Abstract
Objective
Chemerin is an adipocyte-secreted hormone, recently associated with obesity and the metabolic syndrome. Although studies in rodents have outlined aspects of chemerin’s function and expression, its physiology and expression patterns are still to be elucidated in humans.
Methods
To evaluate for any day/night variation in chemerin secretion we analyzed hourly serum samples from six females in the fed state. To examine whether energy deprivation affects chemerin levels, and whether this could be mediated through leptin, we analyzed samples from the same subjects in the fasting state while we were administering either placebo or leptin. To evaluate for any potential dose-effect relationship between leptin and chemerin, we administered increasing metreleptin doses to five females. A tissue array was utilized to study the expression of chemerin in different human tissues. Ex vivo treatment of human fat explants from 3 subjects with leptin was performed to evaluate for any direct effect of leptin on adipocyte chemerin secretion.
Results
Chemerin does not display a day/night variation, while acute energy deprivation resulted in a significant drop in circulating chemerin levels by ~42%. The latter was unaltered by metreleptin administration and leptin administration did not affect secretion of chemerin by human adipose tissue studied ex vivo. Chemerin was expressed primarily in the adrenal gland and liver. Chemerin receptor showed increased expression in lymph nodes and the spleen.
Conclusions
We outline for the first time chemerin expression and physiology in humans which is different from mice.
Keywords: Chemerin, Day/Night Variation, Energy Homeostasis, Leptin, sTNFRII, ex vivo, tissue, mRNA expression
Introduction
With the emergence of obesity as a major health hazard, several adipose tissue secreted proteins and hormones, known as adipokines, have been identified and linked to metabolic regulation. Changes in the secretion of many of these molecules are now thought to play a major role in the development of obesity and obesity related diseases. Chemerin is one such protein that has recently been proposed to be an adipokine. It is secreted as an inactive 18-kDa pro-peptide and is subsequently cleaved to a 16-kDa active protein that binds to the G protein-couple receptor, chemokine-like receptor 1 (CMKLR1) 1. Chemerin was first identified in psoriatic skin lesions 2 and is now known to be involved in the recruitment of immune cells such as immature dendritic cells, macrophages, natural killer cells, and monocytes to sites of tissue inflammation or injury 3. Chemerin is associated with proinflammatory cytokines such as TNF-α both in vitro and in vivo 4, 5. Recently it was demonstrated that chemerin promotes angiogenesis in endothelial cells 6, 7. A major role of chemerin is adipogenesis regulation and it has also been associated with the metabolic syndrome 8–10. Chemerin has been shown to be correlated with body mass index (BMI), homeostasis model assessment of insulin resistance (HOMA-IR), blood pressure, and circulating triglycerides 10, 11 in humans. Furthermore, increased chemerin correlates with other obesity related markers such as leptin, resistin, and C - reactive protein (CRP) as well as glycated hemoglobin (HbA1C) 12–14. In vitro experiments have demonstrated that chemerin is an important regulator of adipocyte differentiation and metabolism, as exhibited by the knockdown of chemerin causing an impaired pre-adipocyte differentiation into mature adipocytes as well as reduced expression of glucose and lipid metabolism genes8, 15. However, little is known about the circulatory or expression patterns of chemerin in humans.
Although chemerin has been proposed to be an adipokine, the expression pattern of chemerin in human tissues has not yet been evaluated comparatively. Furthermore, the physiology of chemerin in humans remains to be fully elucidated. No studies to date have determined whether chemerin displays a day/night pattern of secretion and what the effect of acute and/or chronic energy deprivation on circulating chemerin levels in humans is. It is expected that circulating levels of chemerin, a factor associated with energy homeostasis, would respond appropriately to changes in energy intake. Finally, any potential interactions between chemerin and other molecules affected by energy intake such as leptin have not yet been studied. Leptin is a 167 amino acid adipokine 16 that has a well-established role in the neuroendocrine regulation of energy homeostasis 17, 18. Recent studies have demonstrated an association between leptin levels, obesity, and the metabolic syndrome 17–19. During states of starvation circulating levels decrease and are responsible for changes in the neuroendocrine axis 20. In mouse models, leptin has been shown to act on the hypothalamic pro-opiomelanocortin neurons (POMC) to decrease food intake and maintain glucose homeostasis 21. Studies in patients with conditions characterized by chronic energy deprivation, such as anorexia nervosa and hypothalamic amenorrhea, have demonstrated that low leptin levels are causally related to energy deprivation induced suppression of neuroendocrine axes whereas leptin administration restores these energy deprivation induced changes 20, 22–24.
We utilized herein a human mRNA tissue array to evaluate the expression pattern of chemerin and its receptor in human tissues. We then proceeded to assess whether chemerin has a day/night variation pattern in humans, to determine whether acute energy deprivation (for 3 days) alters chemerin levels, to investigate for potential association with changes in circulating TNF-α as measured by TNFRII as well as leptin mediated effects of energy deprivation in humans, and to examine the tissue expression of chemerin. To these ends, we first utilized frequently collected samples from six healthy female individuals studied while in the isocaloric fed state, after 3 days of fasting and 3 days of fasting plus metreleptin administration; then we used samples collected from another five healthy female subjects while treated with low, physiological, and supraphysiological doses of leptin; and finally, we performed ex vivo experiments involving adipose tissue surgically removed from human subjects.
Methods
Chemerin and CMKLR1 Tissue mRNA Expression
Chemerin and CMKLR1 mRNA expression was measured using human-specific TaqMan® Gene Expression Assays (Assay ID: Chemerin, Hs00161209_g1; CMKLR1, Hs01081979_s1, Applied Biosystems, Foster City, CA) in 7500 Fast Real-Time PCR system using Standard real-time 7500 protocol. Data were analyzed using 7500 system software (Applied Biosystems, Foster City, CA) and relative quantification was done using ΔΔ Ct method with beta-actin as the internal control. Analysis of tissue mRNA expression was done in duplicate with TissueScan™ Real-Time Human Major Tissue Panel from OriGene, Rockville, MD, containing 48 tissue types.
Study 1
Day/Night variation analysis and effect of acute energy deprivation
To evaluate whether chemerin has a day/night variation pattern we collected serum samples from six healthy, lean, eumenorrheic women (age 22.8 ± 3.4 years; BMI 21.7 ± 2.2 kg/m2) initially studied in the isocaloric fed state 25. All female subjects had a regular menstrual cycle and were not taking any medications including oral contraceptive pills. Study visits were standardized at the 6th to 11th day of their menstrual cycle. The study was approved by the Institutional Review Board at the Beth Israel Deaconess Medical Center (BIDMC) and all participants gave informed consent. In order to evaluate whether energy deprivation has any effect on chemerin levels, and whether such a change could be mediated through leptin, we collected samples from the same subjects on two separate admissions, spaced at least 8 weeks apart, in which subjects were randomized to receive either placebo or leptin during complete fasting for three days. Admissions in each state were held in the General Clinical Research Center (GCRC) of BIDMC for 4 days with standardized diet, exercise, and light/dark intervals. Baseline blood samples were taken at 8 a.m. day 1 of each visit before intervention commenced. On day 3 of all 3 visits blood samples were drawn every hour starting at 8 a.m. for 24 hours. For the admission in the fed state subjects were given an isocaloric diet with breakfast at 8 a.m., lunch at 1 p.m., dinner at 6 p.m., and a snack at 10 p.m. The caloric intake was distributed so that 20% of calories were from breakfast, 35% from lunch, 35% from dinner, and 10% from the evening snack. For admissions in the fasting state treatments with placebo or metreleptin were performed in a double-blinded, randomized, cross-over fashion. Replacement doses of metreleptin were given as 4 subcutaneous injections per day every 6 hours starting at 8 a.m. on day 1. Metreleptin was administered at doses of 0.08 mg/kg/day on day 1 and 0.2 mg/kg/day on days 2 and 3. Placebo treatment was carried out with the same schedule as well as the same volume and mode of administration as during leptin treatment.
