Abstract
Severe combined immunodeficiency (SCID) is a syndrome of diverse genetic cause characterized by profound deficiencies of T and B cell function and, in some types, also of NK cells and function. Mutations in thirteen different genes have been found to cause this condition, which is uniformly fatal in the first two years of life unless immune reconstitution can be accomplished. In the 42 years since the first bone marrow transplant was given in 1968, the standard treatment for all forms of SCID has been allogeneic bone marrow transplantation. Both HLA identical unfractionated and T cell depleted HLA haploidentical bone marrow transplants have been very successful in effecting immune reconstitution, especially if performed in the first 3.5 months of life and without pre-transplant chemotherapy. This paper summarizes the longterm outcome, according to molecular type, of 166 consecutive SCID infants given non-conditioned related donor bone marrow transplants at this institution over the past 28.3 years and reviews published reports of longterm outcomes of transplants in SCID performed at other centers.
Keywords: severe combined immunodeficiency, bone marrow transplantation, thymic output, chimerism, survival
INTRODUCTION
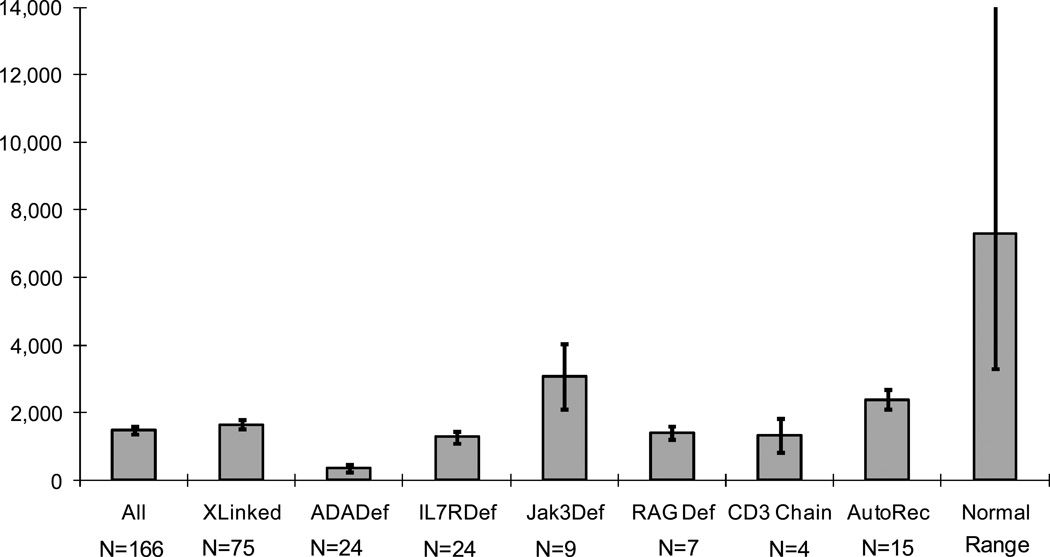
Human severe combined immunodeficiency (SCID) was first reported by ‘Swiss workers more than 60 years ago (1) Since then remarkable progress has been made in understanding the fundamental causes of this syndrome. It is now known that SCID can be caused in humans by mutations in at least 13 different genes (Table 1) (2–11) and the likelihood is that there are other causes yet to be discovered. Regardless of the underlying defect, infants with this syndrome are lymphopenic (Figure 1), lack T cells and have profound deficiencies of T and B cell function (Figures 2a, 3a). In certain types, there are also marked deficiencies of B cells and of natural killer (NK) cells (Table 2;Figure 2a).
Table 1.
Thirteen Abnormal Genes in SCID
| Cytokine Receptor Genes |
| – IL2RG |
| – JAK3 |
| – IL7Rα |
| Antigen Receptor Genes |
| – RAG1 |
| – RAG2 |
| – Artemis |
| – Ligase 4 |
| – DNA-PKcs |
| – CD3δ |
| – CD3ε |
| – CD3ζ |
| Other Genes |
| – ADA |
| – CD45 |
Figure 1.
Means+\-SEM of absolute lymphocyte counts in 166 SCID infants at presentation, showing the lymphopenia characteristic of all forms of SCID.
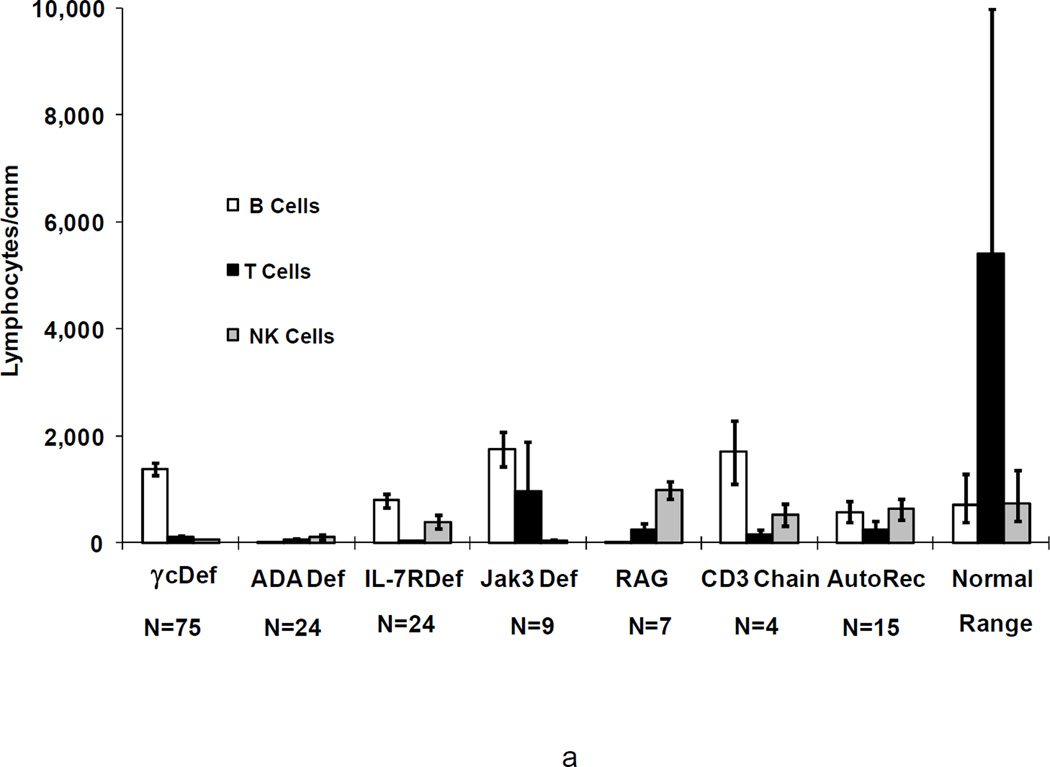
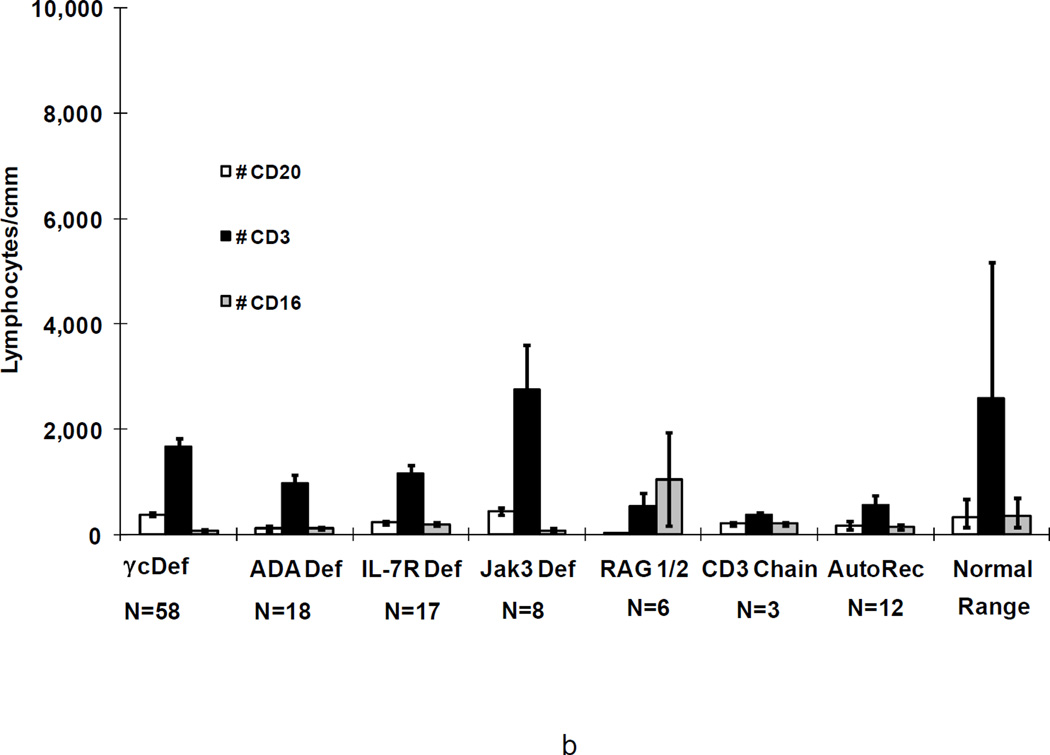
Figure 2.
a. Means+/−SEM of CD20+ B cells, CD3+ T cells, and CD16+ NK cells in 166 SCID patients before (a) and most recently after transplantation (b) in 122 of the 126 survivors according to genetic type, as compared with ranges for normal controls. Not shown are the data for the SCIDs who had Artemis deficiency, cartilage hair hypoplasia, CD45 deficiency or those male SCIDs of unknown molecular type as there is only 1 survivor of each type.
