Abstract
Background
Alterations in the circadian arterial pressure (AP) rhythm predict cardiovascular mortality. We examined the circadian AP rhythm and the effect of renin-angiotensin system blockade in congenic mRen2.Lewis hypertensive rats, a renin-dependent model of hypertension derived from the backcross of transgenic hypertensive [mRen-2]27 rats with Lewis normotensive ones.
Methods
Twenty nine mRen2.Lewis hypertensive rats were randomly assigned to drink tap-water (vehicle; n=9), valsartan (30 mg/kg/day; n=10) or valsartan (30mg/kg/day) combined with aliskiren given s.c. (50 mg/kg/day; n=10) for two weeks treatment. AP, heart rate, and locomotive activity were recorded with chronically implanted radiotelemetry probes. The awake/asleep ratio was calculated as (awake mean AP (MAP) mean – asleep MAP mean)/ (awake MAP mean) × 100. Plasma renin activity (PRA) and concentration (PRC), and plasma and kidney angiotensin II (Ang II) were measured by radioimmunoassays.
Results
Untreated hypertensive rats showed an inverse AP rhythm, higher at day and lower at night, accompanied by normal rhythms of heart rate and locomotive activity. Treatment with valsartan or aliskiren/valsartan normalized both the elevated AP and the AP rhythm with the combination therapy being more effective both in reducing MAP and in restoring the awake/asleep ratio. While PRA and PRC increased with the treatments, addition of aliskiren to valsartan partially reversed the increases in plasma Ang II levels while both valsartan and aliskiren/valsartan markedly reduced renal cortical content of Ang II.
Conclusion
The altered circadian AP rhythm in this renin-dependent hypertension model uncovers a significant role of Ang II on the desynchronization of the circadian rhythm among AP, heart rate, and locomotive activity.
Keywords: angiotensin II, aliskiren, blood pressure, circadian rhythm, direct renin inhibitors, hypertension, valsartan
Introduction
Among the various biological rhythms contributing to normal bodily functions, disturbances in the 24-hour day/night-activity/rest cycle is considered a key chronobiologic factor predisposing to many medical conditions. In most healthy people, the blood pressure biological rhythm is characterized by subjects exhibiting highest levels of arterial pressure from late morning to middle afternoon. A physiologic decline in blood pressure of at least 10% from daytime values occurs during sleep. Both the 24-hour mean level and amplitude of the blood pressure rhythm may be altered in hypertensive subjects including suppression of the normal decreases in arterial pressure during sleep. The loss of a nocturnal fall in blood pressure is associated with increased risk of sleep-apnea, chronic kidney disease, insulin resistance, atherosclerosis, stroke, left ventricular hypertrophy, congestive heart failure, and myocardial infarction (Cuspidi et al., 2004; Della et al., 2005; Fagard et al., 2009; Higashi et al., 2002; Ingelsson et al., 2006; Ohkubo et al., 2002).
Although the brain regulates the mechanisms affecting the “biological clocks”, the neuronal pathways determining the intrinsic activity of these clocks can be altered by extrinsic hormonal and environmental factors. In human hypertension, alterations in sympathetic nerve activity and the renin-angiotensin system (RAS) contribute to loss of the normal pattern of the blood pressure fluctuations. An absence of a blood pressure fall during sleep (non-dippers) has been recorded in African-American hypertensive subjects (Viera et al., 2011) while the presence of non-dipping hypertension has been shown to correlate with target organ damage and increased cardiovascular morbidity and mortality (Cuspidi et al., 2004; Fagard et al., 2009; Hermida et al., 2010; Ohkubo et al., 2002). Early studies in experimental models of hypertension demonstrated that sinoaortic denervation in dogs was associated with increases in nocturnal blood pressure (Ferrario et al., 1969) that in baroreceptor-denervated cats were linked to loss of the carotic chemoreceptor (Baccelli et al., 1976; Del et al., 1985; Guazzi et al., 1968; Kumazawa et al., 1969). The neurogenic form of experimental hypertension induced by chronic administration of subpressor doses of angiotensin II (Ang II) in the dog was also associated with increased blood pressure variability and episodic rises in arterial pressure during sleep (McCubbin et al., 1965). Although the data implicates an interaction among baroreceptor reflexes, Ang II, and sympathetic nerve activity in accounting for the disruption of the blood pressure circadian rhythm, limited information exists as to whether blockade of Ang II activity is associated with restoration of the alterations in the blood pressure rhythm.
In rodents, as in humans, the blood pressure circadian profile correlates with the activity periods but the peak changes in blood pressure and heart rate in this species occurs within the night period given the nocturnal behavior and feeding characteristics of these animals. Studies in a transgenic model of renin-dependent hypertension created by insertion of the mouse Ren-2 gene into the rat genome (the [mRen-2]27 transgenic hypertensive rat (Lee et al., 1996)) showed a reversal of the blood pressure circadian rhythm characterized by higher blood pressure during the day compared to the night (Lemmer et al., 1993; Lemmer et al., 2005). The inversion of the blood pressure circadian rhythm was associated with maintenance of higher heart rates and locomotive activity at night (Lemmer et al., 1993; Lemmer et al., 2005). The dysregulation of the blood pressure circadian rhythm in these m[Ren2]27 transgenic hypertensive rats suggest a critical role of tissue RAS in affecting the neurohormonal mechanisms of the brain biological clock. The brain expresses all of the components of the RAS, and Ang II exists within the suprachiasmatic and hypothalamic nuclei containing the central pacemaker for the biological clocks (Moriguchi et al., 1994a; Moriguchi et al., 1994b; Senanayake et al., 1994; Thomas et al., 2004a; Thomas et al., 2004b). Thomas et al. (Thomas et al., 2004a; Thomas et al., 2004b) concluded that the presence of Ang II immunoreactivity at the luminal face of brain suprachiasmatic parenchyma blood vessels in rats provides a route for actions of circulating Ang II to influence neuronal circuits regulating biological rhythms. The existence of sites where the blood-brain barrier is permeable to the actions of Ang II has been demonstrated previously [see (Ferrario et al., 1972; Ferrario et al., 1979) for review].
The introduction of an orally active direct renin inhibitor for the treatment of hypertension (Ferrario, 2010; Hollenberg, 2002; Ichihara et al., 2010) has provided a prospect to confine the increase in circulating renin and its activity in response to monotherapy with an angiotensin converting enzyme (ACE) inhibitor or an angiotensin II type 1 (AT1) receptor blocker. An additional benefit of renin inhibition results from the blunting of the increase in circulating Ang II due to monotherapy with an AT1 receptor blocker which reportedly limits the blood pressure lowering effects of these antihypertensive agents. The Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET) study revealed that combination of ACE inhibitor and AT1 receptor blocker is not superior to monotherapy of each of them both in reducing blood pressure and preventing organ damage (Yusuf et al., 2008). Therefore, it is important to find other effective combination of RAS blockade.
We developed a congenic model of mRen2.Lewis hypertensive rats, a strain which, derived from the original parent m[Ren2]27 transgenic hypertensive rats, does not share in the genetic variability found in the parent hypertensive rats originally outbred from Sprague Dawley and Wistar Kyoto rats. We reported that the mRen2.Lewis rat exhibits hypertension and target organ injuries equivalent to that found in the original m[Ren2]27 transgenic hypertensive strain (Chappell et al., 2003; Jessup et al., 2006; Jessup et al., 2009). In this study, we describe the effects of combined direct renin inhibition and AT1 receptor blockade in the regulation of the blood pressure oscillations and the levels of Ang II in the blood and kidney of this new model of renin-dependent hypertension.
Methods
Animals
Experiments were conducted in 29 male mRen2.Lewis rats of 10 weeks of age obtained from the Hypertension and Vascular Research Center Congenic colony of Wake Forest University. This congenic strain was developed by our program from the backcross of the original m[Ren2]27 transgenic hypertensive rat with normotensive Lewis rats obtained from Charles River (Wilmington, MA) (Groban et al., 2008; Jessup et al., 2010). All animal procedures were performed in accordance with National Institutes of Health (NIH) guidelines and were approved by the Wake Forest University animal care and use committees. Rats were housed in individual cages under a 12h light (6:00–18:00) −12h darkness (18:00-6:00) cycle, at a constant humidity and temperature, with free access to standard laboratory rat chaw and drinking water.
Arterial pressure, heart rate and locomotive activity measurements
Systolic (SBP), diastolic (DBP), mean arterial pressure (MAP), pulse pressure (PP), heart rate (HR) and locomotive activity were assessed by chronically implanted telemetry probes in conscious rats moving freely in their home cages. Telemetry probes were inserted at 10 weeks of age, as described previously (Schnell and Wood, 1993). Briefly, pressure transmitters were implanted into the peritoneal cavity under aseptic conditions and anesthesia (inhalation of 2% isoflurane). The sensor catheter was placed in the aorta below the renal artery pointing upstream. Before surgery rats were administered 150 mg/kg ampicillin, 0.05 mg/kg buprenorphine subcutaneously and 0.3 mg/kg atropine intramuscularly. Rats were allowed to recover for two weeks prior to the start of experimentation. Rats were then transferred to the quiet telemetry monitoring room where they remained free to move around. Three days of control measurements (C1-C3) were followed by 14 days of treatment (T1-T14). During this period, blood pressure, heart rate and locomotive activity were measured and recorded every 15 minutes throughout the 24 h cycle.
