Abstract
Overexpression of Bcl-2 family proteins has been found in a variety of aggressive human carcinomas, including pancreatic cancer, suggesting that specific agents targeting Bcl-2 family proteins would be valuable for pancreatic cancer therapy. We have previously reported that TW-37, a small-molecule inhibitor of Bcl-2 family proteins, inhibited cell growth and induced apoptosis in pancreatic cancer. However, the precise role and the molecular mechanism of action of TW-37 have not been fully elucidated. In our current study, we found that TW-37 induces cell growth inhibition and S-phase cell cycle arrest, with regulation of several important cell cycle–related genes like p27, p57, E2F-1, cdc25A, CDK4, cyclin A, cyclin D1, and cyclin E. The cell growth inhibition was accompanied by increased apoptosis with concomitant attenuation of Notch-1, Jagged-1, and its downstream genes such as Hes-1 in vitro and in vivo. We also found that down-regulation of Notch-1 by small interfering RNA or γ-secretase inhibitors before TW-37 treatment resulted in enhanced cell growth inhibition and apoptosis. Our data suggest that the observed antitumor activity of TW-37 is mediated through a novel pathway involving inactivation of Notch-1 and Jagged-1.
Introduction
Pancreatic cancer remains one of the most aggressive cancers with a very poor prognosis. More than 33,000 patients die of this deadly disease every year in the United States (1). The vast majority of patients present with gross metastases or micrometastases requiring effective drug therapies. However, conventional chemotherapy has shown only a minimal survival benefit when combined with surgical resection (1). This disappointing outcome suggests that new and alternative approaches to the control of cancer are critically needed. Pancreatic cancer has been shown to overexpress Bcl-2 and its family members (2–4). Therefore, blockade of Bcl-2 activity should become a novel therapeutic strategy for pancreatic cancer. Many groups have been working to develop anticancer drugs that block the function of Bcl-2 members (3, 5–7). TW-37, a recently developed small-molecule inhibitor of Bcl-2, targets multiple members of the Bcl-2 family and attenuates activation of Bcl-2. TW-37 was designed to target the elongated groove of antiapoptotic proteins that normally bind the BH3 domain of proapoptotic effectors such as Bid, Bax, Bim, and others (8). We have found that TW-37 inhibits the growth of a variety of cancer cells, including breast, prostate, lymphoma, and pancreatic cancer (8–11). However, the exact mechanism of action of TW-37 as an antitumor agent has not yet been fully established.
It is well documented that Bcl-2 functions through heterodimerization with proapoptotic members of the Bcl-2 family to prevent mitochondrial pore formation and prevent cytochrome c release and initiation of apoptosis (12). However, there are more evidences showing that Bcl-2 may play an oncogenic role through survival pathways other than its function at the mitochondrial membrane. It has been reported that Bcl-2 activates nuclear factor-κB (NF-κB) by a signaling mechanism that involves Raf-1/MEKK-1–mediated activation of IKKβ (13). Mortenson and colleagues have shown that overexpression of Bcl-2 increased the activity of AKT and IKK as well as NF-κB transcriptional activity in pancreatic cancer (2). Kumar and colleagues found that Bcl-2–induced tumor cell proliferation and tumor cell invasion were significantly mediated by interleukin-8 (14). Recently, Tucker and colleagues reported that Bcl-2 overexpression leading to maintenance of cyclin D1a expression may occur through p38 mitogen-activated protein kinase (MAPK)–mediated signaling pathways in human lymphoma cell lines (15). Moreover, down-regulation of Bcl-2 also could modulate the expression of anhydrase IX (CAIX), vascular endothelial growth factor (VEGF), and pAkt in prostate cancer cell lines (16). In addition, Bcl-2 induces VEGF expression in neovascular endothelial cells through a signal transducer and activator of transcription 3 (STAT3)–mediated pathway (17). These results provide evidence in support of the new functions of Bcl-2 in cancer biology that is beyond its classic role in cell survival (17).
Because Notch signaling also plays important roles in the cellular developmental pathway, including proliferation and apoptosis, alterations in Notch signaling are associated with tumorigenesis (18). Notch-1 has been reported to cross-talk with other pathways, such as AKT and NF-κB (19–22). Thus, given the potential role for Bcl-2 in regulating NF-κB and the known pathway from Notch to NF-κB, we hypothesized that overexpression of Bcl-2 could lead to the activation of Notch signaling pathway in pancreatic cancer and, as such, these pathways would be targeted by the Bcl-2 inhibitor TW-37. Thus, in the present study, we investigated whether TW-37–induced inhibition of pancreatic cancer cell growth could be attributed to Bcl-2 activity and its associated signaling, especially inactivation of Notch-1 activity. We found that the inactivation of Bcl-2 by TW-37 down-regulated the Notch-1 activity, resulting in the inhibition of the growth of pancreatic cancer cells in vitro and in severe combined immunodeficient (SCID) xenograft model.
Materials and Methods
Cell culture and experimental reagents
Human pancreatic cancer cell lines AsPC-1, BxPC-3, Colo-357, HPAC, L3.6pl, MIAPaCa, and PANC-1 were used in this study. Primary antibodies for Bcl-2, Bcl-xL, Mcl-1, Jagged-1, Notch-1, Hes-1, cyclin A, cyclin B, cyclin D1, CDK2, CDK4, CDK6, FoxM1, p21, p57, and E2F-1 were purchased from Santa Cruz Biotechnology. All secondary antibodies were obtained from Pierce. γ-Secretase inhibitors L-685,458 and DAPT were obtained from Calbiochem.
TW-37
Design, synthesis, purification, and chemical characterization of N-[(2-tert-butyl-benzenesulfonyl)-phenyl]-2,3,4-trihydroxy-5-(2-isopropyl-benzyl)-benzamide (TW-37) are described in detail by Wang and colleagues (11); in the inactive congener TW-37a, all three hydroxyl groups in the polyphenolic ring have been substituted with a methyl group, resulting in a 100-fold loss of binding (11).
Cell growth inhibition studies by WST-1 assay
The pancreatic cancer cells (5 × 103) were seeded in a 96-well culture plate. After 12 h, cells were treated with various concentrations of TW-37 for 24, 48, and 72 h. After incubation, the cell growth inhibition studies were performed by WST-1 assay according to the manufacturer’s instructions. In addition to the above assay, we have also done clonogenic assay for assessing the effects of treatment as shown below.
Clonogenic assay
To test the survival of cells treated with TW-37, BxPC-3 and Colo-357 cells were plated (50,000–100,000 per well) in a six-well plate and incubated overnight at 37°C. After 72-h exposure to various concentrations of TW-37, the cells were subjected to a clonogenic assay as described before (23).
Flow cytometry and cell cycle analysis
The TW-37–treated cells, as indicated earlier, were trypsinized, collected, and washed twice with PBS. Cell pellets were fixed in 70% ethanol and the percentage of cells in different phases of the cell cycle was analyzed as described before (24).
Histone/DNA ELISA for detection of apoptosis
The Cell Death Detection ELISA Kit was used for assessing apoptosis according to the manufacturer’s protocol. Briefly, after TW-37 treatment, the cells were lysed and the cell lysates were overlaid and incubated in microtiter plate modules coated with anti-histone antibody for detection of apoptosis as described earlier (10).