Study 2
Effect of increasing Doses of Leptin on Chemerin Circulation
To evaluate for any potential dose-effect relationship of metreleptin administration on chemerin levels, we evaluated five healthy female volunteers in a cross-over study of administration of low (0.01 mg/kg), physiological (0.1 mg/kg), and high (0.3 mg/kg) metreleptin doses on three separate clinical visits26. The mean age and BMI of the subjects was 20.4 ± 0.7 years and 21.9 ± 0.7 kg/m2 accordingly. All female subjects were in the isocaloric fed state had a regular menstrual cycle and were not taking any medications including oral contraceptive pills. The study was approved by the Institutional Review Board at the Beth Israel Deaconess Medical Center (BIDMC) and all participants gave informed consent. One day admissions in each state were held in the General Clinical Research Center (GCRC) of BIDMC with samples taken at 8 a.m., 12, 4, and 8 p.m.. Leptin at all doses was administered once at 8 a.m.
Ex Vivo Treatment of Adipose Tissue
Ex vivo procedures were carried out as previously reported 27. In short, human subcutaneous and omental fat explants from 3 subjects (2 Female, 1 male, age 51.7 ± 9.5 years, BMI 43.8 ± 5.8 kg/m2) were placed into Krebs-Ringer-HEPES buffer (20 mmol/l, pH 7.4) containing 2.5% BSA and 200 nmol/l adenosine in the operating room and immediately taken to the laboratory. Tissue was then minced and all nonadipose tissue was removed by rinsing with fresh buffer. The samples were divided equally and incubated in 37°C shaking water bath with or without leptin treatment (100 ng/mL) for 20 hours. Chemerin secretion was measured via ELISA of the supernatant. Subcutaneous adipose tissue protein levels were measured by western blot analysis.
Western Blotting
Western blotting was done as previously described 27. In short, the proteins were loaded in each lane and underwent SDS-polyacrylamide gel electrophoresis. Proteins were then blotted onto nitrocellulose membranes (Schleicher & Schuell, Inc., Keene, NH). The membranes were blocked for 20 minutes in TBS containing 5 % nonfat dry milk and 0.1 % Tween-20. Primary antibodies were incubated in TBS containing 5 % nonfat dry milk overnight and then incubated with horseradish peroxidase secondary antibodies for 2 hours. After incubation with all antibodies membranes were washed with TBS containing 0.1 % Tween-20. Enhanced chemiluminescence was used for detection. Measurement of signal intensity on nitrocellulose membranes was performed using Image J processing and analysis software.
Hormone Measurements
Serum chemerin was measured by ELISA (Biovendor, Chandler, NC, USA) with a sensitivity of 0.1 ng/mL and intra-assay CV of 5.1 – 7.0% and inter-assay CV of 6.9 – 8.3% 28, 29. Serum sTNFRII was measured by ELISA (R&D Systems, Minneapolis, MN, USA) with a sensitivity of 0.6 pg/mL, intra-assay CV of 2.6 – 4.8% and inter-assay CV of 3.5 – 5.1%. Serum Leptin, Insulin, IGF-1, and FFA were measured as previously reported25.
Statistical Analysis
Statistical analysis was performed with STATA v11.2 (STATA Corp. College Station, TX) and Pulse XP (University of Virginia, Charlottesville, VA) software. Results are presented as means ± standard deviation. Normality of the dependent variables was evaluated with the Shapiro-Wilkes test of normality and P-P plots. Evaluation of the existence of a day/night variation pattern for chemerin was performed by hour to hour comparison across a 24 hour period using repeated measurements analysis of variance. Possible dominant harmonic components were analyzed on a subject to subject basis by spectral domain analysis for confirmation, using stata pergram routine. Chemerin, leptin, and sTNFRII levels were also analyzed for potential day night variation, by 4 parameter, cosine, nonlinear regression models, evaluating the adjusted coefficient of determination (R2). Comparisons of all molecules levels between the three states (fed, fasting + placebo and fasting + leptin) were performed with hierarchical mixed effects linear modeling. Chemerin levels were modeled as a linear function of time, at the level of each individual. Fixed effects of each state on the intercept and slope of the growth trajectories were assessed by introducing “state” as a level-2 dummy variable. Comparisons between day 1 and day 4 between and within each group as well as the study 2 leptin-dose response were performed by repeated measures ANOVA. Correlations between chemerin and insulin levels in the fasting state were evaluated with simple linear regression. Analysis of chemerin levels in ex vivo experiment was done by paired T-Test. All p-values are two sided, alpha criterion was set to 0.05.