Telemetry data were recorded and digitalized using the Dataquest A.R.T.™ System (version 4.3, Datascience Inc). Each animal was sampled for 10 seconds at 15-minute intervals for a period of 17 days. All of recordings were averaged per 12 hours and per 24 hours. Baseline values were calculated using the data from C1 to C3. Post treatment values were calculated using the data from T12 to T14. The changes of mean arterial pressure were calculated as the difference between averages of C1 to C3 and T12 to T14. Awake/asleep ratio (A/S ratio) (O'Brien et al., 1988) was defined as the percent decrease in MAP during the hours of daytime rest (6:00–18:00) relative to the mean MAP obtained during the hours of nighttime activity (18:00-6:00) and calculated as (awake MAP mean – asleep MAP mean)/ (awake MAP mean)× 100.
Treatment protocol
Rats were randomized into three groups at 12 weeks of age: (a) vehicle group, (b) monotherapy with valsartan (30 mg/kg/day, per os, via drinking water); and (c) combination therapy with valsartan (30 mg/kg/day) and the direct renin inhibitor (DRI; aliskiren fumarate, 50 mg/kg/day, s.c.). Aliskiren was delivered by osmotic mini-pumps (ALZET Model 2M2L) implanted in anesthetized rats (inhalation anesthesia, 2% isoflurane) under aseptic conditions on day T1. The pumps containing saline as vehicle were implanted to vehicle and valsartan groups. Rats were put in metabolic cages during C2-C3 and T13-T14 to collect urine. Urine was collected at day C3 and T14. After two weeks of treatment, animals were euthanized by decapitation, and trunk blood and kidney cortex were collected and processed as previously described (Ferrario et al., 2005a; Ferrario et al., 2005b; Jessup et al., 2006). The dose of aliskiren employed in these experiments was shown before to reverse hypertension and cardiac hypertrophy in m[Ren2]27 transgenic hypertensive rats (Habibi et al., 2008; Whaley-Connell et al., 2008; Whaley-Connell et al., 2010; Whaley-Connell et al., 2011). Valsartan and aliskiren were a kind gift from Novartis, Inc.
Biochemistry
Plasma renin activity (PRA), defined as the rate of angiotensin I (Ang I) generation from endogenous substrate, was measured in incubated plasma treated with EDTA and PMSF to prevent the degradation of the generated peptide. The Ang I was quantitated by radioimmunoassay (RIA) (Diosarin Corp, Stillwater, MN, USA). Plasma renin concentration (PRC) was defined as the rate of Ang I generation from renin in the sample incubated at pH 6.5 for 90 min with excess exogenous substrate obtained from nephrectomized rat plasma. The Ang I generated in the sample was quantified by RIA (Diosarin Corp, Stillwater, MN, USA). Ang II levels in plasma and renal tissue were measured by radioimmunoassay (RIA) as described elsewhere (Ferrario et al., 2005a; Ferrario et al., 2005b; Jessup et al., 2006). The minimum detectable level of the Ang II assay was 0.76 fmol/mL. The intra- and interassay coefficients of variability for Ang II were 12% and 22%, respectively.
Statistical analysis
All data are expressed as mean ± SEM. Laboratory data were analyzed by one way analysis of variance (ANOVA) followed by the Turkey’s post hoc test. Changes in mean arterial pressure, heart rate and awake/asleep ratio were analyzed by two way ANOVA followed by the Bonferroni post-test. Other hemodynamic data were analyzed by one way ANOVA followed by the Turkey’s post hoc test. Values of P<0.05 were considered statistically significant.
Results
Hemodynamics
Hemodynamic variables at baseline in each of the treatment groups are presented in Table 1. There are no significant differences in the mean arterial pressure, systolic and diastolic blood pressure, pulse pressure and heart rate of three groups prior to initiation of the treatment regimens. Figure 1 (panels a, b, and c) shows the circadian pattern of mean arterial pressure, heart rate and locomotive activity in all mRen2.Lewis hypertensive rats prior to initiation of treatments (C3). Heart rate and locomotive activity showed normal circadian pattern associated with activities levels (higher in nighttime and lower in daytime) (Figure 1b and 1c). On the other hand, mean arterial pressure showed an inverse circadian pattern, higher in daytime and lower in nighttime in all congenic mRen2.Lewis hypertensive rats (Figure 1a). Figure 2a shows the average 24 h mean arterial pressure at baseline (C1 to C3) and at the end of the study (T12 to T14) in mRen2.Lewis rats medicated with vehicle, valsartan, or the combination therapy. There were no significant differences in 24 h mean arterial pressure in the three groups at baseline. However, after treatment, mean arterial pressure was reduced by valsartan (107 ± 3 mm Hg, p<0.001 vs. 177 ± 3 mm Hg in vehicle) and further reduced in rats receiving the combination therapy (91 ± 1 mm Hg, p < 0.001).
Table 1.
Baseline Hemodynamic Values Prior to Commencement of Treatment in mRen2.Lewis Hypertensive Rats.
| Variable |
Vehicle (n = 9) |
Valsartan (n = 10) |
Combination (n = 10) |
|---|---|---|---|
| Systolic arterial pressure, mm Hg | |||
| 24 h average | 202 ± 4 | 203 ± 2 | 203 ± 4 |
| Diurnal average | 206 ± 4 | 207 ± 2 | 208 ± 4 |
| Nocturnal average | 198 ± 3 | 200 ± 2 | 199 ± 3 |
| Diastolic arterial pressure, mm Hg | |||
| 24 h average | 146 ± 3 | 144 ± 2 | 143 ± 3 |
| Diurnal average | 150 ± 4 | 149 ± 2 | 147 ± 3 |
| Nocturnal average | 141 ± 2 | 140 ± 2 | 138 ± 2 |
| Mean arterial pressure, mm Hg | |||
| 24 h average | 173 ± 3 | 173 ± 2 | 172 ± 3 |
| Diurnal average | 177 ± 4 | 178 ± 2 | 177 ± 3 |
| Nocturnal average | 169 ± 2 | 169 ± 2 | 168 ± 3 |
| Pulse pressure, mm Hg | |||
| 24 h average | 56 ± 4 | 59 ± 2 | 60 ± 3 |
| Diurnal average | 56 ± 4 | 58 ± 2 | 60 ± 3 |
| Nocturnal average | 56 ± 4 | 60 ± 2 | 61 ± 3 |
| Heart Rate, beats/min | |||
| 24 h average | 357 ± 4 | 366 ± 4 | 360 ± 2 |
| Diurnal average | 331 ± 5 | 335 ± 5 | 329 ± 3 |
| Nocturnal average | 383 ± 6 | 396 ± 4 | 390 ± 2 |
| Awake/asleep ratio, (%) | −5 ± 1 | −5 ± 1 | −5 ± 1 |
Values are means ± SEM. Diurnal averages are values between 6:00 and 18:00 h. Nocturnal averages are values between 18:00 and 6:00 h.
Figure 1.
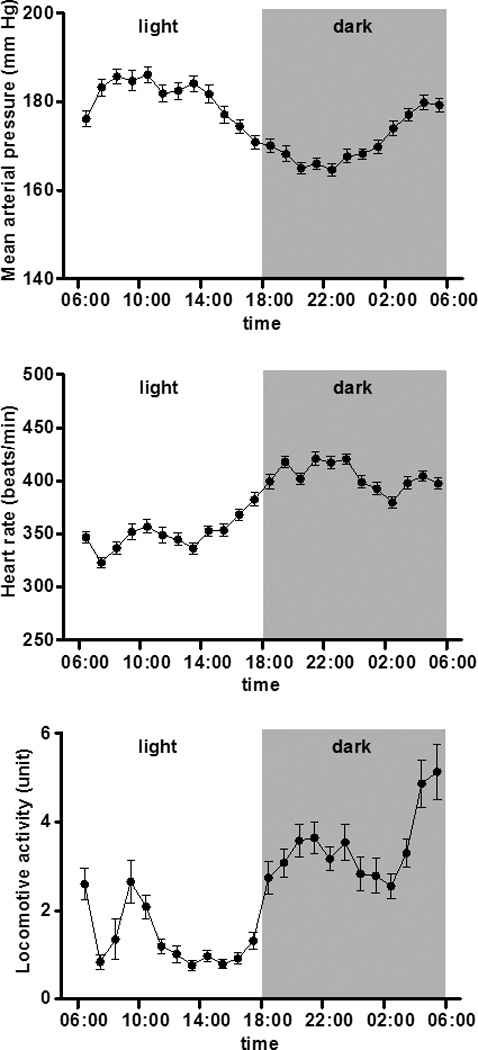
Hourly averages in mean arterial pressure, heart rate, and locomotive activity document a desynchronization of the arterial pressure rhythm - higher in daytime and lower in nighttime- in mRen2.Lewis hypertensive rats at baseline (C3). Heart rate and locomotive activity maintain a normal circadian pattern associated with activities levels (higher in nighttime and lower in daytime).
