Abstract
The Growth Arrest and DNA Damage-inducible 45 (GADD45) proteins have been implicated in regulation of many cellular functions including DNA repair, cell cycle control, senescence and genotoxic stress. However, the pro-apoptotic activities have also positioned GADD45 as an essential player in oncogenesis. Emerging functional evidence implies that GADD45 proteins serve as tumor suppressors in response to diverse stimuli, connecting multiple cell signaling modules. Defects in the GADD45 pathway can be related to the initiation and progression of malignancies. Moreover, induction of GADD45 expression is an essential step for mediating anti-cancer activity of multiple chemotherapeutic drugs and the absence of GADD45 might abrogate their effects in cancer cells. In this review, we present a comprehensive discussion of the functions of GADD45 proteins, linking their regulation to effectors of cell cycle arrest, DNA repair and apoptosis. The ramifications regarding their roles as essential and central players in tumor growth suppression are also examined. We also extensively review recent literature to clarify how different chemotherapeutic drugs induce GADD45 gene expression and how its up-regulation and interaction with different molecular partners may benefit cancer chemotherapy and facilitate novel drug discovery.
Keywords: GADD45 family, cancer, apoptosis, survival
Introduction
The Growth Arrest and DNA damage-inducible 45 (GADD45) gene family encodes three related GADD45 proteins, GADD45α, β, and γ. GADD45α was the first member identified [1] by screening a cDNA library of increased transcripts after ultraviolet (UV) irradiation of Chinese hamster (CHO) cells [1, 2]. GADD45β, also known as Myd118, was identified as a primary response gene transiently induced by IL-6 in myeloid leukemia cell lines [3]. The third member of the family, GADD45γ, was first described as an IL-2-inducible gene and named CR6 [4], but it is also known as OIG37, an oncostatin-M inducible gene [5], and as GRP17, gadd-related protein 17kDa [6].
GADD45 proteins are small (~18kD), with high homology among the multiple members, and localize to both the nucleus and cytoplasm, with low abundance in normal cells [3, 7, 8]. A comparison of the human GADD45 amino acid sequences demonstrates that they have around 55% similarity to each other (Figure 1A), and are evolutionary highly related (Figure 1B).
Figure 1.
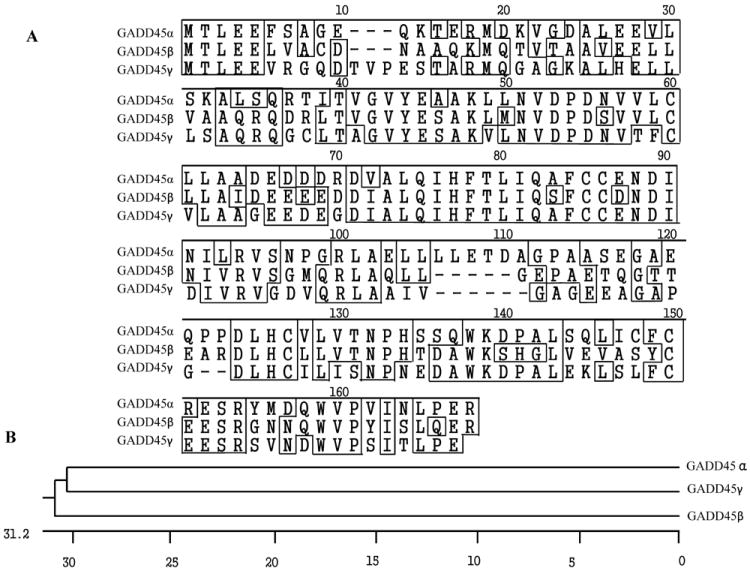
Comparison of the human GADD45α, GADD45β and GADD45γ proteins. (A) Alignment of human GADD45α, GADD45β and GADD45γ amino acid sequences. (B) Phylogenetic tree of GADD45α, GADD45β and GADD45γ proteins.
GADD45 proteins functions are similar, but not identical, and their induction differs under diverse physiological conditions or in different cell types. GADD45 proteins are ubiquitously detected in normal adult and fetal tissues, especially in quiescent cellular populations, and are highly expressed during the G1 phase of the cell cycle, with significantly reduced protein expression during the S phase [9]. Analysis of all GADD45 protein levels in a variety of murine tissues indicates that GADD45γ is highly expressed in skeletal muscle, kidney and liver, with low expression in heart, brain, spleen, lung and testis; GADD45β is detected predominantly in the lungs with moderate levels in skeletal muscle and liver, and low levels in kidney, spleen, brain, heart and testis; and GADD45α is highly expressed in kidney and skeletal muscle, moderately expressed in spleen, heart, lung, brain and liver, with low levels in testis [7]. GADD45 proteins play important roles in cellular genotoxic and non-genotoxic stress responses acting as stress sensors and tumor suppressors. After DNA damage all the GADD45 protein family members are rapidly induced, resulting in cell cycle arrest and/or apoptosis, or they actively participate in DNA repair mechanisms. GADD45 proteins have been extensively investigated due to their relevance in cancer development and progression [10]. Evidence has also been provided that the anti-cancer activity of chemotherapeutic agents [11] and Non-steroidal anti-inflammatory drugs (NSAIDS) [12] rely on GADD45 up-regulation for induction of cell cycle arrest and apoptosis in tumor cells.
In this review, we analyze in-depth the molecular structure of GADD45 proteins and the mechanism(s) by which interactions with their molecular partners modulate cancer cell cycle arrest, apoptosis and DNA repair. We review the relevance of GADD45 deregulation in different types of cancer and the molecular mechanisms involved in the induction of GADD45 expression by several chemotherapeutic drugs.
Protein-protein interactions of GADD45 proteins and the role of structure in mediating GADD45 function and activity
Insights into the function of any given protein are enhanced by elucidation of its molecular structure. In 2008, the structure of the human GADD45α protein was obtained by nuclear magnetic resonance (NMR) [13] and mouse GADD45γ protein was resolved by crystallography [14] demonstrating that these two proteins share similar secondary structures, presenting 5 α-helices and 5 β-sheets (Figure 2). These highly conserved proteins appear to exert similar functions and interact with similar sets of proteins, including CDK1 [15-17], PCNA [18-20], p21waf/cip/mda-6 [17, 21], and MTK1 [22].
Figure 2.
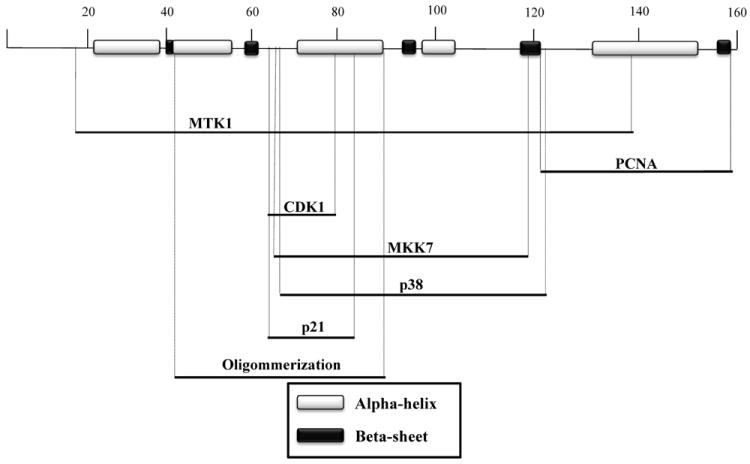
Secondary structure of GADD45 proteins and interaction domains. The structures of GADD45α and GADD45γ proteins were determined by nuclear magnetic resonance and crystallography. α-helices and β-sheet structures are indicated in white and black, respectively. Cross-reference of mutational analysis shows the putative interaction domains of GADD45 proteins with PCNA, CDK1, MTK1, p38, PCNA, MKK7, p21waf/cip/mda-6 and the dimerization domain.
GADD45 dimerization
GADD45 proteins can interact with each other and form homo- and hetero-dimers, and these interactions may play a pivotal role in their functions. Initially the GADD45α region, comprising the amino acids residues 33-61 and 132-165, was shown to be essential for oligomerization [23]. However, crystallography and mutational analyses indicate that mouse GADD45γ α-helices 2 and 3 in the central region (amino acids 43-87) of the protein are essential for dimerization, while mutation of L80, present in α-helix 3, blocks protein dimerization and inhibition of cell growth [14]. Schrag et al. (2008) point out that most of the interaction domains reported in the literature may not be actual interaction regions, but rather the dimerization domain present in the central region of the GADD45 proteins is crucial for protein interactions. As discussed further below, mutational analysis reveals that GADD45 protein interactions with CDK1, MTK1, p21waf/cip/mda-6, p38 and PCNA map to the dimerization region. The relevance of GADD45 oligomerization has been demonstrated in experiments using GADD45 mutants lacking the region between amino acids 62-67 [17]. These mutants exhibit reduced interaction with p21waf/cip/mda-6, CDK1 and PCNA.
Interaction of GADD45 with the upstream kinase MTK1/MEKK4
Interestingly, all three GADD45 family members directly interact with the upstream kinase MTK1/MEKK4 in response to environmental stresses, resulting in apoptosis induction through the p38/JNK pathways [22]. The GADD45β N-terminal amino acid residues (13-132) are essential to disrupt the auto-inhibitory domain of MTK1, leading to dimerization and phosphorylation at Thr1493, which activates the MTK1 kinase domain [22, 24, 25]. Additionally, GADD45α directly interacts with p38 MAPK kinase, which is dependent on the region comprising amino acid residues 71-96, and kinase activation is dependent on the residues between amino acids 71-124 [26].
Interaction of GADD45 with the upstream kinase MKK7
Contrary to its pro-apoptotic activity, GADD45β has been described to participate in pro-survival events through association and inhibition of another MAP kinase, MKK7, which is activated by MTK1/MEKK4, having the JNK kinase as a downstream target. GADD45β putative helices α–3 and α–4 and loops 1 and 2 (comprising amino acid residues 69-113 in the central region) are essential for the inhibition of MKK7. While helix α–3 seems to directly interact with MKK7, loops 1 and 2 and helix α–4 appear to inactivate MKK7 kinase activity by occupying the ATP-binding site, which results in a conformational change and inhibition of MKK7 catalytic function [27].
Interaction of GADD45 with PCNA
GADD45α and β participate in the DNA repair machinery, in nuclear excision repair (NER) through interaction with the Proliferating Cell Nuclear Antigen (PCNA), in recruitment to the DNA lesion sites and in modulating access of DNA to the repair proteins [28, 29]. The GADD45α N-terminal region (comprising amino acid residues 1-94) was identified as essential for binding with PCNA [18]. However, conflicting results from other studies demonstrated that the interaction of GADD45α and β with PCNA is mediated by their C-terminal regions (amino acids 137–165 and 114–156, respectively) [19]. Furthermore, it was shown that the central and C-terminal regions (residues 76-159) of GADD45γ are important for its interaction with PCNA [20]. One important aspect to be considered is that deletion of amino acids residues 1-94 results in disruption of the central portion of the protein responsible for GADD45 dimerization [14], and consequently causes a loss of activity and binding to other proteins, which can explain the differences observed in the various reports.
GADD45 activity is critical for genotoxic stress and DNA repair
The GADD45 family members are regulated by DNA damage mediated by alkylating agents, serum depletion or UV radiation [1, 2]. In this context, GADD45 proteins play a crucial role in preventing transformation of normal into malignant cells [1, 2]. Different molecular contributors either inducing or stabilizing its transcripts regulate GADD45 gene expression. Among these proteins, p53, BRCA1 and several other tumor suppressors have been described to increase GADD45 expression [30-35].
Regulation of GADD45 by p53
Sequence comparison of the human, mouse and hamster GADD45α genes reveals a high level of conservation among the three species within the 1500 bp promoter region (75% homology) and in the third intron (92% homology) [36], where p53 can bind after treatment with UV, the alkylating agent methylmethane sulfonate (MMS) or ionizing radiation (IR) [30, 37-39]. Even though the GADD45α promoter region lacks binding sites for p53 it exhibits reduced levels of activity in cells with a negative p53 status after exposure to MMS, UV radiation and serum depletion [40].
GADD45 induction mediated by p53 appears to be dependent on the specific DNA damaging agent. GADD45α induction by IR is abrogated in myeloid leukemia cell lines with a deleted p53 or heterozygous allele [41], while MMS, UV radiation and serum depletion are still able to induce GADD45 expression in breast and colon cancer cell lines with a negative p53 status [40]. The GADD45 promoter contains binding sites for WT1 and Egr-1 transcription factors that interact with p53. GADD45 promoter activity is augmented by co-expression of p53 and WT1, but not by p53 alone [42].
Mutations in the p53 gene are common and range from 20 to 25% in advanced prostate cancers [43]. While p53 plays an important role in GADD45 induction, it has been demonstrated that activation of JNK-mediated phosphorylation of p53 by GADD45 is essential for the stabilization of a mutant form of p53 (altered in residues 274 and 223) and for mediating an apoptotic response [44]. In this context, GADD45α acts as an upstream effector in stabilizing p53 following DNA damage, thereby defining a positive feedback in the activation of the p53 pathway [45]. The co-dependence of p53 and GADD45α is evidenced by suppression of p53 phosphorylation (Ser15) mediated by zinc in GADD45α null cell lines [46], and reduced GADD45 gene induction mediated by zinc in cells treated with p53 inhibitors [47].
Regulation of GADD45 by BRCA1
The tumor suppressor BRCA1 is a nuclear phosphoprotein with a ring-finger in its N-terminal region and a transcription activation domain in the C-terminal region [48]. BRCA1 is a potent GADD45α inducer [31] relying on at least three essential motifs in the GADD45α gene sequence: (i) a BRCA1 binding site located in the first intron [31], (ii) a ZBRK1 binding site in the third intron [33], and (iii) Oct-1 and CCAAT-box elements in the proximal promoter region of the GADD45α gene between -121 to -75 [32]. GADD45 promoter induction by BRCA1 occurs through a p53-independent mechanism [32].
