Abstract
Daily life functions such as sleep and feeding oscillate with circa 24 h period due to endogenous circadian rhythms generated by circadian clocks. Genetic or environmental disruption of circadian rhythms is associated with various aging-related phenotypes. Circadian rhythms decay during normal aging, and there is a need to explore strategies that could avert age-related changes in the circadian system. Exercise was reported to delay aging in mammals. Here, we investigated whether daily exercise via stimulation of upward climbing movement could improve circadian rest/activity rhythms in aging Drosophila melanogaster. We found that repeated exercise regimen did not strengthen circadian locomotor activity rhythms in aging flies and had no effect on their lifespan. We also tested the effects of exercise on mobility and determined that regular exercise lowered age-specific climbing ability in both wild type and clock mutant flies. Interestingly, the climbing ability was most significantly reduced in flies carrying a null mutation in the core clock gene period, while rescue of this gene significantly improved climbing to wild type levels. Our work highlights the importance of period in sustaining endurance in aging flies exposed to physical challenge.
Keywords: Circadian clock, exercise, endurance, aging
1. Introduction
Aging leads to the weakening of circadian rhythms such as sleep/wake cycles. These rhythms are generated by the circadian clock, which is based on cell-autonomous negative feedback loops involving clock genes that display molecular oscillations with a circa 24 h periodicity. Clock genes are conserved from Drosophila to humans and their oscillatory activities orchestrate rhythms at the molecular, physiological, and behavioral levels (Hardin, 2011). In Drosophila, two transcription factors encoded by the genes Clock (Clk) and cycle (cyc) form CLK-CYC heterodimers, which stimulate the transcription of period (per) and timeless (tim) genes early at night. This leads to periodic increases in per and tim mRNA levels, and subsequently PER and TIM proteins. The latter accumulate in cell nuclei and repress CLK-CYC activator complexes. Subsequent degradation of TIM and PER proteins leads to de-repression of CLK-CYC and the onset of the next molecular oscillator cycle (Hardin, 2011). Circadian rhythms persist in experimental conditions of constant darkness (DD) with a ~24 h free-running period, but are normally entrained to daily light/dark (LD) cycles that serve as Zeitgebers (time givers).
Clock gene oscillations and circadian output rhythms become weaker during aging in mammals (Kondratova and Kondratov, 2012). In addition, chronic disruption of clock functions accelerates physiological aging and late life diseases in mice (Kondratov et al., 2006) suggesting that age-related decline in circadian rhythms may contribute to aging processes. Aging fruit flies also show fragmented sleep/activity patterns and dampened clock gene oscillations (Koh et al., 2006; Luo et al., 2012; Rakshit et al., 2012). Furthermore, disruption of circadian rhythms by a null mutation in the per gene (per01) increases susceptibility to oxidative stress and neurodegeneration in aging flies (Krishnan et al., 2009; Krishnan et al., 2012), providing evidence that the links between circadian desynchrony and aging are conserved from flies to mammals.
Mechanisms underlying age-related decay of the circadian system are not understood, and there is search for treatments that could counteract the loss of rhythmicity. Rest/activity rhythms in old flies can be strengthened by manipulations of light and temperature (Luo et al., 2012). In addition to these strong Zeitgebers, locomotor activity rhythms can be also entrained by other factors repeated every 24 h, such as daily restricted access to food, or periods of physical exercise. Scheduled exercise performed at specific time of the day can shift the phase of rest/activity rhythms in mammals, and access to a running wheel alleviates some age-related changes in the mouse circadian system (Yamanaka et al., 2010; Leise et al., 2013). On the other hand, the free-running period of circadian rhythms appears to be insensitive to scheduled exercise in humans (Cain et al., 2007).
Although Drosophila is an excellent model for circadian biology and aging, the effects of exercise on rhythms in aging flies have not been previously investigated. Only a few studies addressed the role of physical activity on age-related functional decline in Drosophila. One study demonstrated detrimental effects of lifelong voluntary flight activity on lifespan (Magwere et al., 2006). In another study, flies were induced to perform repetitive rapid climbing for several hours each day (Piazza et al., 2009). The latter study reported that flies subjected to a specific exercise-training paradigm showed significant delay in age-related mobility decline relative to flies in which geotaxis was triggered but climbing was prevented by limited space. However, both groups showed decreased climbing compared to the undisturbed control flies (Piazza et al., 2009). With increasing evidence of the beneficial effects of exercise in aging humans, further research is required to determine whether these effects can be modeled in flies.
