (2009). Early-life programming of later-life brain and behavior: a critical role for the immune system. Front. Behav. Neurosci. 3:14. 10.3389/neuro.08.014.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borish L. C., Steinke J. W. (2003). 2. Cytokines and chemokines. J. Allergy Clin. Immunol. 111, S460–S475 10.1067/mai.2003.108 [DOI] [PubMed] [Google Scholar]
- Bornstein S. R., Rutkowski H., Vrezas I. (2004). Cytokines and steroidogenesis. Mol. Cell. Endocrinol. 215, 135–141 10.1016/j.mce.2003.11.022 [DOI] [PubMed] [Google Scholar]
- Borrell J., Vela J. M., Arévalo-Martin A., Molina-Holgado E., Guaza C. (2002). Prenatal immune challenge disrupts sensorimotor gating in adult rats: implications for the Etiopathogenesis of Schizophrenia. Neuropsychopharmacology 26, 204 10.1016/S0893-133X(01)00360-8 [DOI] [PubMed] [Google Scholar]
- Brown A. S., Begg M. D., Gravenstein S., Schaefer C. A., Wyatt R. J., Bresnahan M., et al. (2004a). Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch. Gen. Psychiatry 61, 774–780 10.1001/archpsyc.61.8.774 [DOI] [PubMed] [Google Scholar]
- Brown A. S., Hooton J., Schaefer C. A., Zhang H., Petkova E., Babulas V., et al. (2004b). Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. Am. J. Psychiatry 161, 889–895 10.1176/appi.ajp.161.5.889 [DOI] [PubMed] [Google Scholar]
- Brown A. S., Cohen P., Greenwald S., Susser E. (2000a). Nonaffective psychosis after prenatal exposure to rubella. Am. J. Psychiatry 157, 438–443 10.1176/appi.ajp.157.3.438 [DOI] [PubMed] [Google Scholar]
- Brown A. S., Schaefer C. A., Wyatt R. J., Goetz R., Begg M. D., Gorman J. M., et al. (2000b). Maternal exposure to respiratory infections and adult schizophrenia spectrum disorders: a prospective birth cohort study. Schizophr. Bull. 26, 287–295 10.1093/oxfordjournals.schbul.a033453 [DOI] [PubMed] [Google Scholar]
- Brown A. S., Cohen P., Harkavy-Friedman J., Babulas V., Malaspina D., Gorman J. M., et al. (2001). Prenatal rubella, premorbid abnormalities, and adult schizophrenia. Biol. Psychiatry 49, 473–486 10.1016/S0006-3223(01)01068-X [DOI] [PubMed] [Google Scholar]
- Brown A. S., Schaefer C. A., Quesenberry C. P., Jr. Liu L. Babulas V. P. Susser E. S. (2005). Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am. J. Psychiatry 162, 767–773 10.1176/appi.ajp.162.4.767 [DOI] [PubMed] [Google Scholar]
- Buka S. L., Tsuang M. T., Torrey E. F., Klebanoff M. A., Wagner R. L., Yolken R. H. (2001a). Maternal cytokine levels during pregnancy and adult psychosis. Brain Behav. Immun. 15, 411–420 10.1006/brbi.2001.0644 [DOI] [PubMed] [Google Scholar]
- Buka S. L. S., Tsuang M. T. M. D. P., Torrey E. F. M. D., Klebanoff M. A. M. D., Bernstein D. M. D., Yolken R. H. M. D. (2001b). Maternal infections and subsequent psychosis among offspring. Arch. Gen. Psychiatry 58, 1032–1037 10.1001/archpsyc.58.11.1032 [DOI] [PubMed] [Google Scholar]
- Cheeran M. C., Hu S., Yager S. L., Gekker G., Peterson P. K., Lokensgard J. R. (2001). Cytomegalovirus induces cytokine and chemokine production differentially in microglia and astrocytes: antiviral implications. J. Neurovirol. 7, 135–147 10.1080/13550280152058799 [DOI] [PubMed] [Google Scholar]
- Chess S. (1971). Autism in children with congenital rubella. J. Autism Child Schizophr. 1, 33–47 10.1007/BF01537741 [DOI] [PubMed] [Google Scholar]
- Chess S., Fernandez P., Korn S. (1978). Behavioral consequences of congenital rubella. J. Pediatr. 93, 699–703 10.1016/S0022-3476(78)80921-4 [DOI] [PubMed] [Google Scholar]
