Abstract
OBJECTIVES
To examine the associations of antibiotic exposures during the first 2 years of life and the development of body mass over the first 7 years of life.
DESIGN
Longitudinal birth cohort study.
SUBJECTS
A total of 11532 children born at ≥2500 g in the Avon Longitudinal Study of Parents and Children (ALSPAC), a population-based study of children born in Avon, UK in 1991–1992.
MEASUREMENTS
Exposures to antibiotics during three different early-life time windows (<6 months, 6–14 months, 15–23 months), and indices of body mass at five time points (6 weeks, 10 months, 20 months, 38 months and 7 years).
RESULTS
Antibiotic exposure during the earliest time window (<6 months) was consistently associated with increased body mass (+0.105 and +0.083 s.d. unit, increase in weight-for-length Z-scores at 10 and 20 months, P<0.001 and P=0.001, respectively; body mass index (BMI) Z-score at 38 months +0.067 s.d. units, P=0.009; overweight OR 1.22 at 38 months, P=0.029) in multivariable, mixed-effect models controlling for known social and behavioral obesity risk factors. Exposure from 6 to 14 months showed no association with body mass, while exposure from 15 to 23 months was significantly associated with increased BMI Z-score at 7 years (+0.049 s.d. units, P=0.050). Exposures to non-antibiotic medications were not associated with body mass.
CONCLUSIONS
Exposure to antibiotics during the first 6 months of life is associated with consistent increases in body mass from 10 to 38 months. Exposures later in infancy (6–14 months, 15–23 months) are not consistently associated with increased body mass. Although effects of early exposures are modest at the individual level, they could have substantial consequences for population health. Given the prevalence of antibiotic exposures in infants, and in light of the growing concerns about childhood obesity, further studies are needed to isolate effects and define life-course implications for body mass and cardiovascular risks.
Keywords: antibiotics, human microbiome, body mass, ALSPAC
INTRODUCTION
Since the 1940s, antibiotics have had a crucial part in reducing human morbidity and mortality.1 Antibacterial agents are frequently prescribed worldwide for infants and children, both appropriately2 and inappropriately,3–5 despite a substantial number of expert guidelines advocating more limited use.6–9 Longstanding concerns about overuse primarily reflect the public health threat of increasing antibiotic resistance.4,7,10–12
However, more recently, we have had improved understanding of the human microbiome, composed of thousands of species of resident bacteria that aid in human metabolism, cell differentiation and immune responses.13 Knowledge of the importance of the microbiome in human development raises new issues about antibiotic use in children, as such exposures may disrupt the microbial ecology.14 While the composition of the microbiota of adults appears relatively stable,15 the microbiota of children may be considerably more variable and more vulnerable to antibiotic perturbation. Increased risks from childhood antibiotic exposure for atopic dermatitis,16–18 asthma19,20 and inflammatory bowel disease21 have been reported.
In the womb, the infant is sterile but is colonized early through contact with maternal vaginal,22 fecal23 and cutaneous22 microbiota. These exposures, along with early feeding experiences, establish a gut bacterial community that is the foundation for successive communities into late childhood and adulthood.24 Early life appears to be a critical period for gut colonization,25,26 with interruption of normal colonization (for example, bottle feeding or early administration of antibiotics) disrupting ancient patterns of intestinal colonization.27
Intestinal bacteria participate in metabolism of animals.28 Knowledge that alterations in the microbiota change ‘feed efficiency’ has been exploited since the late 1940s by US farmers,10 who routinely give low-dose antibiotics to domesticated mammalian and avian species, to fatten them for market.29–31 The earlier in life that the animals are exposed, the greater the weight gain;32 however the intermediary mechanisms have not been defined.
The possibility of similar effects in human children, although of clear public health importance, remains relatively unexplored. A recent analysis of the Danish National Birth Cohort is suggestive: children of normal-weight mothers who were exposed to antibiotics during the first 6 months of life33 had increased risk of being overweight when they were 7 years old. However, this study looked only at exposure during the first 6 months, leaving open the question of whether exposures later in infancy have similar associations. Moreover, the study could not examine body mass impacts longitudinally.
Using the Avon Longitudinal Study of Parents and Children (ALSPAC),34 we examine relationships between body mass and exposures to antibiotics during three separate time windows in infancy. This allows us to assess the specificity of the effect in the previously reported <6-month window of vulnerability, and to observe changes in body mass over time.
SUBJECTS AND METHODS
Data source and sample
ALSPAC is a landmark longitudinal birth cohort study to understand the factors influencing child development into adulthood. The study began with the recruitment of 14541 pregnant women resident in Avon, UK with expected dates of delivery 1 April 1991 to 31 December 1992. Methods have been described in detail elsewhere, but 85% of eligible births were captured in a population that is slightly more affluent than the general UK population.35 Over time, data were collected by hospital record, survey and clinical and laboratory examination.
From 14541 pregnancies, 13 988 children were alive at the end of year one. Of these, we selected children born weighing ≥2500 g, to minimize the number of subjects likely to have had health or behavioral characteristics that could confound antibiotic–body mass relationships. We also excluded children for whom information on antibiotic exposure was missing for all three of the time windows. Our analytic sample included 11 532 children.
Measures
Exposures to antibiotics
Parents were asked about children’s exposures to antibiotics in three postal questionnaires that covered a range of topics. The questionnaires referred to exposures at less than 6 months of age (elicited at 6 months), between 6–14 months (elicited at 15 months) and between 15–23 months of age (elicited at 24 months). The question on the 6-month questionnaire was: ‘Children often have accidents or illnesses that need treatment. Please indicate whether antibiotics have been given to your child in the past 6 months’. The question on subsequent versions is identical, except with regard to the referenced time window. Information on type of antibiotic was asked but is of questionable reliability, and data on dosage is not available. Therefore, subjects were classified as either exposed or unexposed to antibiotics, for the relevant time window.