Results
Tissue mRNA Expression of Chemerin and CMKLR1
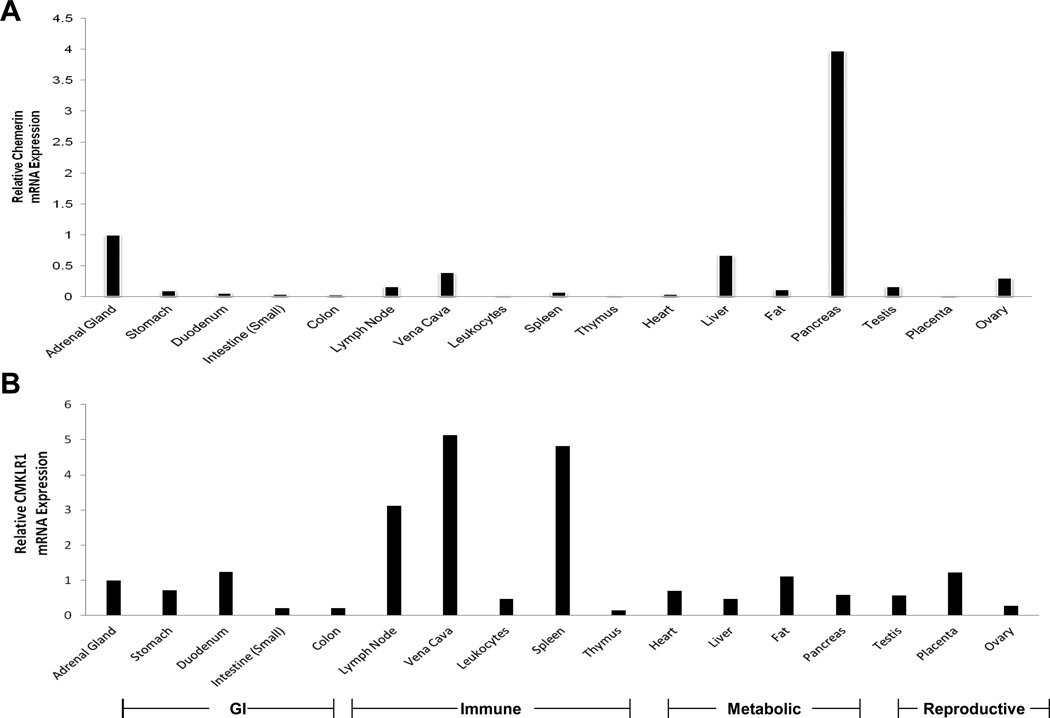
Values of chemerin and CMKLR1 mRNA expression levels are displayed as relative to adrenal gland levels. Chemerin levels are distributed throughout multiple tissues with the highest expression seen in pancreas, liver, adrenal gland, kidney (Fig. 1), and rectum (data not shown). Chemerin is expressed the least in the thymus, brain, plasma leukocytes, and placenta. The expression of CMKLR1 is more widely distributed while the highest levels are seen in tissues important in immune or inflammatory responses such as the spleen, lymph node, and vena cava (Fig. 1). Other tissues analyzed for chemerin and CMKLR1 include muscle, brain, intracranial nerves, retina, optic nerve, urethra, bladder, vagina, penis, uvula, oviduct, seminal vesicles, prostate, cervix, mammary gland, pituitary, thyroid, pericardium, tonsils, esophagus, nasal mucosa, trachea, tongue, spinal cord, epididymis, and skin (data not shown).
Figure 1. Tissue mRNA Expression of Chemerin and CMKLR1.
Relative mRNA expression of Chemerin (A) and CMKLR1 (B) in human tissues utilizing Human mRNA Tissue Array. Specific tissue type for each corresponding bar is labeled in the x-axis of each graph and a legend to group tissues by function in relation to both graphs A and B is located at the bottom of the figure. Methods of experiment are described in detail in Experimental Procedures section.
Chemerin in Circulation
Baseline characteristics including weight, BMI, insulin, Free Fatty Acids (FFA), chemerin, Serum soluble tumor necrosis factor receptor II (sTNFRII), and leptin of the 6 subjects in all three conditions (fed, fasting plus placebo, and fasting plus metreleptin) are outlined in Table 1. Only the fasting plus metreleptin administration state demonstrated a slight decrease in body weight and BMI. Day 4 measurements of insulin, leptin, FFA, sTNFRII and chemerin were not statistically different from day 1 in the fed state. Insulin, leptin, and chemerin all decreased in response to fasting on day 4 compared to day 1. In contrast, FFA increased in response to fasting. Soluble TNFRII was unchanged in all 3 states. Leptin was increased on day 4 in the fasting with metreleptin administration state while all other hormones remained in ranges seen in the fasting with placebo administration condition (Table 1).
Table I. Baseline Characteristics vs. Day 4 of Energy Deprivation.
Characteristics and chemerin levels at 8 AM on Day 1 and Day 4 of fed state, 72-hour fasting with placebo administration, and 72-hour fasting with metreleptin administration. Data were analyzed by ANOVA for repeated variables.
| Fed State | Fasting + Placebo | Fasting + Leptin | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 4 | Day 1 | Day 4 | Day 1 | Day 4 | p trt | p time | p trt*time | |
| Body weight (kg) | 57.1 ± 6.5 | 57.9 ± 6.4 | 56.9 ± 7.5 | 55.4 ± 6.9 | 56.5 ± 6.4 | 54.4 ± 5.8* | 0.002 (0.001) | 0.033 (0.033) | 0.033 (0.048) |
| BMI (kg/m^2) | 21.8 ± 2.1 | 22.2 ± 2.1 | 21.9 ± 2.7 | 21.4 ± 2.6 | 21.7 ± 2.7 | 20.9 ± 2.6* | 0.001 (0.013) | 0.021 (0.021) | 0.019 (0.031) |
| Insulin (µIU/ml) | 5.7 ± 1.8 | 8.1 ± 2.8 | 6.1 ± 2.0 | 1.2 ± 0.6* | 6.9 ± 3.3 | 1.2 ± 0.9* | <0.001 (<0.001) | <0.001 (<0.001) | <0.001 (<0.001) |
| Leptin (ng/ml) | 11.2 ± 4.5 | 16.7 ± 3.3 | 14.7 ± 6.3 | 2.8 ± 0.8* | 12.5 ± 4.6 | 29.6 ± 5.4* | <0.001 (<0.001) | 0.282 (0.282) | <0.001 (<0.001) |
| sTNFRII (pg/ml) | 1753.75 ± 170 | 1706.5 ± 257 | 1775.9 ± 339 | 1810.8 ± 230 | 1596.6 ± 178 | 1865.9 ± 158 | 0.726 (0.726) | 0.644 (0.644) | 0.651 (0.651) |
| Chemerin (ng/ml) | 164.6 ± 37.2 | 155.0 ± 42.4 | 161.1 ± 34.0 | 56.9 ± 26.6* | 151.0 ± 34.5 | 50.9 ± 8.6* | <0.001 (0.003) | <0.001 (<0.001) | 0.006 (0.027) |
Values are means +/− SD. Means with * indicate significant difference between day 1 and day 4 within a state. P trt demonstrates significance of intervention (fed, fasting, leptin); p time demonstrates significance of time between days; p trt*time demonstrates significance of intervention and time interaction.
