Abstract
Gold nanorods have promising applications in drug delivery and cancer treatment and are generally administered via direct injection into circulation. Thus it is necessary to evaluation their potential adverse effects on blood vessels. Herein we use gold nanorods with various surface modifications to evaluate the toxicity and cellular uptake of gold nanorods into vascular endothelial and smooth muscle cells of isolated rat aortic rings. Surfactant-capped gold nanorods (GNRs) were synthesized and either: 1) coated with polyelectrolytes (PE) in order to prepare PE-GNRs; or 2) modified with thiolated polyethylene glycol (PEG) in order to prepare PEG-GNRs. Using toxicity assays, small vessel myography, fluorescence microscopy and electron microscopy, we show that therapeutic concentrations of PE-GNRs but not PEG-GNRs are toxic to the vascular endothelium, which leads to impaired relaxation function of aortic rings. However, no toxicity to smooth muscles was observed. Moreover, electron microscopy analysis confirmed the cellular uptake of PE-GNRs but not PEG-GNRs into the endothelium of exposed aortic rings. The difference in toxicity and cellular uptake for PE-GNRs versus PEG-GNRs is explained and linked to free surfactant molecules and protein adsorption, respectively. Our results indicate that toxicity and cellular uptake in vascular endothelium in blood vessels are potential adverse effects of systemically administered gold nanorod solutions, which can be prevented by appropriate surface functionalization.
Keywords: Gold nanorods, blood vessels, toxicity, endothelial dysfunction, vascular function
1. Introduction
Gold nanoparticles have unique optical and photothermal properties, which make them highly attractive candidates for biomedical applications such as drug/gene delivery, biological imaging, and cancer treatment.[1] Excellent light absorption in the Visible-Near-Infrared (Vis-NIR) spectra coupled with efficient photothermal properties of gold nanorods have been employed in vivo to ablate tumor cells in animal models bearing xenografts.[1a] As is the case for most nanoparticles, intravascular injection is the usual administration route for gold nanorod solutions in cancer treatment and drug delivery applications.[2] Intravascular administration of gold nanorods implies the direct exposure of the blood vessel walls to injected nanomaterials. However, little is known about the toxicity and cellular uptake of gold nanorods in the blood vessels.
The lumen of all blood vessels is lined by a monolayer of endothelial cells (vascular endothelium), which can act as a physical barrier between the blood and underlying smooth muscle cells. Of particular importance, vascular endothelium produces nitric oxide (NO), a potent blood vessel dilator, to maintain necessary vascular blood flow.[3] Vascular endothelium injury or dysfunction is associated with various cardiovascular diseases and results in impaired NO production and altered blood vascular function.[4] Since the endothelium lines the interior of blood vessels, it is directly exposed to circulating agents. In previous reports, it has been shown that particulate matter, diesel nanoparticles and manufactured particles can induce endothelial dysfunction, impairment of blood vessel function, and cardiovascular complications.[5]
To our best knowledge, there have been no studies on the possible adverse effects of engineered gold nanoparticles on the vascular endothelium and smooth muscle cells in blood vessels. Herein, we exposed isolated rat blood vessels to therapeutic concentrations of gold nanorods and evaluated vascular function as well as cellular toxicity/uptake in the vascular endothelium and smooth muscle cells of exposed blood vessels.