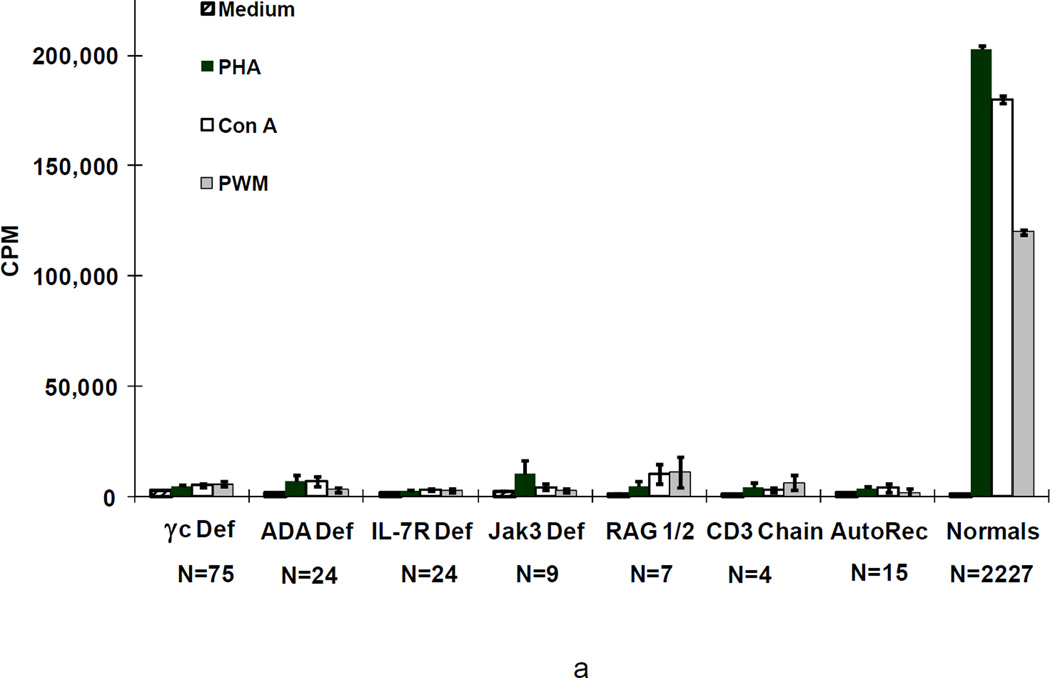
Figure 3.
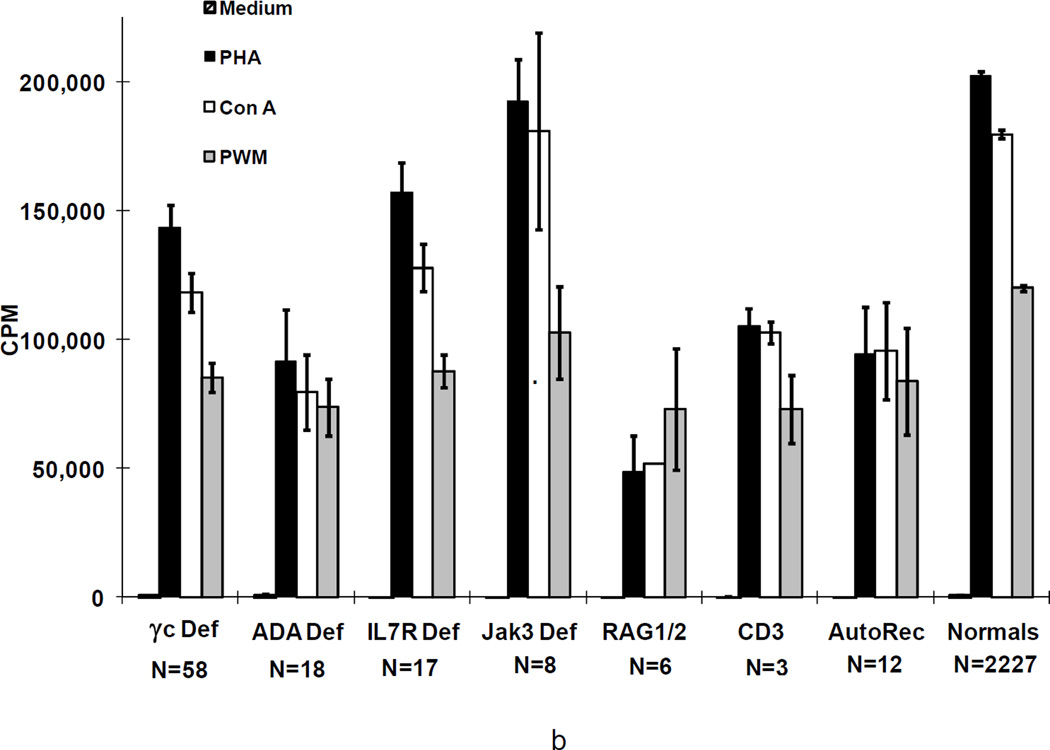
Means+/−SEM cpm [3H] thymidine incorporation by proliferating lymphocytes from the 166 SCIDs before (a) and most recently after transplantation in 122 of the 126 survivors (b) according to genetic type in response to the mitogens, PHA, Con A, and PWM, as compared with means+/−SEM for normal controls. Not shown are the data for the SCIDs who had Artemis deficiency, cartilage hair hypoplasia, CD45 deficiency or those male SCIDs of unknown molecular type as there is only 1 survivor of each type.
Table 2.
Lymphocyte Phenotypes in the Various Molecular Types of SCID
| T-B+NK- | γc-deficient |
| Jak 3-deficient | |
| T-B+NK+ | |
| IL-7Rα-deficient | |
| CD3δ-deficient | |
| CD3ε-deficient | |
| CD3ζ-deficient | |
| CD45-deficient | |
| T-B-NK- | |
| ADA-deficient | |
| T-B-NK+ | |
| RAG1/RAG2-deficient | |
| Artemis-deficient | |
| Ligase 4-deficient | |
| DNA-PKcs deficient |
Shortly after the discovery of HLA in 1968(12), immune function was transferred to an infant with SCID by transplantation of bone marrow from his HLA-identical sister (13). Over the ensuing decade, however, lethal graft-versus-host disease (GVHD) was a major problem when marrow from HLA-mismatched donors was used (14). In the late 1970's, studies in rats (15) and mice (16) revealed that allogeneic marrow or spleen cells that were rigorously depleted of T cells rescued the recipient from lethal irradiation without causing fatal GVHD, despite differences in MHC antigens between donor and host. Techniques developed in the early 1980's for the depletion of T cells from human marrow made it possible to restore immune function in all forms of SCID by marrow transplantation(17–32). Because the molecular defects in infants with SCID lead to a complete absence of T cell function, they cannot reject allografts. Therefore, successful marrow transplantation in SCID does not require pre-transplant chemotherapeutic conditioning, as recently supported by results from the latest follow-up study from Europe(33). Moreover, prophylaxis for GVHD is also not necessary after transplantation of HLA-identical or rigorously T cell-depleted HLA haploidentical marrow, since in the latter case the T cells that cause GVHD have been removed. These circumstances provide a unique opportunity to study the development of T cells from donor hematopoietic stem cells in an unmanipulated recipient, since less than a million T cells per kilogram are given when one uses rigorous T cell depletion techniques (34). At the time of this writing, the author and her colleagues have performed hematopoietic stem cell transplantation in 166 consecutive infants with SCID at our institution over 28.3 years, and 126 of them survive (29;30;32;35;36). Recent progress in identifying the molecular causes of SCID has permitted us to study the outcomes of hematopoietic stem cell transplantation according to the patients’ SCID mutations. Recent reports of longterm outcomes of SCID transplants performed at other centers will also be reviewed.
Patients
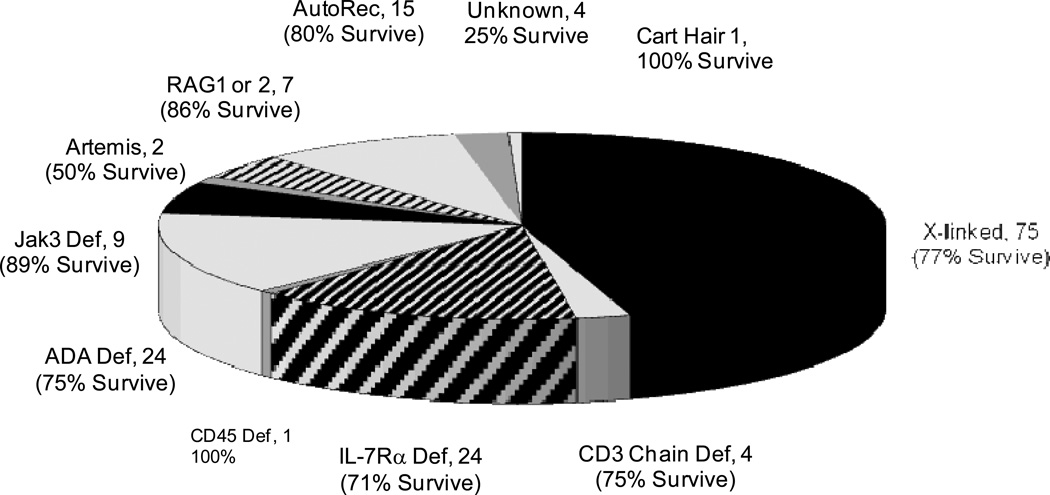
The 166 patients transplanted at our institution over the past 28.3 years ranged in age from newborn to 21 months at diagnosis. They fulfilled the criteria of the World Health Organization for diagnosis of SCID(37).They fit eleven categories of the disease based on family history, sex, other clinical features, and results of enzyme analyses or molecular studies(Figure 4)(38;39) . The largest number of patients-- 75 boys from 70 families or 45%-- had x-linked SCID due to mutations in the gene encoding the common γ chain (γc) (38;40–42). Nine patients from 9 families had SCID due to mutations in the gene encoding Janus kinase 3 (Jak3) (2;38;43;44). Twenty-four patients from 22 families had SCID caused by mutations in the gene encoding IL-7Rα (3). Twenty-four infants from 21 families had SCID due to a deficiency of ADA (45). Fifteen patients from 15 families had autosomal recessive inheritance but unknown mutations; and 4 boys with no family history had SCID of unknown type. Immunologic monitoring was done whenever feasible every 3 weeks until T cell function was established (usually at 3−4 months post-transplantation), then every 3 months for the next 9 months, every 6 months for the next two years, then once a year thereafter. The studies were done with the approval of the Duke University Committee on Human Investigations and written informed consent of the parents.
