Abstract
There have been comparatively few investigations reported of radiation effects in zeolites, although it is known that these materials may be modified substantially by exposure to ionizing radiation. Thus, by exposure to γ-rays or high-energy particles, the charge states of atoms may be changed so to create, and accumulate, lattice point defects, and to form structurally disordered regions. Such a technique may permit the creation, in a controlled fashion, of additionally useful properties of the material while preserving its essential stoichiometry and structure. Accordingly, we present an application, in which the cation-exchange capacity of a natural zeolite (clinoptilolite) is substantially enhanced, for the treatment/decontamination of water contaminated with radionuclides e.g. 134Cs, 137Cs and 90Sr, by its exposure to high-energy (8 MeV) electrons, and to different total doses.
“Rarely in our technological society does the discovery of a new class of inorganic materials result in such a wide scientific interest and kaleidoscopic development of applications as has happened with the zeolite molecular sieves”, Donald W. Breck (1974)1.
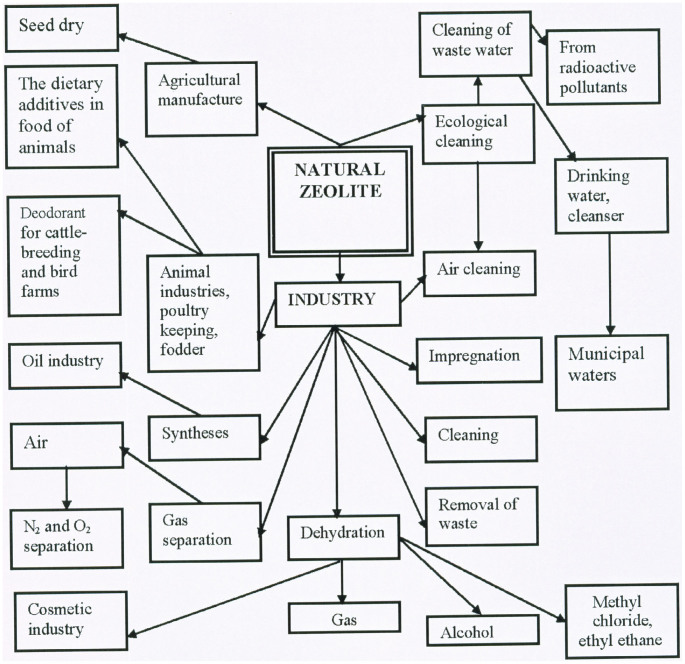
Just some of the manifold applications of zeolites are shown in Fig. 1, the variety and number of which are extended on an annual basis. The study of minerals is a mature science, and a major driving force for investigations of this kind is that, through the understanding of means for their formation, their crystal structure and other salient properties, novel materials may be produced by both a priori and post-synthetic means. Of the broad family of minerals, it is the zeolites which furnish highly effective catalysts, as are used extensively in the petrochemical industry and for other niche applications, e.g. in catalytic convertors, to mitigate local air-pollution from vehicles. The catalytically active sites are located within the internal cavities of a microporous structure, and are associated with cations that are present in sufficient concentration to electrostatically counterbalance the negatively charged, aluminosilicate framework1,2. Many physical and chemical properties of zeolites are determined by the nature and concentrations of those cations that are present in the zeolite unit (elementary) cell.
Figure 1. Schematic diagram for application of natural zeolite in public economy.
Zeolites are high-internal-surface-area crystalline materials with an open, three-dimensional “honeycomb” framework consisting of tetrahedral AlO45− and SiO44− units linked through shared oxygen atoms. There are 40 zeolites known to occur naturally, and over 150 versions which have been prepared by synthetic methods. One might visualize the structure of a zeolite by assuming a neutral SiO2 framework and, periodically, making an isomorphous substitution of an AlO2− unit for SiO2. In consequence, the resulting structure bears a net negative charge on each framework aluminum atom. Accordingly, this negative charge is electrostatically counter-balanced by those cations (e.g., Na+, K+, NH4+) which reside in the pores of the framework. A mobility is hence conferred upon them, so that they are available for exchange with other cations, when placed in contact with a solution of them; indeed, the major volume-use of zeolites currently is in cation-exchange applications, e.g. as “builders” in washing powders, to avoid the ecosystem damage caused by polyphosphates, which were formerly used on a large scale for this purpose.
Some critical properties of zeolites are:
structure;
silica-to-alumina ratio;
pore size; framework density (i.e., atoms per unit cell).
The idealized, stoichiometric formula, given to zeolites is: Mx/n[AlxSiyO2(x + y)]pH2O, where M - (Na, K, Li); (Ca, Mg, Ba, Sr); n - charge of a cation; y/x = 1-6; p/x = 1-4. The oxide formula of zeolites is: M2/nO ·Al2O3 · xSiO2 · yH2O.