Outcomes related to body mass
Birth weight was obtained from the labor ward records and birth length was measured by an ALSPAC researcher. Body mass was assessed by ALSPAC study personnel (in a random 10% subsample) and abstracted from health visitor records (in the remainder) at ages 6–8 weeks, 38–44 weeks (mean 10 months), 77–106 weeks (mean 20 months) and 38 months. At 7 years, body mass was assessed by ALSPAC study personnel in clinic visits. In selecting a metric of body mass, we considered use of International Obesity Task Force thresholds for obese and overweight,36 World Health Organization (WHO) Child Health Growth Standards developed from the 1997–2003 Multicentre Growth Reference Study to derive Z-scores for BMI and weight-for-length,37 ponderal index (weight in kg divided by height/length in meters cubed),38 2000 US Centers for Disease Control and Prevention norms to derive Z-scores for BMI beyond age 2 years,39,40 and 1990 UK population norms for BMI.41 We opted to follow the approach used in the United Kingdom for assessing adiposity, applying WHO weight-for-length Z-score until age 2 years, and 1990 UK BMI Z-score thereafter.
We examined these outcomes: weight-for-length Z-score at 6 weeks, 10 months and 20 months; BMI Z-score at age 38 months and 7 years; and categorical overweight (BMI 85–94th percentile for age and gender) and obese (BMI ≥95th percentile for age and gender) at 38 months and 7 years. Our analyses excluded a small number of outliers (≥ 5 or p ≤ − 5 Z-score or s.d.), which were likely to reflect errors in data recording.
Potential confounders
We adopted a set of social, behavioral and biological early-life predictors of obesity in the ALSPAC cohort described by Reilly et al.,42 in selecting covariates for our analyses. These include maternal parity, race, social class (using the UK Office of Population Census and Survey classifications)43 and education as defined by the UK Office of Qualifications and Examinations Regulation.44 We also included parental BMI (mother pre-pregnancy overweight/obese, father overweight/obese and both overweight/obese), first trimester smoking, breastfeeding categorized as never/breastfed cessation before 6 months/continuous breastfeeding through 6 months and timing of introduction of complementary foods as covariates. From Reilly et al., we also drew on a set of childhood ‘lifestyle’ variables (time spent per day watching television, in car on weekdays, in car on weekends),42 and dietary pattern classifications, based on a food frequency questionnaire at 38 months, developed for the ALSPAC cohort,45 and duration of nighttime sleep at 7 years. All models also adjusted for birth weight.
Statistical analysis
We began by characterizing the sample in terms of the prevalence of key exposures and outcomes, and then examined the relationship between exposures and potential confounders, using a chi-square test of association.
Our analysis of the relationship between exposures and outcomes took advantage of repeated measures of body mass over time. We used mixed models with random effects for subjects, analyzing the impact of exposure in each of the three windows separately. Differences between exposed and unexposed subjects were tested at each of the five time points. In addition to these formal statistical tests, we looked for patterns of association that would be consistent with causality. For example, an increase in body mass at a time point (for example, 6 weeks) before the exposure window assessed (6–14 months) would be consistent with spurious association. Multivariable models included all of the potential confounders mentioned above. Linear models were used for continuous (Z-score) outcomes, and logistic models were used for dichotomous outcomes (overweight/obese). Regression models were tested for collinearity using the variance inflation factor (VIF) statistic.46 For the logistic models, we examined VIF for the corresponding linear probability model. The key antibiotic exposure variables showed no sign of multicollinearity (maximum VIF for exposure in any model ≤1.5). As there was moderate collinearity for some potential confounders, we tested the robustness of estimates of the impact of antibiotic exposure in a series of models by sequentially excluding individual potential confounders; estimates from those models were consistent with those reported here (data not shown). Stata 12 (College Station, TX, USA) was used for the analyses.
Interactions with maternal weight
The previous report on antibiotic exposure at <6 months33 showed increased odds of obesity in children of normal-weight women, but decreased odds in children of overweight and obese women. Accordingly, for each of the three exposure windows, in addition to the main effect of exposure, we examined associations in subsamples stratified by maternal weight.
Missing data
While the follow-up rate in the ALSPAC sample was generally good, covariates were missing for some subjects in this longitudinal data set. Consistent with a prior analysis of the ALSPAC data, we included a ‘missing’ category for each of the covariates,42 to maximize sample size for multivariable analysis. Therefore, multivariable models omitted only those cases for which the exposure was missing. Sample sizes for each analysis are shown in tables.
Tests for spurious association
As antibiotic use might reflect unmeasured parental receptivity to administration of medications to children, we identified two medications that were commonly used in the study population: antipyretics and eye ointment. Multivariable analyses were run for exposure to these two medications separately, to see if either exposure was associated with increased body mass.
Human subjects
This study was reviewed and approved by the New York University Washington Square and Medical School campuses’ Institutional Review Boards. Ethical approval for the study was obtained from the ALSPAC Ethics and Law Committee and the Local Research Ethics Committees.
Reporting
The reporting of this study conforms to the STROBE statement.47
RESULTS
Description of the cohort
Of the 11 532 children studied, the mean birth weight was 3477±460 g (Table 1). Nearly a third of the children received antibiotics in the first 6 months of life, with cumulative use increasing with age. However, by 2 years of age, 25.7% of the sample remained unexposed to antibiotic medications. Birth weights of children who had been exposed by 2 years were not significantly different from those of unexposed children (3494 g vs 3478 g; P=0.223), nor were birth weight-for-length Z-scores different for the two groups (−0.295 s.d. units vs −0.294 s.d. units; P=0.988). Antibiotic use at <6 months was significantly associated with birth weight (3506g vs 3468g, P<0.001) and birth-weight Z-score (+ 0.338 vs + 0.393, P=0.004), but for weight-for-length Z-score, the exposed group tended to have lower body mass (− 0.287 s.d. units vs − 0.321 s.d. units, P=0.193).
Table 1.