Figure 2.
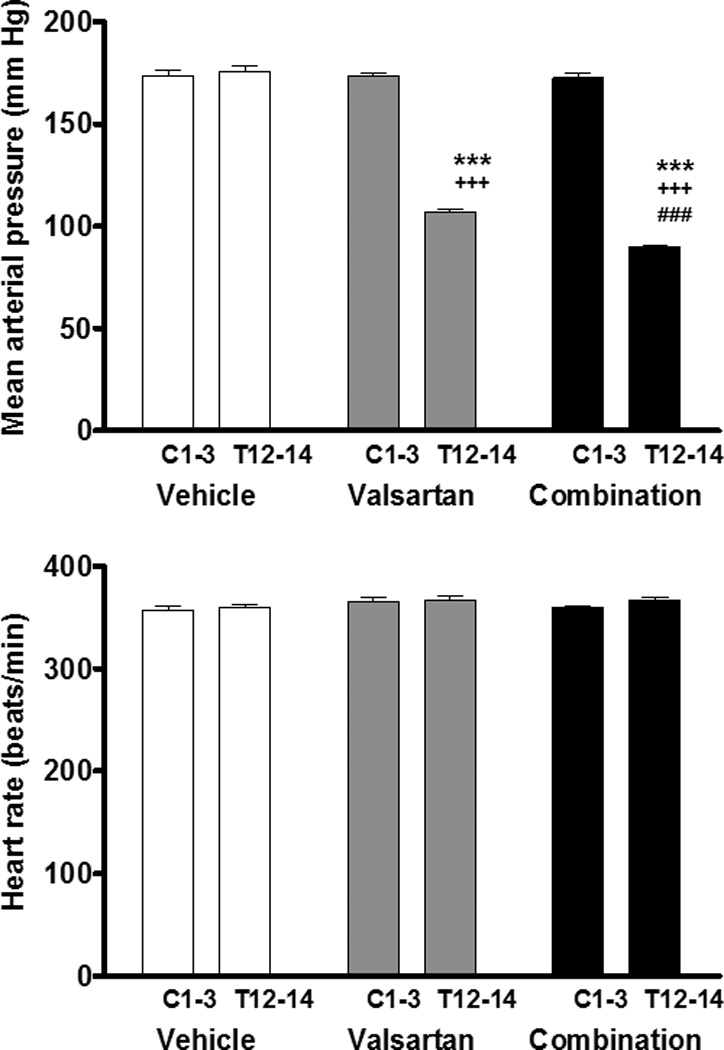
Changes in mean arterial pressure and heart rate in the three groups. There are no significant differences of mean arterial pressure in three groups at baseline (C1–C3). However, after treatment (T12–T14), mean arterial pressure was reduced by valsartan and further reduced by the addition of aliskiren to the valsartan treatment. There were no significant differences of heart rate in three groups both at baseline (C1–C3) and after treatment (T12–T14). ***: P<0.001 vs. C1 to C3, +++: P<0.001 vs. Vehicle, ###: P<0.001 vs. Valsartan.
Figure 2b shows the 24 h heart rate at baseline (C1 to C3) and at the end of the study (T12 to T14). There were no significant differences in average 24 h heart rate in the three groups both at baseline and at the end of the study. These findings indicate that both valsartan and combination therapy with valsartan and aliskiren reduced blood pressure without affecting heart rate and locomotive activities although the combination therapy was significantly more effective in reducing blood pressure than valsartan alone in mRen2.Lewis rats.
Urinary sodium and potassium excretion
Table 2 shows the 24 hours urinary sodium and potassium excretion of each group at baseline and after treatment. There were no significant differences in these variables in the three groups both at baseline and after treatment.
Table 2.
Urinary sodium and potassium excretion
| Variable |
Vehicle (n = 8) |
Valsartan (n = 10) |
Combination (n = 10) |
|---|---|---|---|
| Urinary sodium (mmol/24h) | |||
| Baseline (C3) | 1.84 ± 0.13 | 1.89 ± 0.08 | 2.06 ± 0.17 |
| After treatment (T14) | 1.75 ± 0.17 | 2.06 ± 0.11 | 2.02 ± 0.12 |
| Urinary potassium (mmol/24h) |
|||
| Baseline (C3) | 4.81 ± 0.23 | 4.69 ± 0.12 | 4.96 ± 0.42 |
| After treatment (T14) | 4.86 ± 0.24 | 5.21 ± 0.24 | 5.15 ± 0.21 |
Values are means ± SEM.
Effects of treatment on circadian rhythms in mRen2.Lewis rats
Figure 3 (panels a, b, and c) shows the circadian patterns of mean arterial pressure, heart rate and locomotive activity in three groups at the day T14. The antihypertensive effect of both valsartan and valsartan combined with aliskiren in mRen2.Lewis rats was associated with a marked diminution of the circadian blood pressure variability found before treatment and the disappearance of the inverse blood pressure rhythm (Figure 3a). The circadian patterns of heart rate and locomotive activity in the three groups were similar to those at baseline and not different among the three groups (Figure 3b and 3c).
Figure 3.
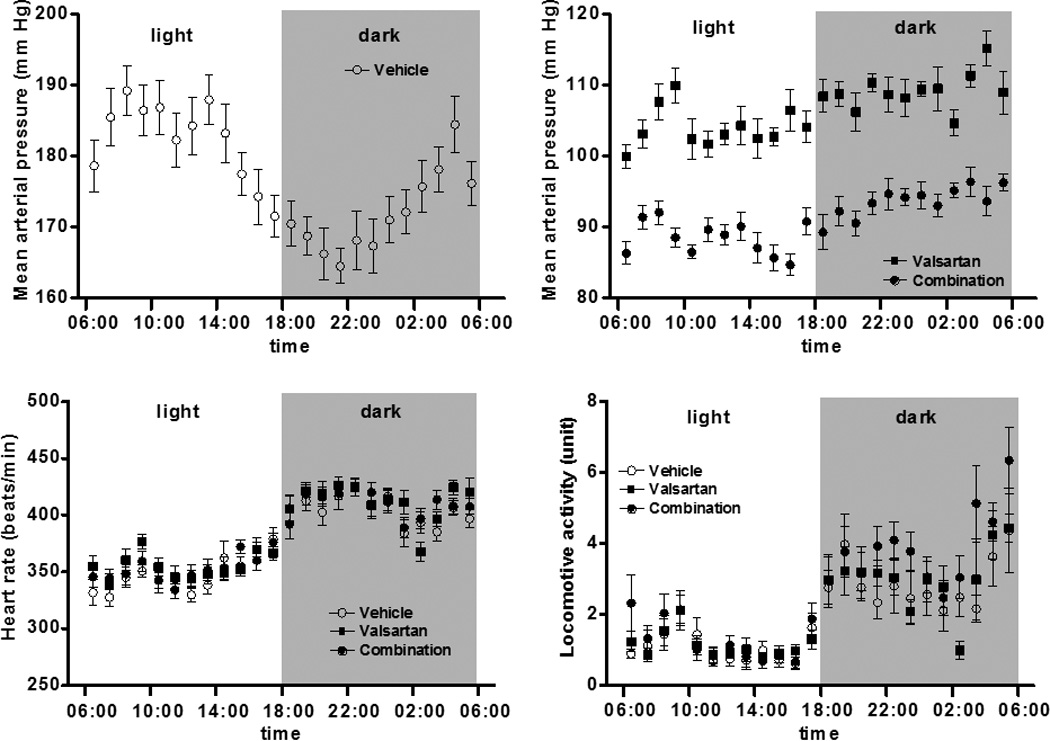
Circadian rhythms in three groups after treatment (T14). The circadian rhythm of mean arterial pressure in the vehicle-treated group exhibits an inverse rhythm (panel a) which is essentially normalized in the mRen2.Lewis rats medicated with either valsartan or the combination of valsartan/aliskiren (panel b). The circadian rhythms of heart rate (panel c) and locomotive activity (panel d) are not different from vehicle-treated rats.
As shown in Figure 4a, valsartan reduced mean blood pressure more during daytime (−78 ± 2 mm Hg) than in nighttime (−62 ± 13 mm Hg, p < 0.05) while in the animals medicated with combination of valsartan and aliskiren the reductions in mean arterial pressure averaged −93 ± 2 mm Hg vs. −77 ± 27 mm Hg, p<0.01, respectively. On the other hand, there was no significant difference in the change in mean arterial pressure between day and night in vehicle treated rats (3 ± 2 mm Hg vs. 2 ± 2 mm Hg). Changes in awake/asleep ratios in the three groups are shown in Figure 4b. At baseline (C3), awake/asleep ratios in all groups were negative. The ratios in both valsartan and combination groups turned positive at day T14 of treatment although the ratio in vehicle was still negative. These differences in the awake/asleep ratio between vehicle and mRen2.Lewis rats medicated with valsartan or valsartan/aliskiren were already revealed at day T5 (data not shown). Furthermore, the awake/asleep ratio of the combination group is significantly higher than that of the valsartan monotherapy group (6.19 ± 0.33 % vs. 3.74 ± 0.55 %, P<0.05, respectively).
