Abstract
Objective:
Semantic memory impairment is common in both Mild Cognitive Impairment (MCI) and early Alzheimer’s disease (AD), and the ability to recognize familiar people is particularly vulnerable. A time-limited temporal gradient (TG) in which well known people from decades earlier are better recalled than those learned recently is also reported in both AD and MCI. In this study, we hypothesized that the TG pattern on a famous name recognition task (FNRT) administered to cognitively intact elders would predict future episodic memory decline, and would also show a significant correlation with hippocampal volume.
Methods:
78 healthy elders (ages 65-90) with normal cognition and episodic memory at baseline were administered a FNRT. Follow-up episodic memory testing 18 months later produced two groups: Declining (≥ 1 SD reduction in episodic memory) and Stable (< 1 SD).
Results:
The Declining group (N=27) recognized fewer recent famous names than the Stable group (N=51), while recognition for remote names was comparable. Baseline MRI volumes for both the left and right hippocampus was significantly smaller in the Declining group than the Stable group. Smaller baseline hippocampal volume was also significantly correlated with poorer performance for recent, but not remote famous names. Logistic regression analyses indicated that baseline TG performance was a significant predictor of group status (Declining versus Stable) independent of chronological age and APOE ε4 inheritance.
Conclusions:
Famous name recognition may serve as an early pre-clinical cognitive marker of episodic memory decline in older individuals.
Keywords: Famous names, episodic memory, hippocampus, semantic memory, temporal gradient
Introduction
Alterations in brain integrity are evident prior to the manifestation of the cognitive symptoms associated with Alzheimer’s dementia (AD), and also predate the symptoms associated with the prodromal stage of Mild Cognitive Impairment (MCI) (Albert et al., 2011). Therefore, considerable research effort is currently focused on finding reliable markers that identify individuals at high risk for AD, even before clinical symptoms are evident. The apolipoprotein E (APOE) ε4 allele is considered a reliable biomarker for increased risk of conversion from MCI to AD, and for the transition from asymptomatic to MCI (Brainerd, Reyna, Petersen, Smith, & Taub, 2012; Corder et al., 1993; Petersen et al., 1995). APOE status is also associated with distinct patterns of structural (e.g., MRI, DTI), and functional neuroimaging findings (fMRI, PET) in both healthy elders and in the prodormal MCI stage (Bookheimer et al., 2000; Bondi et al., 2005; Gold et al., 2010; Reiman et al., 2005; Woodard et al., 2009).
From a neuropsychological perspective, the goal is to identify cognitive domains and specific measures that can identify cognitively intact individuals at increased risk for subsequently developing MCI and AD. Episodic memory tasks (particularly delayed recall) have generally been considered the most consistent cognitive measure predictive of progression from age-appropriate memory performance to MCI and AD (Backman et al., 2005; Bondi et al., 1999; Caselli et al., 2004). However, it has become increasingly evident that the pre-clinical period is also be characterized by prominent difficulties in other cognitive domains including executive functions (attention), language, and working memory (Winoblad et al., 2004). In addition, the deleterious impact for ε4 allele carriers is evident in several cognitive domains as well (Twamley et al., 2005).
The presence of semantic memory impairment in AD has been well-established (Chertkow et al., 2008; Salmon, Nelson, & Chan, 1999). More recently, several studies have suggested that the inclusion of semantic memory tasks is important in the assessment of cognitive decline in the non-demented elderly (Carter et al., 2012; Spaan, Raaijmakers, & Jonker, 2005). Jacova et al. (2008) reported that 80% of subjects who showed cognitive impairment but no dementia also showed impairment on measures of semantic memory and had MRI evidence of MTL atrophy. Mickes et al. (2007) found deficits in both episodic and semantic memory three years prior to the progression to AD. Similar findings have been reported in other studies (Wilson, et al., 2011). These studies used measures of general semantic memory, such as category fluency and object naming.
In this study, we examined a person-identity semantic memory test, recognition accuracy for famous names, as a potential early marker for identification of increased risk of episodic memory decline in asymptomatic individuals. The selection of a famous name recognition accuracy task was based on converging evidence showing a disproportionate impairment for person identity knowledge impairment in MCI and AD compared to general semantic memory (Ahmed et al., 2008; Clague et al., 2011; Joubert et al., 2010). Furthermore, the disruption of effective semantic memory encoding processes negatively impacts episodic memory performance (Craik & Lockhart, 1972; Mayeux et al., 1980; Nebes, 1988)) Findings from functional magnetic resonance imaging (fMRI) studies also indicate that recognition of famous names consistently produced patterns of BOLD signal activity in a neural network that overlaps with the default network, and includes regions known to be affected early in the AD process (Sugarmen et al., 2011; Woodard et al., 2010).
AD typically (though not always) shows better performance for recognition of remote memory than recent memory, and this pattern is referred to as a time-limited temporal gradient (TG) (Beatty et al., 1988; Greene & Hodges, 1996; Westmacott et al. 2004). A time-limited TG has also been reported in MCI, and with people who convert from MCI to AD (Bizzozero et al., 2009; Estevez-Gonzales et al., 2004; Seidenberg et al. (2009). However, different temporal gradient patterns (TG) (flat, reversed) have been reported for other types of dementia (Kopelman, 2000; Sagar et al., 1988; Graham & Hodges, 1997; Beatty et al., 1988: Hodges & Hurd, 1994). It has been hypothesized that damage restricted to the hippocampus is more likely to produce a time-limited TG, but damage extending beyond the MTL region, and particularly into the anterior temporal lobe, is likely to produce a flat temporal gradient of remote memory impairment (Bright et al., 2006; Reed & Squire, 1998; Westmacott et al., 2004). In addition, fMRI studies have reported increased MTL signal activation for recent famous names compared to remote famous names in healthy controls (Leveroni et al., 2000; Haist et al., 2000 Smith & Squire, 2009).
In this study, TG findings from a famous name recognition task (recent and remote) and MRI hippocampal volumes were examined in 78 cognitively intact individuals, classified into a Stable or a Declining group based on their 18-month follow-up performance on a multi-trial list-learning episodic memory task. The Declining group was expected to show poorer baseline recognition accuracy for recent famous names compared to the Stable group, but not for remote famous names. The Declining group was also predicted to have smaller baseline hippocampal volumes than the Stable group, and the extent of the TG (remote > recent) was expected to co-vary with hippocampal volume. We also examined the contribution of famous name memory performance for predicting the probability of memory decline compared to other known predictor variables including chronological age, and presence/absence of the Apolipoprotein E (APOE) ε4 allele.
Methods
All participants in this study signed an informed consent in accordance with the guidelines of the Institutional Review Board of Rosalind Franklin University. Participants were paid for their participation.