The pore size refers to a two-dimensional “window” within the zeolite structure and is determined by the number of tetrahedral units that are mutually connected. This structure is further extended by connecting the tetrahedral units to form a three-dimensional array, which provides a series of more voluminous internal cavities that are interconnected by the pore openings. In some zeolites there are no cavities at all, but rather, a series of one-, two-, or three- dimensional channels which permeate the structure. In addition, various post-synthetic modifications have been developed, including hydrothermal treatments, coating techniques, by selectively admitting molecules with selected properties, and through molecular impregnation, which serve to provide certain further adjustments to the catalytic and adsorptive characteristics of chosen zeolites.
Of the 40 known designated natural zeolites, the three that are of greatest economic importance are clinoptilolite, mordenite and chabazite. Clinoptilolite presents a unique combination of high cation-exchange capacity, and stability to environmental attrition, rendering it a particularly effective material for the removal of toxic pollutants from water and from soil.
There are about 50 million metric tons of natural clinoptilolite present in different regions of Armenia - the best known being the Noyemberyan region - which may be used in a wide range of applications (Table 1).
Table 1. General zeolite properties.
| Property | Range |
|---|---|
| Channels | 2.2–8 Å |
| Cavities | 6.6–11.8 Å |
| Thermal stability | 500–1.000°C |
| Ion-exchange capacity | Up to 700 milliequivalents/100 g |
| Surface area | Up to 900 m2/g |
| Water capacity | <1 to ~ 25 wt.% |
| Water affinity | Hydrophilic to hydrophobic |
The oxide formula of clinoptilolite is:
{K, Na, 1/2Ca}2O · Al2O3 · 10SiO2 · 8H2O
Crystal data: Space group: C12/ml (#12)
a = 17.662 Å b = 17.911 Å c = 7.407 Å
α = 90° β = 116.40° γ = 90°
Comment: unique axis b, cell choice 1.
Density: 2.16 g/cm3
Hardness on the Mohs scale: 3.5 – 4.
There have been many articles published, concerning both pure and applied aspects, on the zeolite, clinoptilolite3,4; however, the use of radiation to modify the properties of zeolites is relatively unreported5,6,7,8. The present communication focuses on a specific application, which is the employment of an initially irradiated clinoptilolite for the absorption of radioactive cations from aqueous media, as in the treatment (“clean-up”) of radioactive wastewater from the Armenian Nuclear Power Plant (ANPP). Indeed, this is one of the first reports of the use of radiation-modified clinoptilolite samples for this purpose, which is shown to be a highly effective material in reducing of the overall radioactivity of the water. Since the liquid radioactive wastes from nuclear power plants (NPP) contain far more cesium than other radioactive elements, our focus is on the removal of this particularly, by sorption into the zeolite cages through cation-exchange, during the cleaning process.
Results
Radioactive wastewater from the Armenian Nuclear Power Plant with a low concentration of Na+ and K+, was chosen for this investigation. The additional chemical elements present were: Cl - 0.15 mg/kg, NH4+ - 0.5 mg/kg, Na - 0.15 mg/kg, K - 0.2 mg/kg, B - 12.6 g/L. The initial pH of the solution was 5.9, but this was increased to pH 12 by the addition of aqueous NaOH. The initial radioactivity of the water was: 137Cs – 2.9·104 Bq/L, 134Cs – 2.4·104 Bq/L, 60Co – 4.7·103 Bq/L.
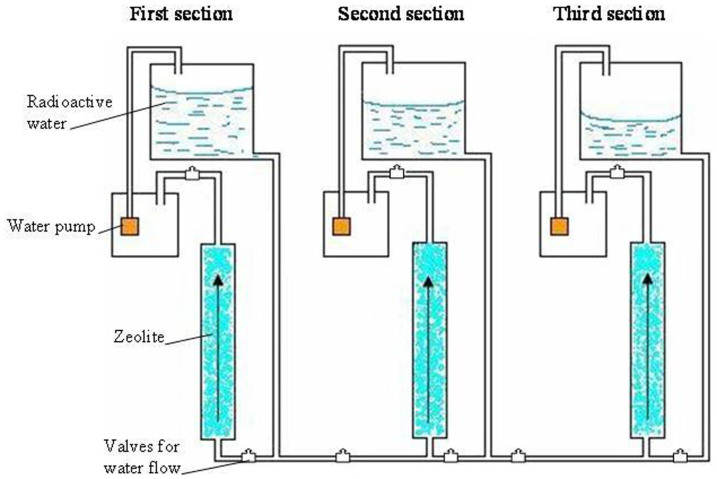
Semiconductor detectors with a high efficiency were applied to measure the water radioactivity. The average count time of the detector was about an hour at the background level of 30–40 counts/min and the “Genie” program was used. At least 10 different zeolite samples were counted to obtain sufficient statistics and a good correspondence of results (80–90%) was found. The three column system applied (Fig. 2) in these experiments proved to be a useful and efficient laboratory installation. In order to determine the efficiency of the entire 3-column installation, all 3 columns were connected consecutively: described as columns N° 1, 2, 3.