In this study, we used D. melanogaster to investigate the relationships between physical activity, circadian rest/activity rhythms, and lifespan. First, we sought to determine whether daily exercise in the form of climbing, could increase the locomotor activity rhythm strength during aging and affect lifespan. Second, we investigated the effects of scheduled physical activity on age-related loss of mobility in wild-type flies and core clock gene mutants with disrupted circadian rhythms.
2. Methods
2.1. Fly rearing and stocks
Flies were reared on yeast-cornmeal-molasses-agar diet (35 g yeast/l) at 25°C in 12 h light: 12 h dark (LD 12:12) cycles with light levels of approximately 800 lux. All experiments were performed between 1 and 4 h after lights-on, which is a natural morning activity period in flies. s. Clock mutants per01 (Konopka and Benzer, 1971), tim01 (Sehgal et al., 1994) and ClkJrk (Allada et al., 1998) were backcrossed by genotyping to the wild-type Canton S (CS) flies for eight generations to equalize the genetic background. Control flies designated a s CSp for per01, CSt for tim01, and CSJ for ClkJrk, were derived from heterozygous siblings of respective mutant lines. Since CSp, CSt, and CSJ flies showed similar climbing, they were pooled together for greater statistical power in experiment where effects of exercise in wild type were tested. To rescue per function, we used transgenic flies carrying a wild type copy of per on the third chromosome (Cheng et al., 1998) combined with X carrying per01 and the second chromosome of our per01 mutant stock. To determine lifespan, 3–4 cohorts of 25 flies of each genotype were housed in Drosophila vials capped with cotton and containing diet on the bottom. Flies were transferred to fresh vials every 2–3 days without anesthesia, and mortality was recorded at this time.
2.2. Exercise training device and protocols
In building the exercise device, we took advantage of the flies’ instinctive negative geotaxis behavior to induce upward walking. Vials with diet housing 25 flies each were loaded vertically into a transparent box that could be rotated about its horizontal axis by a 12V electric motor with a gear reducing its shaft speed to about 1/2 revolution per second. A cam mounted on the motor’s shaft activated an electromechanical micro switch at two positions 180° apart. The 12 V DC supply for the motor passed through an electronic gate that could be set in “on” or “off” states. It was set “on” by a pulse generated every 10 s by an electronic timer based on standard 555 chip, and “off” by a signal from the micro switch activated by the cam. Thus, each timing pulse caused a flip of the box by 180°, which stimulated flies to climb (Supplemental movie 1). Most flies continued to respond by climbing throughout the exercise period; a few which failed to climb were actively walking at the bottom of the vial. In the initial experiment, 20 day-old flies were exercised for 1 h, five times a week for 2 weeks and then their locomotor activity and longevity were recorded after the conclusion of exercise. In the subsequent experiment, we used the published exercise regime that was reported to increase the climbing vigor of flies (Piazza et al., 2009). Namely, 7–8 day-old flies were exercised for 3 weeks in a ramping protocol with 2 h of exercise in week 1, 2.5 h in week 2, and 3 h in week 3.
2.3. Rapid Iterative Negative Geotaxis (RING) assay
Age-specific mobility was tested using the RING assay at room temperature (25 ± 1°C) (Gargano et al., 2005). Three groups of 25 flies were transferred into empty narrow vials that were loaded into the RING apparatus. After 3 min rest, the apparatus was tapped to initiate a negative geotaxis response. The flies’ movement in tubes was videotaped and digital images captured 4 s after initiating the behavior. Five consecutive trials were interspersed with a 30 s rest. Average height climbed by all flies in each vial during the 4 s interval was calculated, and the climbing performance was averaged for three vials of a given genotype and age. We also calculated the decline time (DT) for loss of climbing function to 50 or 75% of its peak value, interpolated from second-order polynomial curve fits as previously described (Martin et al., 2005).
