Abstract
Reactive oxygen species (ROS) have been shown to be a contributor to aging and disease. ROS also serve as a trigger switch for signaling cascades leading to corresponding cellular and molecular events. In the central nervous system, microglial cells are likely the main source of ROS production. However, activated astrocytes also appear to be capable of generating ROS. In this study we investigated ROS production in human astrocytes stimulated with interleukin (IL)-1β and interferon (IFN)-γ and its potential harmful effects. Although IFN-γ alone had no effect, it potentiated IL-1β-induced ROS production in a time-dependent manner. One of the sources of ROS in IL-1β-activated astrocytes was from increased superoxide production in mitochondria accompanied by enhanced manganese superoxide dismutase and inhibited catalase expression. NADPH oxidase (NOX) may also contribute to ROS production as astrocytes express NOX isoforms. Glutamate uptake, which represents one of the most important methods of astrocytes to prevent excitotoxity, was down-regulated in IL-1β-activated astrocytes, and was further suppressed in the presence of IFN-γ; IFN-γ itself exerted minimal effect. Elevated levels of 8-isoprostane in IL-1β ± IFN-γ-activated human astrocytes indicate downstream lipid peroxidation. Pretreatment with DPI (diphenyleneiodonium) abolished the IL-1β ± IFN-γ-induced ROS production, restored glutamate uptake function and reduced 8-isoprostane to near control levels suggesting that ROS contributes to the dysfunction of activated astrocytes. These results support the notion that dampening activated human astrocytes to maintain the redox homeostasis is vital to preserve their neuroprotective potential in the CNS.
Keywords: Astrocytes, Interleukin 1 beta, Reactive Oxygen Species, Mitochondria, 8-isoprostane, lipid peroxidation
Background
Free radicals, naturally produced by aerobic organisms, are highly reactive with many cellular components, leading to potential damage. Besides normal aerobic respiration, high level of reactive oxygen species (ROS) can also be generated under various conditions, including inflammation. It has been shown that ROS contribute to several neurodegenerative diseases such as Parkinson’s disease, Alzheimer’s disease, HIV-1-associated dementia (HAD) and amyotrophic lateral sclerosis (ALS) [1–4]. ROS were also found to limit the lifespan of hematopoietic stem cells [5]. On the other hand, ROS also serve as important immune defense weapons and as trigger switches for signaling cascade pathways and gene expression. Studies have also shown that ROS production is essential for downstream signaling pathways [6, 7] and perhaps foster communication among various cellular processes to maintain homeostasis [8]. A shortage of ROS, termed reductive stress, can also disrupt many physiological signaling processes [9]. Reaction of ROS with target molecules can induce further ROS production in a chain reaction [10] and this can present dire consequences unless the ROS are removed in a timely fashion. Hence, the antioxidant system, including both enzymes and specific molecules, acts as scavengers of ROS and its downstream products to minimize the buildup of ROS and lessen the potential inflicted damage. As such, the balance between ROS and the antioxidant system plays a critical role in determining the fate of cells.
ROS can be generated from many sources, involving enzymatic and non-enzymatic processes [11]; mitochondria are considered to be one of the main contributors to ROS production [12]. ROS production has been demonstrated in macrophages and microglial cells through the activity of NADPH oxidase (NOX) [13–15], as well as in human astrocytic cell lines U-87 [16, 17], CRT-MG [18], astrocytes differentiated from human brain-derived progenitor cells [19] and in primary rat astrocytes [20]. However, in primary human astrocyte cultures, expression of NOX isoform NOX4, but not NOX2, has been reported [21] and NOX4 is the most widely distributed isoform [9]. Diphenyleneiodonium (DPI) is often used as an inhibitor of NADPH oxidase [13, 22–24], which catalyzes superoxide anion (O2−) production, although it is not specific for NOX.
Astrocytes are the most abundant glial cells in the central nervous system (CNS) and they play a major role in cross-talk with neurons, provide physical and functional support of neurons, and ultimately afford a level of protection for neurons, such as preventing glutamate excitotoxicity [25]. Damage to astrocytes could therefore be deleterious to neuronal function, resulting in apoptosis and neurotoxicity. The proinflammatory cytokine interleukin 1 (IL)-1β, which is primarily released from activated microglial cells during inflammation, injury or other pathological conditions, is a robust activator of human astrocytes leading to inflammatory mediator production such as cytokines, chemokines, nitric oxide (NO) and ROS [19, 26–31] and to down-regulation of glutamate uptake [32]. Some of the downstream effects of ROS accumulation, including lipid peroxidation of membrane arachidonic acid leading to isoprostane formation in astrocytes, has also been demonstrated [33]. Interferon (IFN)-γ, which is largely secreted by lymphocytes trafficking into the brain during CNS infection and inflammation, potentiates IL-1β-induced production of inflammatory mediators derived from astrocytes [27, 34], which could trigger unfavorable and injurious autocrine and paracrine conditions for the surrounding neuronal and glial cells. Therefore, dampening astrocytes activated by IL-1β could be important in minimizing neurotoxicity. Because glutamate uptake has been shown to be inhibited by ROS in rodent astrocytes [35–37] and we have reported down-regulation of glutamate uptake by IL-1β in human astrocytes [32], in this study we sought to investigate the production of ROS in IL-1β + IFN-γ-activated human astrocytes and characterize the damage inflicted by ROS.
Materials and Methods
Reagents
The following reagents were purchased from the indicated sources: Dulbecco’s modified Eagle’s medium (DMEM), bovine serum albumin (BSA), Hanks’ balanced salts (HBSS), penicillin, streptomycin, trypsin, Tween 20, phosphate buffered saline (PBS), 3,3’-diaminobenzidine, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H–tetrazolium bromide (MTT), diphenyleneiodonium (DPI) (Sigma-Aldrich, St. Louis, MO); heat-inactivated fetal bovine serum (FBS, Hyclone, Logan, UT); 3H-glutamate (GE Healthcare, formerly Amersham Biosciences, Piscataway, NJ); acrylamide/bis-acrylamide gel and protein assay (Bio-Rad, Hercules, CA); gentamicin, Fungizone®, RNase inhibitor, SuperScript™ III reverse transcriptase, Hoechst 33342, MitoSOX Red mitochondrial superoxide indicator (Invitrogen, Carlsbad, CA); RNeasy mini kit (Qiagen, Valencia, CA); DNase (Ambion, Austin, TX); random hexmer and oligo (dT)12–18 (Gene Link, Hawthorne, NY); SYBR® Advantage® qPCR premix (ClonTech, Mountain View, CA); dNTPs (GE Healthcare, Piscataway, NJ); CDP-Star substrate (Applied Biosystems, Foster City, CA); anti-rabbit IgG-alkaline phosphatase (AP) conjugate (Promega, Madison, WI); human recombinant IL-1β and IFN-γ (R&D Systems, Minneapolis, MN); anti-p38, anti-extracellular signal-regulated kinase 1 and 2 (ERK1/2 or p44/42) MAPK, anti-Stat1(Tyr701) and (Ser727) and anti-β-actin antibodies (Cell Signaling, Beverly, MA); anti-SOD2 and anti-catalase antibodies (Santa Cruz Biotechnology, Santa Cruz, CA); H2DCFDA, SB203580 (an inhibitor of p38 MAPK), SB202474 (negative control of SB203580), U0126 (an inhibitor of MAP kinase [MEK]1/2, upstream of ERK1/2), U0124 (negative control of U0126) and NG-monomethyl-L-arginine (NGMA, inhibitor of nitric oxide synthase) (EMD Biosciences, La Jolla, CA); 8-isoprostane EIA kit (Cayman Chemical, Ann Arbor, MI).
Astrocyte cultures
Astrocytes were prepared from 16- to 22-week-old aborted human fetal brain tissues obtained under a protocol approved by the Human Subjects Research Committee at our institution. Brain tissues were dissociated and resuspended in DMEM containing FBS (6%), penicillin (100 U/ml), streptomycin (100 µg/ml), gentamicin (50 µg/ml) and Fungizone® (250 pg/ml) and plated onto poly-L-lysine (20 µg/ml)-coated 75-cm2 flasks at a density of 80–100 × 106 cells/flask and incubated in a 6% CO2 incubator. Culture medium was changed after 24h and at a weekly interval. On day 21, flasks were shaken at 180–200 rpm for 16h followed by trypsinization with 0.25% trypsin in HBSS for 30 min. After adding FBS (final concentration 10%), centrifugation and washing, cells were seeded into new flasks with DMEM, as shown above, followed by media change after 24h. The subculture procedure was repeated four times at a weekly interval to achieve highly purified astrocyte cultures (99% of cells stained positive with anti-GFAP antibody) which were plated onto 4-well chamber slide (2×104 cells/well) for staining or MitoSOX Red mitochondrial superoxide indicator, 12-well (106 cells/well) for RNA extraction or Western Blot, 24-well (1×105 cells/well) for 3H-glutamate uptake assay or 96-well (104 cells/well) plates for ROS production or 8-isoprostane assay. The number of cells plated for each culture vessel achieves 100% confluence. Two days after cells plating, experiments were initiated.
3H-Glutamate uptake
Previously, we have determined the 3H-glutamate concentration and optimal incubation time in astrocyte cultures [32] and adopted the procedure [38] to determine total 3H-glutamate uptake vs. nonspecific value. In this study, 3H-glutamate (specific activity: 50 Ci/mmol) 10 nM in DMEM was added to cell cultures in 24-well plates (1×105 cells/well, 300 µl DMEM) for 10 min at 37°C after treatment followed by washing with media and cell lysis with 2N NaOH. Cell lysates were added to scintillation cocktail to measure 3H-glutamate uptake activity. Values were adjusted to protein content.