Figure 2. Automatic cleaning installation of radioactive waste water from nuclear reactor.
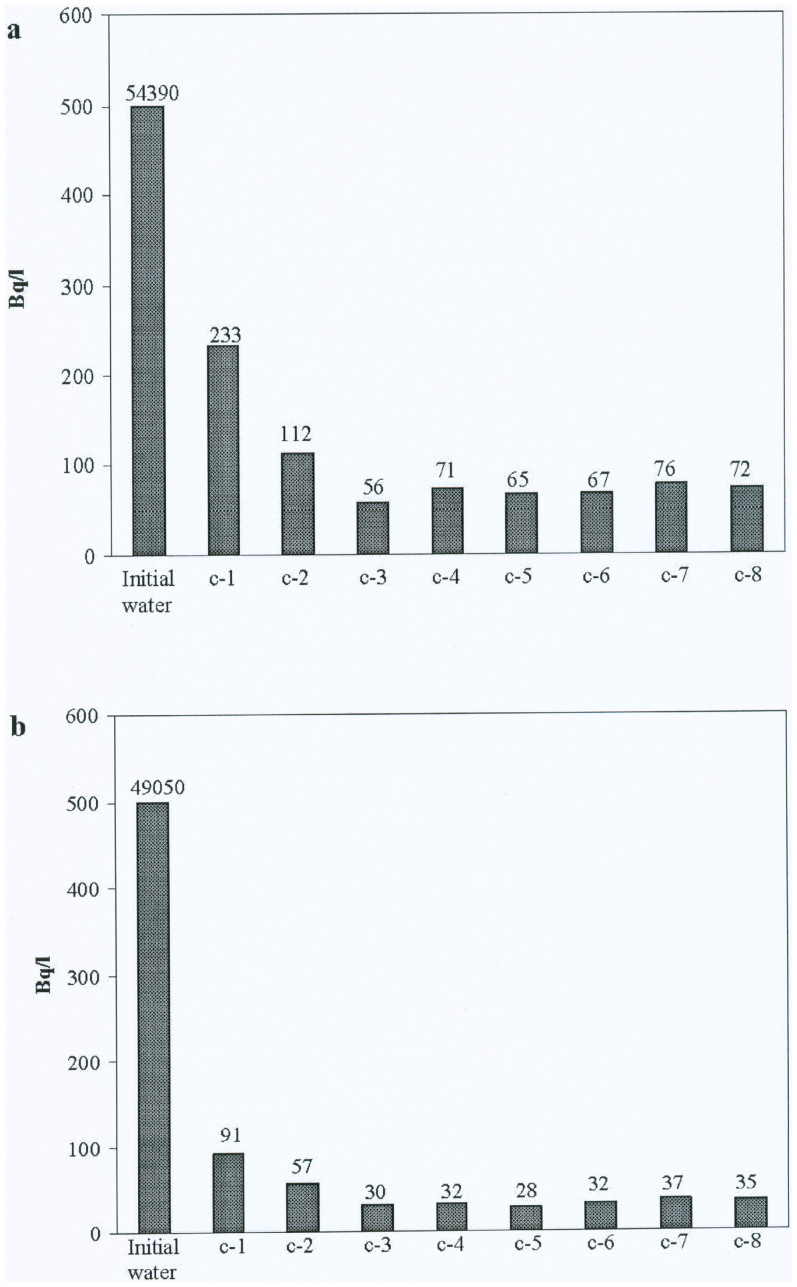
Typical values are presented in the Tables 2, 3 below and also graphically in Fig. 3. The water radioactivity was determined following each cycle after passing through the 3 columns of the installation. Each measurement was repeated some 8–10 times to ensure statistical consistency. Electron-irradiation of each zeolite sample was performed at five different doses, totaling around 100 separate experiments.
Table 2. Water cleaning scheme – 3 cycles thorough column 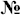 1 (natural clinoptilolite).
1 (natural clinoptilolite).