2. Results
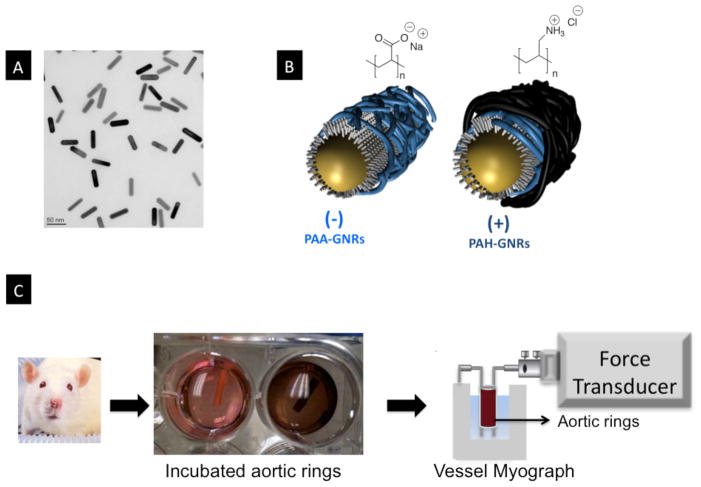
A wet chemical seed-mediated method was used to prepare gold nanorods with excellent shape and size distribution.[6] TEM images (Figure 1A) confirmed the rod-shape of these nanoparticles and indicated an average length of 47.5±2.9 nm and average diameter of 13.5±1.6 nm (aspect ratio as defined by the length/width is 3.5±0.35). As prepared, gold nanorods have a bilayer of positively charged surfactant (cetyltrimethylamoniumm bromide, CTAB) on their surfaces.[7] The well-documented polyelectrolyte coating of CTAB-capped gold nanorods (CTAB-GNRs) was then used to enhance nanorod stability and biocompatibility.[8] CTAB-GNRs were coated with negatively-charged polyelectrolytes (polyacrylic acid, PAA) to prepare anionic GNRs (PAA-GNRs, Figure 1B).[8a] The PAA-GNRs were overcoated with positively-charged polyelectrolytes (Polyallylamine hydrochloride, PAH) to prepare cationic GNRs (PAH-GNRs, Figure 1B).[8a] Zeta potential analysis for PAA-GNRs and PAH-GNRs in water confirmed their negative and positive surface charges, respectively (Figure S1). The UV-Vis spectra of coated gold nanorods did not exhibit significant broadening in the transverse and longitudinal plasmon peaks (λmax≈520 & 750 nm respectively), which indicates that coating did not result in nanorod aggregation (Figure S2A). Dynamic light scattering analysis of gold nanorods after coating with polyelectrolytes indicates a slight increase of the nanorods hydrodynamic diameter, which is consistent with the deposition of polymer layers on nanorod surface without aggregation (Figure S2B).
Figure 1.
A) Transmission electron microscope image of gold nanorods, scale bar=50 nm. B) Cartoon demonstrates coating of CTAB-GNRs with either anionic (PAA) or cationic (PAH) polyelectrolytes. Chemical structures of PAA and PAH are shown. C) Rat aorta rings were isolated, cut into rings and incubated with media contains gold nanorods (brown color in the right well is due to the presence of gold nanorods). Aortic rings were mounted on vessel myograph to examine vascular function. CTAB: Cetyltrimethylammonium bromide; PAA: poly(acrylic acid, sodium salt); PAH: Poly(allylamine hydrochloride).
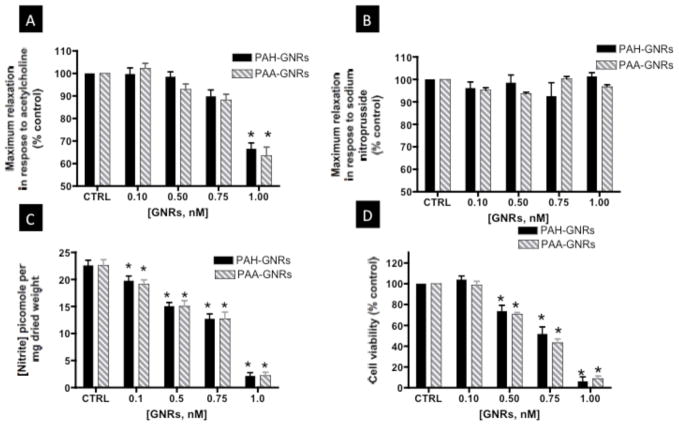
Rat aorta were freshly isolated, cut into rings, incubated with either PAA- or PAH-GNRs for eight hours, and evaluated for vascular function using a vessel myography (Figure 1C). We evaluated the ability of pre-constricted aortic rings to relax in response to the endothelium-dependent vasorelaxant acetylcholine (ACh). ACh activates nitric oxide synthase (NOS) in vascular endothelial cells of aortic rings to produce NO, which relaxes smooth muscle cells and induces vessel relaxation. At a concentration of 1.0 nM of gold nanorods, we observed significant impairment of endothelium-dependent relaxation in aortic rings exposed to either PAA- or PAH-GNRs (Figure 2A&B). The impairment of endothelium-dependent relaxation was dose-dependent and similar for both PAA- and PAH-GNRs despite the difference in their surface charge (Figure 3A). Interestingly, NO production by endothelial cells in exposed aortic rings to polyelectrolytes-coated gold nanorods decreased in a dose-dependent manner (Figure 3C), which correlates with impairment of vascular function.