Figure 4.
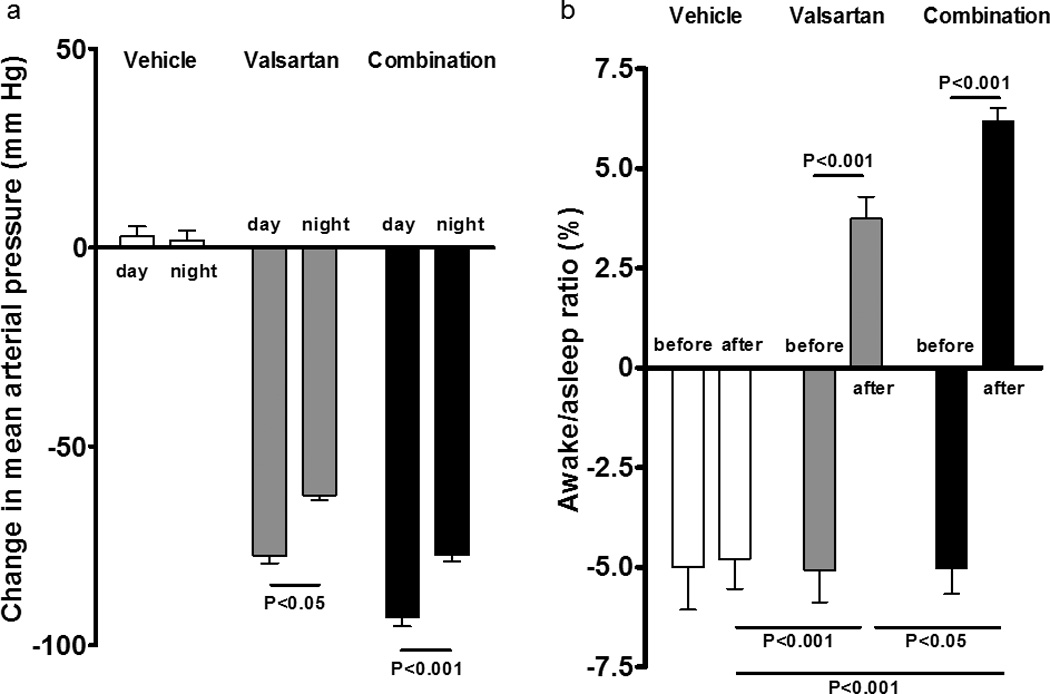
Day-night difference of changes in mean arterial pressure and the awake/asleep ratio in three groups. a) There was no significant difference of changes of mean arterial pressure between day and night in vehicle group. However, both valsartan and combination treatments reduced mean blood pressure more in daytime than in nighttime; b) At baseline (C3), awake/asleep ratios in all groups were negative. The ratios in both valsartan and combination groups turned positive at the day T14 although the ratio in vehicle was still negative.
Effects of treatment on Renin Activity and Ang II Levels
Table 3 and Figure 5 show the effects of the treatments on PRA and Ang II plasma and renal tissue concentrations. Monotherapy with valsartan or the combination of valsartan and aliskiren increased PRA compared to vehicle (7.54 ± 1.22 ng/mL/h in valsartan, P<0.001 vs 1.82 ± 0.33 ng/mL/h in vehicle, 9.64 ± 4.40 ng/mL/h in combination group, P<0.0001 vs vehicle). There was no significant difference in PRA between valsartan and combination groups. Although valsartan monotherapy tended to increase PRC compared to vehicle (56.60 ± 12.50 ng/mL/h vs 18.80 ± 1.71 ng/mL/h), this difference was not statistically significant. PRC in mRen2.Lewis rats medicated with the combined therapy was much higher than the values found in either vehicle-treated or valsartan-treated rats (260.2 ± 23.6 ng/mL/h, P<0.0001 vs vehicle, P<0.0001 vs valsartan). In contrast, the PRA/PRC ratio was not different in mRen2.Lewis rats given valsartan (0.14 ± 0.02) and the vehicle (0.10 ± 0.02) while the PRA/PRC ratio was significantly lower in mRen2.Lewis rats medicated with aliskiren/valsartan (0.04 ± 0.01, P<0.05 vs vehicle, P<0.0001 vs valsartan). Likewise, the addition of aliskiren to rats medicated with valsartan blunted the increases in plasma Ang II levels (74.9 ± 9.2 fmol/mL, p<0.01 vs 28.8 ± 4.9 fmol/mL in vehicle) induced by blockade of AT1 receptors. Figure 5 also shows the effect of the treatments on kidney cortex Ang II. As shown in Figure 5b, both monotherapy with valsartan and combination therapy significantly reduced renal Ang II levels. Figure 6 shows that there was a robust and negative correlation between awake/asleep ratio and kidney Ang II (r2=0.64, P<0.0001).
Table 3.
Effects of treatment on PRA, PRC and PRA/PRC
| Variable |
Vehicle (n = 8) |
Valsartan (n = 9) |
Combination (n = 9) |
|---|---|---|---|
| PRA (ng/ml/h) | 1.82 ± 0.35 | 7.54 ±1.22** | 9.64 ± 1.48*** |
| PRC (ng/ml/h) | 18.8 ± 1.7 | 56.6 ± 12.5 | 260.2 ± 23.6***,+++ |
| PRA/PRC ratio | 0.10 ± 0.02 | 0.14 ± 0.02 | 0 .04 ± 0.01*,+++ |
Values are means ± SEM.
P<0.05 vs. Vehicle,
P<0.01 vs. Vehicle,
P<0.001 vs. Vehicle,
P<0.001 vs. Valsartan
Figure 5.
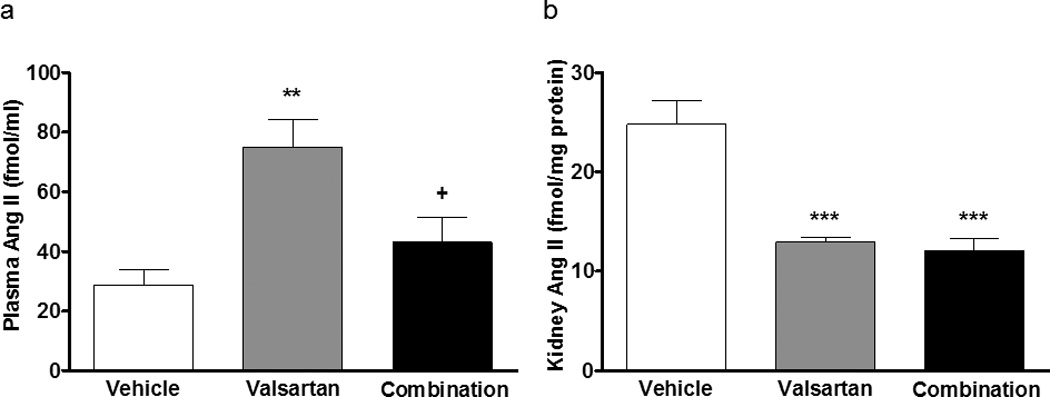
Effects of treatment on plasma and kidney Ang II. a) The addition of aliskiren to rats medicated with valsartan blunted the increases in plasma Ang II levels induced by blockade of AT1 receptors while both treatments reduce renal Ang II levels. b) Both treatments reduced kidney Ang II levels.
Figure 6.
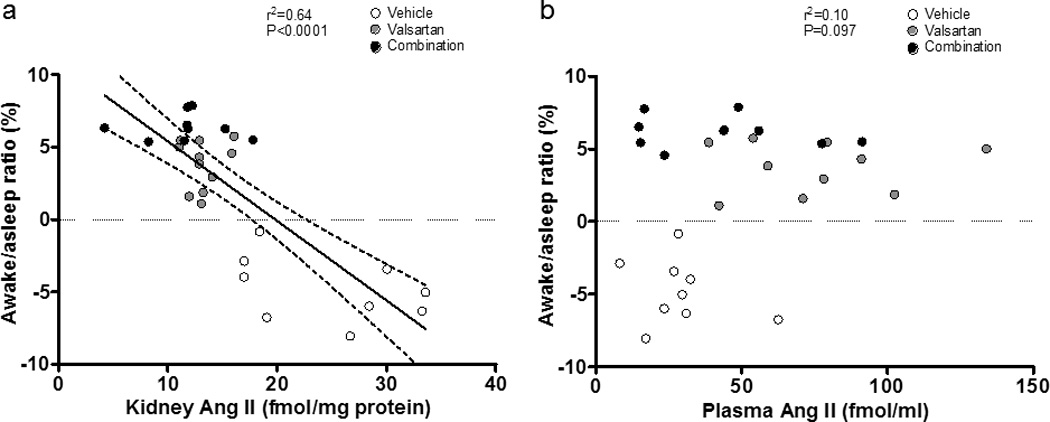
Correlation between kidney or plasma Ang II level and awake/asleep ratio. a) There was negative correlation between awake/asleep ratio and kidney Ang II. b) There was no significant correlation between awake/asleep ratio and plasma Ang II.