2.4. Locomotor Activity Analysis
Flies were entrained in LD 12:12 at 25°C, and their locomotor activity was recorded for 3 days in LD 12:12, followed by 7 days in constant darkness (DD) using the Trikinetics locomotor activity monitor (Waltham, MA) and analyzed using ClockLab software (Actimetrics; Coulbourn Instruments, Whitehall, PA) as described (Rakshit et al., 2012). For a quantitative measure of circadian rhythmicity in DD, Fast Fourier Transform (FFT) analysis was conducted. Flies with FFT values <0.04 were classified as arrhythmic, ones with values of 0.04–0.08 were classified as weakly rhythmic, whereas flies with FFT values >0.08 were considered strongly rhythmic. Flies with both weak and strong rhythms that showed a single peak in the periodogram were included in the calculation of the free running period.
2.5. Statistical Analysis
Data were statistically analyzed with GraphPad Prism (v.5.0) and GraphPad Instat (v.3.0; San Diego, CA). The RING data were evaluated by two-way analysis of variance (ANOVA) with Bonferroni’s post hoc test. Life span and survival curves were plotted following Kaplan Meier survival analysis and statistical significance of curves assessed using the Log-Rank (Mantel-Cox) test.
3. Results and Discussion
3.1. Scheduled exercise does not improve circadian rhythms or lifespan in wild type flies
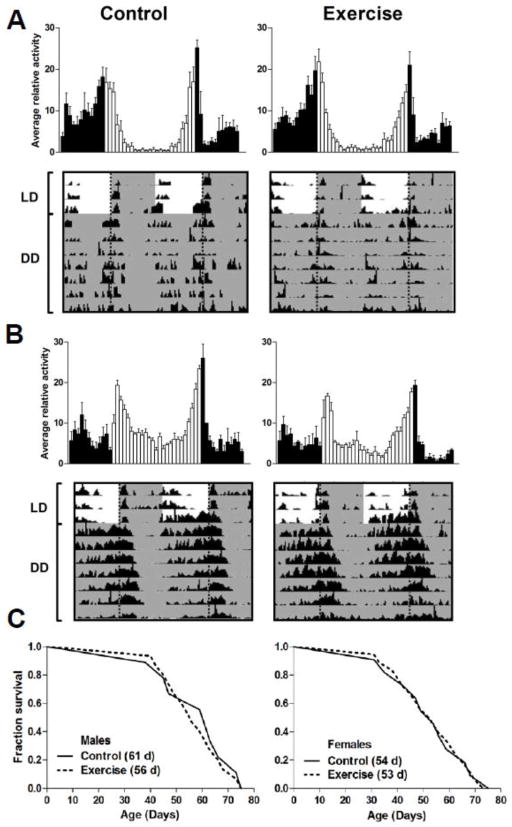
Given that exercise was suggested as a rhythm-entraining stimulus in mammals, we tested the effects of induced climbing on several parameters of circadian rest/activity rhythms. Day 20 flies reared in LD 12:12 were placed in the exercise apparatus 1 h after lights-on (ZT 1). The apparatus was flipped 180° every 10 s, initiating upward movement of flies. After 2 weeks of daily exercise, individual flies were loaded into the activity monitor and their rest/activity rhythms were recorded. Both control and exercised males showed typical bimodal activity rhythms in LD with morning and evening peaks of activity, and there was no obvious difference in the activity profiles between the two groups (Fig. 1A). Analysis of free-running rhythms in DD determined that both control and exercised males showed age-related rhythm weakening. More than 50% of males in both groups became arrhythmic with age, and the average rhythm strength in the remaining rhythmic males was low (Fig. 1A and Table 1). Both groups had similar free-running period and similar daily average activity counts (Table 1). In comparison to males, females had stronger activity rhythms, but again no difference was apparent between control and exercised females (Fig. 1B), except that average daily activity counts were significantly lower in exercised females (Table 1). Together, these data demonstrate that 1 h of exercise at the time that flies are normally active had no discernible effect on the rest/activity pattern in LD or the free-running period in aging flies. We cannot exclude that different amount or timing of exercise schedule could affect these rhythm parameters; however, congruent with our data, scheduled exercise did not alter the circadian period in humans (Cain et al., 2007).
Figure 1. Exercise regime does not improve circadian rhythms or lifespan in wild type flies.