Figure 4.
Survival following bone marrow transplantation of 126 of 166 SCIDs according to genetic type of SCID.
All 149 HLA-haploidentical and 9 of the 17 HLA-identical transplants from related donors were T cell-depleted (17;19;29). Forty-nine patients received from one to three additional T cell-depleted marrow transplants, from either the original donor or another haploidentical relative. None of the HLA-identical or haploidentical related marrow recipients received any pre-transplant conditioning or post-transplant prophylaxis against GVHD. Two patients were given cyclosporine for 1 month because of cutaneous GVHD from transplacentally-transferred maternal T cells at presentation. Five of the infants who received haploidentical marrow transplants also received unrelated placental blood transplants. Four of the latter received pre-transplant conditioning and they were also given post-transplant prophylaxis against GVHD.
Results of Bone Marrow Transplantation at Duke
Factors Influencing Survival
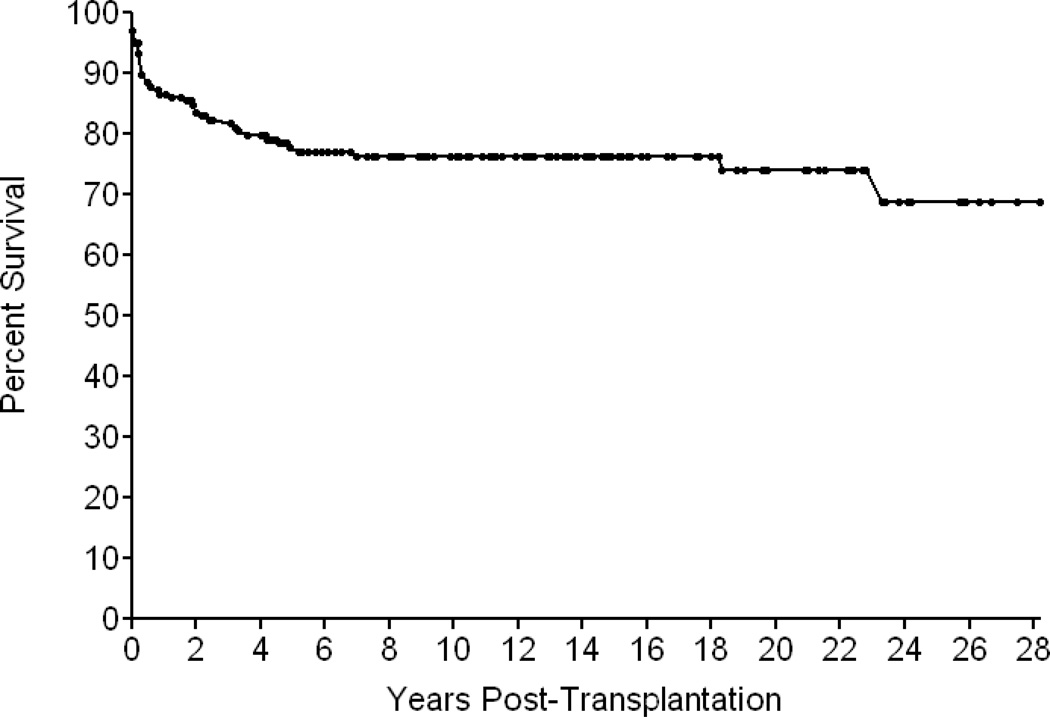
Of the 166 SCID patients, 126 (76 percent) are alive from 2 months to 28.3 years after transplantation (Figure 5). None show any evidence of susceptibility to opportunistic infections and most are in good general health(36). Of these 126 patients, 125 have survived 1 or more years after transplantation, 110 are alive 5 or more years, and 83 have survived for 10 or more years. Median follow-up of surviving patients is 10 years. All 17 recipients of marrow from HLA-identical donors, 109 of the 149 recipients (73 percent) given T cell-depleted haploidentical parental bone marrow, and 2 of 5 infants (40 percent) from the latter group also given unrelated placental blood transplants are among the survivors. The survival rates are similar for the different genetic types of SCID, except that only 1 of 2 infants with Artemis deficiency survives and only 1 of the small group of 4 male infants with SCID of unknown type survives (Figure 4). Influences on survival include race (more who were Caucasian survive) and age at the time of transplant. Of the 48 infants transplanted during the first 3.5 months of life, 44 (94 percent) survive, compared to 82/118 (69 percent) who were transplanted after that age (p<0.001). Thirty of the 40 deaths occurred from viral infections: CMV,9; adenovirus,9; EBV, 6; enteroviruses and rotavirus,4; parainfluenza 3, 4; Herpes zoster, 2; Herpes simplex,1 and respiratory syncitial virus 1(Table 3). Some infants had more than one viral infection at death. Ten others died of miscellaneous causes but none died from GVHD, despite the fact that 149 of them received mismatched parental bone marrow transplants (Table 3). Two ADA-deficient patients who had been treated with polyethylene glycol modified bovine ADA died at 7 years of age of a parainfluenza 3 lung infection and at 18 years of pulmonary hypertension, respectively.
Figure 5.
Kaplan Meier survival curve of 166 SCID infants transplanted at Duke University Medical Center from 1982 to 2010 without pre-transplant chemotherapy or post-transplantation GVHD prophylaxis; 149 received rigorously T cell-depleted haploidentical parental marrow and only 17 had HLA-identical donors.
Table 3.
Causes of Death in 40 SCIDs After Marrow Transplantation
| Cause: | Number: |
|---|---|
| CMV | 9 |
| Adenovirus | 9 |
| EBV /Lymphoma | 6 |
| Enterovirus, Rotovirus | 4 |
| Parainfluenza 3 | 4 |
| Varicella | 2 |
| Herpes simplex | 1 |
| RSV | 1 |
| Pulmonary disease | 4 |
| Candida sepsis | 2 |
| Mitochondrial defect | 1 |
| CNS Infection | 1 |
| Nephrotic syndrome/chemo | 1 |
| VOD | 1 |
| GVHD | 0 |
Graft-versus-host Disease
GVHD occurred in 45/149 patients given T cell-depleted haploidentical parental marrow, 8/17 given HLA-identical marrow, and 4/5 given placental blood. In 45/57 cases, this complication occurred when there was persistence of transplacentally-transferred maternal T cells. Most of the GVHD after administration of T cell-depleted marrow was mild (Grade I or II) and required no treatment(46). Ten patients from the entire group had Grade III GVHD involving the skin, gastrointestinal tract and/or marrow; 9/10 were given corticosteroids and cyclosporine as treatment, one corticosteroids alone. One neonate with IL7Rα-deficient SCID who received a T cell depleted haploidentical maternal marrow transplant developed Grade 4 GVHD with autoimmune hemolytic anemia, bone marrow suppression, diarrhea, and cholestatic liver disease. He subsequently received a segmental liver transplant from his mother and is now completely off of all immunosuppressive agents with normal liver function at 11 years post-transplantation. He is a complete hematopoietic chimera, does not require IVIG, is growing and developing normally and is very healthy. One of the recipients of unrelated placental blood had severe acute GVHD, requiring longterm steroid and cyclosporine therapy. No patient died of GVHD.
Engraftment and Chimerism
Genetic analyses of blood lymphocytes at the survivors’ latest evaluations have shown that all of the T cells in 121/126 are of donor origin. Of note, 13/19 ADA-deficient patients given T cell-depleted haploidentical marrow and all 5 of those given HLA identical marrow (all without pre-transplant chemotherapy or post-transplant GVHD prophylaxis) are alive at 1.5−25.8 years after transplantation, with T cell chimerism in 14. Two of the 5 infants who failed to become chimeric received successful gene therapy in Italy(47), one was given a chemoablated MUD bone marrow transplant elsewhere, and the other two are receiving polyethylene glycol-modified bovine ADA after rejecting haploidentical parental hematopoietic stem cell transplants.
By contrast to the development of donor T cells in all but the above 5 patients, the B cells in a majority of the cases remain those of the recipient. However, 5/17 recipients of HLA-identical marrow and 33/109 haploidentical marrow recipients have donor B cells despite the lack of conditioning (unpublished data). Of note, one third of all of the X-linked SCIDs have donor B cells.
Lymphocyte Phenotypes
Patients with the various genetic types of SCID had distinct lymphocyte phenotypes before transplantation (Figure 2a) (29;35;38). All patients had a profound deficiency of T cells at presentation and, when T cells were present, they were usually transplacentally-transferred maternal T cells. In one Jak3-deficient patient, there were 8,268 circulating maternal T cells/mm3 (48). B cells were elevated in γc-deficient, CD3 chain deficient and Jak3-deficient SCIDs, normal in IL-7Rα-deficient SCIDs, CD45 deficiency and in autosomal recessive SCIDs of unknown molecular type, extremely low in ADA deficient SCIDs and absent in cartilage hair hypoplasia, RAG1, RAG2 and Artemis-deficient SCIDs. Numbers of NK cells were lowest in γ c-deficient, Jak3-deficient (T-B+NK-) SCIDs, and in ADA-deficient (T-B-NK-) SCIDs, but were elevated in RAG1, RAG2 and Artemis-deficient SCIDs (T-B-NK+), normal in IL-7Rα-deficient, CD45 deficient, CD3 chain deficient SCIDs (all T-B+NK+), in autosomal recessive SCIDs of unknown molecular type and in the 4 males with unknown inheritance (Figure 2a) (29;35;38). At the most recent evaluation following transplantation, the mean number of T cells in the 126 surviving patients was within the normal range for the γ c-deficient , ADA-deficient, IL-7Rα-deficient, CD45 deficient and Jak3-deficient SCIDs and below the normal range in the CD3 chain, RAG1 and RAG2-deficient SCIDs and in the autosomal recessive SCIDs of unknown molecular cause (Figure 2b). The mean number of B cells was still elevated in γ c-deficient and Jak3-deficient SCIDs, very low in the RAG1 or RAG2-deficient SCIDs but normal in all others. The mean number of NK cells remained low in the γ c-deficient and Jak3-deficient groups but was normal in the others.