ROS assay
After treatment, cell culture media were removed and cultures in 96-well plates (104 cells/well, 100 µl DMEM) were washed with HBSS (with Ca2+) once followed by incubation in H2DCFDA (20 µM in HBSS with Ca2+) for 45 min in the dark before the culture plate being read in a fluorescence plate reader (Molecular Devices, Sunnyvale, CA) at Ex485nm and Em538nm.
MitoSOX Red mitochondrial superoxide indicator
After treatment, MitoSox reagent was applied to astrocyte cultures on chamber slide (2×104 cells/well, 500 µl DMEM) and incubated for 10 min according to the manufacturer’s protocol. After washing cells were counterstained with Hoechst 33342, washed and mounted in warm buffer to be microphotographed under fluorescent microscope.
NO assay
After treatment, astrocyte culture supernatants from 96-well plates (104 cells/well, 100 µl DMEM) were collected to measure nitrite (NO2−) release using Griess reagent, which reflects NO production in cultures as previously described [34]. In brief, Griess reagent (freshly mixed before use) consisting of equal volumes of 0.1% naphthylenediamine dihydrochloride in distilled H2O and 1% sulfanilamide and 6% H3PO4 in distilled H2O, was added in equal volume to astrocyte culture supernatants. After 10 min incubation at room temperature, the mixtures were read with a microplate reader at 550 nm and NO2− level was extrapolated from a standard curve generated with a series of concentrations of sodium nitrite (NaNO2, 1–62.5 µM). The detection limit for NO2− was 0.5 µM.
Cell viability assay
To determine the effect of treatment on astrocyte viability a MTT assay, which provides quantitative assessment of mitochondrial integrity [transformation of tetrazolium salt via mitochondrial succinic dehydrogenases to formazan crystals (purple)], was used. After treatment of astrocytes MTT (final concentration of 1 mg/ml) was added to cell cultures for 4h followed by addition of lysis buffer (20% SDS [w/v] in 50% N,N-dimethyl formamide, pH 4.7, adjusted with 2.5% acetic acid and 1 N HCl [32:1]) for 16h. Cell lysate was collected and absorbance was read at 600 nm (Molecular Devices, Sunnyvale, CA) to reflect possible cytotoxicity caused by treatment. Another assay using trypan blue exclusion test, in which live cells with intact cell membranes exclude certain dyes like trypan blue while dead cells do not, was used to verify cell viability. Cell suspension was mixed with 0.4% trypan blue and examined under light microscope to visualize if blue cells (i.e. dead cells) were present.
Real-time polymerase chain reaction
Total RNA extracted from astrocytes in 12-well plates (106 cells/well, 600 µl DMEM) with RNeasy mini kit after treatment was treated with DNase and reverse transcribed to cDNA with oligo (dT)12–18, random hexmer, dNTPs, RNase inhibitor and SuperScript™ III reverse transcriptase. Mixtures of diluted cDNA, primers and SYBR® Advantage® qPCR premix were subjected to real-time PCR (Stratagene, La Jolla, CA) according to manufacture’s protocol. Primer sequences were sense 5’-TGGCACCCTTTTACACTGACATCC −3’ and antisense 5’-CTCCCACTAACATCACCACCTCATAG −3’ for NOX2 (gp91phox, 217 bp), sense 5’-ACCCTGTTGGATGACTGGAAAC −3’ and antisense 5’-CCCATCTGTTTGACTGAGGTACAG −3’ for NOX4 (180 bp), sense 5’-GAGCCTGCTGACTAAACTGGAGAT −3’ and antisense 5’-CCCGTGATGGAGTCTTTCTTCT −3’ for NOX5 (174 bp), sense 5’-TTCTGGACAAACCTCAGCCCTAAC −3’ and antisense 5’-CCTTATTGAAACCAAGCCAACC −3’ for SOD2 (MnSOD, 163 bp), sense 5’-CCTTTTTGCCTATCCTGACACTC −3’ and antisense 5’-GTTGAATCTCCGCACTTCTCCAG −3’ for catalase (238 bp), sense 5’-GACCTGCTGGATTACATCAAAGCACT −3’ and antisense 5’-CTTTGGATTATACTGCCTGACCAAGG −3’ for HPRT (hypoxanthine phosphoribosyltransferase, 240 bp).
Western Blot
Cell lysates collected from 12-well plates (106 cells/well, 600 µl DMEM) after treatment were electrophoresed in 12% acrylamide/bis-acrylamide, electrotransfered onto nitrocellulose membrane and probed with antibodies for SOD2, catalase, MAPKs (p38 or p44/42), Stat1 and β-actin followed by AP-conjugated secondary antibodies with chemiluminescence detection using image station (Carestream Health (formerly Kodak), New Heaven, CT).
8-isoprostane assay
After treatment, astrocyte culture supernatants from 96-well plates (104 cells/well, 100 µl DMEM) were collected for 8-isoprostane detection according to manufacturer’s protocol. Elevated level of 8-isoprostance was projected as oxidative stress marker.
Statistical analysis
Data are expressed as mean ± MSE as indicated. For comparison of means of multiple groups, analysis of variance (ANOVA) was used, followed by Tukey’s test.
Results
3H-glutamate uptake activity and ROS
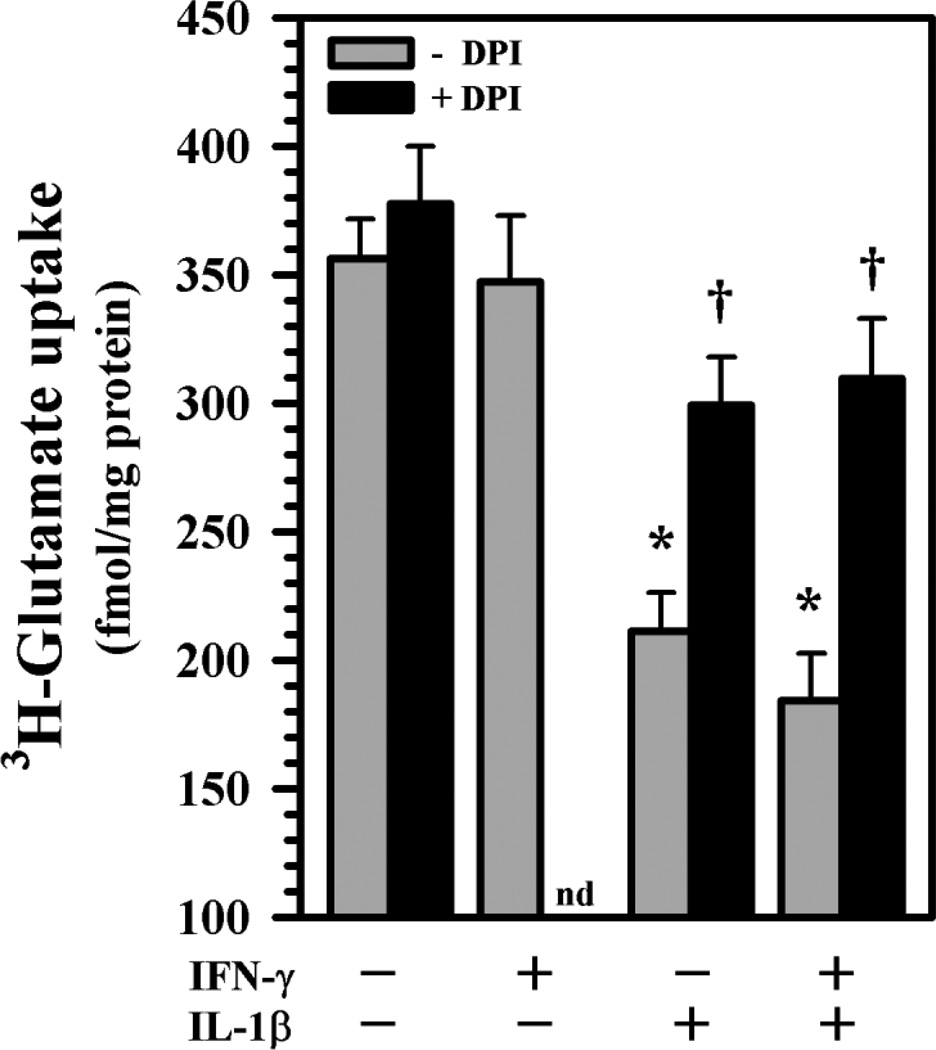
Reports of inhibition of glutamate uptake by ROS in rodent astrocytes [35–37] led us to conduct experiments using DPI, a classic but non-specific NOX inhibitor, prior to cytokine exposure to verify if ROS was involved in our human astrocyte cultures. We confirm that exposure to IL-1β ± IFN-γ exerts significant suppression of 3H-glutamate uptake activity in human astrocytes [32], while IFN-γ alone shows no effect (Fig. 1). Pretreatment with DPI produces a remarkable blockade of the suppression of 3H-glutamate uptake activity (Fig. 1) suggesting the involvement of ROS in IL-1β ± IFN-γ’s inhibitory effect. This observation led us to investigate ROS production by human astrocytes using the same treatment paradigm. In our astrocyte cultures, IL-1β ± IFN-γ treatment at 24h did not induce cell loss, reduced viability or apoptosis verified by trypan dye exclusion and MTT assay (data not shown).
Fig. 1.