Discussion
The present study demonstrated that congenic mRen2.Lewis hypertensive rat derived from the backcross of m[Ren2]27 transgenic hypertensive rat with the normotensive Lewis rat has are characterized by a shift of the peak blood pressure elevation from the nocturnal activity phase of the diurnal cycle into the resting day-light phase. The inversion of the blood pressure rhythm in these congenic mRen2.Lewis hypertensive rats’ results in a desynchronization of the 24 h blood pressure variability compared to the cycling changes of the heart rate and locomotive activity. We further demonstrate that the shift in the blood pressure circadian rhythm is normalized by blockade of the actions of Ang II by monotherapy with the AT1 receptor blocker, valsartan or by combination therapy of valsartan and DRI, aliskiren. Moreover, the data documents that the combination therapy is superior to blockade of AT1 receptors in the normalization of the blood pressure and in restoring the blood pressure circadian rhythm. The marked reductions in plasma Ang II achieved with the mixture of valsartan/aliskiren are associated with more pronounced effects on correcting the inversion of the blood pressure circadian rhythm.
Although m[Ren2]27 transgenic hypertensive rats are an established model for hypertension studies (Brosnihan et al., 1994; Lee et al., 1996; Lemmer et al., 1993; Lemmer et al., 2005; Monosikova et al., 2007; Moriguchi et al., 1994a; Moriguchi et al., 1994b; Moriguchi et al., 1995; Pons et al., 1996; Senanayake et al., 1994; Witte and Lemmer, 1999), the development of fulminant hypertension at an earlier age and the non-homogenous genetic background from which these rats were outbred has constrained the usefulness of this model for the extrapolation of identified mechanisms to human hypertension. The creation of a congenic model of increased tissue renin transgene expression derived from the backcross of the original m[Ren2]27 transgenic hypertensive rat with Lewis normotensive rats has markedly reduced the occurrence of fulminant hypertension while retaining main features of the model such as sex differences in the magnitude of blood pressure elevation, target organ damage and renal injury, normalization of blood pressure and reversal of cardiac hypertrophy by blockade of Ang II expression or activity, and changes in cardiac and renal tissue expression of RAS components (Chappell et al., 2008; Jessup et al., 2009; Pendergrass et al., 2006; Pendergrass et al., 2008). In the pursuit of the further characterization of this experimental model of hypertension we now show that mRen2.Lewis hypertensive rats retain the parent trait of an inverse blood pressure circadian rhythm, uncoupled from the circadian oscillations in heart rate and motility.
The inclusion of an experimental arm combining AT1 receptor blockade with suppression of renin activity was designed to exclude the possibility that the large increases in plasma Ang II associated with blockade of AT1 receptors may still be capable of maintaining the altered blood pressure rhythm through incomplete blockade of Ang II actions in the brain due to low penetrability of the antagonist or Ang II action on AT2 receptors. The demonstration that both the antihypertensive response and the degree of restoration of the awake/sleep ratios were significantly greater in mRen2.Lewis rats medicated with valsartan/aliskiren demonstrates an incomplete suppression of Ang II activity in rats given valsartan alone. Whether this greater effect of the combination therapy resulted from the decrease in circulating Ang II levels, as shown in the current experiments, or an additional effect of aliskiren in the brain remains to be investigated. Nevertheless, the more robust effect of the combination of valsartan/aliskiren in restoring the circadian rhythm of the blood pressure shows that the desynchronization of the blood pressure rhythm in terms of both heart rate and locomotive activity is primarily driven by Ang II.
As discussed above, the antihypertensive effect of valsartan in mRen2.Lewis rats was further potentiated in the presence of aliskiren. These data agree with the observation in humans of a greater antihypertensive action of the combined administration of these agents (Oparil et al., 2007). Although aliskiren is associated with decreases in PRA in humans (Azizi et al., 2007; Krone et al., 2011; Moriyama et al., 2011; Nussberger et al., 2007; Stanton et al., 2009), we found that in mRen2.Lewis rats blood pressure normalization with the dual combination therapy was associated with increases in both PRA and PRC. Although aliskiren is less specific in inhibiting rat renin, the dose of aliskiren employed here was shown before to elicit significant antihypertensive effects in m[Ren2]27 transgenic hypertensive rats that were associated with reversal or cardiac hypertrophy, improved insulin resistance, and reduced renal oxidative stress (Habibi et al., 2008; Lastra et al., 2009; Whaley-Connell et al., 2008; Whaley-Connell et al., 2010; Whaley-Connell et al., 2011). The finding that in our experiments the addition of aliskiren resulted in decreases in blood pressure greater than those obtained with valsartan alone provided further evidence that aliskiren neutralized the reactive increase in renin produced by blockade of AT1 receptors. These data agree with the concomitant finding that the addition of aliskiren to valsartan was associated with blunting of the increases in plasma Ang II concentrations resulting from the administration of valsartan. Previous studies in normal healthy volunteers and essential hypertensive subjects showed that aliskiren is accompanied by large increases in PRC (Jordan et al., 2007; Smith et al., 2007; Verdecchia et al., 2008), a measure of the amount of renin protein present in the sample (Nussberger et al., 2007; Sealey and Laragh, 2007). On the other hand, PRA measures the rate of conversion of angiotensinogen to Ang I by renin (Sealey, 1991). The characteristics of the procedures utilized in the assessment of PRA in the rat assay may cause dissociation of aliskiren from renin in the sample. This will need further investigation.
In SHR, chronic elevations in arterial pressure do not result in changes in the circadian rhythm of blood pressure, heart rate, and motility when compared to normotensive Wistar-Kyoto and Sprague-Dawley rats (Lemmer et al., 1993). Importantly, an inversion of the blood pressure circadian rhythm but not of heart rate and locomotive activity results from the chronic administration of Ang II in Sprague Dawley rats (Baltatu et al., 2001) while administration of enalapril in m[Ren2]27 transgenic hypertensive rats is associated with normalization of the blood pressure circadian rhythm (Lemmer et al., 1994) underlining the important contributory role of RAS in the desynchronization of the blood pressure rhythm.
An important factor influencing the circadian rhythm of blood pressure is the daily fluctuation in renal function, such as renal blood flow, glomerular filtration rate (GFR), and the excretion of electrolytes (Stow and Gumz, 2011). In this regard, Pons et al. reported that m[Ren2]27 transgenic hypertensive rats have a normal circadian pattern of urinary water and electrolytes excretion, and of GFR despite having an inverse circadian rhythm of the blood pressure. In agreement with those studies we found that the 24 hours sodium and potassium excretion rates in mRen2.Lewis rats were not changed by the treatments.
The first demonstration of a reversal of the awake/sleep ratio associated with reduced renal Ang II content by either valsartan or valsartan-aliskiren combination provides a new and novel clue for the unraveling of the mechanism accounting for the control of the circadian blood pressure oscillations. We showed previously increased content of Ang II associated with downregulation of AT1 receptors in the kidneys of mRen2.Lewis hypertensive rats (Pendergrass et al., 2006). These data suggest that the reduction of renal Ang II content may be a contributing mechanism to the normalization of the blood pressure circadian rhythm most likely related to a decrease in neural input from renal afferent nerves reaching either the brainstem or the hypothalamus. Renal afferent nerves provide information to both hypothalamic structures and the nucleus of the solitary tract (nTS) region of the dorsomedial medulla oblongata known to be involved in the regulation of arterial pressure and fluid balance (Calaresu and Ciriello, 1981; Ciriello and Calaresu, 1980; Felder, 1986). Moreover, selective removal of renal afferent nerves attenuates the development of deoxycorticosterone acetate–salt hypertension (Oparil et al., 1987) while bilateral rhizotomy delayed development of two-kidney two-clip (2K-2C) hypertension and prevented vascular hypertrophy; contents of norepinephrine and epinephrine in the medulla oblongata, adrenal gland and plasma were all decreased, whereas hypothalamic catecholamine content was increased (Wang et al., 1995). One possible site for the inversion of the blood pressure circadian rhythm may be the suprachiasmatic nuclei (SCN) of the hypothalamus, the site for the generation of neuronal and hormonal regulation of 24 h body cycles. Although ablation of the SCN abolished the inversion of the blood pressure rhythmicity in m[Ren2]27 transgenic rats, the loss was also associated with disappearance of the heart rate and locomotive day/night oscillations (Witte et al., 1998; Witte and Lemmer, 1999). These data agrees with the findings of Janssen et al. (Janssen et al., 1994) who showed that the circadian rhythmicity in blood pressure, heart rate, and food intake were abolished in SCN-lesioned rats. These findings indicate that other anatomical sites within the central nervous system may contribute to the selective desynchronization of the blood pressure rhythm.