Figure 2.
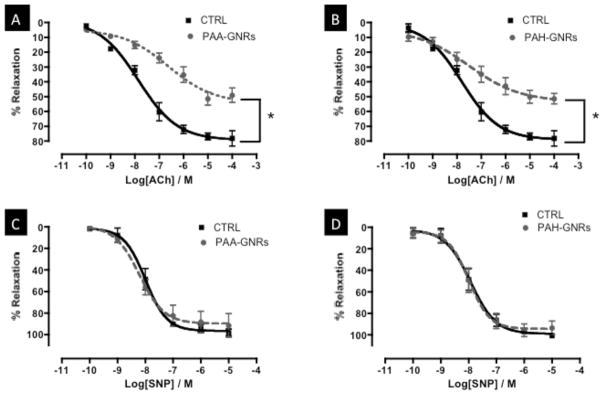
Dose-response relaxation curves of aortic rings exposed to 1.0 nM gold nanorods (dashed-gray lines) versus controls (solid-black lines). Relaxation curves of aortic rings exposed to PAA-GNRs (A) and PAH-GNRs (B) in response to ACh (endothelium-dependent vasorelaxant). Relaxation curves of aortic rings exposed to PAA-GNRs (C) and PAH-GNRs (D) in response to SNP (endothelium-independent vasorelaxant). n=6 in each group, * indicates differences between groups (p<0.05). PAA: poly(acrylic acid, sodium salt); PAH: Poly(allylamine hydrochloride); ACh=acetylcholine; SNP=sodium nitroprusside.
Figure 3.
(A) Endothelium-dependent maximum relaxation, (B) endothelium-independent maximum relaxation, and C) Nitric oxide production of aortic rings exposed to PAA-GNRs (stripped-gray bars) or PAH-GNRs (black bars) for 8 hours. (D) Cell viability of BAEC incubated with PAA-GNRs or PAH-GNRs for 8 hours. n=6 in each group, * indicates differences from control (CTRL) group (p<0.05). BAEC: Bovine aortic endothelial cells, PAA: poly(acrylic acid, sodium salt); PAH: Poly(allylamine hydrochloride).
In parallel, we evaluated the ability of aortic rings to relax in response to endothelium-independent vasorelaxant (sodium nitroprusside, SNP). SNP is a NO-donor and thus directly relaxes vascular smooth muscles without an endothelium contribution. No significant impairment of endothelium-independent relaxation was observed upon incubation with either PAA- or PAH-GNRs (Figure 2C&D and Figure 3B), indicating no impairment of smooth muscle cell function.