3H-glutamate uptake activity in human astrocytes. Human astrocyte cultures in 24-well plates (1×105 cells/well, 300 µl DMEM) were untreated or pretreated with DPI (300 nM) for 3h prior to IL-1β ± IFN-γ exposure for 24h followed by addition of 3H-glutamate (10 nM) for 10 min before harvesting for quantification. Data presented are mean ± MSE of triplicates from four separate experiments using astrocytes derived from different brain tissue specimens. nd: not done; *p<0.05 vs. untreated control, †p<0.05 vs. IL-1β or IL-1β+IFN-γ correspondingly.
ROS production in human astrocytes
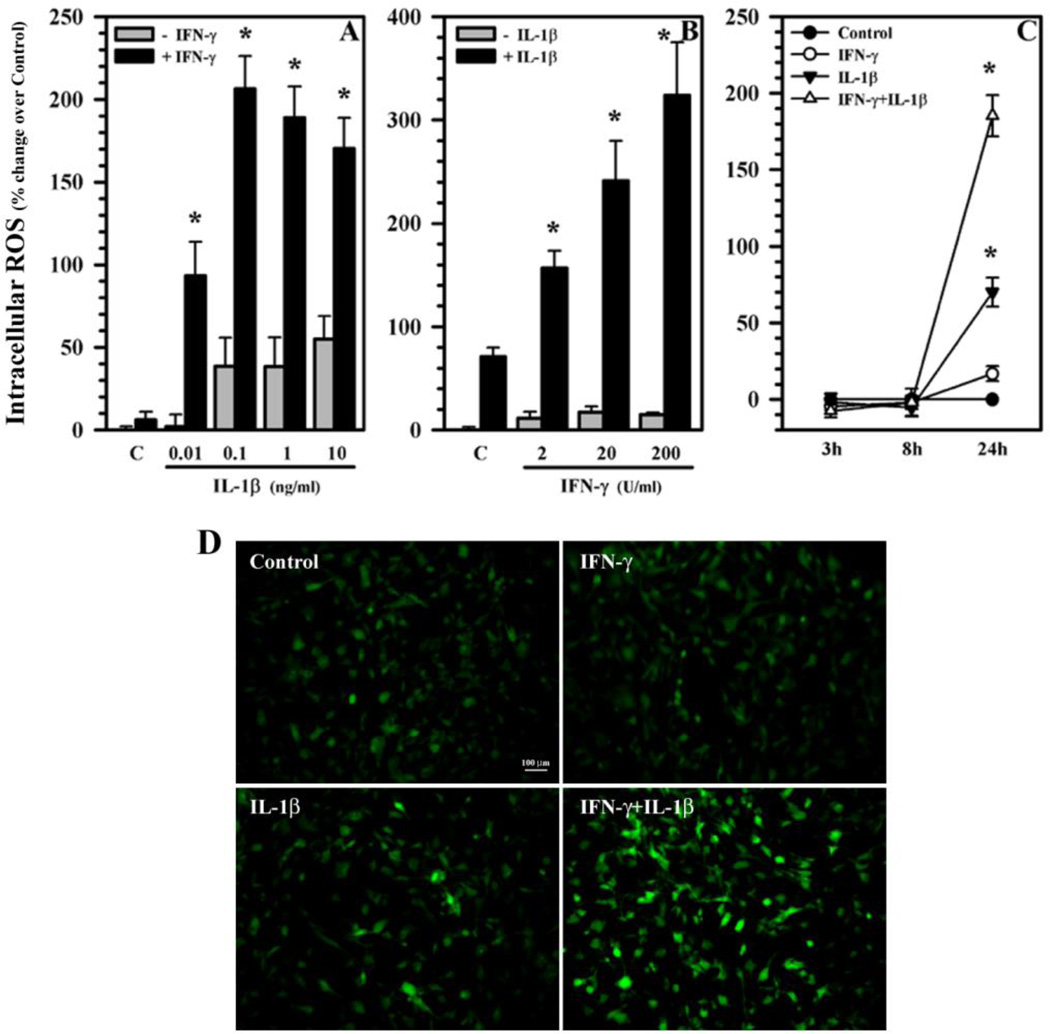
Human astrocytes treated with IL-1β alone (0.01 to 10 ng/ml) induce peak levels of ROS starting at 0.1 ng/ml (Fig. 2A) while minimal ROS induction is observed by IFN-γ treatment alone (Fig. 2A, B) at 24h. However, the combination of IL-1β and IFN-γ robustly potentiates ROS production (Fig. 2A, B). It appears that IFN-γ at 200 U/ml maximizes the potentiation of IL-1β-induced ROS at 0.1 ng/ml, while IL-1β at 10 ng/ml potentiates IFN-γ-induced ROS in a concentration-dependent manner (Fig. 2B). By the same token, levels of ROS production were undetectable for all treatments at 3h and 8h (Fig. 2C). By 24h, only IL-1β or IL-1β + IFN-γ-treated astrocytes show a marked increase of ROS (Fig. 2C). Therefore, the 24h treatment time was selected for the subsequent experiments.
Fig. 2.
ROS production in human astrocytes. Human astrocyte cultures in 96-well plates (104 cells/well, 100 µl DMEM) were untreated (C) or treated with either A) IL-1β (0.01 to 10 ng/ml) ± IFN-γ (200 U/ml); B) IFN-γ (2 to 200 U/ml) ± IL-1β (10 ng/ml) for 24h;C) IL-1β (10 ng/ml) alone or in combination with IFN-γ (200 U/ml) for 3, 8 and 24h; or D) IFN-γ (200 U/ml), IL-1β (10 ng/ml) or IL-1β + IFN-γ for 24h. After washing, cultures were incubated with H2DCFDA (20 µM) for 45 min and read at Ex485nm and Em538nm (A–C) or microphotographed under fluorescent microscope (D). Data presented are mean ± MSE of triplicates of three separate experiments using astrocytes derived from different brain tissue specimens. *p<0.05 vs. respective (A) IL-1β, (B) IFN-γ alone or (C) untreated control.
To microphotograph ROS production, astrocytes untreated or treated with IFN-γ, IL-1β or IL-1β + IFN-γ for 24h were washed and incubated with H2DCFDA (20 µM) followed by visualization under fluorescent microscope. Minimal ROS fluorescence is observed in untreated control or IFN-γ-treated cells, while IL-1β exposure induces enhanced ROS fluorescence (Fig. 2D). In IL-1β + IFN-γ-activated astrocytes, ROS production is dramatically increased, as demonstrated by enhanced fluorescence (Fig. 2D).
NADPH oxidase (NOXs) in astrocytes
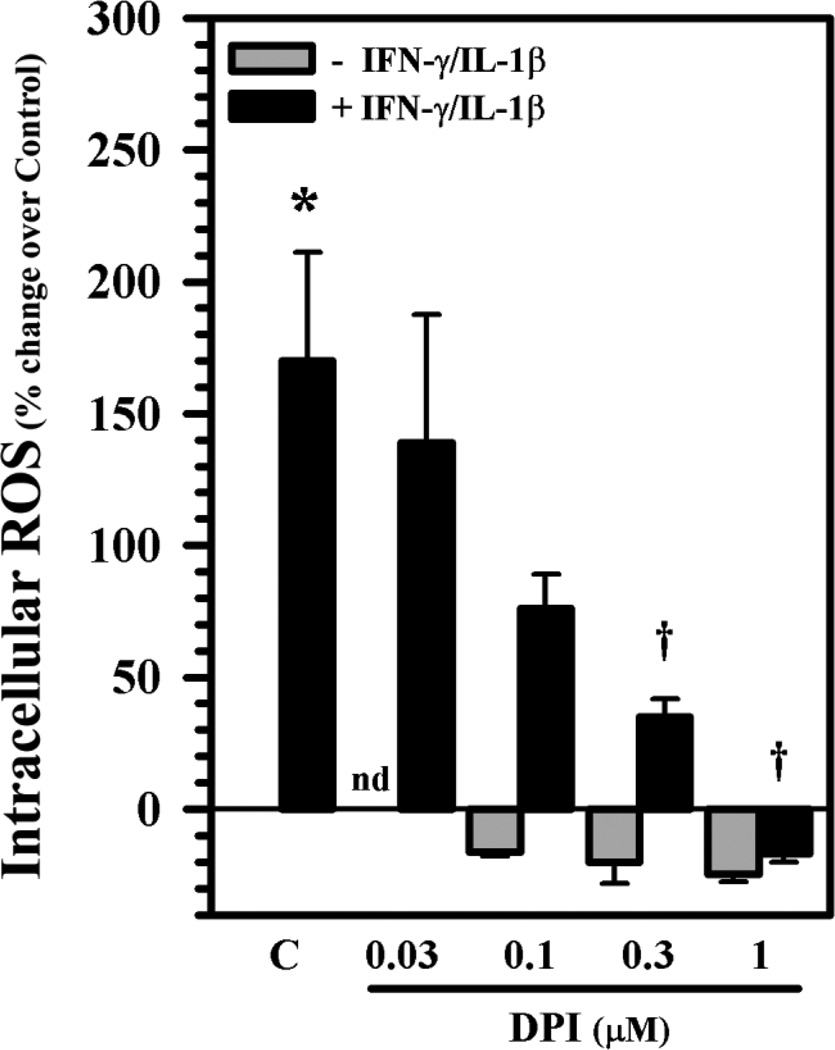
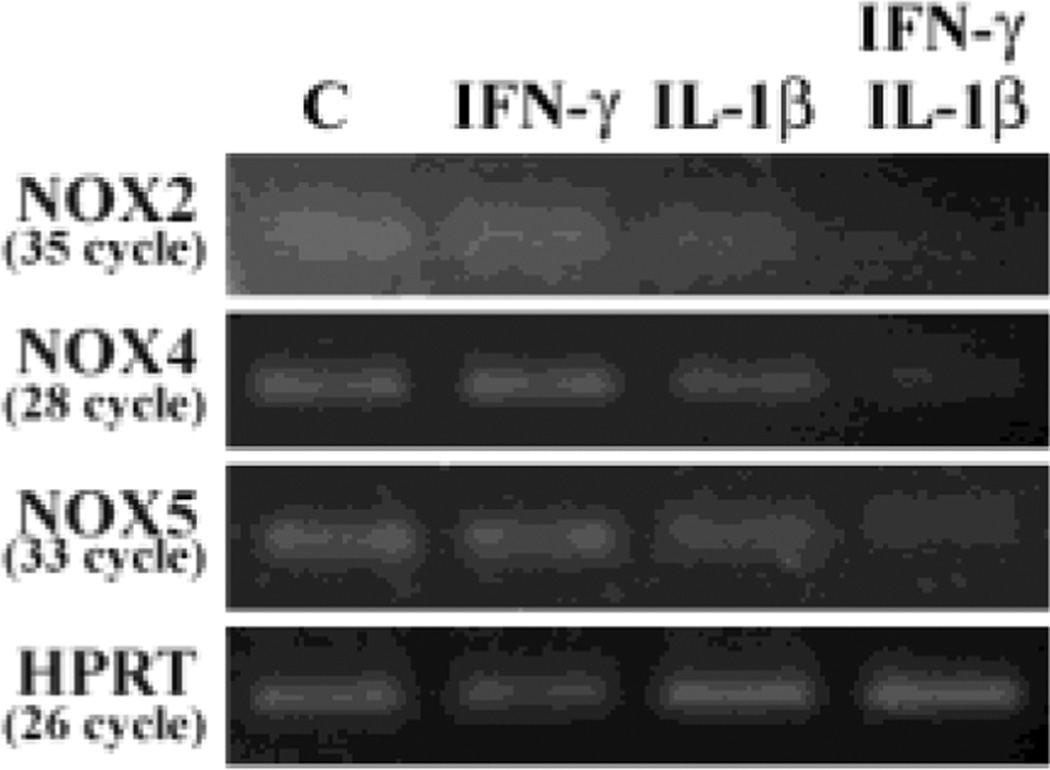