Characteristics of the sample
| Characteristicsa | Mean (s.d.) or % |
|---|---|
| Birth weight (g) (n=11532) | 3477 ± 460 |
| Weight-for-length Z-score at 6 weeks (n=11 814) | −0.55±1.24 |
| Weight-for-length Z-score at 15 months (n=11 065) | 0.44 ± 1.04 |
| Weight-for-length Z-score at 23 months (n=10 183) | 0.63 ± 1.03 |
| BMI Z-score at 38 months (n=9443) | −0.31 ±1.09 |
| BMI Z-score at 7 years (n=8210) | 0.15 ±1.03 |
| Overweight at 38 months (%) (n=9443) | 18.0 |
| Overweight at 7 years (%) (n=8210) | 23.9 |
| Obese at 38 months (%) (n=9443) | 8.4 |
| Obese at 7 years (%) (n=8210) | 8.6 |
| Antibiotic use between 0–5 months (%) (n=10 745) | 31.9 |
| Antibiotic use between 6–14 months (%) (n=10 388) | 53.6 |
| Antibiotic use between 15–23 months (%) (n=9789) | 47.5 |
| Antibiotic use between 0–23 months (%) (n=8881) | 74.3 |
| Antipyretic use between 0–5 months (%) (n=10 745) | 86.7 |
| Eye ointment use between 0–5 months (%) (n=10 745) | 44.9 |
Statistics are derived from subjects with non-missing data, for each characteristic.
Although the risk factor profile of the ALSPAC population with respect to obesity has been described,42 pertinent for this analysis is that the population is largely white (92.6%) and has less parental obesity than current prevalence in the United Kingdom,48 and 26% breastfeeding at least through 6 months, which is consistent with the heavily white population sampled here. By the age of 7 years, 17.6% of children were overweight and 8.3% were obese, using contemporaneous norms.49
Factors associated with antibiotic exposures
Antibiotic exposures during the first 2 years of life were unassociated with many characteristics known to be associated with obesity, including maternal overweight, socioeconomic status and education (Table 2). There were modest associations with maternal behaviors including smoking during the first trimester (more exposures among children of smokers) and breastfeeding (more exposures among children who were never breastfed). ‘Lifestyle’ variables were also modestly associated with exposures, with children spending more time traveling in cars more likely to be exposed. There was no association of antibiotic exposure with dietary patterns at 38 months.
Table 2.
Description of the sample by characteristics potentially associated with obesity, and comparison of children who were exposed and not exposed to antibiotics during the first 2 years of life, with respect to those characteristics
| Characteristics | Sample | By exposure statusa | |
|---|---|---|---|
| Exposed to antibiotics 0–2 years | Not exposed to antibiotics 0–2 years | ||
|
|
|||
| n=11532 | n=6598 | n=2283 | |
| Family characteristics | |||
| Mother overweight | |||
| No (n=8607) | 74.6 | 78.2 | 77.4 |
| Yes (n=1554) | 13.5 | 13.2 | 14.1 |
| Missing (n=1371) | 11.9 | 8.1 | 8.5 |
| Father overweight | |||
| No (n=5129) | 44.5 | 48.3 | 48.3 |
| Yes (n=2340) | 20.3 | 21.9 | 21.8 |
| Missing (n=4063) | 35.2 | 29.8 | 29.7 |
| Both parents obese | |||
| No (n=6924) | 60.0 | 65.4 | 66.0 |
| Yes (n=54) | 0.5 | 0.5 | 0.4 |
| Missing (n=4554) | 39.5 | 34.0 | 33.6 |
| Socioeconomic statusb | |||
| I (highest) (n=543) | 4.7 | 5.6 | 5.0 |
| II (n=2887) | 25.0 | 28.7 | 26.3 |
| III (n=4551) | 39.5 | 40.7 | 42.3 |
| IV (n=868) | 7.5 | 7.1 | 7.5 |
| V (n=180) | 1.6 | 1.4 | 1.6 |
| Missing (n=2503) | 21.7 | 16.9 | 16.9 |
| Educationc | |||
| CSE/vocational (n=3086) | 26.8 | 23.2 | 25.7 |
| O level (n=3847) | 33.4 | 35.3 | 34.1 |
| A level (n=2534) | 22.0 | 24.6 | 23.7 |
| Degree (n=1486) | 12.9 | 14.8 | 14.5 |
| Missing (n=579) | 5.0 | 2.0 | 2.0 |
| Race/ethnicity | |||
| White (n=10674) | 92.6 | 96.1 | 95.8 |
| Non-white (n=233) | 2.0 | 1.6 | 1.7 |
| Missing (n=625) | 5.4 | 2.3 | 2.5 |
| Maternal factors | |||
| Parity | * | ||
| 0 prior (n=4856) | 42.1 | 43.3 | 43.7 |
| 1 prior (n=3934) | 34.1 | 36.3 | 34.4 |
| 2 prior (n=1592) | 13.8 | 14.0 | 13.2 |
| 3 or more (n=615) | 5.3 | 5.1 | 5.1 |
| Missing (n=535) | 4.6 | 2.4 | 3.6 |
| Maternal smoking first trimester | * | ||
| No (n=8626) | 74.8 | 78.9 | 80.6 |
| Yes (n=2561) | 22.2 | 20.3 | 17.6 |
| Missing (n=345) | 3.0 | 1.0 | 1.8 |
| Infant feeding | |||
| Breastfeeding at 6 months | ** | ||
| Still breastfeeding (n=3098) | 26.9 | 30.6 | 32.5 |
| Has stopped (n=4978) | 43.1 | 46.3 | 45.3 |