Participants
Seventy-eight healthy cognitively intact older participants (mean age = 73 years, SD = 3.4) were administered measures of general cognitive and episodic memory functioning that included the Mini-Mental State Exam (MMSE) (Folstein, Folstein, & McHugh, 1975), Mattis Dementia Rating Scale-2 (DRS-2) (Jurica, Leitten, & Mattis, 2001; Mattis, 1988), and the Rey Auditory Verbal Learning Test (RAVLT) (Rey, 1958). These same measures were repeated a second time 18-month later. Participants were recruited from a larger sample of 459 community-dwelling adults via newspaper advertisements. A phone screen was used to determine eligibility for inclusion in the study. Individuals reporting a previous or current history of neurological disease, major psychiatric disturbance or substance abuse meeting DSM-IV Axis I criteria, or current use of psychoactive medications were excluded. In addition, all study participants agreed to undergo APOE genotyping and to have an MRI scan performed. APOE genotyping used a PCR method (Saunders et al., 1996) and DNA was isolated with the Gentra Systems Autopure LS for Large Sample Nucleic Acid Purification. 78 of the 84 subjects tested at baseline (93%) were also seen at the eighteen month re-testing. Reasons provided by the six individuals tested at baseline who did not return were: medical illness (2), lost interest (2), loss contact (1), and deceased (1).
Baseline Cognitive Status
At baseline, all participants were considered to be asymptomatic based on the scores obtained from the MMSE, DRS-2, and RAVLT at baseline. Inclusion in the current study required a minimum MMSE score of 27, a minimum DRS-2 total score of 130, and scores no less than 1.5 standard deviations below the mean of a local sample (n=91) on either the delayed recall or immediate learning indices of the RAVLT.
Definition of Memory Decline
The definition of memory decline over the 18-month follow-up period was based on the extent of reduction from baseline performance on three outcome indices: DRS-2 Total Score, RAVLT Sum of Trials 1-5, and RAVLT Delayed Recall. Alternate forms of the DRS-2 and RAVLT were used for baseline and 18-month follow-up. Residualized change scores were computed for each cognitive measure by predicting Time 2 (follow-up) scores from Time 1 (baseline) scores. This procedure adjusted for baseline performance, practice effects, and regression to the mean. Participants with standardized residuals of −1.0 or lower were assigned to the Declining group (n = 27) and the remaining participants were classified as Stable (n=51). Table 2 provides the baseline and eighteen month follow-up scores on the cognitive measures for the Stable and Decline groups.
Table 2.
Raw Score Performance on Cognitive Measures.
| Baseline | Change from Baseline | Group x Time Interaction |
||||
|---|---|---|---|---|---|---|
| Measure (Raw Score Range) |
Stable M (SD) |
Declining M (SD) |
p-value (d) | Stable M (SD) |
Declining M (SD) |
P-value (ηp2) |
| MMSE (0−30) |
29.4 (0.8) | 28.9 (1.2) | .04 (.50) | −.14 (1.2) | −.11 (.85) | .92 (.000) |
| DRS−2 Attention (0−37) |
36.6 (0.6) | 36.4 (0.7) | .30 (.25) | −.12 (.77) | −.19 (.68) | .70 (.002) |
| DRS−2 Initiation/Perseveration (0−37) |
36.5 (1.5) | 36.5 (0.8) | .92 (.02) | −.27 (1.6) | .44 (2.0) | .09 (.037) |
| DRS−2 Construction (0−6) |
5.9 (0.2) | 6.0 (0.0) | .08 (.42) | −.04 (.28) | .00 (.00) | .47 (.007) |
| DRS−2 Conceptualization (0−39) |
37.2 (2.7) | 37.0 (2.0) | .74 (.08) | 1.4 (3.2) | 2.4 (2.4) | .17 (.025) |
| DRS−2 Memory (0−25) |
24.3 (1.0) | 23.7 (1.9) | .13 (.37) | .20 (1.4) | 1.5 (1.7) | .001 (.142) |
| DRS−2 Total (0−144) |
140.7 (3.2) | 139.7 (3.8) | .25 (.27) | 1.2 (3.4) | 4.1 (3.9) | .001 (.136) |
| RAVLT TR (0−75) |
50.6 (8.8) | 46.8 (8.1) | .07 (.44) | 1.1 (6.0) | 6.7 (6.2) | <.001 (.164) |
| RAVLT DR (0−15) |
10.1 (2.6) | 9.0 (2.8) | .08 (.42) | −.14 (2.1) | 3.0 (2.4) | <.001 (.315) |
MMSE = Mini-Mental State Examination; DRS−2 = Dementia Rating Scale−2; RAVLT TR = Rey Auditory Verbal Learning Test Total Words Recalled; RAVLT DR = Rey Auditory Verbal Learning Test Delayed Recall
Famous Name Stimuli
Name stimuli were selected through a carefully standardized procedure and normative study (Douville et al., 2005). An initial corpus of 784 famous names selected from the internet, trivia books, magazines, and newspapers, and non-famous names from a metropolitan telephone directory, were used in a normative study of 25 healthy older adults (mean age = 68 years, range 65-90) and 25 healthy young control adults (mean age = 27 years, range = 25-40). Based on these normative findings, we selected 30 recent famous names, 30 remote famous names, and 60 non-famous names. Specific criteria for categorizing a famous name as recent or remote were used: (1) recent famous names: people who achieved public prominence in the 1995-2005 period and were correctly identified by 90% of both older and younger participants of the normative study, (2) remote famous names: persons who achieved prominence in the 1950-1965 time period, but who have been out of the public eye and are not likely to have appeared recently in the news or entertainment media. These famous name stimuli were correctly identified by at least 90% of older but only 30% of younger participants, and (3) non-famous names: names correctly identified as non-famous by at least 90% of older and younger participants. Three separate lists of the famous names were prepared composed of 10 recent names, 10 remote names, 10 enduring names and 30 non-famous names. Each list included a unique set of famous and non-famous names. In this study, one set of 10 names of people who achieved prominence during the 1950-1965 period but were well-recognized by both younger and older individuals (enduring famous names; Frank Sinatra) was not included in the current analyses to ensure that memory age for names from the remote and recent epochs did not overlap. Appendix 1 provides a listing of the recent and remote famous names used in this study.
Procedure
The presentation of famous names occurred during an event-related fMRI scanning session. The fMRI findings have been previously published, but the famous name behavioral data presented here have neither been published nor discussed in our previous papers (Seidenberg, et al., 2009; Woodard et al., 2010). Each name was visually presented for 4 seconds and accuracy and reaction time was collected through an e-prime computer program. Participants were instructed to make a right index finger (i.e., dominant hand) key press if the name was famous and a right middle finger key press if the name was non-famous. Any trial in which a response was not made during the 4 second presentation time was considered incorrect. Names were presented is a pseudo-random format. Testing of participants on the famous name recognition task took place between April 30, 2005 and November 22, 2006.