- Chez M. G., Dowling T., Patel P. B., Khanna P., Kominsky M. (2007). Elevation of tumor necrosis factor-alpha in cerebrospinal fluid of autistic children. Pediatr. Neurol. 36, 361–365 10.1016/j.pediatrneurol.2007.01.012 [DOI] [PubMed] [Google Scholar]
- Chow S. S., Craig M. E., Jones C. A., Hall B., Catteau J., Lloyd A. R., et al. (2008). Differences in amniotic fluid and maternal serum cytokine levels in early midtrimester women without evidence of infection. Cytokine 44, 78–84 10.1016/j.cyto.2008.06.009 [DOI] [PubMed] [Google Scholar]
- Chrousos G. P., Kino T. (2005). Ikaros transcription factors: flying between stress and inflammation. J. Clin. Invest. 115, 844–848 10.1172/JCI200524886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow T. J., Done D. J. (1992). Prenatal exposure to influenza does not cause schizophrenia. Br. J. Psychiatry 161, 390–393 10.1192/bjp.161.3.390 [DOI] [PubMed] [Google Scholar]
- Curry A. E., Vogel I., Skogstrand K., Drews C., Schendel D. E., et al. (2008). Maternal plasma cytokines in early- and mid-gestation of normal human pregnancy and their association with maternal factors. J. Reprod. Immunol. 77, 152–160 10.1016/j.jri.2007.06.051 [DOI] [PubMed] [Google Scholar]
- Czeh M., Gressens P., Kaindl A. M. (2011). The yin and yang of microglia. Dev. Neurosci. 33, 199–209 10.1159/000328989 [DOI] [PubMed] [Google Scholar]
- Dahlgren J., Samuelsson A. M., Jansson T., Holmang A. (2006). Interleukin-6 in the maternal circulation reaches the rat fetus in mid-gestation. Pediatr. Res. 60, 147–151 10.1203/01.pdr.0000230026.74139.18 [DOI] [PubMed] [Google Scholar]
- Deverman B. E., Patterson P. H. (2009). Cytokines and CNS Development. Neuron 64, 61–78 10.1016/j.neuron.2009.09.002 [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. (2000). Proinflammatory cytokines. Chest 118, 503–508 10.1378/chest.118.2.503 [DOI] [PubMed] [Google Scholar]
- Dong Y., Benveniste E. N. (2001). Immune function of astrocytes. Glia 36, 180–190 10.1002/glia.1107 [DOI] [PubMed] [Google Scholar]
- Dunn A. J. (2000). Cytokine activation of the HPA axis. Ann. N. Y. Acad. Sci. 917, 608–617 10.1111/j.1749-6632.2000.tb05426.x [DOI] [PubMed] [Google Scholar]
- Dziegielewska K. M., Moller J. E., Potter A. M., Ek J., Lane M. A., Saunders N. R. (2000). Acute-phase cytokines IL-1beta and TNF-alpha in brain development. Cell Tissue Res. 299, 335–345 10.1007/s004419900157 [DOI] [PubMed] [Google Scholar]
- Falkai P., Honer W. G., David S., Bogerts B., Majtenyi C., Bayer T. A. (1999). No evidence for astrogliosis in brains of schizophrenic patients. A post-mortem study. Neuropathol. Appl. Neurobiol. 25, 48–53 10.1046/j.1365-2990.1999.00162.x [DOI] [PubMed] [Google Scholar]
- Fatemi S. H., Folsom T. D., Rooney R. J., Mori S., Kornfield T. E., Reutiman T. J., et al. (2012). The viral theory of schizophrenia revisited: abnormal placental gene expression and structural changes with lack of evidence for H1N1 viral presence in placentae of infected mice or brains of exposed offspring. Neuropharmacology 62, 1290–1298 10.1016/j.neuropharm.2011.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest C. M., Khalil O. S., Pisar M., Darlington L. G., Stone T. W. (2013). Prenatal inhibition of the tryptophan-kynurenine pathway alters synaptic plasticity and protein expression in the rat hippocampus. Brain Res. 1504, 1–15 10.1016/j.brainres.2013.01.031 [DOI] [PubMed] [Google Scholar]
- Franchimont D., Kino T., Galon J., Meduri G. U., Chrousos G. (2002). Glucocorticoids and inflammation revisited: the state of the art. NIH clinical staff conference. Neuroimmunomodulation 10, 247–260 10.1159/000069969 [DOI] [PubMed] [Google Scholar]