| Radioactivity (Rad.) | ||||||
|---|---|---|---|---|---|---|
| Nuclide | Energy keV | Intensity | error% | Ci/l | [Bq/L] | Rad. reduction factor, 0/n |
| Initial activity of water | ||||||
| 137Cs | 662 | 3.55 × 101 | 3 | 7.84 × 10−7 | 29030 | |
| 134Cs | 796 | 1.84 × 101 | 4 | 6.49 × 10−7 | 24060 | |
| 60Co | 1173 | 1.22 × 100 | 6.5 | 1.3 × 10−7 | 4720 | |
| Cycle – 1 | ||||||
| 137Cs | 662 | 1.47 × 100 | 6 | 0.23 × 10−7 | 860 | 33.7 |
| 134Cs | 796 | 1.83 × 10−1 | 6 | 0.12 × 10−7 | 474 | 50.7 |
| 60Co | 1173 | 2.3 × 10−1 | 9 | 0.14 × 10−7 | 535 | 8.82 |
| 4051 Cycle – 2 | ||||||
| 137Cs | 662 | 1.12 × 100 | 7 | 0.16 × 10−7 | 611 | 47.5 |
| 134Cs | 796 | 2.09 × 10−1 | 6 | 0.11 × 10−7 | 397 | 60.6 |
| 60Co | 1173 | 2.29 × 10−1 | 11 | 0.14 × 10−7 | 535 | 8.82 |
| Cycle – 3 | ||||||
| 137Cs | 662 | 9.27 × 10−1 | 8 | 0.11 × 10−7 | 405 | 71.7 |
| 134Cs | 796 | 1.68 × 10−1 | 10 | 0.086 × 10−7 | 320 | 75.2 |
| 60Co | 1173 | 2.26 × 10−1 | 9 | 0.14 × 10−7 | 535 | 8.82 |
Table 3. Water cleaning scheme – 8 cycles thorough columns  1,2,3 consequently.
1,2,3 consequently.
| Radioactivity (Rad.) | ||||||
|---|---|---|---|---|---|---|
| Nuclide | Energy keV | Intensity | error% | Ci/L | [Bq/L] | Rad. reduction factor 0/n |
| Initial activity of water | ||||||
| 137Cs | 662 | 3.19 × 101 | 4 | 14.46 × 10−7 | 54390 | |
| 134Cs | 796 | 2.58 × 101 | 6 | 13.26 × 10−7 | 49050 | |
| 60Co | 1173 | 1.74 × 100 | 8 | 1.09 × 10−7 | 4051 | |
| Cycle1 | ||||||
| 137Cs | 662 | 3.89 × 10−1 | 9 | 0.06 × 10−7 | 233 | 233 |
| 134Cs | 796 | 4.8 × 10−2 | 14 | 0.024 × 10−7 | 91 | 539 |
| 60Co | 1173 | 7.33 × 10−2 | 15 | 0.046 × 10−7 | 170 | 23.8 |
| Cycle 2 | ||||||
| 137Cs | 662 | 2.14 × 10−1 | 14 | 0.03 × 10−7 | 112 | 485 |
| 134Cs | 796 | 3.01 × 10−2 | 10 | 0.015 × 10−7 | 57 | 860 |
| 60Co | 1173 | 7.8 × 10−2 | 12 | 0.05 × 10−7 | 181 | 22.4 |
| Cycle 3 | ||||||
| 137Cs | 662 | 2.86 × 10−1 | 9 | 0.015 × 10−7 | 56 | 971 |
| 134Cs | 796 | 1.65 × 10−2 | 11 | 0.009 × 10−7 | 30 | 1635 |
| 60Co | 1173 | 6.78 × 10−2 | 16 | 0.042 × 10−7 | 158 | 25.6 |
| Cycle 4 | ||||||
| 137Cs | 662 | 1.84 × 10−1 | 17 | 0.019 × 10−7 | 71 | 766 |
| 134Cs | 796 | 1.68 × 10−2 | 12 | 0.009 × 10−7 | 32 | 153.2 |
| 60Co | 1173 | 5.68 × 10−2 | 14 | 0.036 × 10−7 | 132 | 30.7 |
| Cycle 5 | ||||||
| 137Cs | 662 | 1.97 × 10−1 | 16 | 0.018 × 10−7 | 65 | 837 |
| 134Cs | 796 | 1.49 × 10−2 | 14 | 0.0075 × 10−7 | 28 | 1751.8 |
| 60Co | 1173 | 8.67 × 10−2 | 16 | 0.054 × 10−7 | 201 | 20.15 |
| Cycle 6 | ||||||
| 137Cs | 662 | 1.95 × 10−1 | 15 | 0.0181 × 10−7 | 67 | 811 |
| 134Cs | 796 | 1.51 × 10−2 | 14 | 0.009 × 10−7 | 32 | 1532.8 |
| 60Co | 1173 | 8.527 × 10−2 | 17 | 0.067 × 10−7 | 190 | 21.3 |
| Cycle7 | ||||||
| 137Cs | 662 | 1.71 × 10−1 | 14 | 0.02 × 10−7 | 76 | 715 |
| 134Cs | 796 | 1.9 × 10−2 | 17 | 0.01 × 10−7 | 37 | 1325 |
| 60Co | 1173 | 8.0 × 10−2 | 16 | 0.050 × 10−7 | 192 | 21.1 |
| Cycle 8 | ||||||
| 137Cs | 662 | 1.62 × 10−1 | 13 | 0.002 × 10−7 | 72 | 755 |
| 134Cs | 796 | 1.87 × 10−2 | 15 | 0.0096 × 10−7 | 35 | 1401 |
| 60Co | 1173 | 9.62 × 10−2 | 18 | 0.058 × 10−7 | 217 | 18.7 |
Figure 3. Cleaning dynamics of 137Cs (a) and 134Cs (b) thorough columns  1, 2, 3 connected in sequence (water specific activity, Bq/L).