T Cell Function
Figure 3 shows in vitro responses to the mitogens (phytohemagglutinin, concanavalin A and pokeweed mitogen) by T cells from patients with the various types of SCID, before (3a) and after (3b) transplantation, as compared to such responses by T cells from normal adults. Remarkably, mean responses to all 3 mitogens were normal in all groups after transplantation as compared with no or extremely low responses before transplantation. Moreover, T cells from all patients with SCID responded poorly to allogeneic cells before transplantation; however, after transplantation T cells from all groups responded normally to allogeneic cells, candida and tetanus antigens (not shown).
Role of the Thymus
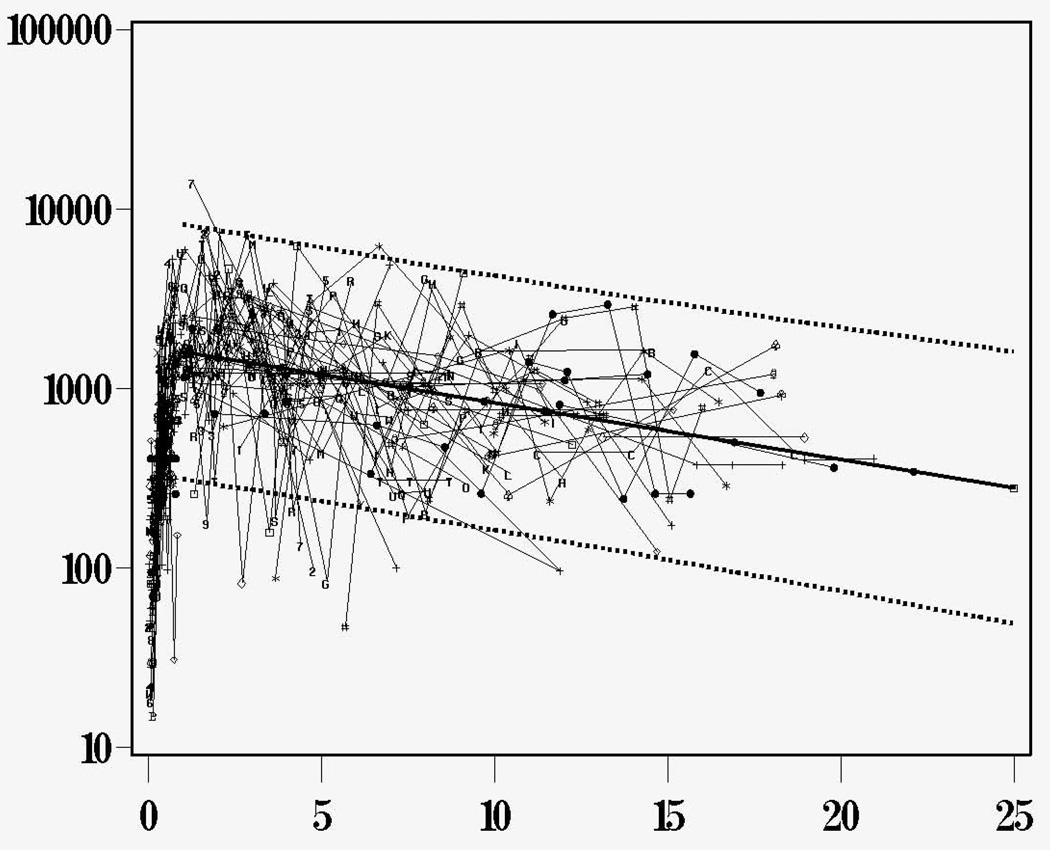
Because very few T cells were present in the T cell depleted donor marrow, it was initially surprising that genetically donor T cells emerged in these SCID infants at 90−120 days post-transplantation, considering that they all had vestigial thymi and no T cells prior to transplantation. To clarify whether the donor stem cells actually developed into T cells within the infants’ thymuses, we analyzed signal joint T-cell-receptor recombination excision circles (TRECs) in 83 of the patients with SCID given marrow transplants (49). We found that T cell numbers were low before and early after transplantation, with a predominance of CD45RO+ T cells (primarily due to transplacentally transferred maternal cells), and TRECs were undetectable in blood mononuclear cells. A majority of infants given either T cell-depleted marrow or unfractionated HLA-identical marrow developed genetically donor TREC +, CD45RA+, CD62L+ T cells (recent thymic emigrants) by 3−6 months post-transplantation. Thymic output peaked (5,525±1,502 TRECs /µg PBMC DNA) 1−2 years after transplantation and declined gradually after that (not statistically different from the decline seen in normal individuals). More importantly, we were able to demonstrate sustained thymic output into the third decade post-transplantation (Figure 6)(35). From these studies, we conclude that the vestigial thymus of infants with SCID can produce enough genetically donor T cells to confer T cell function by 3−6 months after transplantation, regardless of whether the donor is HLA-identical or haploidentical (35;49). The sustained thymic output into the third decade after transplantation provides strong evidence that donor stem cells have engrafted.
Figure 6.
TREC values (N=597) were observed over time in 115 patients for whom samples were available for analysis and the combined results of all 115 patients are shown here. The slope of decline with time is not significantly different from that in normal individuals. Reproduced with permission from: Sarzotti-Kelsoe M, Win CM, Parrott RE, Cooney M, Moser BK, Roberts JL, Sempowski GD, Buckley RH: Blood, 114: 1445−1453, 2009.
T Cell Diversity
Immunoscope analysis of the TCR Vβ repertoire was performed on 36 SCID patients given bone marrow transplants (BMT)(35;50). Before and within the first 100 days after BMT, patients’ PBMC displayed an oligoclonal or skewed T cell repertoire, low TREC values and a predominance of CD45RO+ T cells. In contrast, the presence of high numbers of CD45RA+ cells in the circulation of SCID patients >100 days post-BMT correlated with active T cell output by the thymus, as revealed by high TREC values, and a polyclonal T cell repertoire demonstrated by a Gaussian distribution of Vβ-specific peaks . In a recent study we demonstrated that most patients developed and maintained a diverse TCR Vβ repertoire with time(35). We conclude that a normal T cell repertoire develops in SCID patients as a result of thymic output, and the repertoire remains highly diverse well into the third decade post-transplantation. The TCR diversity positively correlated with TREC levels (50).
B Cell Function
B cell function did not develop to the extent that T cell function did. Serum IgG prior to transplantation was in most cases maternal or from intravenous immunoglobulin, but paraproteins were present in some. One of the IL-7Rα-deficient patients had both IgG and IgA paraproteins prior to transplantation, as has been noted previously in SCID(51–53). At their latest evaluation, 62 patients have normal serum IgA, 98 normal IgM and 52 have isohemagglutinins appropriate for host red blood cell type. Sixty-three of the 126 (50%) patients are currently receiving immunoglobulin replacement to prevent bacterial and common viral infections. All patients who are not receiving immunoglobulin infusions have demonstrated the capacity to produce antibodies to one or more vaccine antigens (unpublished data). The best B cell function is in the IL7Rα-deficient , the CD3 chain and ADA-deficient SCIDs, despite the fact that most of the children in those groups do not have donor B cells. This indicates that the molecular type of SCID is the principal determinant as to whether good B cell function will develop.
NK Cell Function
Before engraftment, NK cell numbers were lowest in γ c-deficient and Jak3-deficient SCIDs (p<.001)(Figure 2a), whereas they were higher than normal in all other types except ADA deficiency. Following transplantation, many γ c- and Jak3-deficient SCIDs continued to have low NK cell numbers and function, while the numbers of NK cells and NK function were normal in all other types. The NK cells in the IL-7Rα-deficient, CD3 chain deficient, and CD45-deficient SCIDs did not interfere with engraftment of T cell depleted parental bone marrow stem cells despite no pre-transplant chemotherapy. The NK cells in the RAG-and Artemis-deficient patients may have been responsible for the longer time course to development of T cell function but did not prevent engraftment even though no chemotherapy was given.
Transplants Performed in the First Three and One-Half Months of Life
As already noted, survival of the SCIDs who were transplanted in the first 3.5 months of life was superior to that in those performed later. We hypothesized that the kinetics of immune reconstitution would be different for those transplanted in the neonatal period when compared to those transplanted after that time. We compared immune function in 21 SCIDs transplanted in the neonatal period with that in 70 SCIDs transplanted after that (30). We measured lymphocyte phenotypes, proliferative responses to mitogens, immunoglobulin levels and T-cell antigen receptor excision circles (TRECs) pre-transplantation and sequentially post-transplantation. Infants transplanted in the newborn period developed higher lymphocyte responses to phytohemagglutinin and higher numbers of CD3+ and CD45RA+ T cells in the first 3 years of life than those transplanted late (p<0.05). TRECs peaked earlier and with higher values (p<0.01) in the neonatal transplants (181 days-1 year) than in the late transplants (1–3 years). Thus, SCID recipients of allogeneic, related hematopoietic stem cells in the neonatal period had higher levels of T cell reconstitution and thymic output and a higher survival rate than those transplanted after 28 days of life (30).
Booster Transplants
In an attempt to overcome poor B or T cell function or resistance to engraftment, ‘booster’ transplants were performed in 49 (29 percent) of the 166 patients, and 33 (67percent) of them survive. All of these were done without pre-transplant chemotherapy and with the same T cell depletion method as used for the initial transplants. Twenty-nine patients received ‘booster’ transplants from the same parental donor; and 7 of them died of opportunistic viral infections. Fourteen also received ‘booster’ transplants from the other parent; 5 of them died. One patient received a blood transfusion from an identical twin who had accepted a marrow transplant from their father and he survives. Five others received matched unrelated cord blood transplants; 3 of them died. Immune function improved in all of the survivors who received booster transplants.