Annexin V assay
Characterization of apoptosis was carried out after propidium iodide and Annexin V–FITC staining with apoptosis detection kit (Pharmingen) followed by flow cytometric analysis after 48 h of 500 nmol/L TW-37 treatment of BxPC-3 and Colo-357 according to the manufacturer’s instructions.
Hoechst staining and terminal deoxynucleotidyltransferase-mediated nick end labeling assay for detection of apoptosis
Cells were treated with TW-37 for 72 h, as described above. After treatment, cells were washed with cold PBS and fixed in ethanol for 1 h. The cells were then stained with 5 µg/mL Hoechst for 30 min and visualized under a fluorescence microscope. Bright condensed, punctuate, or granular nuclei were considered apoptotic. Moreover, terminal deoxynucleotidyltransferase-mediated nick end labeling (TUNEL) was assayed with a commercial apoptosis detection kit (Promega Corp.).
Western blot analysis
Cells were lysed in lysis buffer by incubating for 20 min at 4°C. The protein concentration was determined using the Bio-Rad assay system (Bio-Rad). Total proteins were fractionated using SDS-PAGE and transferred onto a nitrocellulose membrane for Western blotting as described earlier (10).
Real-time reverse transcription-PCR analysis for gene expression studies
The total RNA from treated cells was isolated by Trizol (Invitrogen) and purified by RNeasy Mini Kit and RNase-free DNase Set (Qiagen) according to the manufacturer’s protocols. The primers used in the PCR reaction for Notch-1, Jagged-1, Hes-1, p21, p27, p57, E2F-1, Survivin, Cdc25A, FoxM1, and β-actin were described before (19, 24). Real-time PCR amplifications were performed as described earlier (24).
Immunofluorescence staining
The cells were plated on coverslips in each well of an eight-well chamber for 24 h. After treatment of TW-37 for 72 h, cells were then fixed with paraformaldehyde for 15 min, rinsed with PBS, and incubated with 5% goat serum for 30 min. The cells were then incubated with anti–Notch-1 and anti–Jagged-1 antibody for 2 h, respectively. After washing with PBS, the cells were incubated with FITC-conjugated secondary antibody for 45 min and washed with PBS. Cell images were observed under a fluorescent microscope.
Plasmids and transfections
Bcl-2 siRNA, Notch-1 siRNA, and siRNA control were obtained from Santa Cruz Biotechnology. The Bcl-2 cDNA plasmid was generated as described earlier (25). The Notch-1 cDNA plasmid encoding the Notch-1 intracellular domain was a kind gift from L. Miele (Department of Biopharmaceutical Sciences and Cancer Center, University of Illinois at Chicago, Chicago, IL; ref. 26). Human pancreatic cancer cells, BxPC-3 and Colo-357, were transfected with the Bcl-2 plasmid using Lipofectamine 2000 as described earlier (27). Cells were stably transfected with human Notch-1 ICN or vector alone (pcDNA3) and maintained under neomycin selection.
Colo-357 xenografts
Four-week-old female ICR-SCID mice were obtained from Taconic Laboratory. The mice were adapted to animal housing and Colo-357 xenografts were developed as described earlier (10). Using this model, we have previously shown the antitumor activity of TW-37. Tumor tissues harvested from this experiment were used for histologic, immunohistochemical, and Western blotting analyses. All studies involving mice were performed under Animal Investigation Committee–approved protocols.
Immunohistochemical expression of Ki67, proliferating cell nuclear antigen, and phospho-p65
The expression of Ki67, proliferating cell nuclear antigen (PCNA; proliferative marker), and phospho-p65 was detected in histologic sections of tumor xenografts as described before (28). Apoptotic cells were identified by terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) staining as recommended by the manufacturer (Chemicon, Inc.).
Statistical analysis
Data are represented as mean ± SD for the absolute values or percentage of controls as indicated in the vertical axis legend of figures. The statistical significance of differential findings between experimental groups and control was determined by the Student’s t test as implemented by GraphPad StatMate software (GraphPad Software, Inc.). P values lower than 0.05 were considered statistically significant.
Results
Effects of TW-37 on the viability of pancreatic cancer cells
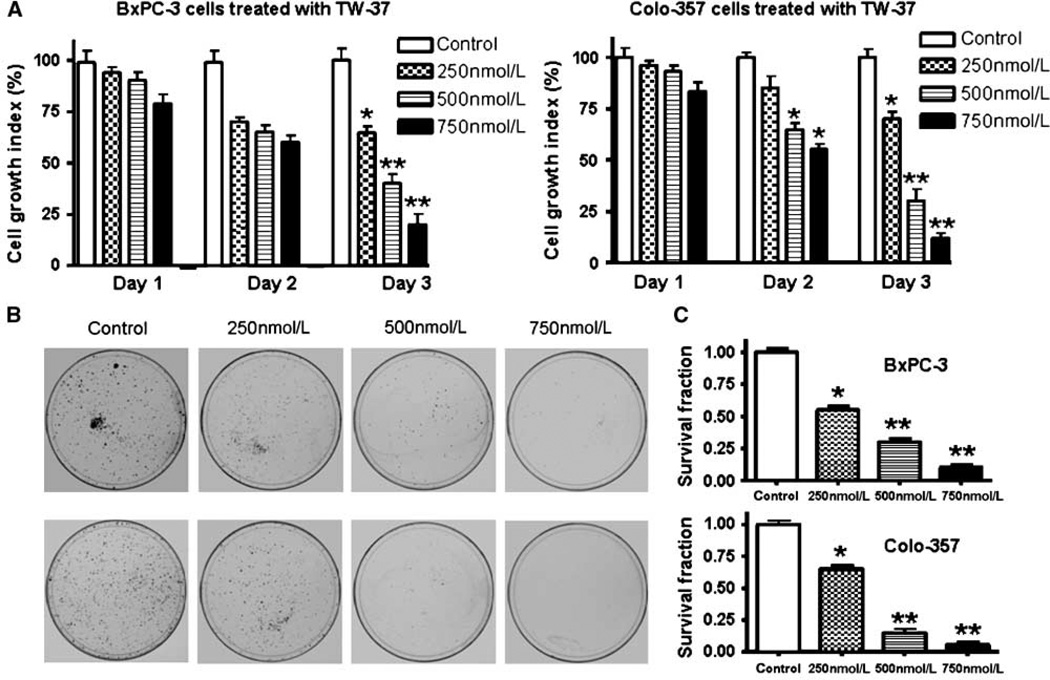
The baseline expression of Bcl-2 family proteins was determined in a panel of human pancreatic cancer cell lines that included AsPC-1, BxPC-3, Colo-357, HPAC, L3.6pl, MIA PaCa, and PANC-1. The results showed that Bcl-2, Bcl-xL, and Mcl-1 were frequently but differentially expressed in different human pancreatic cancer cell lines (Supplementary Fig. S1B). BxPC-3 and Colo-357 were chosen for this study based on their constitutive levels of Bcl-2 family proteins. Viability of BxPC-3 and Colo-357 cells treated with TW-37 was determined by the WST assay, and the data are presented in Fig. 1. The treatment of pancreatic cancer cells for 1 to 3 days with 250, 500, and 750 nmol/L of TW-37 resulted in cell growth inhibition in a dose- and time-dependent manner in both BxPC-3 and Colo-357 pancreatic cancer cell lines (Fig. 1A). In addition, we have also tested the effects of treatment on cell viability by clonogenic assay as shown below.