Oct-1 and NF-YA transcription factors have been shown to be important in GADD45 promoter activation by UV, MMS [34, 49], and Trichostatin A [50]. GADD45 third intron activation mediated by BRCA1 is regulated by Zinc finger and BRCA1 interacting protein with a KRAB domain 1 (ZBRK1), which encodes a 60-KD protein containing a BRCA1 binding site, a zinc finger domain, and a KRAB (Krüppel-associated box) domain. ZBRK1 interacts directly with BRCA1 and represses the transcriptional activation of GADD45 [33]. ZBRK1 is degraded in the presence of UV and MMS, establishing a link between environmental stress and GADD45 expression during both UV exposure and MMS treatment [51]. Similar to ZBRK1, BRCA1 associated RING domain (BARD1), another inhibitor of BRCA1 transcriptional activity, represses BRCA1-mediated trans-activation of the GADD45 promoter, even though BARD1 increases the accumulation of BRCA1 in the nucleus [35].
Regulation of GADD45 by NF-κB
NF-κB has been implicated in tumorigenesis, cancer cell survival, invasion and metastasis [52]. Regulation of GADD45α and γ expression by NF-κB is mediated by upregulation of c-myc, another oncogene and transcription factor frequently overexpressed or translocated in a variety of cancers [53]. C-myc inhibits the GADD45 promoter by its interaction with the GC-rich region, which contains the WT1 and Egr motifs [54], by a post-RNA polymerase II recruitment mechanism [55]. C-myc also inhibits induction of GADD45 mediated by CCAAT/enhancer-binding protein alpha (C/EBPa) [56]. In addition, inhibition of NF-κB has also been demonstrated to increase GADD45α mRNA stability by interacting with nucleolin [57]. The main players involved in GADD45 expression are depicted in Figure 3.
Figure 3.
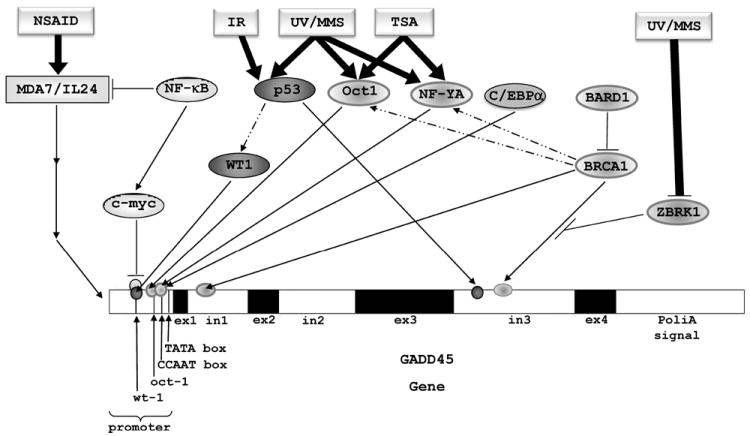
GADD45 modulation and activation. The GADD45 promoter region contains Oct-1, CCAAT-box, WT1, and Egr-1 elements, which are activated by direct interaction with Oct-1/NF-YA/CEBPα, or through indirect interaction of p53/WT1/BRCA1 with Oct-1/NF-YA. The first intron of GADD45 gene contains a BRCA1 element, while the third intron contains p53 and ZBRK1 elements. Using the third intron of the GADD45 gene, UV, MMS and IR agents may activate the expression of GADD45 gene by the induction of p53, through its direct interaction with the p53 element or ZBRK1 degradation through the proteasome pathway, resulting in the release of BRCA1 inhibition by ZBRK1. IR activation relies on p53; UV, MMS, TSA, and other drugs activate expression by means of other transcription factors. NSAIDs activate the expression of GADD45 genes through activation of the mda-7/IL-24 gene, which can be inhibited by NF-κB. NF-κB can also induce c-myc, which inhibits GADD45 genes expression through interaction with Oct-1 and NF-YA.
In contrast to GADD45α and γ, GADD45β expression is induced by NF-κB via direct interaction within NF-κβ elements present in the GADD45β promoter [58]. However, GADD45β gene expression is also induced by other transcription factors. Analysis of colon cancer cell lines showed that activation of the GADD45β gene by MMS relies on the transcription factors NFY, SP1 and Egr1 [59].
GADD45 and DNA repair
The role of GADD45 proteins in the DNA repair machinery is still not clear. It has been reported that GADD45 null mice display increased sensitivity to radiation carcinogenesis, genomic instability and chromosome abnormalities, characterized by aneuploidy, centrosome amplification, aberrant mitosis and cytokinesis [60]. Additionally, GADD45 null mice exhibit a reduction in NER activity, an increase in mutation levels and enhanced sensitivity to chemically-induced carcinogenesis [61]. These deleterious features are postulated to be related to the lack of GADD45-mediated DNA repair.
GADD45-mediated DNA repair events may involve the interaction of GADD45 proteins with PCNA. PCNA changes its nuclear localization from DNA replication sites to DNA damage sites after exposure to DNA damaging agents [62], provoking changes in NER, mismatch repair (MMR) and base excision repair (BER) machinery [63]. While GADD45 interaction with PCNA stimulates the process [29], silencing of GADD45 genes abrogates PCNA presence in the DNA damage sites and reduces DNA-repair [28].
In another mechanism, GADD45 is recruited to mononucleosomes, which have been altered by histone acetylation or UV radiation, where it interacts with core histones causing alteration in DNA accessibility and facilitating the relaxation and cleavage activity of topoisomerase [64].
GADD45 effects on methylation
The role of the GADD45 family of genes in demethylation processes is controversial and appears linked to NER. The DNA demethylation process occurs by conversion of the cytosine group to thymine, and excision of the mismatched base followed by DNA repair [65]. GADD45 activates the expression of methylation-silenced reporter genes and induces global DNA hypomethylation, while GADD45 inhibition induces DNA hypermethylation. Interestingly, GADD45-mediated DNA demethylation is an active mechanism, which does not rely on cell replication. GADD45 interacts with the DNA repair endonuclease XPG, which acts as an endonuclease during NER activating methylation-silenced reporter genes [66]. Analysis of DNA demethylation in a Zebrafish model enhanced understanding of this process. The Zebrafish contains 6 GADD45 family members, GADD45α, β, and γ, and GADD45α, β, and γ-like proteins. Overexpression of GADD45α increased the level of demethylation, while silencing of the GADD45 and GADD45-like proteins reduced demethylation [65].
Activation induced deaminase (AID) and Apolipo-protein B RNA-editing catalytic component-1 (Apobec-1) are members of the cytidine deaminase family. These proteins participate in the deamination of 5-meC, generating a thymine in just one of the DNA strands and causing a mismatch that should be repaired [67]. Co-expression of GADD45, AID and methyl-binding domain protein 4 (Mdb4) increases the levels of demethylation, and the overexpression of GADD45 upregulates the expression of the AID/Apobec family [65]. Rai et al. (2008) proposed a model of DNA demethylation, in which GADD45 interacts with AID, which deaminates the methylated cytosine and converts it into a thymine. After Mdb4 excises the mismatched base, the DNA repair machinery promotes the replacement of the unmethylated base [65]. Recruitment of GADD45α to methylation sites may involve TAF12, a TATA-binding protein (TBP)-associated factor, which has been shown to recruit GADD45α to rDNA-methylated sites. GADD45α then recruits the DNA repair machinery promoting demethylation [68]. A role for GADD45 in mediating demethylation is still an area of debate, since neither the demethylation activity of GADD45 nor the correlation between expression of GADD45 and DNA demethylation could be reproduced in subsequent studies [69, 70].
The role of GADD45 proteins in cell cycle arrest
Cell cycle arrest is an essential cell-defense mechanism to preserve genome integrity and prevent replication of damaged DNA and propagation of misleading information. Moreover, genomic instability due to loss of cell cycle checkpoints is frequently associated with tumorigenesis [71, 72]. The progression of cell cycle through G1, S, G2 and M phases occurs in an orderly fashion and is controlled by regulatory proteins, such as the Ser/Thr kinases, cyclin-dependent kinases (CDK) and Cyclins [73]. While CDK protein expression is maintained at a stable rate, cyclin levels fluctuate during cell cycle, activating the CDKs and promoting phosphorylation of proteins that lead to progression of cell cycle [74-77].
Interaction of GADD45 with the CDK1/Cyclin B1 complex induces G2/M cell cycle arrest
The CDK1/Cyclin B1 complex, which is responsible for the G2/M checkpoint [78], is the main target of GADD45-mediated cell cycle arrest. GADD45 induction of cell cycle arrest leads to a reduced cell proliferation rate, in both normal and cancer cells. GADD45α, β [79] and γ [7] have been shown to be firmly involved in this process. Overexpression of GADD45 α, β and γ suppresses cell proliferation in numerous cell lines, without evidence of apoptosis induction [5, 80].
Silencing of the GADD45α gene in human and murine cell lines leads to an impaired G2/M arrest mediated by UV or MMS, but not by IR. Indeed, the most prominent action of GADD45 proteins in regulation of the cell cycle is observed at the G2/M checkpoint by disrupting the CDK1/Cyclin B1 complex [81]. GADD45 proteins interact directly with CDK1, but not with Cyclin B1, inhibiting the kinase activity of the CDK1/Cyclin B1 complex, but not the CDK2/Cyclin E complex [15]. GADD45α and β disrupt the CDK1/CyclinB1 complex, while GADD45γ inhibits the complex without disrupting interaction [8]. A GADD45α mutant lacking the central region (amino acids 71-124) of the protein is unable to interact with the CDK1/Cyclin B1 complex and inhibit its kinase activity [15]. It was later found using GADD45 deletion mutants that interaction of GADD45 with the CDK1/CyclinB1 complex involves a central region (amino acids 65-84) of the GADD45 proteins, which is also essential for interaction with p21waf/cip/mda-6 [21]. Additionally, GADD45 proteins lacking the region between amino acid residues 50-76 are unable to induce G2/M arrest in fibroblast cells and lack inhibitory effects on the CDK1/CyclinB1 complex [17]. However, further work is necessary to comprehend the molecular mechanisms used by GADD45 proteins to inhibit CDK1/CyclinB1 complex activity and induce G2/M arrest. Initial studies indicated that this process was p38-independent [80]. However, conflicting reports demonstrated that GADD45α- and γ-mediated cell cycle arrest depends on the activation of the JNK and p38 pathways [82]. Furthermore, GADD45β and γ have been shown neither to induce cell cycle arrest nor to inhibit CDK1 kinase activity [17]. The different results observed may be due to disparate experimental conditions as well as dissimilar cell types employed by the investigators.
GADD45 induces G1/S cell cycle arrest through CRIF1
The modulation of the G1/S transition by GADD45 proteins relies on a different mechanism. CR6 interacting factor 1 (CRIF1) co-localizes in the nucleus with GADD45γ, and its overexpression inhibits cell cycle progression at the G1/S phase, and increases GADD45α- and γ mediated inhibition of the CDK1/Cyclin B1 complex [83]. CRIF1 has also been found to inhibit androgen-induced proliferation and cell cycle progression at the G1/S phase in prostate cancer cells [84].
The cyclin-dependent kinase p21waf/cip/mda-6 has been implicated in cell cycle checkpoint regulation through interaction with GADD45 proteins. p21waf/cip/mda-6 interacts with CDK/Cyclin complexes [85], activating the G1/S checkpoint. GADD45α, β and γ [9, 86, 87] interact with p21waf/cip/mda-6 leading to cell cycle arrest at both the G1/S and G2/M transitions.
Apoptosis and cell survival are regulated by GADD45 proteins
Cells exposed to stresses that impair cell growth or cause damage to DNA, normally undergo growth arrest until the damage is repaired. Nevertheless, if the damage cannot be repaired, cells will undergo apoptosis. As already highlighted in this review, GADD45 proteins also play an important role in apoptosis induction.
GADD45 effects on JNK and p38 signaling
In breast cancer, GADD45 induction by BRCA1 leads to programmed cell death through the JNK pathway via interaction with the upstream kinase MTK1/MEKK4 [31]. JNK has been shown to be involved in the induction of apoptosis after genotoxic stress and UV radiation [88]. However, JNK and p38 may exert antagonistic effects depending on cell context, cross-talk with other signaling pathways and intensity and duration of the stimulus [89]. The importance of JNK in induction of apoptosis is controversial. Originally, JNK activation and inhibition experiments indicated a pro-apoptotic role for JNK, such as in UV-induced apoptosis [90, 91]. However, other studies provide evidence for an anti-apoptotic role for JNK [92, 93]. This controversy about JNK may be related to the different systems and inducers being used in the various studies.
GADD45α null mouse skin and keratinocyte cell lines demonstrate impaired activation of p38, JNK and p53 and are resistant to UV-induced apoptosis substantiating JNK and GADD45 proteins pro-apoptotic activity [94]. Induction of apoptosis mediated by TGF-β relies on GADD45β expression in a Smad 2, 3 and 4 dependent manner [95]. Silencing the GADD45β gene delays TGF-β-mediated apoptosis in myeloid leukemia and pancreatic cancer cell lines and mouse hepatocytes [95-97].
The induction of GADD45α and γ expression is regulated by NF-κB. NF-κB is a dimeric transcription factor activated by inflammatory cytokines (such as tumor necrosis factor-α interleukin-1 and lipopolysaccharide) and environmental stresses (such as UV light and γ-irradiation). NF-κB is found constitutively activated in different cancer cell types, and has been implicated in tumorigenesis, invasion, angiogenesis, metastasis, and as a mechanism triggered to allow tumor evasion from the host immune system [98]. NF-κB increases c-myc expression that in turn inhibits GADD45α and γ gene expression, but not GADD45β expression. In this scenario, JNK-mediated apoptosis can be blocked by silencing of GADD45α and γ, demonstrating the important role of NF-κB regulation in GADD45 expression and cancer cell survival [53].
