Abstract
Cell therapy is currently considered as a potential therapeutic alternative to traditional treatments of diabetes. Islet and whole pancreas transplantations provided the proof- of-concept of glucose homeostasis restoration after replenishment of the deficiency of β cells responsible for the disease. Current limitations of these procedures have led to the search for strategies targeting replication of pre-existing β cells or transdifferentiation of progenitors and adult cells. These investigations revealed an unexpected plasticity towards β cells of adult cells residing in pancreatic epithelium (e.g. acinar, duct and α cells). Here we discuss recent developments in β-cell replication and β-cell transdifferentiation of adult epithelial pancreatic cells, with an emphasis on techniques with a potential for clinical translation.
Keywords: diabetes, pancreas, replication, progenitors, transdifferentiation, β cells, adult cells
Introduction
Anomalies of glucose metabolism appear after quantitative or functional decline of the pancreatic β cells, leading eventually to the diagnosis of diabetes, whether type 1 (T1D), type 2 (T2D) or monogenic. The replacement of functional pancreatic β cells essentially cures diabetes as was demonstrated first with whole pancreas transplantation and then with islet transplantation; they are currently associated with insulin independence respectively in up to 30% and 44% of T1D patients 5 and 3 years after transplantation [1, 2]. However, the indications and availability of both techniques are hampered by scarcity of donors and graft failure within a few years. Pluripotent stem cells, namely embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), have been regarded as alternative sources because of their theoretically unlimited supply and differentiation potential. Indeed, pancreatic progenitors derived from ESCs and iPSCs acquire glucose-responsive insulin secretion in vivo [3–5] and can normalize glycemia when transplanted into diabetic animals. However, the translational application of pluripotent stem cells through transplantation faces important barriers with the risk of tumor formation in vivo and the need to be protected from immune attack.
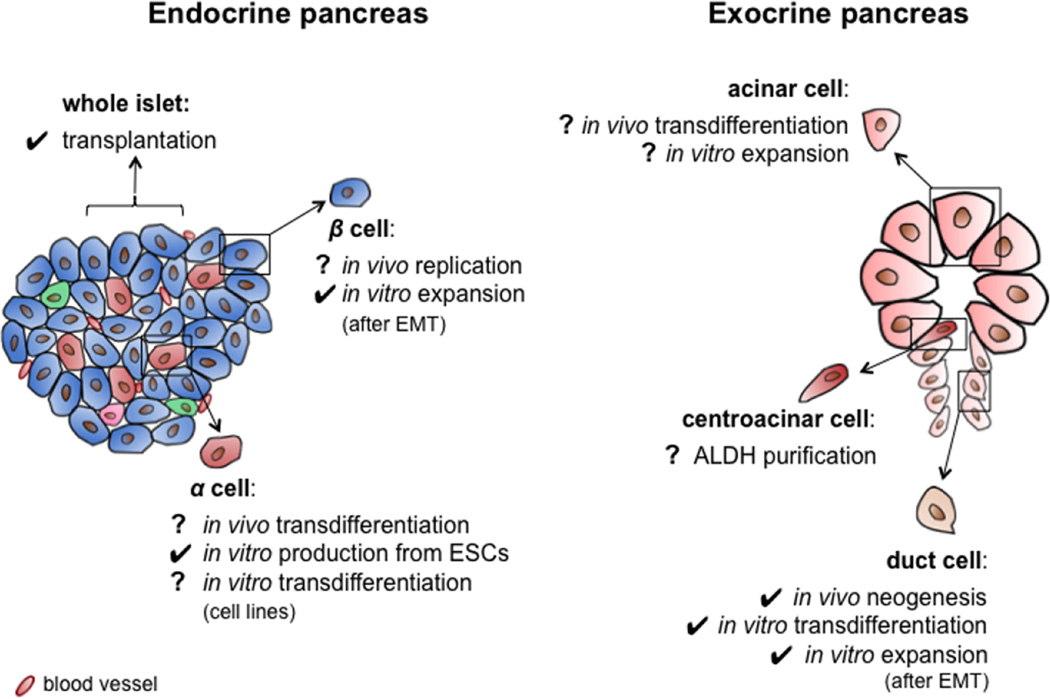
In this context, pancreatic epithelial cells (duct, acinar, α and β cells) emerge as a potential alternative to pluripotent stem cells because of their demonstrated β-cell differentiation capacities and their likelihood of fewer safety concerns. Besides identifying candidate cell sources, cell therapy for diabetes requires further developments for protection of the new β cells from autoimmune destruction and/or rejection. While the complexities of immunoprotection have been described elsewhere [6], herein we discuss recent progress in exploiting the potential of the pancreas itself as a source of β cells for replacement therapy (Figure 1).
Figure 1.
Potential cell sources in the human pancreas for diabetes cell therapy. Check marks indicate procedures or phenomena (i.e. neogenesis) that were validated or observed with human cells or tissues. Question marks refer to experiments achieved in rodents and that await translation to human.
Why choose cells within the pancreas?
Regeneration and cell plasticity have both been demonstrated as occurring in rodent pancreas under specific conditions. As discussed below, new islet cells can arise from preexisting pancreatic cells of varied origin. Furthermore, the existence of facultative progenitors with in vivo or ex vivo β-cell engineering potential has been reported. Together these observations suggest the possibility that the formation of new β cells from cells residing within the adult pancreas has therapeutic potential. Having a reservoir of endocrine progenitor cells in the organ itself allows for either in situ or ex vivo expansion and transdifferentiation approaches to increase β-cell mass. Since pancreatic epithelial cells all arise from a common progenitor [7], they share similar epigenetic profiles [8, 9] that could facilitate their transdifferentiation towards β cells.
Pancreatic epithelial cells have a natural advantage over pluripotent stem cells due to the stability of their differentiation status after isolation or in vitro culture. Experience with transplantation of epithelial cells (hepatocytes [10], islets [11], corneal cells [12]) confirms this stability even after years of follow-up. In contrast, clinical translation of pluripotent stem cell-derived β-like cells awaits better definition of the differentiated products [13, 14] to avoid the transplantation of precursor cells with tumorigenic potential. For all expanded cells, attention must be paid to chromosomal abnormalities and epigenetic changes associated with risk of transformation that might occur after their expansion in culture as described with cells of mesenchymal origin [15].
What is a good candidate for β-cell engineering?