Figure 1.
Effect of TW-37 on pancreatic cancer cell growth. A, dose and time responses of TW-37 on growth of pancreatic cancer cells. Cells were seeded in 96-well plates at 5,000 per well and treated with varied concentrations of TW-37 for different times. After treatment, cell densities were determined by the WST assay. Columns, mean (n = 6) of three independent experiments; bars, SD. *P < 0.05, **P < 0.01, compared with the control. B and C, cell survival of the human pancreatic cancer cell lines BxPC-3 and Colo-357. Cells treated with varied concentrations of TW-37 for 72 h were evaluated by the clonogenic assay. Photomicrographic difference in colony formation in cells untreated and treated with TW-37 (B). There was a significant reduction in the colony formation in BxPC-3 and Colo-357 cells treated with TW-37 compared with control cells (C). P values represent comparisons between cells treated by TW-37 and control using the paired t test.
Inhibition of cell growth/survival by clonogenic assay
To determine the effect of TW-37 on cell growth, cells were treated with TW-37 and assessed for cell viability by clonogenic assay. TW-37 resulted in a significant inhibition of colony formation of BxPC-3 and Colo-357 cells when compared with control (Fig. 1B and C). Overall, the results from clonogenic assay was consistent with the WST data as shown in Fig. 1A, suggesting that TW-37 inhibited cell growth in BxPC-3 and Colo-357 pancreatic cancer cells. Next, we examined whether the inhibition of cell growth was also accompanied by the induction of apoptosis induced by TW-37.
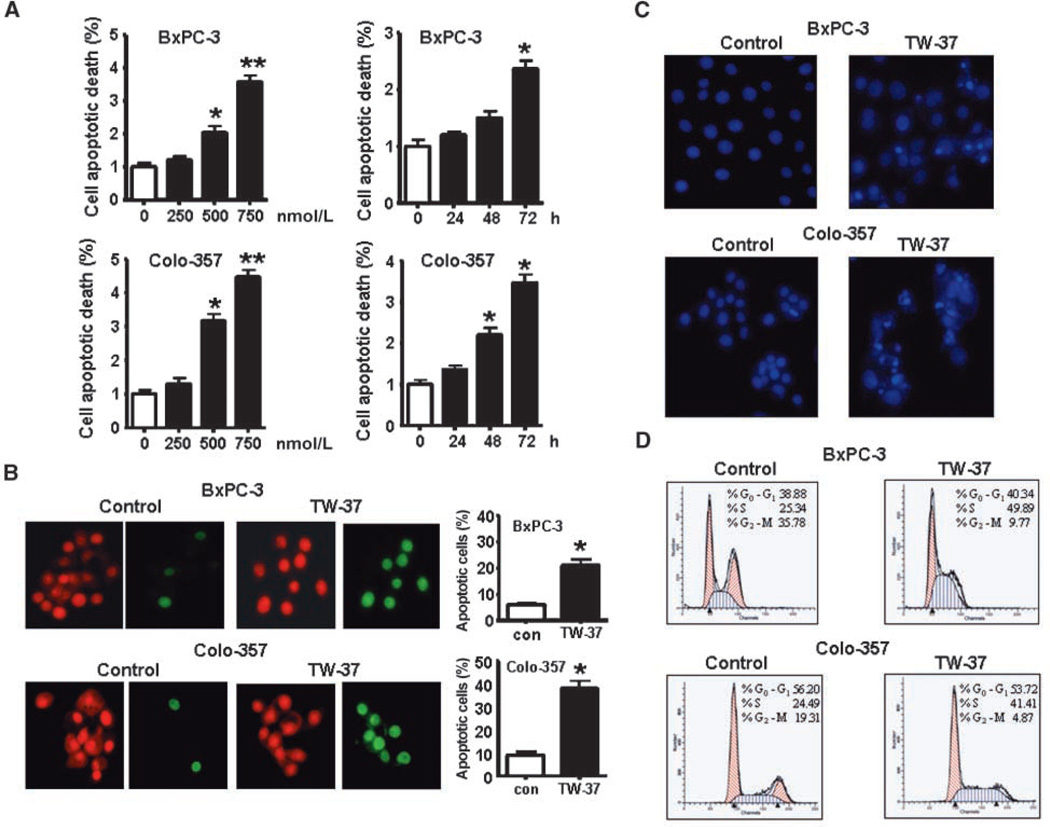
TW-37 induced apoptosis in pancreatic cancer cell lines
To quantitatively measure apoptotic cell death after different treatment, we conducted a histone/DNA enzyme-linked immunosorbent apoptosis assay. We found that TW-37 induced apoptosis in a dose- and time-dependent manner (Fig. 2A). To confirm this result, we also used other methods to detect apoptosis: BxPC-3 and Colo-357 cells were treated with 500 nmol/L TW-37 for 48 h. By staining cells with Annexin V–FITC and propidium iodide, we found that the percentage of apoptotic cells increased from 5% to 6% in the control to 12% to 14% in both BxPC-3 and Colo-357 cell lines (Supplementary Fig. S2A). Our TUNEL assay also showed that TW-37 induced apoptosis in BxPC-3 and Colo-357 cells (Fig. 2B). In addition, by Hoechst staining for testing apoptotic cells, we observed more bright condensed and granular stained nuclei in TW-37–treated cells compared with control (Fig. 2C), suggesting that TW-37 could induce apoptosis.
Figure 2.
Effect of TW-37 on pancreatic cancer cell apoptotic death. A, BxPC-3 and Colo-357 cells were exposed to different concentrations of TW-37 for different times. Apoptosis was measured by histone DNA ELISA. Columns, mean; bars, SD. *P < 0.05, **P < 0.01, compared with the control. B, TUNEL was performed in BxPC-3 and Colo-357 cells treated with 500 nmol/L TW-37 for 72 h using an apoptosis detection kit. Propidium iodide stains both apoptotic and nonapoptotic cells red. Fluorescein-12-dUTP incorporation results in localized green fluorescence within the nucleus of apoptotic cells only. C, BxPC-3 and Colo-357 cells were treated with 500 nmol/L TW-37 for 48 h. After treatment, cells were washed with cold PBS and fixed in ethanol for 1 h. The cells were then stained with 5 µg/mL Hoechst for 30 min and visualized under a fluorescence microscope. Bright condensed, punctuate, or granular nuclei were considered apoptotic. We observed more bright condensed and granular stained nuclei in TW-37–treated cells compared with control. D, effect of TW-37 on cell cycle distribution. The 500 nmol/L TW-37–treated BxPC-3 and Colo-357 cells were harvested for cell cycle analysis using propidium iodide staining. X axis, DNA content; Y axis, the number of nuclei.