- Fuller Torrey E., Rawlings R., Waldman I. N. (1988). Schizophrenic births and viral diseases in two states. Schizophr. Res. 1, 73–77 10.1016/0920-9964(88)90043-6 [DOI] [PubMed] [Google Scholar]
- Gibb J., Hayley S., Poulter M. O., Anisman H. (2011). Effects of stressors and immune activating agents on peripheral and central cytokines in mouse strains that differ in stressor responsivity. Brain Behav. Immun. 25, 468–482 10.1016/j.bbi.2010.11.008 [DOI] [PubMed] [Google Scholar]
- Gilmore J., Jarskog L. F. (1997). Exposure to infection and brain development: cytokines in the pathogenesis of schizophrenia. Schizophr. Res. 24, 365–367 10.1016/S0920-9964(96)00123-5 [DOI] [PubMed] [Google Scholar]
- Gilmore J. H., Jarskog L. F., Vadlamudi S. (2005). Maternal poly I:C exposure during pregnancy regulates TNF[alpha], BDNF, and NGF expression in neonatal brain and the maternal-fetal unit of the rat. J. Neuroimmunol. 159, 106–112 10.1016/j.jneuroim.2004.10.008 [DOI] [PubMed] [Google Scholar]
- Giovanoli S., Engler H., Engler A., Richetto J., Voget M., Willi R., et al. (2013). Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science 339, 1095–1099 10.1126/science.1228261 [DOI] [PubMed] [Google Scholar]
- Giulian D., Young D. G., Woodward J., Brown D. C., Lachman L. B. (1988). Interleukin-1 is an astroglial growth factor in the developing brain. J. Neurosci. 8, 709–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graciarena M., Depino A. M., Pitossi F. J. (2010). Prenatal inflammation impairs adult neurogenesis and memory related behavior through persistent hippocampal TGFbeta1 downregulation. Brain Behav. Immun. 24, 1301–1309 10.1016/j.bbi.2010.06.005 [DOI] [PubMed] [Google Scholar]
- Hagberg H., Gressens P., Mallard C. (2012). Inflammation during fetal and neonatal life: implications for neurologic and neuropsychiatric disease in children and adults. Ann. Neurol. 71, 444–457 10.1002/ana.22620 [DOI] [PubMed] [Google Scholar]
- Hanada T., Yoshimura A. (2002). Regulation of cytokine signaling and inflammation. Cytokine Growth Factor Rev. 13, 413–421 10.1016/S1359-6101(02)00026-6 [DOI] [PubMed] [Google Scholar]
- Hanisch U. K. (2002). Microglia as a source and target of cytokines. Glia 40, 140–155 10.1002/glia.10161 [DOI] [PubMed] [Google Scholar]
- Heikkinen J., Mottonen M., Pulkki K., Lassila O., Alanen A. (2001). Cytokine levels in midtrimester amniotic fluid in normal pregnancy and in the prediction of pre-eclampsia. Scand. J. Immunol. 53, 310–314 10.1046/j.1365-3083.2001.00872.x [DOI] [PubMed] [Google Scholar]
- Howerton C. L., Bale T. L. (2012). Prenatal programing: at the intersection of maternal stress and immune activation. Horm. Behav. 62, 237–242 10.1016/j.yhbeh.2012.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua L. L., Lee S. C. (2000). Distinct patterns of stimulus-inducible chemokine mRNA accumulation in human fetal astrocytes and microglia. Glia 30, 74–81 [DOI] [PubMed] [Google Scholar]
- Imanishi J. (2000). Expression of cytokines in bacterial and viral infections and their biochemical aspects. J. Biochem. 127, 525–530 10.1093/oxfordjournals.jbchem.a022636 [DOI] [PubMed] [Google Scholar]
- Irving W. L., James D. K., Stephenson T., Laing P., Jameson C., Oxford J. S., et al. (2000). Influenza virus infection in the second and third trimesters of pregnancy: a clinical and seroepidemiological study. BJOG 107, 1282–1289 10.1111/j.1471-0528.2000.tb11621.x [DOI] [PubMed] [Google Scholar]