Average daily activity in LD 12:12 (top panel) and representative actograms (bottom panel) of 35 day-old undisturbed controls and exercised wild type CS A) males and B) females that were subject to 1 h morning exercise for 2 weeks starting on day 20. C) Kaplan-Meier survival curves showing similar lifespan of CS flies subjected to the exercise regimen compared to the undisturbed controls.
Table 1.
Locomotor activity analysis of exercised and control flies
| Genotype & Treatment | n | % Rhythmic (Strong + Weak)* | Rhythm Strength (Avg. FFT) | Period (DD) | Avg. Daily Activity (LD) |
|---|---|---|---|---|---|
| CS (Males) | |||||
| Control | 25 | 44% (4% + 40%) | 0.06 ± 0.01 | 24.6 ± 0.2 | 305 ± 29 |
| Exercised | 38 | 47% (11% + 36%) | 0.07 ± 0.01 | 24.8 ± 0.3 | 307 ± 22 |
|
| |||||
| CS (Females) | |||||
| Control | 11 | 100% (91% + 9%) | 0.26 ± 0.04 | 24.8 ± 0.2 | 392 ± 46 |
| Exercised | 11 | 100% (100% + 0%) | 0.26 ± 0.02 | 24.5 ± 0.1 | 275 ± 42 |
Flies with FFT values >0.08 were considered strongly rhythmic, 0.04–0.08 as weakly rhythmic, while <0.04 were classified as arrhythmic.
Because exercise may affect longevity in humans (Moore et al., 2012), we also monitored survival in parallel cohorts of control or exercised males and females. Calculation of median lifespan determined that longevity did not differ between cohorts that underwent 2 weeks exercise versus the undisturbed controls (Fig. 1C). In the previous study on D. melanogaster, exercise in the form of continuous flying ability throughout life was associated with reduced lifespan (Magwere et al., 2006). In our experiments, the lack of detrimental effects on longevity may be attributed to a different type of exercise (flight versus walking), less strenuous activity, and age of flies. In any case, neither exercise regime extended lifespan in flies suggesting that the emerging links between physical activity and longevity in mammals may not extend to Drosophila.
3.2. Effect of exercise on functional decline in aging clock mutant flies
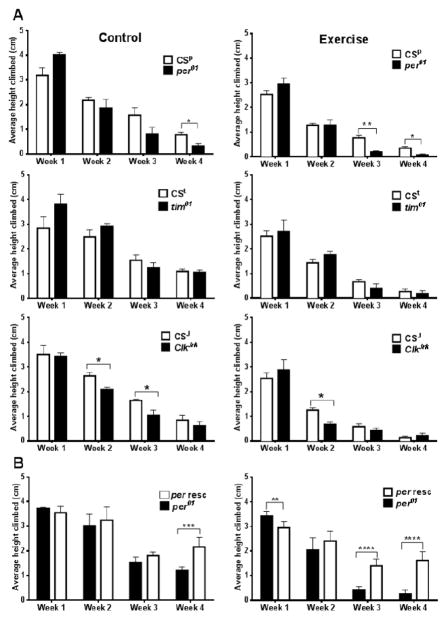
Because the previous report suggested that a specific paradigm of exercise training in a “power tower” improved the climbing speed in flies (Piazza et al., 2009), we used the same exercise regime in our exercise machine, exposing wild-type and clock mutant flies to 2, 2.5, and 3 h of exercise respectively during three consecutive weeks. Control flies were left undisturbed in their vials in the same room. RING assay was conducted at the end of exercise weeks 1–3, and also after 1 week of rest (week 4). Mutants in core clock genes per01, tim01, and ClkJrk, along with genetically matched wild type CS flies were tested using this protocol. As expected, the climbing ability declined with age in all genotypes (Fig. 2). Interestingly, the climbing ability was significantly reduced with age in both undisturbed and exercised per-null flies compared to age matched CSp flies. Climbing ability was also significantly reduced in unexercised ClkJrk flies on week 2 and 3, and in exercised ones on the second week of the experiment, compared to age matched CSJ controls. No significant difference was observed between tim01 and control CSt flies. Differential effects of different clock gene mutants have been previously reported with regard to other phenotypes; for example, the ability to combat pathogenic infection was diminished in per01 flies but not in mutants with impairment in other core clock genes (Lee and Edery, 2008). Thus, it is possible that period has pleiotropic fitness-related functions in addition to its role as an essential clock gene. For example, it could be involved in the regulation of geotaxis, or protection of organism from accumulation of oxidative damage (Krishnan et al., 2009; Krishnan et al., 2012).