TW-37 induced S-phase arrest
To further investigate the effect of TW-37 on cell growth in more detail, we analyzed the effects of 500 nmol/L TW-37 on the cell cycle distribution of BxPC-3 and Colo-357 cells. The cell cycle distribution was monitored by flow cytometry (fluorescence-activated cell sorting) analysis after propidium iodide staining of the cellular DNA. As seen in Fig. 2D, in comparison with untreated control cells, TW-37 induced an accumulation of cells in the S-phase fractions. The S-phase fraction increased from 25.34% in control cells to 45.89% and from 24.49% in control cells to 41.41% in TW-37–treated BxPC-3 cells and Colo-357 cells, respectively.
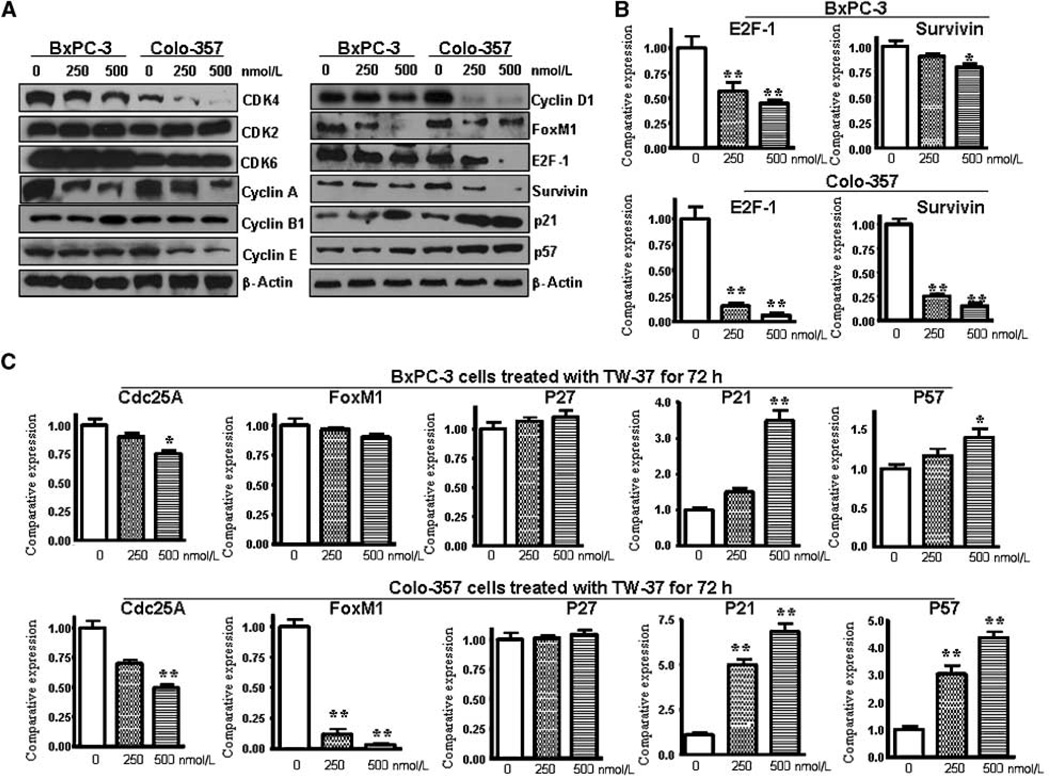
To further characterize the S-phase arrest, we examined the level of expression of several known S-phase cell cycle regulatory factors. Consistent with cell cycle arrest, the expression of cyclin A, E, D1, and CDK4 levels was found to be decreased, whereas p21 and p57 expression was increased (Fig. 3A and C), suggesting the mechanistic roles of these molecules during TW-37–induced cell cycle progression and cell cycle arrest by TW-37. To further confirm our data, we found that the expression of cell cycle regulatory factors involved in cell proliferation and survival, such as E2F-1, Survivin, and cdc25A, was down-regulated in TW-37–treated cells (Fig. 3A–C). This observation suggests that the S-phase arrest by TW-37 is in part due to profound alterations in the expression of positive and negative regulatory cell cycle–related proteins. To further understand the molecular mechanism involved in TW-37–induced apoptosis of pancreatic cancer cells, alterations in the cell survival pathway were investigated. Because Notch signaling plays important roles in the cellular proliferation and apoptosis, we investigated whether TW-37 could regulate Notch signaling pathway.
Figure 3.
Effect of TW-37 on the expression of several known cell cycle regulatory factors. A, the protein levels of several cell cycle regulatory factors were detected by Western blotting in BxPC-3 and Colo-357 pancreatic cancer cells treated with TW-37 for 72 h. B and C, the mRNA levels of cell cycle regulatory factors were investigated by real-time RT-PCR in BxPC-3 and Colo-357 pancreatic cancer cells treated with TW-37 for 72 h.
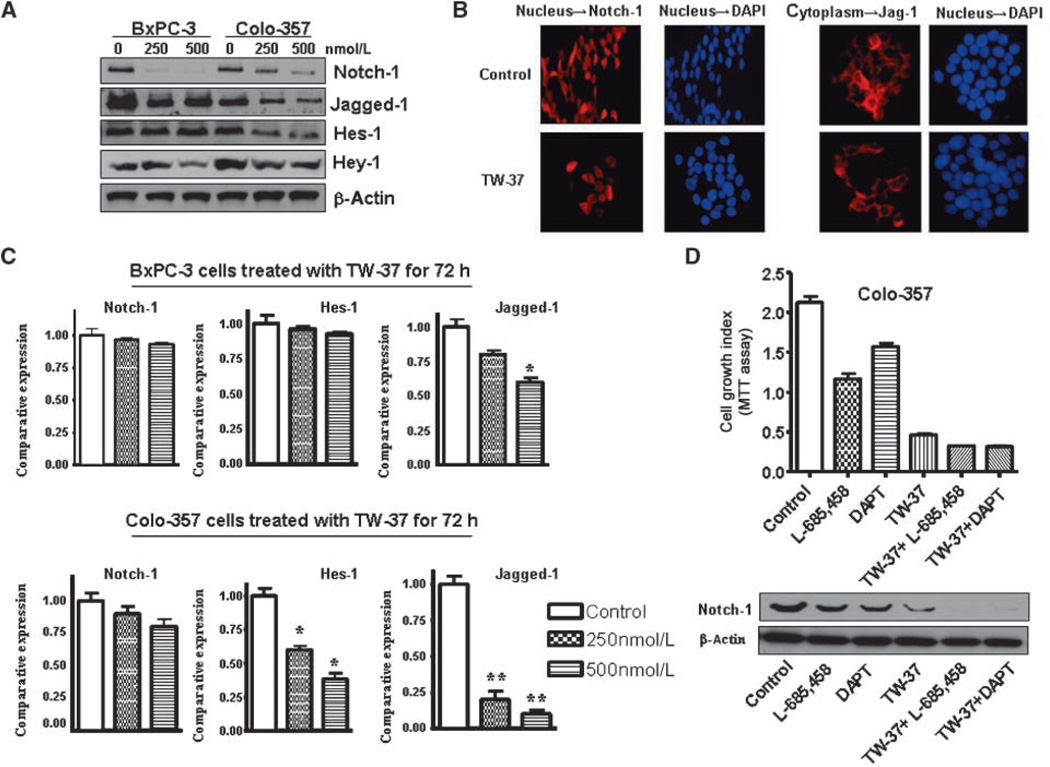
Down-regulation of the Notch-1 expression by TW-37
Notch-1, Jagged-1, and Hes-1 mRNA and protein expression in BxPC-3 and Colo-357 cell lines treated with TW-37 for 72 hours were assessed using real-time reverse transcription-PCR (RT-PCR) and Western blotting analysis, respectively. We found that Notch-1 (ICN) and Jagged-1 were down-regulated by TW-37 in both cell lines (Fig. 4A). To confirm our results, we also did immunofluorescent staining. Indeed, we observed a lower level of Notch-1 protein in the nucleus and Jagged-1 in the cytoplasm in the TW-37–treated cells (Fig. 4B). We also found that the expression of the Jagged-1 gene at the mRNA level was down-regulated after TW-37 treatment in both the cell lines, suggesting transcriptional inactivation of Jagged-1 gene expression in pancreatic cancer cells (Fig. 4C). However, the Notch-1 mRNA level was not affected by TW-37 in both the cell lines. Interestingly, Hes-1 mRNA and protein expression were decreased in Colo-357 cell lines but not in BxPC-3 cells (Fig. 4C). The mechanisms of such differences need further investigation in the future. To further confirm this result, we also treated BxPC-3 and Colo-357 cells with another Bcl-2 inhibitor, ApoG2. We found that ApoG2 also inhibited the expression of Notch and Jagged-1 (Supplementary Fig. S1D).