GADD45 effects on mitochondria-mediated cell death
GADD45α is also linked to mitochondria-mediated cell death. GADD45α interacts with elongation factor 1-α (EF1-α) and disrupts cytoskeletal stability, which causes Bim dissociation from microtubules-associated components and translocation to the mitochondria. Furthermore, its interaction with Bcl-2 relieves Bax, increasing cytochrome-c into the cytoplasm and consequently inducing apoptosis of HeLa cells [99].
Even with evidence supporting pro-apoptotic activity and a well-established mechanism of GADD45 induction of apoptosis, there are still some controversies. Shaulian and Karin (1999) demonstrated that induction of GADD45α transcripts mediated by cellular stress or DNA damaging agents lagged behind the activation of JNK in 3T3 fibroblasts [100]. Furthermore, Wang et al. (1999) were not able to detect differences in JNK activation after cellular stress in GADD45α null MEFs compared to GADD45 wild type cells [101]. Likewise, the apoptosis induction after UV treatment is not impaired in GADD45α null lymphocytes or MEFs [60], suggesting that activation of the JNK pathway is GADD45-independent. A pro-survival activity of GADD45β in hematopoietic cell lines has also been shown [102].
Cross-talk between NF-κB and JNK
GADD45β, X-chromosome linked IAP (XIAP), A20 and blockers of reactive oxygen species (ROS) have been linked in the cross-talk between NF-κB and JNK [98]. GADD45β up-regulation is induced by NF-κB, and is involved in the down-regulation of JNK activation mediated by TNF-α [103]. The cytokine TNF-α regulates immune responses, inflammation and apoptosis [104]. JNK activation induced by TNF-α is prolonged in NF-κB deficient cells and triggers apoptosis [103]. In contrast to GADD45α and GADD45γ pro-apoptotic function, GADD45β plays a role in inhibiting JNK activation of apoptosis mediated by TNF-α by binding to and inhibiting MKK7 [103]. In addition, GADD45β is essential for suppression of TNF-α-induced cytotoxicity [105, 106]. In hematopoietic cells, both GADD45α and β have a distinct role in cell survival. GADD45β plays a role in JNK pathway inactivation by interaction with MKK4, while GADD45α activates p38 and subsequently inhibit IKβ, releasing NF-κB, and consequently leading to cell survival [107].
Pro-survival role of GADD45β
Several reports validate the pro-survival role of GADD45β protein. Bone marrow cells from GADD45β deficient mice are more sensitive to UVC-, VP-16- and daunorubicin-induced apoptosis [108]. Infiltrating Type 1 T helper (Th1) cells in the synovial fluid of patients with rheumatoid arthritis express high levels of GADD45β and are resistant to apoptosis, which was abrogated by silencing GADD45β gene expression [109]. Indeed, GADD45β overexpression in fibroblasts protects cells from programmed cell death [110]. However, MEFs from normal or GADD45β null mice are not susceptible to TNF-α-mediated apoptosis and do not demonstrate differences in JNK activity [111].
GADD45 proteins respond to environmental stresses mediating the activation of both p38 and JNK pathways, and are considered crucial mediators of cellular stress responses. However, the cell’s decision to undergo survival or apoptosis is complex, and dependent not only on GADD45 proteins activation, but also on the environmental stimuli and the cell type involved. MAPK pathways may act together in apoptosis induction or play different roles depending on the molecular participants involved. In cancer cells, the activation of GADD45 proteins induces pro-apoptotic pathways, while in some normal and particularly in hematopoietic cell lines, GADD45 proteins play a role in pro-survival pathways. In sum, these studies demonstrate the heterogeneity of mechanisms involved in GADD45 protein activation. Our current understanding of pro-survival and apoptotic activities of GADD45 proteins are summarized in Figures 4 and 5.
Figure 4.
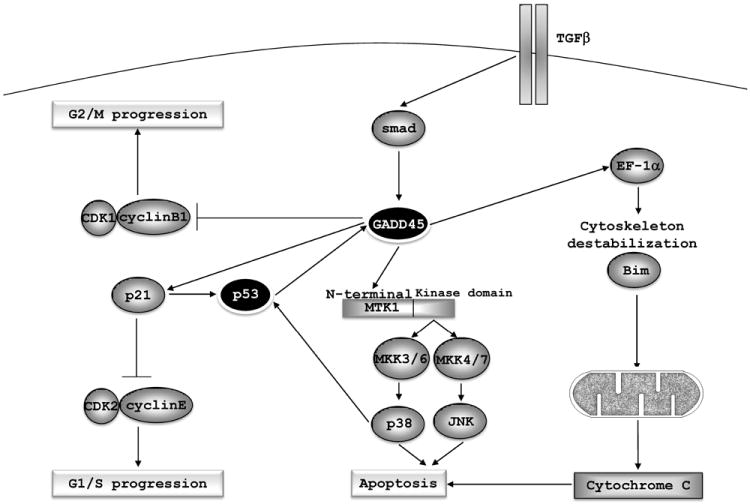
GADD45-mediated cell cycle arrest and apoptosis. GADD45 inhibits the kinase activity of the CDK1/CyclinB1 complex and promotes G2/M arrest; interaction with p21waf/cip/mda-6 contributes to G1/S arrest. Interaction of GADD45 proteins with the N-terminal domain of MTK1 leads to relieve of its auto-inhibitory domain and activation of the kinase domain, which activates both p38 and JNK pathways and induces apoptosis. GADD45 proteins also participate in mitochondria-mediated apoptosis by interaction with EF1-α, which leads to cytoskeleton destabilization and to the release of Bim. As a consequence, Bim translocates into the mitochondria promoting release of cytochrome-c, resulting in the induction of apoptosis. GADD45 proteins inhibition is an essential step in the NF-κB-mediated cell survival.
Figure 5.
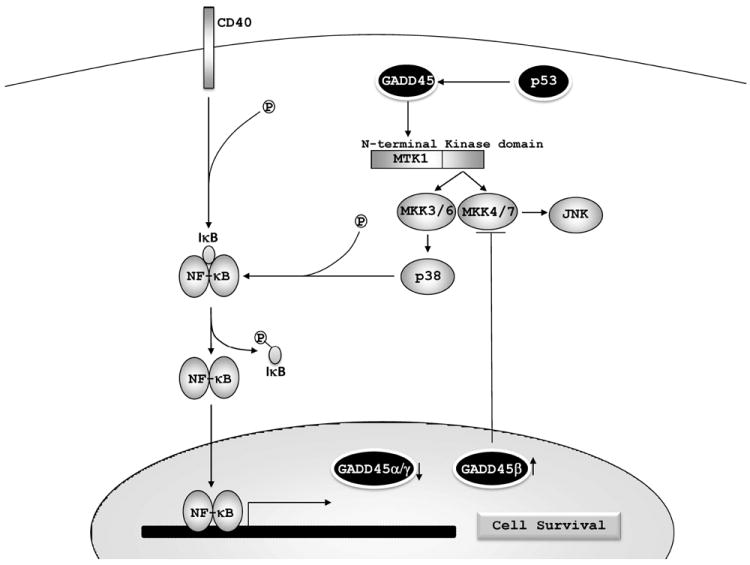
GADD45 proteins and cell survival. NF-κB represses GADD45α and GADD45γ, while it induces GADD45β expression. NF-κB mediates cell survival and GADD45β activation leading to inhibition of MKK4/7 and JNK-mediated apoptosis. GADD45 proteins may also play a role in cell survival by induction of p38, which promotes degradation of IκB and activation of the NF-κB pathway.
GADD45 expression is deregulated in various types of cancer
Mutations of GADD45 in cancer
Mutation and gene expression analyses of the GADD45 family have been performed in different types of tumors to identify sequence alterations and/or deregulated expression. GADD45α is located on chromosome 1p31.1-21.2 [36], which has been shown to contain a high incidence of deletions in breast cancer [112]. No mutations in the GADD45 gene have been observed in familial breast cancer [113, 114]. Different tumor cell lines, including lung, breast, bladder, testis, cervix, leukemia, ovarian, epidermis and sarcoma also do not contain mutations in the GADD45α gene [115], and no mutation is present in atypical fibroxanthoma [116]. However, 13% of the clinical samples from patients with invasive pancreatic ductal carcinomas harbor point mutations in exon 4 between codons 141-159 of the GADD45α gene [117]. Furthermore, 9.1% of Egyptian and 4.5% of American pancreatic adenocarcinoma patients were found with GADD45 gene mutations in exons 1 and 4 [118]. GADD45β is located at chromosome 19p13.3. This locus was found to be a common integration site in polyclonal tumors developed by retroviral insertion, and this integration leads to increased levels of GADD45β expression and inhibition of apoptosis in tumor cells [110]. GADD45γ is located at chromosome 9q22.1-q22.2 and at the present time no mutations have been found in this gene. The current information regarding GADD45 gene mutations in human cancer cells is summarized in Table 1.
Table 1.
GADD45 mutations in human cancer cells.
| Cancer cell type | GADD45 mutations |
|---|---|
| Atypical fibroxanthoma | No mutations in GADD45α [116] |
| Breast cancer | No mutations in GADD45α [113, 114] |
| Breast, bladder, cervix, epidermis, leukemia, lung, ovarian, sarcoma and testis | No mutations in GADD45α [115] |
| Pancreas invasive ductal carcinomas | Point mutations in exon 4 of GADD45α in 13% of the patients analyzed [117] |
| Pancreatic adenocarcinoma | GADD45α mutations in 9.1% of the Egyptian patients and 4.5% of the American patients analyzed [118] |
Methylation of GADD45 promoters in cancer
Comparison of normal and tumor cell lines as well as evaluation of clinical samples have provided new insights about GADD45 deregulation in cancer. Analysis of hepatocellular carcinoma clinical samples indicates down-regulation of GADD45γ mRNA in 65% of the cases [119] and GADD45α mRNA in 77% of the cases [120]. The expression of GADD45α is 10 times lower in non-small cell lung carcinoma (NSCLC) compared to normal lung tissues [121] and is also down-regulated in pituitary tumors [122].
Down-regulation of GADD45 genes is usually, but not always, correlated with methylation of the gene’s promoter region. The DNA methylation process consists of the addition of a methyl moiety to a DNA cytosine base in its fifth position (5-methycytosine), leading to the insertion of a new coding element which may play a role in transfer of genomic information through modulation and alteration in gene expression. The region where most of the methylation events occur in mammals are in CpG dinucleotides. The DNA methylation may take place in both silenced and actively transcribed genomic regions and, in general, is not an event readily reversible [123].
Methylation frequencies in NSCLC are 1.4% for GADD45α, 7.2% for GADD45β, and 31.6% for GADD45γ [124]. In breast cancer cell lines DNA demethylation mediated by 5-azacytidine (5-Aza) induces cell growth arrest, which does not occur in normal breast cell lines [125]. 5-Aza induces DNA hypomethylation [126] and has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of all subtypes of myelodysplastic syndromes [127]. Using a methylation-sensitive transcriptome approach, GADD45α was one of the genes identified to be expressed after DNA demethylation in breast cancer cells [125]. In addition, reduced GADD45α expression in prostate cancer cell lines correlates with methylation of a 4CpG region upstream of the GADD45α proximal promoter [128].
GADD45β expression is restored by 5-Aza in hepatocellular carcinoma cell lines [129]. Decreased GADD45β expression is demonstrated in individuals infected by hepatitis C virus, and is associated with hypermethylation of the GADD45β promoter upon viral infection [130].
GADD45γ is heavily methylated in cancer cell lines. GADD45γ CpG island methylation is present in 75% of carcinoma and lymphoma cell lines, 85% of non-Hodgkin’s lymphomas, 50% of Hodgkin’s lymphomas, 73% of nasopharyngeal carcinomas, 50% of cervical carcinomas, 29% of esophageal carcinomas, and 40% of lung carcinomas, while no methylation is present in any immortalized normal epithelial cell line, normal tissue, or peripheral blood mononuclear cells [131, 132]. Furthermore, recent findings demonstrated that methylation of the GADD45γ gene is also more frequent in gastric, colorectal and pancreatic cancers cells compared to normal cells [133], and the loss of GADD45γ expression in pituitary tumors is associated with methylation of CpG islands in the GADD45γ gene [134].
In contrast, clinical samples of colon carcinoma demonstrate down-regulation of ZBRK1 and up-regulation of the BRCA1 and GADD45 genes [10]. Although these genes are deregulated, there are neither promoter hypermethylation in GADD45 and BRCA1 genes, or mutations in GADD45 and ZBRK1 interaction regions [10]. GADD45α and p53 expression in clinical samples of invasive pancreatic ductal carcinomas correlated with lower survival rates for patients with GADD45α (+) subgroup compared to GADD45α (-) subgroup, suggesting a link between GADD45α and p53 expression and poor prognosis for pancreatic cancer patients [117]. Supporting this data, GADD45 proteins are highly detected in pancreatic cancer samples and those patients with positive expression of p53 and GADD45α have shorter post-operative survival rates [135]. Moreover, in pancreatic ductal adenocarcinoma, GADD45α knockdown reduces cell proliferation and induces apoptosis [136]. In these cases, the high malignant potential of the tumor cells might be able to overcome the high levels of GADD45α and p53 expression. The alterations of GADD45 gene expression in different types of human cancers are summarized in Table 2.
Table 2.