Image Acquisition
MRI scans were obtained on a General Electric (Waukesha, WI) Signa Excite 3.0 Tesla short bore scanner equipped with a quad split quadrature transmit/receive head coil. High-resolution, three-dimensional spoiled gradient-recalled at steady-state (SPGR) anatomical images were acquired (TE = 3.9 ms; TR = 9.5 ms; inversion recovery (IR) preparation time = 450 ms; flip angle = 12 degrees; number of excitations (NEX) = 2; slice thickness = 1.0 mm; FOV = 24 cm; resolution = 256 × 224). Foam padding was used to reduce head movement within the coil.
Hippocampal Volume Measurement
For the current study, baseline left and right hippocampal volumes were obtained by manual editing by two raters blinded to participant group membership using the T1-weighted SPGR images and initial parcellations provided by Freesurfer version 4.02 (http://surfer.nmr.mgh.harvard.edu) (Dale, Fischl, & Sereno, 1999; Fischl, Liu, & Dale, 2001). Using coronal views, the mask was further refined by excluding the fimbria and alveus and retaining the hippocampus (uncal apex, cornu ammonis, subiculum, gyrus of retzius and fasciola cinerea). Intraclass correlation for the two raters was 0.87. Hippocampal volumes were normalized within-subjects by dividing by the total intracranial volume (ICV).
Image Analysis
The MRI scans were analyzed using Freesurfer version 4.0.2 which is freely available online (http://surfer.nmr.mgh.harvard.edu). Previous studies have reported on the validation of the Freesurfer software (Dale, Fischl, & Sereno, 1999; Fischl, Liu, & Dale, 2001). For the current study, baseline left and right hippocampal volumes were obtained by manual editing by two raters blinded to participant group membership using the T1-weighted SPGR images provided by Freesurfer. Using coronal views, the mask was further refined by excluding the fimbria and alveus and retaining the hippocampus (uncal apex, cornu ammonis, subiculum, gyrus of retzius and fasciola cinerea). Intraclass correlation for the two raters was 0.87. Hippocampal volumes were normalized within-subjects by dividing by the total intracranial volume (ICV).
Data Analyses
A 2 (Group) × 2 (Time Epoch) mixed design ANOVA was used to compare baseline famous name recognition performance for recent and remote names between the Stable and Declining groups. Episodic memory performance and general cognitive status were examined with a mixed design ANOVA comparing performance at baseline to the 18-month follow-up (Group × Testing Session). A TG ratio index score was calculated for each subject ((remote-recent)/ (remote + recent)). Higher TG ratio scores indicate a propensity to show higher recognition accuracy for remote famous names compared to recent famous names. In contrast, lower TG ratio scores represent higher recognition accuracy for recent famous names compared to remote famous names. A t-test was used to compare hippocampal volumes (ICV corrected) between the Declining and Stable groups. Pearson product-moment correlations were used to examine the relationship between baseline hippocampal volumes, baseline famous name recognition, and baseline episodic memory (AVLT). Logistic regression analysis was used to determine the relative contribution of baseline famous name recognition performance (TG ratio) and APOE - ε4 inheritance for predicting the probability of falling in the Decline or Stable group. For each model, Table 4 provides the Nagelkerke R2 and C values along with the coefficients, standard errors, and significance levels for each predictor within the models. R2 provides a measure of the importance of the predictors in a given model and the C index indicates the proportion of all pairs of subjects in which the predicted group status was correct. The C index is a summary statistic of the ROC and is considered the best measure of the strength of the logistic regression model. We present the bootstrapped values for C and R2to correct for over fitting the data.
Table 4.
Results of Logistic Regressions.
| Variables | Likelihood Ratio |
R2 | C Index1 |
Coefficient | SE | p-value | |
|---|---|---|---|---|---|---|---|
| Model | TG Ratio | 7.26 | .123 | .599 | 4.93 | 2.2 | .024 |
| 1 | Age | .010 | .05 | .852 | |||
| Education | −.062 | .10 | .518 | ||||
| Gender | −.232 | .56 | .681 | ||||
| Model | TG Ratio | 12.21 | .200 | .701 | 5.14 | 2.2 | .020 |
| 2 | APOE | 1.25 | .53 | .019 |
Bootstrapped C index
TG Ratio = temporal gradient ratio; correct recognition of (remote – recent)/(remote + recent); APOE = Apolipoprotein ε4 inheritance
Results
Demographic and hippocampal volume data for the Declining and Stable groups are shown in Table 1. There were no significant group differences in age, education, gender distribution, or time interval between the baseline and follow-up testing (p’s > .05). Mean baseline and change from baseline scores on the MMSE, RAVLT, and DRS-2 are shown in Table 2. As expected, given the group inclusion criteria, a 2 (Group) × 2 (Testing Session) ANOVA revealed a significant interaction for the RAVLT sum of words recalled across Trials 1-5 [F (1, 76) = 14.95, p < .001, partial η2 = .164]; RAVLT delayed recall, [F (1, 76) = 34.9, p < .001, partial η2 = .315]; and DRS-2 Total Score, [F (1, 76) = 11.99, p = .001, partial η2 = .136]. For each of the RAVLT indices, the baseline performance of the Declining and Stable groups were not significantly different (p’s > .05), but the average episodic memory decline in the Declining group was substantial (see Table 2). Two of the 27 Declining subjects (7.4%) would be considered MCI converters based on the criteria we have used in several earlier studies (Seidenberg, et al., 2009; Woodard, et al., 2010). The DRS-2 scales other than memory and MMSE did not show a significant interaction effect (p’s > .05).
Table 1.