1, 2, 3 connected in sequence (water specific activity, Bq/L).
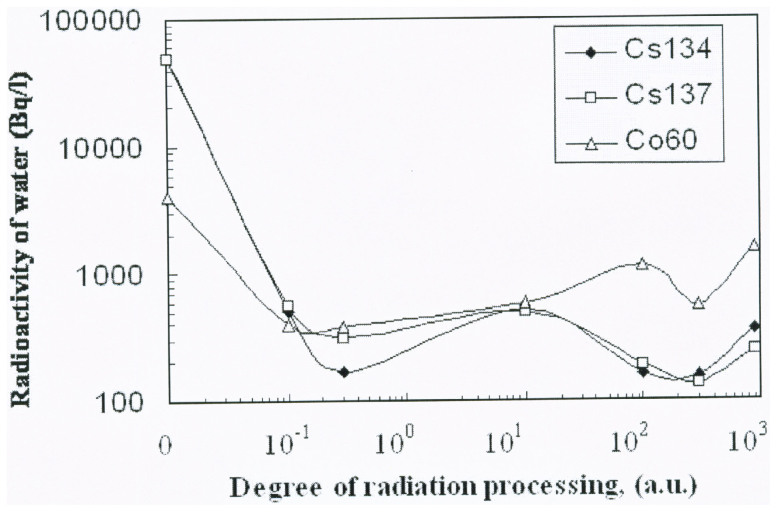
The reduction of radioactivity from ANPP waste water by means of electron irradiated clinoptilolite, as a function of the degree of electron irradiation (dose) is presented in Fig. 4 for the principal radionuclides: 134Cs, 137Cs and 60Co.
Figure 4. Reduction of waste water radioactivity from ANPP depending on clinoptilolite sorbent electron irradiation processing degree.
The results show the absorption of radioactive cations as is specified for the prevailing degree of radiation modification, and which, at a maximum, corresponds to a reduction in radioactivity from 137Cs by a factor of more than 800 and 134Cs by a factor of more than1750, indicating that an isotope fractionation operates and which serves to preferentially absorb the lighter isotope. Accordingly, the maximum initial radioactivity of the water was 2.9·104 Bq/L, but after processing this was reduced to 30 Bq/L. This may be compared with the acceptable level for which water may be employed in technical applications, i.e. 3 Bq/L. When the untreated (non irradiated) zeolite was used, the reduction in the radioactivity level of the water was reduced by a similar factor to that reported in the literature12. However, while the different degrees of reduction (72 for 137Cs and 75 for 134Cs) indicate that an isotope fractionation serves for the untreated clinoptilolite samples, it is clear that the effect is markedly enhanced when the zeolite is modified by the radiation treatment.
Discussion
From the results obtained for cleaning radioactive wastewater from the ANPP (summarized in Fig. 4), it is clear that the radiation modification of clinoptilolite leads to a considerable enhancement in its capacity to sorp radioactive cations. This is due to an increase in the sorption capacity at the surface of the zeolite grains, caused by the energy delivered by the radiation. The effect is more pronounced for cations with a higher ionic radius (rCs+ ~ 1.67 Å, rSr2+ ~ 1.44 Å) than for those cations with a smaller ionic radius (rCo3+ ~ 0.63 Å; rAg+ = 1.13 Å). While another fission radionuclide (I) has a larger ionic radius (rI− = 2.2 Å), it is not sorped into the zeolite by cation exchange, since it carries a negative rather than a positive charge and is effectively repelled by the negatively charged zeolite framework. There are different effects8,13,14 that can occur when zeolites are exposed to radiation, but which may be grouped broadly as (i) ionization (radiolysis) and (ii) atomic displacements by nuclear collisions (“knock on” effects). The latter are expected to contribute more greatly when high-energy radiation is being employed, as in the present study. Thus, radiation shaking, may lead to the removal of different molecular species that were originally contained in the zeolite cage pores, and changes in the charge-states of sub-lattices may occur to provide sites for the capture of radioactive Cs+ and other cations. It is likely that part of the mechanism by which the cation-exchange capacity of clinoptilolite toward Cs+ is so markedly improved by prior electron-irradiation involves the radiolysis and displacement of water molecules that are entrapped in the zeolite micropores, along with the creation of other point-defects and (charged) radiation damage centres where Cs+ (along with other radionuclide cations) can be trapped, although there is much to be understood about the process as yet. It was proposed previously that hydroxyl radicals, formed by the radiolysis of water, might be able to break Si-O-Si or Si-O-Al bonds in the zeolite framework, and that the radiolytic production of hydrated protons [H+(H2O)n] can also induce the cleavage of these bonds15. A variety of charged defect centres have also been characterized in zeolites and related materials formed by exposure to radiation of various kinds, e.g. A- and A'-centres (trapped holes on oxygen from Si-O bonds), and B-centres, which are sites where an electron has been captured at an Al-O unit. Positively charged (hole) sites of the type, Si-O○-Si and Si-O○-Al have been identified by E.P.R. spectroscopy in irradiated zeolite samples15, and a wide range of radical cations formed by charge-transfer from adsorbed organic molecules to the zeolite, have also been characterized16. However, the nature of the corresponding electron capture site is less clear16. In a recent study, the catalytic performance of the zeolite SSZ-13 was shown to be enhanced by neutron irradiation, in which Si-O-O• peroxyl radicals and nonbridging oxygen hole centres (NBOHC: Si-O○) were identified and are proposed to be the active defects responsible for the catalyst modification15. Most noteworthy is the fact that in previous studies of zeolites that were exposed to high-energy electrons, a partial or complete amorphization of the initially crystalline structure occurred, accompanied by a dramatic reduction (by up to 95%)13,14 in the cation-exchange and absorption/desorption capacities of the zeolite for radionuclides, such as Cs+ and Sr2+. Our present results of an enhanced sorption of Cs+ and Sr2+ (and significantly so) caused by exposure of high-energy electrons are the exact opposite of this. More comprehensive irradiation dose dependence measurements are necessary to determine the highest possible enhancement parameter.