GADD45 gene expression deregulation and promoter methylation in human cancer cells.
| Cancer cell type | Alterations in GADD45 gene |
|---|---|
| Carcinoma and lymphoma cell lines | GADD45γ promoter hypermethylation frequency of 75% [131] |
| Cervical carcinoma cell lines | GADD45γ promoter hypermethylation frequency of 50% [131] |
| Colon carcinomas clinical samples | Up-regulation of GADD45α gene in colon carcinoma clinical samples compared to normal tissue [10] |
| Esophageal carcinoma cell lines | GADD45γ promoter hypermethylation frequency of 29% [131] |
| Hepatocellular carcinoma clinical samples | Down-regulation of GADD45γ in 65% of the patients analyzed compared to their adjacent normal tissue [119] |
| Hodgkin’s lymphoma cell lines | GADD45γ promoter hypermethylation frequency of 50% [131] |
| Lung carcinoma cell lines | GADD45γ promoter hypermethylation frequency of 40% [131] |
| Nasopharyngeal carcinoma cell lines | GADD45γ promoter hypermethylation frequency of 73% [131] |
| Non-Hodgkin’s lymphoma cell lines | GADD45γ promoter hypermethylation frequency of 85% [131] |
| Non-small cell lung carcinoma clinical samples | GADD45α expression is 10 times lower compared to normal lung tissue [121] |
| Methylation frequencies of 1.4% in GADD45α, 7.2% in GADD45β, and 31.6% in GADD45γ [124] | |
| Pancreatic cancer cell lines and pancreatic ductal adenocarcinoma | GADD45α is overexpressed compared to normal pancreatic cell lines or tissues [117, 135, 136] |
| Pituitary tumor clinical samples | GADD45γ is down regulated compared to normal pituitary tissue [122] |
Inhibition of GADD45 family of genes through promoter methylation or NF-κB activation is considered a critical step in cancer development, with essential implications in survival, and resistance to environmental stresses and DNA damage, through inhibition of cell cycle arrest and diminished DNA excision repair.
GADD45 proteins are new targets for antitumor therapy
Several chemotherapeutic drugs induce the expression of GADD45 proteins in vivo and in vitro, either by promoting transcription or by stabilizing the transcripts, which may contribute to the observed drug effect on cell growth and survival.
CD437 effects on GADD45
A great number of drugs have been shown to induce cell cycle arrest and inhibit cancer cell growth by targeting GADD45α expression. Among these compounds, CD437 (6-[3-adamantyl-4-hydroxyphenyl]-2-naphthalene) causes G1 arrest and apoptosis in breast cancer cell lines [137, 138]. The mechanism underlying CD437’s effect in breast cancer depends on increased expression of GADD45α through stabilization of its transcripts [11]. CD437 has also been shown to induce GADD45α mRNA stabilization in other carcinoma cell lines, including fibrosarcomas and lung, mammary, bladder and colorectal carcinomas [139]. A similar mechanism of action has been described for arsenic chloride. In human bronchial epithelial cell lines, arsenic chloride stabilizes GADD45α transcripts through the RNA binding protein, nucleolin [140], promoting GADD45 mRNA translation through an internal ribosome entry site located in the 5′ –untranslated region of the GADD45 gene [141].
Genistein effects on GADD45
Genistein is an isoflavone that has been linked to inhibition of tyrosine kinases [142] and to decreased risk of mortality for prostate and breast cancer [143, 144]. Genistein’s growth arrest in prostate cancer cell lines relies on the induction of the GADD45α gene through the CCAAT-box motif present in the GADD45α promoter region [145].
Trichostatin A effects on GADD45
Trichostatin A (TSA) inhibits the histone deacetylase (HDAC) family, which regulates expression of key genes involved in apoptosis, cell differentiation and cell cycle arrest [146]. In human osteosarcoma cell lines, TSA induces GADD45α expression and causes G2/M arrest [50]. GADD45α and γ induction by TSA requires the Oct-1 and CCAAT-box motifs in the promoter region of these genes [147]. Other histone deacetylase inhibitors demonstrate additive effects in inducing GADD45α expression and inhibiting cancer cell growth in a p53-dependent manner [148].
(-)-Xanthatin effects on GADD45
(-)-Xanthatin is an exo-Methylene lactone group-containing compound, which is present in a large variety of biologically active natural products and shows anti-inflammatory, antimalarial and cytotoxic activities in cancer cells [149]. In a farnesyltransferase-inhibitor (FTI) resistant breast cancer cell line (-)-Xanthatin induced GADD45γ and consequently promoted p38 and JNK activation causing reduced proliferation and caspase-independent apoptosis [150].
NSAID effects on GADD45
NSAIDs have emerged as potential drugs for chemoprevention in cancer. Several epidemiological studies indicated that the use of NSAIDs at clinically relevant concentrations reduce colorectal [151], breast [152] and ovarian cancer risk [153-155], although there are still some controversies [156, 157]. One major target of NSAID action is through inhibition of cyclooxygenases (COX), which are responsible for the conversion of arachidonic acids into prostaglandins. The two COX genes, COX-1 and COX-2, are almost identical; however, one relevant difference is that COX-1 expression is constitutive, whereas COX-2 expression is induced by growth factors and pro-inflammatory stimuli [158].
High COX-1 expression in ovarian cancer strongly correlates with high levels of Vascular Endothelial Growth Factor (VEGF) [159-161] and NSAIDs inhibit VEGF production in ovarian cancer cell lines [162, 163] indicating that COX-1 may regulate VEGF expression. Angiogenesis and VEGF expression are implicated in ascites formation [164] and metastasis of ovarian cancer [165], while its inhibition prevents ascites formation and inhibits disseminated cancer growth [166].
Along with COX and VEGF inhibition, NSAIDs mechanism of action involves induction of GADD45 proteins [12]. The intraperitoneal administration of NSAIDs, such as Sulindac and Indomethacin, results in the up-regulation of GADD45α gene expression, leading to gastric mucosal injury and apoptosis in mice, while the administration of NSAIDs in GADD45-null mice shows reduced cell apoptosis [167]. Moreover, the suppression of the GADD45α gene reduces caspase 9 activation-induced by NSAIDs in human gastric mucosal cells [167].
We have shown that in ovarian, prostate, renal, breast and stomach cancer cell lines structurally diverse NSAIDs induce apoptosis through activation of melanoma differentiation associated gene-7/Interleukin-24 (mda-7/IL-24) [12, 168-170]. mda-7/IL-24 induction and activation by NSAIDs leads to upregulation of GADD45α and GADD45γ that is essential for cancer programmed cell death via c-Jun NH(2)-terminal kinase (JNK) activation [12]. mda-7/IL-24 is expressed in cells of the immune system and normal melanocytes [171]. However, when mda-7/IL-24 is overexpressed it induces apoptosis only in cancer cells sparing the normal cells and therefore has been highlighted as a “magic bullet” for cancer [172-174]. Some promising studies indicate that induction of mda-7/IL-24 by a recombinant adenovirus results in apoptosis of non-small cell lung carcinoma [175], lung carcinoma [176], malignant gliomas [177-179], renal carcinomas [180], pancreatic carcinoma [181] and ovarian cancer [182, 183].
Effect of the thymidine kinase (TK) gene coupled with the antiviral drug gancyclovir (GCV) on GADD45
The efficacy of another therapy that combines a recombinant adenovirus expressing the thymidine kinase (TK) gene coupled with the antiviral drug gancyclovir (GCV) in human pancreatic adenocarcinomas relies on CCNE1 and GADD45 genes to induce cell death [184]. In another recombinant adenoviral approach, the combination of adenoviral-mediated expression of GADD45α and anticancer drugs such as etoposide, cisplatin, and 5-fluorouracil, resulted in chemosensitivity in pancreatic ductal adenocarcinoma cancer-derived cell lines [185].
Effect of Fucoxanthin and curcumin on GADD45
The carotenoid Fucoxanthin exhibits tumor cell growth inhibition by cell cycle arrest and apoptosis induction as a result of GADD45α and β gene activation [186, 187]. In hepatocellular carcinoma cells the inhibition of the MAPKs, p38 and ERK enhances the expression of GADD45α and GADD45β, respectively, mediated by Fucoxanthin, while the inhibition of JNK suppresses GADD45α gene expression induced by Fucoxanthin in prostate cancer cell lines [186]. Another recent finding indicates that Curcumin, a molecule isolated from the plant Curcuma longa (LINN), increases expression of GADD45α in a p53-independent manner, inducing cell cycle arrest and concurrently apoptosis in human lung cancer cell lines [188].
Effect of docetaxel on GADD45
Docetaxel is a chemotherapeutic drug that binds to microtubules and inhibits cancer cell proliferation [189]. The sensitivity of prostate cancer cell lines to docetaxel is enhanced by overexpression of GADD45α or treatment with the demethylation agent 5-Aza [128]. Another demethylation agent, decitabine, also induces GADD45α expression and apoptosis in osteosarcoma cell lines, and silencing of GADD45α gene expression abrogates its apoptotic response [190]. Multidrug resistant human osteosarcoma cell lines have been shown to have direct links with GADD45 gene expression. The authors demonstrated that Paclitaxel or Doxorubicin impairment of apoptosis induction due to multidrug resistance is dependent of GADD45 defective gene expression. However, drug resistance can be overcome by transient expression of GADD45α, increasing cell sensitivity to chemotherapeutic drugs [191]. The effect of different drugs in GADD45 expression in several cancer cell lines is listed in Table 3.
Table 3.
Effect of different drugs in different cancer cell lines on GADD45 expression.
| Drug | Cancer cell type | Effect in GADD45 expression |
|---|---|---|
| Adenovirus TK/GCV | Human pancreatic adenocarcinoma | GADD45 and apoptosis induction [184] |
| Adenovirus GADD45α + Etoposide, Cisplatin and 5-Fluorouracil | Human pancreatic ductal adenocarcinoma | Increases sensitivity to chemotherapy [185] |
| Arsenic chloride | Human bronchial epithelial cell line | GADD45 mRNA stabilization through nucleolin [140] |
| Promotes GADD45 mRNA translation through an internal ribosome entry site (IRES) [141] | ||
| CD437 (6-[3-adamantyl-4-hydroxyphenyl]-2-naphthalene) | Human fibrosarcoma, lung, mammary, bladder and colorectal carcinomas | GADD45 mRNA stabilization [139] |
| Breast cancer | Increased GADD45 expression [11] | |
| Curcumin | Human lung cancer cell line | Increased GADD45α expression, cell cycle arrest and apoptosis [188] |
| Fucoxanthin | Human hepatocarcinoma cell line | GADD45α and β activation, cancer cell growth inhibition and cell cycle arrest [186, 187] |
| Human prostate cancer cell line | GADD45α up-regulation and G1 cell cycle arrest [186, 187] | |
| Genistein | Human prostate cancer cell line | GADD45α induction and G2/M cell cycle arrest [50] |
| NSAIDs (Ibuprofen) | Human gastric cancer cells | GADD45α and G1 blockage [194] |
| NSAIDs (Sulindac and Indomethacin) | GADD45-/- B6129F1 mice or matching w/t mice. Human gastric carcinoma epithelial cell line | Up-regulation of GADD45α and apoptosis [167] |
| NSAIDs (Sulindac sulfide, Aspirin, Ibuprofen, Sulindac sulfone, Acetaminophen, Naproxen, NS-398, Celecoxib, Diclofenac, Finasteride, Flufenamic acid, Meloxicam, Ebselen, and Flurbiprofen) | Human prostate, renal, breast and stomach cancer cell lines | GADD45α and GADD45γ induction mediated by MDA-7/IL-24 [12] |
| Paclitaxel and Doxorubicin | Human osteosarcoma multidrug resistant cell line | Multidrug resistant cell line present defects in GADD45 expression and low levels of apoptosis mediated by the drugs [191] |
| Peptidylarginine deaminase 4 and histone deacetylase inhibitors | Human osteosarcoma cell line | GADD45α induction and cancer cell growth inhibition [148] |
| Trichostatin A | Human osteosarcoma cell line | GADD45α induction and G2/M cell cycle arrest [50] |
| Zinc | Human bronchial epithelial cell lines | GADD45α induction and G2/M cell cycle arrest [46, 47] |
| (-)-Xanthatin | FTI resistant breast cancer cell line | GADD45γ induction and reduced proliferation and induction of apoptosis [150] |
Some of the drugs mentioned above are currently used as treatment options for several forms of cancer. Docetaxel, for instance increases the mean survival time of patients with castration-resistant prostate cancer (CRPC) and was approved by the FDA in 2004 and has been used since then for the treatment of CRCP [192]; 5-AZA has been used for the therapy of all subtypes of myelodysplastic syndromes [127]. Some natural compounds, like Genistein and curcumin have been studied for both chemoprevention and treatment of cancer; soy food has high content of isoflavones, like Genistein and its intake has been hypothesized to contribute to the lower incidence of breast cancer in Asian populations [193]. Development of viral vectors expressing tumor supressors genes that induce GADD45 expression, like MDA-7/IL-24 and p53, have produced promising results and represent an alternative to traditional drug therapies. The dependence of several natural and synthetic drugs and tumor suppressor genes on activation of GADD45 proteins highlights the importance of this family of proteins in cancer therapy.
Concluding remarks
GADD45 proteins have been shown to mediate the activation of several molecules involved in crucial steps of tumorigenesis. Our current understanding of the mechanism of action of GADD45 proteins supports the suggestion that these molecules may serve as potential targets to be utilized in novel cancer therapeutic strategies. Therapies involving activation of GADD45 expression, mRNA stabilization and modulation of any of its upstream or downstream effectors, may provide the requisite promising strategies. Future work, resulting in a better understanding of GADD45 pathways regulated by the tumor microenvironment and in cancer stem cells, will provide additional entry points to interfere with GADD45 function. Additionally, high throughput screening strategies with natural and synthetic libraries to identify and develop small molecule drugs with potential to selectively induce GADD45 pathways in cancer cells is clearly an avenue of research worth exploring.
Acknowledgments
R.E.T. was supported by an International Centre for Genetic Engineering and Biotechnology fellowship. J.F.V. was recipient of a CAPES international fellowship (1102-08-7) and FAPESP 06/01158-3. This work was supported by NIH grants 1R01 CA85467, P50 CA090381, P50 CA105009 and the Hershey Foundation (T.A.L.); NIH grants 1R01 CA097318, 1R01 CA127641, P01 CA104177 and grants from the Samuel Waxman Cancer Research Foundation and the National Foundation for Cancer Research (P.B.F.); NIH grant R01 CA138540 (D.S.); and Department of Defense grants PC051217 and OC0060439 (L.F.Z.). D.S. is a Harrison Endowed Scholar in Cancer Research and a Blick Scholar. P.B.F. holds the Thelma Newmeyer Corman Chair in Cancer Research in the VCU Massey Cancer Center and is a SWCRF and a NFCR Investigator.