The plasma membrane- and membrane of phagosome-associated NOXs are the main sources of O2−. To verify whether NOXs play a role in ROS production, we first pretreated astrocytes with DPI overnight, followed by IL-1β + IFN-γ exposure for 24h. A robust blockade of ROS production by DPI is evident in a concentration-dependent fashion (Fig. 3). Cytotoxicity by DPI was not found at the concentrations used in these experiments as measured by trypan blue exclusion assay (data not shown). We then used real-time RT-PCR to assess expression of different NOX isoforms in human astrocytes and found medium NOX4 (Ct 24 ∼26) to moderate NOX5 (Ct 28 ∼ 30) to low NOX2 (Ct 30 ∼ 33) mRNA expression in astrocytes (Fig. 4). These results suggest that NOXs may contribute to ROS production in IL-1β ± IFN-γ-activated astrocytes.
Fig. 3.
Inhibition of ROS production in human astrocytes. Human astrocyte cultures in 96-well plates (104 cells/well, 100 µl DMEM) were untreated (C) or pretreated with DPI (0.03 – 1 µM) overnight prior to IL-1β (10 ng/ml) + IFN-γ (200 U/ml) exposure for 24h before being washed and incubated with H2DCFDA (20 µM) for ROS measurement. Data presented are mean ± MSE of triplicates of three separate experiments using astrocytes derived from different brain tissue specimens. nd: not done; *p<0.05 vs. untreated control, tp<0.05 vs. IL-1β + IFN-γ.
Fig. 4.
Expression of NOX isoforms in human astrocytes. After treatment total RNA (1 µg) collected from astrocyte cultures in 12-well plates (106 cells/well, 600 µl DMEM) was treated with DNase and reverse transcribed to cDNA followed by qPCR for NOX isoforms and housekeeping gene HPRT. PCR products were electrophoresed in 4% NuSieve 3:1 agarose gel and stained with ethidium bromide. Data presented are representative of three separated experiments using astrocytes derived from different brain tissue specimens.
Mitochondria and ROS production
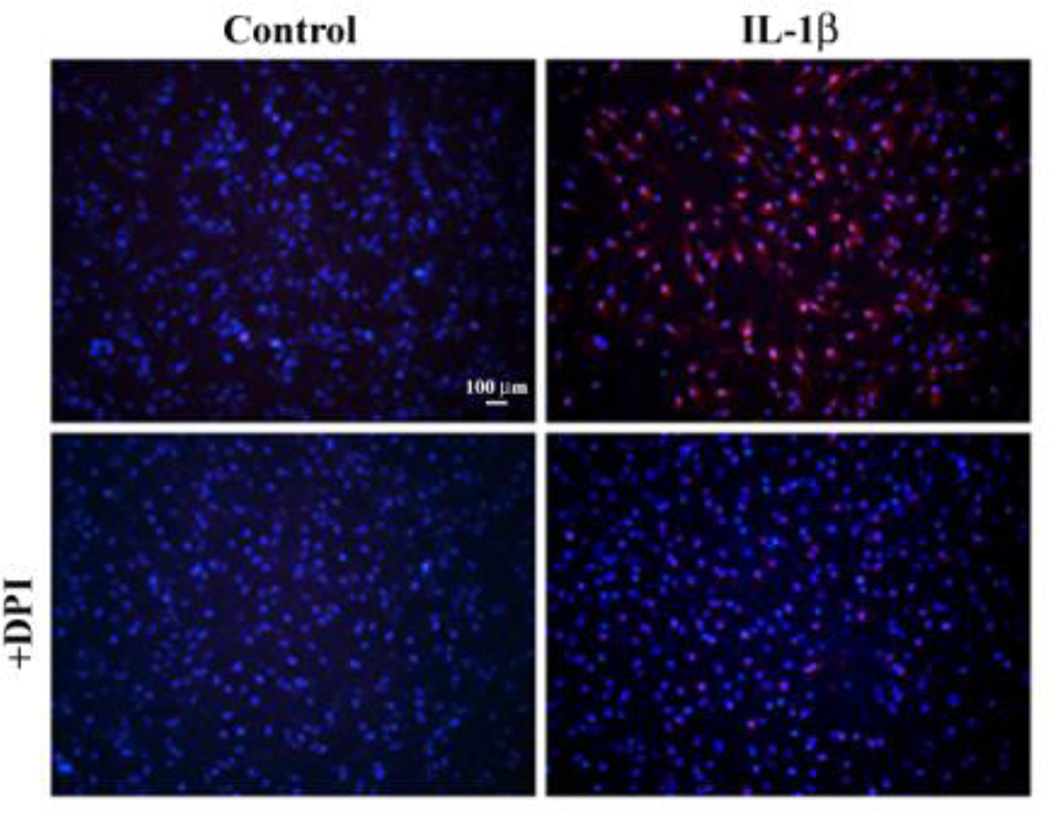
Mitochondria are the primary sources of energy and ATP for any cell via the electron transport chain, which generates ROS as a natural byproduct. We used the MitoSOX™ Red mitochondrial superoxide indicator to stain and image untreated (control) or DPI pretreated astrocyte cultures prior to IL-1 β exposure for 24h. Treatment with IL-1 β dramatically increases superoxide production (substantial red stain) (Fig. 5) while the untreated control or IFN-γ alone (data not shown) show minimal superoxide production (no positive red stain). Pretreatment with DPI appears to abolish the superoxide production (minimized to almost no red stain) suggesting the inhibitory action on flavoproteins in mitochondria. Similar findings in IL-1β + IFN-γ-activated astrocytes were also observed (data not shown). These results suggest that mitochondria also contribute to ROS release in IL-1β ± IFN-γ-activated astrocytes.
Fig. 5.
Mitochondrial ROS production in human astrocytes. Human astrocyte cultures in 4-well chamber slide (2×104 cells/well, 500 µl DMEM) were untreated (Control) or pretreated with DPI (1 µM) overnight prior to IL-1β (10 ng/ml) exposure for 24h before being stained with MitoSOX™ Red mitochondrial superoxide indicator. After washing, cells were counterstained with Hoechst 33342, washed and mounted in warm buffer to be microphotographed under a fluorescent microscope.