Even though the acquisition of bona fide β-cell functionality is the ultimate goal of β-cell engineering procedures, additional issues must be addressed before a cell source can be considered for cell therapy. These include the need to isolate the candidate cells in a reliable and minimally invasive manner to collect or expand the cells to produce a clinically relevant mass, to cryopreserve the cells in a cell bank for elective procedures, to maintain genetic stability of the expanded cells during in vitro expansion and after transplantation [16], and to prepare the cells in a good manufacturing practice-compliant facility. The need to have full β-cell functionality is perhaps the most stringent prerequisite but it might not be absolute. Indeed providing patients with diabetes with cells capable of insulin secretion, even without fine-tuned glucose regulation, might be helpful for lowering daily insulin requirements and improving glycemic control in some difficult to control individuals.
What cell types are candidates?
A. β cells
Replication of endogenous β cells
The β cell has proven to be a major determinant of the regeneration potential of the pancreas in rodents after birth [17]. In humans, an important increase in β cell mass occurs by replication of preexisting β cells in the neonate but fades after 2 years of age [18, 19]. In the adult, β-cell replication in some studies appeared to be a rare event [19, 20] although Reers et al. systematically observed proliferative Ki67+ β-cells in human donor pancreases [21] with the number of Ki67+ β cells decreasing with age. In a recent study [22], we found 0.22±0.03% Ki67+/insulin+ cells in surgically resected pancreases from older humans, suggesting that Ki67 positivity may be artifactually low in autopsy and cadaver donor pancreas, meaning that β-cell turnover may be much higher and important than generally thought.
As seen in rodents, the human endocrine pancreas seems to respond with a compensatory increase in β-cell mass to conditions with higher metabolic demand and insulin resistance, such as obesity [23, 24] and pregnancy [25, 26]. In the latter, β-cell turnover is believed to result partly from replication, increased insulin secretion, and neogenesis [25, 26]. Activation of serotonin synthesis (through activity of tryptophan hydroxylase 1) is one of the pathways implicated in the changes in β-cell growth during pregnancy [27]. Obesity and pregnancy appear to be associated with similar molecular controls of β-cell replication, including upregulation of FoxM1 expression [28, 29] and inhibition of menin [30], each playing opposite roles in cell cycle activity. Recently, decreased miR-338-3p levels were also identified as a β-cell expansion stimulus in both obese and pregnant rodents [31].
Thorough understanding of the mechanisms that govern the β-cell cycle in human pancreas is needed to identify a pathway that can be selectively targeted for therapeutic purposes [32, 33]. The feasibility of inducing human β-cell proliferation has been shown using virus-based over-expression of cyclin-dependent kinase (cdk)-4 and cyclin D1 in vitro [34], or cdk-6, with or without cyclin D1, in vitro and in grafts [35]. Although such strategies offer opportunities for ex vivo islet expansion, specific β cell targets are needed for in vivo interventions. Also, non-viral stimulating agents are sought for clinical application, as small molecules having the advantage of being inexpensive and readily available, making them suitable for high-throughput screening methods [36, 37]. Accordingly, recent screens identified new compounds promoting β-cell replication through interactions with the adenosine pathway [38, 39].
Besides cell cycle proteins, other metabolic pathway gatekeepers are currently considered as putative targets for molecular induction; among these are the enzymes glycogen synthase kinase-3 β (GSK-3β) and glucokinase (GCK). GSK-3β is a widely expressed and constitutively active serine/threonine kinase implicated in signaling pathways, such as the Wnt/β-catenin- and the phosphoinositide 3-kinase (PI3K)/Akt, both of which have important roles in β-cell function and proliferation [40, 41]. For example, GSK-3β activity indirectly induces proteasome degradation of β-catenin, leading to reduced β-cell replication [42], so its inhibition may have stimulating effects on β-cell mass. Since GSK-3β inhibitors such as paullones tend to interact with CDK1/cyclin B, more selective inhibitors are being developed, such as 9-cyano-1-aza-paullone or CHIR99021 that induced proliferation of primary rat β cells [43]. Since GSK-3β has such important roles in the entire organism, the development of β-cell specific GSK-3β stimulators for in vivo use will be challenging.
GCK is the β-cell glucose sensor for insulin secretion because it is the rate-limiting enzyme for glycolysis thereby regulating mitochondrial oxidation and ATP production. Increased GCK activity can stimulate islet proliferation, as shown by a child with a GCK (V91L) mutation coding for a variant with 8.5 fold higher than normal glucose affinity [44]. The child’s pancreas had hyperplastic islets (larger and with more Ki67+ cells) compared to age-matched controls. The role of GCK in the regulation of β-cell mass has been further studied in mice, in which the loss of one GCK allele led to the loss of a compensatory increase in β-cell replication in response to a high-fat diet [45]; the proliferation could be restored with a glucokinase activator (GKA) [46]. Furthermore, this GKA augmented β-cell proliferation in wild-type mice, an effect that was blunted in Irs2−/− mice and by oxidative stress [47]. GKAs have a broader impact on glucose metabolism not only by stimulating insulin secretion but also by enhancing hepatic glucose uptake [48]. Some GKAs, including LY2599506, are being evaluated in clinical trials for their therapeutic potential in diabetes [49].
Glucagon-like peptide (GLP)-1 receptor agonists and DPPIV inhibitors that limit GLP-1 degradation are already widely used in T2D patients for their beneficial effects on glucose metabolism [50]. In rodents, use of these agonists, e.g. exendin-4 and liraglutide, resulted in increased β-cell mass [51, 52] leading to hopes for similar beneficial effects on β-cell mass in humans. However, the potential effect of exendin-4 as a proliferation stimulus of transplanted human islets has been observed only with young donors [53] and was not confirmed in our series [22]. As of yet, no lasting effect after drug washout suggesting significant restitution of β-cell mass has been reported for this class of drugs.
Studies on β-cell fate during development of diabetes may provide new clues for β cell mass restoration. Recently, Talchai et al. described β-cell dedifferentiation in diabetic mouse models (db/db and insulin-resistant GIRKO mice) and proposed a central role of this mechanism in the β-cell failure observed in diabetes [54]. These authors observed an increased proportion of ‘empty’ chromogranin A+/hormone− cells in hyperglycemic mice and suggested that re-differentiation of these empty endocrine cells might be as useful as replication of pre-existing β cells for increasing the β-cell mass in type 2 diabetes. Previously impressive changes in expression of genes key to the β-cell phenotype (e.g. transcription factors, glucose metabolism genes, stress genes) have been shown to result from exposure to chronic modest hyperglycemia and correlated with β-cell dysfunction [55, 56], but it is unclear whether any of these β cells reverted to an endocrine progenitor as recently suggested [54]. Moreover, it remains to be seen if such dedifferentiated cells can be found in islets of individuals with T2D.