Figure 4.
Effect of TW-37 on Notch-1 expression in human pancreatic cancer cells. A, the expression of Notch-1, its ligand Jagged-1, and its target gene Hes-1 was detected by Western blotting. B, the Colo-357 pancreatic cancer cells treated with 500 nmol/L TW-37 for 72 h were subjected to immunofluorescent staining using anti–Notch-1 antibody and anti–Jagged-1 antibody. C, the Notch-1 mRNA level was detected in BxPC-3 and Colo-357 cell lines treated with TW-37 for 72 h as measured by real-time RT-PCR. D, top, GSI (L-685,458 and DAPT) significantly inhibited Colo-357 cell growth. TW-37 plus GSI inhibited Colo-357 cell growth to a greater degree compared with TW-37. Bottom, the expression of Notch-1 was detected by Western blotting to check the GSI efficacy of down-regulation of Notch-1.
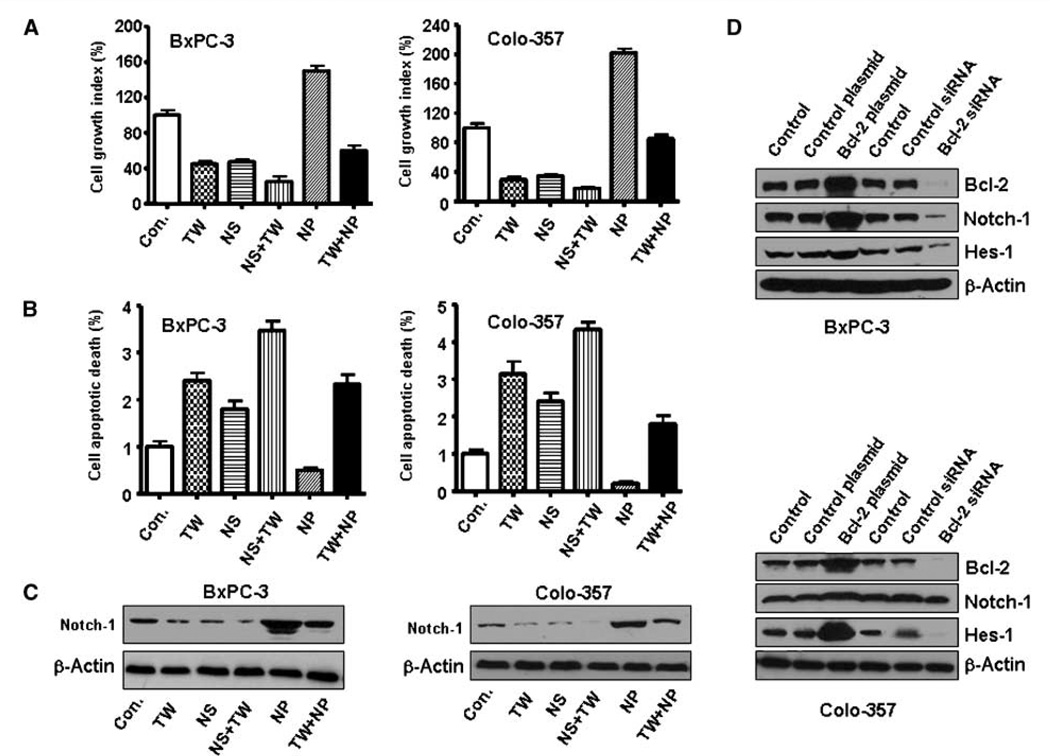
Down-regulation of Notch-1 expression by small interfering RNA (siRNA) or γ-secretase inhibitor (GSI) potentiates TW-37– induced cell growth inhibition and apoptosis. Next, we observed that down-regulation of Notch-1 expression by siRNA or GSI significantly inhibited cell growth in TW-37 treated cells (Figs. 4D and 5A). Notch-1 siRNA-transfected cells were significantly more sensitive to spontaneous and TW-37–induced apoptosis (Fig. 5B). However, overexpression of Notch-1 by cDNA transfection rescued TW-37–induced cell growth inhibition and abrogated TW-37–induced apoptosis to a certain degree (Fig. 5B).
Figure 5.
Pancreatic cancer cell growth inhibition and cell death induced by GSI or Notch-1 siRNA and TW-37. Con, control; TW, TW-37; NS, Notch-1 siRNA; NS+TW, TW-37+Notch-1 siRNA; NP, Notch-1 plasmid; TW+NP, TW-37+Notch-1 plasmid. A, down-regulation of Notch-1 by siRNA significantly inhibited BxPC-3 and Colo-357 cell growth. TW-37 plus Notch-1 siRNA inhibited cell growth to a greater degree compared with TW-37 alone. B, pancreatic cancer cell death induced by Notch-1 siRNA and TW-37. Notch-1 siRNA–transfected cells were significantly more sensitive to spontaneous and TW-37–induced apoptosis. C, the expression of Notch-1 was detected by Western blotting to check the Notch-1 plasmid transfection efficacy. D, Notch-1 and Hes-1 expression was up-regulated by Bcl-2 cDNA. However, Notch-1 and Hes-1 expression was down-regulated by Bcl-2 siRNA.
Overexpression of Bcl-2 by cDNA transfection increased Notch-1 expression in cancer cells
To detect whether Bcl-2 regulates the Notch-1 expression, we did Bcl-2 cDNA transfection experiment. Indeed, we found that overexpression of Bcl-2 by cDNA transfection increased Notch-1 ICN expression. However, down-regulation of Bcl-2 by siRNA inhibited the Notch-1 expression in BxPC-3 and Colo-357 cells (Fig. 5D). We found similar results in PC-3 prostate cancer cells and MCF-7 breast cancer cells (Supplementary Fig. S2B), suggesting that Bcl-2 regulates Notch-1 activity in many different cell lines.