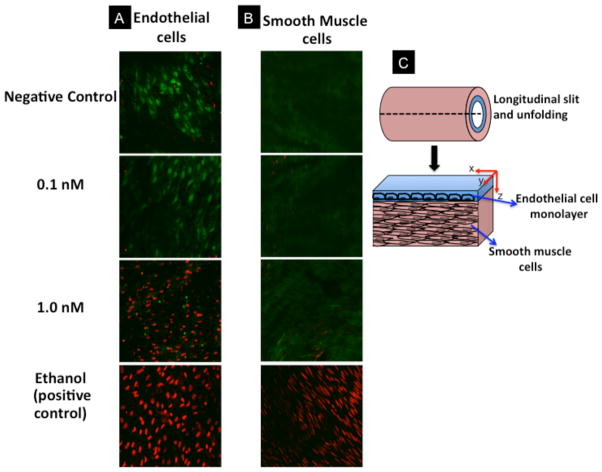
To explain the observed impairment of endothelium-dependent relaxation in aortic rings exposed to polyelectrolytes-coated gold nanorods, we hypothesized that gold nanorods caused endothelial dysfunction. To test our hypothesis, we examined the toxicity of PAA- or PAH-GNRs on cultured bovine aortic endothelial cells (BAEC) using an in-vitro viability test (WST-1 assay).[9] We observed that PAA- and PAH-GNRs caused a dose-dependent toxicity on cultured BAEC (Figure 3D). These results motivated us to evaluate toxicity of PAA- or PAH-GNRs on endothelial cells in aortic rings. After incubation with gold nanorods, aortic rings were treated with two fluorescent dyes: calcein AM and ethidium homodimer-1 to label live and dead cells, respectively.[10] Fluorescence confocal microscopy images of endothelial cells in aortic rings exposed to 1.0 nM PAH-GNRs show a significant cellular death (Figure 4A). At a concentration of 0.1 nM, that neither induced toxicity to BAEC nor impaired vascular function, we did not observe toxicity to vascular endothelium in exposed aortic rings (Figure 4A). To evaluate the toxicity of gold nanorods to smooth muscle cells in the same aortic rings (beneath the endothelium), we collected fluorescent confocal microscopy images along the z-axis of opened aortic ring (Figure 4B & 4C). Upon exposure to the highest concentration of polyelectrolyte-coated gold nanorods (1.0 nM), we did not observe any significant death of smooth muscle cells in aortic rings (Figure 4B). Fluorescence images of endothelial and smooth muscle cells in exposed aortic rings suggest that the toxicity of gold nanorods is mostly confined to the endothelium and not to smooth muscle.
Figure 4.
Fluorescence confocal microscopy images of aortic flat mounts at different z-axis depths show (A) endothelial and (B) smooth muscle cells. Aortic rings stained with green (labels living cells) and red (labels dead cells) dyes. Smooth muscle images were taken in a plane about 50 microns deep in the z-axis from the x,y endothelium horizontal plane. Green dye: calcein AM; red dye: ethidium homodimer-1; each image is 230×230 micron. C) Cartoon demonstrates the imaging planes for both endothelial and smooth muscles layers in imaged aortic rings.
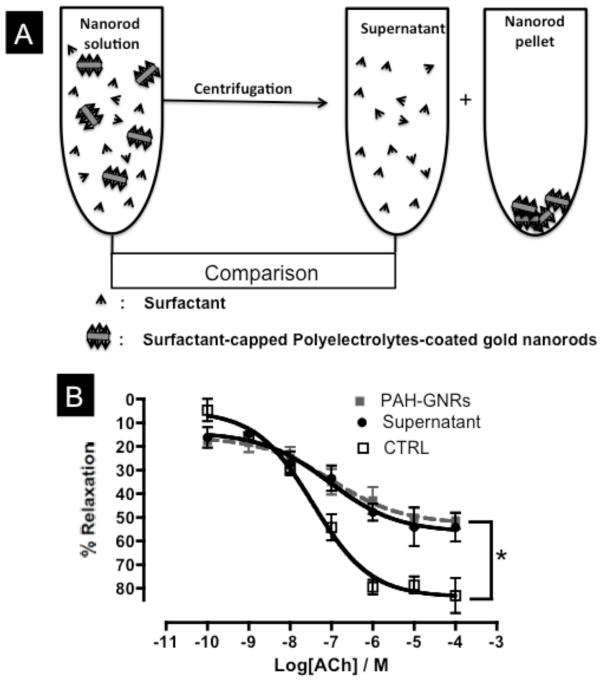
To determine if the toxic effect of gold nanorod solutions on the vascular endothelium is due to gold nanorods themselves or free CTAB surfactants in the solution, we compared the toxicity to bovine aortic endothelial cells (BAEC) and the vascular function impairment in aortic rings upon incubation with either gold nanorod solutions or their correspondent supernatants (after removing all nanorods by aggressive centrifugation, Figure 5A).[8b] Interestingly, PAA- and PAH-GNRs solutions induced a similar toxicity on cultured BAEC compared to their supernatant solutions (cell viability ~10% for PAA-GNRs, PAH-GNRs and their supernatants at concentration of 1.0 nM original gold nanorods solution). Moreover, aortic rings exposed to polyelectrolytes-coated gold nanorod solutions or their supernatant solutions exhibited similar endothelium-dependent relaxation impairment, suggesting that the origin of toxicity is free CTAB molecules in gold nanorod solutions (Figure 5).[8b]
Figure 5.