In T1D, in situ replication of β cells would require immunomodulation to limit destruction of newly formed β cells. However, even with ongoing autoimmunity, the possibility to maintain the β cell mass to a minimum level could help stabilize the disease, as seen during the partial remission phase. Subjects with T2D and monogenic diabetes, not having a threat from autoimmunity, should represent better candidates for β-cell replication strategies. One concern is that the metabolic syndrome in T2D would be expected to make the β cells work harder, but failure from such stress should take years to cause problems.
Ex vivo expansion of β cells
Under specific in vitro culture conditions, β cells appear to revert to an undifferentiated mesenchymal-like phenotype. The team of Efrat has exploited this propensity to expand cells from the human β-cell lineage [57, 58]. The epithelial-mesenchymal transition (EMT) cells thought to be of β-cell origin showed high proliferation capacity (up to 16 population doublings) and could be re-differentiated by inhibition of notch signaling, aggregation in serum-free media with a cocktail of nicotinamide, exendin-4, activin A, N2 and B27 supplements [59, 60]. Using lineage marking, previously dedifferentiated β cells were shown to be responsible for the re-differentiation capacity, which was attributed to their epigenetic memory [8]. The authors reported a re-differentiation yield of up to 25% of all EMT cells, which exceeds the yields described with ESCs [3, 4] and represents an 8–32 fold increase in the number of insulin+ cells starting from the uncultured islets. Preliminary studies on transplantation of these re-differentiated EMT cells showed an in vivo functional ability to partially reduce the blood glucose of streptozotocin-diabetic mice.
B. Duct cells
During pancreas development, the ductal epithelium gives rise to all pancreatic epithelial lineages, i.e. duct, acinar and endocrine cells. As shown in rodent studies particularly with lineage-tracing techniques, new islet cells can develop from progenitors through a process called neogenesis in postnatal life and in models of pancreas injury [61]. The existence of facultative β-cell progenitors in the duct epithelium was elegantly shown by Xu et al. [62], with the induction of Ngn3+cytokeratin+ cells as soon as 3 days after partial duct ligation (PDL) in mice. Seven days after PDL in Ngn3-nlacZ reporter mice, cells co-stained with the lineage marker β-gal+ and islet hormones, indicating endocrine differentiation of the Ngn3+ progenitors. Then using Ngn3-eGFP mice and FACS-sorted eGFP+ cells 7 days after PDL, the eGFP+ non-granulated cells or Ngn3+ progenitors, which represented only 0.04% of the total number of sorted pancreatic cells, had an expression profile of pancreatic progenitors and were shown to differentiate into glucose-responsive β-like cells when cultured with Ngn3null embryonic pancreas explants.
The capacity of duct cells to give rise to new β cells in the adult mouse has become controversial with contradictory results even when similar Cre-loxP lineage tracing strategies were employed [63–69]. While several studies [63–65] found no endocrine cells marked for duct lineage after birth or after injury, other studies did [66–68]. Lineage tracing is considered the “gold-standard” for tracing progeny, but there are caveats as with any technique, including cell heterogeneity within the ductal tree [70], distinct reprogramming potential of subpopulations, perdurance of tamoxifen [71], or a low efficiency of some tracing strategies. The discrepancy of findings from two well-done studies using Sox9 to drive inducible Cre illustrate this point: in one [63] Kopp et al. found a few non-β-endocrine cells derived from the Sox9+ ducts in early postnatal life but no labeled acinar cells, in the other Furuyama et al. observed plentiful labeling of acinar cells after the pulse-chase was initiated during postnatal period [64] and only 1% labeled pancreatic endocrine cells when tracing was initiated on the first postnatal day. Both studies traced the fate of Sox9+ populations, stressing that accuracy and interpretability of current lineage tracing approaches are not without issues.
In the human pancreas, it is much more difficult to assess neogenesis but using the definition of neogenesis as hormone-positive cells “budding” from ducts, the frequency of insulin positive cells within the duct has been reported as about 0.5% insulin+ duct cell in healthy patients [18, 21] and tends to increase in situations of high metabolic demand, as obesity [72] and pregnancy [26]. However, there is considerable uncertainty about how this natural propensity for duct cells to transdifferentiate into β cells might be exploited in vivo for clinical purposes.
Ex vivo, unequivocal demonstration of the β-cell differentiation potential of human duct cells has been made with purified CA19-9+ populations [73]. The ex vivo exploitation of duct cells has several advantages over other pancreatic epithelial cells: duct cells are relatively more resistant to handling and shipping, the cells can be reliably purified based on cell surface markers, and they attach in culture, facilitating the possibility of 2D differentiation and enhancement of proliferation signals. However, as with many epithelial cell types, duct cells lack the capacity for sustained proliferation and tend to lose their phenotype in vitro [74, 75]. We developed a system to induce proliferation of purified CA19-9+ duct cells by forcing their natural tendency to dedifferentiate [76]. Using specific culture conditions, we obtained highly proliferative cultures that showed typical features of EMT. These undifferentiated EMT cells were found to respond to in vitro β-cell differentiation strategies by acquiring β-cell specific characteristics in gene expression and human insulin secretion, even though glucose-stimulated insulin secretion (GSIS) was lacking. Whereas further work is needed to provide a full picture of the capacity of these duct-derived cells for β-cell engineering, our work suggests that these expanded human duct cells can be differentiated into cells with a β cell-like phenotype.
Another aspect of research on duct cell differentiation is their potential to give rise to β-cell lineage. In their work on Pax4-induced α-to-β transdifferentiation, Collombat et al. showed the capacity of duct cells to reprogram to α cells through the activation of Ngn3 expression [77]. Pax4-induced α-to-β conversion led to a glucagon deficiency that triggered neogenesis of α cells from the duct epithelium. Lentiviral-based knockdown strategies confirmed the central role of Ngn3 in this duct-to-α cell neogenesis.