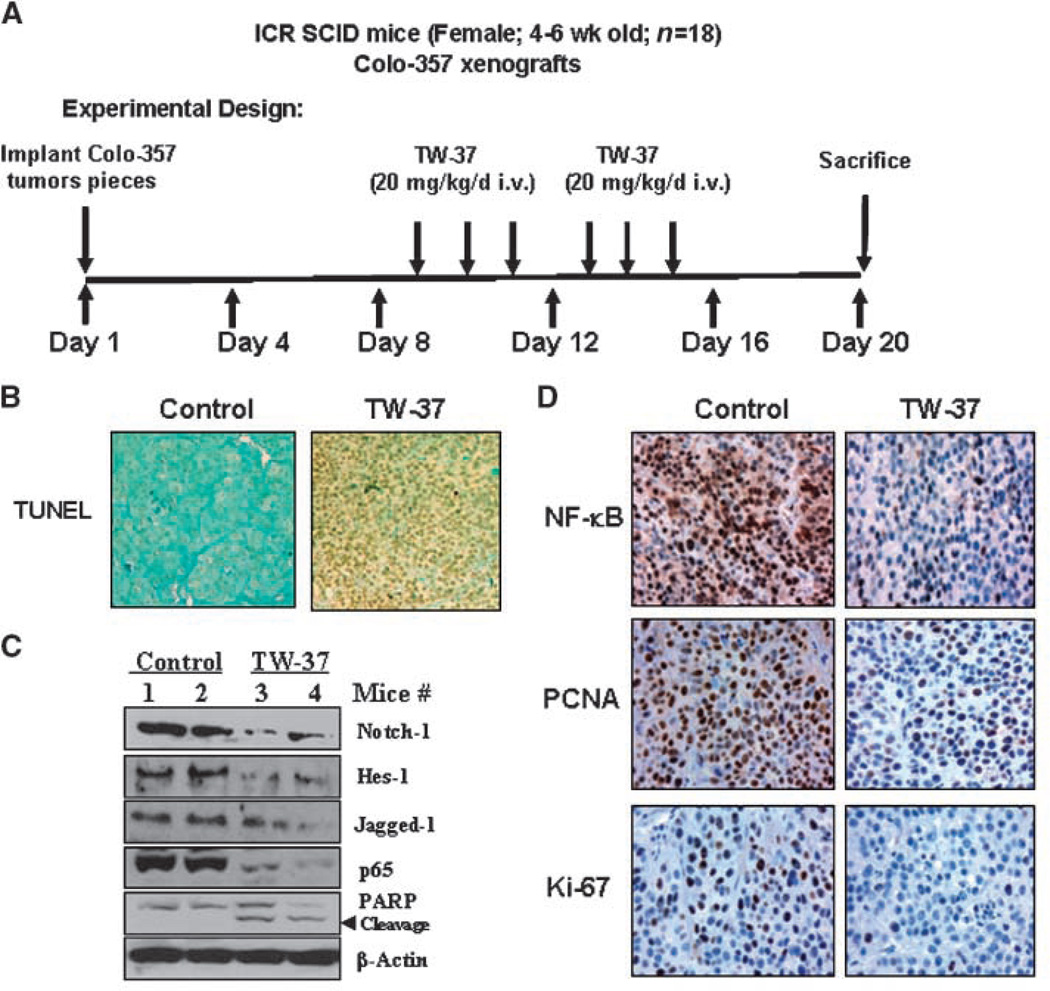
Effect of TW-37on Notch-1 expression in vivo
We have previously found that TW-37 treatment significantly inhibited pancreatic tumor growth in vivo (10). TW-37 also did not show any toxicity or caused any loss in the body weight of the animals during the course of the treatment (up to 20 days). To further investigate whether TW-37 could down-regulate Notch-1 in vivo, we examined the Notch-1 expression in tumor tissues obtained from tumor-bearing mouse treated with TW-37 as published earlier (10). Western blot analysis showed that the expression level of Notch-1 was significantly lower in tumors from the TW-37–treated mice than those from vehicle-treated control mice (Fig. 6C), suggesting that TW-37 could down-regulate Notch-1 in vivo, similar to those observed in vitro. In addition, we found that the expression of Jagged-1 and Notch-1 downstream target gene, Hes-1, was also down-regulated in TW-37–treated tumors (Fig. 6C).
Figure 6.
TW-37 inhibits the expression of Notch-1, Jagged-1, and Hes-1 in vivo. A, the schedule for animal experiments. B, immunohistochemical demonstration of apoptosis by TUNEL in tissues harvested from tumor-bearing SCID mouse. Significant differences in the percentage of TUNEL-positive cells were noted in tumors derived from the TW-37 treatment group relative to control group. C, the expression of Notch-1, Jagged-1, Hes-1, p65, and PARP was detected by Western blotting of tumor tissue extracts. D, immunohistochemical staining of Colo-357 tumor xenografts. Tumors were resected and processed for routine histologic analysis, and the 5 µmol/L tissue sections were stained with antibodies to phospho-p65, Ki-67, and PCNA.
The PCNA and Ki-67 nuclear labeling indices, as determined by immunohistochemical staining, were decreased in the TW-37–treated tumors compared with control tumors (Fig. 6D), suggesting inhibition of tumor cell proliferation. In our earlier report, we showed that TW-37 could down-regulate the DNA-binding activity of NF-κB in vitro. To determine whether TW-37 could affect the NF-κB gene in vivo, we also examined the expression of p65 and the phosphorylated form of p65 (active NF-κB) in tumor tissues. We found that the expression of p65 and phospho-p65 was downregulated in TW-37–treated animal tissues (Fig. 6C and D). To determine TW-37 induces apoptosis, we assessed activation of poly(ADP)-ribose polymerase (PARP), an important mediator of apoptosis, in animal tissues by Western immunoblotting. We found the increased expression of cleaved PARP in TW-37–treated animal tissues. In addition, significant differences in the percentage of TUNEL-positive cells were also noted in tumors derived from the TW-37 treatment group relative to control group (Fig. 6B). These results are consistent with our in vitro data showing that TW-37 is a powerful agent for the inhibition of cell growth and induction of apoptosis, which is mediated by inhibition of Bcl-2 family of proteins and its downstream genes, especially Notch-1 and NF-κB.
Discussion
The Bcl-2 family of proteins plays critical roles in human cancers, including pancreatic cancer. The activation of Bcl-2 has been shown to enhance tumor growth, invasion, motility, tumor spreading and metastasis, and inhibition of apoptosis. The overexpression of Bcl-2 family proteins in pancreatic cancer may also play important roles in resistance to a wide spectrum of chemotherapeutic agents (29). Therefore, identification of an inhibitor targeting Bcl-2 family of proteins is likely to provide a therapeutic benefit for pancreatic cancer. Our laboratory and others have extensively studied a number of small-molecule inhibitors such as gossypol, apogossypolone, as well as TW-37 for their antitumor activity in various cancers (4, 8, 10, 30, 31). The present study shows that TW-37 inhibits tumor growth and induces apoptosis of pancreatic cancer cells, which was partly mediated through inactivation of Notch-1 and NF-κB signal pathways that are downstream of Bcl-2.