Demographic and Hippocampal Volume Statistics for Stable and Declining Groups.
| Stable (n=51) |
Declining (n=27) |
p-value | Effect Size (d) |
|
|---|---|---|---|---|
| Demographics | Mean (SD) | Mean (SD) | ||
| Age (yrs) | 72.7 (5.0) | 73.6 (4.7) | .40 | .20 |
| Education (yrs) | 15.1 (2.4) | 14.5 (3.2) | .33 | .23 |
| Sex (M/F)1 | 13/38 | 8/19 | .79 | .06 |
| Retest Interval (days) | 551.7 (43.5) | 560.6 (47.0) | .41 | .20 |
| APOE-ε4 positive1 | 23.5% | 51.9% | .02 | .54 |
|
Hippocampal
Volume2 |
||||
| Left Hippocampus | 2.23 (.26) | 2.04 (.27) | .005 | .69 |
| Right Hippocampus | 2.31 (.31) | 2.14 (.34) | .03 | .53 |
Fisher Exact Test;
Hippocampal volumes are measured in cm3
Famous Name Recognition
Table 3 provides the baseline performance for the two groups on the famous name recognition task for the recent and remote time epochs. A 2 (Group) × 2 (Time Epoch) yielded a significant main effect of Group [F (1, 76) = 3.91, p = .05, partial η2 = .049], demonstrating that the Stable group correctly recognized more famous names than the Declining group. A significant main effect of Time Epoch was also observed [F (1, 76) = 76.5, p < .001, partial η2 = .502], demonstrating that remote famous names were recognized more accurately than recent names. Most important, however, the predicted Group x × Time Epoch interaction was also significant [F (1, 76) = 6.90, p = .010, partial η2 = .083]. Both groups recognized a similar number of remote famous names, [t (76) = −.42, p = .67, d = .10], but the Declining group recognized fewer famous names from the recent time epoch than the Stable group, [t (76) = 2.37, p =.02, d =.57]. There was also no significant group difference for correct rejection of the non-famous names, [t (33.9) = .93, p = .36, d = .22]. However, the Stable group did show significantly better discriminability (d’) for the recent time epoch, [t (76) = 2.3, p= .02, d= .55] compared to the Decline group, but not for the remote famous names [t (76) = .34, p= .74, d = .08].
Table 3.
Famous Name Recognition Performance at Baseline for Stable and Declining Groups.
| Stable (n=51) |
Declining (n=27) |
p-value | Effect Size (d) |
|
|---|---|---|---|---|
| Accuracy (% correct) | Mean (SD) | Mean (SD) | ||
| Recent | 84.7 (15.7) | 74.8 (20.6) | .02 | .57 |
| Remote | 96.9 (5.8) | 97.4 (4.5) | .67 | .10 |
| Unfamiliar | 96.9 (4.6) | 95.2 (8.8) | .36 | .22 |
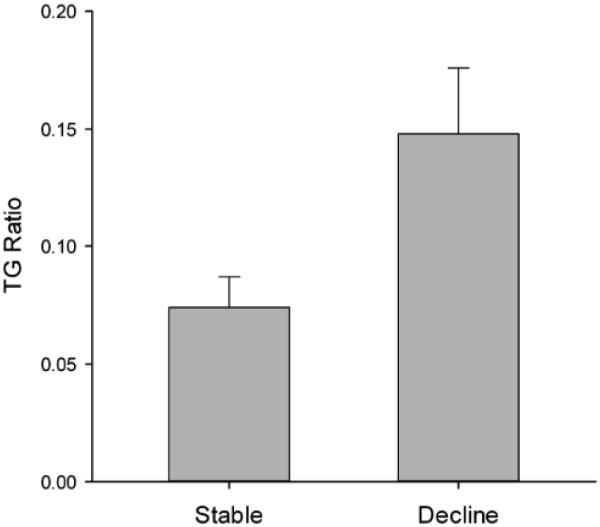
| TG Ratio1 | .07 (.09) | .15 (.15) | .03 | .54 |
| Reaction Time2 (ms) | ||||
| Recent | 1482.83(297.15) | 1516.01 (284.35) | .64 | .11 |
| Remote | 1263.10 (255.17) | 1270.04 (206.91) | .90 | .03 |
| Unfamiliar | 1669.67 (354.46) | 1610.99 (385.49) | .50 | .16 |
| Sensitivity Index (d') | ||||
| Recent | 2.95 (.66) | 2.57 (.77) | .02 | .55 |
| Remote | 3.39 (.45) | 3.35 (.57) | .74 | .08 |
TG Ratio = correct recognition of famous names (remote-recent)/(remote + recent). Positive scores reflect greater accuracy for remote compared to recent famous names.
Reaction times are presented for correct trials only.
We also calculated a TG ratio index score for each subject ((remote-recent)/ (remote + recent), which provided the relative proportion of accurate recognition for remote famous names from compared to recent famous names. Higher positive TG scores indicate better recognition accuracy for remote than recent names while below zero scores indicate the opposite pattern. Consistent with the results from the ANOVA, the Declining group showed a higher TG ratio score indicating a greater propensity than the Stable group to recognize more remote famous names than recent famous names [t (37.5) = −2.3, p = .03, d = .54]. Examination of reaction time for correct responses shown in Table 3 indicated no significant differences between the two groups for either the recent or remote famous names (p’s > .05). For both groups, reaction time for remote famous names was faster than for recent names.
Hippocampal Volumes
Baseline hippocampal volumes were smaller for the Declining group than the Stable group on both the left side [t (76) = 2.88, p = .005, d = .69], and right side [t (76) = 2.21, p = .03, d = .53] (see Table 1). However, there was no group difference in total intracranial volume (ICV), [t (76) = 1.41, p = .16]. Right hippocampal volume was significantly correlated with recognition accuracy for recent famous names (r = .22, p < .05), but not for the remote names (r = −.06, p = .59). The TG ratio score was significantly correlated with both right hippocampus volume (r = −.28, p = .01), and left hippocampus volume (r = −.24, p = .04). Individuals with smaller hippocampal volumes had a greater propensity to recall more remote famous names than recent famous names. In contrast, baseline RAVLT indices were not significantly correlated with baseline hippocampal volumes (all p’s > .05), or with performance on recent and remote famous name recognition accuracy (p’s < .05).
Logistic Regression Analyses
Logistic regression models were evaluated to determine the contribution of the famous name TG ratio score for classifying participants into Stable and Declining groups. We examined two models. Model 1 included the TG ratio and three demographic variables; age, gender and education. This model produced an R2 (bootstrapped) = .0132, C (bootstrapped) =.5986. None of the demographic variables produced a significant coefficient (p’s > .05), however, the TG ratio coefficient was significant, (p = .024).Thus, the TG ratio contributed significantly to prediction even after accounting for the demographic variables. The addition of APOE ε4 inheritance as a predictor variable (Model 2) yielded a prediction model with R2 (bootstrapped) = .1568, C (bootstrapped) = .7013. The TG index ratio (p = .02) remained a significant predictor in the model independent of APOE status.
Discussion
The current findings suggest that a person-identity semantic memory task comparing recent and remote famous names was able to identify cognitively intact elders most likely to exhibit future episodic memory decline. Compared to the Stable group, the Declining group had poorer baseline performance for recent famous names, but not remote famous names. Baseline MRI volumes of both the left and right hippocampus were significantly smaller in the Declining group. In addition, the steepness of the TG ratio (i.e., propensity for better accuracy for remote than recent names) was related to smaller hippocampal volumes. Finally, the baseline TG pattern made a significant contribution in a prediction model of episodic memory performance eighteen months later, and its effect was independent of the contribution of APOE ε4 allele inheritance and demographic variables. These findings also cannot be accounted for by group differences in age, education, gender, or baseline ICV. Episodic memory performance at baseline also did not differ between the two groups, and scores fell within the average range for age.