Day/Night Variation of Chemerin
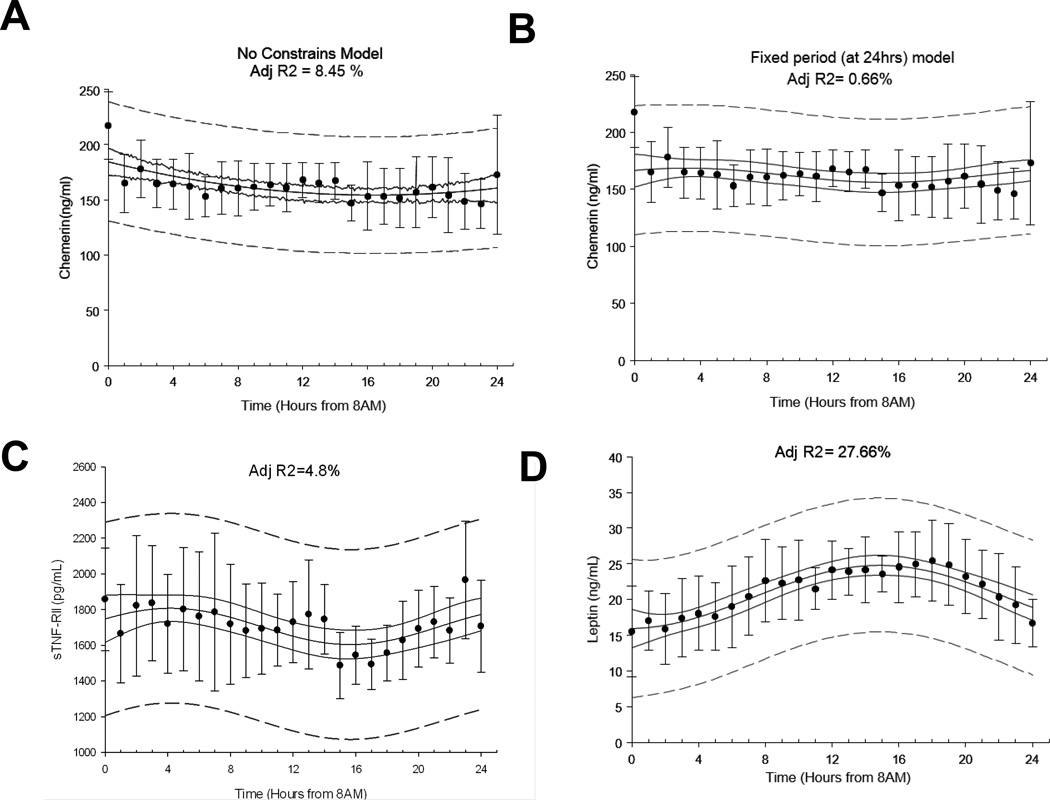
Repeated measurements analysis of variance on normalized chemerin levels revealed no statistically significant difference between hourly time points (p=0.169). The nonlinear cosinor regression analysis of chemerin over the 24 hour period demonstrated a statistically significant day/night variation in both chemerin and sTNFRII circulating levels during the fed state (adjusted coefficients of determination: R2= 8.45%, p<0.01 and R2=4.8%, p=0.02, accordingly). Although statistically significant, we believe that the coefficient of determination calculated for chemerin and sTNFRII was of not clinically significant magnitude. There are no available guidelines on what level of coefficient of determination, derived from non-linear regression models, should serve as a threshold to characterize a model as “physiologically” or “clinically” significant and the statistical consensus recommends that this decision should be made on a study-to-study base, based on the pre-exiting literature. Cortisol and leptin are hormones with established day/night variability secretion patterns and thus using them as a reference is relevant. Previous studies from our group have demonstrated that cortisol, yields an adjusted coefficient of determination of more than 30% in similar statistical models30, while in the present study leptin yielded an adjusted coefficient of determination of 27.66% (p<0.01). Given the fact that the coefficient of determination for hormones with well-established day/night variation patterns is in the 25-30% range we support the notion that the 4.8-8.45% level observed in chemerin and sTNFRII circulating levels is not of clinical significance. Leptin levels displayed a significant day/night rhythm during the fed state with a peak during the night (Figure 2)
Figure 2. Day/Night Variation of Chemerin.
Average 24-hour (A) chemerin (ng/ml) with no constrains, (B) chemerin (ng/ml) with 24-hour fixed period, (C) sTNFRII (pg/ml) and (D) leptin (ng/ml) levels (non-linear adjusted R2 is displayed on top center of each panel). Error bars represent SD, continuous line represents 95% confidence band, and dashed line represents 95% prediction bands. Adj-R^2, Adjusted coefficient of determination.
Effects of Acute Energy Deprivation on Chemerin
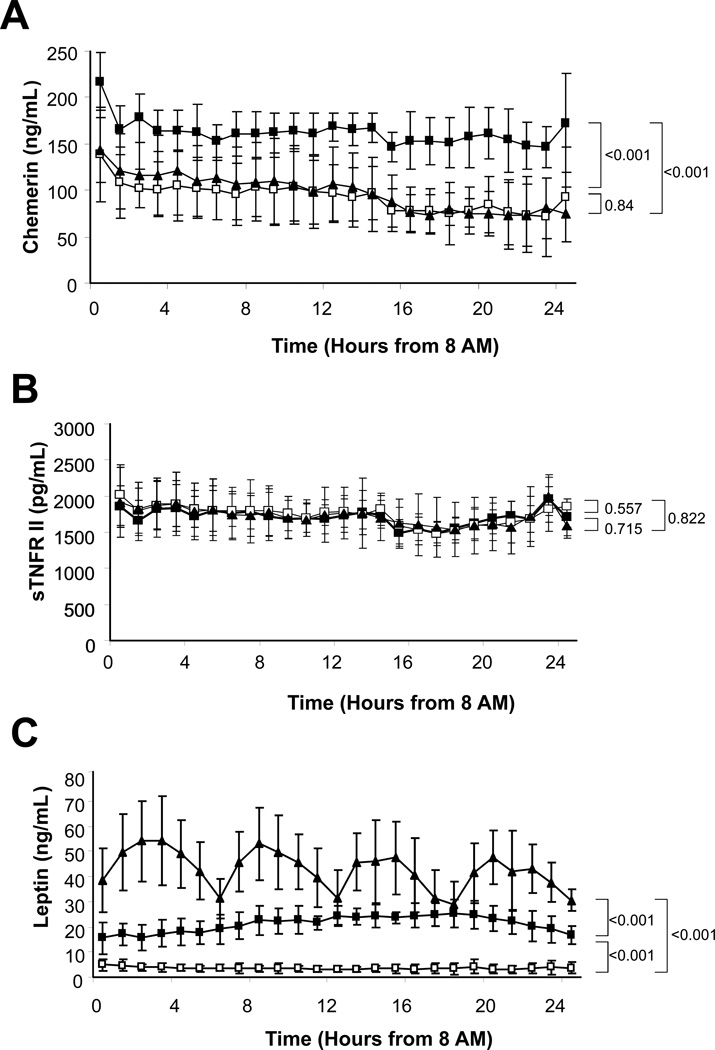
Circulating chemerin levels were significantly lower after a 72-hour fasting than during the fed state (p<0.001). Similarly, leptin displayed decreased circulating levels in the fasting state compared to the fed state (p<0.001). However, sTNFRII levels were not affected by a 72-hour fast (p=0.557) (Fig. 3).
Figure 3. Effects of Acute Energy Deprivation.
Average 24-hour (A) chemerin (ng/ml), (B) sTNFRII (pg/ml), and (C) Leptin (ng/ml) levels of fed state (■), fasting with placebo administration (□), and fasting with metreleptin administration (▲). P-values are shown next to brackets.
Leptin Effects on Chemerin
Leptin levels showed a significant drop during the fasting state as compared to the fed state. Leptin replacement in the fasting state by metreleptin administration completely restored serum leptin levels to the physiological range (Table 1). On the contrary, chemerin levels were significantly lower in fasting state during metreleptin administration compared to during the fed state (p<0.001). Also, levels of chemerin during leptin replacement were not statistically different from levels during fasting plus placebo (p=0.84). Furthermore, levels of sTNFRII were unaffected by treatment with recombinant leptin (p=0.715) (Fig. 3).