Figure 2. Exercise leads to accelerated loss of climbing ability with age in some circadian clock mutants compared to wild type controls.
A) Bar graphs showing the average height climbed by undisturbed (control) and exercised clock mutants (per01, tim01, ClkJrk) and respective wild type (CS) flies subjected to 1, 1.5, and 2 h exercise on weeks 1, 2, and 3, followed by rest on week 4. B) Climbing activity can be significantly restored to wild type levels in exercised per-rescue flies in weeks 3 and 4. Average values for each mutant and respective CS control were analyzed by unpaired T-test separately for each week. Asterisks mark significantly different values (*p<0.05; **p<0.01; ***p<0.001).
Since the loss of per function reduced climbing ability in both unexercised and exercised flies more severely than loss of other clock genes (Fig. 2A), we next tested whether this phenotype can be reversed by the rescue of per function. We used transgenic flies carrying a wild-type copy of per in a per01 background (Cheng et al., 1998). Males (designated as per-rescue) were compared with homozygous per01 flies exposed to 2, 2.5, and 3 h exercise during three consecutive weeks followed by a week’s rest. We observed that climbing ability was significantly higher in undisturbed aging per-rescue flies in week 4 and in exercised per-rescue flies in weeks 3 and 4, compared to age-matched per01 mutants (Fig. 2B). Interestingly, young per01 flies climb significantly higher than the controls while old ones climbed significantly lower. These data are consistent with our previous report (Krishnan et al., 2009) and suggest that per may be an antagonistic pleiotropy gene with regard to aging. To elucidate effects of 3 week ramped exercise on lifespan, the mortality of undisturbed and exercised per01 and per-rescue flies was monitored after endurance tests were completed. The median lifespan did not significantly differ between cohorts of the same genotype that underwent 3 weeks of exercise regime versus controls that were left undisturbed. The median lifespan was 63 days for undisturbed and 63 days for exercised per01 (p value = 0.68). The median lifespan was 78 days for undisturbed and 80 days for exercised per-rescue flies (p=value 0.66).
To better discern the effects of different clock mutants on endurance in undisturbed and exercised flies, we interpolated the time required for climbing function to decline to 50% and 75% of its original peak value for every genotype. Because the CS flies used as controls with different mutants had similar behavior, we combined them for better statistical power. The fastest decline in climbing speed (DT 50) was observed among undisturbed per01 flies (Fig. 3A). This suggests that the poorest performance of these flies after exercise (Fig. 2A) stems from diminished endurance of undisturbed mutants, which we also reported previously (Krishnan et al., 2009). Apparently, exercise cannot increase climbing ability in these flies.