Why would a measure of famous name recognition, typically viewed as a semantic memory measure, predict subsequent episodic memory decline? Westmacott & Moscovitch (2003) suggested that some famous names carry an autobiographical significance (AS), and can therefore be considered to have both an episodic component as well as a semantic component. They found that patients with semantic dementia and healthy controls, but not AD patients, showed a benefit on reading speed and episodic recall for high AS famous names compared to low AS famous names. They argued that the hippocampal pathology typically evident in AD underlies the inability to benefit from the AS episodic component of famous names. Viewed from this perspective, the findings reported here (remote names > recent names) could represent the presence of an early and subtle difficulties in “episodic memory” in the Declining group. Of interest, several recent (but not all) fMRI studies have reported greater hippocampal activation for recent famous names or faces compared to remote names or faces (Douville, et al., 2005; Haist, Bowden, Gore, & Mao, 2001; Smith & Squire, 2009). Alternatively, a number of studies have shown that semantic memory measures without a temporal dimension (e.g., category fluency) may also be a sensitive cognitive marker during the pre-clinical period (Rosen et al., 2005; Henry, Crawford, & Phillips, 2004; Monsch et al., 1992). In addition, the baseline scores on the AVLT, a much often used clinical measure of episodic memory, was not different between the Declining and Stable groups.
More broadly speaking, there is an increased focus on characterizing the interdependence of semantic and episodic memory, and the degree of involvement of the hippocampus (and under what conditions) in each type of memory (Greenberg & Verfaellie, 2010). Several earlier lesion studies examined the possibility that the hippocampus plays a role in semantic memory as well as episodic memory (MacKay, Stewart, & Burke, 1998; Manns, Hopkins, & Squire, 2003; Schmolck, Stefanacci, & Squire, 2000). Recent fMRI studies have reported evidence indicating that hippocampal activation co-varied with the category cue used (e.,g., things in your garage, things worn on feet, things that are red) while performing a fluency task (Ryan et al. 2008). Sheldon & Moscovitch (2011) suggested that MTL activation on fluency tasks is related to the extent that episodic memory is used to help generate specific items. Thus, the general distinction between episodic and semantic memory tasks may not fully capture the extent to which engagement of the hippocampus occurs. Additional neuroimaging and lesion studies of “semantic memory” tasks are needed to establish a better understanding of the task demands, and stimulus features that engage the hippocampus and it’s connectivity with other neocortical areas.
MRI atrophy in the hippocampus is considered a reliable biomarker for AD, MCI, and pre-clinical AD (Frisoni et al., 2010). We found that the Declining group had smaller baseline hippocampal volumes than the Stable group. Thus, at the point (baseline) that participants were “asymptomatic” based on a list-learning task (RAVLT) and two cognitive screen measures (DRS-2 and MMSE), subtle hippocampal volume differences were detected between the two groups Baseline hippocampal volume was also significantly correlated with performance for the TG pattern (TG ratio). In contrast, the RAVLT (delayed memory and percent retention), generally considered a prototypical episodic memory measure did not differ between the two groups at baseline and was not significantly correlated with baseline hippocampal volumes. It is possible that the study inclusion criteria reduced the range of scores on the RAVLT. Alternatively, the two measures RAVLT and Famous Name Recognition may be measuring different memory constructs and cognitive processes with somewhat different neural underpinnings.
In this study, 34.6% of the sample showed a minimum decline of one standard deviation on at least one of three measures, the RAVLT Delay Recall, RAVLT Percent Retention, and DRS-2 Total score. Within the Declining group, 56% were APOE ε4 allele carriers compared to 26% of the individuals in the Stable group. This finding is consistent with previous research indicating that the APOE ε4 allele is over-represented in both AD and MCI, and is also thought to increase the frequency and rate of cognitive decline in cognitively intact elderly individuals (Boyle et al., 2010; Bretsky et al., 2003; Caselli et al., 2009; Raber, Huang, & Ashford, 2004). The Stable group did not show any evidence of unusual protection against normal age-related decline (e.g. education level, ε2 allele). At follow-up testing, only 2 of the 27 participants in the Declining group met study criterion for diagnosis of MCI, although the degree of episodic memory decline as measured by the RAVLT was quite evident. Nevertheless, longer term tracking of these participants is necessary to determine if conversion to MCI or AD occurs.
A recent paper from our lab using the same study sample reported on here, focused on the predictive value of neuroimaging findings including MRI hippocampal volume, and fMRI cortical and hippocampal activation (during the famous name task), predicted future memory decline. They found that a model which included a combination of cortical fMRI activation, hippocampal fMRI activation, MRI total hippocampus volume, and APOE status produced the strongest model with a C Index of .789 and R2 value of .293 (Woodard et al., 2010). In the current study, the focus was on the potential utility of a cognitive measure, the TG for recent and famous names, for the prediction of decline. We found that demographic variables did not significantly predict group status either alone or in combination, but the TG ratio was significant even when demographic variable were included in the model. When APOE was included in a model together with the TG ratio, the TG ratio provided a significant amount of unique predicted value independent of APOE status.
There are several methodological and interpretive issues in the current study that warrant additional comment. First, the study participants were volunteers from the community, and local norms were used to identify individuals who were asymptomatic when they entered the study. The sample as a whole was highly educated and was predominately female (i.e., approximately 75%) which may limit generalizability. In addition, because recognition accuracy was very high for remote famous names for both groups, as the task was designed, there is a possibility of ceiling effects for the remote famous names. However, as noted in the Methods section, both the recent and remote famous names were specifically selected only if normative data indicated an accuracy rate of 90% or higher based on an independent sample. Thus, names were selected to be highly recognized for both time epochs. Consistent with this selection criterion, both groups had mean accuracy scores over 90% for the remote names. However, the Stable group performed much closer to that level for the recent famous names than did the Declining group. In addition, reaction time, which is often considered a measure of task difficulty, did not differ between the two groups for the recent or remote famous names. Both groups also had a similar difference in reaction time between recent and remote names. Thus, the difference between the recent and remote famous names is unlikely to be attributable to ceiling effects or other stimulus limitations. Nevertheless, it would be helpful to examine names with a greater range of difficulty levels in future studies.