| Never breastfed (n=2572) | 22.3 | 22.2 | 20.7 |
| Missing (n=884) | 7.7 | 0.8 | 1.4 |
| Timing of introduction of solids | |||
| 0–2 months (n=1740) | 15.1 | 15.7 | 14.3 |
| 3 months (n=6068) | 52.6 | 56.7 | 58.0 |
| 4–6 months (n=2827) | 24.5 | 26.7 | 26.6 |
| Missing (n=897) | 7.8 | 1.0 | 1.1 |
| Lifestyle factors in childhood | |||
| Television watching at 38 months | |||
| 0–4h (n=1995) | 17.3 | 10.7 | 20.6 |
| 4.1–8 h (n=4241) | 36.8 | 42.9 | 40.5 |
| >8h (n=3047) | 26.4 | 28.4 | 30.2 |
| Missing (n=2249) | 19.5 | 8.9 | 8.7 |
| Time in car at 38 months (weekday) | * | ||
| Not at all (n=828) | 7.2 | 7.5 | 9.0 |
| <1h (n=7482) | 64.9 | 73.7 | 73.8 |
| ≥1h (n=1079) | 9.4 | 10.9 | 9.2 |
| Missing (n=2143) | 18.6 | 8.0 | 7.9 |
| Time in car at 38 months (weekend) | * | ||
| Not at all (n=421) | 3.7 | 3.5 | 4.3 |
| <1h (n=6130) | 53.2 | 60.5 | 62.9 |
| ≥1h (n=2771) | 24.0 | 27.5 | 24.4 |
| Missing (n=2210) | 19.2 | 8.6 | 8.4 |
| Nighttime sleep at 7 years | |||
| <10.5h (n=1061) | 9.2 | 10.6 | 10.3 |
| 10.5–11.4 h (n=3357) | 29.1 | 33.7 | 32.2 |
| 11.5–11.9 h (n=2202) | 19.1 | 21.5 | 22.1 |
| ≥11.9h (n=2590) | 22.4 | 27.3 | 24.8 |
| Missing (n=2322) | 20.1 | 9.4 | 9.6 |
| Dietary patterns at 38 months (Groups of foods consumed) | |||
| Food group 1: junk | |||
| Quartile 1 (low) (n=2347) | 20.4 | 24.4 | 24.1 |
| Quartile 2 (n=2339) | 20.3 | 23.3 | 23.3 |
| Quartile 3 (n=2339) | 20.3 | 22.7 | 23.7 |
| Quartile 4 (high) (n=2312) | 20.1 | 21.0 | 21.0 |
| Missing (n=2195) | 19.0 | 8.5 | 7.9 |
| Food group 2: healthy | |||
| Quartile 1 (low) (n=2309) | 20.0 | 22.6 | 22.9 |
| Quartile 2 (n=2333) | 20.2 | 23.1 | 22.7 |
| Quartile 3 (n=2357) | 20.4 | 23.2 | 23.2 |
| Quartile 4 (high) (n=2338) | 20.3 | 22.6 | 23.4 |
| Missing (n=2195) | 19.0 | 8.5 | 7.9 |
| Food group 3: traditional | |||
| Quartile 1 (low) (n=2327) | 20.2 | 22.4 | 24.8 |
| Quartile 2 (n=2346) | 20.3 | 23.3 | 22.7 |
| Quartile 3 (n=2347) | 20.4 | 22.9 | 23.0 |
| Quartile 4 (high) (n=2317) | 20.1 | 23.0 | 21.7 |
| Missing (n=2195) | 19.0 | 8.5 | 7.9 |
| Food group 4: snack/fussy | |||
| Quartile 1 (low) (n=2305) | 20.0 | 21.3 | 22.5 |
| Quartile 2 (n=2339) | 20.3 | 22.5 | 23.4 |
| Quartile 3 (n=2352) | 20.4 | 23.9 | 22.5 |
| Quartile 4 (high) (n=2341) | 20.3 | 23.8 | 22.6 |
| Missing (n=2195) | 19.0 | 8.5 | 7.8 |
Comparison of characteristics by exposure status includes only those subjects for whom exposure status is non-missing for all three exposure windows (< 6 months, 6–15 months, 15–24 months).
Social class, assessed using the UK Office of Population Census and Survey classifications (see text).
Level defined using the UK Office of Qualifications and Examinations Regulation (see text).
P<0.05
P<0.01 for chi-square test of association between this characteristic and exposure status.
Antibiotic exposures and morphometric outcomes
In univariate analysis, exposure at <6 months was consistently associated with elevations in body mass index and with overweight and obesity from ages 10 to 38 months (see Appendix Table 1 for the univariate findings, along with a comparison with the multivariable analyses). In contrast, exposures at later time windows were not associated with subsequent increases in body mass, with the exception of exposure at 15–23 months, which was significantly associated with elevations in Z-score BMI at 7 years.
Findings from full multivariable analysis are shown in Figures 1 and 2, with separate panels for each of the three exposure windows. For the body mass Z-score outcomes (Figure 1), exposure during the <6-month window was consistently associated with elevations in Z-score body mass in post-exposure period, with increased body mass from age of 10 to 38 months (weight-for-length Z-scores at 10 and 20 months +0.106 increase in s.d. units, P<0.001 and + 0.083 s.d. units, P<0.001, respectively; BMI Z-score at 38 months + 0.067 s.d. units, P=0.009). In contrast, exposure during the 6–14 month window was not associated with elevations in Z-score body mass at any subsequent time point. The pattern of association for exposure 15–23 months was less clear. The lack of association with elevated body mass at 38 months did not support an association with exposure, but the sole elevation in BMI Z-score for exposed children (at 7 years; + 0.049 s.d. units, P=0.050) was consistent with an association.
Figure 1.