- Jack C. S., Arbour N., Manusow J., Montgrain V., Blain M., McCrea E., et al. (2005). TLR signaling tailors innate immune responses in human microglia and astrocytes. J. Immunol. 175, 4320–4330 [DOI] [PubMed] [Google Scholar]
- Jonakait G. M. (1997). Cytokines in neuronal development. Adv. Pharmacol. 37, 35–67 10.1016/S1054-3589(08)60947-3 [DOI] [PubMed] [Google Scholar]
- Juckel G., Manitz M. P., Brune M., Friebe A., Heneka M. T., Wolf R. J. (2011). Microglial activation in a neuroinflammational animal model of schizophrenia–a pilot study. Schizophr. Res. 131, 96–100 10.1016/j.schres.2011.06.018 [DOI] [PubMed] [Google Scholar]
- Kapoor A., Dunn E., Kostaki A., Andrews M. H., Matthews S. G. (2006). Fetal programming of hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids. J. Physiol. 572, 31–44 10.1113/jphysiol.2006.105254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A., Leen J., Matthews S. G. (2008). Molecular regulation of the hypothalamic-pituitary-adrenal axis in adult male guinea pigs after prenatal stress at different stages of gestation. J. Physiol. 586, 4317–4326 10.1113/jphysiol.2008.153684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendell R. E., Kemp I. W. (1989). Maternal influenza in the etiology of schizophrenia. Arch. Gen. Psychiatry 46, 878–882 10.1001/archpsyc.1989.01810100020004 [DOI] [PubMed] [Google Scholar]
- Kershman J. (1939). Genesis of microglia in the human brain. Arch. Neurol. Psychiatry 41, 24–50 10.1001/archneurpsyc.1939.02270130034002 [DOI] [Google Scholar]
- Kruse N., Greif M., Moriabadi N. F., Marx L., Toyka K. V., Rieckmann P. (2000). Variations in cytokine mRNA expression during normal human pregnancy. Clin. Exp. Immunol. 119, 317–322 10.1046/j.1365-2249.2000.01123.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence J. A., Fatemi S. H. (2005). Glial fibrillary acidic protein is elevated in superior frontal, parietal and cerebellar cortices of autistic subjects. Cerebellum 4, 206–210 10.1080/14734220500208846 [DOI] [PubMed] [Google Scholar]
- Lee Y. B., Nagai A., Kim S. U. (2002). Cytokines, chemokines, and cytokine receptors in human microglia. J. Neurosci. Res. 69, 94–103 10.1002/jnr.10253 [DOI] [PubMed] [Google Scholar]
- Lokensgard J. R., Hu S., Sheng W., van Oijen M., Cox D., Cheeran M. C., et al. (2001). Robust expression of TNF-alpha, IL-1beta, RANTES, and IP-10 by human microglial cells during nonproductive infection with herpes simplex virus. J. Neurovirol. 7, 208–219 10.1080/13550280152403254 [DOI] [PubMed] [Google Scholar]
- Machón R. A., Huttunen M. O., Mednick S. A., Sinivuo J., Tanskanen A., Bunn Watson. J., et al. (2002). Adult schizotypal personality characteristics and prenatal influenza in a Finnish birth cohort. Schizophr. Res. 54, 7–16 10.1016/S0920-9964(01)00346-2 [DOI] [PubMed] [Google Scholar]
- Maes M., Bocchio Chiavetto L., Bignotti S., Battisa Tura G. J., Pioli R., Boin F., et al. (2002). Increased serum interleukin-8 and interleukin-10 in schizophrenic patients resistant to treatment with neuroleptics and the stimulatory effects of clozapine on serum leukemia inhibitory factor receptor. Schizophr. Res. 54, 281–291 10.1016/S0920-9964(00)00094-3 [DOI] [PubMed] [Google Scholar]
- Matthews S. G. (2000). Antenatal glucocorticoids and programming of the developing CNS. Pediatr. Res. 47, 291–300 10.1203/00006450-200003000-00003 [DOI] [PubMed] [Google Scholar]
- Matthews S. G. (2002). Early programming of the hypothalamo-pituitary-adrenal axis. Trends Endocrinol. Metab. 13, 373–380 10.1016/S1043-2760(02)00690-2 [DOI] [PubMed] [Google Scholar]