Master switches that in the brain regulate the activity of biological clocks are influenced by the intrinsic and extrinsic activities of neurotransmitters and neuromodulators expressed by the autonomic system and RAS. The interplay between the RAS and sympathetic nervous system, both peripherally and centrally, is recognized to play a critical role in the evolution of hypertension in part due to the fact that Ang II has a major influence in the regulation of central and peripheral vasomotor tone (Ferrario et al., 1972; Ferrario, 1983; Ferrario et al., 1990) as well as acting within the nTS to inhibit baroreceptor reflexes (Averill et al., 1987; Diz et al., 1997). An age-dependent altered brain and adrenal gland norepinephrine/epinephrine content and turnover has been reported in m[Ren2]27 transgenic rats with loss of increased cardiac norepinephrine turnover rate in the dark phase (Lemmer et al., 2005). Since the rhythm of the circadian variations in heart rate and locomotive activity do not occur in either m[Ren2]27 transgenic rats (Lemmer et al., 1993) or in our experiments in mRen2.Lewis hypertensive rats, a direct loss of sympathetic nerve activity may not explain our findings. On the other hand, normalization of the circadian blood pressure rhythm in mRen2.Lewis rats following blockade of Ang II receptors or the combined inhibition of Ang II activity and renin suggest a deterministic role of the peptide in the desynchronization of the central control of arterial pressure, heart rate and locomotive activity. While our experiments did not explore the site and specific mechanism accounting for these changes, both increased brain and circulating Ang II may contribute. All RAS components exist in the brain and circulating Ang II exerts powerful modulatory influences on brain vasomotor centers through its actions on circumventricular organs (Ferrario, 1983; Ferrario et al., 1987; Ferrario et al., 1990). In that regard, an increased plasma Ang II in response to valsartan monotherapy was abolished by addition of aliskiren uncovering the constrain of elevated plasma Ang II not only on blood pressure lowering effect but also on reversal of the awake/sleep ratio in valsartan treated rats. Further studies are warrant to examine whether the superior restoration of the awake/sleep ratio by the combined administration of an Ang II receptor blockers and renin inhibitors correlates with better control of target organ damage and reduced cardiovascular morbidity and mortality.
In summary, we showed that blockade of the actions of Ang II in a renin-dependent model of experimental hypertension results in the resynchronization of the circadian rhythm of arterial pressure with respect to the accompanying circadian variations in heart rate and locomotive activity. The observation that this reversal of the blood pressure circadian rhythm was associated with decreases in the renal content of Ang II suggest that local tissue actions of Ang II may modulate renal afferent traffic activity to brainstem and hypothalamic circuits regulating the activity of the circadian blood pressure rhythm. Moreover, the greater effects of the combined approach to blockade of Ang II actions, as demonstrated by blood pressure normalization, restoration of the blood pressure circadian rhythm, and the corresponding awake/sleep ratio when compared to monotherapy with valsartan alone are new findings that need to be further investigated as they may lead to a further understanding of the mechanisms associated with blood pressure dysregulation and an ultimate better management of hypertensive target organ injuries and reduced cardiovascular morbidity and mortality.
Acknowledgments
Sources of funding
This research was supported by an unrestricted research grant provided by Novartis, Inc. and the National Heart, Lung and Blood Institutes of the National Institutes of Health (2PO1 HL-051952 and R01 HL-56973). We also acknowledge partial support provided by the Farley-Hudson Foundation, Jacksonville, NC.
Footnotes
Conflict of interests
The work reported here was supported by an unrestricted research grant provided by Novartis, Inc. to Carlos M Ferrario, MD. The sponsor had no direct input on either the design of the study, data analysis and interpretation, as well as the writing of the paper.
References
- 1.Averill DB, Diz DI, Barnes KL, Ferrario CM. Pressor responses of angiotensin II microinjected into the dorsomedial medulla of the dog. Brain Res. 1987;414:294–300. doi: 10.1016/0006-8993(87)90009-6. [DOI] [PubMed] [Google Scholar]
- 2.Azizi M, Menard J, Bissery A, Guyene TT, Bura-Riviere A. Hormonal and hemodynamic effects of aliskiren and valsartan and their combination in sodium-replete normotensive individuals. Clin J Am Soc Nephrol. 2007;2:947–955. doi: 10.2215/CJN.00360107. [DOI] [PubMed] [Google Scholar]
- 3.Baccelli G, Albertini R, Mancia G, Zanchetti A. Interplay of sino-aortic reflexes and haemodynamic changes during natural sleep in the cat. Clin Sci Mol Med Suppl. 1976;3:373s–375s. doi: 10.1042/cs051373s. [DOI] [PubMed] [Google Scholar]
- 4.Baltatu O, Janssen BJ, Bricca G, Plehm R, Monti J, Ganten D, et al. Alterations in blood pressure and heart rate variability in transgenic rats with low brain angiotensinogen. Hypertension. 2001;37:408–413. doi: 10.1161/01.hyp.37.2.408. [DOI] [PubMed] [Google Scholar]
- 5.Brosnihan KB, Moriguchi A, Nakamoto H, Dean RH, Ganten D, Ferrario CM. Estrogen augments the contribution of nitric oxide to blood pressure regulation in transgenic hypertensive rats expressing the mouse Ren-2 gene. Am J Hypertens. 1994;7:576–582. doi: 10.1093/ajh/7.7.576. [DOI] [PubMed] [Google Scholar]
- 6.Calaresu FR, Ciriello J. Renal afferent nerves affect discharge rate of medullary and hypothalamic single units in the cat. J Auton Nerv Syst. 1981;3:311–320. doi: 10.1016/0165-1838(81)90072-2. [DOI] [PubMed] [Google Scholar]
- 7.Chappell MC, Gallagher PE, Averill DB, Ferrario CM, Brosnihan KB. Estrogen or the AT1 antagonist olmesartan reverses the development of profound hypertension in the congenic mRen2. Lewis rat. Hypertension. 2003;42:781–786. doi: 10.1161/01.HYP.0000085210.66399.A3. [DOI] [PubMed] [Google Scholar]
- 8.Chappell MC, Westwood BM, Yamaleyeva LM. Differential effects of sex steroids in young and aged female mRen2.Lewis rats: a model of estrogen and salt-sensitive hypertension. Gend Med. 2008;(5 Suppl A):S65–S75. doi: 10.1016/j.genm.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciriello J, Calaresu FR. Hypothalamic projections of renal afferent nerves in the cat. Can J Physiol Pharmacol. 1980;58:574–576. doi: 10.1139/y80-095. [DOI] [PubMed] [Google Scholar]
- 10.Cuspidi C, Meani S, Salerno M, Valerio C, Fusi V, Severgnini B, et al. Cardiovascular target organ damage in essential hypertensives with or without reproducible nocturnal fall in blood pressure. J Hypertens. 2004;22:273–280. doi: 10.1097/00004872-200402000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Del BA, Baccelli G, Cellina G, Fea F, Ferrari A, Zanchetti A. Carotid sinus reflexes during postural changes, naturally elicited fighting behaviour, and phases of sleep in the cat. Cardiovasc Res. 1985;19:762–769. doi: 10.1093/cvr/19.12.762. [DOI] [PubMed] [Google Scholar]
- 12.Della MP, Lupia M, Bandolin V, Guzzon S, Sonino N, Vettor R, et al. Adiponectin, insulin resistance, and left ventricular structure in dipper and nondipper essential hypertensive patients. Am J Hypertens. 2005;18:30–35. doi: 10.1016/j.amjhyper.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 13.Diz DI, Falgui B, Bosch SM, Westwood BM, Kent J, Ganten D, et al. Hypothalamic substance P release. Attenuated angiotensin responses in mRen2(27) transgenic rats. Hypertension. 1997;29:510–513. doi: 10.1161/01.hyp.29.1.510. [DOI] [PubMed] [Google Scholar]
- 14.Fagard RH, Thijs L, Staessen JA, Clement DL, De Buyzere ML, De Bacquer DA. Night-day blood pressure ratio and dipping pattern as predictors of death and cardiovascular events in hypertension. J Hum Hypertens. 2009;23:645–653. doi: 10.1038/jhh.2009.9. [DOI] [PubMed] [Google Scholar]