C. Acinar cells
Being by far the major population of cells in the pancreas, acinar cells must be examined for their capacity to generate new β cells. Acinar-to-β cell differentiation was suggested in rodents with in vitro differentiation assays [78] and staining studies showing cells expressing both amylase and insulin in rat pancreas after PDL [79], and in zebrafish with lineage tracing studies [80]. This potential was however challenged by a report showing no acinar-to-β cell transdifferentiation after 70% pancreatectomy, PDL, or caerulein-induced pancreatitis in mice [81]. However, with in vitro lineage tracing, cultured human acinar cells were shown to dedifferentiate or transdifferentiate to a ductal phenotype within a week [82], thus presenting the possibility that they could be induced to differentiate to endocrine cells.
Zhou et al. [83] showed the possibility to reprogram exocrine cells to β cells in vivo by adenoviral-mediated over-expression of key β-cell transcription factors (TFs) Pdx1, Ngn3, and MafA. Adenoviruses (Ad) were chosen as vectors due to their supposedly preferential infection of exocrine cells as compared to islet cells [84]. By 3 days after a single intrapancreatic injection of the 3 Ad-TF constructs co-expressing the reporter green fluorescent protein (GFP) in Rag1−/− non-diabetic animals, insulin-positive GFP-positive cells were found; by 10 days the reprogrammed cells had many of the characteristics of bona fide β cells in their expression profile and immunostaining of β-cell proteins. In streptozotocin (STZ)-treated animals, these newly formed β cells led to a significant decrease of hyperglycemia, which did not reach true normoglycemia. It still remains to be shown that these have glucose-stimulated insulin secretion and are fully reprogrammed. Others have shown the feasibility of reprogramming cells using TFs without viruses using protein transduction [85] or modified RNA transfection [86], albeit at low efficiency and by means of technically demanding protocols. With these possibilities, acinar cells remain an attractive source for new β cell formation.
Our understanding of the network of TFs governing pancreas development is growing [7], with new players being regularly identified [87]. However, there are theoretically only a few combinations of TFs exploitable for β cell engineering, as elegantly shown by Zhou et al in 2007 [88]. They identified using a genome-wide expression study of >1100 TFs in the developing pancreas, only 30 TFs being localized in pancreatic and endocrine progenitors by in situ hybridization. Even though TF-based reprogramming is intuitively powerful and specific, difficulties may arise in terms of accessibility of binding sites, influence of context-specific co-factors and presence of epigenetic remodeling complexes [89]. As for clinical translation perspectives, the cost/effectiveness of TF-based reprogramming will need to be weighed against small molecule- or growth factor-based protocols.
D. α cells
The concept of plasticity of mature islet cells, particularly a cells, has led to excitement about the therapeutic potential of converting α cells to β cells. Using genetic manipulation in mice, Collombat et al. first showed successful conversion of adult mouse β cells into α- and PP-derivatives by misexpression of Arx coding for a transcription factor that specifies α cell fate [90]. Then, they showed that Pax4 over-expression in the embryonic Pdx1+ pancreatic progenitor cells or in mature glucagon+ endocrine cells were sufficient for their specification into functional β cells capable of reversing STZ-induced diabetes [77]. Herrera’s group confirmed these results showing that when almost all (> 99%) β cells were destroyed by selective expression of diphtheria toxin receptor downstream of the rat insulin promoter, α cells spontaneously reprogrammed into β cells [91]. In this study, the combined ablation of β and α cells prevented the reprogramming. Differential α-to-β reprogramming between Pdx1-enforced expression in Ngn3+ endocrine progenitors and in mature hormone-expressing cells suggests a context-dependent receptiveness that may reflect prior epigenetic markings [92].
It should be pointed out, however, that while the proportion of β cells marked as having come from α cells was significant, the actual number of these cells as a function of normal β-cell mass was very low because there were so few β cells left after the killing by diphtheria toxin. It is also noteworthy that there are as yet no convincing studies showing that such conversion occurs after β-cell killing with either STZ or alloxan.
In human pancreas, similar plasticity has not yet been demonstrated even though insulin+glucagon+ cells have been reported in fibrotic pancreases [93]. For therapeutic purposes, α cells may represent a putative reservoir of new β cells in vivo or may require ex vivo expansion for cell therapy. In vivo strategies might benefit from the natural microenvironment of α cells as islet cells already networking with each other and with other endocrine cells. In vitro derivation of α cells has been reported from human ESCs [94] and the feasibility to implement α-to-β reprogramming in vitro has been shown with a murine α-cell line that was driven towards insulin-producing cells by the small molecule BRD-7389 [95]. At this point there are only a few studies showing apparent conversion of α cells to small numbers of β cells raising concerns that this reprogramming may not prove to be a fruitful path to generating a useful number of β cells. Of note, a recent study in non-human primates found neither α-cell replication nor β-cell regeneration after STZ-provoked β-cell ablation [96].
E. Progenitors characterized in vitro
In the quest for pancreatic stem cells/progenitors, intracellular expression of aldehyde dehydrogenase (ALDH) has emerged as a new area of investigation after the demonstration that human bone marrow ALDH+ populations are enriched in hematopoietic progenitors [97]. The association between ALDH expression and stemness is thought to result from the capacity to detoxify potentially cytotoxic metabolic products [98]. Rovira et al. studied the staining pattern of ALDH1 protein in adult mouse pancreas and found its expression in centroacinar cells, terminal duct epithelial cells and mesenchymal cells surrounding endocrine islets and exocrine acini [99]. They further studied the centroacinar cells, an elusive cell in the pancreas that actively proliferates after pancreas injury [100]. Centroacinar cells are small cells (10 µm in diameter) within the pancreatic epithelium residing at the junction of the terminal duct and the acinus. The function of these cells and whether they represent a unique phenotype or reflect several cell types (e.g. terminal duct cells) residing in the centroacinar position, is largely unknown. Using a fluorogenic ALDH1 substrate to sort the central acinar/terminal duct cells from exocrine tissue depleted from islets and large ducts, the authors observed that the ALDH1+ population accounting for 0.5% of the FACS-sorted mouse pancreatic cells was enriched for progenitor markers (Sca1, Sdf1, c-Met), and expanded to form spheres in vitro, either in suspension or after plating in clonal dilution. Within 5 to 7 days, 30% of the pancreatospheres expressed C-peptide; after injection into cultured embryonic dorsal pancreatic buds, ALDH1+ cells contributed to both pancreatic exocrine and endocrine lineages. Furthermore, Ioannou et al. identified in the adult mouse rare ALDH1B1+peanut agglutinin+ cells identified as centroacinar cells and able to proliferate extensively and rapidly after regeneration stimuli (caerulein and STZ treatments) [101]. Both studies suggest an important role for centroacinar cells in pancreas regeneration, and further work is required to confirm this potential in humans.