Effects of IL-1β and IFN-γ on antioxidant enzyme expression
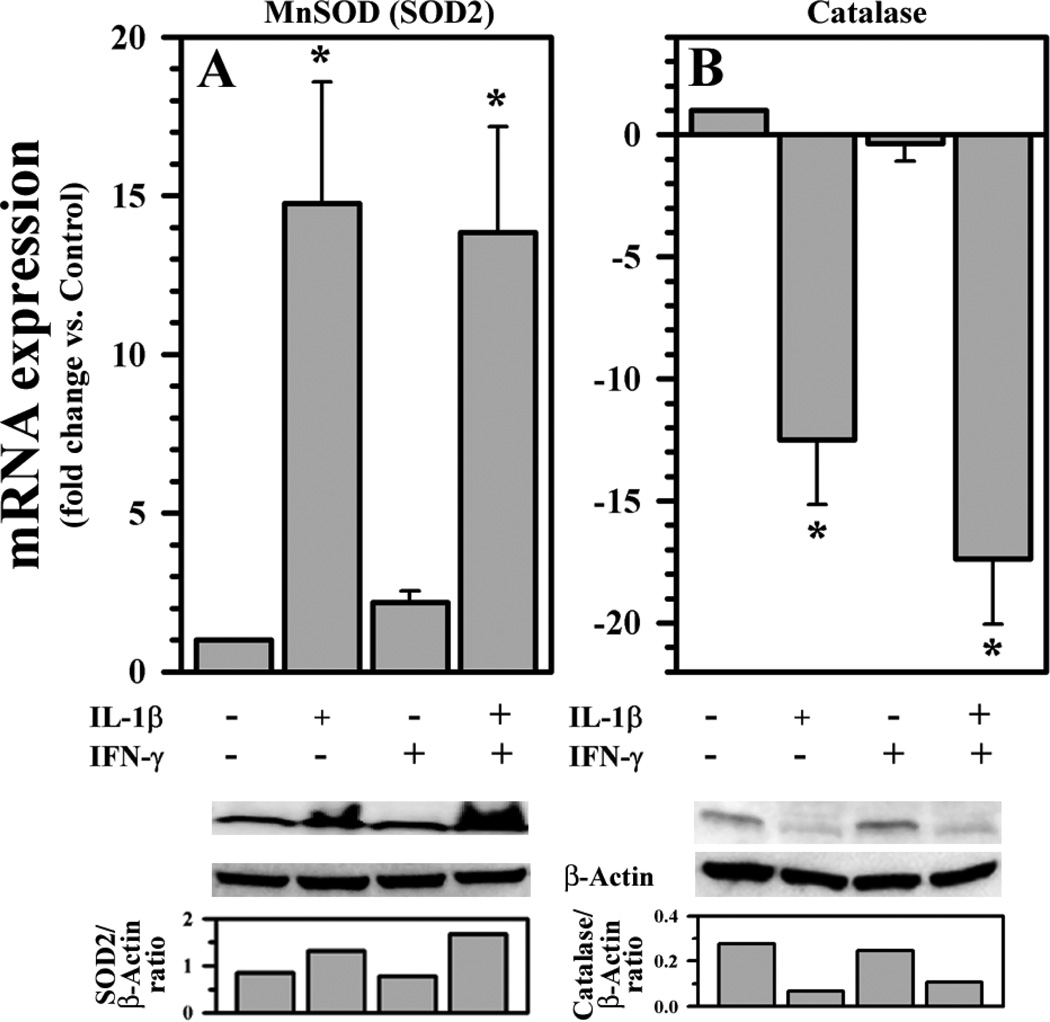
ROS production by activated human astrocytes led us to investigate whether antioxidant enzymes were affected by IL-1β ± IFN-γ treatment. We found that expression of superoxide dismutase 2 (SOD2 or MnSOD) is up-regulated and catalase (CAT) is down-regulated by IL-1β ± IFN-γ treatment, while there is no effect by IFN-γ alone (Fig. 6A, B). The result of increased MnSOD (SOD2) expression could be a response to increased superoxide production in mitochondria. With suppressed CAT expression, we speculate that H2O2 generated from MnSOD reaction may not be removed efficiently and its accumulation could impose further deleterious effects on astrocytes already under oxidative stress.
Fig. 6.
Effects of IL-1β and IFN-γ on antioxidant enzymes in human astrocytes. Total RNA (1 µg) or cell culture lysates collected from human astrocyte cultures in 12-well plates (106 cells/well, 600 µl DMEM) untreated or treated with IL-1β (10 ng/ml) ± IFN-γ (200 U/ml) for 24h were DNase treated and reverse transcribed to cDNA followed by qPCR or electrophoresed, transblotted and probed, respectively, to assess A) SOD2 and B) catalase expression. Densitometric measurement (normalized to β-actin) of the bands was shown. Data presented are mean ± MSE of three separate experiments using astrocytes derived from different brain tissue specimens. *p<0.05 vs. untreated control.
IL-1β and IFN-γ induced signaling pathways in human astrocytes
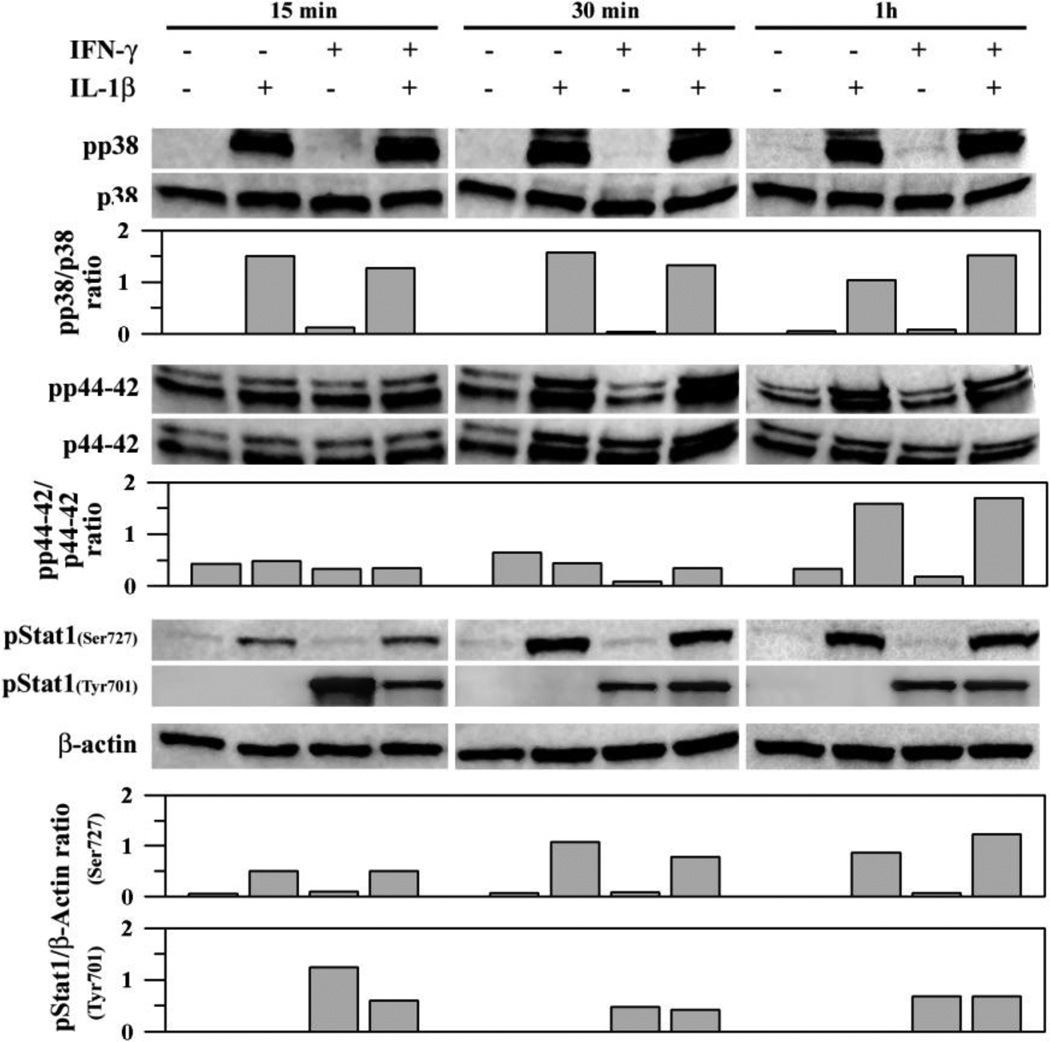
Many reports have demonstrated the involvement of ROS in MAPK pathways [5, 6, 15, 39]. In this study, we first investigated several signaling pathways in human astrocytes exposed to IL-1β ± IFN-γ. We found that within 15 min of IL-1β treatment, phosphorylation of p38, p44/42 MAPK and Stat1(Ser727) is induced and the activation continues for at least 1h (Fig. 7). There is no activation of these signaling pathways by IFN-y exposure alone, except that of pStat1(Tyr701), nor is there any potentiation of IL-1p–induced signaling by IFN-γ (Fig. 7). Quantitation and normalization of band density was performed for each target for visual assessment (Fig. 7).
Fig. 7.
IL-1β- and IFN-γ-induced signaling pathways in human astrocytes. Human astrocyte cultures in 12-well plates (106 cells/well, 600 µl DMEM) were untreated (as control) or treated with IL-1β (10 ng/ml) ± IFN-γ(200 U/ml) for 15, 30 and 60 min before collecting cell lysates to be electrophoresed, transblotted to nitrocellulose membrane and probed for p38 or p44/42 MAPK, Stat1 or β-actin (as internal control). Densitometric measurement (normalized to total p38 or p44/42 or β-actin) of the bands was shown. Data presented are representative of three to five separate experiments using astrocytes derived from different brain tissue specimens. All targets of the same experiment were electrophoresed and analyzed in individual blot without re-probing.