Footnotes
Conflict of interest
The authors have no conflict of interest to declare.
References
- 1.Fornace AJ, Jr, Alamo I, Jr, Hollander MC. DNA damage-inducible transcripts in mammalian cells. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8800–4. doi: 10.1073/pnas.85.23.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fornace AJ, Jr, Nebert DW, Hollander MC, et al. Mammalian genes coordinately regulated by growth arrest signals and DNA-damaging agents. Mol Cell Biol. 1989 Oct;9(10):4196–203. doi: 10.1128/mcb.9.10.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdollahi A, Lord KA, Hoffman-Liebermann B, Liebermann DA. Sequence and expression of a cDNA encoding MyD118: a novel myeloid differentiation primary response gene induced by multiple cytokines. Oncogene. 1991 Jan;6(1):165–7. [PubMed] [Google Scholar]
- 4.Beadling C, Johnson KW, Smith KA. Isolation of interleukin 2-induced immediate-early genes. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2719–23. doi: 10.1073/pnas.90.7.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakayama K, Hara T, Hibi M, Hirano T, Miyajima A. A novel oncostatin M-inducible gene OIG37 forms a gene family with MyD118 and GADD45 and negatively regulates cell growth. J Biol Chem. 1999 Aug 27;274(35):24766–72. doi: 10.1074/jbc.274.35.24766. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki M, Watanabe TK, Fujiwara T, et al. Molecular cloning, expression, and mapping of a novel human cDNA, GRP17, highly homologous to human gadd45 and murine MyD118. J Hum Genet. 1999;44(5):300–3. doi: 10.1007/s100380050164. [DOI] [PubMed] [Google Scholar]
- 7.Zhang W, Bae I, Krishnaraju K, et al. CR6: A third member in the MyD118 and Gadd45 gene family which functions in negative growth control. Oncogene. 1999 Sep 2;18(35):4899–907. doi: 10.1038/sj.onc.1202885. [DOI] [PubMed] [Google Scholar]
- 8.Vairapandi M, Balliet AG, Hoffman B, Liebermann DA. GADD45b and GADD45g are cdc2/cyclinB1 kinase inhibitors with a role in S and G2/M cell cycle checkpoints induced by genotoxic stress. J Cell Physiol. 2002 Sep;192(3):327–38. doi: 10.1002/jcp.10140. [DOI] [PubMed] [Google Scholar]
- 9.Kearsey JM, Coates PJ, Prescott AR, Warbrick E, Hall PA. Gadd45 is a nuclear cell cycle regulated protein which interacts with p21Cip1. Oncogene. 1995 Nov 2;11(9):1675–83. [PubMed] [Google Scholar]
- 10.Garcia V, Garcia JM, Pena C, et al. The GADD45, ZBRK1 and BRCA1 pathway: quantitative analysis of mRNA expression in colon carcinomas. J Pathol. 2005 May;206(1):92–9. doi: 10.1002/path.1751. [DOI] [PubMed] [Google Scholar]
- 11.Rishi AK, Sun RJ, Gao Y, et al. Post-transcriptional regulation of the DNA damage-inducible gadd45 gene in human breast carcinoma cells exposed to a novel retinoid CD437. Nucleic Acids Res. 1999 Aug 1;27(15):3111–9. doi: 10.1093/nar/27.15.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zerbini LF, Czibere A, Wang Y, et al. A novel pathway involving melanoma differentiation associated gene-7/interleukin-24 mediates nonsteroidal anti-inflammatory drug-induced apoptosis and growth arrest of cancer cells. Cancer Res. 2006 Dec 15;66(24):11922–31. doi: 10.1158/0008-5472.CAN-06-2068. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez R, Pantoja-Uceda D, Torres D, et al. NMR assignment and secondary structure of human growth arrest and DNA damage alpha protein (Gadd45 alpha) Biomol NMR Assign. 2008 Dec;2(2):139–42. doi: 10.1007/s12104-008-9105-9. [DOI] [PubMed] [Google Scholar]
- 14.Schrag JD, Jiralerspong S, Banville M, Jaramillo ML, O’Connor-McCourt MD. The crystal structure and dimerization interface of GADD45gamma. Proc Natl Acad Sci U S A. 2008 May 6;105(18):6566–71. doi: 10.1073/pnas.0800086105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhan Q, Antinore MJ, Wang XW, et al. Association with Cdc2 and inhibition of Cdc2/Cyclin B1 kinase activity by the p53-regulated protein Gadd45. Oncogene. 1999 May 6;18(18):2892–900. doi: 10.1038/sj.onc.1202667. [DOI] [PubMed] [Google Scholar]
- 16.Jin S, Antinore MJ, Lung FD, et al. The GADD45 inhibition of Cdc2 kinase correlates with GADD45-mediated growth suppression. J Biol Chem. 2000 Jun 2;275(22):16602–8. doi: 10.1074/jbc.M000284200. [DOI] [PubMed] [Google Scholar]
- 17.Yang Q, Manicone A, Coursen JD, et al. Identification of a functional domain in a GADD45-mediated G2/M checkpoint. J Biol Chem. 2000 Nov 24;275(47):36892–8. doi: 10.1074/jbc.M005319200. [DOI] [PubMed] [Google Scholar]
- 18.Hall PA, Kearsey JM, Coates PJ, et al. Characterisation of the interaction between PCNA and Gadd45. Oncogene. 1995 Jun 15;10(12):2427–33. [PubMed] [Google Scholar]
- 19.Vairapandi M, Azam N, Balliet AG, Hoffman B, Liebermann DA. Characterization of MyD118, Gadd45, and proliferating cell nuclear antigen (PCNA) interacting domains. PCNA impedes MyD118 AND Gadd45-mediated negative growth control. J Biol Chem. 2000 Jun 2;275(22):16810–9. doi: 10.1074/jbc.275.22.16810. [DOI] [PubMed] [Google Scholar]
- 20.Azam N, Vairapandi M, Zhang W, Hoffman B, Liebermann DA. Interaction of CR6 (GADD45gamma) with proliferating cell nuclear antigen impedes negative growth control. J Biol Chem. 2001 Jan 26;276(4):2766–74. doi: 10.1074/jbc.M005626200. [DOI] [PubMed] [Google Scholar]
- 21.Zhao H, Jin S, Antinore MJ, et al. The central region of Gadd45 is required for its interaction with p21/WAF1. Exp Cell Res. 2000 Jul 10;258(1):92–100. doi: 10.1006/excr.2000.4906. [DOI] [PubMed] [Google Scholar]
- 22.Takekawa M, Saito H. A family of stress-inducible GADD45-like proteins mediate activation of the stress-responsive MTK1/MEKK4 MAPKKK. Cell. 1998 Nov 13;95(4):521–30. doi: 10.1016/s0092-8674(00)81619-0. [DOI] [PubMed] [Google Scholar]
- 23.Kovalsky O, Lung FD, Roller PP, Fornace AJ., Jr Oligomerization of human Gadd45a protein. J Biol Chem. 2001 Oct 19;276(42):39330–9. doi: 10.1074/jbc.M105115200. [DOI] [PubMed] [Google Scholar]
- 24.Mita H, Tsutsui J, Takekawa M, Witten EA, Saito H. Regulation of MTK1/MEKK4 kinase activity by its N-terminal autoinhibitory domain and GADD45 binding. Mol Cell Biol. 2002 Jul;22(13):4544–55. doi: 10.1128/MCB.22.13.4544-4555.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyake Z, Takekawa M, Ge Q, Saito H. Activation of MTK1/MEKK4 by GADD45 through induced N-C dissociation and dimerization-mediated trans autophosphorylation of the MTK1 kinase domain. Mol Cell Biol. 2007 Apr;27(7):2765–76. doi: 10.1128/MCB.01435-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bulavin DV, Kovalsky O, Hollander MC, Fornace AJ., Jr Loss of oncogenic H-ras-induced cell cycle arrest and p38 mitogen-activated protein kinase activation by disruption of Gadd45a. Mol Cell Biol. 2003 Jun;23(11):3859–71. doi: 10.1128/MCB.23.11.3859-3871.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papa S, Monti SM, Vitale RM, et al. Insights into the structural basis of the GADD45beta-mediated inactivation of the JNK kinase, MKK7/JNKK2. J Biol Chem. 2007 Jun 29;282(26):19029–41. doi: 10.1074/jbc.M703112200. [DOI] [PubMed] [Google Scholar]
- 28.Smith ML, Ford JM, Hollander MC, et al. p53-mediated DNA repair responses to UV radiation: studies of mouse cells lacking p53, p21, and/or gadd45 genes. Mol Cell Biol. 2000 May;20(10):3705–14. doi: 10.1128/mcb.20.10.3705-3714.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith ML, Chen IT, Zhan Q, et al. Interaction of the p53-regulated protein Gadd45 with proliferating cell nuclear antigen. Science. 1994 Nov 25;266(5189):1376–80. doi: 10.1126/science.7973727. [DOI] [PubMed] [Google Scholar]
- 30.Kastan MB, Zhan Q, el-Deiry WS, et al. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992 Nov 13;71(4):587–97. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- 31.Harkin DP, Bean JM, Miklos D, et al. Induction of GADD45 and JNK/SAPK-dependent apoptosis following inducible expression of BRCA1. Cell. 1999 May 28;97(5):575–86. doi: 10.1016/s0092-8674(00)80769-2. [DOI] [PubMed] [Google Scholar]
- 32.Jin S, Zhao H, Fan F, et al. BRCA1 activation of the GADD45 promoter. Oncogene. 2000 Aug 17;19(35):4050–7. doi: 10.1038/sj.onc.1203759. [DOI] [PubMed] [Google Scholar]
- 33.Zheng L, Pan H, Li S, et al. Sequence-specific transcriptional corepressor function for BRCA1 through a novel zinc finger protein, ZBRK1. Mol Cell. 2000 Oct;6(4):757–68. doi: 10.1016/s1097-2765(00)00075-7. [DOI] [PubMed] [Google Scholar]
- 34.Jin S, Fan F, Fan W, et al. Transcription factors Oct-1 and NF-YA regulate the p53-independent induction of the GADD45 following DNA damage. Oncogene. 2001 May 10;20(21):2683–90. doi: 10.1038/sj.onc.1204390. [DOI] [PubMed] [Google Scholar]
- 35.Fabbro M, Henderson BR. BARD1 regulates BRCA1-mediated transactivation of the p21WAF1/CIP1 and Gadd45 promoters. Cancer Lett. 2008 May 18;263(2):189–96. doi: 10.1016/j.canlet.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Hollander MC, Alamo I, Jackman J, et al. Analysis of the mammalian gadd45 gene and its response to DNA damage. J Biol Chem. 1993 Nov 15;268(32):24385–93. [PubMed] [Google Scholar]
- 37.Zhan Q, Carrier F, Fornace AJ., Jr Induction of cellular p53 activity by DNA-damaging agents and growth arrest. Mol Cell Biol. 1993 Jul;13(7):4242–50. doi: 10.1128/mcb.13.7.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su ZZ, Fisher PB. Early events in methyl methanesulfonate enhancement of adenovirus transformation of cloned rat embryo fibroblast cells. Mol Carcinog. 1989;2(5):252–60. doi: 10.1002/mc.2940020505. [DOI] [PubMed] [Google Scholar]
- 39.Su ZZ, Zhang PQ, Geard C, Fisher PB. Enhancement of adenovirus transformation of cloned rat embryo fibroblast cells by gamma irradiation. Mol Carcinog. 1990;3(3):141–9. doi: 10.1002/mc.2940030307. [DOI] [PubMed] [Google Scholar]
- 40.Zhan Q, Fan S, Smith ML, et al. Abrogation of p53 function affects gadd gene responses to DNA base-damaging agents and starvation. DNA Cell Biol. 1996 Oct;15(10):805–15. doi: 10.1089/dna.1996.15.805. [DOI] [PubMed] [Google Scholar]
- 41.Carrier F, Smith ML, Bae I, et al. Characterization of human Gadd45, a p53-regulated protein. J Biol Chem. 1994 Dec 23;269(51):32672–7. [PubMed] [Google Scholar]
- 42.Zhan Q, Chen IT, Antinore MJ, Fornace AJ., Jr Tumor suppressor p53 can participate in transcriptional induction of the GADD45 promoter in the absence of direct DNA binding. Mol Cell Biol. 1998 May;18(5):2768–78. doi: 10.1128/mcb.18.5.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bookstein R, MacGrogan D, Hilsenbeck SG, Sharkey F, Allred DC. p53 is mutated in a subset of advanced-stage prostate cancers. Cancer research. 1993 Jul 15;53(14):3369–73. [PubMed] [Google Scholar]
- 44.Zerbini LF, Wang Y, Correa RG, Cho JY, Libermann TA. Blockage of NF-kappaB induces serine 15 phosphorylation of mutant p53 by JNK kinase in prostate cancer cells. Cell cycle (Georgetown, Tex) 2005 Sep;4(9):1247–53. doi: 10.4161/cc.4.9.1966. [DOI] [PubMed] [Google Scholar]
- 45.Jin S, Mazzacurati L, Zhu X, et al. Gadd45a contributes to p53 stabilization in response to DNA damage. Oncogene. 2003 Nov 20;22(52):8536–40. doi: 10.1038/sj.onc.1206907. [DOI] [PubMed] [Google Scholar]
- 46.Shih RS, Wong SH, Schoene NW, Lei KY. Suppression of Gadd45 alleviates the G2/M blockage and the enhanced phosphorylation of p53 and p38 in zinc supplemented normal human bronchial epithelial cells. Exp Biol Med (Maywood) 2008 Mar;233(3):317–27. doi: 10.3181/0708-RM-220. [DOI] [PubMed] [Google Scholar]
- 47.Shih RS, Wong SH, Schoene NW, Zhang JJ, Lei KY. Enhanced Gadd45 expression and delayed G2/M progression are p53-dependent in zinc-supplemented human bronchial epithelial cells. Exp Biol Med (Maywood) 2010 Aug;235(8):932–40. doi: 10.1258/ebm.2010.010076. [DOI] [PubMed] [Google Scholar]