A) Cartoon demonstrates the “supernatant control” experiment. Gold nanorods were separated from free surfactants by centrifugation. B) Endothelium-dependent relaxation curves for aortic rings exposed to gold nanorod solution (dashed-gray line), supernatant solution (black line, closed circles) or control (black line, open squares) in response to acetylcholine (ACh). n=6 in each group, * indicates statistical difference from control (CTRL) group (p<0.05).
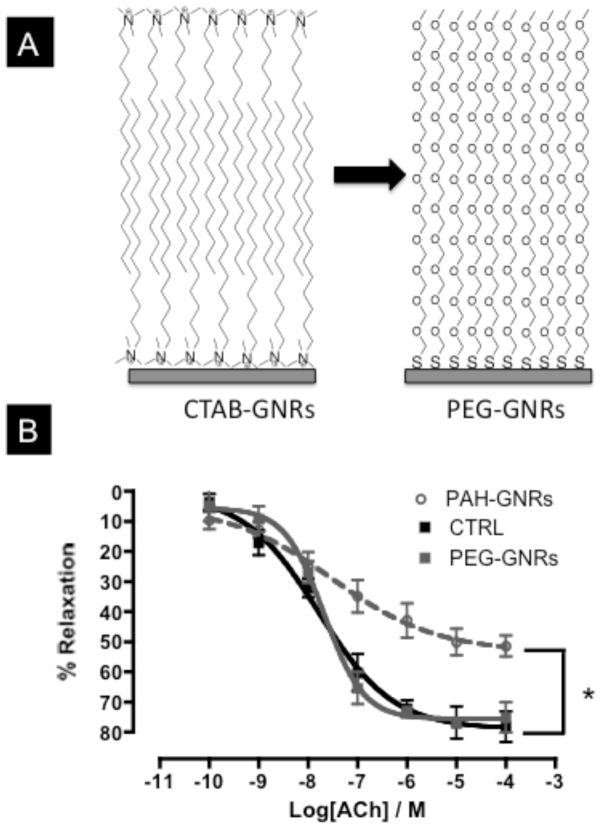
In other experiments, CTAB molecules at the surface of gold nanorods were displaced with thiolated-polyethylene glycol (PEG) to prepare PEG-GNRs (Figure 6A).[11] The CTAB bilayer on the surface of gold nanorods is stable and prevents molecules from adsorbing to the nanorod surface.[11] We found that aggressive centrifugation to remove CTAB from the surface of gold nanorods resulted in nanorods aggregation.
Figure 6.
A) Cartoon demonstrates “PEGylation” of gold nanorods by displacing CTAB bilayer with thiolated-polyethylene glycol (PEG-SH). B) Endothelium-dependent relaxation curves of aortic rings exposed to PAH-GNRs (dashed-gray line), PEG-GNRs (solid-gray line), or control (black line). n=6 in each group, * indicates statistical difference from control (CTRL) group (p<0.05). CTAB: Cetyltrimethylammonium bromide; PEG: polyethylene glycol; PAH: Poly(allylamine hydrochloride); ACh=acetylcholine.