Longterm Clinical Outcomes
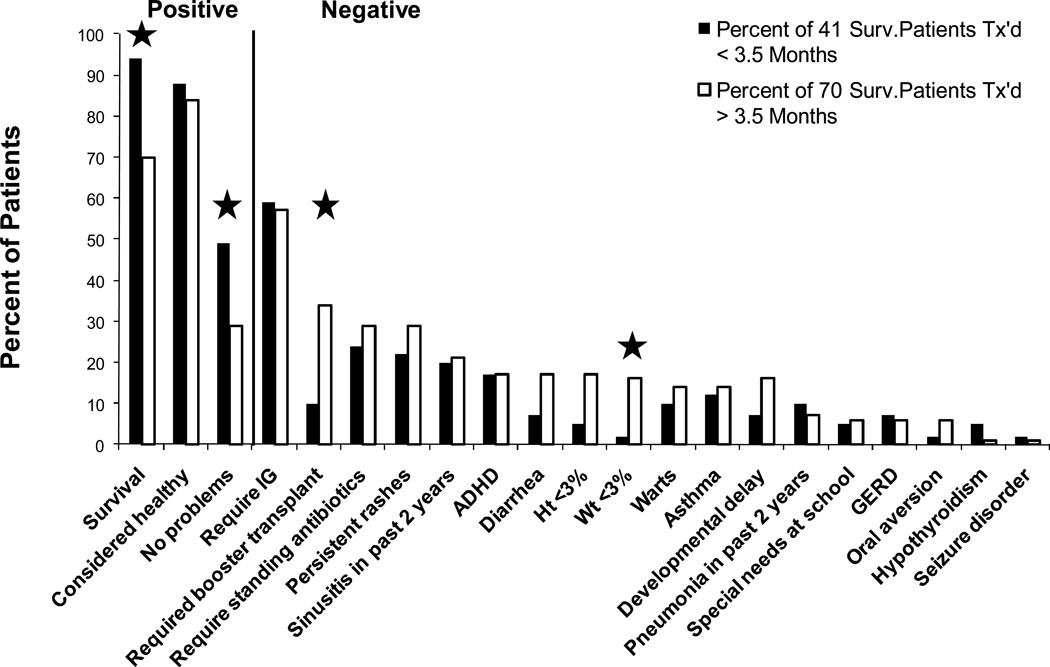
In 2008, all 124 survivors at that time were sent questionnaires about their current clinical statuses. Details from clinic visits were also compiled. One hundred eleven patients (90%) were reached (36). We compared outcomes of patients transplanted before and after 3.5 months of life and by molecular defect. As seen in Figure 7, one or more clinical problems were reported to have been present in the past two years in 71 (64%) of the survivors, although 95 (86%) were considered healthy by their families. Several have gone to college and one is in medical school. At least 4 are the parents of young children. Most SCID patients transplanted with related donor marrow without pre-transplant chemotherapy have done well long-term, but those transplanted early had a superior survival rate, a lower rate of clinical problems, less need for booster transplants and better nutritional status.
Figure 7.
A comparison of clinical features reported as percentages of 41 surviving SCID patients who were transplanted prior to 3.5 months of life with the same features reported as percentages of 70 surviving SCID patients who were transplanted after 3.5 months of life and followed longterm for up to 26 years after transplantation. Significant differences are indicated by the asterisks. Reproduced with permission from: Railey MD, Lokhnygina Y, Buckley RH: Journal of Pediatrics, 155:834−840, 2009.
Results of Bone Marrow Transplantation at Other Centers
Although the approaches used to attempt immune reconstitution by bone marrow transplantation in SCID infants have differed from center to center, much has been learned about what factors influence success or failure. In general, the mortality has been much higher at centers that use pre-transplant chemotherapy(54). Most of the published results of bone marrow transplantation for SCID at other centers have come from the European Group for Blood and Marrow Transplantation and the European Society for Immunodeficiency(31;33;55;56). The transplants were done at many different centers in Europe and, therefore, the approaches were not all the same. In a report published in 1998 of a long-term retrospective study of immune reconstitution in 193 SCIDs transplanted in Europe with T cell-depleted HLA-non-identical marrow between January 1, 1983 and December 31, 1993, the overall survival at the time of publication was 92 patients or 47%. Eighty-nine of the 116 (77%) patients who survived for at least 6 months had been given pre-transplant chemotherapy. Seventy- seven of the deaths occurred within 6 months after transplantation, and 24 more occurred after 6 months (55). A publication from that group in 2003 covered 475 SCID patients transplanted with all marrow types, of which pre-transplant conditioning was given to 275 or 57% of the patients(31). Three year survival rates with sustained engraftment were better for HLA-identical (77%) than for mismatched transplants (54%). However, this report found that there was significant overall improvement in the outcomes of SCID transplants over time, with an improvement of from around 40% survival of mismatched transplants performed from 1968 to 1985 to around 78% in those performed from 1996−1999. The latter was attributed to the development of rigorous T cell depletion techniques, more effective antibiotics, and perhaps earlier recognition of SCID. Another finding was that the outcome was significantly better in the B cell+ SCIDs than in the B cell- SCIDS (31).
In the most recent report published from Europe in 2010, covering 699 SCID patients transplanted between 1968 and 2005, the ten-year survival in patients with SCID had improved since 1995 being 71% (n=338) vs 56% (n=361) prior to 1995, although there was no difference in survival in the period between 1995 and 2000 when compared to the period between 2000 and 2005(33). In none of the European reports were the results reported according to molecular type of SCID, but in the most recent report they were again divided into B+ SCIDs (49%), B- SCID (29%) and other (22%) and the report again found that those with B- SCID had a poorer survival rate (51%) when compared to those with either B+ (70%) or other (71%) types of SCID. In this report they also found that 10 year survival using a mismatched relative had improved to 66%, similar to that using an unrelated donor (69%)(33). SCID infants transplanted before 6 months of age had a better overall survival rate (68%) than those transplanted >12 months old (51%). Significantly this report found that the use of pre-transplant chemotherapy did not significantly affect survival (chemotherapy, n = 280 [61% survival]; no chemotherapy, n = 399 [63% survival]; P = 0.53. However, there was no quality of life assessment in this report, so it is unknown whether those who received chemotherapy had long term toxicities from this treatment(33) .
In the U. S., sixteen infants with Athabascan SCID due to Artemis mutations were given bone marrow transplants at the University of California in San Francisco between 1984 and 1999.(57) Seven of them received HLA-identical sibling marrow, and nine received T cell-depleted parental marrow. All but 2 of the infants received pre-transplant chemotherapy. All 7 of those who received HLA-identical marrow are surviving, whereas only 5 of the 9 who received parental marrow survived. Three of the 4 children who died received radiation or busulfan and 2 of the 8 long-term survivors who were also recipients of cytototoxic chemotherapy failed to develop secondary teeth. Thus, children with this radiation sensitive form of SCID had a poor outcome if they were given pre-transplant chemotherapy (57). At Children’s Hospital of Los Angeles, between November of 1984 and December of 1997, 48 SCID infants were given bone marrow transplants(58). Eleven received HLA-identical related bone marrow transplants, and 37 T cell depleted haploidentical parental marrow. All recipients received pre-transplant conditioning except one who received HLA-identical sibling marrow. At the time of the report, all of the 11 who received HLA-identical marrow were surviving, but only 17 of the 37 (47%) who received T cell depleted parental marrow were surviving (58).
Few studies have been reported to date on long-term T cell reconstitution after hematopoietic stem cell transplantation (HSCT) in SCID patients(59–65). The findings described in the patients at our center are in keeping with those of Borghans et al.(59), who found that 11/19 SCID patients followed up to 25 years after conditioned BMT had no evidence for an accelerated decline of T cell-immunity or thymus output; however, those SCIDs who had low thymic output soon after transplantation continued to have decreased long-term T-cell reconstitution. Mazzolari et al.(61) reported on the long-term immune reconstitution and clinical outcome of 40 patients with severe T-cell immunodeficiency who survived for up to 16.3 years after HSCT. Thirty-five percent of the 40 patients had low TREC levels at their last follow-up and oligoclonality of the T-cell repertoire was demonstrated at the last follow-up in 27.5% of the patients. Cavazzana-Calvo et al.(60), and Friedrich et al.(62), have reported findings similar to those of Mazzolari et al.(61) and speculated that use of a conditioning regimen before HSCT for SCID was necessary for thymic output at greater than 10 years after transplantation. However, Patel et al.(64) have recently reported sustained T cell function in 15 long-term SCID survivors (up to 26 years) of non-conditioned bone marrow transplants. Our data confirm that finding and provide evidence for continued thymic output at >10 years post-transplantation in the absence of both pre-transplant conditioning and post-transplantation GVHD prophylaxis.
There have been few published reports that have addressed the long term clinical outcome of these patients. Borghans et al reported an increased incidence of infections and autoimmune disorders late after transplantation.(59) Honig et al(66) reported that SCID patients with adenosine deaminase deficiency had a high incidence of CNS complications such as mental retardation, motor dysfunction, and sensorineural hearing deficits. Mazzolari et al(61) and Slatter et al(63) analyzed the long-term follow up of 36 and 40 patients, respectively, with severe T-lymphocyte immunodeficiency. These groups found relatively high incidences of endocrine, autoimmune and behavioral problems, as well as human papilloma virus infections post-transplantation. More recently, Neven et al (65) reported the long term outcomes of 90 surviving patients with SCID from 149 transplanted in Paris, France from 1972 to 2004. Overall survival was 63%. Chronic GVHD was found in 11%, 13% percent had autoimmune or inflammatory complications, and 26% had HPV infections, nine of which were severe. Finally, a study at Great Ormond Street Hospital in 105 primary immunodeficiency children after transplantation revealed an increased risk of long term cognitive difficulties and associated emotional and behavioral difficulties, particularly in ADA-deficient SCIDs.(67) All six groups followed patients who had received pre-transplant chemotherapy and post-transplant GVHD prophylaxis. Only one longterm follow-up study has been reported on non-conditioned bone marrow transplants in SCID infants and, in that study, 15 of 25 (60%) recipients were surviving from 10 to 26 years post-transplantation.(64)
Discussion
The experience at our center clearly demonstrates that transplantation of rigorously T cell-depleted HLA-identical or HLA-haploidentical bone marrow is highly effective in reconstituting T cell immunity in all of the known genetic types of SCID. No chemotherapeutic conditioning is required to achieve engraftment, because the recipient is virtually devoid of T cells at the time of transplantation. This advantage eliminates adverse effects caused by these toxic agents, including neutropenia, red cell and platelet transfusion-dependency, mucositis, veno-occlusive disease, busulfan lung disease, growth suppression, sterility and a 15% risk of later malignancy(68). Although GVHD prophylaxis was not used at our center except for 1 month of cyclosporine given to two infants who presented with GVHD from transplacentally transferred maternal T cells and for the placental blood transplants, clinically significant GVHD was seldom seen. The omission of GVHD prophylaxis with cyclosporine permitted the infants to develop T cell function without hindrance.