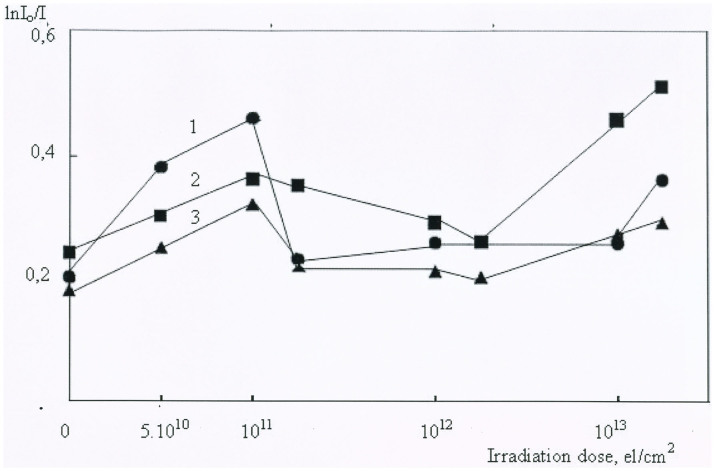
To obtain further insight into the present case, we have measured the infrared absorption spectra (IR) and the dielectric permittivity (fig. 5) of the irradiated clinoptilolite samples. Fig. 6 shows the variation in the intensity of the λ = 1.91 μm band as a function of radiation dose, for (i) the zeolite treated with acetic acid, prior to the irradiation, (ii) the untreated zeolite, and (iii) the zeolite treated with aqueous ammonium chloride, prior to the irradiation. The 1.91 μm wavelength corresponds to 5236 cm−1 which accords with water residing in the zeolite pores17, and we note that when this became a minimum (for irradiation doses of 1012–1013 e/cm2), the intensity of the λ = 2.94 μm band (3450 cm−1) became a maximum. The latter frequency accords with one of the vibrational signatures for free water18. We interpret these changes in terms of both ionization and knock on effects on water molecules confined in the zeolite pores. Water molecules can be displaced easily, either by direct collision, or as constituent fragments separated by radiolysis. Thus, the structural water may be either displaced as an intact molecule or in the form of separate ions and radicals.
Figure 5. Electron irradiation dose dependence of natural clinoptilolite dielectric constant ε′ for some frequencies of the electric field.
Figure 6. Electron irradiation dose dependence of the relative intensity of an absorption band at λ = 1.91 μm for the chemically treated and raw samples: 1 - treated by CH3COOH; 2-raw zeolite (clinoptilolite); 3 - treated by NH4Cl.
The intensity of the 1100 cm−1 (9 μm) band was observed to decrease and a new absorption band of 830 cm−1 (12 μm) appeared to increase following a radiation dose in the region of 1013 e/cm2. This can probably be attributed to a change in the oxygen state from an interstitial to a lattice site because the irradiation creates many more vacancies of Si and Al atoms and releases new sites for mobile oxygen atoms. The situation resembles the so-called “A-center” in silicon crystals following irradiation, where interstitial oxygen atoms move to silicon atom vacancies generated by irradiation, and then form oxygen (hole) vacancy complexes, which have an IR absorption band at 12 μm (833 cm−1).