Figure 3. Age-related functional decline is greater in both controls and exercised clock mutants compared to wild type flies.
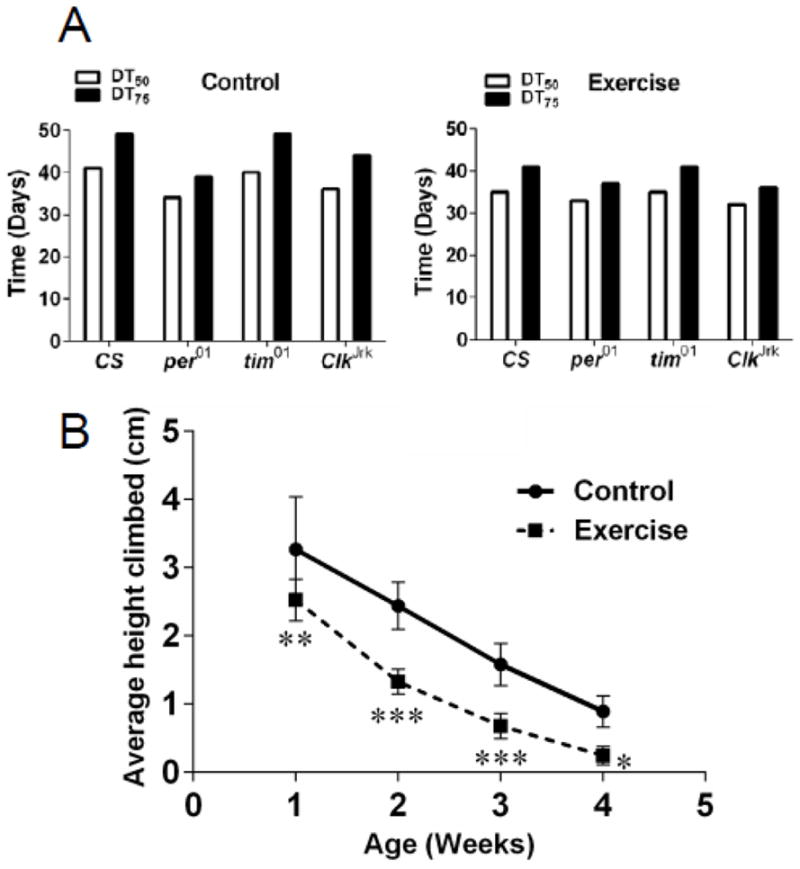
A) Bar graphs showing time of decline of climbing ability to 50% (DT50) and 75% (DT75) of its initial value, interpolated from second order polynomial curves in different fly genotypes both exercised and controls. B) Average height climbed by wild type CS (CSp, CSt, CSJ pooled) flies that were undisturbed and exercised suggest that exercise negatively affects vertical mobility in flies. Data were analyzed by 2-way ANOVA. Asterisks mark significantly different values (*p<0.05; **p<0.01; ***p<0.001).
Finally, we compared climbing performance between cohorts of undisturbed and exercised CS flies. Climbing ability declined faster in exercised flies, and this difference was evident even in young flies after first week of exercise (Fig. 3B). Our results appear to differ from the previous study in which a similar exercise routine led to increased climbing speed in exercised flies (Piazza et al., 2009). However in this study, exercised flies were compared to unexercised flies placed in the power tower with a sponge stopper to prevent the flies from climbing (Piazza et al., 2009), while we used undisturbed flies kept next to the exercise machine as control. We note that in the previous study, flies that were left in the incubator showed significantly better climbing performance than both exercised and unexercised flies in the power tower (Piazza et al., 2009), which is consistent with the data shown here.
In conclusion, our study suggests that exercise for one hour each morning does not improve circadian rest/activity rhythms or longevity in wild type flies. We also demonstrate that longer and more intense exercise reduces climbing performance compared to undisturbed control flies. Finally, we show that some arrhythmic clock mutants, especially per01 have significant reduction in climbing abilities both without and after exercise, suggesting important role of circadian clocks or specific clock genes in maintaining physical fitness as part of healthy aging.
Acknowledgments
We thank Eileen S. Chow for helping with fly rearing and the graphing software. This research was supported by NIH R21-AG038989 and R01-AG045830 grants to JMG. KR was supported by NSF IGERT in Aging Sciences Fellowship at Oregon State University (DGE 0965820). RW was supported by CHAR LIFE and URISC scholarships at OSU.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kuntol Rakshit, Email: rakshitk@science.oregonstate.edu.
Rebecca Wambua, Email: wambuar@onid.orst.edu.
Tomasz M. Giebultowicz, Email: tgiebult@physics.oregonstate.edu.
Jadwiga M. Giebultowicz, Email: giebultj@science.oregonstate.edu.