A general limitation inherent in all studies of remote memory is the substantial individual variability in the time frame and context of the initial encoding of a person’s name or historical event, as well as the differences in frequency or recency of subsequent exposure. We carefully selected the recent and remote famous names based on recognition accuracy scores derived from a normative sample of young and older subjects. In this way, we could be confident that the remote names were not likely to have been updated during the recent time period, and recent famous names were likely to be recognized by people over the age of 65 (see Methods section for details). In this study, the memory age for remote famous names spanned the previous 35-40 years, whereas the names for the recent time period spanned the previous 10-year interval. It is possible that a more discrete breakdown of time epoch intervals would provide additional information about the nature of the TG. We also cannot dismiss the possibility that remote famous names carry greater AS than recent famous names. Interestingly, the high AS names used in the Westmacott & Moscovitch (2003) study, included persons with extended periods of fame over the 20th century (e.g., Adolf Hitler, Martin Luther King, Lucille Ball, and John Kennedy). None of these names would meet the criteria for the remote famous name category used in the current study. In addition, factors that tend to co-vary with memory age (frequency of exposure, age of initial exposure) may impact the extent of the observed TG (Leyhe et al., 2010). Finally, recognition accuracy was examined in the current study, but there are additional “levels” of semantic knowledge for person-identity (e.g., reason for fame, associative relationships, specific events or attributes) that reflect the richness of the memory representation. Further investigation examining access to details of knowledge in the person identity system may provide additional information about subtle disruption in semantic memory.
In summary, findings from this study suggest that separation of famous names into recent and remote time epochs may be quite informative in predicting the course of episodic memory in the pre-clinical phase. If these findings can be replicated and extended, several clinical implications may ensue. First, the examination of the person identity semantic memory system, a low-cost and easily available behavioral measure might provide a readily acquired early cognitive marker for assessing risk of cognitive decline in older individuals. It also could be used as an independent outcome measure used in clinical trial studies and combined with findings from neuroimaging techniques. In addition, investigation of the TG pattern for famous names across the continuum from health to disease, particularly when combined with neuroimaging techniques, may provide important data for current viewpoints on the interaction between the hippocampus and neocortical regions in long-term memory consolidation.
Figure 1.
Temporal gradient ratio for famous names calculated as (remote-recent/remote + recent). Higher scores indicates a greater proportion of remote names recognized compared to recent names.
| Recent | Remote |
|---|---|
| Pam Anderson | June Allyson |
| Tony Blair | Kitty Carlisle |
| George Clooney | Alan Funt |
| Rudolph Guilianni | Johnny Weissmuller |
| Jude Law | Jack Paar |
| Jennifer Lopez | Brigitte Bardot |
| Catherine Zeta-Jones | Leo Durocher |
| Winona Ryder | Gina Lollobrigida |
| Jessica Simpson | Alan Shepard |
| Linda Tripp | Mike Todd |
| David Koresh | Don Ameche |
| Gwyneth Paltrow | John Cameron Swayze |
| Barack Obama | Benny Goodman |
| Ben Affleck | Vincent Price |
| Justin Timberlake | Rex Harrison |
| Sammy Sosa | Walter Brennan |
| Jeffery Dahmer | Warren Spahn |
| Connie Chung | Vic Damone |
| Hilary Swank | Groucho Marx |
| Chelsea Clinton | Eddie Fisher |
| Jennifer Aniston | Mamie Van Doren |
| Kato Kaelin | Ernie Kovacs |
| Britney Spears | Sirhan Sirhan |
| Tiger Woods | Steve Allen |
| Hugh Grant | Jimmy Durante |
| Martha Stewart | Sid Caesar |
| Leonardo DiCaprio | Stan Musial |
| Paris Hilton | Gene Autry |
| Kobe Bryant | Adlai Stevenson |
| Kate Winslet | Lenny Bruce |
Acknowledgements
This project was supported by the National Institute on Aging (R01 AG022304) to SMR, Medical College of Wisconsin General Clinical Research Center (M01 RR00058), and the Medical College of Wisconsin Advancing a Healthier Wisconsin Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health.
Appendix 1. List of Recent and Remote Famous Names
References
- Ahmed S, Arnold R, Thompson SA, Graham KS, Hodges JR. Naming of objects, faces and buildings in mild cognitive impairment. Cortex. 2008;44(6):746–752. doi: 10.1016/j.cortex.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's and Dementia. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amieva H, Le Goff M, Millet X, Orgogozo JM, Peres K, Barberger-Gateau P, Jacqmin-Gadda H, Dartigues JF. Prodromal Alzheimer's disease: successive emergence of the clinical symptoms. Annals of Neurology. 2008;64(5):492–498. doi: 10.1002/ana.21509. [DOI] [PubMed] [Google Scholar]
- Backman L, Jones S, Berger AK, Laukka EJ, Small BJ. Cognitive impairment in preclinical Alzheimer's disease: a meta-analysis. Neuropsychology. 2005;19(4):520–531. doi: 10.1037/0894-4105.19.4.520. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Salmon DP, Butters N, Heindel WC, Granholm EL. Retrograde amnesia in patients with Alzheimer's disease or Huntington's disease. Neurobiology of Aging. 1988;9(2):181–186. doi: 10.1016/s0197-4580(88)80048-4. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzozero I, Lucchelli F, Saetti MC, Spinnler H. Mild cognitive impairment does entail retrograde amnesia for public events. Journal of Clinical and Experimental Neuropsychology. 2009;31(1):48, 56. doi: 10.1080/13803390801978864. [DOI] [PubMed] [Google Scholar]
- Blacker D, Lee H, Muzikansky A, Martin EC, Tanzi R, McArdle JJ, Moss M, Albert M. Neuropsychological measures in normal individuals that predict subsequent cognitive decline. Archives of Neurology. 2007;64(6):862–871. doi: 10.1001/archneur.64.6.862. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Salmon DP, Galasko D, Thomas RG, Thal LJ. Neuropsychological function and apolipoprotein E genotype in the preclinical detection of Alzheimer's disease. Psychology and Aging. 1999;14(2):295–303. doi: 10.1037//0882-7974.14.2.295. [DOI] [PubMed] [Google Scholar]