To understand the effect of electron irradiation on the zeolite framework structure, we assume that in the clinoptilolite crystal sample there is a dominant and sufficient concentration of oxygen atoms (1019 cm−3–1021 cm−3) which can form bonds with vacancies (V) or interstitial atoms (I) and also form capture centers:
where V, I are Si and Al vacancy interstitials, respectively, O is the oxygen atom. The concentration of V or I is defined by the irradiation dose:
where σ is the probability (cross section) of defect production (cm2), N0 is the concentration of Si or Al atoms (cm−3) in clinoptilolite, ϕ is the irradiation dose (cm−2). Since the ratio of SiO2/Al2O3 = 9. 6, in the clinoptilolite samples, to a degree of approximation one can apply the results obtained for silicon crystals11, i. e. σSi ≅ 10−23 cm2; N0 = 6·1023 cm−3. For the ϕ = 1013 e/cm2 dose one can derive from (2): NI,V = 10−23 cm2·6·1023 cm−3·1013 cm−2 ≅ 6·1013 e/cm−3. These A-centers act as acceptors and are able to capture positively charged radionuclides in a practically useful amount from NPP waste waters.
The results presented herein are in accord with our previous findings for electro-physical and optical properties of zeolites, and in particular, an enhanced sorption of Cs+ cations6,7.
Both the fundamental scientific, and potential practical significance of these results makes it desirable to further the present studies and moreover to develop and design pilot industrial installations, taking into account factors such as: zeolite grain size, dimensions (diameter and length) of the column, water flow rate, optimum pH, to optimize the final cleaning technology for radioactive liquid waste and also municipal sewage processing.
Methods
Radiation-modified natural zeolites (mainly clinoptilolite) were tested for the treatment of waste water taken from the Armenian Nuclear Power Plant (ANPP). The radiation modification was performed by irradiating the zeolite samples at room temperature with 8 MeV electrons, and at various doses in the range 1·1012 e/cm2–3*1016 e/cm2 with an error close to 15%, as produced by the linear electron accelerator ELU-8 at the Yerevan Physics Institute. By way of information, we note that for a zeolite, an irradiation dose of 1013 e/cm2 corresponds to an absorption dose in the range of 1 Mrad. All experiments were performed using untreated (raw) zeolite samples, i.e. as mined from the ground, which were then selected and granulated. An energy of 8 MeV was chosen according to previous experiments, in which we employed electrons with energies in the range 3 MeV–10 MeV, and discovered that 8 MeV electrons are optimal for the generation of point structural defects, while simultaneously avoiding any disruption in the stoichiometry of the material, permitting a full penetration of electrons through the body of the sample and avoiding the induction of residual radioactivity. The clinoptilolite concentration in all the samples used was in the range 83−85%.
XRD analyses were applied to determine the clinoptilolite concentration in the samples, yielding crystallographic characteristics from which a pore size of 3.6–4.4 Å was defined. Samples containing up to 85% clinoptilolite, with the highest absorption capacity and a Si/Al ratio of 9.8, were selected from more than different 30 raw specimens. Other contaminations in the raw materials were determined, on the basis of chemical, I.R. and thermogravimetric analysis, to be: feldspar – 5%, quartz – 5%, mica – 2%, clays – 3%, with an error of 1%. A water absorption capacity of ca 20 wt% was obtained for a range of raw and chemically treated zeolite samples.
We have shown that the irradiation up to a dose of 3.1016 electron/cm2 significantly influences the properties of clinoptilolite. However, the corresponding changes in the dielectric and optical properties of these materials are non-monatonic which indicates the complexity of the underlying process (Fig. 5 and Fig. 6). The radiolytic and displacement effects on adsorbed water must also be considered. A radiation dose of 3·1016 electron/cm2 at an energy of 8 MeV appears critical to promote structural changes in these natural zeolite samples, which is signified by a pronounced variation in the dielectric properties and optical characteristics of the materials9,10.
Most of the radioactivity in wastewaters from nuclear power plants is from Cs+ cations, and consequently, our main focus was upon decreasing the concentration of the isotopes 134Cs and 137Cs, although the radioactivity from secondary elements I−, Ag+, Co3+ was also measured, which are also present in NPP wastewater. However the concentrations of I−, Ag+ nuclides are comparatively small and the present strategy of employing a radiation modified zeolite is less effective in removing them.
An automatic installation consisting of a three-column stainless steel tube system was designed and employed in the present study (Fig. 2). Column 1 was filled with natural granular clinoptilolites, while columns 2 and 3 were filled with the radiation modified zeolite. The radioactive water enters the column from the bottom and, after passing through the clinoptilolite sorbent, enters into an intermediate tank, fitted with a small pump. When the water reaches a certain level, the pump is automatically activated, so to transfer the cleaned water to a storage tank for further processing, by passage either through the same column or another column in the series.
This equipment acts in two regimes: step-by-step and effectively as an autonomous cleaning system (Fig. 2). The parameters are:
A three column system which can be operated separately and in combination. Each column is of the following dimensions: length −50 cm, diameter −4 cm.