- 48.Chapman MS, Verma IM. Transcriptional activation by BRCA1. Nature. 1996 Aug 22;382(6593):678–9. doi: 10.1038/382678a0. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi S, Saito S, Ohtani N, Sakai T. Involvement of the Oct-1 regulatory element of the gadd45 promoter in the p53-independent response to ultraviolet irradiation. Cancer Res. 2001 Feb 1;61(3):1187–95. [PubMed] [Google Scholar]
- 50.Hirose T, Sowa Y, Takahashi S, et al. p53-independent induction of Gadd45 by histone deacetylase inhibitor: coordinate regulation by transcription factors Oct-1 and NF-Y. Oncogene. 2003 Oct 30;22(49):7762–73. doi: 10.1038/sj.onc.1207091. [DOI] [PubMed] [Google Scholar]
- 51.Yun J, Lee WH. Degradation of transcription repressor ZBRK1 through the ubiquitin-proteasome pathway relieves repression of Gadd45a upon DNA damage. Mol Cell Biol. 2003 Oct;23(20):7305–14. doi: 10.1128/MCB.23.20.7305-7314.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002 Apr;2(4):301–10. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 53.Zerbini LF, Wang Y, Czibere A, et al. NF-kappa B-mediated repression of growth arrest- and DNA-damage-inducible proteins 45alpha and gamma is essential for cancer cell survival. Proc Natl Acad Sci U S A. 2004 Sep 14;101(37):13618–23. doi: 10.1073/pnas.0402069101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amundson SA, Zhan Q, Penn LZ, Fornace AJ., Jr Myc suppresses induction of the growth arrest genes gadd34, gadd45, and gadd153 by DNA-damaging agents. Oncogene. 1998 Oct 29;17(17):2149–54. doi: 10.1038/sj.onc.1202136. [DOI] [PubMed] [Google Scholar]
- 55.Barsyte-Lovejoy D, Mao DY, Penn LZ. c-Myc represses the proximal promoters of GADD45a and GADD153 by a post-RNA polymerase II recruitment mechanism. Oncogene. 2004 Apr 22;23(19):3481–6. doi: 10.1038/sj.onc.1207487. [DOI] [PubMed] [Google Scholar]
- 56.Constance CM, Morgan JIt, Umek RM. C/EBPalpha regulation of the growth-arrest-associated gene gadd45. Mol Cell Biol. 1996 Jul;16(7):3878–83. doi: 10.1128/mcb.16.7.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng X, Zhang Y, Chen YQ, et al. Inhibition of NF-kappaB stabilizes gadd45alpha mRNA. Biochem Biophys Res Commun. 2005 Apr 1;329(1):95–9. doi: 10.1016/j.bbrc.2005.01.105. [DOI] [PubMed] [Google Scholar]
- 58.Jin R, De Smaele E, Zazzeroni F, et al. Regulation of the gadd45beta promoter by NF-kappaB. DNA Cell Biol. 2002 Jul;21(7):491–503. doi: 10.1089/104454902320219059. [DOI] [PubMed] [Google Scholar]
- 59.Zumbrun SD, Hoffman B, Liebermann DA. Distinct mechanisms are utilized to induce stress sensor gadd45b by different stress stimuli. J Cell Biochem. 2009 Dec 1;108(5):1220–31. doi: 10.1002/jcb.22354. [DOI] [PubMed] [Google Scholar]
- 60.Hollander MC, Sheikh MS, Bulavin DV, et al. Genomic instability in Gadd45a-deficient mice. Nat Genet. 1999 Oct;23(2):176–84. doi: 10.1038/13802. [DOI] [PubMed] [Google Scholar]
- 61.Hollander MC, Kovalsky O, Salvador JM, et al. Dimethylbenzanthracene carcinogenesis in Gadd45a-null mice is associated with decreased DNA repair and increased mutation frequency. Cancer Res. 2001 Mar 15;61(6):2487–91. [PubMed] [Google Scholar]
- 62.Essers J, Theil AF, Baldeyron C, et al. Nuclear dynamics of PCNA in DNA replication and repair. Molecular and cellular biology. 2005 Nov;25(21):9350–9. doi: 10.1128/MCB.25.21.9350-9359.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stoimenov I, Helleday T. PCNA on the crossroad of cancer. Biochemical Society transactions. 2009 Jun;37(Pt 3):605–13. doi: 10.1042/BST0370605. [DOI] [PubMed] [Google Scholar]
- 64.Carrier F, Georgel PT, Pourquier P, et al. Gadd45, a p53-responsive stress protein, modifies DNA accessibility on damaged chromatin. Mol Cell Biol. 1999 Mar;19(3):1673–85. doi: 10.1128/mcb.19.3.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rai K, Huggins IJ, James SR, et al. DNA demethylation in zebrafish involves the coupling of a deaminase, a glycosylase, and gadd45. Cell. 2008 Dec 26;135(7):1, 201–12. doi: 10.1016/j.cell.2008.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barreto G, Schafer A, Marhold J, et al. Gadd45a promotes epigenetic gene activation by repair-mediated DNA demethylation. Nature. 2007 Feb 8;445(7128):671–5. doi: 10.1038/nature05515. [DOI] [PubMed] [Google Scholar]
- 67.Morgan HD, Dean W, Coker HA, Reik W, Petersen-Mahrt SK. Activation-induced cytidine deaminase deaminates 5-methylcytosine in DNA and is expressed in pluripotent tissues: implications for epigenetic reprogramming. J Biol Chem. 2004 Dec 10;279(50):52353–60. doi: 10.1074/jbc.M407695200. [DOI] [PubMed] [Google Scholar]
- 68.Schmitz KM, Schmitt N, Hoffmann-Rohrer U, et al. TAF12 recruits Gadd45a and the nucleotide excision repair complex to the promoter of rRNA genes leading to active DNA demethylation. Mol Cell. 2009 Feb 13;33(3):344–53. doi: 10.1016/j.molcel.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 69.Jin SG, Guo C, Pfeifer GP. GADD45A does not promote DNA demethylation. PLoS genetics. 2008 Mar;4(3):e1000013. doi: 10.1371/journal.pgen.1000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Engel N, Tront JS, Erinle T, et al. Conserved DNA methylation in Gadd45a(-/-) mice. Epigenetics. 2009 Feb 16;4(2):98–9. doi: 10.4161/epi.4.2.7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hartwell LH, Kastan MB. Cell cycle control and cancer. Science (New York, NY) 1994 Dec 16;266(5192):1821–8. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- 72.Paulovich AG, Toczyski DP, Hartwell LH. When checkpoints fail. Cell. 1997 Feb 7;88(3):315–21. doi: 10.1016/s0092-8674(00)81870-x. [DOI] [PubMed] [Google Scholar]
- 73.Vermeulen K, Van Bockstaele DR, Berneman ZN. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003 Jun;36(3):131–49. doi: 10.1046/j.1365-2184.2003.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Evans T, Rosenthal ET, Youngblom J, Distel D, Hunt T. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell. 1983 Jun;33(2):389–96. doi: 10.1016/0092-8674(83)90420-8. [DOI] [PubMed] [Google Scholar]
- 75.Morgan DO. Principles of CDK regulation. Nature. 1995 Mar 9;374(6518):131–4. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 76.Pines J. Cyclins and cyclin-dependent kinases: a biochemical view. Biochem J. 1995 Jun 15;308(Pt 3):697–711. doi: 10.1042/bj3080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pines J. Cyclins: wheels within wheels. Cell Growth Differ. 1991 Jun;2(6):305–10. [PubMed] [Google Scholar]
- 78.Nurse P. The central role of a CDK in controlling the fission yeast cell cycle. Harvey lectures. 1996;92:55–64. [PubMed] [Google Scholar]
- 79.Zhan Q, Fan S, Bae I, et al. Induction of bax by genotoxic stress in human cells correlates with normal p53 status and apoptosis. Oncogene. 1994 Dec;9(12):3743–51. [PubMed] [Google Scholar]
- 80.Jin S, Tong T, Fan W, et al. GADD45-induced cell cycle G2-M arrest associates with altered subcellular distribution of cyclin B1 and is independent of p38 kinase activity. Oncogene. 2002 Dec 12;21(57):8696–704. doi: 10.1038/sj.onc.1206034. [DOI] [PubMed] [Google Scholar]
- 81.Wang XW, Zhan Q, Coursen JD, et al. GADD45 induction of a G2/M cell cycle checkpoint. Proc Natl Acad Sci U S A. 1999 Mar 30;96(7):3706–11. doi: 10.1073/pnas.96.7.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhu N, Shao Y, Xu L, Yu L, Sun L. Gadd45-alpha and Gadd45-gamma utilize p38 and JNK signaling pathways to induce cell cycle G2/M arrest in Hep-G2 hepatoma cells. Mol Biol Rep. 2009 Nov;36(8):2075–85. doi: 10.1007/s11033-008-9419-9. [DOI] [PubMed] [Google Scholar]
- 83.Chung HK, Yi YW, Jung NC, et al. CR6-interacting factor 1 interacts with Gadd45 family proteins and modulates the cell cycle. J Biol Chem. 2003 Jul 25;278(30):28079–88. doi: 10.1074/jbc.M212835200. [DOI] [PubMed] [Google Scholar]
- 84.Suh JH, Shong M, Choi HS, Lee K. CR6-interacting factor 1 represses the transactivation of androgen receptor by direct interaction. Mol Endocrinol. 2008 Jan;22(1):33–46. doi: 10.1210/me.2007-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang H, Xiong Y, Beach D. Proliferating cell nuclear antigen and p21 are components of multiple cell cycle kinase complexes. Mol Biol Cell. 1993 Sep;4(9):897–906. doi: 10.1091/mbc.4.9.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vairapandi M, Balliet AG, Fornace AJ, Jr, Hoffman B, Liebermann DA. The differentiation primary response gene MyD118, related to GADD45, encodes for a nuclear protein which interacts with PCNA and p21WAF1/CIP1. Oncogene. 1996 Jun 20;12(12):2579–94. [PubMed] [Google Scholar]
- 87.Fan W, Richter G, Cereseto A, Beadling C, Smith KA. Cytokine response gene 6 induces p21 and regulates both cell growth and arrest. Oncogene. 1999 Nov 11;18(47):6573–82. doi: 10.1038/sj.onc.1203054. [DOI] [PubMed] [Google Scholar]
- 88.Chen YR, Wang X, Templeton D, Davis RJ, Tan TH. The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and gamma radiation. Duration of JNK activation may determine cell death and proliferation. J Biol Chem. 1996 Dec 13;271(50):31929–36. doi: 10.1074/jbc.271.50.31929. [DOI] [PubMed] [Google Scholar]
- 89.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009 Aug;9(8):537–49. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 90.Verheij M, Bose R, Lin XH, et al. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996 Mar 7;380(6569):75–9. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 91.Tournier C, Hess P, Yang DD, et al. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000 May 5;288(5467):870–4. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- 92.Sabapathy K, Jochum W, Hochedlinger K, et al. Defective neural tube morphogenesis and altered apoptosis in the absence of both JNK1 and JNK2. Mech Dev. 1999 Dec;89(1-2):115–24. doi: 10.1016/s0925-4773(99)00213-0. [DOI] [PubMed] [Google Scholar]
- 93.Potapova O, Gorospe M, Dougherty RH, et al. Inhibition of c-Jun N-terminal kinase 2 expression suppresses growth and induces apoptosis of human tumor cells in a p53-dependent manner. Molecular and cellular biology. 2000 Mar;20(5):1713–22. doi: 10.1128/mcb.20.5.1713-1722.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hildesheim J, Bulavin DV, Anver MR, et al. Gadd45a protects against UV irradiation-induced skin tumors, and promotes apoptosis and stress signaling via MAPK and p53. Cancer Res. 2002 Dec 15;62(24):7305–15. [PubMed] [Google Scholar]
- 95.Yoo J, Ghiassi M, Jirmanova L, et al. Transforming growth factor-beta-induced apoptosis is mediated by Smad-dependent expression of GADD45b through p38 activation. J Biol Chem. 2003 Oct 31;278(44):43001–7. doi: 10.1074/jbc.M307869200. [DOI] [PubMed] [Google Scholar]
- 96.Selvakumaran M, Lin HK, Sjin RT, et al. The novel primary response gene MyD118 and the proto-oncogenes myb, myc, and bcl-2 modulate transforming growth factor beta 1-induced apoptosis of myeloid leukemia cells. Mol Cell Biol. 1994 Apr;14(4):2352–60. doi: 10.1128/mcb.14.4.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Takekawa M, Tatebayashi K, Itoh F, et al. Smad-dependent GADD45beta expression mediates delayed activation of p38 MAP kinase by TGF-beta. EMBO J. 2002 Dec 2;21(23):6473–82. doi: 10.1093/emboj/cdf643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Basseres DS, Baldwin AS. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene. 2006 Oct 30;25(51):6817–30. doi: 10.1038/sj.onc.1209942. [DOI] [PubMed] [Google Scholar]
- 99.Tong T, Ji J, Jin S, et al. Gadd45a expression induces Bim dissociation from the cytoskeleton and translocation to mitochondria. Mol Cell Biol. 2005 Jun;25(11):4488–500. doi: 10.1128/MCB.25.11.4488-4500.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shaulian E, Karin M. Stress-induced JNK activation is independent of Gadd45 induction. J Biol Chem. 1999 Oct 15;274(42):29595–8. doi: 10.1074/jbc.274.42.29595. [DOI] [PubMed] [Google Scholar]