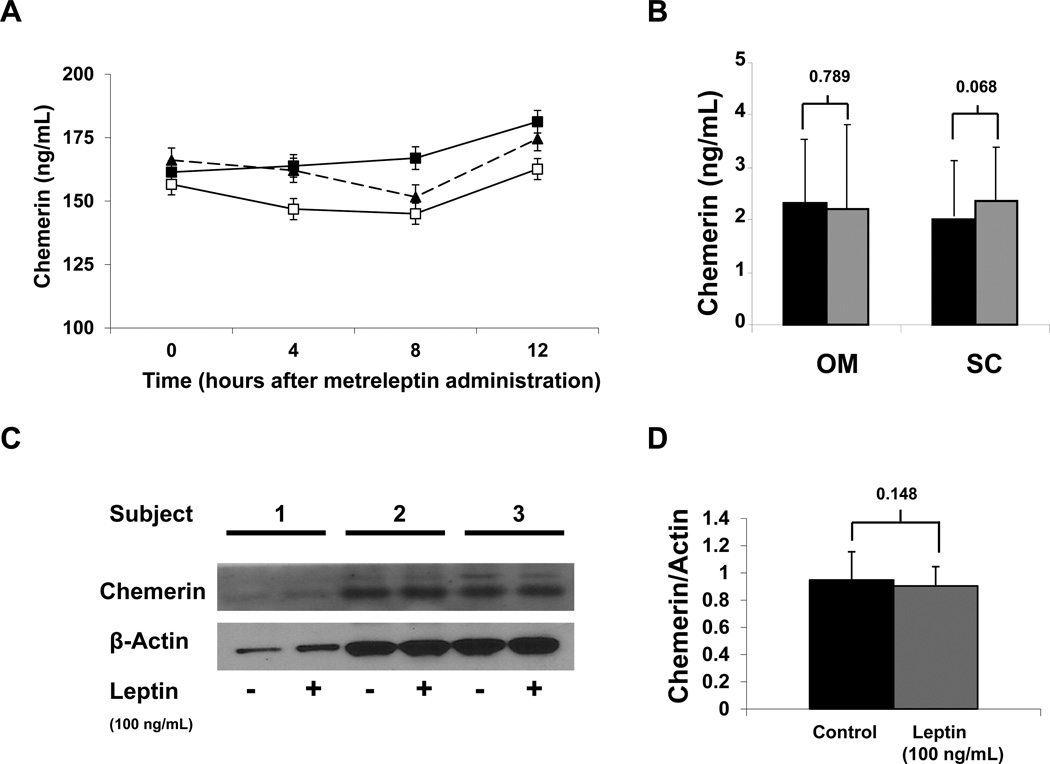
Leptin treatment at high, physiological, and low doses showed no difference in chemerin circulating levels (Fig. 4A). Similarly, treatment of subcutaneous and omental adipose tissue explants showed with leptin at 100 ng/mL for 20 hours showed no effect on chemerin secretion and protein levels (Fig. 4B–D).
Figure 4. Leptin Effects on Chemerin.
Average 16-hour (A) chemerin (ng/mL) levels after treatment with three doses of leptin (0.01, 0.1, and 0.3 mg/Kg). Chemerin levels in supernatant (B) with ( ) or without (■)100 ng/mL leptin treatment for 20 hours in subcutaneous and omental adipose tissue. Chemerin protein expression (C – D) in subcutaneous adipose tissue after leptin treatment. (B–D) Results are the mean of 3 experiments. Data were analyzed by paired T-Test. Values are means +/− SD.
) or without (■)100 ng/mL leptin treatment for 20 hours in subcutaneous and omental adipose tissue. Chemerin protein expression (C – D) in subcutaneous adipose tissue after leptin treatment. (B–D) Results are the mean of 3 experiments. Data were analyzed by paired T-Test. Values are means +/− SD.
Correlations of Chemerin with Insulin in the Fasting state
Correlations of chemerin with insulin were also evaluated in the fasting state. In the beginning of the fasting period, the standardized regression coefficient between chemerin and insulin was β=0.74 (p=0.11) while after 4 days of fasting it was β=−0.7 (p=0.86). Regression of the change of insulin levels on chemerin levels also yielded a non-significant association (β=0.24, p=0.35)
Discussion
Previous studies in mice have demonstrated that chemerin is a molecule almost exclusively expressed in adipose tissue and liver 8, 10. Furthermore, studies in humans have evaluated either the expression of chemerin in some tissues/cells 31 or in just adipose tissue 32, but a relative comparison of adipose tissue expression to all other tissue types has not been done. We thus comparatively evaluated for the first time chemerin expression in several human tissue types with that of adipose tissue, by use of human tissue mRNA array. While liver still displays an increased relative expression of chemerin in humans, fat seems to express less chemerin when compared to other tissues such as pancreas and the adrenal gland. Furthermore, upon evaluation of the receptor of chemerin, CMKLR1, we show that it is almost evenly dispersed through multiple human tissues including fat, lung, the adrenal gland, and the duodenum. Furthermore, it appears that the highest expression of CMKLR1 levels are in tissues involved in inflammatory or immune processes such as the lymph nodes, spleen, and the vena cava. This data suggest that chemerin in humans might not be an adipocytokine and may rather play a more prominent role in immune/inflammatory response. The latter is consistent with initial studies demonstrating chemerin as a chemoactractant signal in psoriatic skin lesions 2.
Subsequently, we studied the physiology of chemerin in humans, specifically evaluating for any potential day/night variation of chemerin circulating levels and for chemerin’s response to starvation and leptin administration in humans. We demonstrate herein, for the first time in humans, that, in contrast to leptin, chemerin does not show a significant day/night oscillation. Although no prior human studies have evaluated this, a 20 hour long study of mice with free access to food and water, and blood drawn every 4 hours, has previously demonstrated that chemerin exhibits a day/night pattern with a trough exhibited during the night and a peak during the day 4. This is another property of chemerin’s physiology that we fail to replicate in humans. The human transcriptome displays a day/night variation 33 and many adipokines, such as leptin and high molecular weight adiponectin, display a diurnal variation in humans 34, 35. Interestingly, both the pancreas and liver, which we demonstrate to be one of the prominent sites of chemerin expression, have a vast number of genes, such the ones encoding proteins essential for glucose metabolism, that express a circadian pattern 36. In our study we demonstrated absence of any significant day/night oscillation in chemerin circulating levels. We could thus speculate that either chemerin gene expression is not regulated in a circadian fashion in the pancreas and the liver, or that constant, non-circardian secretion from other tissues masks such a pattern. Further studies are encouraged to elucidate this aspect of chemerin’s phsysiology.
We also demonstrate, for the first time in humans, that fasting dramatically affects the levels of circulating chemerin. Fasting for 72-hour reduces chemerin levels to ~42% of that seen during the isocaloric fed state. This suggests that chemerin demonstrates an acute response to changes in energy homeostasis such as acute caloric restriction. Interestingly chemerin is highly expressed in tissues that are either direct nutrient sensors, such as the pancreas, or in tissues important for relaying nutrient availability such as the adrenal gland, which produces catecholamines during energy deprivation, and the liver. Therefore, chemerin might be another signal of nutrient status, directed towards the parts of the immune tissues that are expressing the highest CMKLR1, indicating prolonged energy deprivation is occurring, and therefore decreased activity or energy expenditure from those tissues is required. The latter however is only a reasonable speculation derived from chemerin’s expression patters as well as its response to acute energy deprivation; further translational studies are needed to confirm this. Although sTNFRII levels did not change with fasting, this does not exclude chemerin-induced immune modification in a TNF-a independent fashion.