- Mednick S. A., Machon R. A., Huttunen M. O., Bonett D. (1988). Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch. Gen. Psychiatry 45, 189–192 10.1001/archpsyc.1988.01800260109013 [DOI] [PubMed] [Google Scholar]
- Meeuwsen S., Persoon-Deen C., Bsibsi M., Ravid R., van Noort J. M. (2003). Cytokine, chemokine and growth factor gene profiling of cultured human astrocytes after exposure to proinflammatory stimuli. Glia 43, 243–253 10.1002/glia.10259 [DOI] [PubMed] [Google Scholar]
- Meyer U. (2011). Anti-inflammatory signaling in schizophrenia. Brain Behav. Immun. 25, 1507–1518 10.1016/j.bbi.2011.05.014 [DOI] [PubMed] [Google Scholar]
- Meyer U., Feldon J., Dammann O. (2011). Schizophrenia and autism: both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatr. Res. 69, 26R–33R. 10.1203/PDR.0b013e318212c196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U., Feldon J., Yee B. K. (2009). A review of the fetal brain cytokine imbalance hypothesis of schizophrenia. Schizophr. Bull. 35, 959–972 10.1093/schbul/sbn022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U., Feldon J., Schedlowski M., Yee B. K. (2006a). Immunological stress at the maternal-foetal interface: a link between neurodevelopment and adult psychopathology. Brain Behav. Immun. 20, 378–388 10.1016/j.bbi.2005.11.003 [DOI] [PubMed] [Google Scholar]
- Meyer U., Nyffeler M., Engler A., Urwyler A., Schedlowski M., Knuesel I., et al. (2006b). The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J. Neurosci. 26, 4752–4762 10.1523/JNEUROSCI.0099-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U., Murray P. J., Urwyler A., Yee B. K., Schedlowski M., Feldon J. (2008a). Adult behavioral and pharmacological dysfunctions following disruption of the fetal brain balance between pro-inflammatory and IL-10-mediated anti-inflammatory signaling. Mol. Psychiatry 13, 208–221 10.1038/sj.mp.4002042 [DOI] [PubMed] [Google Scholar]
- Meyer U., Nyffeler M., Yee B. K., Knuesel I., Feldon J. (2008b). Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav. Immun. 22, 469–486 10.1016/j.bbi.2007.09.012 [DOI] [PubMed] [Google Scholar]
- Monier A., Adle-Biassette H., Delezoide A. L., Evrard P., Gressens P., Verney C. (2007). Entry and distribution of microglial cells in human embryonic and fetal cerebral cortex. J. Neuropathol. Exp. Neurol. 66, 372–382 10.1097/nen.0b013e3180517b46 [DOI] [PubMed] [Google Scholar]
- Morgan J. T., Chana G., Pardo C. A., Achim C., Semendeferi K., Buckwalter J., et al. (2010). Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol. Psychiatry 68, 368–376 10.1016/j.biopsych.2010.05.024 [DOI] [PubMed] [Google Scholar]
- Morgan V., Castle D., Page A., Fazio S., Gurrin L., Burton P., et al. (1997). Influenza epidemics and incidence of schizophrenia, affective disorders and mental retardation in Western Australia: no evidence of a major effect. Schizophr. Res. 26, 25–39 10.1016/S0920-9964(97)00033-9 [DOI] [PubMed] [Google Scholar]
- Mortensen P. B., Nørgaard-Pedersen B., Waltoft B. L., Sørensen T. L., Hougaard D., Torrey E. F., et al. (2007). Toxoplasma gondii as a risk factor for early-onset schizophrenia: analysis of filter paper blood samples obtained at birth. Biol. Psychiatry 61, 688–693 10.1016/j.biopsych.2006.05.024 [DOI] [PubMed] [Google Scholar]
- Mousa A., Seiger A., Kjaeldgaard A., Bakhiet M. (1999). Human first trimester forebrain cells express genes for inflammatory and anti-inflammatory cytokines. Cytokine 11, 55–60 10.1006/cyto.1998.0381 [DOI] [PubMed] [Google Scholar]