In addition, the adult mouse has been shown to contain a progenitor population that can be obtained from islet or duct tissues in vitro using single-cell clonal culture [102]. In serum-free media, these pancreas-derived multipotent precursors (PMPs) formed colonies expressing markers of both pancreatic and neural lineages. Upon culture with Matrigel, PMPs developed into β-like cells. More recently [103], the authors identified the pancreas and not the neural crest as the source of PMPs, using Wnt1-Cre;Z/EG and PDX-1-Cre;Z/EG mice, respectively marking progeny of neural and pancreatic origin. Using MIP-GFP mice, they showed mouse PMPs were predominantly insulin+Glut2low. Similarly human PMPs were derived with an efficiency of about 2.6 generated spheres/10,000 isolated cells and expressed neural and endocrine progenitor markers. After Matrigel-induced differentiation, human PMPs contained 11.6% insulin+/PDX-1+ β-like cells, and showed modest (2 fold) higher insulin release to high glucose than low glucose. After transplantation under the kidney capsule of STZ-treated diabetic NOD-SCID mice, human PMPs lowered the hyperglycemia somewhat. The composition of grafts was not described, so it is unclear the fate of the predominant (≈ 88.4%) non-β cells present before transplantation. To facilitate prospective isolation of such progenitors, efforts are being made to identify cell surface markers for the various stages of β cell progenitors [104], much as is being done with different stages of embryonic stem cell development [13, 14].
Although these cell clusters could teach us something important about the development of islet cells, much more work must be done to show that these cells have therapeutic potential.
Conclusion
In coming years, the pancreas may serve as a source of cells for β-cell replacement therapy. Major hurdles remain for targeting cells in vivo for inducing proliferation and/or transdifferentiation and for isolating and expanding cells with tropism towards the β-cell lineage. However, new technologies (e.g. protein or RNA transduction, small molecule screening, EMT-based cell expansion) offer a broad spectrum of applications, both in vitro and in vivo that may facilitate the efforts. As we learn more of the intricate molecular mechanisms that control β-cell fate and the factors that are responsible for cellular plasticity, we will have new clues for β-cell engineering.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Philippe A. Lysy, Gordon C. Weir, Susan Bonner-Weir declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1. Barton FB, et al. Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes care. 2012;35(7):1436–1445. doi: 10.2337/dc12-0063.This paper describes the recent progresses of islet transplantation: increased insulin independence at 3 years post-transplant (44%) and islet graft function (c-peptide secretion), and decreased adverse events in the years 2007–2010.
- 2.Maglione M, Ploeg RJ, Friend PJ. Donor risk factors, retrieval technique, preservation and ischemia/reperfusion injury in pancreas transplantation. Current opinion in organ transplantation. 2013;18(1):83–88. doi: 10.1097/MOT.0b013e32835c29ef. [DOI] [PubMed] [Google Scholar]
- 3.Kroon E, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nature biotechnology. 2008;26(4):443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 4.Rezania A, et al. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes. 2012;61(8):2016–2029. doi: 10.2337/db11-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schroeder IS. Potential of pluripotent stem cells for diabetes therapy. Current diabetes reports. 2012;12(5):490–498. doi: 10.1007/s11892-012-0292-5. [DOI] [PubMed] [Google Scholar]
- 6.O'Sullivan ES, et al. Islets transplanted in immunoisolation devices: a review of the progress and the challenges that remain. Endocrine Reviews. 2011 doi: 10.1210/er.2010-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pan FC, Wright C. Pancreas organogenesis: from bud to plexus to gland. Developmental dynamics : an official publication of the American Association of Anatomists. 2011;240(3):530–565. doi: 10.1002/dvdy.22584.Thorough overview of pancreas embryogenesis with insights into molecular and cellular aspects of organ development.
- 8. Bar-Nur O, et al. Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet Beta cells. Cell stem cell. 2011;9(1):17–23. doi: 10.1016/j.stem.2011.06.007.This work highlights the importance of epigenetic memory in cell reprogramming and the benefits of using of pancreatic cells for β-cell differentiation.
- 9.Avrahami D, Kaestner KH. Epigenetic regulation of pancreas development and function. Seminars in cell & developmental biology. 2012;23(6):693–700. doi: 10.1016/j.semcdb.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sokal EM. From hepatocytes to stem and progenitor cells for liver regenerative medicine: advances and clinical perspectives. Cell proliferation. 2011;44(Suppl 1):39–43. doi: 10.1111/j.1365-2184.2010.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shapiro AM. State of the Art of Clinical Islet Transplantation and Novel Protocols of Immunosuppression. Current diabetes reports. 2011 doi: 10.1007/s11892-011-0217-8. [DOI] [PubMed] [Google Scholar]
- 12.Tan DT, et al. Corneal transplantation. Lancet. 2012;379(9827):1749–1761. doi: 10.1016/S0140-6736(12)60437-1. [DOI] [PubMed] [Google Scholar]
- 13.Naujok O, Lenzen S. A critical re-evaluation of CD24-positivity of human embryonic stem cells differentiated into pancreatic progenitors. Stem cell reviews. 2012;8(3):779–791. doi: 10.1007/s12015-012-9362-y. [DOI] [PubMed] [Google Scholar]
- 14. Kelly OG, et al. Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nature biotechnology. 2011;29(8):750–756. doi: 10.1038/nbt.1931.This paper shows the possibility to obtain glucose-responsive β-like cells in vivo after transplantation of FACS-sorted CD149+ pancreatic endoderm cells derived from human ESCs. Interestingly, purified endocrine cells preferentially differentiated in vivo in α cells.