References
- Allada R, White NE, So WV, Hall JC, Rosbash M. A mutant Drosophila homolog of mammalian Clock disrupts circadian rhythms and transcription of period and timeless. Cell. 1998;93:791–804. doi: 10.1016/s0092-8674(00)81440-3. [DOI] [PubMed] [Google Scholar]
- Cain SW, Rimmer DW, Duffy JF, Czeisler CA. Exercise distributed across day and night does not alter circadian period in humans. J Biol Rhythms. 2007;22:534–541. doi: 10.1177/0748730407306884. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Gvakharia B, Hardin PE. Two alternatively spliced transcripts from the Drosophila period gene rescue rhythms having different molecular and behavioral characteristics. Mol Cell Biol. 1998;18:6505–6514. doi: 10.1128/mcb.18.11.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargano JW, Martin I, Bhandari P, Grotewiel MS. Rapid iterative negative geotaxis (RING): a new method for assessing age-related locomotor decline in Drosophila. Exp Gerontol. 2005;40:386–395. doi: 10.1016/j.exger.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Hardin PE. Molecular genetic analysis of circadian timekeeping in Drosophila. Adv Genet. 2011;74:141–173. doi: 10.1016/B978-0-12-387690-4.00005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh K, Evans JM, Hendricks JC, Sehgal A. A Drosophila model for age-associated changes in sleep: wake cycles. Proc Natl Acad Sci U S A. 2006;103:13843–13847. doi: 10.1073/pnas.0605903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev. 2006;20:1868–1873. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratova AA, Kondratov RV. The circadian clock and pathology of the ageing brain. Nat Rev Neurosci. 2012;13:325–335. doi: 10.1038/nrn3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka RJ, Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci USA. 1971;68:2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan N, Kretzschmar D, Rakshit K, Chow E, Giebultowicz J. The circadian clock gene period extends healthspan in aging Drosophila melanogaster. Aging. 2009;1:937–948. doi: 10.18632/aging.100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan N, Rakshit K, Chow ES, Wentzell JS, Kretzschmar D, Giebultowicz JM. Loss of circadian clock accelerates aging in neurodegeneration-prone mutants. Neurobiol Dis. 2012;45:1129–1135. doi: 10.1016/j.nbd.2011.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Edery I. Circadian regulation in the ability of Drosophila to combat pathogenic infections. Curr Biol. 2008;18:195–199. doi: 10.1016/j.cub.2007.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leise TL, Harrington ME, Molyneux PC, Song I, Queenan H, Zimmerman E, Lall GS, Biello SM. Voluntary exercise can strengthen the circadian system in aged mice. Age. 2013 doi: 10.1007/s11357-012-9502-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Chen WF, Yue Z, Chen D, Sowcik M, Sehgal A, Zheng X. Old flies have a robust central oscillator but weaker behavioral rhythms that can be improved by genetic and environmental manipulations. Aging Cell. 2012;11:428–438. doi: 10.1111/j.1474-9726.2012.00800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magwere T, Pamplona R, Miwa S, Martinez-Diaz P, Portero-Otin M, Brand MD, Partridge L. Flight activity, mortality rates, and lipoxidative damage in Drosophila. J Gerontol A Biol Sci Med Sci. 2006;61:136–145. doi: 10.1093/gerona/61.2.136. [DOI] [PubMed] [Google Scholar]
- Martin I, Gargano JW, Grotewiel MS. A proposed set of descriptors for functional senescence data. Aging Cell. 2005;4:161–164. doi: 10.1111/j.1474-9726.2005.00155.x. [DOI] [PubMed] [Google Scholar]
- Moore SC, Patel AV, Matthews CE, Berrington de Gonzalez A, Park Y, Katki HA, Linet MS, Weiderpass E, Visvanathan K, Helzlsouer KJ, Thun M, Gapstur SM, Hartge P, Lee IM. Leisure time physical activity of moderate to vigorous intensity and mortality: a large pooled cohort analysis. PLoS medicine. 2012;9:e1001335. doi: 10.1371/journal.pmed.1001335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza N, Gosangi B, Devilla S, Arking R, Wessells R. Exercise-training in young Drosophila melanogaster reduces age-related decline in mobility and cardiac performance. PLoS One. 2009;4:e5886. doi: 10.1371/journal.pone.0005886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakshit K, Krishnan N, Guzik EM, Pyza E, Giebultowicz JM. Effects of aging on the molecular circadian oscillations in Drosophila. Chronobiol Int. 2012;29:1–10. doi: 10.3109/07420528.2011.635237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal A, Price J, Man B, Youngs M. Loss of circadian behavioral rhythms and per RNA oscillations in the Drosophila mutant timeless. Science. 1994;263:1603–1606. doi: 10.1126/science.8128246. [DOI] [PubMed] [Google Scholar]
- Yamanaka Y, Hashimoto S, Tanahashi Y, Nishide SY, Honma S, Honma K. Physical exercise accelerates reentrainment of human sleep-wake cycle but not of plasma melatonin rhythm to 8-h phase-advanced sleep schedule. American journal of physiology Regulatory, integrative and comparative physiologyss. 2010;298:R681–691. doi: 10.1152/ajpregu.00345.2009. [DOI] [PubMed] [Google Scholar]