However, and as adapted from previous reports, dialysis of a mixture of CTAB-GNRs and thiolated PEG (in the same dialysis tube) against large volume of water for 24 hours resulted in gold nanorods that have a neutral surface charge.[11] The dialysis tube was selected to have a pore size large enough to allow CTAB molecules to diffuse out from the dialysis tube but small enough to retain PEG molecules (Dialysis cutoff=3500 Da, CTAB MW= 364.45 Da, PEG MW=5000 Da). After PEGylation, the surface charge of gold nanorods became −2±1.01 mV (CTAB-GNRs =+42±2.03 mV), indicating successful displacement of cationic CTAB bilayer by neutral PEG molecules. However, zeta potential analysis is a qualitative analysis and does not confirm a complete removal of CTAB molecules from the surface of gold nanorods. UV-Vis spectra of gold nanorods after PEGylation did not exhibit a broadening to either transverse or longitudinal plasmon peak, which indicates no nanorods aggregation occurred during the displacement reaction (Figure S3A). Moreover, the severe aggregation, as judged by UV-Vis spectra and dynamic light scattering, of CTAB-GNRs but not PEG-GNRs upon transfer from water to ethanol is another indication of surface modification with PEG molecules (Figure S3 A&B).[11b] Interestingly, exposure to PEG-GNRs (1.0 nM) did not cause impairment of endothelium-dependent relaxation in exposed aortic rings or toxicity in cultured BAEC (Figure 6B and S4).
Cellular uptake of gold nanorods by endothelial and/or smooth muscle cells in aortic rings was evaluated using transmission electron microscopy after exposure to a non-toxic concentration (0.1 nM) of PAA-, PAH-, or PEG-GNRs for 8 hours. Polyelectrolytes-coated gold nanorods (PAH- and PAA-GNRs) were taken up by endothelium cells of aortic rings and accumulated in cytoplasmic vesicles (Figure 7). However, nanorods did not cross the endothelium barrier as none were found in the smooth muscle layers. In contrast to polyelectrolytes-coated gold nanorods, PEG-GNRs entered neither endothelial nor smooth muscle cells in aortic rings even at higher concentrations (1.0 nM), as no nanorods were found in 200 randomly tested endothelial and smooth muscle cells.
Figure 7.
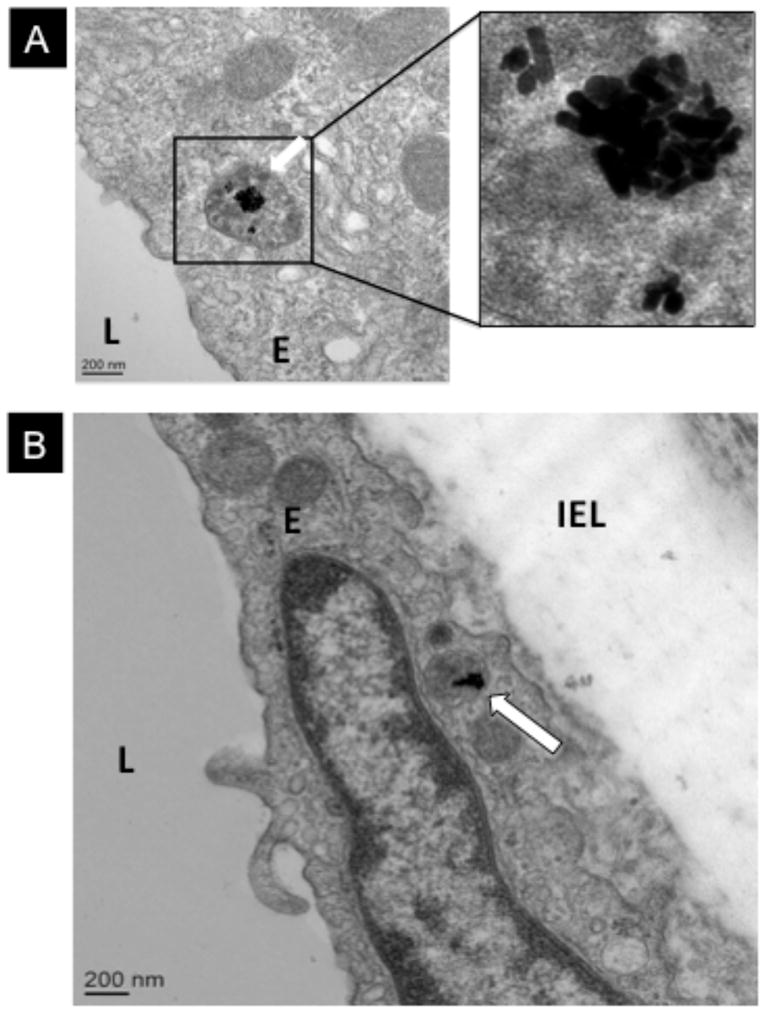
TEM images of aortic ring exposed to A) PAH-GNRs and B) PAA-GNRs. Square in right, panel A, is a magnified image of PAH-GNRs in endocytic vesicle within the cytoplasm of endothelial cell. Panel B shows PAA-GNRs inside endocytic vesicle in the endothelium (white arrow). L: aortic lumen; E: endothelial cell; IEL: Internal elastic lamina.
3. Discussion
Possible adverse effects of nanoparticles using biological systems are important issues.[12] Since intravascular injection is the most frequently used route of administration for nanoparticles, blood vessels are among the first compartments with which nanoparticles will interact. Therefore, we evaluated the toxicity and cellular uptake of engineered gold nanorods to exposed blood vessels.