- Mueller B. R., Bale T. L. (2008). Sex-specific programming of offspring emotionality after stress early in pregnancy. J. Neurosci. 28, 9055–9065 10.1523/JNEUROSCI.1424-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller N., Riedel M., Ackenheil M., Schwarz M. J. (1999). The role of immune function in schizophrenia: an overview. Eur. Arch. Psychiatry Clin. Neurosci. 249(Suppl. 4), 62–68 10.1007/PL00014187 [DOI] [PubMed] [Google Scholar]
- Myint A. M., Schwarz M. J., Muller N. (2012). The role of the kynurenine metabolism in major depression. J. Neural. Transm. 119, 245–251 10.1007/s00702-011-0741-3 [DOI] [PubMed] [Google Scholar]
- Nemeroff C. B. (1992). New vistas in neuropeptide research in neuropsychiatry: focus on corticotropin-releasing factor. Neuropsychopharmacology 6, 69–75 [PubMed] [Google Scholar]
- Ng P. C. (2000). The fetal and neonatal hypothalamic-pituitary-adrenal axis. Arch. Dis. Child. Fetal Neonatal Ed. 82, F250–F254 10.1136/fn.82.3.F250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A., Kirchhoff F., Helmchen F. (2005). Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318 10.1126/science.1110647 [DOI] [PubMed] [Google Scholar]
- Opal S. M., DePalo V. A. (2000). Anti-inflammatory cytokines. Chest 117, 1162–1172 10.1378/chest.117.4.1162 [DOI] [PubMed] [Google Scholar]
- Pardo C. A., Vargas D. L., Zimmerman A. W. (2005). Immunity, neuroglia and neuroinflammation in autism. Int. Rev. Psychiatry 17, 485–495 10.1080/02646830500381930 [DOI] [PubMed] [Google Scholar]
- Patterson P. H. (2009). Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav. Brain Res. 204, 313–321 10.1016/j.bbr.2008.12.016 [DOI] [PubMed] [Google Scholar]
- Perry V. H., Nicoll J. A., Holmes C. (2010). Microglia in neurodegenerative disease. Nat. Rev. Neurol. 6, 193–201 10.1038/nrneurol.2010.17 [DOI] [PubMed] [Google Scholar]
- Ponzio N. M., Servatius R., Beck K., Marzouk A., Kreider T. (2007). Cytokine levels during pregnancy influence immunological profiles and neurobehavioral patterns of the offspring. Ann. N. Y. Acad. Sci. 1107, 118–128 10.1196/annals.1381.013 [DOI] [PubMed] [Google Scholar]
- Potvin S., Stip E., Sepehry A. A., Gendron A., Bah R., Kouassi E. (2008). Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol. Psychiatry 63, 801–808 10.1016/j.biopsych.2007.09.024 [DOI] [PubMed] [Google Scholar]
- Radewicz K., Garey L. J., Gentleman S. M., Reynolds R. (2000). Increase in HLA-DR immunoreactive microglia in frontal and temporal cortex of chronic schizophrenics. J. Neuropathol. Exp. Neurol. 59, 137–150 [DOI] [PubMed] [Google Scholar]
- Ratnayake U., Quinn T. A., Castillo-Melendez M., Dickinson H., Walker D. W. (2012). Behaviour and hippocampus-specific changes in spiny mouse neonates after treatment of the mother with the viral-mimetic Poly I:C at mid-pregnancy. Brain Behav. Immun. 26, 1288–1299 10.1016/j.bbi.2012.08.011 [DOI] [PubMed] [Google Scholar]
- Rehn A. E., Rees S. M. (2005). Investigating the neurodevelopmental hypothesis of Schizophrenia. Clin. Exp. Pharmacol. Physiol. 32, 687–696 10.1111/j.1440-1681.2005.04257.x [DOI] [PubMed] [Google Scholar]
- Rezaie P., Male D. (1999). Colonisation of the developing human brain and spinal cord by microglia: a review. Microsc. Res. Tech. 45, 359–382 [DOI] [PubMed] [Google Scholar]
- Rezaie P., Trillo-Pazos G., Everall I. P., Male D. K. (2002a). Expression of beta-chemokines and chemokine receptors in human fetal astrocyte and microglial co-cultures: potential role of chemokines in the developing CNS. Glia 37, 64–75 10.1002/glia.1128 [DOI] [PubMed] [Google Scholar]