Chemerin’s response to caloric restriction is similar to that seen by other hormones important in energy homeostasis, such as leptin 35. Leptin is a key molecule signaling information on the amount of energy stored in the adipose tissue and has been shown to be a regulator of metabolic and neuroendocrine adjustment to starvation 20, 35. Previous studies from our group have demonstrated that the decrease of leptin levels observed during acute energy deprivation is causally linked to the decrease of other hormones such as luteinizing hormone (LH) 18, 35, while replacement of leptin to physiologic levels corrects starvation-induced changes of neuroendocrine systems 35, 37. Cross sectional studies have also demonstrated a statistically significant correlation between circulating chemerin and leptin levels in lean, overweight and type-2 diabetic humans 12. No study to date has evaluated for any causal relationship between leptin and chemerin. Given the fact that in our study fasting had the same effect on the direction of change in both chemerin and leptin circulating levels we wanted to further evaluate for potential regulation of chemerin circulating levels by leptin. We thus performed an interventional cross-over study of metreleptin and placebo administration in the fasting state on the same subjects that we utilized to study for potential day/night variability of chemerin’s secretion. We demonstrated that metreleptin replacement has no effect on chemerin levels, implying the lack of any causal relationship between leptin and chemerin. This was also confirmed by our escalating metreleptin dose experiment where administration of low, physiological, and supraphysiological doses of metreleptin during the fed state failed to elicit any dose-response relationship between administered metreleptin dose and measured circulating chemerin levels. Furthermore, we analyzed the effect of 100 ng/mL leptin treatment on subcutaneous and omental adipose tissue explants. Chemerin levels in the supernatant of both subcutaneous and omental fat tissue as well as its protein expression levels in the subcutaneous adipose tissue were unaltered after overnight treatment with leptin. We thus can report with confidence that leptin does not affect directly or indirectly circulating chemerin levels or expression, suggesting that a mechanism other than leptin controls the acute response of chemerin to decreased energy intake.
Insulin is another hormone that has been suggested to affect chemerin levels in ex vivo human studies. Specifically prolonged insulin infusions increase chemerin secretion in human adipose tissue explants 32. Although the drop in chemerin levels observed during the fasting state could theoretically be explained by the expected drop in insulin levels, failure of chemerin levels to increase with meals, when insulin levels are expected to increase, surrogates against insulin secretion regulating chemerin levels in vivo. In addition, in our study, chemerin and insulin levels were not correlated in the fasting state at baseline or at day 4, and there was no significant association in regression of the change of insulin levels between fed and fasting, on chemerin levels. Future studies utilizing long term insulin administration, possibly through hyperinsulinemic euglycemic clamps, are needed to definitively rule in or out an effect of dropping insulin levels on chemerin during energy deprivation.
Proinflammatory cytokines such as TNF-α have been demonstrated to induce chemerin production in vitro and in vivo 4, 5. Production of TNF-α by peripheral blood mononuclear cells increases during acute energy deprivation, while spontaneous TNF-α production has been noted in patients with anorexia nervosa which is a model disease of chronic energy deprivation38, 39. We thus wanted to evaluate whether the decrease of circulating chemerin observed in our study during acute energy deprivation could be attributed to a starvation-induced elevation of circulating TNF-α levels. We thus measured sTNFRII, a reliable marker of TNF-α system activity 40, 41, in the subjects undergoing acute energy deprivation. Although we did not measure TNF-α directly, it has been demonstrated that sTNFRII, is a more easily and readily detected marker of activation of the TNF-α system that is highly correlated with TNF-α levels 42. Levels of sTNFRII were unaltered by a 72-hour fast, indicating that the TNF system is not likely responsible for chemerin’s changes during short term energy deprivation.
Our study has several strengths: this is the first study in humans examining tissue expression of chemerin and its receptor. It is also the first study in humans to evaluate for day night variability patterns of chemerin secretion and its association with short term changes in energy homeostasis. The temporal resolution of our sampling intervals is high, enabling us to infer the absence of any potential day/night variation patterns with great confidence. In addition, methods were fully standardized and the subjects were seen at the same period of their menstrual cycle, received the same isocaloric diet, were instructed to keep their exercise levels stable and were exposed to the same light/dark intervals throughout the study. Furthermore the randomized cross-over design of this study enables us to control for potential confounding. The ≥8 week interval between study visits enabled adequate wash out of the medication as well as restoration of the subject’s hematocrit. The four day stay in the GCRC enabled proper standardization of the study conditions. And finally the use of multiple study groups as well as the data from ex vivo experiments using tissue explants strongly confirms the findings.
One limitation of this study is the small sample size (n=11) of the physiology studies. However, we overcame this limitation by the frequent sampling approach and the cross-over design of the study, which increases study power. This or similar study samples have been proven to be adequate to detect leptin day/night variation patterns and leptin replacement was able to restore hormonal levels that the 4 day fasting had induced as previously described 25. Also, leptin served as a positive control in this study by demonstrating significant day/night rhythm as well as significant effects due to fasting. Thus, the lack of statistically significant changes in the chemerin day night variation could not be attributed to lack of power. Similarly, we confirmed some important findings with other experiments including ex vivo adipose tissue leptin treatment. Furthermore, this study is limited by the use of only female subjects for the analysis of Day/Night variation and effect of acute energy deprivation, as well as a dosage response to leptin. Future studies must be carried out in a male population to confirm our results are not limited by gender.
In conclusion, our findings show, for the first time, that chemerin has a different tissue-specific expression pattern compared to rodents, that it is distributed throughout several human tissues with higher expression in pancreas and liver suggesting that it may not be functioning as an adipocytokine in humans. Its receptor, CMKLR1, displays greatest expression in immune tissues such as the spleen and lymph nodes. Furthermore, we demonstrated that, contrary to rodents, chemerin does not exhibit a day/night variation in humans, but is responsive to acute changes in energy homeostasis. We thus recommend clinical studies investigating human circulating chemerin should consider the fasting state of subjects but not necessarily the time of day during blood draws. We also demonstrated that the decreasing levels of chemerin due to starvation are mediated through leptin or the TNF-α system. These data contribute towards elucidation of chemerin biology in humans and suggest that chemerin physiology in humans is different from rodents and extreme caution should be applied to any attempt to translate findings regarding chemerin from rodent models to human physiology and pathophysiology. Further studies are needed to elucidate the role of chemerin and its regulation in humans.
Acknowledgments
Grant Support:
The project described was supported by Grant Number UL1 RR025758-Harvard Clinical and Translational Science Center, from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health The Mantzoros Laboratory is supported by the National Institute of Diabetes and Digestive and Kidney Diseases grants 58785, 79929 and 81913 as well as Award Number 1I01CX000422-01A1 from the Clinical Science Research and Development Service of the VA Office of Research and Development. Amylin Pharmaceuticals, Inc. supplied metreleptin for this study but had no role in the study design; conduct of the study; collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Footnotes
Disclosure summary:
The authors have nothing to disclose.