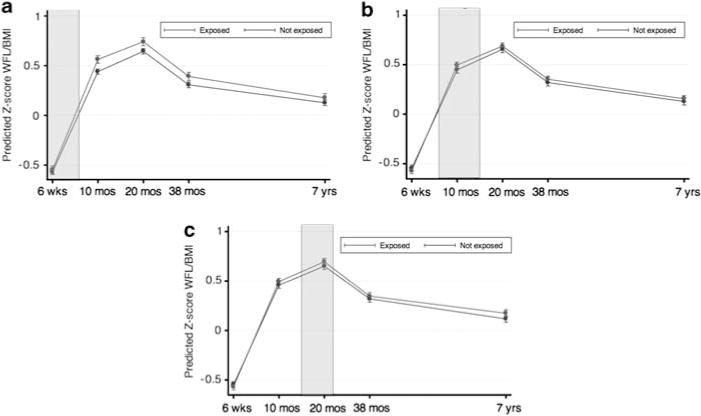
Multivariable associations of <6 months (a), 6–14 months (b) and 15–23 months (c) antibiotic exposure and body mass outcomes (WHO weight-for-length Z-score <2 years; BMI Z-score ≥2 years). Exposure window shaded for contemporaneous comparison of children who were exposed and not exposed to antibiotics, adjusting for birth weight and other factors associated with antibiotic exposures. 95% confidence intervals represented in lines, and point estimate represented as bullet.
Figure 2.
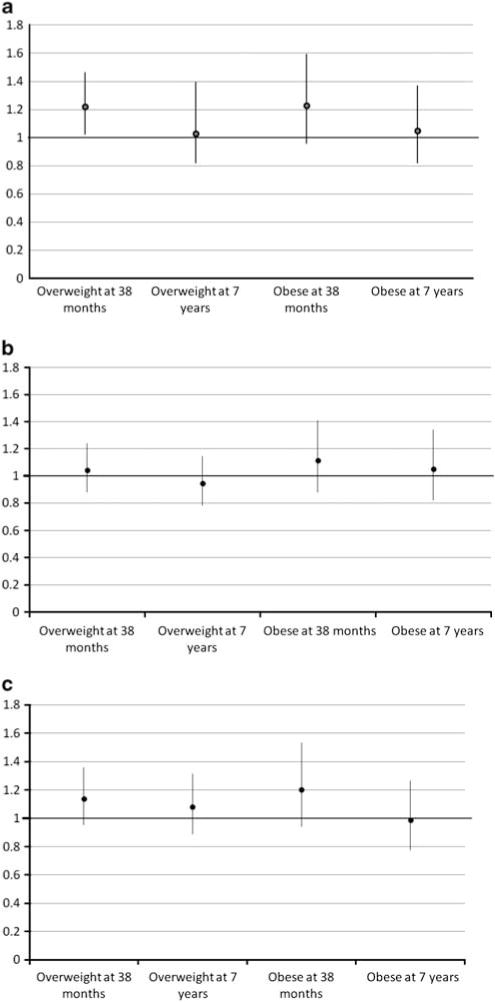
Adjusted odds ratios of <6 months (a), 6–14 months (b) and 15–23 months (c) antibiotic exposure for overweight and obesity at 38 months and 7 years. 95% confidence intervals represented in lines, and point estimate represented as bullet. All multivariable models included birth weight, maternal parity, race, social class, education, parental BMI (categorized as mother pre-pregnancy overweight/obese, father overweight/obese and both overweight/obese), first trimester smoking, breastfeeding (categorized as never/breastfed cessation before 6 months/continuous breastfeeding through 6 months), timing of introduction of complementary foods, time spent per day watching television, in car on weekdays, in car on weekends, dietary pattern classifications at 38 months and duration of nighttime sleep at 7 years.
Figure 2 shows associations with overweight and obesity at 38 months and 7 years, for exposures during each of the time windows. Adjusting for the potential confounders, antibiotic exposure at <6 months was associated with significantly elevated odds of overweight at 38 months (OR 1.22, P=0.029) and near-significantly elevated odds of obesity at 38 months (OR 1.23, P=0.097), but not at 7 years. Neither exposure at 6–14 months nor exposure at 15–23 months had a significant association with overweight or obesity at 38 months or 7 years.
Interaction with maternal weight
When interactions with maternal weight were examined in stratified analysis, we found evidence of greater effects of exposure among normal-weight mothers than among mothers who were obese or overweight (data not shown). At 38 months, <6-month exposure was associated with a 29.4% increase in overweight among normal-weight mothers (P=0.032), with no increase among overweight and obese mothers (OR 1.07, P=0.752). However, in contrast to the previous report,33 we did not find a significant association of exposure at <6 months with child overweight or obesity at 7 years, even in the sub-sample of children of normal-weight mothers.
Tests for spurious association
Neither exposure to antipyretics nor to eye ointment showed patterns of association with increased body mass, in any of the three time windows.
DISCUSSION
This longitudinal study found that early-life antibiotic exposure was associated with subsequent increases in body mass. Of the three time windows analyzed, only exposure during the period before 6 months of age was consistently associated with increases in body mass. At 38 months, children who had been exposed to antibiotics during this earliest period had significantly higher standardized BMI scores, and were 22% more likely to be overweight than children who had not been exposed. In contrast, exposures after 6 months were not consistently associated with body mass increases. Those in the 6- to 14-month period showed no association, while those in the period 15–23 months were significantly associated only with elevated standardized BMI score at 7 years, but not with consistently elevated scores in the interim.
Our finding of an association of antibiotic exposure at <6 months with later-life body mass is consistent with a prior report. It adds important evidence that exposure timing matters. We also add a test of spuriousness, with the finding that exposure to non-antibiotic medications during the same early windows is not associated with elevations in body mass. This makes it less plausible that exposure to antibiotics reflects a greater receptivity to medications, which is also correlated with increases in body mass. Unlike the previous study, however, we did not find an association with antibiotic exposure at <6 months persisting to 7 years of age. Perhaps this reflects differences in the antibiotics used in the two samples, or different doses. Given that intravenous antibiotics are used in these first 6 months of life (often for neonatal sepsis), antibiotic type (that is, Gram-positive or Gram-negative/anaerobic coverage) and route of administration (intravenous or orally administered antibiotics) might have differential effects on gut microbiota composition and development. This is consistent with a recent analysis finding associations of intravenous vancomycin, but not amoxicillin, treatment in adults with the development of obesity.50 Alternatively, our failure to find a significant association may simply reflect our somewhat smaller sample size.