- Rezaie P., Trillo-Pazos G., Greenwood J., Everall I. P., Male D. K. (2002b). Motility and ramification of human fetal microglia in culture: an investigation using time-lapse video microscopy and image analysis. Exp. Cell Res. 274, 68–82 10.1006/excr.2001.5431 [DOI] [PubMed] [Google Scholar]
- Rothwell N. J., Luheshi G. N. (2000). Interleukin 1 in the brain: biology, pathology and therapeutic target. Trends Neurosci. 23, 618–625 10.1016/S0166-2236(00)01661-1 [DOI] [PubMed] [Google Scholar]
- Schmidt E. D., Aguilera G., Binnekade R., Tilders F. J. (2003). Single administration of interleukin-1 increased corticotropin releasing hormone and corticotropin releasing hormone-receptor mRNA in the hypothalamic paraventricular nucleus which paralleled long-lasting (weeks) sensitization to emotional stressors. Neuroscience 116, 275–283 10.1016/S0306-4522(02)00555-9 [DOI] [PubMed] [Google Scholar]
- Schwarcz R., Bruno J. P., Muchowski P. J., Wu H. Q. (2012). Kynurenines in the mammalian brain: when physiology meets pathology. Nat. Rev. Neurosci. 13, 465–477 10.1038/nrn3257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckl J. R. (2004). Prenatal glucocorticoids and long-term programming. Eur. J. Endocrinol. 151(Suppl. 3), U49–U62 10.1530/eje.0.151U049 [DOI] [PubMed] [Google Scholar]
- Selten J. P., Slaets J. P. (1994). Evidence against maternal influenza as a risk factor for schizophrenia. Br. J. Psychiatry 164, 674–676 10.1192/bjp.164.5.674 [DOI] [PubMed] [Google Scholar]
- Selten J.-P., Frissen A., Lensvelt-Mulders G., Morgan V. A. (2009). Schizophrenia and 1957 pandemic of influenza: meta-analysis. Schizophr. Bull. 36, 219–228 10.1093/schbul/sbp147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L., Tu N., Patterson P. H. (2005). Maternal influenza infection is likely to alter fetal brain development indirectly: the virus is not detected in the fetus. Int. J. Dev. Neurosci. 23, 299–305 10.1016/j.ijdevneu.2004.05.005 [DOI] [PubMed] [Google Scholar]
- Shobokshi A., Shaarawy M. (2002). Maternal serum and amniotic fluid cytokines in patients with preterm premature rupture of membranes with and without intrauterine infection. Int. J. Gynaecol. Obstet. 79, 209–215 10.1016/S0020-7292(02)00238-2 [DOI] [PubMed] [Google Scholar]
- Short S. J., Lubach G. R., Karasin A. I., Olsen C. W., Styner M., Knickmeyer R. C., et al. (2010). Maternal influenza infection during pregnancy impacts postnatal brain development in the rhesus monkey. Biol. Psychiatry 67, 965–973 10.1016/j.biopsych.2009.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M. N., Pearce B. D., Biron C. A., Miller A. H. (2005). Immune modulation of the hypothalamic-pituitary-adrenal (HPA) axis during viral infection. Viral Immunol. 18, 41–78 10.1089/vim.2005.18.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. E. P., Li J., Garbett K., Mirnics K., Patterson P. H. (2007). Maternal immune activation alters fetal brain development through Interleukin-6. J. Neurosci. 27, 10695–10702 10.1523/JNEUROSCI.2178-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner J., Bielau H., Brisch R., Danos P., Ullrich O., Mawrin C., et al. (2008). Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J. Psychiatr. Res. 42, 151–157 10.1016/j.jpsychires.2006.10.013 [DOI] [PubMed] [Google Scholar]
- Steiner J., Mawrin C., Ziegeler A., Bielau H., Ullrich O., Bernstein H. G., et al. (2006). Distribution of HLA-DR-positive microglia in schizophrenia reflects impaired cerebral lateralization. Acta Neuropathol. 112, 305–316 10.1007/s00401-006-0090-8 [DOI] [PubMed] [Google Scholar]