Normal T cell function appeared within two weeks after transplantation of unfractionated HLA-identical marrow, due to the adoptive transfer of mature donor T cells. By contrast, it did not develop until 3−4 months after administration of T cell-depleted marrow, whether HLA-identical or haploidentical (19;29). The latter is the average time required for the donor stem cells to become phenotypically and functionally mature T cells in the recipient(19;29). T cell function often developed much earlier in neonatal recipients(30) and in patients in whom transplacental transfer of maternal T cells had occurred(69), but it developed later in some patients who had high numbers of NK cells at presentation. In the case of the unrelated placental blood transplants, T cells were present immediately, but T cell function was suppressed by the large doses of corticosteroids and cyclosporine needed to prevent or treat GVHD.
As for B cells, only 5/17 survivors of HLA-identical and 33/109 survivors of haploidentical transplants have donor B cells; 63/126 are currently receiving immunoglobulin replacement therapy to prevent bacterial and common viral infections, because the capacity to produce protective antibodies has not yet been demonstrated. However, several of these patients may be able to discontinue immunoglobulin treatment, because they are now producing IgA and isohemagglutinins.
Most of the γ c-deficient and Jak3-deficient patients who do not have any evidence of donor-derived B cells continue to have poor B cell function, as demonstrated by failure of isotype-switching following immunization with bacteriophage ϕ X174 and the inability to produce IgA, IgM and IgE or isohemagglutinins normally in vivo (unpublished data). Thus, normal stem cells that mature in γ c- and Jak3-deficient patients develop into normal T cells, but much less frequently into normal B cells; the host B cells in these patients most likely fail to function because they lack normal cytokine receptors. By contrast, a majority of the ADA-, CD3 chain- and IL-7Rα-deficient patients and some of those with autosomal recessive SCID of unknown molecular cause have good host B cell function, indicating that those mutations do not adversely affect B cell function.
Before transplantation, NK cell numbers and function were also lowest in γ c and Jak3-deficient patients, whereas they were higher than normal in most other types of SCID. Following transplantation, profoundly low NK cell numbers and function persisted in most γ c and Jak3-deficient patients, while these were normal in all other types of SCID.
Although the ability to give half-matched T cell-depleted parental marrow to patients with SCID has been a remarkable therapeutic advance, it is not a perfect treatment. During the 3−4 months needed for donor stem cells to develop into mature functioning T cells, the infant is susceptible to viral infections. However, this is also true if one uses pre-transplant chemotherapy and post-transplantation GVHD prophylactic immunosuppressive drugs. Pre-transplant chemotherapy fails to accelerate immune reconstitution, heightens the susceptibility of the recipient to infection because it removes innate immunity and the use of cyclosporine post-transplantation prolongs the T cell deficiency(55). The poor B cell function in γ c-deficient and Jak3-deficient patients in whom donor B cells do not develop has led some to use pre-transplant conditioning. However, chemotherapy does not guarantee that donor B cells will develop, and the risks outweigh the potential for development of B cell function(54). Indeed, in one study from Europe, there was no better B cell function in those who received pre-transplant chemotherapy versus those who did not(55).
Finally, resistance to engraftment is a problem that was overcome in most of our center’s cases by booster or second parent T cell-depleted transplants, again without conditioning.
Placental blood transplantation from unrelated donors is fraught with problems due to GVHD in patients with SCID. In most institutions performing placental blood transplants for SCID, pre-transplant chemotherapy is given and prolonged (6 months) GVHD prophylaxis with cyclosporine is required (70–72). All of this heightens the risk of infection. In utero stem cell transplants from related donors do not appear to offer any advantage over such transplants done soon after birth. The mother would probably not be used as a donor for an in utero transplant, because of the risks of anesthesia during pregnancy. The invasive procedures required in in utero stem cell administration carry risks, and one would also not be able to detect or treat either a graft-versus-graft reaction or GVHD in utero (73;74).
SCID is a pediatric emergency, and the potential exists to diagnose this condition routinely at birth (38). Cord blood white cell counts and manual differentials can detect the lymphopenia that is almost invariably present, and appropriate immunologic tests could then be done. Prenatal diagnosis can often be made when the mutation has been determined because there is a family history of SCID. More recently, TREC analyses have been recommended for newborn screening for SCID because these analyses can be performed on dried blood spots collected at the time of discharge of the newborn. The Secretary’s Advisory Committee for Heritable Disorders of Newborns and Children recently unanimously recommended that SCID be added to the list of conditions routinely screened for in newborns, and the Secretary of Health and Human Services has approved that recommendation. If a stem cell transplant from a relative can be done in the first 3.5 months of life without pre-transplant chemotherapy or post-transplantation GVHD immunosuppressive drugs and before infections develop, there is a high (94 percent) probability of success. The risks of chemotherapeutic agents are much higher for very young infants, who could be the main age group transplanted in the future when newborn screening becomes established.
In summary, T cell-depleted haploidentical marrow transplantation provides life-saving therapy for all forms of SCID. The prospect of gene therapy offers hope that the remaining defects in these chimeras will eventually be correctable by that means.
Acknowledgements
This research was supported by Grants AI042951 and AI47605 from the National Institute of Allergy and Infectious Diseases. The author wishes to thank all of those who have participated in the care and study of the SCID infants over the past 3 decades. In particular, she wants to express her gratitude to the research colleagues who have participated in this work, including Marcella Sarzotti-Kelsoe, Ph.D., Roberta E. Parrott, Sherrie E. Schiff, Chan M. Win, Elisa Sajaroff, Zermeena Marshall, Joseph L. Roberts, M.D./Ph.D and Myriah Cooney,
References
- 1.Glanzmann E, Riniker P. Essentielle lymphocytophtose. Ein neues krankeitsbild aus der Sauglingspathologie. Ann Paediat. 1950;174:1–5. [PubMed] [Google Scholar]
- 2.Russell SM, Tayebi N, Nakajima H, Riedy MC, Roberts JL, Aman MJ, et al. Mutation of Jak3 in a patient with SCID: Essential role of Jak3 in lymphoid development. Sci. 1995;270:797–800. doi: 10.1126/science.270.5237.797. [DOI] [PubMed] [Google Scholar]
- 3.Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T(-)B(+)NK(+) severe combined immunodeficiency. Nat Genet. 1998;20(4):394–397. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- 4.Schwarz K, Gauss GH, Ludwig L, Pannicke U, Li Z, Lindner D, et al. RAG mutations in human B cell-negative SCID. Sci. 1996;274:97–99. doi: 10.1126/science.274.5284.97. [DOI] [PubMed] [Google Scholar]
- 5.Moshous D, Callebaut I, de Chasseval R, Corneo B, Cavazzana-Calvo M, Le Deist F, et al. Artemis, a novel DNA double-strand break repair/V(D)J recombination protein, is mutated in human severe combined immune deficiency. Cell. 2001;105(2):177–186. doi: 10.1016/s0092-8674(01)00309-9. [DOI] [PubMed] [Google Scholar]
- 6.Kung C, Pingel JT, Heikinheimo M, Klemola T, Varkila K, Yoo LI, et al. Mutations in the tyrosine phosphatase CD45 gene in a child with severe combined immunodeficiency disease. Nat Med. 2000;6(3):343–345. doi: 10.1038/73208. [DOI] [PubMed] [Google Scholar]
- 7.Buck D, Moshous D, de Chasseval R, Ma Y, Le Deist F, Cavazzana-Calvo M, et al. Severe combined immunodeficiency and microcephaly in siblings with hypomorphic mutations in DNA ligase IV. Eur J Immunol. 2006;36(1):224–235. doi: 10.1002/eji.200535401. [DOI] [PubMed] [Google Scholar]
- 8.van der Burg M, Ijspeert H, Verkaik NS, Turul T, Wiegant WW, Morotomi-Yano K, et al. A DNA-PKcs mutation in a radiosensitive T-B- SCID patient inhibits Artemis activation and nonhomologous end-joining. J Clin Invest. 2009;119(1):91–98. doi: 10.1172/JCI37141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dadi HK, Simon AJ, Roifman CM. Effect of CD3delta deficiency on maturation of alpha/beta and gamma/delta T-cell lineages in severe combined immunodeficiency. N Engl J Med. 2003;349(19):1821–1828. doi: 10.1056/NEJMoa031178. [DOI] [PubMed] [Google Scholar]