That early-life antibiotic exposure can lead to increased body mass is consistent with a evidence on the farm of antibiotic-induced weight gain,10,25,26 and with more recent studies in laboratory animals elucidating a link between early antibiotic exposure and changed development in controlled environments.51 Many of these studies also find that the earliest months of life are periods of unique vulnerability to antibiotic disruption.
There are important limitations to our work. The relationship between antibiotic exposure and body mass is potentially confounded by multiple social, behavioral and biological factors. For example, BMI in infancy and childhood is associated with biological factors, such as parental BMI and maternal smoking in utero, social class and education; feeding (breastfeeding, later family food environment) and childhood ‘lifestyle’,42 including prevalence of sedentary behavior and regularity of sleep patterns. Early-life antibiotic use also is potentially correlated to each of these, as parental receptivity to antibiotics varies by social class and ethnicity,52,53 and children in larger families or those who attend daycare also have greater exposure to contagious diseases. Fortunately, the ALSPAC data are rich, and we were able to control for many confounders in multivariable analyses. We found remarkably weak associations between antibiotic exposure and the potential confounders that we examined. However, there may be other variables which, if included, would have attenuated the reported association. Exposure was measured by parental recall for events over a 3- to 9-month period. Although such memories may be imprecise, there is no a priori reason why exposure recall bias would be associated with child body mass.
No method to assess adiposity (BMI, ponderal index, weight-for-height or the Quetelet index) is uniquely suited to represent adiposity and risk for subsequent cardiovascular disease,54 and we accept that ponderal index and BMI Z-scores as outcomes of adiposity may be more clinically meaningful insofar (as Howe et al.55 have suggested) they represent greater fat mass and cardiovascular risk later in life. Dual-energy X-ray absorptiometry data, which better represent fat mass deposition,56 are later data points available for further study of the ALSPAC data.57
Also limiting generalizability is that we only examined effects among children born at ≥2500 g. Even so, our sample of infants with normal birth weight likely included children who had substantial illness and were treated with antibiotics. To the extent that children with such illnesses are likely to be thinner, the magnitude of the association between exposure and body mass we identified may underestimate effects that occur in healthy children. Given that high birth weight is also a possible risk factor for obesity in later life, our effect size may have been diminished by inclusion of a high-risk subpopulation who is more likely to develop obesity for other reasons.58 Future work can examine exclusion of this subpopulation. Finally, the ALSPAC sample dates from the early 1990s in the United Kingdom, when antibiotic exposure was probably less frequent than that found today, given the average increase in use of 4.3% annually since 2000 in the United Kingdom.59 The effects of today’s more frequent antibiotic exposure in early life may therefore be greater, and transgenerational impacts of continued antibiotic use could compound effects.13,14
The effect sizes that we report are modest. Translated into weight increments for the average 38-month-old, a +0.067 s.d. unit increase in BMI Z-score corresponds to a +90 g increase in weight. Although such increases are likely to have small impacts on individuals, they may be important for population health: assuming a normal distribution, shifts of 0.067 s.d. units in BMI Z-score (the change noted at 38 months in our final model) would increase overweight by 1.62% and obesity by 0.72%, in the population. As obesity is multifactorial and early exposure to antibiotics is common, such increases in overweight and obesity due to small shifts in means may be potentially important.60
CONCLUSION
This study reinforces concerns that early-life antibiotic exposure may cause increases in body mass in later life. It also points to the period from birth to 6 months as a window of special vulnerability to exposure. Further study is needed to disaggregate the effect of early exposures to antibiotics from those occurring in the prenatal and perinatal periods, and to quantify the life-course implications for body mass and cardiovascular risks, at the population level.
Supplementary Material
Acknowledgments
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. The UK Medical Research Council, the Wellcome Trust (Grant ref: 092731) and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors and Drs Trasande and Blustein will serve as guarantors for the contents of this paper and does not reflect the views of the ALSPAC executive. Support for our preliminary work with the ALPSAC database was provided through a pilot grant from the NYU Global Public Health Research Challenge Fund, and by NIH grants RO1GM090989 and 1UL1RR029893. We are grateful for support of Joseph Conigliaro, John Billings and Teresa Attina for their critical review of early results of our analysis.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on International Journal of Obesity website (http://www.nature.com/ijo)
References
- 1.Jayachandran S, Lleras-Muney A, Smith KV. Modern Medicine and the 20th Century Decline in Mortality: Evidence on the Impact of Sulfa Drugs. The National Bureau of Economic Research; 2009. (NBER Working Paper Series No 15089). [Google Scholar]
- 2.McCaig LF, Besser RE, Hughes JM. Trends in antimicrobial prescribing rates for children and adolescents. JAMA. 2002;287:3096–3102. doi: 10.1001/jama.287.23.3096. [DOI] [PubMed] [Google Scholar]