TW-37, a recently developed small-molecule inhibitor of Bcl-2, is capable of antagonizing the function of pan-Bcl-2 family and thereby may have greater therapeutic potential as an entirely new class of antitumor agent. We have found that TW-37 inhibits the growth of a variety of cancer cells, including pancreatic cancer cells (8, 10, 11). Here, we investigated the mechanism by which TW-37 elicits its biological effects on pancreatic cancer cells. In this study, we used two human pancreatic cancer cell lines, BxPC-3 and Colo-357. Both cell lines have high expression of Bcl-2, Bcl-xL, and Mcl-1. We found that TW-37 was capable of inducing significant growth inhibition in both BxPC-3 and Colo-357 cells as detected by the WST assay and the clonogenic assay. Moreover, TW-37 also induced apoptotic cell death in both cell lines, suggesting that blocking Bcl-2 is sufficient to trigger apoptosis in pancreatic cancer cells overexpressing these molecules. To further elucidate the mechanism of action, we detected whether cell cycle arrest was related to the cell growth inhibition. Indeed, we found that TW-37 increased cell population in the Sphase. Moreover, we observed a marked decrease in cyclin D1, cyclin A, and Cdk4 and the increased expression of CdkI proteins, including p21CIP and p57KIP2, in TW-37–treated cells.
Recent reports have shown that Bcl-2 may play an oncogenic role by regulating important proteins in the survival pathway, such as AKT, NF-κB, MAPK, and STAT3 (2, 15, 17). It has been reported that AKT and NF-κB cross-talk with Notch-1 (20–22). We have reported that Bcl-2 regulated the NF-κB activity in pancreatic cancer (10). In this study, we further tested whether Bcl-2 could also regulate NF-κB upstream signaling pathway, namely Notch-1. Indeed, we found that TW-37 inhibits the activation of Notch-1 and its ligand Jagged-1 in vitro and in vivo in pancreatic cancer. We also found that TW-37 inhibited the expression of the Notch-1 target gene Hes-1. Recently, it has been reported that the Notch pathway is known to play critical roles in the processes of tumor cell proliferation and apoptosis in pancreatic cancer (18). Therefore, TW-37–mediated cell growth inhibition could be partly mediated via inactivation of Notch-1 activity. Indeed, we found that down-regulation of Notch-1 by siRNA or GSI together with TW-37 treatment inhibited cell growth and induced apoptosis to a greater degree in pancreatic cancer cells compared with TW-37 treatment alone. In view of these findings, we strongly believe that inactivation of Bcl-2 by TW-37 results in the down-regulation of Notch-1 and subsequently inactivates NF-κB, which are believed to be mechanistically linked with TW-37–induced apoptotic processes.
Recently, it has been documented that activation of Notch-1 leads to the activation of NF-κB, which has been shown to be activated in a variety of cancers (22). Increasing evidence of dysregulated NF-κB–associated pathways has been found in various human pancreatic cancer cell lines and primary tumors, which supports the role of NF-κB in pancreatic cancer (32). In our previous study, we found that TW-37 inhibits NF-κB activation in pancreatic cancer. In this study, our results show, for the first time, that NF-κB activity is significantly inhibited in the tumors of TW-37–treated animals compared with untreated controls. Moreover, TW-37 treatment significantly inhibited pancreatic cancer cell growth in vivo in the SCID xenograft model (10), which could in part be attributed to decreased proliferation as evidenced by reduced Ki-67 and PCNA immunoreactivity in the tumors of TW-37–treated animals.
In summary, we presented experimental evidence that strongly supports the role of TW-37 as an antitumor agent. On the basis of our results, we propose a hypothetical pathway by which TW-37 inhibits cell growth of pancreatic cancer cells, partly mediated through inactivation of Notch-1 and NF-κB signaling pathways (Supplementary Fig. S3). However, further in-depth studies are needed to ascertain the precise molecular regulation of Bcl-2, Notch-1, and FoxM1 and their cross-talks with NF-κB in elucidating the role of TW-37 in cell growth inhibition and apoptosis of pancreatic cancer cells and its antitumor activity in animal models before translating our findings for the treatment of human pancreatic cancer.
Supplementary Material
Acknowledgments
Grant support: National Cancer Institute, NIH grant R01CA109389 (R.M. Mohammad), NIH grant 5R01CA101870 (F.H. Sarkar), and NIH grant U19CA113317 (S. Wang).
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
The University of Michigan has filed a patent on TW-37, which has been licensed by Ascenta Therapeutics, Inc. The University of Michigan and S. Wang own equity in Ascenta. S. Wang also serves as a consultant for Ascenta and is the principal investigator on a research contract from Ascenta to The University of Michigan. The other authors disclosed no potential conflicts of interest.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Mortenson MM, Galante JG, Gilad O, et al. BCL-2 functions as an activator of the AKT signaling pathway in pancreatic cancer. J Cell Biochem. 2007;102:1171–1179. doi: 10.1002/jcb.21343. [DOI] [PubMed] [Google Scholar]
- 3.Huang S, Sinicrope FA. BH3 mimetic ABT-737 potentiates TRAIL-mediated apoptotic signaling by unsequestering Bim and Bak in human pancreatic cancer cells. Cancer Res. 2008;68:2944–2951. doi: 10.1158/0008-5472.CAN-07-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohammad RM, Wang S, Banerjee S, Wu X, Chen J, Sarkar FH. Nonpeptidic small-molecule inhibitor of Bcl-2 and Bcl-XL, (−)-Gossypol, enhances biological effect of genistein against BxPC-3 human pancreatic cancer cell line. Pancreas. 2005;31:317–324. doi: 10.1097/01.mpa.0000179731.46210.01. [DOI] [PubMed] [Google Scholar]
- 5.Vaux DL. ABT-737, proving to be a great tool even before it is proven in the clinic. Cell Death Differ. 2008;15:807–808. doi: 10.1038/cdd.2008.31. [DOI] [PubMed] [Google Scholar]