References
- 1.Meder W, Wendland M, Busmann A, Kutzleb C, Spodsberg N, John H, Richter R, Schleuder D, Meyer M, Forssmann WG. Characterization of human circulating TIG2 as a ligand for the orphan receptor ChemR23. FEBS Lett. 2003;555:495–499. doi: 10.1016/s0014-5793(03)01312-7. [DOI] [PubMed] [Google Scholar]
- 2.Nagpal S, Patel S, Jacobe H, DiSepio D, Ghosn C, Malhotra M, Teng M, Duvic M, Chandraratna RA. Tazarotene-induced gene 2 (TIG2), a novel retinoid-responsive gene in skin. J Invest Dermatol. 1997;109:91–95. doi: 10.1111/1523-1747.ep12276660. [DOI] [PubMed] [Google Scholar]
- 3.Ernst MC, Sinal CJ. Chemerin: at the crossroads of inflammation and obesity. Trends Endocrinol Metab. 2010;21:660–667. doi: 10.1016/j.tem.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Parlee SD, Ernst MC, Muruganandan S, Sinal CJ, Goralski KB. Serum Chemerin Levels Vary with Time of Day and Are Modified by Obesity and Tumor Necrosis Factor-a. Endocrinology. 2010;151:2590–2602. doi: 10.1210/en.2009-0794. [DOI] [PubMed] [Google Scholar]
- 5.Kralisch S, Weise S, Sommer G, Lipfert J, Lossner U, Bluher M, Stumvoll M, Fasshauer M. Interleukin-1beta induces the novel adipokine chemerin in adipocytes in vitro. Regul Pept. 2009;154:102–106. doi: 10.1016/j.regpep.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Bozaoglu K, Curran JE, Stocker CJ, Zaibi MS, Segal D, Konstantopoulos N, Morrison S, Carless M, Dyer TD, Cole SA, Goring HH, Moses EK, Walder K, Cawthorne MA, Blangero J, Jowett JB. Chemerin, a novel adipokine in the regulation of angiogenesis. J Clin Endocrinol Metab. 2010;95:2476–2485. doi: 10.1210/jc.2010-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaur J, Adya R, Tan BK, Chen J, Randeva HS. Identification of chemerin receptor (ChemR23) in human endothelial cells: chemerin-induced endothelial angiogenesis. Biochem Biophys Res Commun. 2010;391:1762–1768. doi: 10.1016/j.bbrc.2009.12.150. [DOI] [PubMed] [Google Scholar]
- 8.Goralski KB, McCarthy TC, Hanniman EA, Zabel BA, Butcher EC, Parlee SD, Muruganandan S, Sinal CJ. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem. 2007;282:28175–28188. doi: 10.1074/jbc.M700793200. [DOI] [PubMed] [Google Scholar]
- 9.Roh SG, Song SH, Choi KC, Katoh K, Wittamer V, Parmentier M, Sasaki S. Chemerin--a new adipokine that modulates adipogenesis via its own receptor. Biochem Biophys Res Commun. 2007;362:1013–1018. doi: 10.1016/j.bbrc.2007.08.104. [DOI] [PubMed] [Google Scholar]
- 10.Bozaoglu K, Bolton K, McMillan J, Zimmet P, Jowett J, Collier G, Walder K, Segal D. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology. 2007;148:4687–4694. doi: 10.1210/en.2007-0175. [DOI] [PubMed] [Google Scholar]
- 11.Bozaoglu K, Segal D, Shields KA, Cummings N, Curran JE, Comuzzie AG, Mahaney MC, Rainwater DL, VandeBerg JL, MacCluer JW, Collier G, Blangero J, Walder K, Jowett JB. Chemerin is associated with metabolic syndrome phenotypes in a Mexican-American population. J Clin Endocrinol Metab. 2009;94:3085–3088. doi: 10.1210/jc.2008-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weigert J, Neumeier M, Wanninger J, Filarsky M, Bauer S, Wiest R, Farkas S, Scherer MN, Schaffler A, Aslanidis C, Scholmerich J, Buechler C. Systemic chemerin is related to inflammation rather than obesity in type 2 diabetes. Clin Endocrinol (Oxf) 2010;72:342–348. doi: 10.1111/j.1365-2265.2009.03664.x. [DOI] [PubMed] [Google Scholar]
- 13.Sell H, Divoux A, Poitou C, Basdevant A, Bouillot JL, Bedossa P, Tordjman J, Eckel J, Clement K. Chemerin correlates with markers for fatty liver in morbidly obese patients and strongly decreases after weight loss induced by bariatric surgery. J Clin Endocrinol Metab. 2010;95:2892–2896. doi: 10.1210/jc.2009-2374. [DOI] [PubMed] [Google Scholar]
- 14.Tonjes A, Fasshauer M, Kratzsch J, Stumvoll M, Bluher M. Adipokine pattern in subjects with impaired fasting glucose and impaired glucose tolerance in comparison to normal glucose tolerance and diabetes. PLoS One. 2010;5:e13911. doi: 10.1371/journal.pone.0013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi M, Takahashi Y, Takahashi K, Zolotaryov FN, Hong KS, Kitazawa R, Iida K, Okimura Y, Kaji H, Kitazawa S, Kasuga M, Chihara K. Chemerin enhances insulin signaling and potentiates insulin-stimulated glucose uptake in 3T3-L1 adipocytes. FEBS Lett. 2008;582:573–578. doi: 10.1016/j.febslet.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 17.Kelesidis T, Kelesidis I, Chou S, Mantzoros CS. Narrative review: the role of leptin in human physiology: emerging clinical applications. Ann Intern Med. 2010;152:93–100. doi: 10.1059/0003-4819-152-2-201001190-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mantzoros CS. Role of leptin in reproduction. Ann N Y Acad Sci. 2000;900:174–183. doi: 10.1111/j.1749-6632.2000.tb06228.x. [DOI] [PubMed] [Google Scholar]
- 19.Hamnvik OP, Liu X, Petrou M, Gong H, Chamberland JP, Kim EH, Christophi CA, Kales SN, Christiani DC, Mantzoros CS. Soluble leptin receptor and leptin are associated with baseline adiposity and metabolic risk factors, and predict adiposity, metabolic syndrome, and glucose levels at 2-year follow-up: the Cyprus Metabolism Prospective Cohort Study. Metabolism. 2011;60:987–993. doi: 10.1016/j.metabol.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Chan JL, Mantzoros CS. Role of leptin in energy-deprivation states: normal human physiology and clinical implications for hypothalamic amenorrhoea and anorexia nervosa. Lancet. 2005;366:74–85. doi: 10.1016/S0140-6736(05)66830-4. [DOI] [PubMed] [Google Scholar]