- 3.Finkelstein JA, Metlay JP, Davis RL, Rifas-Shiman SL, Dowell SF, Platt R. Antimicrobial use in defined populations of infants and young children. Arch Pediatr Adolesc Med. 2000;154:395–400. doi: 10.1001/archpedi.154.4.395. [DOI] [PubMed] [Google Scholar]
- 4.Hicks LA, Chien YW, Taylor TH, Jr, Haber M, Klugman KP. Outpatient antibiotic prescribing and nonsusceptible Streptococcus pneumoniae in the United States, 1996–2003. Clin Infect Dis. 2011;53:631–639. doi: 10.1093/cid/cir443. [DOI] [PubMed] [Google Scholar]
- 5.Paul IM, Maselli JH, Hersh AL, Boushey HA, Nielson DW, Cabana MD. Antibiotic prescribing during pediatric ambulatory care visits for asthma. Pediatrics. 2011;127:1014–1021. doi: 10.1542/peds.2011-0218. [DOI] [PubMed] [Google Scholar]
- 6.Coco A, Vernacchio L, Horst M, Anderson A. Management of acute otitis media after publication of the 2004 AAP and AAFP clinical practice guideline. Pediatrics. 2010;125:214–220. doi: 10.1542/peds.2009-1115. [DOI] [PubMed] [Google Scholar]
- 7.Finkelstein JA, Stille C, Nordin J, Davis R, Raebel MA, Roblin D, et al. Reduction in antibiotic use among US children, 1996–2000. Pediatrics. 2003;112:620–627. doi: 10.1542/peds.112.3.620. [DOI] [PubMed] [Google Scholar]
- 8.Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302:758–766. doi: 10.1001/jama.2009.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson PL, Gilbert RE, Long PF, Saxena S, Sharland M, Wong IC, et al. Guidance affected general practitioner antibiotic prescribing for otitis media in children? J Public Health. 2008;30:479–486. doi: 10.1093/pubmed/fdn072. [DOI] [PubMed] [Google Scholar]
- 10.Jukes TH. Antibiotics in animal feeds and animal production. Bioscience. 1972;22:526–534. [Google Scholar]
- 11.Millar MR, Walsh TR, Linton CJ, Zhang S, Leeming JP, Bennett PM. Carriage of antibiotic-resistant bacteria by healthy children. J Antimicrob Chemother. 2001;47:605–610. doi: 10.1093/jac/47.5.605. [DOI] [PubMed] [Google Scholar]
- 12.Sjolund M, Wreiber K, Andersson DI, Blaser MJ, Engstrand L. Long-term persistence of resistant Enterococcus species after antibiotics to eradicate Helicobacter pylori. Ann Intern Med. 2003;139:483–487. doi: 10.7326/0003-4819-139-6-200309160-00011. [DOI] [PubMed] [Google Scholar]
- 13.Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nat Rev Microbiol. 2009;7:887–894. doi: 10.1038/nrmicro2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blaser M. Antibiotic overuse: stop the killing of beneficial bacteria. Nature. 2011;476:393–394. doi: 10.1038/476393a. [DOI] [PubMed] [Google Scholar]
- 15.Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci USA. 2011;108:4554–4561. doi: 10.1073/pnas.1000087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strachan DP. Family size, infection and atopy: the first decade of the “hygiene hypothesis”. Thorax. 2000;55:S2–10. doi: 10.1136/thorax.55.suppl_1.s2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor AL, Dunstan JA, Prescott SL. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: a randomized controlled trial. J Allergy Clin Immunol. 2007;119:184–191. doi: 10.1016/j.jaci.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 18.Viljanen M, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T, et al. Probiotics in the treatment of atopic eczema/dermatitis syndrome in infants: a double-blind placebo-controlled trial. Allergy. 2005;60:494–500. doi: 10.1111/j.1398-9995.2004.00514.x. [DOI] [PubMed] [Google Scholar]
- 19.Kozyrskyj AL, Ernst P, Becker AB. Increased risk of childhood asthma from antibiotic use in early life. Chest. 2007;131:1753–1759. doi: 10.1378/chest.06-3008. [DOI] [PubMed] [Google Scholar]
- 20.Wickens K, Pearce N, Crane J, Beasley R. Antibiotic use in early childhood and the development of asthma. Clin Exp Allergy. 1999;29:766–771. doi: 10.1046/j.1365-2222.1999.00536.x. [DOI] [PubMed] [Google Scholar]
- 21.Hviid A, Svanstrom H, Frisch M. Antibiotic use and inflammatory bowel diseases in childhood. Gut. 2011;60:49–54. doi: 10.1136/gut.2010.219683. [DOI] [PubMed] [Google Scholar]
- 22.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spor A, Koren O, Ley R. Unravelling the effects of the environment and host genotype on the gut microbiome. Nat Rev Microbiol. 2011;9:279–290. doi: 10.1038/nrmicro2540. [DOI] [PubMed] [Google Scholar]
- 25.Dubos R, Savage D, Schaedler R. Biological Freudianism: lasting effects of early environmental influences. 1966. Int J Epidemiol. 2005;34:5–12. doi: 10.1093/ije/dyh309. [DOI] [PubMed] [Google Scholar]
- 26.Dubos R, Schaedler RW, Stephens M. The effect of antibacterial drugs on the fecal flora of mice. J Exp Med. 1963;117:231–243. doi: 10.1084/jem.117.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bedford Russell AR, Murch SH. Could peripartum antibiotics have delayed health consequences for the infant? BJOG. 2006;113:758–765. doi: 10.1111/j.1471-0528.2006.00952.x. [DOI] [PubMed] [Google Scholar]
- 28.Ley RE, Lozupone CA, Hamady M, Knight R, Gordon JI. Worlds within worlds: evolution of the vertebrate gut microbiota. Nat Rev Microbiol. 2008;6:776–788. doi: 10.1038/nrmicro1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cromwell GL. Why and how antibiotics are used in swine production. Anim Biotechnol. 2002;13:7–27. doi: 10.1081/ABIO-120005767. [DOI] [PubMed] [Google Scholar]
- 30.Gaskins HR, Collier CT, Anderson DB. Antibiotics as growth promotants: mode of action. Anim Biotechnol. 2002;13:29–42. doi: 10.1081/ABIO-120005768. [DOI] [PubMed] [Google Scholar]
- 31.Libby DA, Schaible PJ. Observations on growth responses to antibiotics and arsonic acids in poultry feeds. Science. 1955;121:733–734. doi: 10.1126/science.121.3151.733. [DOI] [PubMed] [Google Scholar]
- 32.Lassiter CA. Antibiotics as growth stimulant for dairy cattle: a review. J Dairy Science. 1955;38:1102–1138. [Google Scholar]