- Boyle PA, Buchman AS, Wilson RS, Kelly JF, Bennett DA. The APOE epsilon4 allele is associated with incident mild cognitive impairment among community-dwelling older persons. Neuroepidemiology. 2010;34(1):43–49. doi: 10.1159/000256662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretsky P, Guralnik JM, Launer L, Albert M, Seeman TE. The role of APOE-epsilon4 in longitudinal cognitive decline: MacArthur Studies of Successful Aging. Neurology. 2003;60(7):1077–1081. doi: 10.1212/01.wnl.0000055875.26908.24. [DOI] [PubMed] [Google Scholar]
- Bright P, Buckman J, Fradera A, Yoshimasu H, Colchester AC, Kopelman MD. Retrograde amnesia in patients with hippocampal, medial temporal, temporal lobe, or frontal pathology. Learning and Memory. 2006;13(5):545–557. doi: 10.1101/lm.265906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter SF, Caine D, Burns A, Herholz K, Lambon Ralph MA. Staging of the cognitive decline in Alzheimer's disease: insights from a detailed neuropsychological investigation of mild cognitive impairment and mild Alzheimer's disease. International Journal of Geriatric Psychiatry. 2012;27(4):423–432. doi: 10.1002/gps.2738. [DOI] [PubMed] [Google Scholar]
- Caselli RJ, Reiman EM, Osborne D, Hentz JG, Baxter LC, Hernandez JL, Alexander GG. Longitudinal changes in cognition and behavior in asymptomatic carriers of the APOE e4 allele. Neurology. 2004;62(11):1190–1995. doi: 10.1212/01.wnl.0000129533.26544.bf. [DOI] [PubMed] [Google Scholar]
- Chen P, Ratcliff G, Belle SH, Cauley JA, DeKosky ST, Ganguli M. Cognitive tests that best discriminate between presymptomatic AD and those who remain nondemented. Neurology. 2000;55(12):1847–1853. doi: 10.1212/wnl.55.12.1847. [DOI] [PubMed] [Google Scholar]
- Clague F, Graham KS, Thompson SA, Hodges JR. Is knowledge of famous people compromised in mild cognitive impairment? Cogn Behav Neurol. 2011;24(3):134–144. doi: 10.1097/WNN.0b013e318234315a. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Sperling RA, Hyman BT, Albert MS, Blacker D. Clinical prediction of Alzheimer disease dementia across the spectrum of mild cognitive impairment. Archives of General Psychiatry. 2007;64(12):1443–1450. doi: 10.1001/archpsyc.64.12.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douville K, Woodard JL, Seidenberg M, Miller SK, Leveroni CL, Nielson KA, Franczak M, Antuono P, Rao SM. Medial temporal lobe activity for recognition of recent and remote famous names: an event-related fMRI study. Neuropsychologia. 2005;43(5):693–703. doi: 10.1016/j.neuropsychologia.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Estevez-Gonzalez A, Garcia-Sanchez C, Boltes A, Otermin P, Pascual-Sedano B, Gironell A, Kulisevsky J. Semantic knowledge of famous people in mild cognitive impairment and progression to Alzheimer's disease. Dementia and Geriatric Cognitive Disorders. 2004;17(3):188–195. doi: 10.1159/000076355. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Greenberg DL, Verfaellie M. Interdependence of episodic and semantic memory: evidence from neuropsychology. Journal of the International Neuropsychological Society. 2010;16(5):748–753. doi: 10.1017/S1355617710000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene JD, Hodges JR. Identification of famous faces and famous names in early Alzheimer's disease. Relationship to anterograde episodic and general semantic memory. Brain. 1996;119(Pt 1):111–128. doi: 10.1093/brain/119.1.111. [DOI] [PubMed] [Google Scholar]
- Haist F, Bowden Gore J, Mao H. Consolidation of human memory over decades revealed by functional magnetic resonance imaging. Nature Neuroscience. 2001;4(11):1139–1145. doi: 10.1038/nn739. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Graham KS. A reversal of the temporal gradient for famous person knowledge in semantic dementia: implications for the neural organisation of long-term memory. Neuropsychologia. 1998;36(8):803–825. doi: 10.1016/s0028-3932(97)00126-7. [DOI] [PubMed] [Google Scholar]
- Jacova C, Peters KR, Beattie BL, Wong E, Riddehough A, Foti D, Scheltens P, Li DK, Feldman HH. Cognitive impairment no dementia - neuropsychological and neuroimaging characterization of an amnestic subgroup. Dementia and Geriatric Cognitive Disorders. 2008;25(3):238–247. doi: 10.1159/000115848. [DOI] [PubMed] [Google Scholar]
- Johnson DK, Storandt M, Morris JC, Galvin JE. Longitudinal study of the transition from healthy aging to Alzheimer disease. Archives of Neurology. 2009;66(10):1254–1259. doi: 10.1001/archneurol.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert S, Brambati SM, Ansado J, Barbeau EJ, Felician O, Didic M, Lacombe J, Goldstein R, Chayer C, Kergoat MJ. The cognitive and neural expression of semantic memory impairment in mild cognitive impairment and early Alzheimer's disease. Neuropsychologia. 2010;48(4):978–988. doi: 10.1016/j.neuropsychologia.2009.11.019. [DOI] [PubMed] [Google Scholar]
- Jurica P, Leitten C, Mattis S. Psychological Assessment Resources; Odessa, FL: 2001. Dementia Rating Scale-2 (DRS-2) [Google Scholar]
- Leyhe T, Muller S, Eschweiler GW, Saur R. Deterioration of the memory for historic events in patients with mild cognitive impairment and early Alzheimer's disease. Neuropsychologia. 2010;48(14):4093–4101. doi: 10.1016/j.neuropsychologia.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Lu PH, Thompson PM, Leow A, Lee GJ, Lee A, Yanovsky I, Parikshak N, Khoo T, Wu S, Geschwind D, Bartzokis G. Apolipoprotein E genotype is associated with temporal and hippocampal atrophy rates in healthy elderly adults: a tensor-based morphometry study. Journal of Alzheimer's Disease. 2011;23(3):433–442. doi: 10.3233/JAD-2010-101398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay DG, Stewart R, Burke DM. H.M. revisited: relations between language comprehension, memory, and the hippocampal system. Journal of Cognitive Neuroscience. 1998;10(3):377–394. doi: 10.1162/089892998562807. [DOI] [PubMed] [Google Scholar]
- Manns JR, Hopkins RO, Squire LR. Semantic memory and the human hippocampus. Neuron. 2003;38(1):127–133. doi: 10.1016/s0896-6273(03)00146-6. [DOI] [PubMed] [Google Scholar]
- Mattis S. Psychological Assessment Resources; Odessa, FL: 1988. Dementia Rating Scale (DRS) [Google Scholar]
- Mickes L, Wixted JT, Fennema-Notestine C, Galasko D, Bondi MW, Thal LJ, Salmon DP. Progressive impairment on neuropsychological tasks in a longitudinal study of preclinical Alzheimer's disease. Neuropsychology. 2007;21(6):696–705. doi: 10.1037/0894-4105.21.6.696. [DOI] [PubMed] [Google Scholar]