- 15.Wagner W. Implications of long-term culture for mesenchymal stem cells: genetic defects or epigenetic regulation? Stem cell research & therapy. 2012;3(6):54. doi: 10.1186/scrt145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prockop DJ, Olson SD. Clinical trials with adult stem/progenitor cells for tissue repair: let's not overlook some essential precautions. Blood. 2007;109(8):3147–3151. doi: 10.1182/blood-2006-03-013433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desgraz R, Bonal C, Herrera PL. beta-cell regeneration: the pancreatic intrinsic faculty. Trends in endocrinology and metabolism: TEM. 2011;22(1):34–43. doi: 10.1016/j.tem.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Meier JJ, et al. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes. 2008;57(6):1584–1594. doi: 10.2337/db07-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gregg BE, et al. Formation of a human beta-cell population within pancreatic islets is set early in life. The Journal of clinical endocrinology and metabolism. 2012;97(9):3197–3206. doi: 10.1210/jc.2012-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perl S, et al. Significant human beta-cell turnover is limited to the first three decades of life as determined by in vivo thymidine analog incorporation and radiocarbon dating. The Journal of clinical endocrinology and metabolism. 2010;95(10):E234–E239. doi: 10.1210/jc.2010-0932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reers C, et al. Impaired islet turnover in human donor pancreata with aging. European journal of endocrinology / European Federation of Endocrine Societies. 2009;160(2):185–191. doi: 10.1530/EJE-08-0596. [DOI] [PubMed] [Google Scholar]
- 22.Caballero F, et al. Birth and death of human beta-cells in pancreas from cadaver donors, autopsies, surgical specimens, and islets transplanted into mice. Cell transplantation. 2013 doi: 10.3727/096368912X659916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahier J, et al. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes, obesity & metabolism. 2008;10(Suppl 4):32–42. doi: 10.1111/j.1463-1326.2008.00969.x. [DOI] [PubMed] [Google Scholar]
- 24.Hanley SC, et al. {beta}-Cell mass dynamics and islet cell plasticity in human type 2 diabetes. Endocrinology. 2010;151(4):1462–1472. doi: 10.1210/en.2009-1277. [DOI] [PubMed] [Google Scholar]
- 25.Rieck S, Kaestner KH. Expansion of beta-cell mass in response to pregnancy. Trends in endocrinology and metabolism: TEM. 2010;21(3):151–158. doi: 10.1016/j.tem.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butler AE, et al. Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia. 2010;53(10):2167–2176. doi: 10.1007/s00125-010-1809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim H, et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nature medicine. 2010;16(7):804–808. doi: 10.1038/nm.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H, et al. Gestational diabetes mellitus resulting from impaired beta-cell compensation in the absence of FoxM1, a novel downstream effector of placental lactogen. Diabetes. 2010;59(1):143–152. doi: 10.2337/db09-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis DB, et al. FoxM1 is up-regulated by obesity and stimulates beta-cell proliferation. Molecular endocrinology. 2010;24(9):1822–1834. doi: 10.1210/me.2010-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karnik SK, et al. Menin controls growth of pancreatic beta-cells in pregnant mice and promotes gestational diabetes mellitus. Science. 2007;318(5851):806–809. doi: 10.1126/science.1146812. [DOI] [PubMed] [Google Scholar]
- 31. Jacovetti C, et al. MicroRNAs contribute to compensatory beta cell expansion during pregnancy and obesity. The Journal of clinical investigation. 2012;122(10):3541–3551. doi: 10.1172/JCI64151.• This paper is the first report of the role of microRNAs in regulation of β-cell proliferation, offering a new area of investigation.
- 32.Cozar-Castellano I, et al. Molecular control of cell cycle progression in the pancreatic beta-cell. Endocrine reviews. 2006;27(4):356–370. doi: 10.1210/er.2006-0004. [DOI] [PubMed] [Google Scholar]
- 33.Kulkarni RN, et al. Human beta-cell proliferation and intracellular signaling: driving in the dark without a road map. Diabetes. 2012;61(9):2205–2213. doi: 10.2337/db12-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cozar-Castellano I, et al. Induction of beta-cell proliferation and retinoblastoma protein phosphorylation in rat and human islets using adenovirus-mediated transfer of cyclin-dependent kinase-4 and cyclin D1. Diabetes. 2004;53(1):149–159. doi: 10.2337/diabetes.53.1.149. [DOI] [PubMed] [Google Scholar]
- 35.Fiaschi-Taesch N, et al. Survey of the human pancreatic beta-cell G1/S proteome reveals a potential therapeutic role for cdk-6 and cyclin D1 in enhancing human beta-cell replication and function in vivo. Diabetes. 2009;58(4):882–893. doi: 10.2337/db08-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Firestone AJ, Chen JK. Controlling destiny through chemistry: small-molecule regulators of cell fate. ACS chemical biology. 2010;5(1):15–34. doi: 10.1021/cb900249y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vetere A, Wagner BK. Chemical methods to induce Beta-cell proliferation. International journal of endocrinology. 2012;2012:925143. doi: 10.1155/2012/925143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Annes JP, et al. Adenosine kinase inhibition selectively promotes rodent and porcine islet beta-cell replication. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(10):3915–3920. doi: 10.1073/pnas.1201149109.The authors show a successful high-throughput small molecule screening for stimulating proliferation of primary mammalian β cells through modulation of adenosine pathway.
- 39.Andersson O, et al. Adenosine signaling promotes regeneration of pancreatic beta cells in vivo. Cell metabolism. 2012;15(6):885–894. doi: 10.1016/j.cmet.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rulifson IC, et al. Wnt signaling regulates pancreatic beta cell proliferation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(15):6247–6252. doi: 10.1073/pnas.0701509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elghazi L, et al. Regulation of beta-cell mass and function by the Akt/protein kinase B signalling pathway. Diabetes, obesity & metabolism. 2007;9(Suppl 2):147–57. doi: 10.1111/j.1463-1326.2007.00783.x. [DOI] [PubMed] [Google Scholar]
- 42.Welters HJ, Kulkarni RN. Wnt signaling: relevance to beta-cell biology and diabetes. Trends in endocrinology and metabolism: TEM. 2008;19(10):349–355. doi: 10.1016/j.tem.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 43.Stukenbrock H, et al. 9-cyano-1-azapaullone (cazpaullone), a glycogen synthase kinase-3 (GSK-3) inhibitor activating pancreatic beta cell protection and replication. Journal of medicinal chemistry. 2008;51(7):2196–2207. doi: 10.1021/jm701582f. [DOI] [PubMed] [Google Scholar]
- 44. Kassem S, et al. Large islets, beta-cell proliferation, and a glucokinase mutation. The New England journal of medicine. 2010;362(14):1348–1350. doi: 10.1056/NEJMc0909845.This paper is an example of translation from bedside to bench that offers possibilities to study islet proliferation.