- 101.Wang X, Gorospe M, Holbrook NJ. gadd45 is not required for activation of c-Jun N-terminal kinase or p38 during acute stress. J Biol Chem. 1999 Oct 15;274(42):29599–602. doi: 10.1074/jbc.274.42.29599. [DOI] [PubMed] [Google Scholar]
- 102.Zazzeroni F, Papa S, Algeciras-Schimnich A, et al. Gadd45 beta mediates the protective effects of CD40 costimulation against Fas-induced apoptosis. Blood. 2003 Nov 1;102(9):3270–9. doi: 10.1182/blood-2003-03-0689. [DOI] [PubMed] [Google Scholar]
- 103.De Smaele E, Zazzeroni F, Papa S, et al. Induction of gadd45beta by NF-kappaB downregulates pro-apoptotic JNK signalling. Nature. 2001 Nov 15;414(6861):308–13. doi: 10.1038/35104560. [DOI] [PubMed] [Google Scholar]
- 104.Sethu S, Melendez AJ. New developments on the TNFalpha-mediated signalling pathways. Biosci Rep. 2010 Oct;31(1):63–76. doi: 10.1042/BSR20100040. [DOI] [PubMed] [Google Scholar]
- 105.Papa S, Zazzeroni F, Bubici C, et al. Gadd45 beta mediates the NF-kappa B suppression of JNK signalling by targeting MKK7/JNKK2. Nat Cell Biol. 2004 Feb;6(2):146–53. doi: 10.1038/ncb1093. [DOI] [PubMed] [Google Scholar]
- 106.Tornatore L, Marasco D, Dathan N, et al. Gadd45 beta forms a homodimeric complex that binds tightly to MKK7. J Mol Biol. 2008 Apr 18;378(1):97–111. doi: 10.1016/j.jmb.2008.01.074. [DOI] [PubMed] [Google Scholar]
- 107.Gupta M, Gupta SK, Hoffman B, Liebermann DA. Gadd45a and Gadd45b protect hematopoietic cells from UV-induced apoptosis via distinct signaling pathways, including p38 activation and JNK inhibition. J Biol Chem. 2006 Jun 30;281(26):17552–8. doi: 10.1074/jbc.M600950200. [DOI] [PubMed] [Google Scholar]
- 108.Gupta M, Gupta SK, Balliet AG, et al. Hematopoietic cells from Gadd45a- and Gadd45b-deficient mice are sensitized to genotoxic-stress-induced apoptosis. Oncogene. 2005 Nov 3;24(48):7170–9. doi: 10.1038/sj.onc.1208847. [DOI] [PubMed] [Google Scholar]
- 109.Du F, Wang L, Zhang Y, et al. Role of GADD45 beta in the regulation of synovial fluid T cell apoptosis in rheumatoid arthritis. Clin Immunol. 2008 Aug;128(2):238–47. doi: 10.1016/j.clim.2008.03.523. [DOI] [PubMed] [Google Scholar]
- 110.Engelmann A, Speidel D, Bornkamm GW, Deppert W, Stocking C. Gadd45 beta is a pro-survival factor associated with stress-resistant tumors. Oncogene. 2008 Feb 28;27(10):1429–38. doi: 10.1038/sj.onc.1210772. [DOI] [PubMed] [Google Scholar]
- 111.Amanullah A, Azam N, Balliet A, et al. Cell signalling: cell survival and a Gadd45-factor deficiency. Nature. 2003 Aug 14;424(6950):741. doi: 10.1038/424741b. discussion 2. [DOI] [PubMed] [Google Scholar]
- 112.Hoggard N, Brintnell B, Howell A, Weissenbach J, Varley J. Allelic imbalance on chromosome 1 in human breast cancer. II. Microsatellite repeat analysis. Genes Chromosomes Cancer. 1995 Jan;12(1):24–31. doi: 10.1002/gcc.2870120105. [DOI] [PubMed] [Google Scholar]
- 113.Sensi E, Tancredi M, Aretini P, et al. Clinicopathological significance of GADD45 gene alterations in human familial breast carcinoma. Breast Cancer Res Treat. 2004 Sep;87(2):197–201. doi: 10.1023/B:BREA.0000041625.60280.4a. [DOI] [PubMed] [Google Scholar]
- 114.Blaszyk H, Hartmann A, Sommer SS, Kovach JS. A polymorphism but no mutations in the GADD45 gene in breast cancers. Hum Genet. 1996 Apr;97(4):543–7. doi: 10.1007/BF02267084. [DOI] [PubMed] [Google Scholar]
- 115.Campomenosi P, Hall PA. Gadd45 mutations are uncommon in human tumour cell lines. Cell Prolif. 2000 Oct;33(5):301–6. doi: 10.1046/j.1365-2184.2000.00187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sakamoto A, Akieda S, Oda Y, Iwamoto Y, Tsuneyoshi M. Mutation analysis of the Gadd45 gene at exon 4 in atypical fibroxanthoma. BMC Dermatol. 2009;9:1. doi: 10.1186/1471-5945-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yamasawa K, Nio Y, Dong M, Yamaguchi K, Itakura M. Clinicopathological significance of abnormalities in Gadd45 expression and its relationship to p53 in human pancreatic cancer. Clin Cancer Res. 2002 Aug;8(8):2563–9. [PubMed] [Google Scholar]
- 118.Soliman AS, Bondy M, Webb CR, et al. Differing molecular pathology of pancreatic adenocarcinoma in Egyptian and United States patients. Int J Cancer. 2006 Sep 15;119(6):1455–61. doi: 10.1002/ijc.21986. [DOI] [PubMed] [Google Scholar]
- 119.Sun L, Gong R, Wan B, et al. GADD45gamma, down-regulated in 65% hepatocellular carcinoma (HCC) from 23 chinese patients, inhibits cell growth and induces cell cycle G2/M arrest for hepatoma Hep-G2 cell lines. Mol Biol Rep. 2003 Dec;30(4):249–53. doi: 10.1023/a:1026370726763. [DOI] [PubMed] [Google Scholar]
- 120.Gramantieri L, Chieco P, Giovannini C, et al. GADD45-alpha expression in cirrhosis and hepatocellular carcinoma: relationship with DNA repair and proliferation. Hum Pathol. 2005 Nov;36(11):1154–62. doi: 10.1016/j.humpath.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 121.Higashi H, Vallbohmer D, Warnecke-Eberz U, et al. Down-regulation of Gadd45 expression is associated with tumor differentiation in non-small cell lung cancer. Anticancer Res. 2006 May-Jun;26(3A):2143–7. [PubMed] [Google Scholar]
- 122.Zhang X, Sun H, Danila DC, et al. Loss of expression of GADD45 gamma, a growth inhibitory gene, in human pituitary adenomas: implications for tumorigenesis. J Clin Endocrinol Metab. 2002 Mar;87(3):1262–7. doi: 10.1210/jcem.87.3.8315. [DOI] [PubMed] [Google Scholar]
- 123.Schar P, Fritsch O. DNA repair and the control of DNA methylation. Prog Drug Res. 2011;67:51–68. doi: 10.1007/978-3-7643-8989-5_3. [DOI] [PubMed] [Google Scholar]
- 124.Na YK, Lee SM, Hong HS, et al. Hypermethylation of growth arrest DNA-damage-inducible gene 45 in non-small cell lung cancer and its relationship with clinicopathologic features. Mol Cells. 2010 Jul;30(1):89–92. doi: 10.1007/s10059-010-0092-1. [DOI] [PubMed] [Google Scholar]
- 125.Wang W, Huper G, Guo Y, et al. Analysis of methylation-sensitive transcriptome identifies GADD45a as a frequently methylated gene in breast cancer. Oncogene. 2005 Apr 14;24(16):2705–14. doi: 10.1038/sj.onc.1208464. [DOI] [PubMed] [Google Scholar]
- 126.Jones PA, Taylor SM, Wilson VL. Inhibition of DNA methylation by 5-azacytidine. Recent Results Cancer Res. 1983;84:202–11. doi: 10.1007/978-3-642-81947-6_15. [DOI] [PubMed] [Google Scholar]
- 127.Kaminskas E, Farrell AT, Wang YC, Sridhara R, Pazdur R. FDA drug approval summary: azacitidine (5-azacytidine, Vidaza) for injectable suspension. Oncologist. 2005 Mar;10(3):176–82. doi: 10.1634/theoncologist.10-3-176. [DOI] [PubMed] [Google Scholar]
- 128.Ramachandran K, Gopisetty G, Gordian E, et al. Methylation-mediated repression of GADD45alpha in prostate cancer and its role as a potential therapeutic target. Cancer Res. 2009 Feb 15;69(4):1527–35. doi: 10.1158/0008-5472.CAN-08-3609. [DOI] [PubMed] [Google Scholar]
- 129.Qiu W, Zhou B, Zou H, et al. Hypermethylation of growth arrest DNA damage-inducible gene 45 beta promoter in human hepatocellular carcinoma. Am J Pathol. 2004 Nov;165(5):1689–99. doi: 10.1016/s0002-9440(10)63425-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Higgs MR, Lerat H, Pawlotsky JM. Downregulation of Gadd45beta expression by hepatitis C virus leads to defective cell cycle arrest. Cancer Res. 2010 Jun 15;70(12):4901–11. doi: 10.1158/0008-5472.CAN-09-4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ying J, Srivastava G, Hsieh WS, et al. The stress-responsive gene GADD45G is a functional tumor suppressor, with its response to environmental stresses frequently disrupted epigenetically in multiple tumors. Clin Cancer Res. 2005 Sep 15;11(18):6442–9. doi: 10.1158/1078-0432.CCR-05-0267. [DOI] [PubMed] [Google Scholar]
- 132.Zerbini LF, Libermann TA. GADD45 deregulation in cancer: frequently methylated tumor suppressors and potential therapeutic targets. Clin Cancer Res. 2005 Sep 15;11(18):6409–13. doi: 10.1158/1078-0432.CCR-05-1475. [DOI] [PubMed] [Google Scholar]
- 133.Zhang W, Li T, Shao Y, et al. Semi-quantitative detection of GADD45-gamma methylation levels in gastric, colorectal and pancreatic cancers using methylation-sensitive high-resolution melting analysis. J Cancer Res Clin Oncol. 2010 Aug;136(8):1267–73. doi: 10.1007/s00432-010-0777-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bahar A, Bicknell JE, Simpson DJ, Clayton RN, Farrell WE. Loss of expression of the growth inhibitory gene GADD45gamma, in human pituitary adenomas, is associated with CpG island methylation. Oncogene. 2004 Jan 29;23(4):936–44. doi: 10.1038/sj.onc.1207193. [DOI] [PubMed] [Google Scholar]
- 135.Dong M, Dong Q, Zhang H, et al. Expression of Gadd45a and p53 proteins in human pancreatic cancer: potential effects on clinical outcomes. J Surg Oncol. 2007 Mar 15;95(4):332–6. doi: 10.1002/jso.20684. [DOI] [PubMed] [Google Scholar]
- 136.Schneider G, Weber A, Zechner U, et al. GADD45alpha is highly expressed in pancreatic ductal adenocarcinoma cells and required for tumor cell viability. Int J Cancer. 2006 May 15;118(10):2405–11. doi: 10.1002/ijc.21637. [DOI] [PubMed] [Google Scholar]
- 137.Shao ZM, Dawson MI, Li XS, et al. p53 independent G0/G1 arrest and apoptosis induced by a novel retinoid in human breast cancer cells. Oncogene. 1995 Aug 3;11(3):493–504. [PubMed] [Google Scholar]
- 138.Hsu CA, Rishi AK, Su-Li X, et al. Retinoid induced apoptosis in leukemia cells through a retinoic acid nuclear receptor-independent pathway. Blood. 1997 Jun 15;89(12):4470–9. [PubMed] [Google Scholar]
- 139.Sakaue M, Adachi H, Jetten AM. Post-transcriptional regulation of MyD118 and GADD45 in human lung carcinoma cells during 6-[3-(1-adamantyl)-4-hydroxyphenyl]-2- naphthalene carboxylic acid-induced apoptosis. Mol Pharmacol. 1999 Apr;55(4):668–76. [PubMed] [Google Scholar]
- 140.Zhang Y, Bhatia D, Xia H, et al. Nucleolin links to arsenic-induced stabilization of GADD45alpha mRNA. Nucleic Acids Res. 2006;34(2):485–95. doi: 10.1093/nar/gkj459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chang Q, Bhatia D, Zhang Y, et al. Incorporation of an internal ribosome entry site-dependent mechanism in arsenic-induced GADD45 alpha expression. Cancer Res. 2007 Jul 1;67(13):6146–54. doi: 10.1158/0008-5472.CAN-07-0867. [DOI] [PubMed] [Google Scholar]
- 142.Akiyama T, Ishida J, Nakagawa S, et al. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987 Apr 25;262(12):5592–5. [PubMed] [Google Scholar]
- 143.Hwang YW, Kim SY, Jee SH, Kim YN, Nam CM. Soy food consumption and risk of prostate cancer: a meta-analysis of observational studies. Nutr Cancer. 2009;61(5):598–606. doi: 10.1080/01635580902825639. [DOI] [PubMed] [Google Scholar]
- 144.Lee HP, Gourley L, Duffy SW, et al. Dietary effects on breast-cancer risk in Singapore. Lancet. 1991 May 18;337(8751):1197–200. doi: 10.1016/0140-6736(91)92867-2. [DOI] [PubMed] [Google Scholar]
- 145.Oki T, Sowa Y, Hirose T, et al. Genistein induces Gadd45 gene and G2/M cell cycle arrest in the DU145 human prostate cancer cell line. FEBS Lett. 2004 Nov 5;577(1-2):55–9. doi: 10.1016/j.febslet.2004.09.085. [DOI] [PubMed] [Google Scholar]
- 146.Vanhaecke T, Henkens T, Kass GE, Rogiers V. Effect of the histone deacetylase inhibitor trichostatin A on spontaneous apoptosis in various types of adult rat hepatocyte cultures. Biochem Pharmacol. 2004 Aug 15;68(4):753–60. doi: 10.1016/j.bcp.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 147.Campanero MR, Herrero A, Calvo V. The histone deacetylase inhibitor trichostatin A induces GADD45 gamma expression via Oct and NF-Y binding sites. Oncogene. 2008 Feb 21;27(9):1263–72. doi: 10.1038/sj.onc.1210735. [DOI] [PubMed] [Google Scholar]