The “therapeutic” dose of gold nanorods varies among studies.[2] We started with a concentration of 1.0 nM, a level of nanorods used previously in vivo to effectively treat cancer after intravascular injection.[11b] The expected circulation half-time (t1/2) of gold nanoparticles in blood varies and depends on the size, shape, and surface chemistry of nanoparticles.[2] Reported t1/2 values for gold nanorods, with similar dimensions to the gold nanorods used in this study, range from 0.5 to 17 hours based on their surface chemistry.[11b, 13] With this in mind, we selected eight hours incubation time as a midpoint and expected duration of nanoparticle-vessel interactions. Two widely used surface modifications for CTAB-capped gold nanorods were examined in this study:1) overcoating with polyelectrolytes; 2) replacing the surfactant from the surface and displacing it with thiolated PEG molecules. Both modifications have been extensively used to enhance the stability and biocompatibility of gold nanoparticles and have potential use in gold nanorod formulations for intravascular administration.[8b, c, 11] However, the former modification provides a CTAB bilayer underneath the coating polymer, where the later modification completely removes the CTAB molecules from the surface of gold nanorods.
Our first aim was to assess the toxicity of gold nanorods to endothelial and smooth muscle cells in blood vessels using vessel function and toxicity screening assays. Small vessel myography is a tool widely used to evaluate the function of blood vessels by monitoring their ability to contract and relax in response to vasoactive compounds.[14] With injury to vascular endothelium, we expect impaired endothelium-dependent relaxation in pre-contracted aortic rings.
Our results indicated that exposure to either PAA- or PAH-GNRs at 1.0 nM resulted in significant toxicity to endothelial cells in both cultured cells and aorta rings. Toxicity to endothelium resulted in decreased NO production and impaired endothelium-dependent relaxation. Interestingly, toxicity of polyelectrolyte-coated gold nanorod solutions was confined to the endothelium without any significant toxicity to vascular smooth muscle cells in exposed aortic rings. Toxicity of polyelectrolytes-coated gold nanorods to the vascular endothelium and not smooth muscle cells in aortic rings could be explained by the fact the endothelium covers the inner surface of blood vessels and it is directly exposed to nanoparticle solutions, whereas smooth muscle cells are separated from the nanorod solutions by the endothelium layer and sub-endothelial connective tissues. Indeed, one of the roles of the vascular endothelium is to form a barrier between blood and the underlying tissue layers.[4] Moreover, connective tissues and fibroblasts cells in the outer layer of exposed aorta are also a barrier and should protect smooth muscle cells from exposure to the nanoparticle solution.