P38 MAPK in ROS production and glutamate uptake
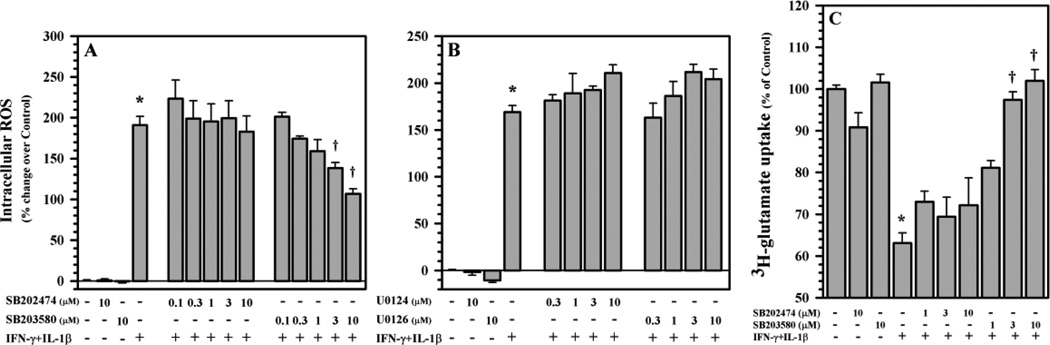
Since IFN-γ did not potentiate IL-1β-induced signaling, we then used inhibitors to analyze the signaling pathways involved in IL-1β + IFN-γ-induced ROS production. After pretreatment with SB203580 (p38 MAPK inhibitor), SB202474 (negative control of SB203580), U0126 (p44/42 MAPK inhibitor) and U0124 (negative control of U0126) prior to IL-1β + IFN-γ exposure, ROS production is suppressed only by the p38 MAPK inhibitor SB 203580 in a concentration-dependent fashion (Fig. 8A), while the p44/42 MAPK inhibitor U0126 shows no effect (Fig. 8B). This finding suggests that p38 MAPK is required for IL-1β + IFN-γ-induced ROS production in astrocytes. Using a similar pretreatment paradigm, SB203580 but not SB202474 reverses the inhibition on 3H-glutamate uptake activity (Fig. 8C) indicating that p38 MAPK mediates this inhibitory effect of IL-1β + IFN-γ.
Fig. 8.
Involvement of p38 MAPK in ROS production and glutamate uptake in human astrocytes. Human astrocyte cultures in 96-well plates (104 cells/well, 100 µl DMEM) were untreated (as control) or pretreated with A) SB203580 (p38 MAPK inhibitor) and SB202474 (as negative control) or B) U0126 (ERK1/2 MAPK inhibitor) and U0124 (as negative control) for 1h prior to IL-1β (10 ng/ml) + IFN-γ (200 U/ml) exposure for 24h before being washed and incubated with H2DCFDA (20 µM) for ROS measurement. C) Human astrocyte cultures in 24-well plates (1×105 cells/well, 300 µl DMEM) were untreated (as control) or pretreated with SB203580 (p38 MAPK inhibitor) and SB202474 (as negative control) for 1h prior to IL-1β (10 ng/ml) + IFN-γ (200 U/ml) exposure for 24h followed by addition of 3H-glutamate (10 nM) for 10 min before harvesting for quantification. Data presented are mean ± MSE of triplicates of three to five (A & B) and three (C) separate experiments using astrocytes derived from different brain tissue specimens. *p<0.05 vs. untreated control; †p<0.05 vs. IL-1β+IFN-γ.
Effects of IL-1β and IFN-γ on lipid peroxidation
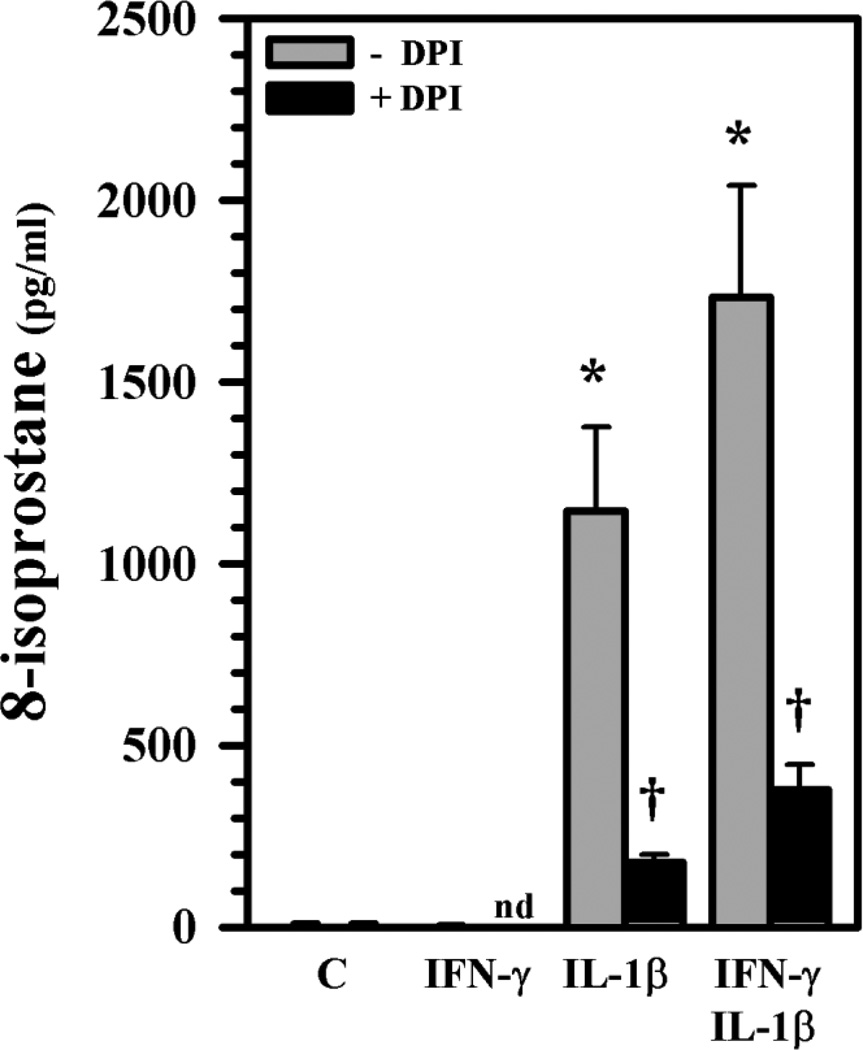
Lipid peroxidation is one of the end results of free-radical-induced injury and 8-isoprostane, a prostaglandin (PG)-F2-like compound (8-epi PGF2α) produced by peroxidation of arachidonic acid, has been shown to have biological activity [40]. Although 8-isoprostane is present in normal healthy subjects, an elevated 8-isoprostane level is a signal of oxidative stress. After exposure for 24h, 8-isoprostane levels in culture supernatants are markedly enhanced in IL-1β ± IFN-γ-treated human astrocytes and are significantly suppressed by pretreatment with DPI (Fig. 9), suggesting the contribution of ROS to the lipid peroxidation. Although no detectable NO was present at 24h by Griess reagent (detecting presence of nitrite indicative of NO production), we used the NOS inhibitor NGMA as pretreatment to see if NO might also play a role in 8-isoprostane production. No significant reduction of 8-isoprostane level was found (data not show), suggesting that NO is not involved in IL-1β ± IFN-γ-induced 8-isoprostane release in human astrocytes at 24h.
Fig. 9.
ROS-induced 8-isoprostane production in human astrocytes. Human astrocyte cultures in 96-well plates (104 cells/well, 100 µl DMEM) were untreated (C) or pretreated with DPI (1 µM) overnight prior to IL-1β ± IFN-γ exposure for 24h before harvesting culture supernatants for 8-isoprostane measurement. Data presented are mean ± MSE of triplicates of three separate experiments using astrocytes derived from different brain tissue specimens. nd: not done; *p<0.05 vs. untreated control; †p<0.05 vs. IL-1β or IL-1β+IFN-γ correspondingly.
Discussion
In coordination with antioxidant enzymes and molecules, ROS produced under normal condition can be managed and homeostasis can be maintained without triggering injury to the host. However, ROS can damage cells under conditions such as aging, inflammation, or other pathological circumstances. In the CNS, activated microglial cells, the resident macrophages of the brain, are the main source of phagocytic ROS production, primarily through NADPH-oxidase activity as demonstrated by studies using DPI or siRNA treatment and knock-out animal models [41–43]. Human astrocyte cell lines have also been shown to produce ROS through NADPH-oxidase activity [17, 18] while no ROS source was identified in astrocytes differentiated from human neural progenitor cells [19]. Recently, we reported induced heme oxygenase 1 expression in IL-1β-activated astrocytes is likely due to oxidative stress [44]. In this study IL-1β ± IFN-γ are able to induce ROS in astrocytes but not in primary microglial cells (unpublished observation) suggesting different regulatory mechanisms between these two types of glial cells. In IL-1β-activated astrocytes some IFN-stimulated genes (ISG) were induced and the phosphorylation and nuclear translocation of IFN regulatory factor 3 (IRF3) and the expression of IFN-β and chemokines (CXCL10 and CCL5) were demonstrated in IL-1β signaling [31]. Also, IFN-γ might prime astrocytes for heightened responses to IL-1β as it was reported in macrophages exposed to LPS [45]. These effects might reflect on the exacerbated ROS production in IL-1β + IFN-γ compared to IL-1β exposure alone in this study.
Previously, mRNA expression of NOX4 was demonstrated in primary human astrocytes [21] and NOX4 was also found to localize to mitochondria [46]. Here we found medium to moderate to low expression of NOX isoforms NOX4, NOX5 and NOX2, respectively, in human astrocytes (NOX1 and NOX3 were undetectable) suggesting that NADPH-oxidase may contribute to ROS production in IL-1β ± IFN-γ-activated astrocytes.