- 10.de Saint Basile G, Geissmann F, Flori E, Uring-Lambert B, Soudais C, Cavazzana-Calvo M, et al. Severe combined immunodeficiency caused by deficiency in either the delta or the epsilon subunit of CD3. J Clin Invest. 2004;114(10):1512–1517. doi: 10.1172/JCI22588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts JL, Lauritsen JHP, Cooney M, Parrott RE, Sajaroff EO, Win CM, et al. T-B+NK+ severe combined immunodeficiency caused by complete deficiency of the CD3 zeta subunit of the T cell antigen receptor complex. Blood. 2007;109:3198–3206. doi: 10.1182/blood-2006-08-043166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amos DB, Bach FH. Phenotypic expressions of the major histocompatibility locus in man (HL-A): leukocyte antigens and mixed leukocyte culture reactivity. J Exp Med. 1968;128:623–637. doi: 10.1084/jem.128.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gatti RA, Meuwissen HJ, Allen HD, Hong R, Good RA. Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet. 1968;2:1366–1369. doi: 10.1016/s0140-6736(68)92673-1. [DOI] [PubMed] [Google Scholar]
- 14.Bortin MM, Rimm AA. Severe combined immunodeficiency disease. Characterization of the disease and results of transplantation. JAMA. 1977;238:591–600. [PubMed] [Google Scholar]
- 15.Muller-Ruchholtz W, Wottge HU, Muller-Hermelink HK. Bone marrow transplantation in rats across strong histocompatibility barriers by selective elimination of lymphoid cells in donor marrow. Transplant Proc. 1976;8:537–541. [PubMed] [Google Scholar]
- 16.Reisner Y, Itzicovitch L, Meshorer A, Sharon N. Hematopoietic stem cell transplantation using mouse bone marrow and spleen cells fractionated by lectins. Proc Nat Acad Sci USA. 1978;75:2933–2936. doi: 10.1073/pnas.75.6.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reisner Y, Kapoor N, Kirkpatrick D, Pollack MS, Cunningham-Rundles S, Dupont B, et al. Transplantation for severe combined immunodeficiency with HLA-A, B, D, DR incompatible parental marrow cells fractionated by soybean agglutinin and sheep red blood cells. Blood. 1983;61:341–348. [PubMed] [Google Scholar]
- 18.Friedrich W, Goldmann SF, Ebell W, Blutters-Sawatzki R, Gaedecke G, Raghavachar A, et al. Severe combined immunodeficiency: treatment by bone marrow transplantation in 15 infants using HLA-haploidentical donors. Eur J Pediatr. 1985;144:125–130. doi: 10.1007/BF00451897. [DOI] [PubMed] [Google Scholar]
- 19.Buckley RH, Schiff SE, Sampson HA, Schiff RI, Markert ML, Knutsen AP, et al. Development of immunity in human severe primary T cell deficiency following haploidentical bone marrow stem cell transplantation. J Immunol. 1986;136:2398–2407. [PubMed] [Google Scholar]
- 20.O'Reilly RJ, Brochstein J, Collins N, Keever C, Kapoor N, Kirkpatrick D, et al. Evaluation of HLA-haplotype disparate parental marrow grafts depleted of T lymphocytes by differential agglutination with a soybean lectin and E rosette depletion for the treatment of severe combined immunodeficiency. Vox Sang. 1986;51:81–86. doi: 10.1111/j.1423-0410.1986.tb02013.x. [DOI] [PubMed] [Google Scholar]
- 21.Moen RC, Horowitz SD, Sondel PM, Borcherding WR, Trigg ME, Billing R, et al. Immunologic reconstitution after haploidentical bone marrow transplantation for immune deficiency disorders: treatment of bone marrow cells with monoclonal antibody CT-2 and complement. Blood. 1987;70:664–669. [PubMed] [Google Scholar]
- 22.O'Reilly RJ, Keever CA, Small TN, Brochstein J. The use of HLA-non-identical T-cell-depleted marrow transplants for correction of severe combined immunodeficiency disease. Immunodefic Rev. 1989;1(4):273–309. [PubMed] [Google Scholar]
- 23.Wijnaendts L, LeDeist F, Griscelli C, Fischer A. Development of immunologic functions after bone marrow transplantation in 33 patients with severe combined immunodeficiency. Blood. 1989;74:2212–2219. [PubMed] [Google Scholar]
- 24.Fischer A, Landais P, Friedrich W, Morgan G, Gerritsen B, Fasth A, et al. European experience of bone marrow transplantation for severe combined immunodeficiency. Lancet. 1990;336:850–854. doi: 10.1016/0140-6736(90)92348-l. [DOI] [PubMed] [Google Scholar]
- 25.Dror Y, Gallagher R, Wara DW, Colombe BW, Merino A, Benkerrou M, et al. Immune reconstitution in severe combined immunodeficiency disease after lectin-treated, T cell depleted haplocompatible bone marrow transplantation. Blood. 1993;81:2021–2030. [PubMed] [Google Scholar]
- 26.Stephan JL, Vlekova V, Le Deist F, Blanche S, Donadieu J, de Saint-Basile G, et al. Severe combined immunodeficiency: a retrospective single-center study of clinical presentation and outcome in 117 cases. J Pediatr. 1993;123:564–572. doi: 10.1016/s0022-3476(05)80951-5. [DOI] [PubMed] [Google Scholar]
- 27.Giri N, Vowels M, Ziegler JB, Ford D, Lam-Po-Tang R. HLA non-identical T-cell-depleted bone marrow transplantation for primary immunodeficiency diseases. Aust N Z J Med. 1994;24(1):26–30. doi: 10.1111/j.1445-5994.1994.tb04421.x. [DOI] [PubMed] [Google Scholar]
- 28.Buckley RH. Bone marrow transplantation in primary immunodeficiency. In: Rich RR, editor. Clinical immunology: principles and practice. St. Louis: C.V. Mosby; 1995. pp. 1813–1830. [Google Scholar]
- 29.Buckley RH, Schiff SE, Schiff RI, Markert L, Williams LW, Roberts JL, et al. Hematopoietic stem cell transplantation for the treatment of severe combined immunodeficiency. N Engl J Med. 1999;340:508–516. doi: 10.1056/NEJM199902183400703. [DOI] [PubMed] [Google Scholar]
- 30.Myers LA, Patel DD, Puck JM, Buckley RH. Hematopoietic stem cell transplantation for severe combined immunodeficiency in the neonatal period leads to superior thymic output and improved survival. Blood. 2002;99(3):872–878. doi: 10.1182/blood.v99.3.872. [DOI] [PubMed] [Google Scholar]
- 31.Antoine C, Muller S, Cant A, Cavazzana-Calvo M, Veys P, Vossen J, et al. Long-term survival and transplantation of haemopoietic stem cells for immunodeficiencies: report of the European experience 1968−99. Lancet. 2003;361:553–560. doi: 10.1016/s0140-6736(03)12513-5. [DOI] [PubMed] [Google Scholar]
- 32.Buckley RH. Molecular defects in human severe combined immunodeficiency and approaches to immune reconstitution. Ann Rev Immunol. 2004;55:625–656. doi: 10.1146/annurev.immunol.22.012703.104614. [DOI] [PubMed] [Google Scholar]
- 33.Gennery AR, Slatter MA, Grandin L, Taupin P, Cant AJ, Veys P, et al. Transplantation of hematopoietic stem cells and long-term survival for primary immunodeficiencies in Europe: entering a new century, do we do better? J Allergy Clin Immunol. 2010;126(3):602–610. doi: 10.1016/j.jaci.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 34.Schiff SE, Kurtzberg J, Buckley RH. Studies of human bone marrow treated with soybean lectin and sheep erythrocytes: stepwise analysis of cell morphology, phenotype and function. Clin Exp Immunol. 1987;68:685–693. [PMC free article] [PubMed] [Google Scholar]
- 35.Sarzotti-Kelsoe M, Win CM, Parrott RE, Cooney M, Moser BK, Roberts JL, et al. Thymic output, T-cell diversity, and T-cell function in long-term human SCID chimeras. Blood. 2009;114(7):1445–1453. doi: 10.1182/blood-2009-01-199323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Railey MD, LoKhnygina Y, Buckley RH. Long Term Clinical Outcome of Patients with Severe Combined Immunodeficiency who Received Related Donor Bone Marrow Transplants without Pre-transplant Chemotherapy or Post-transplant GVHD Prophylaxis. J Pediatr. 2009;155:834–840. doi: 10.1016/j.jpeds.2009.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Notarangelo LD, Fischer A, Geha RS, Casanova JL, Chapel H, Conley ME, et al. Primary immunodeficiency: 2009 update. J Allergy Clin Immunol. 2009;124(6):1161–1178. doi: 10.1016/j.jaci.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buckley RH, Schiff RI, Schiff SE, Markert ML, Williams LW, Harville TO, et al. Human severe combined immunodeficiency (SCID): Genetic, phenotypic and functional diversity in 108 infants. J Pediatr. 1997;130:378–387. doi: 10.1016/s0022-3476(97)70199-9. [DOI] [PubMed] [Google Scholar]
- 39.Buckley RH. The multiple causes of human SCID. J Clin Invest. 2004;114(10):1409–1411. doi: 10.1172/JCI23571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noguchi M, Yi H, Rosenblatt HM, Filipovich AH, Adelstein S, Modi WS, et al. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147–157. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- 41.Puck JM, Deschenes SM, Porter JC, Dutra AS, Brown CJ, Willard HF, et al. The interleukin-2 receptor gamma chain maps to Xq13.1 and is mutated in X-linked severe combined immunodeficiency, SCIDX1. Human Molecular Genetics. 1993;2:1099–1104. doi: 10.1093/hmg/2.8.1099. [DOI] [PubMed] [Google Scholar]
- 42.Puck JM. Molecular and genetic basis of X-linked immunodeficiency disorders. J Clin Immunol. 1994;14:81–89. doi: 10.1007/BF01541340. [DOI] [PubMed] [Google Scholar]
- 43.Macchi P, Villa A, Gillani S, Sacco MG, Frattini A, Porta F, et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID) Nature. 1995;377:65–68. doi: 10.1038/377065a0. [DOI] [PubMed] [Google Scholar]