- 6.Tse C, Shoemaker AR, Adickes J, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 7.Hann CL, Daniel VC, Sugar EA, et al. Therapeutic efficacy of ABT-737, a selective inhibitor of BCL-2, in small cell lung cancer. Cancer Res. 2008;68:2321–2328. doi: 10.1158/0008-5472.CAN-07-5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohammad RM, Goustin AS, Aboukameel A, et al. Preclinical studies of TW-37, a new nonpeptidic small-molecule inhibitor of Bcl-2, in diffuse large cell lymphoma xenograft model reveal drug action on both Bcl-2 and Mcl-1. Clin Cancer Res. 2007;13:2226–2235. doi: 10.1158/1078-0432.CCR-06-1574. [DOI] [PubMed] [Google Scholar]
- 9.Zeitlin BD, Joo E, Dong Z, et al. Antiangiogenic effect of TW37, a small-molecule inhibitor of Bcl-2. Cancer Res. 2006;66:8698–8706. doi: 10.1158/0008-5472.CAN-05-3691. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Song W, Aboukameel A, et al. TW-37, a small-molecule inhibitor of Bcl-2, inhibits cell growth and invasion in pancreatic cancer. Int J Cancer. 2008;123:958–966. doi: 10.1002/ijc.23610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Wang G, Nikolovska-Coleska Z, Yang CY, et al. Structure-based design of potent small-molecule inhibitors of anti-apoptotic Bcl-2 proteins. J Med Chem. 2006;49:6139–6142. doi: 10.1021/jm060460o. [DOI] [PubMed] [Google Scholar]
- 12.Scorrano L, Korsmeyer SJ. Mechanisms of cytochrome c release by proapoptotic BCL-2 family members. Biochem Biophys Res Commun. 2003;304:437–444. doi: 10.1016/s0006-291x(03)00615-6. [DOI] [PubMed] [Google Scholar]
- 13.Regula KM, Ens K, Kirshenbaum LA. IKKβ is required for Bcl-2-mediated NF-κB activation in ventricular myocytes. J Biol Chem. 2002;277:38676–38682. doi: 10.1074/jbc.M206175200. [DOI] [PubMed] [Google Scholar]
- 14.Kumar P, Ning Y, Polverini PJ. Endothelial cells expressing Bcl-2 promotes tumor metastasis by enhancing tumor angiogenesis, blood vessel leakiness and tumor invasion. Lab Invest. 2008;88:740–749. doi: 10.1038/labinvest.2008.46. [DOI] [PubMed] [Google Scholar]
- 15.Tucker CA, Kapanen AI, Chikh G, et al. Silencing Bcl-2 in models of mantle cell lymphoma is associated with decreases in cyclin D1, nuclear factor-κB, p53, bax, and p27 levels. Mol Cancer Ther. 2008;7:749–758. doi: 10.1158/1535-7163.MCT-07-0302. [DOI] [PubMed] [Google Scholar]
- 16.Anai S, Shiverick K, Medrano T, et al. Downregulation of BCL-2 induces downregulation of carbonic anhydrase IX, vascular endothelial growth factor, and pAkt and induces radiation sensitization. Urology. 2007;70:832–837. doi: 10.1016/j.urology.2007.06.1118. [DOI] [PubMed] [Google Scholar]
- 17.Kaneko T, Zhang Z, Mantellini MG, et al. Bcl-2 orchestrates a cross-talk between endothelial and tumor cells that promotes tumor growth. Cancer Res. 2007;67:9685–9693. doi: 10.1158/0008-5472.CAN-07-1497. [DOI] [PubMed] [Google Scholar]
- 18.Miele L. Notch signaling. Clin Cancer Res. 2006;12:1074–1079. doi: 10.1158/1078-0432.CCR-05-2570. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z, Zhang Y, Li Y, Banerjee S, Liao J, Sarkar FH. Down-regulation of Notch-1 contributes to cell growth inhibition and apoptosis in pancreatic cancer cells. Mol Cancer Ther. 2006;5:483–493. doi: 10.1158/1535-7163.MCT-05-0299. [DOI] [PubMed] [Google Scholar]
- 20.Palomero T, Dominguez M, Ferrando AA. The role of the PTEN/AKT pathway in NOTCH1-induced leukemia. Cell Cycle. 2008;7:965–970. doi: 10.4161/cc.7.8.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gutierrez A, Look AT. NOTCH and PI3K-AKT pathways intertwined. Cancer Cell. 2007;12:411–413. doi: 10.1016/j.ccr.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 22.Osipo C, Golde TE, Osborne BA, Miele LA. Off the beaten pathway: the complex cross talk between Notch and NF-κB. Lab Invest. 2008;88:11–17. doi: 10.1038/labinvest.3700700. [DOI] [PubMed] [Google Scholar]
- 23.Ali S, Banerjee S, Ahmad A, El-Rayes BF, Philip PA, Sarkar FH. Apoptosis-inducing effect of erlotinib is potentiated by 3,3′-diindolylmethane in vitro and in vivo using an orthotopic model of pancreatic cancer. Mol Cancer Ther. 2008;7:1708–1719. doi: 10.1158/1535-7163.MCT-08-0354. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Wang Z, Banerjee S, Kong D, Li Y, Sarkar FH. Down-regulation of Forkhead Box M1 transcription factor leads to the inhibition of invasion and angiogenesis of pancreatic cancer cells. Cancer Res. 2007;67:8293–8300. doi: 10.1158/0008-5472.CAN-07-1265. [DOI] [PubMed] [Google Scholar]
- 25.Xu L, Kong D, Zhu L, Zhu W, Andrews DW, Kuo TH. Suppression of IP3-mediated calcium release and apoptosis by Bcl-2 involves the participation of protein phosphatase 1. Mol Cell Biochem. 2007;295:153–165. doi: 10.1007/s11010-006-9285-5. [DOI] [PubMed] [Google Scholar]
- 26.Weijzen S, Rizzo P, Braid M, et al. Activation of Notch-1 signaling maintains the neoplastic phenotype in human Ras-transformed cells. Nat Med. 2002;8:979–986. doi: 10.1038/nm754. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Banerjee S, Li Y, Rahman KM, Zhang Y, Sarkar FH. Down-regulation of Notch-1 inhibits invasion by inactivation of nuclear factor-{κ}B, vascular endothelial growth factor, and matrix metalloproteinase-9 in pancreatic cancer cells. Cancer Res. 2006;66:2778–2784. doi: 10.1158/0008-5472.CAN-05-4281. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Banerjee S, Wang Z, et al. Antitumor activity of epidermal growth factor receptor-related protein is mediated by inactivation of ErbB receptors and nuclear factor-κB in pancreatic cancer. Cancer Res. 2006;66:1025–1032. doi: 10.1158/0008-5472.CAN-05-2968. [DOI] [PubMed] [Google Scholar]
- 29.Schniewind B, Christgen M, Kurdow R, et al. Resistance of pancreatic cancer to gemcitabine treatment is dependent on mitochondria-mediated apoptosis. Int J Cancer. 2004;109:182–188. doi: 10.1002/ijc.11679. [DOI] [PubMed] [Google Scholar]
- 30.Arnold AA, Aboukameel A, Chen J, et al. Preclinical studies of Apogossypolone: a new nonpeptidic pan small-molecule inhibitor of Bcl-2, Bcl-XL and Mcl-1 proteins in follicular small cleaved cell lymphoma model. Mol Cancer. 2008;7:20. doi: 10.1186/1476-4598-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohammad RM, Wang S, Aboukameel A, et al. Preclinical studies of a nonpeptidic small-molecule inhibitor of Bcl-2 and Bcl-X(L) [(−)-gossypol] against diffuse large cell lymphoma. Mol Cancer Ther. 2005;4:13–21. [PubMed] [Google Scholar]
- 32.Wang Z, Zhang Y, Banerjee S, Li Y, Sarkar FH. Inhibition of nuclear factor κB activity by genistein is mediated via Notch-1 signaling pathway in pancreatic cancer cells. Int J Cancer. 2006;118:1930–1936. doi: 10.1002/ijc.21589. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.