- Nielson KA, Douville KL, Seidenberg M, Woodard JL, Miller SK, Franczak M, Antuono P, Rao SM. Age-related functional recruitment for famous name recognition: an event-related fMRI study. Neurobiology of Aging. 2006;27(10):1494–1504. doi: 10.1016/j.neurobiolaging.2005.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nature Reviews Neuroscience. 2007;8(12):976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Raber J, Huang Y, Ashford JW. ApoE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiology of Aging. 2004;25(5):641–650. doi: 10.1016/j.neurobiolaging.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Reed JM, Squire LR. Retrograde amnesia for facts and events: findings from four new cases. Journal of Neuroscience. 1998;18(10):3943–3954. doi: 10.1523/JNEUROSCI.18-10-03943.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A. Presses Universitaires de France; Paris, France: 1958. L’examin clinique en psychologie. [Google Scholar]
- Ribot T. London: 1906. Diseases of memory: An essay in positive psychology. [Google Scholar]
- Ryan L, Cox C, Hayes SM, Nadel L. Hippocampal activation during episodic and semantic memory retrieval: comparing category production and category cued recall. Neuropsychologia. 2008;46(8):2109–2121. doi: 10.1016/j.neuropsychologia.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar HJ, Cohen NJ, Sullivan EV, Corkin S, Growdon JH. Remote memory function in Alzheimer's disease and Parkinson's disease. Brain. 1988;111(Pt 1):185–206. doi: 10.1093/brain/111.1.185. [DOI] [PubMed] [Google Scholar]
- Sartori G, Lombardi L. Semantic relevance and semantic disorders. Journal of Cognitive Neuroscience. 2004;16(3):439–452. doi: 10.1162/089892904322926773. [DOI] [PubMed] [Google Scholar]
- Saunders AM, Hulette O, Welsh-Bohmer KA, Schmechel DE, Crain B, Burke JR, Alberts MJ, Strittmatter WJ, Breitner JC, Rosenberg C. Specificity, sensitivity, and predictive value of apolipoprotein-E genotyping for sporadic Alzheimer's disease. Lancet. 1996;348(9020):90–93. doi: 10.1016/s0140-6736(96)01251-2. [DOI] [PubMed] [Google Scholar]
- Schmolck H, Stefanacci L, Squire LR. Detection and explanation of sentence ambiguity are unaffected by hippocampal lesions but are impaired by larger temporal lobe lesions. Hippocampus. 2000;10(6):759–770. doi: 10.1002/1098-1063(2000)10:6<759::AID-HIPO1013>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Seidenberg M, Guidotti L, Nielson KA, Woodard JL, Durgerian S, Antuono P, Zhang Q, Rao SM. Semantic memory activation in individuals at risk for developing Alzheimer disease. Neurology. 2009a;73(8):612–620. doi: 10.1212/WNL.0b013e3181b389ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenberg M, Guidotti L, Nielson KA, Woodard JL, Durgerian S, Zhang Q, Gander A, Antuono P, Rao SM. Semantic knowledge for famous names in mild cognitive impairment. Journal of the International Neuropsychological Society. 2009b;15(1):9–18. doi: 10.1017/S1355617708090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon S, Moscovitch M. The nature and time-course of medial temporal lobe contributions to semantic retrieval: An fMRI study on verbal fluency. Hippocampus. 2011 doi: 10.1002/hipo.20985. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Martin A. The anterior temporal lobes and the functional architecture of semantic memory. Journal of the International Neuropsychological Society. 2009;15(5):645–649. doi: 10.1017/S1355617709990348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CN, Squire LR. Medial temporal lobe activity during retrieval of semantic memory is related to the age of the memory. Journal of Neuroscience. 2009;29(4):930–938. doi: 10.1523/JNEUROSCI.4545-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaan PE, Raaijmakers JG, Jonker C. Early assessment of dementia: the contribution of different memory components. Neuropsychology. 2005;19(5):629–640. doi: 10.1037/0894-4105.19.5.629. [DOI] [PubMed] [Google Scholar]
- Squire LR, Bayley PJ. The neuroscience of remote memory. Current Opinion in Neurobiology. 2007;17(2):185–196. doi: 10.1016/j.conb.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugarman MA, Woodard JL, Nielson KA, Seidenberg M, Smith JC, Durgerian S, Rao SM. Functional magnetic resonance imaging of semantic memory as a presymptomatic biomarker of Alzheimer's disease risk. Biochimica et Biophysica Acta. 2012;1822(3):442–456. doi: 10.1016/j.bbadis.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twamley EW, Ropacki SA, Bondi MW. Neuropsychological and neuroimaging changes in preclinical Alzheimer's disease. Journal of the International Neuropsychological Society. 2006;12(5):707–735. doi: 10.1017/S1355617706060863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westmacott R, Moscovitch M. The contribution of autobiographical significance to semantic memory. Memory and Cognition. 2003;31(5):761–774. doi: 10.3758/bf03196114. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Leurgans SE, Boyle PA, Bennett DA. Cognitive decline in prodromal Alzheimer disease and mild cognitive impairment. Archives of Neurology. 2011;68(3):351–356. doi: 10.1001/archneurol.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Barnes LL, Beckett LA, Aggarwal NT, Cochran EJ, Berry-Kravis E, Bach J, Fox JH, Evans DA, Bennett DA. The apolipoprotein E epsilon 4 allele and decline in different cognitive systems during a 6-year period. Archives of Neurology. 2002;59(7):1154–1160. doi: 10.1001/archneur.59.7.1154. [DOI] [PubMed] [Google Scholar]
- Winocur G, Moscovitch M. Memory transformation and systems consolidation. Journal of the International Neuropsychological Society. 2011;17(5):766–780. doi: 10.1017/S1355617711000683. [DOI] [PubMed] [Google Scholar]
- Wong C, Gallate J. The function of the anterior temporal lobe: a review of the empirical evidence. Brain Research. 2012;1449:94–116. doi: 10.1016/j.brainres.2012.02.017. [DOI] [PubMed] [Google Scholar]
- Woodard JL, Seidenberg M, Nielson KA, Antuono P, Guidotti L, Durgerian S, Zhang Q, Lancaster M, Hantke N, Butts A, Rao SM. Semantic memory activation in amnestic mild cognitive impairment. Brain. 2009;132(Pt 8):2068–2078. doi: 10.1093/brain/awp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodard JL, Seidenberg M, Nielson KA, Smith JC, Antuono P, Durgerian S, Guidotti L, Zhang Q, Butts A, Hantke N, Lancaster M, Rao SM. Prediction of cognitive decline in healthy older adults using fMRI. Journal of Alzheimer's Disease. 2010;21(3):871–885. doi: 10.3233/JAD-2010-091693. [DOI] [PMC free article] [PubMed] [Google Scholar]