- 15.Felder RB. Excitatory and inhibitory interactions among renal a cardiovascular afferent nerves in dorsomedial medulla. Am J Physiol. 1986;250:R580–R588. doi: 10.1152/ajpregu.1986.250.4.R580. [DOI] [PubMed] [Google Scholar]
- 16.Ferrario CM. Neurogenic actions of angiotensin II. Hypertension. 1983;5:V73–V79. doi: 10.1161/01.hyp.5.6_pt_3.v73. [DOI] [PubMed] [Google Scholar]
- 17.Ferrario CM. Addressing the theoretical and clinical advantages of combination therapy with inhibitors of the renin-angiotensin-aldosterone system: antihypertensive effects and benefits beyond BP control. Life Sci. 2010;86:289–299. doi: 10.1016/j.lfs.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrario CM, Barnes KL, Block CH, Brosnihan KB, Diz DI, Khosla MC, et al. Pathways of angiotensin formation and function in the brain. Hypertension. 1990;15:I13–I19. doi: 10.1161/01.hyp.15.2_suppl.i13. [DOI] [PubMed] [Google Scholar]
- 19.Ferrario CM, Barnes KL, Diz DI, Block CH, Averill DB. Role of area postrema pressor mechanisms in the regulation of arterial pressure. Can J Physiol Pharmacol. 1987;65:1591–1597. doi: 10.1139/y87-250. [DOI] [PubMed] [Google Scholar]
- 20.Ferrario CM, Barnes KL, Szilagyi JE, Brosnihan KB. Physiological and pharmacological characterization of the area postrema pressor pathways in the normal dog. Hypertension. 1979;1:235–245. doi: 10.1161/01.hyp.1.3.235. [DOI] [PubMed] [Google Scholar]
- 21.Ferrario CM, Gildenberg PL, McCubbin JW. Cardiovascular effects of angiotensin mediated by the central nervous system. Circ Res. 1972;30:257–262. doi: 10.1161/01.res.30.3.257. [DOI] [PubMed] [Google Scholar]
- 22.Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005a;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 23.Ferrario CM, Jessup J, Gallagher PE, Averill DB, Brosnihan KB, Ann TE, et al. Effects of renin-angiotensin system blockade on renal angiotensin-(1–7) forming enzymes and receptors. Kidney Int. 2005b;68:2189–2196. doi: 10.1111/j.1523-1755.2005.00675.x. [DOI] [PubMed] [Google Scholar]
- 24.Ferrario CM, McCubbin JW, Page IH. Hemodynamic characteristics of chronic experimental neurogenic hypertension in unanesthetized dogs. Circ Res. 1969;24:911–922. doi: 10.1161/01.res.24.6.911. [DOI] [PubMed] [Google Scholar]
- 25.Groban L, Yamaleyeva LM, Westwood BM, Houle TT, Lin M, Kitzman DW, et al. Progressive diastolic dysfunction in the female mRen(2). Lewis rat: influence of salt and ovarian hormones. J Gerontol A Biol Sci Med Sci. 2008;63:3–11. doi: 10.1093/gerona/63.1.3. [DOI] [PubMed] [Google Scholar]
- 26.Guazzi M, Baccelli G, Zanchetti A. Reflex chemoceptive regulation of arterial pressure during natural sleep in the cat. Am J Physiol. 1968;214:969–978. doi: 10.1152/ajplegacy.1968.214.5.969. [DOI] [PubMed] [Google Scholar]
- 27.Habibi J, Whaley-Connell A, Hayden MR, DeMarco VG, Schneider R, Sowers SD, et al. Renin inhibition attenuates insulin resistance, oxidative stress, and pancreatic remodeling in the transgenic Ren2 rat. Endocrinology. 2008;149:5643–5653. doi: 10.1210/en.2008-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hermida RC, Ayala DE, Mojon A, Fernandez JR. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiol Int. 2010;27:1629–1651. doi: 10.3109/07420528.2010.510230. [DOI] [PubMed] [Google Scholar]
- 29.Higashi Y, Nakagawa K, Kimura M, Noma K, Hara K, Sasaki S, et al. Circadian variation of blood pressure and endothelial function in patients with essential hypertension:a comparison of dippers and non-dippers. J Am Coll Cardiol. 2002;40:2039–2043. doi: 10.1016/s0735-1097(02)02535-4. [DOI] [PubMed] [Google Scholar]
- 30.Hollenberg NK. Angiotensin II suppression in humans by the orally active renin inhibitor aliskiren (SPP100). Comparison with enalapril. Curr Hypertens Rep. 2002;4:269–270. [PubMed] [Google Scholar]
- 31.Ichihara A, Sakoda M, Kurauchi-Mito A, Narita T, Kinouchi K, Bokuda K, et al. New approaches to blockade of the renin-angiotensin-aldosterone system: characteristics and usefulness of the direct renin inhibitor aliskiren. J Pharmacol Sci. 2010;113:296–300. doi: 10.1254/jphs.10r04fm. [DOI] [PubMed] [Google Scholar]
- 32.Ingelsson E, Bjorklund-Bodegard K, Lind L, Arnlov J, Sundstrom J. Diurnal blood pressure pattern and risk of congestive heart failure. JAMA. 2006;295:2859–2866. doi: 10.1001/jama.295.24.2859. [DOI] [PubMed] [Google Scholar]
- 33.Janssen BJ, Tyssen CM, Duindam H, Rietveld WJ. Suprachiasmatic lesions eliminate 24-h blood pressure variability in rats. Physiol Behav. 1994;55:307–311. doi: 10.1016/0031-9384(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 34.Jessup JA, Gallagher PE, Averill DB, Brosnihan KB, Tallant EA, Chappell MC, et al. Effect of angiotensin II blockade on a new congenic model of hypertension derived from transgenic Ren-2 rats. Am J Physiol Heart Circ Physiol. 2006;291:H2166–H2172. doi: 10.1152/ajpheart.00061.2006. [DOI] [PubMed] [Google Scholar]
- 35.Jessup JA, Lindsey SH, Wang H, Chappell MC, Groban L. Attenuation of salt-induced cardiac remodeling and diastolic dysfunction by the GPER agonist G-1 in female mRen2.Lewis rats. PLoS One. 2010;5:e15433. doi: 10.1371/journal.pone.0015433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jessup JA, Westwood BM, Chappell MC, Groban L. Dual ACE-inhibition and AT1 receptor antagonism improves ventricular lusitropy without affecting cardiac fibrosis in the congenic mRen2.Lewis rat. Ther Adv Cardiovasc Dis. 2009;3:245–257. doi: 10.1177/1753944709338489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jordan J, Engeli S, Boye SW, Le BS, Keefe DL. Direct Renin inhibition with aliskiren in obese patients with arterial hypertension. Hypertension. 2007;49:1047–1055. doi: 10.1161/HYPERTENSIONAHA.106.084301. [DOI] [PubMed] [Google Scholar]
- 38.Krone W, Hanefeld M, Meyer HF, Jung T, Bartlett M, Yeh CM, et al. Comparative efficacy and safety of aliskiren and irbesartan in patients with hypertension and metabolic syndrome. J Hum Hypertens. 2011;25:186–195. doi: 10.1038/jhh.2010.38. [DOI] [PubMed] [Google Scholar]
- 39.Kumazawa T, Baccelli G, Guazzi M, Mancia G, Zanchetti A. Hemodynamic patterns during desynchronized sleep in intact cats and in cats with sinoaortic deafferentation. Circ Res. 1969;24:923–927. doi: 10.1161/01.res.24.6.923. [DOI] [PubMed] [Google Scholar]
- 40.Lastra G, Habibi J, Whaley-Connell AT, Manrique C, Hayden MR, Rehmer J, et al. Direct renin inhibition improves systemic insulin resistance and skeletal muscle glucose transport in a transgenic rodent model of tissue renin overexpression. Endocrinology. 2009;150:2561–2568. doi: 10.1210/en.2008-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee MA, Bohm M, Paul M, Bader M, Ganten U, Ganten D. Physiological characterization of the hypertensive transgenic rat TGR(mREN2)27. Am J Physiol. 1996;270:E919–E929. doi: 10.1152/ajpendo.1996.270.6.E919. [DOI] [PubMed] [Google Scholar]
- 42.Lemmer B, Mattes A, Bohm M, Ganten D. Circadian blood pressure variation in transgenic hypertensive rats. Hypertension. 1993;22:97–101. doi: 10.1161/01.hyp.22.1.97. [DOI] [PubMed] [Google Scholar]
- 43.Lemmer B, Schiffer S, Witte K, Gorbey S. Inverse blood pressure rhythm of transgenic hypertensive TGR(mREN2)27 rats: role of norepinephrine and expression of tyrosine-hydroxylase and reuptake1-transporter. Chronobiol Int. 2005;22:473–488. doi: 10.1081/CBI-200062360. [DOI] [PubMed] [Google Scholar]
- 44.Lemmer B, Witte K, Makabe T, Ganten D, Mattes A. Effects of enalaprilat on circadian profiles in blood pressure and heart rate of spontaneously and transgenic hypertensive rats. J Cardiovasc Pharmacol. 1994;23:311–314. [PubMed] [Google Scholar]
- 45.McCubbin JW, DeMoura RS, Page IH, Olmsted F. Arterial hypertension elicited by subpressor amounts of angiotensin. Science. 1965;149:1394–1395. doi: 10.1126/science.149.3690.1394. [DOI] [PubMed] [Google Scholar]