- Stern H., Booth J. C., Elek S. D., Fleck D. G. (1969). Microbial causes of mental retardation. The role of prenatal infections with cytomegalovirus, rubella virus, and toxoplasma. Lancet 2, 443–448 10.1016/S0140-6736(69)90162-7 [DOI] [PubMed] [Google Scholar]
- Suvisaari J., Haukka J., Tanskanen A., Hovi T., Lonnqvist J. (1999). Association between prenatal exposure to poliovirus infection and adult schizophrenia. Am. J. Psychiatry 156, 1100–1102 [DOI] [PubMed] [Google Scholar]
- Takeda K., Akira S. (2005). Toll-like receptors in innate immunity. Int. Immunol. 17, 1–14 10.1093/intimm/dxh186 [DOI] [PubMed] [Google Scholar]
- Takeuchi O., Akira S. (2007). Recognition of viruses by innate immunity. Immunol. Rev. 220, 214–224 10.1111/j.1600-065X.2007.00562.x [DOI] [PubMed] [Google Scholar]
- Tandon R., Keshavan M. S., Nasrallah H. A. (2008). Schizophrenia, “Just the Facts”: what we know in 2008 part 1: overview. Schizophr. Res. 100, 4–19 10.1016/j.schres.2008.01.022 [DOI] [PubMed] [Google Scholar]
- Tremblay M. E., Stevens B., Sierra A., Wake H., Bessis A., Nimmerjahn A. (2011). The role of microglia in the healthy brain. J. Neurosci. 31, 16064–16069 10.1523/JNEUROSCI.4158-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullian E. M., Harris B. T., Wu A., Chan J. R., Barres B. A. (2004). Schwann cells and astrocytes induce synapse formation by spinal motor neurons in culture. Mol. Cell. Neurosci. 25, 241–251 10.1016/j.mcn.2003.10.011 [DOI] [PubMed] [Google Scholar]
- Urakubo A., Jarskog L. F., Lieberman J. A., Gilmore J. H. (2001). Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain. Schizophr. Res. 47, 27–36 10.1016/S0920-9964(00)00032-3 [DOI] [PubMed] [Google Scholar]
- Vargas D. L., Nascimbene C., Krishnan C., Zimmerman A. W., Pardo C. A. (2005). Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 57, 67–81 10.1002/ana.20315 [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Someya T., Nawa H. (2010). Cytokine hypothesis of schizophrenia pathogenesis: evidence from human studies and animal models. Psychiatry Clin. Neurosci. 64, 217–230 10.1111/j.1440-1819.2010.02094.x [DOI] [PubMed] [Google Scholar]
- Webster M. J., O'Grady J., Kleinman J. E., Weickert C. S. (2005). Glial fibrillary acidic protein mRNA levels in the cingulate cortex of individuals with depression, bipolar disorder and schizophrenia. Neuroscience 133, 453–461 10.1016/j.neuroscience.2005.02.037 [DOI] [PubMed] [Google Scholar]
- Welberg L. A., Seckl J. R. (2001). Prenatal stress, glucocorticoids and the programming of the brain. J. Neuroendocrinol. 13, 113–128 10.1111/j.1365-2826.2001.00601.x [DOI] [PubMed] [Google Scholar]
- Williams J. G., Higgins J. P. T., Brayne C. G. (2006). The epidemiology of autistic spectrum disorders: is the prevalence rising. Arch. Dis. Child 91, 8–15 10.1136/adc.2004.062083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaretsky M. V., Alexander J. M., Byrd W., Bawdon R. E. (2004). Transfer of inflammatory cytokines across the placenta. Obstet. Gynecol. 103, 546–550 10.1097/01.AOG.0000114980.40445.83 [DOI] [PubMed] [Google Scholar]
- Zheng R., Qin X., Li Y., Yu X., Wang J., Tan M., et al. (2012). Imbalanced anti-H1N1 immunoglobulin subclasses and dysregulated cytokines in hospitalized pregnant women with 2009 H1N1 influenza and pneumonia in Shenyang, China. Hum. Immunol. 73, 906–911 10.1016/j.humimm.2012.06.005 [DOI] [PubMed] [Google Scholar]