- 45.Terauchi Y, et al. Glucokinase and IRS-2 are required for compensatory beta cell hyperplasia in response to high-fat diet-induced insulin resistance. The Journal of clinical investigation. 2007;117(1):246–257. doi: 10.1172/JCI17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakamura A, et al. Impact of small-molecule glucokinase activator on glucose metabolism and beta-cell mass. Endocrinology. 2009;150(3):1147–1154. doi: 10.1210/en.2008-1183. [DOI] [PubMed] [Google Scholar]
- 47.Nakamura A, et al. Control of beta cell function and proliferation in mice stimulated by small-molecule glucokinase activator under various conditions. Diabetologia. 2012;55(6):1745–1754. doi: 10.1007/s00125-012-2521-5. [DOI] [PubMed] [Google Scholar]
- 48.Matschinsky FM, et al. Research and development of glucokinase activators for diabetes therapy: theoretical and practical aspects. Handbook of experimental pharmacology. 2011(203):357–401. doi: 10.1007/978-3-642-17214-4_15. [DOI] [PubMed] [Google Scholar]
- 49.Zhang X, et al. Dose selection using a semi-mechanistic integrated glucose-insulin-glucagon model: designing phase 2 trials for a novel oral glucokinase activator. Journal of pharmacokinetics and pharmacodynamics. 2012 doi: 10.1007/s10928-012-9286-9. [DOI] [PubMed] [Google Scholar]
- 50.Garber AJ. Incretin effects on beta-cell function, replication, and mass: the human perspective. Diabetes care. 2011;34(Suppl 2):S258–S263. doi: 10.2337/dc11-s230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu G, et al. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48(12):2270–2276. doi: 10.2337/diabetes.48.12.2270. [DOI] [PubMed] [Google Scholar]
- 52.Sturis J, et al. GLP-1 derivative liraglutide in rats with beta-cell deficiencies: influence of metabolic state on beta-cell mass dynamics. British journal of pharmacology. 2003;140(1):123–132. doi: 10.1038/sj.bjp.0705397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tian L, et al. Comparison of exendin-4 on beta-cell replication in mouse and human islet grafts. Transplant international : official journal of the European Society for Organ Transplantation. 2011;24(8):856–864. doi: 10.1111/j.1432-2277.2011.01275.x. [DOI] [PubMed] [Google Scholar]
- 54.Talchai C, et al. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell. 2012;150(6):1223–1234. doi: 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jonas JC, et al. Chronic hyperglycemia triggers loss of pancreatic beta cell differentiation in an animal model of diabetes. The Journal of biological chemistry. 1999;274(20):14112–14121. doi: 10.1074/jbc.274.20.14112. [DOI] [PubMed] [Google Scholar]
- 56.Laybutt DR, et al. Critical reduction in beta-cell mass results in two distinct outcomes over time. Adaptation with impaired glucose tolerance or decompensated diabetes. The Journal of biological chemistry. 2003;278(5):2997–3005. doi: 10.1074/jbc.M210581200. [DOI] [PubMed] [Google Scholar]
- 57.Russ HA, et al. In vitro proliferation of cells derived from adult human beta-cells revealed by cell-lineage tracing. Diabetes. 2008;57(6):1575–1583. doi: 10.2337/db07-1283. [DOI] [PubMed] [Google Scholar]
- 58.Russ HA, et al. Epithelial-mesenchymal transition in cells expanded in vitro from lineage-traced adult human pancreatic beta cells. PloS one. 2009;4(7):e6417. doi: 10.1371/journal.pone.0006417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Russ HA, et al. Insulin-producing cells generated from dedifferentiated human pancreatic beta cells expanded in vitro. PloS one. 2011;6(9):e25566. doi: 10.1371/journal.pone.0025566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bar Y, et al. Redifferentiation of expanded human pancreatic beta-cell-derived cells by inhibition of the NOTCH pathway. The Journal of biological chemistry. 2012;287(21):17269–17280. doi: 10.1074/jbc.M111.319152.This paper continues the work of the Efrat group here showing high levels of β-cell differentiation of epithelial-mesenchymal transition cells obtained after expansion of human β cells.
- 61.Bonner-Weir S, et al. Beta-cell growth and regeneration: replication is only part of the story. Diabetes. 2010;59(10):2340–2348. doi: 10.2337/db10-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu X, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132(2):197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 63.Kopp JL, et al. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development. 2011;138(4):653–665. doi: 10.1242/dev.056499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Furuyama K, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nature genetics. 2011;43(1):34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- 65.Solar M, et al. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Developmental cell. 2009;17(6):849–860. doi: 10.1016/j.devcel.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 66.Inada A, et al. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(50):19915–19919. doi: 10.1073/pnas.0805803105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Criscimanna A, et al. Duct Cells Contribute to Regeneration of Endocrine and Acinar Cells Following Pancreatic Damage in Adult Mice. Gastroenterology. 2011 doi: 10.1053/j.gastro.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakamura K, et al. Pancreatic beta-cells are generated by neogenesis from non-beta-cells after birth. Biomedical research. 2011;32(2):167–174. doi: 10.2220/biomedres.32.167. [DOI] [PubMed] [Google Scholar]
- 69.Pan FC, et al. Spatiotemporal patterns of multipotentiality in Ptf1a–expressing cells during pancreas organogenesis and injury-induced facultative restoration. Development. 2013;140(4):751–764. doi: 10.1242/dev.090159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ouziel-Yahalom L, et al. Ductal heterogeneity suggests a subpopulation serves as postantal pancreatic progenitors. Diabetes. 2010;59 [Google Scholar]
- 71. Reinert RB, et al. Tamoxifen-Induced Cre-loxP Recombination Is Prolonged in Pancreatic Islets of Adult Mice. PloS one. 2012;7(3):e33529. doi: 10.1371/journal.pone.0033529.This paper points out the need for some caution in interpreting inducible Cre-Lox results since there is perdurance of tamoxifen often for weeks.
- 72.Meier JJ, et al. Hyperinsulinemic hypoglycemia after gastric bypass surgery is not accompanied by islet hyperplasia or increased beta-cell turnover. Diabetes care. 2006;29(7):1554–1559. doi: 10.2337/dc06-0392. [DOI] [PubMed] [Google Scholar]
- 73.Yatoh S, et al. Differentiation of affinity-purified human pancreatic duct cells to beta-cells. Diabetes. 2007;56(7):1802–1809. doi: 10.2337/db06-1670. [DOI] [PubMed] [Google Scholar]
- 74.Baertschiger RM, et al. Mesenchymal stem cells derived from human exocrine pancreas express transcription factors implicated in beta-cell development. Pancreas. 2008;37(1):75–84. doi: 10.1097/MPA.0b013e31815fcb1e. [DOI] [PubMed] [Google Scholar]
- 75.Seeberger KL, et al. Expansion of mesenchymal stem cells from human pancreatic ductal epithelium. Laboratory investigation; a journal of technical methods and pathology. 2006;86(2):141–153. doi: 10.1038/labinvest.3700377. [DOI] [PubMed] [Google Scholar]
- 76.Lysy P, et al. Partial transition towards a mesenchymal phenotype allows human panreatic duct cells to proliferate while showing a beta-cell differentiation potential in vitro. Hormone Research in Pediatrics. 2011;76(Suppl.2):34. [Google Scholar]
- 77. Collombat P, et al. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell. 2009;138(3):449–462. doi: 10.1016/j.cell.2009.05.035.An important paper illustrating the plasticity of islet endocrine cells. The expression of a single transcription factor in α cells drives them to form β cells, which in turn provokes hypoglycemia that then drives the formation of new α cells.