Mitochondrial ROS formation is a natural product and can be potentiated by exogenous factors such as oxidants [47]. Our results show enhanced superoxide production in mitochondria by IL-1β ± IFN-γ activation, which is abated by DPI, since flavoproteins are present in mitochondria. Involvement of other processes in ROS production, such as free iron, cyclooxygenase and monoamine oxidase, however, cannot be ruled out. Future experiments using specific inhibitors may clarify the contribution of these processes to ROS generation in activated human astrocytes. With enhanced superoxide production, increased MnSOD expression and suppressed catalase expression found in this study, it is logical to speculate that H2O2 formed may not be removed promptly and its accumulation could be damaging; an example of this includes inhibition of glutamate uptake in rodent astrocytes [35–37]. Others have shown increased H2O2 production with enhanced MnSOD activity [48–50]. However, one must bear in mind that catalase is not the only antioxidant molecule capable of removing H2O2, as glutathione peroxidases and peroxiredoxins are also able to convert H2O2 to water [8]. Although we did not find glutathione levels to be affected until 72h, we did observe that mRNA expression of enzymes involved in glutathione synthesis and recycling were suppressed to an extent in IL-1β ± IFN-γ-activated astrocytes (unpublished observation).
Reports have shown that there is an interaction between MAPK signaling and ROS [5, 15, 39, 51] and both are required in normal physiological and biological processes. Phosphorylation of p38 and p44/42 MAPK within 15 min seems to occur much earlier than the H2DCFDA-detectable ROS at 24h and the ROS production was dampened only by the p38 inhibitor SB203580, but not by the p44/42 MAPK inhibitor U0126 in IL-1β + IFN-γ-activated astrocytes. Therefore, activation of p38 MAPK appears to be required for ROS production in IL-1β + IFN-γ-activated astrocytes. Results showing the reversal of glutamate uptake suppression by p38 inhibitor SB203580 further supported the belief of p38 MAPK activation playing a role in ROS production leading to the inhibitory effect of IL-1β + IFN-γ on 3H-glutamate uptake of astrocytes.
Elevated 8-isoprostane levels from lipid peroxidation is considered an indicator of ROS damage [52] and increased 8-isoprostane levels in serum correlate negatively with pulmonary function in patients with systemic sclerosis [53]. In rat astrocyte cultures, isoprostane levels increase by many fold after mechanical trauma or injury by free radicals [33]. Because 8-isoprostane exerts biological functions such as vasoconstriction and reducing blood flow [40], the increased isoprostane levels in injured astrocytes might be deleterious to the nearby cerebral vessels [33]. Stimulation of 8-isoprostane production in IL-1β ± IFN-γ-activated astrocytes and its suppression by DPI, but not by the NOS inhibitor NGMA, further implicates ROS production in activated human astrocytes as a source of lipid peroxidation.
Previously, we have shown induction of apoptosis and inhibition of glutamate transporter expression/activity and glutamine synthetase activity in human astrocytes exposed to IL-1β [28, 32, 54]. It was also reported that increased mitochondrial ROS in CA1 astrocytes contributed to the loss of glutamate transporter activity leading to CA1 neuronal injury [55]. These results, together with the finding of increased lipid peroxidation in this study, suggest that ROS production in activated astrocytes may either set the stage for functional impairment of astrocytes indirectly leading to neuronal injury, or possibly lead to direct damage to the surrounding neurons.
In summary, we have demonstrated that the inflammatory cytokines IL-1β and IL-1β + IFN-γ induce ROS in human astrocytes and that NADPH oxidase and mitochondria may contribute to ROS production. A hallmark of ROS damage in IL-1β ± IFN-γ-activated astrocytes is the significantly suppressed 3H-glutamate uptake and elevated 8-isoprostane level indicative of oxidative stress-induced lipid peroxidation, which may have deleterious consequences for the surrounding cells and tissues. With ROS being shown to contribute to many neurodegenerative diseases and astrocytes being capable of ROS production it will be vital to maintain astrocyte function and integrity at a homeostasis state to be neuroprotective. Further detailed studies are needed to study the downstream effects of ROS generated by IL-1β ± IFN-γ-activated human astrocytes on neurotoxicity.
Acknowledgement
We thank Dr. Phillip K. Peterson for guidance. This study was supported in part by United States Public Health Service Grants DA00924 and DA025525.
References
- 1.Patten DA, Germain M, Kelly MA, Slack RS. Reactive oxygen species: stuck in the middle of neurodegeneration. J Alzheimers Dis. 2010;20(Suppl 2):S357–S367. doi: 10.3233/JAD-2010-100498. [DOI] [PubMed] [Google Scholar]
- 2.Tabner BJ, Turnbull S, El-Agnaf O, Allsop D. Production of reactive oxygen species from aggregating proteins implicated in Alzheimer's disease, Parkinson's disease and other neurodegenerative diseases. Curr Top Med Chem. 2001;1:507–517. doi: 10.2174/1568026013394822. [DOI] [PubMed] [Google Scholar]
- 3.Henkel JS, Beers DR, Zhao W, Appel SH. Microglia in ALS: the good, the bad, and the resting. J Neuroimmune Pharmacol. 2009;4:389–398. doi: 10.1007/s11481-009-9171-5. [DOI] [PubMed] [Google Scholar]
- 4.Sacktor N, Haughey N, Cutler R, Tamara A, Turchan J, Pardo C, Vargas D, Nath A. Novel markers of oxidative stress in actively progressive HIV dementia. J Neuroimmunol. 2004;157:176–184. doi: 10.1016/j.jneuroim.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 5.Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y, Suda T. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 6.Guyton KZ, Liu Y, Gorospe M, Xu Q, Holbrook NJ. Activation of mitogen-activated protein kinase by H2O2. Role in cell survival following oxidant injury. J Biol Chem. 1996;271:4138–4142. doi: 10.1074/jbc.271.8.4138. [DOI] [PubMed] [Google Scholar]
- 7.Lo YY, Wong JM, Cruz TF. Reactive oxygen species mediate cytokine activation of c-Jun NH2-terminal kinases. J Biol Chem. 1996;271:15703–15707. doi: 10.1074/jbc.271.26.15703. [DOI] [PubMed] [Google Scholar]
- 8.Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012;48:158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altenhofer S, Kleikers PW, Radermacher KA, Scheurer P, Rob Hermans JJ, Schiffers P, Ho H, Wingler K, Schmidt HH. The NOX toolbox: validating the role of NADPH oxidases in physiology and disease. Cell Mol Life Sci. 2012;69:2327–2343. doi: 10.1007/s00018-012-1010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 11.Nathan C, Cunningham-Bussel A. Beyond oxidative stress: an immunologist's guide to reactive oxygen species. Nat Rev Immunol. 2013;13:349–361. doi: 10.1038/nri3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qian L, Gao X, Pei Z, Wu X, Block M, Wilson B, Hong JS, Flood PM. NADPH oxidase inhibitor DPI is neuroprotective at femtomolar concentrations through inhibition of microglia over-activation. Parkinsonism Relat Disord. 2007;13(Suppl 3):S316–S320. doi: 10.1016/S1353-8020(08)70023-3. [DOI] [PubMed] [Google Scholar]
- 14.Cheret C, Gervais A, Lelli A, Colin C, Amar L, Ravassard P, Mallet J, Cumano A, Krause KH, Mallat M. Neurotoxic activation of microglia is promoted by a nox1-dependent NADPH oxidase. J Neurosci. 2008;28:12039–12051. doi: 10.1523/JNEUROSCI.3568-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu S, Sheng WS, Schachtele SJ, Lokensgard JR. Reactive oxygen species drive herpes simplex virus (HSV)-1-induced proinflammatory cytokine production by murine microglia. J Neuroinflammation. 2011;8:123. doi: 10.1186/1742-2094-8-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams R, Yao H, Peng F, Yang Y, Bethel-Brown C, Buch S. Cooperative induction of CXCL10 involves NADPH oxidase: Implications for HIV dementia. Glia. 2010;58:611–621. doi: 10.1002/glia.20949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mishra MK, Kumawat KL, Basu A. Japanese encephalitis virus differentially modulates the induction of multiple pro-inflammatory mediators in human astrocytoma and astroglioma cell-lines. Cell Biol Int. 2008;32:1506–1513. doi: 10.1016/j.cellbi.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Song HY, Ryu J, Ju SM, Park LJ, Lee JA, Choi SY, Park J. Extracellular HIV-1 Tat enhances monocyte adhesion by up-regulation of ICAM-1 and VCAM-1 gene expression via ROS-dependent NF-kappaB activation in astrocytes. Exp Mol Med. 2007;39:27–37. doi: 10.1038/emm.2007.4. [DOI] [PubMed] [Google Scholar]