- 44.Roberts JL, Lengi A, Brown SM, Chen M, Zhou Y-J, O'Shea JJ, et al. Janus Kinase 3 (JAK3) deficiency: clinical, immunologic and molecular analyses of 10 patients and outcomes of stem cell transplantation. Blood. 2004;103:209–218. doi: 10.1182/blood-2003-06-2104. [DOI] [PubMed] [Google Scholar]
- 45.Hirschhorn R. Immunodeficiency diseases due to deficiency of adenosine deaminase. In: Ochs HD, Smith CIE, Puck JM, editors. Primary Immunodeficiency Diseases: A Molecular and Genetic Approach. New York and Oxford: Oxford University Press; 1999. pp. 121–139. [Google Scholar]
- 46.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. Consensus conference on acute GVHD grading. Bone Marrow Transplantation. 1995;15:825–828. [PubMed] [Google Scholar]
- 47.Aiuti A, Cattaneo F, Galimberti S, Benninghoff U, Cassani B, Callegaro L, et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med. 2009;360(5):447–458. doi: 10.1056/NEJMoa0805817. [DOI] [PubMed] [Google Scholar]
- 48.Friedman NJ, Schiff SE, Ward FE, Schiff RI, Buckley RH. Graft-versus-graft and graft-versus-host reactions after HLA-identical bone marrow transplantation in a patient with severe combined immunodeficiency with transplacentally acquired lymphoid chimerism. Pediatr Allerg Immunol. 1991;2:111–116. [Google Scholar]
- 49.Patel DD, Gooding ME, Parrott RE, Curtis KM, Haynes BF, Buckley RH. Thymic function after hematopoietic stem-cell transplantation for the treatment of severe combined immunodeficiency. N Engl J Med. 2000;342(18):1325–1332. doi: 10.1056/NEJM200005043421804. [DOI] [PubMed] [Google Scholar]
- 50.Sarzotti M, Patel DD, Li X, Ozaki DA, Cao S, Langdon S, et al. T cell repertoire development in humans with SCID after nonablative allogeneic marrow transplantation. J Immunol. 2003;170(5):2711–2718. doi: 10.4049/jimmunol.170.5.2711. [DOI] [PubMed] [Google Scholar]
- 51.Ghory P, Schiff S, Buckley R. Appearance of multiple benign paraproteins during early engraftment of soy lectin T cell-depleted haploidentical bone marrow cells in severe combined immunodeficiency. J Clin Immunol. 1986;6:161–169. doi: 10.1007/BF00918749. [DOI] [PubMed] [Google Scholar]
- 52.Kent EF, Crawford J, Cohen HJ, Buckley RH. Development of multiple monoclonal serum immunoglobulins (multiclonal gammopathy) following both HLA-identical unfractionated and T cell-depleted haploidentical bone marrow transplantation in severe combined immunodeficiency. J Clin Immunol. 1990;10:106–114. doi: 10.1007/BF00918192. [DOI] [PubMed] [Google Scholar]
- 53.Gerritsen EJA, van Tol MJD, Lankester AC, van der Weijden-Rajas CPM, Jol-van der Zjide CM, Oudeman-Gruber NJ, et al. Immunoglobulin levels and monoclonal gammopathies in children after bone marrow transplantation. Blood. 1993;82:3493–3502. [PubMed] [Google Scholar]
- 54.Buckley RH. B cell function in severe combined immunodeficiency after stem cell or gene therapy: A review. J Allergy Clin Immunol. 2010;125 doi: 10.1016/j.jaci.2010.02.012. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haddad E, Landais P, Friedrich W, Gerritsen B, Cavazzana-Calvo M, Morgan G, et al. Long-term immune reconstitution and outcome after HLA-nonidentical T- cell-depleted bone marrow transplantation for severe combined immunodeficiency: a European retrospective study of 116 patients. Blood. 1998;91(10):3646–3653. [PubMed] [Google Scholar]
- 56.Bertrand Y, Landais P, Friedrich W, Gerritsen B, Morgan G, Fasth A, et al. Influence of severe combined immunodeficiency phenotype on the outcome of HLA non-identical T cell-depleted bone marrow transplantation. J Pediatr. 1999;134:740–748. doi: 10.1016/s0022-3476(99)70291-x. [DOI] [PubMed] [Google Scholar]
- 57.O'Marcaigh AS, DeSantes K, Hu D, Pabst H, Horn B, Li L, et al. Bone marrow transplantation for T-B- severe combined immunodeficiency disease in Athabascan-speaking native Americans. Bone Marrow Transplant. 2001;27(7):703–709. doi: 10.1038/sj.bmt.1702831. [DOI] [PubMed] [Google Scholar]
- 58.Smogorzewska EM, Brooks J, Annett G, Kapoor N, Crooks GM, Kohn DB, et al. T cell depleted haploidentical bone marrow transplantation for the treatment of children with severe combined immunodeficiency. Arch Immunol Ther Exp (Warsz ) 2000;48(2):111–118. [PubMed] [Google Scholar]
- 59.Borghans JA, Bredius RG, Hazenberg MD, Roelofs H, Jol-van der Zijde EC, Heidt J, et al. Early determinants of long-term T-cell reconstitution after hematopoietic stem cell transplantation for severe combined immunodeficiency. Blood. 2006;108(2):763–769. doi: 10.1182/blood-2006-01-009241. [DOI] [PubMed] [Google Scholar]
- 60.Cavazzana-Calvo M, Carlier F, Le Deist F, Morillon E, Taupin P, Gautier D, et al. Long-term T-cell reconstitution after hematopoietic stem-cell transplantation in primary T-cell-immunodeficient patients is associated with myeloid chimerism and possibly the primary disease phenotype. Blood. 2007;109(10):4575–4581. doi: 10.1182/blood-2006-07-029090. [DOI] [PubMed] [Google Scholar]
- 61.Mazzolari E, Forino C, Guerci S, Imberti L, Lanfranchi A, Porta F, et al. Long-term immune reconstitution and clinical outcome after stem cell transplantation for severe T-cell immunodeficiency. J Allergy Clin Immunol. 2007;120(4):892–899. doi: 10.1016/j.jaci.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 62.Friedrich W, Honig M, Muller SM. Long-term follow-up in patients with severe combined immunodeficiency treated by bone marrow transplantation. Immunol Res. 2007;38(1−3):165–173. doi: 10.1007/s12026-007-0030-2. [DOI] [PubMed] [Google Scholar]
- 63.Slatter MA, Brigham K, Dickinson AM, Harvey HL, Barge D, Jackson A, et al. Long-term immune reconstitution after anti-CD52-treated or anti-CD34-treated hematopoietic stem cell transplantation for severe T-lymphocyte immunodeficiency. J Allergy Clin Immunol. 2007;121:361–367. doi: 10.1016/j.jaci.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 64.Patel NC, Chinen J, Rosenblatt HM, Hanson IC, Brown BS, Paul ME, et al. Long-term outcomes of nonconditioned patients with severe combined immunodeficiency transplanted with HLA-identical or haploidentical bone marrow depleted of T cells with anti-CD6 mAb. J Allergy Clin Immunol. 2008;122(6):1185–1193. doi: 10.1016/j.jaci.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 65.Neven B, Leroy S, Decaluwe H, Le Deist F, Picard C, Moshous D, et al. Long-term outcome after haematopoietic stem cell transplantation of a single-centre cohort of 90 patients with severe combined immunodeficiency: Long-term outcome of HSCT in SCID. Blood. 2009 doi: 10.1182/blood-2008-09-177923. [DOI] [PubMed] [Google Scholar]
- 66.Honig M, Albert MH, Schulz A, Sparber-Sauer M, Schutz C, Belohradsky B, et al. Patients with adenosine deaminase deficiency surviving after hematopoietic stem cell transplantation are at high risk of CNS complications. Blood. 2007;109(8):3595–3602. doi: 10.1182/blood-2006-07-034678. [DOI] [PubMed] [Google Scholar]
- 67.Titman P, Pink E, Skucek E, O'Hanlon K, Cole TJ, Gaspar J, et al. Cognitive and behavioural abnormalities in children following haematopoietic stem cell transplantation for severe congenital immunodeficiencies. Blood. 2008;112:3907–3913. doi: 10.1182/blood-2008-04-151332. [DOI] [PubMed] [Google Scholar]
- 68.Clement-De Boers A, Oostdijk W, Van Weel-Sipman MH, Van den Broeck J, Wit JM, Vossen JM. Final height and hormonal function after bone marrow transplantation in children. J Pediatr. 1996;129:544–550. doi: 10.1016/s0022-3476(96)70119-1. [DOI] [PubMed] [Google Scholar]
- 69.Barrett MJ, Buckley RH, Schiff SE, Kidd PC, Ward FE. Accelerated development of immunity following transplantation of maternal marrow stem cells into infants with severe combined immunodeficiency and transplacentally acquired lymphoid chimerism. Clin Exp Immunol. 1988;72:118–123. [PMC free article] [PubMed] [Google Scholar]
- 70.Kurtzberg J, Laughlin M, Graham ML, Smith C, Olson JF, Halperin EC, et al. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. New Engl J Med. 1996;335(3):157–166. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- 71.Gluckman E, Rocha V, Boyer-Chammard A, Locatelli F, Arcese W, Pasquini R, et al. Outcome of cord blood transplantation from related and unrelated donors. New Engl J Med. 1997;337:373–381. doi: 10.1056/NEJM199708073370602. [DOI] [PubMed] [Google Scholar]
- 72.Knutsen AP, Wall DA. Umbilical cord blood transplantation in severe T-cell immunodeficiency disorders: two-year experience. J Clin Immunol. 2000;20(6):466–476. doi: 10.1023/a:1026463900925. [DOI] [PubMed] [Google Scholar]
- 73.Flake AW, Roncarolo MG, Puck JM, Almeida-Porada G, Evans MI, Johnson MP, et al. Treatment of X-linked severe combined immunodeficiency by in utero transplantation of paternal bone marrow. N Engl J Med. 1996;335:1806–1810. doi: 10.1056/NEJM199612123352404. [DOI] [PubMed] [Google Scholar]
- 74.Wengler GS, Lanfranchi A, Frusca T, Verardi R, Neva A, Brugnoni D, et al. In-utero transplantation of parental CD34 haematopoietic progenitor cells in a patient with X-linked severe combined immunodeficiency (SCIDX1) Lancet. 1996;348:1484–1487. doi: 10.1016/s0140-6736(96)09392-0. [DOI] [PubMed] [Google Scholar]