- 46.Monosikova J, Herichova I, Mravec B, Kiss A, Zeman M. Effect of upregulated renin-angiotensin system on per2 and bmal1 gene expression in brain structures involved in blood pressure control in TGR(mREN-2)27 rats. Brain Res. 2007;1180:29–38. doi: 10.1016/j.brainres.2007.08.061. [DOI] [PubMed] [Google Scholar]
- 47.Moriguchi A, Brosnihan KB, Kumagai H, Ganten D, Ferrario CM. Mechanisms of hypertension in transgenic rats expressing the mouse Ren-2 gene. Am J Physiol. 1994a;266:R1273–R1279. doi: 10.1152/ajpregu.1994.266.4.R1273. [DOI] [PubMed] [Google Scholar]
- 48.Moriguchi A, Ferrario CM, Brosnihan KB, Ganten D, Morris M. Differential regulation of central vasopressin in transgenic rats harboring the mouse Ren-2 gene. Am J Physiol. 1994b;267:R786–R791. doi: 10.1152/ajpregu.1994.267.3.R786. [DOI] [PubMed] [Google Scholar]
- 49.Moriguchi A, Tallant EA, Matsumura K, Reilly TM, Walton H, Ganten D, et al. Opposing actions of angiotensin-(1–7) and angiotensin II in the brain of transgenic hypertensive rats. Hypertension. 1995;25:1260–1265. doi: 10.1161/01.hyp.25.6.1260. [DOI] [PubMed] [Google Scholar]
- 50.Moriyama T, Tsuruta Y, Kojima C, Itabashi M, Sugiura H, Takei T, et al. Beneficial effect of aliskiren combined with olmesartan in reducing urinary protein excretion in patients with chronic kidney disease. Int Urol Nephrol. 2011 doi: 10.1007/s11255-011-9991-0. [DOI] [PubMed] [Google Scholar]
- 51.Nussberger J, Gradman AH, Schmieder RE, Lins RL, Chiang Y, Prescott MF. Plasma renin and the antihypertensive effect of the orally active renin inhibitor aliskiren in clinical hypertension. Int J Clin Pract. 2007;61:1461–1468. doi: 10.1111/j.1742-1241.2007.01473.x. [DOI] [PubMed] [Google Scholar]
- 52.O'Brien E, Sheridan J, O’Malley K. Dippers and non-dippers. Lancet. 1988;2:397. doi: 10.1016/s0140-6736(88)92867-x. [DOI] [PubMed] [Google Scholar]
- 53.Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens. 2002;20:2183–2189. doi: 10.1097/00004872-200211000-00017. [DOI] [PubMed] [Google Scholar]
- 54.Oparil S, Sripairojthikoon W, Wyss JM. The renal afferent nerves in the pathogenesis of hypertension. Can J Physiol Pharmacol. 1987;65:1548–1558. doi: 10.1139/y87-244. [DOI] [PubMed] [Google Scholar]
- 55.Oparil S, Yarows SA, Patel S, Fang H, Zhang J, Satlin A. Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: a randomised, double-blind trial. Lancet. 2007;370:221–229. doi: 10.1016/S0140-6736(07)61124-6. [DOI] [PubMed] [Google Scholar]
- 56.Pendergrass KD, Averill DB, Ferrario CM, Diz DI, Chappell MC. Differential expression of nuclear AT1 receptors and angiotensin II within the kidney of the male congenic mRen2. Lewis rat. Am J Physiol Renal Physiol. 2006;290:F1497–F1506. doi: 10.1152/ajprenal.00317.2005. [DOI] [PubMed] [Google Scholar]
- 57.Pendergrass KD, Pirro NT, Westwood BM, Ferrario CM, Brosnihan KB, Chappell MC. Sex differences in circulating and renal angiotensins of hypertensive mRen(2). Lewis but not normotensive Lewis rats. Am J Physiol Heart Circ Physiol. 2008;295:H10–H20. doi: 10.1152/ajpheart.01277.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pons M, Schnecko A, Witte K, Lemmer B, Waterhouse JM, Cambar J. Circadian rhythms in renal function in hypertensive TGR(mRen-2)27 rats and their normotensive controls. Am J Physiol. 1996;271:R1002–R1008. doi: 10.1152/ajpregu.1996.271.4.R1002. [DOI] [PubMed] [Google Scholar]
- 59.Schnell CR, Wood JM. Measurement of blood pressure and heart rate by telemetry in conscious, unrestrained marmosets. Am J Physiol. 1993;264:H1509–H1516. doi: 10.1152/ajpheart.1993.264.5.H1509. [DOI] [PubMed] [Google Scholar]
- 60.Sealey JE. Plasma renin activity and plasma prorenin assays. Clin Chem. 1991;37:1811–1819. [PubMed] [Google Scholar]
- 61.Sealey JE, Laragh JH. Aliskiren, the first renin inhibitor for treating hypertension: reactive renin secretion may limit its effectiveness. Am J Hypertens. 2007;20:587–597. doi: 10.1016/j.amjhyper.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 62.Senanayake PD, Moriguchi A, Kumagai H, Ganten D, Ferrario CM, Brosnihan KB. Increased expression of angiotensin peptides in the brain of transgenic hypertensive rats. Peptides. 1994;15:919–926. doi: 10.1016/0196-9781(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 63.Smith TR, Philipp T, Vaisse B, Bakris GL, Wernsing M, Yen J, et al. Amlodipine and valsartan combined and as monotherapy in stage 2, elderly, and black hypertensive patients: subgroup analyses of 2 randomized, placebo-controlled studies. J Clin Hypertens (Greenwich ) 2007;9:355–364. doi: 10.1111/j.1524-6175.2007.06689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stanton AV, Dicker P, O'Brien ET. Aliskiren monotherapy results in the greatest and the least blood pressure lowering in patients with high- and low-baseline PRA levels, respectively. Am J Hypertens. 2009;22:954–957. doi: 10.1038/ajh.2009.114. [DOI] [PubMed] [Google Scholar]
- 65.Stow LR, Gumz ML. The circadian clock in the kidney. J Am Soc Nephrol. 2011;22:598–604. doi: 10.1681/ASN.2010080803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thomas MA, Fleissner G, Stohr M, Hauptfleisch S, Lemmer B. Localization of components of the renin-angiotensin system in the suprachiasmatic nucleus of normotensive Sprague-Dawley rats: part A. angiotensin I/II, a light and electron microscopic study. Brain Res. 2004a;1008:212–223. doi: 10.1016/j.brainres.2004.01.086. [DOI] [PubMed] [Google Scholar]
- 67.Thomas MA, Fleissner G, Stohr M, Hauptfleisch S, Lemmer B. Localization of components of the renin-angiotensin system in the suprachiasmatic nucleus of normotensive Sprague-Dawley rats: part B. angiotensin II (AT1)-receptors, a light and electron microscopic study. Brain Res. 2004b;1008:224–235. doi: 10.1016/j.brainres.2004.01.085. [DOI] [PubMed] [Google Scholar]
- 68.Verdecchia P, Angeli F, Mazzotta G, Gentile G, Reboldi G. The renin angiotensin system in the development of cardiovascular disease: role of aliskiren in risk reduction. Vasc Health Risk Manag. 2008;4:971–981. doi: 10.2147/vhrm.s3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Viera AJ, Zhu S, Hinderliter AL, Shimbo D, Person SD, Jacobs DR., Jr Diurnal blood pressure pattern and development of prehypertension or hypertension in young adults: the CARDIA study. J Am Soc Hypertens. 2011;5:48–55. doi: 10.1016/j.jash.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Q, Fan XP, Chen Z, Zhao QH, Chen SQ, Wan ZH. [Role of afferent renal nerves in 2K2C Goldblatt hypertension] Sheng Li Xue Bao. 1995;47:366–372. [PubMed] [Google Scholar]
- 71.Whaley-Connell A, Habibi J, Cooper SA, DeMarco VG, Hayden MR, Stump CS, et al. Effect of renin inhibition and AT1R blockade on myocardial remodeling in the transgenic Ren2 rat. Am J Physiol Endocrinol Metab. 2008;295:E103–E109. doi: 10.1152/ajpendo.00752.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Whaley-Connell A, Habibi J, Panfili Z, Hayden MR, Bagree S, Nistala R, et al. Angiotensin II Activation of mTOR Results in Tubulointerstitial Fibrosis through Loss of N-Cadherin. Am J Nephrol. 2011;34:115–125. doi: 10.1159/000329327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Whaley-Connell A, Nistala R, Habibi J, Hayden MR, Schneider RI, Johnson MS, et al. Comparative effect of direct renin inhibition and AT1R blockade on glomerular filtration barrier injury in the transgenic Ren2 rat. Am J Physiol Renal Physiol. 2010;298:F655–F661. doi: 10.1152/ajprenal.00373.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Witte K, Lemmer B. Development of inverse circadian blood pressure pattern in transgenic hypertensive TGR(mREN2)27 rats. Chronobiol Int. 1999;16:293–303. doi: 10.3109/07420529909116859. [DOI] [PubMed] [Google Scholar]
- 75.Witte K, Schnecko A, Buijs RM, van d V, Scalbert E, Delagrange P, et al. Effects of SCN lesions on circadian blood pressure rhythm in normotensive and transgenic hypertensive rats. Chronobiol Int. 1998;15:135–145. doi: 10.3109/07420529808998678. [DOI] [PubMed] [Google Scholar]
- 76.Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]