- 19.Sharma V, Mishra M, Ghosh S, Tewari R, Basu A, Seth P, Sen E. Modulation of interleukin-1beta mediated inflammatory response in human astrocytes by flavonoids: implications in neuroprotection. Brain Res Bull. 2007;73:55–63. doi: 10.1016/j.brainresbull.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Zhu D, Hu C, Sheng W, Tan KS, Haidekker MA, Sun AY, Sun GY, Lee JC. NAD(P)H oxidase-mediated reactive oxygen species production alters astrocyte membrane molecular order via phospholipase A2. Biochem J. 2009;421:201–210. doi: 10.1042/BJ20090356. [DOI] [PubMed] [Google Scholar]
- 21.Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 22.Beak SM, Lee YS, Kim JA. NADPH oxidase and cyclooxygenase mediate the ultraviolet B-induced generation of reactive oxygen species and activation of nuclear factor-kappaB in HaCaT human keratinocytes. Biochimie. 2004;86:425–429. doi: 10.1016/j.biochi.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Trush MA. Diphenyleneiodonium, an NAD(P)H oxidase inhibitor, also potently inhibits mitochondrial reactive oxygen species production. Biochem Biophys Res Commun. 1998;253:295–299. doi: 10.1006/bbrc.1998.9729. [DOI] [PubMed] [Google Scholar]
- 24.Cross AR, Jones OT. The effect of the inhibitor diphenylene iodonium on the superoxide-generating system of neutrophils. Specific labelling of a component polypeptide of the oxidase. Biochem J. 1986;237:111–116. doi: 10.1042/bj2370111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugiyama K, Brunori A, Mayer ML. Glial uptake of excitatory amino acids influences neuronal survival in cultures of mouse hippocampus. Neuroscience. 1989;32:779–791. doi: 10.1016/0306-4522(89)90298-4. [DOI] [PubMed] [Google Scholar]
- 26.Lee SC, Dickson DW, Liu W, Brosnan CF. Induction of nitric oxide synthase activity in human astrocytes by interleukin-1 beta and interferon-gamma. J Neuroimmunol. 1993;46:19–24. doi: 10.1016/0165-5728(93)90229-r. [DOI] [PubMed] [Google Scholar]
- 27.Hu S, Sheng WS, Peterson PK, Chao CC. Differential regulation by cytokines of human astrocyte nitric oxide production. Glia. 1995;15:491–494. doi: 10.1002/glia.440150412. [DOI] [PubMed] [Google Scholar]
- 28.Ehrlich LC, Peterson PK, Hu S. Interleukin (IL)-1beta-mediated apoptosis of human astrocytes. Neuroreport. 1999;10:1849–1852. doi: 10.1097/00001756-199906230-00009. [DOI] [PubMed] [Google Scholar]
- 29.Thornton P, Pinteaux E, Gibson RM, Allan SM, Rothwell NJ. Interleukin-1-induced neurotoxicity is mediated by glia and requires caspase activation and free radical release. J Neurochem. 2006;98:258–266. doi: 10.1111/j.1471-4159.2006.03872.x. [DOI] [PubMed] [Google Scholar]
- 30.Lee SC, Liu W, Dickson DW, Brosnan CF, Berman JW. Cytokine production by human fetal microglia and astrocytes. Differential induction by lipopolysaccharide and IL-1 beta. J Immunol. 1993;150:2659–2667. [PubMed] [Google Scholar]
- 31.Rivieccio MA, John GR, Song X, Suh HS, Zhao Y, Lee SC, Brosnan CF. The cytokine IL-1beta activates IFN response factor 3 in human fetal astrocytes in culture. J Immunol. 2005;174:3719–3726. doi: 10.4049/jimmunol.174.6.3719. [DOI] [PubMed] [Google Scholar]
- 32.Hu S, Sheng WS, Ehrlich LC, Peterson PK, Chao CC. Cytokine effects on glutamate uptake by human astrocytes. Neuroimmunomodulation. 2000;7:153–159. doi: 10.1159/000026433. [DOI] [PubMed] [Google Scholar]
- 33.Hoffman SW, Rzigalinski BA, Willoughby KA, Ellis EF. Astrocytes generate isoprostanes in response to trauma or oxygen radicals. J Neurotrauma. 2000;17:415–420. doi: 10.1089/neu.2000.17.415. [DOI] [PubMed] [Google Scholar]
- 34.Chao CC, Hu S, Sheng WS, Bu D, Bukrinsky MI, Peterson PK. Cytokine-stimulated astrocytes damage human neurons via a nitric oxide mechanism. Glia. 1996;16:276–284. doi: 10.1002/(SICI)1098-1136(199603)16:3<276::AID-GLIA10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 35.Piani D, Frei K, Pfister HW, Fontana A. Glutamate uptake by astrocytes is inhibited by reactive oxygen intermediates but not by other macrophage-derived molecules including cytokines, leukotrienes or platelet-activating factor. J Neuroimmunol. 1993;48:99–104. doi: 10.1016/0165-5728(93)90063-5. [DOI] [PubMed] [Google Scholar]
- 36.Volterra A, Trotti D, Tromba C, Floridi S, Racagni G. Glutamate uptake inhibition by oxygen free radicals in rat cortical astrocytes. J Neurosci. 1994;14:2924–2932. doi: 10.1523/JNEUROSCI.14-05-02924.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sorg O, Horn TF, Yu N, Gruol DL, Bloom FE. Inhibition of astrocyte glutamate uptake by reactive oxygen species: role of antioxidant enzymes. Mol Med. 1997;3:431–440. [PMC free article] [PubMed] [Google Scholar]
- 38.Sher PK, Hu SX. Increased glutamate uptake and glutamine synthetase activity in neuronal cell cultures surviving chronic hypoxia. Glia. 1990;3:350–357. doi: 10.1002/glia.440030506. [DOI] [PubMed] [Google Scholar]
- 39.McCubrey JA, Lahair MM, Franklin RA. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid Redox Signal. 2006;8:1775–1789. doi: 10.1089/ars.2006.8.1775. [DOI] [PubMed] [Google Scholar]
- 40.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A. (2nd) 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Block ML, Li G, Qin L, Wu X, Pei Z, Wang T, Wilson B, Yang J, Hong JS. Potent regulation of microglia-derived oxidative stress and dopaminergic neuron survival: substance P vs. dynorphin. Faseb J. 2006;20:251–258. doi: 10.1096/fj.05-4553com. [DOI] [PubMed] [Google Scholar]
- 42.Huo Y, Rangarajan P, Ling EA, Dheen ST. Dexamethasone inhibits the Nox-dependent ROS production via suppression of MKP-1-dependent MAPK pathways in activated microglia. BMC Neurosci. 2011;12:49. doi: 10.1186/1471-2202-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dohi K, Ohtaki H, Nakamachi T, Yofu S, Satoh K, Miyamoto K, Song D, Tsunawaki S, Shioda S, Aruga T. Gp91phox (NOX2) in classically activated microglia exacerbates traumatic brain injury. J Neuroinflammation. 2010;7:41. doi: 10.1186/1742-2094-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheng WS, Hu S, Nettles AR, Lokensgard JR, Vercellotti GM, Rock RB. Hemin inhibits NO production by IL-1beta-stimulated human astrocytes through induction of heme oxygenase-1 and reduction of p38 MAPK activation. J Neuroinflammation. 2010;7:51. doi: 10.1186/1742-2094-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 46.Block K, Gorin Y, Abboud HE. Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci U S A. 2009;106:14385–14390. doi: 10.1073/pnas.0906805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jou MJ. Pathophysiological and pharmacological implications of mitochondria-targeted reactive oxygen species generation in astrocytes. Adv Drug Deliv Rev. 2008;60:1512–1526. doi: 10.1016/j.addr.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 48.Wenk J, Brenneisen P, Wlaschek M, Poswig A, Briviba K, Oberley TD, Scharffetter-Kochanek K. Stable overexpression of manganese superoxide dismutase in mitochondria identifies hydrogen peroxide as a major oxidant in the AP-1-mediated induction of matrix-degrading metalloprotease-1. J Biol Chem. 1999;274:25869–25876. doi: 10.1074/jbc.274.36.25869. [DOI] [PubMed] [Google Scholar]
- 49.Rodriguez AM, Carrico PM, Mazurkiewicz JE, Melendez JA. Mitochondrial or cytosolic catalase reverses the MnSOD-dependent inhibition of proliferation by enhancing respiratory chain activity, net ATP production, and decreasing the steady state levels of H(2)O(2) Free Radic Biol Med. 2000;29:801–813. doi: 10.1016/s0891-5849(00)00362-2. [DOI] [PubMed] [Google Scholar]
- 50.Ranganathan AC, Nelson KK, Rodriguez AM, Kim KH, Tower GB, Rutter JL, Brinckerhoff CE, Huang TT, Epstein CJ, Jeffrey JJ, Melendez JA. Manganese superoxide dismutase signals matrix metalloproteinase expression via H2O2-dependent ERK1/2 activation. J Biol Chem. 2001;276:14264–14270. doi: 10.1074/jbc.M100199200. [DOI] [PubMed] [Google Scholar]
- 51.Torres M. Mitogen-activated protein kinase pathways in redox signaling. Front Biosci. 2003;8:d369–d391. doi: 10.2741/999. [DOI] [PubMed] [Google Scholar]
- 52.Morrow JD. The isoprostanes - unique products of arachidonate peroxidation: their role as mediators of oxidant stress. Curr Pharm Des. 2006;12:895–902. doi: 10.2174/138161206776055985. [DOI] [PubMed] [Google Scholar]
- 53.Ogawa F, Shimizu K, Muroi E, Hara T, Hasegawa M, Takehara K, Sato S. Serum levels of 8-isoprostane, a marker of oxidative stress, are elevated in patients with systemic sclerosis. Rheumatology (Oxford) 2006;45:815–818. doi: 10.1093/rheumatology/kel012. [DOI] [PubMed] [Google Scholar]
- 54.Chao CC, Hu S, Ehrlich L, Peterson PK. Interleukin-1 and tumor necrosis factor-alpha synergistically mediate neurotoxicity: involvement of nitric oxide and of N-methyl-D-aspartate receptors. Brain Behav Immun. 1995;9:355–365. doi: 10.1006/brbi.1995.1033. [DOI] [PubMed] [Google Scholar]
- 55.Ouyang YB, Voloboueva LA, Xu LJ, Giffard RG. Selective dysfunction of hippocampal CA1 astrocytes contributes to delayed neuronal damage after transient forebrain ischemia. J Neurosci. 2007;27:4253–4260. doi: 10.1523/JNEUROSCI.0211-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]