- 33.Ajslev TA, Andersen CS, Gamborg M, Sorensen TI, Jess T. Childhood overweight after establishment of the gut microbiota: the role of delivery mode, pre-pregnancy weight and early administration of antibiotics. Int J Obes. 2011;35:522–529. doi: 10.1038/ijo.2011.27. [DOI] [PubMed] [Google Scholar]
- 34.Headley J, Northstone K. Medication administered to children from 0 to 7.5 years in the Avon Longitudinal Study of Parents and Children (ALSPAC) Eur J Clin Pharmacol. 2007;63:189–195. doi: 10.1007/s00228-006-0231-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Golding J, Pembrey M, Jones R. ALSPAC—the Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatr Perinat Epidemiol. 2001;15:74–87. doi: 10.1046/j.1365-3016.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- 36.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Onis M, WHO Multicentre Growth Reference Study Group WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. 2006;95:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 38.Barker DJ, Godfrey KM, Osmond C, Bull A. The relation of fetal length, ponderal index and head circumference to blood pressure and the risk of hypertension in adult life. Paediatr Perinat Epidemiol. 1992;6:35–44. doi: 10.1111/j.1365-3016.1992.tb00741.x. [DOI] [PubMed] [Google Scholar]
- 39.Grummer-Strawn LM, Reinold C, Krebs NF. Use of World Health Organization and CDC growth charts for children aged 0–59 months in the United States. MMWR Recomm Rep. 2010;59:1–15. [PubMed] [Google Scholar]
- 40.Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 41.Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med. 1998;17:407–429. [PubMed] [Google Scholar]
- 42.Reilly JJ, Armstrong J, Dorosty AR, Emmett PM, Ness A, Rogers I, et al. Early life risk factors for obesity in childhood: cohort study. BMJ. 2005;330:1357. doi: 10.1136/bmj.38470.670903.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.OPCS. Standard Occupational Classification. Vol. 3. HMSO; London: p. 1991. [Google Scholar]
- 44.Office of Qualifications and Examinations Regulations. Explaining the National Qualifications Framework. Available at http://www.ofqual.gov.uk/ (accessed 23 December 2011)
- 45.North K, Emmett P. Multivariate analysis of diet among three-year-old children and associations with socio-demographic characteristics. The Avon Longitudinal Study of Pregnancy and Childhood (ALSPAC) Study Team. Eur J Clin Nutr. 2000;54:73–80. doi: 10.1038/sj.ejcn.1600896. [DOI] [PubMed] [Google Scholar]
- 46.O’Brien RMA. Caution regarding rules of thumb for variance inflation factors. Qual Quant. 2006;41:673–690. [Google Scholar]
- 47.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 48.Zaninotto P, Head J, Stamatakis E, Wardle H, Mindell J. Trends in obesity among adults in England from 1993 to 2004 by age and social class and projections of prevalence to 2012. J Epidemiol Community Health. 2009;63:140–146. doi: 10.1136/jech.2008.077305. [DOI] [PubMed] [Google Scholar]
- 49.Cole TJ, Freeman JV, Preece MA. Body mass index reference curves for the UK, 1990. Arch Dis Child. 1995;73:25–29. doi: 10.1136/adc.73.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thuny F, Richet H, Casalta J-P, Angelakis E, Habib G, Raoult D. Vancomycin treatment of infective endocarditis is linked with recently acquired obesity . PLoS ONE. 2010;5:e9074. doi: 10.1371/journal.pone.0009074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cho I, Yamanishi S, Cox LM, Methe B, Zavadil J, Li K, et al. Early-life antibiotics alter the murine colonic microbiome and adiposity. Nature. doi: 10.1038/nature11400. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mangrio E, Wremp A, Moghaddassi M, Merlo J, Bramhagen AC, Rosvall M. Antibiotic use among 8-month-old children in Malmo, Sweden—in relation to child characteristics and parental sociodemographic, psychosocial and lifestyle factors. BMC Pediatr. 2009;9:31. doi: 10.1186/1471-2431-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thrane N, Olesen C, Schonheyder HC, Sorensen HT. Socioeconomic factors and prescription of antibiotics in 0- to 2-year-old Danish children. J Antimicrob Chemother. 2003;51:683–689. doi: 10.1093/jac/dkg118. [DOI] [PubMed] [Google Scholar]
- 54.Billewicz WZ, Kemsley WF, Thomson AM. Indices of adiposity. Br J Prev Soc Med. 1962;16:183–188. doi: 10.1136/jech.16.4.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Howe LD, Tilling K, Benfield L, Logue J, Sattar N, Ness AR, et al. Changes in ponderal index and body mass index across childhood and their associations with fat mass and cardiovascular risk factors at age 15. PLoS One. 2010;5:e15186. doi: 10.1371/journal.pone.0015186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gutin B, Litaker M, Islam S, Manos T, Smith C, Treiber F. Body-composition measurement in 9–11-y-old children by dual-energy X-ray absorptiometry, skinfold-thickness measurements, and bioimpedance analysis. Am J Clin Nutr. 1996;63:287–292. doi: 10.1093/ajcn/63.3.287. [DOI] [PubMed] [Google Scholar]
- 57.Toschke AM, Martin RM, von Kries R, Wells J, Smith GD, Ness AR. Infant feeding method and obesity: body mass index and dual-energy X-ray absorptiometry measurements at 9–10 y of age from the Avon Longitudinal Study of Parents and Children (ALSPAC) Am J Clin Nutr. 2007;85:1578–1585. doi: 10.1093/ajcn/85.6.1578. [DOI] [PubMed] [Google Scholar]
- 58.Oken E, Gillman MW. Fetal origins of obesity. Obes Res. 2003;11:496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- 59.Schneider-Lindner V, Quach C, Hanley JA, Suissa S. Secular trends of antibacterial prescribing in UK paediatric primary care. J Antimicrob Chemother. 2011;66:424–433. doi: 10.1093/jac/dkq452. [DOI] [PubMed] [Google Scholar]
- 60.Trasande L. How much should we invest in preventing childhood obesity? Health Aff. 2010;29:372–378. doi: 10.1377/hlthaff.2009.0691. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