- 78.Baeyens L, et al. In vitro generation of insulin-producing beta cells from adult exocrine pancreatic cells. Diabetologia. 2005;48(1):49–57. doi: 10.1007/s00125-004-1606-1. [DOI] [PubMed] [Google Scholar]
- 79.Bertelli E, Bendayan M. Intermediate endocrine-acinar pancreatic cells in duct ligation conditions. The American journal of physiology. 1997;273(5 Pt 1):C1641–C1649. doi: 10.1152/ajpcell.1997.273.5.C1641. [DOI] [PubMed] [Google Scholar]
- 80.Hesselson D, Anderson RM, Stainier DY. Suppression of Ptf1a activity induces acinar-to-endocrine conversion. Current biology : CB. 2011;21(8):712–717. doi: 10.1016/j.cub.2011.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Desai BM, et al. Preexisting pancreatic acinar cells contribute to acinar cell, but not islet beta cell, regeneration. The Journal of clinical investigation. 2007;117(4):971–977. doi: 10.1172/JCI29988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Houbracken I, et al. Lineage tracing evidence for transdifferentiation of acinar to duct cells and plasticity of human pancreas. Gastroenterology. 2011;141(2):731–741. doi: 10.1053/j.gastro.2011.04.050. 741 e1–4. [DOI] [PubMed] [Google Scholar]
- 83.Zhou Q, et al. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455(7213):627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang AY, et al. Comparison of adenoviral and adeno-associated viral vectors for pancreatic gene delivery in vivo. Human gene therapy. 2004;15(4):405–413. doi: 10.1089/104303404322959551. [DOI] [PubMed] [Google Scholar]
- 85.Kim D, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell stem cell. 2009;4(6):472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Warren L, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell stem cell. 2010;7(5):618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Smith SB, et al. Rfx6 directs islet formation and insulin production in mice and humans. Nature. 2010;463(7282):775–780. doi: 10.1038/nature08748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou Q, et al. A multipotent progenitor domain guides pancreatic organogenesis. Developmental cell. 2007;13(1):103–114. doi: 10.1016/j.devcel.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 89.Gifford CA, Meissner A. Epigenetic obstacles encountered by transcription factors: reprogramming against all odds. Current opinion in genetics & development. 2012;22(5):409–415. doi: 10.1016/j.gde.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Collombat P, et al. Embryonic endocrine pancreas and mature beta cells acquire alpha and PP cell phenotypes upon Arx misexpression. The Journal of clinical investigation. 2007;117(4):961–970. doi: 10.1172/JCI29115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Thorel F, et al. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464(7292):1149–1154. doi: 10.1038/nature08894.This study confirms the plasticity of islet endocrine cells in the context of extensive β-cell failure.
- 92.Yang YP, et al. Context-specific alpha- to-beta-cell reprogramming by forced Pdx1 expression. Genes & development. 2011;25(16):1680–1685. doi: 10.1101/gad.16875711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gianani R. Beta cell regeneration in human pancreas. Seminars in immunopathology. 2011;33(1):23–27. doi: 10.1007/s00281-010-0235-7. [DOI] [PubMed] [Google Scholar]
- 94.Rezania A, et al. Production of functional glucagon-secreting alpha-cells from human embryonic stem cells. Diabetes. 2011;60(1):239–247. doi: 10.2337/db10-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fomina-Yadlin D, et al. Small-molecule inducers of insulin expression in pancreatic alpha-cells. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(34):15099–15104. doi: 10.1073/pnas.1010018107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Saisho Y, et al. Ongoing beta-cell turnover in adult nonhuman primates is not adaptively increased in streptozotocin-induced diabetes. Diabetes. 2011;60(3):848–856. doi: 10.2337/db09-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jones RJ, et al. Assessment of aldehyde dehydrogenase in viable cells. Blood. 1995;85(10):2742–2746. [PubMed] [Google Scholar]
- 98.Gasparetto M, et al. Aldehyde dehydrogenases are regulators of hematopoietic stem cell numbers and B-cell development. Experimental hematology. 2012;40(4):318–329. doi: 10.1016/j.exphem.2011.12.006. e2. [DOI] [PubMed] [Google Scholar]
- 99. Rovira M, et al. Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(1):75–80. doi: 10.1073/pnas.0912589107.This paper suggest s role of centroacinar cells in the adult rodent pancreas as facultative progenitors with β-cell differentiation potential.
- 100.Hayashi KY, et al. Differentiation and proliferation of endocrine cells in the regenerating rat pancreas after 90% pancreatectomy. Archives of histology and cytology. 2003;66(2):163–174. doi: 10.1679/aohc.66.163. [DOI] [PubMed] [Google Scholar]
- 101.Ioannou M, et al. ALDH1B1 is a potential stem/progenitor marker for multiple pancreas progenitor pools. Developmental biology. 2013;374(1):153–163. doi: 10.1016/j.ydbio.2012.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Seaberg RM, et al. Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nature biotechnology. 2004;22(9):1115–1124. doi: 10.1038/nbt1004. [DOI] [PubMed] [Google Scholar]
- 103. Smukler SR, et al. The adult mouse and human pancreas contain rare multipotent stem cells that express insulin. Cell stem cell. 2011;8(3):281–293. doi: 10.1016/j.stem.2011.01.015.This paper extends previous work of this group on the isolation from both islet and exocrine tissues of a progenitor cell with stem-like characteristics, in adult mouse and human pancreas.
- 104.Hald J, et al. Pancreatic islet and progenitor cell surface markers with cell sorting potential. Diabetologia. 2012;55(1):154–165. doi: 10.1007/s00125-011-2295-1. [DOI] [PubMed] [Google Scholar]