The multiplicity and velocity of radioactive water flow was defined by the time intervals measured over a period of a few hours, after which measurements were made of levels of radioactivity and chemical concentrations.
Different clinoptilolite containing zeolite samples were tested and those with a high sorption ability (85%) were selected.
The clinoptilolite grains have a diameter range: 1.6–2.2 mm.
This study shows the highly efficient nature of radiation modification of zeolites in removing radionuclides from the liquid wastes generated by nuclear reactors. We believe that these preliminary results demonstrate a unique phenomenon which, in view of its potential practical importance, demands a wider awareness and further detailed investigation. Since we have applied the method to a real situation, i.e. to clean wastewaters taken directly from the Armenian Nuclear Power Plant, there is the clear potential for such radiation-modified zeolites to be applied to this and other similar installations, on a practical scale.
Author Contributions
H.Y. supervised all works, A.S. and A.H. carried out electron irradiation in accelerator, V.H. and V.A. carried out works in Armenian nuclear Power Plant, E.H. and N.G. prepared zeolite samples, S.N. and C.R. performed data analysis, Y.K. took part in chemical analyses of samples.
References
- Breck D. W. Zeolite Molecular Sieves: Structure, Chemistry and Use. (J, Wiley & sons, New York, 1974).
- Armbruster & Gunter M. E. Crystal Structure of Natural Zeolites. Rev. in Miner. and Geoch., Nat. Zeol.: Occur., Prop., Appl. 45, 1–57 (2001). [Google Scholar]
- Denes Applications of Natural Zeolites in Water and Waste Water Treatment. Rev. in Miner. and Geoch., Nat. Zeol.: Occur., Prop., Appl. 45, 519–550 (2001). [Google Scholar]
- Petrakakis Y., Mylona E., Georgantas D. & Grigoropoulau H. Leaching of lead from clinoptilolite at axidic conditions. J. Global NEST. 9, 207–213 (2007). [Google Scholar]
- Wang L. M., Wang S. X. & Ewing R. C. Radiation Effects in Zeolite. Proceedings of the 9-th Annual International Radioactive Waste Management, American Nuclear Society, Las Vegas, May 11–14, 772–775 (1998).
- Gevorgyan R. G. et al. Study of Absorption Properties of Modified Zeolites. Chemie der Erde Geochemitry 62, 237–342 (2002). [Google Scholar]
- Akhalbedashvili L. et al. Ion exchange properties of irradiated and chemically modified clinoptilolite regarding to Cs+ and Sr+2. International Symposium Devoted to the 80th Anniversary of Academician O.O.Chuiko, Modern Problems of Surface Chemistry and Physics, 18–21 May, Kiev-Ukraine, 45–46 (2010).
- Dikiy N. P. et al. The Influence of Radiation and Temperature on Diffusion of Radionuclide 22Na from Tuff and Clinoptilolite. J. Visnik Kharkivskogo Universitetu 946, 29–36 (2011). [Google Scholar]
- Yeritsyan H. N. et al. Electrophysical and optical properties of natural zeolites (clinoptilolite) from Armenia and USA, Zeolite 06, 7th International Conference on the Occurrence, Properties, and Utilization of Natural Zeolites, Book of Abstracts 25, Ed. Robert, S. Bowman & Susan, E. Delap), Socorro, New Mexico, USA, July2006.
- Yeritsyan H. N. et al. The effect of electron irradiation on the optical properties of the natural armenian zeolite-clinoptilolite. Central European Journal of Physics, CEJP 3, 623–635 (2005). [Google Scholar]
- Vavilov V. S. The influence of irradiation on the Semiconductors. Moscow, (1963).
- Pabalan R. T. Natural zeolites in nuclear waste management. Book of Abstracts. Zeolite 02, 6-th International Conference on the occurrence, properties and utilization of Natural Zeolite, Thesaloniki, Greece, 8, June2002.
- Wang S. X., Wang L. M. & Ewing R. C. Electron and ion irradiation of zeolites. J. Nuclear Materials 278, 233–241 (2000). [Google Scholar]
- Wang L. M., Chen J. & Ewing R. C. Radiation and thermal effects on porous and layer structured materials as getters of radionuclides. Current Opinion in Solid State and Materials Science 8, 405–418 (2004). [Google Scholar]
- Sommer L. et al. Enhanced catalyst performance of zeolite SSZ-13 in the methanol to olefin reaction after neutron irradiation. J. Phys. Chem. C 115, 6521–6530 (2011). [Google Scholar]
- Rhodes C. J. Spectroscopic Characterisation of Molecules Adsorbed at Zeolite Surfaces. Annu. Rep. Prog. Chem. 106, 36–76 (2010). [Google Scholar]
- Karge H. G. & Weitkamp J. (Eds.). Molecular Sieves (Springer, Berlin 2003).
- Nakamoto K. Infrared and Raman spectra of inorganic and coordination compounds (5th edition), 170–171 (Wiley, 1997).