- 148.Li P, Wang D, Yao H, et al. Coordination of PAD4 and HDAC2 in the regulation of p53-target gene expression. Oncogene. 2010 May 27;29(21):3153–62. doi: 10.1038/onc.2010.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang S, Won YK, Ong CN, Shen HM. Anti-cancer potential of sesquiterpene lactones: bioactivity and molecular mechanisms. Curr Med Chem Anticancer Agents. 2005 May;5(3):239–49. doi: 10.2174/1568011053765976. [DOI] [PubMed] [Google Scholar]
- 150.Takeda S, Matsuo K, Yaji K, et al. (-)-Xanthatin Selectively Induces GADD45gamma and Stimulates Caspase-Independent Cell Death in Human Breast Cancer MDA-MB-231 Cells. Chem Res Toxicol. 2011 Jun 20;24(6):855–65. doi: 10.1021/tx200046s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Berkel H, Holcombe RF, Middlebrooks M, Kannan K. Nonsteroidal antiinflammatory drugs and colorectal cancer. Epidemiologic reviews. 1996;18(2):205–17. doi: 10.1093/oxfordjournals.epirev.a017926. [DOI] [PubMed] [Google Scholar]
- 152.Coogan PF, Rao SR, Rosenberg L, et al. The relationship of nonsteroidal anti-inflammatory drug use to the risk of breast cancer. Prev Med. 1999 Aug;29(2):72–6. doi: 10.1006/pmed.1999.0518. [DOI] [PubMed] [Google Scholar]
- 153.Cramer DW, Harlow BL, Titus-Ernstoff L, et al. Over-the-counter analgesics and risk of ovarian cancer. Lancet. 1998 Jan 10;351(9096):104–7. doi: 10.1016/S0140-6736(97)08064-1. [DOI] [PubMed] [Google Scholar]
- 154.Wernli KJ, Newcomb PA, Hampton JM, Trentham-Dietz A, Egan KM. Inverse association of NSAID use and ovarian cancer in relation to oral contraceptive use and parity. Br J Cancer. 2008 Jun 3;98(11):1781–3. doi: 10.1038/sj.bjc.6604392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rodriguez-Burford C, Barnes MN, Oelschlager DK, et al. Effects of nonsteroidal anti-inflammatory agents (NSAIDs) on ovarian carcinoma cell lines: preclinical evaluation of NSAIDs as chemopreventive agents. Clin Cancer Res. 2002 Jan;8(1):202–9. [PubMed] [Google Scholar]
- 156.Lacey JV, Jr, Sherman ME, Hartge P, Schatzkin A, Schairer C. Medication use and risk of ovarian carcinoma: a prospective study. Int J Cancer. 2004 Jan 10;108(2):281–6. doi: 10.1002/ijc.11538. [DOI] [PubMed] [Google Scholar]
- 157.Pinheiro SP, Tworoger SS, Cramer DW, Rosner BA, Hankinson SE. Use of nonsteroidal antiinflammatory agents and incidence of ovarian cancer in 2 large prospective cohorts. Am J Epidemiol. 2009 Jun 1;169(11):1378–87. doi: 10.1093/aje/kwp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Vane JR, Botting RM. Mechanism of action of antiinflammatory drugs. Int J Tissue React. 1998;20(1):3–15. [PubMed] [Google Scholar]
- 159.Boocock CA, Charnock-Jones DS, Sharkey AM, et al. Expression of vascular endothelial growth factor and its receptors flt and KDR in ovarian carcinoma. Journal of the National Cancer Institute. 1995 Apr 5;87(7):506–16. doi: 10.1093/jnci/87.7.506. [DOI] [PubMed] [Google Scholar]
- 160.Abu-Jawdeh GM, Faix JD, Niloff J, et al. Strong expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in ovarian borderline and malignant neoplasms. Laboratory investigation; a journal of technical methods and pathology. 1996 Jun;74(6):1105–15. [PubMed] [Google Scholar]
- 161.Paley PJ, Staskus KA, Gebhard K, et al. Vascular endothelial growth factor expression in early stage ovarian carcinoma. Cancer. 1997 Jul 1;80(1):98–106. doi: 10.1002/(sici)1097-0142(19970701)80:1<98::aid-cncr13>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 162.Gupta RA, Tejada LV, Tong BJ, et al. Cyclooxygenase-1 is overexpressed and promotes angiogenic growth factor production in ovarian cancer. Cancer research. 2003 Mar 1;63(5):906–11. [PubMed] [Google Scholar]
- 163.Urick ME, Giles JR, Johnson PA. VEGF expression and the effect of NSAIDs on ascites cell proliferation in the hen model of ovarian cancer. Gynecologic oncology. 2008 Sep;110(3):418–24. doi: 10.1016/j.ygyno.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 164.Mesiano S, Ferrara N, Jaffe RB. Role of vascular endothelial growth factor in ovarian cancer: inhibition of ascites formation by immunoneutralization. The American journal of pathology. 1998 Oct;153(4):1249–56. doi: 10.1016/S0002-9440(10)65669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nagy JA, Masse EM, Herzberg KT, et al. Pathogenesis of ascites tumor growth: vascular permeability factor, vascular hyperpermeability, and ascites fluid accumulation. Cancer research. 1995 Jan 15;55(2):360–8. [PubMed] [Google Scholar]
- 166.Byrne AT, Ross L, Holash J, et al. Vascular endothelial growth factor-trap decreases tumor burden, inhibits ascites, and causes dramatic vascular remodeling in an ovarian cancer model. Clin Cancer Res. 2003 Nov 15;9(15):5721–8. [PubMed] [Google Scholar]
- 167.Chiou SK, Hodges A, Hoa N. Suppression of Growth Arrest and DNA Damage-Inducible 45{alpha} Expression Confers Resistance to Sulindac and Indomethacin-Induced Gastric Mucosal Injury. J Pharmacol Exp Ther. 2010 Sep;334(3):693–702. doi: 10.1124/jpet.110.168153. [DOI] [PubMed] [Google Scholar]
- 168.Jiang H, Lin JJ, Su ZZ, Goldstein NI, Fisher PB. Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene. 1995 Dec 21;11(12):2477–86. [PubMed] [Google Scholar]
- 169.Jiang H, Su ZZ, Lin JJ, et al. The melanoma differentiation associated gene mda-7 suppresses cancer cell growth. Proceedings of the National Academy of Sciences of the United States of America. 1996 Aug 20;93(17):9160–5. doi: 10.1073/pnas.93.17.9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zerbini LF, Tamura RE, Correa RG, et al. Combinatorial effect of non-steroidal anti-inflammatory drugs and NF-kappaB inhibitors in ovarian cancer therapy. PLoS One. 2011;6(9):e24285. doi: 10.1371/journal.pone.0024285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Huang EY, Madireddi MT, Gopalkrishnan RV, et al. Genomic structure, chromosomal localization and expression profile of a novel melanoma differentiation associated (mda-7) gene with cancer specific growth suppressing and apoptosis inducing properties. Oncogene. 2001 Oct 25;20(48):7051–63. doi: 10.1038/sj.onc.1204897. [DOI] [PubMed] [Google Scholar]
- 172.Emdad L, Lebedeva IV, Su ZZ, et al. Historical perspective and recent insights into our understanding of the molecular and biochemical basis of the antitumor properties of mda-7/IL-24. Cancer biology & therapy. 2009 Mar;8(5):391–400. doi: 10.4161/cbt.8.5.7581. [DOI] [PubMed] [Google Scholar]
- 173.Fisher PB. Is mda-7/IL-24 a “magic bullet” for cancer? Cancer research. 2005 Nov 15;65(22):10128–38. doi: 10.1158/0008-5472.CAN-05-3127. [DOI] [PubMed] [Google Scholar]
- 174.Dash R, Bhutia SK, Azab B, et al. mda-7/IL-24: A unique member of the IL-10 gene family promoting cancer-targeted toxicity. Cytokine Growth Factor Rev. 2010 Oct 4; doi: 10.1016/j.cytogfr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kawabe S, Nishikawa T, Munshi A, et al. Adenovirus-mediated mda-7 gene expression radiosensitizes non-small cell lung cancer cells via TP53-independent mechanisms. Mol Ther. 2002 Nov;6(5):637–44. [PubMed] [Google Scholar]
- 176.Oida Y, Gopalan B, Miyahara R, et al. Sulindac enhances adenoviral vector expressing mda-7/IL-24-mediated apoptosis in human lung cancer. Molecular cancer therapeutics. 2005 Feb;4(2):291–304. [PubMed] [Google Scholar]
- 177.Su ZZ, Lebedeva IV, Sarkar D, et al. Melanoma differentiation associated gene-7, mda-7/IL-24, selectively induces growth suppression, apoptosis and radiosensitization in malignant gliomas in a p53-independent manner. Oncogene. 2003 Feb 27;22(8):1164–80. doi: 10.1038/sj.onc.1206062. [DOI] [PubMed] [Google Scholar]
- 178.Yacoub A, Mitchell C, Lebedeva IV, et al. mda-7 (IL-24) Inhibits growth and enhances radiosensitivity of glioma cells in vitro via JNK signaling. Cancer biology & therapy. 2003 Jul-Aug;2(4):347–53. doi: 10.4161/cbt.2.4.422. [DOI] [PubMed] [Google Scholar]
- 179.Yacoub A, Mitchell C, Lister A, et al. Melanoma differentiation-associated 7 (interleukin 24) inhibits growth and enhances radiosensitivity of glioma cells in vitro and in vivo. Clin Cancer Res. 2003 Aug 15;9(9):3272–81. [PubMed] [Google Scholar]
- 180.Yacoub A, Mitchell C, Brannon J, et al. MDA-7 (interleukin-24) inhibits the proliferation of renal carcinoma cells and interacts with free radicals to promote cell death and loss of reproductive capacity. Molecular cancer therapeutics. 2003 Jul;2(7):623–32. [PubMed] [Google Scholar]
- 181.Lebedeva IV, Su ZZ, Sarkar D, et al. Induction of reactive oxygen species renders mutant and wild-type K-ras pancreatic carcinoma cells susceptible to Ad.mda-7-induced apoptosis. Oncogene. 2005 Jan 20;24(4):585–96. doi: 10.1038/sj.onc.1208183. [DOI] [PubMed] [Google Scholar]
- 182.Emdad L, Sarkar D, Lebedeva IV, et al. Ionizing radiation enhances adenoviral vector expressing mda-7/IL-24-mediated apoptosis in human ovarian cancer. Journal of cellular physiology. 2006 Aug;208(2):298–306. doi: 10.1002/jcp.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gopalan B, Shanker M, Chada S, Ramesh R. MDA-7/IL-24 suppresses human ovarian carcinoma growth in vitro and in vivo. Molecular cancer. 2007;6:11. doi: 10.1186/1476-4598-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Abate-Daga D, Garcia-Rodriguez L, Sumoy L, Fillat C. Cell cycle control pathways act as conditioning factors for TK/GCV sensitivity in pancreatic cancer cells. Biochim Biophys Acta. 2010 Oct;1803(10):1175–85. doi: 10.1016/j.bbamcr.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 185.Li Y, Qian H, Li X, et al. Adenoviral-mediated gene transfer of Gadd45a results in suppression by inducing apoptosis and cell cycle arrest in pancreatic cancer cell. J Gene Med. 2009 Jan;11(1):3–13. doi: 10.1002/jgm.1270. [DOI] [PubMed] [Google Scholar]
- 186.Satomi Y, Nishino H. Implication of mitogen-activated protein kinase in the induction of G1 cell cycle arrest and gadd45 expression by the carotenoid fucoxanthin in human cancer cells. Biochim Biophys Acta. 2009 Apr;1790(4):260–6. doi: 10.1016/j.bbagen.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 187.Satomi Y, Nishino H. Fucoxanthin, a natural carotenoid, induces G1 arrest and GADD45 gene expression in human cancer cells. In Vivo. 2007 Mar-Apr;21(2):305–9. [PubMed] [Google Scholar]
- 188.Saha A, Kuzuhara T, Echigo N, et al. Apoptosis of human lung cancer cells by curcumin mediated through up-regulation of “growth arrest and DNA damage inducible genes 45 and 153”. Biol Pharm Bull. 2010;33(8):1291–9. doi: 10.1248/bpb.33.1291. [DOI] [PubMed] [Google Scholar]
- 189.Fulton B, Spencer CM. Docetaxel. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the management of metastatic breast cancer. Drugs. 1996 Jun;51(6):1075–92. doi: 10.2165/00003495-199651060-00011. [DOI] [PubMed] [Google Scholar]
- 190.Al-Romaih K, Sadikovic B, Yoshimoto M, et al. Decitabine-induced demethylation of 5’ CpG island in GADD45A leads to apoptosis in osteosarcoma cells. Neoplasia. 2008 May;10(5):471–80. doi: 10.1593/neo.08174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yang C, Yang S, Wood KB, et al. Multidrug resistant osteosarcoma cell lines exhibit deficiency of GADD45alpha expression. Apoptosis. 2009 Jan;14(1):124–33. doi: 10.1007/s10495-008-0282-x. [DOI] [PubMed] [Google Scholar]
- 192.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004 Oct 7;351(15):1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 193.Adlercreutz H. Epidemiology of phytoestrogens. Baillieres Clin Endocrinol Metab. 1998 Dec;12(4):605–23. doi: 10.1016/s0950-351x(98)80007-4. [DOI] [PubMed] [Google Scholar]
- 194.Bonelli P, Tuccillo FM, Calemma R, et al. Changes in the gene expression profile of gastric cancer cells in response to ibuprofen: a gene pathway analysis. Pharmacogenomics J. 2010 Jun 15; doi: 10.1038/tpj.2010.55. [DOI] [PubMed] [Google Scholar]