- 21.Hill JW, Elias CF, Fukuda M, Williams KW, Berglund ED, Holland WL, Cho YR, Chuang JC, Xu Y, Choi M, Lauzon D, Lee CE, Coppari R, Richardson JA, Zigman JM, Chua S, Scherer PE, Lowell BB, Bruning JC, Elmquist JK. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab. 2010;11:286–297. doi: 10.1016/j.cmet.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004;351:987–997. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- 23.Chou SH, Chamberland JP, Liu X, Matarese G, Gao C, Stefanakis R, Brinkoetter MT, Gong H, Arampatzi K, Mantzoros CS. Leptin is an effective treatment for hypothalamic amenorrhea. Proc Natl Acad Sci U S A. 2011;108:6585–6590. doi: 10.1073/pnas.1015674108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sienkiewicz E, Magkos F, Aronis KN, Brinkoetter M, Chamberland JP, Chou S, Arampatzi KM, Gao C, Koniaris A, Mantzoros CS. Long-term metreleptin treatment increases bone mineral density and content at the lumbar spine of lean hypoleptinemic women. Metabolism: clinical and experimental. doi: 10.1016/j.metabol.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 25.Chan JL, Matarese G, Shetty GK, Raciti P, Kelesidis I, Aufiero D, De Rosa V, Perna F, Fontana S, Mantzoros CS. Differential regulation of metabolic, neuroendocrine, and immune function by leptin in humans. Proc Natl Acad Sci U S A. 2006;103:8481–8486. doi: 10.1073/pnas.0505429103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan JL, Wong SL, Mantzoros CS. Pharmacokinetics of subcutaneous recombinant methionyl human leptin administration in healthy subjects in the fed and fasting states: regulation by gender and adiposity. Clin Pharmacokinet. 2008;47:753–764. doi: 10.2165/00003088-200847110-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moon HS, Chamberland JP, Diakopoulos KN, Fiorenza CG, Ziemke F, Schneider B, Mantzoros CS. Leptin and amylin act in an additive manner to activate overlapping signaling pathways in peripheral tissues: in vitro and ex vivo studies in humans. Diabetes Care. 2010;34:132–138. doi: 10.2337/dc10-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chakaroun R, Raschpichler M, Kloting N, Oberbach A, Flehmig G, Kern M, Schon MR, Shang E, Lohmann T, Dressler M, Fasshauer M, Stumvoll M, Bluher M. Effects of weight loss and exercise on chemerin serum concentrations and adipose tissue expression in human obesity. Metabolism: clinical and experimental. 2012;61:706–714. doi: 10.1016/j.metabol.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 29.El-Mesallamy HO, El-Derany MO, Hamdy NM. Serum omentin-1 and chemerin levels are interrelated in patients with Type 2 diabetes mellitus with or without ischaemic heart disease. Diabetic medicine : a journal of the British Diabetic Association. 2011;28:1194–1200. doi: 10.1111/j.1464-5491.2011.03353.x. [DOI] [PubMed] [Google Scholar]
- 30.Vamvini MT, Aronis KN, Chamberland JP, Mantzoros CS. Energy deprivation alters in a leptin- and cortisol-independent manner circulating levels of activin A and follistatin but not myostatin in healthy males. The Journal of clinical endocrinology and metabolism. 2011;96:3416–3423. doi: 10.1210/jc.2011-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wittamer V, Franssen JD, Vulcano M, Mirjolet JF, Le Poul E, Migeotte I, Brezillon S, Tyldesley R, Blanpain C, Detheux M, Mantovani A, Sozzani S, Vassart G, Parmentier M, Communi D. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med. 2003;198:977–985. doi: 10.1084/jem.20030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan BK, Chen J, Farhatullah S, Adya R, Kaur J, Heutling D, Lewandowski KC, O'Hare JP, Lehnert H, Randeva HS. Insulin and metformin regulate circulating and adipose tissue chemerin. Diabetes. 2009;58:1971–1977. doi: 10.2337/db08-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loboda A, Kraft WK, Fine B, Joseph J, Nebozhyn M, Zhang C, He Y, Yang X, Wright C, Morris M, Chalikonda I, Ferguson M, Emilsson V, Leonardson A, Lamb J, Dai H, Schadt E, Greenberg HE, Lum PY. Diurnal variation of the human adipose transcriptome and the link to metabolic disease. BMC Med Genomics. 2009;2:7. doi: 10.1186/1755-8794-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scheer FA, Chan JL, Fargnoli J, Chamberland J, Arampatzi K, Shea SA, Blackburn GL, Mantzoros CS. Day/night variations of high-molecular-weight adiponectin and lipocalin-2 in healthy men studied under fed and fasted conditions. Diabetologia. 2010;53:2401–2405. doi: 10.1007/s00125-010-1869-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mullington JM, Chan JL, Van Dongen HP, Szuba MP, Samaras J, Price NJ, Meier-Ewert HK, Dinges DF, Mantzoros CS. Sleep loss reduces diurnal rhythm amplitude of leptin in healthy men. J Neuroendocrinol. 2003;15:851–854. doi: 10.1046/j.1365-2826.2003.01069.x. [DOI] [PubMed] [Google Scholar]
- 36.Kalsbeek A, Ruiter M, La Fleur SE, Cailotto C, Kreier F, Buijs RM. The hypothalamic clock and its control of glucose homeostasis. Prog Brain Res. 2006;153:283–307. doi: 10.1016/S0079-6123(06)53017-1. [DOI] [PubMed] [Google Scholar]
- 37.Mantzoros CS, Cramer DW, Liberman RF, Barbieri RL. Predictive value of serum and follicular fluid leptin concentrations during assisted reproductive cycles in normal women and in women with the polycystic ovarian syndrome. Hum Reprod. 2000;15:539–544. doi: 10.1093/humrep/15.3.539. [DOI] [PubMed] [Google Scholar]
- 38.Vaisman N, Schattner A, Hahn T. Tumor necrosis factor production during starvation. Am J Med. 1989;87:115. doi: 10.1016/s0002-9343(89)80497-8. [DOI] [PubMed] [Google Scholar]
- 39.Vaisman N, Hahn T. Tumor necrosis factor-alpha and anorexia--cause or effect? Metabolism. 1991;40:720–723. doi: 10.1016/0026-0495(91)90090-j. [DOI] [PubMed] [Google Scholar]
- 40.Diez-Ruiz A, Tilz GP, Zangerle R, Baier-Bitterlich G, Wachter H, Fuchs D. Soluble receptors for tumour necrosis factor in clinical laboratory diagnosis. Eur J Haematol. 1995;54:1–8. doi: 10.1111/j.1600-0609.1995.tb01618.x. [DOI] [PubMed] [Google Scholar]
- 41.Mantzoros CS, Moschos S, Avramopoulos I, Kaklamani V, Liolios A, Doulgerakis DE, Griveas I, Katsilambros N, Flier JS. Leptin concentrations in relation to body mass index and the tumor necrosis factor-alpha system in humans. J Clin Endocrinol Metab. 1997;82:3408–3413. doi: 10.1210/jcem.82.10.4323. [DOI] [PubMed] [Google Scholar]
- 42.Aderka D. The potential biological and clinical significance of the soluble tumor necrosis factor receptors. Cytokine Growth Factor Rev. 1996;7:231–240. doi: 10.1016/s1359-6101(96)00026-3. [DOI] [PubMed] [Google Scholar]