Coating with polyelectrolytes is a simple surface modification for CTAB-GNRs, which has been used to complex siRNA for in vivo delivery and to attach recognition ligand to the surface of gold nanorods for in vivo targeting.[1b] However, polyelectrolyte-coated GNRs contain a CTAB bilayer at the surface of gold nanorods underneath the polyelectrolyte polymers (Figure 1B).[8a] CTAB is a surfactant with quaternary ammonium head group, which is known to be toxic to cells.[8b] CTAB molecules in the bilayer are held via weak hydrophobic interactions and thus can desorb from the surface of PAA- and PAH-GNRs into the solution bulk.[8b]
To check if free CTAB molecules are responsible for the observed toxicity, we centrifuged gold nanorod solution to pull all nanorods down and then we compared the toxicity of gold nanorod supernatant with the original gold nanorod solution.[12a] In vitro toxicity results and endothelium-dependent relaxation curves for aortic rings exposed to either PAH-GNRs solution or its corresponding supernatant solutions were similar, indicating that toxicity of polyelectrolytes-coated gold nanorod solutions is mainly due to the free CTAB molecules and not the nanorods themselves (Figure 5). This finding agrees with previous reports, in which supernatant of PAA-GNRs and PAH-GNRs contained free CTAB molecules and were found to be as toxic as their original nanorod solutions.[8b, c]
Despite the fact the PAA-GNRs and PAH-GNRs have different surface charge, it is interesting that they induce similar adverse effects to the vascular endothelium. Our explanation for this similarity is that free CTAB molecules may be present in both preparations at similar levels. In our previous work, we found that the concentration of free CTAB in PAA-GNRs and PAH-GNRs is similar and in the range of 0.2–0.3 μM.[12b] Moreover, PAA-GNRs and PAH-GNRs have similar negative surface charge in the culture media due to the formation of a protein corona on the surface of both nanorods (Figure S1).[8b] The similarity in the level of free CTAB molecules and the surface charge in culture media may explain the similar side effects upon exposure to either anionic PAA-GNRs or cationic PAH-GNRs.
Since CTAB molecules appear to be the source of toxicity, we displaced the CTAB on the surface of gold nanorods with thiolated-PEG. PEGylation is a versatile surface modification for various classes of nanoparticles, which enhance their biocompatibility and physical stability.[11, 15] Interestingly, we found that endothelium-dependent relaxation curves for aortic rings exposed to 1.0 nM PEG-GNRs were similar to non-exposed rings, which indicate that PEG-GNRs did not induce toxicity to vascular endothelial cells in aortic rings and did not alter the aortic function. Moreover, we found that treatment of cultured BAEC with 1.0 nM of PEG-GNRs did not induce toxicity as measured by WST-1 assay (Figure S4), whereas PAH-GNRs resulted in less than 10% cell viability. These results indicate that PEG-GNRs are superior to either PAA- or PAH-GNRs in term of biocompatibility to vascular endothelium and highlight the importance of surface modification to prevent the toxicity of nanoparticles to vascular endothelium in blood vessels. In our case, the CTAB molecules in polyelectrolytes-coated gold nanorod solutions were the origin of toxicity and upon displacing CTAB with PEG molecules; toxicity of gold nanorods was completely eliminated.
The second aim of this study was to determine if cellular uptake of nanoparticles by vascular endothelium and smooth muscle cells occurred in aortic rings after exposure to gold nanorods. Transmission electron microscopy imaging of aortic rings incubated with PAA- or PAH-GNRs (non-lethal concentration) show significant accumulation of gold nanorods in single membrane-endocytic vesicles within the cytoplasm of the endothelium (Figure 7 and Figure S5). However, no nanorods were found in smooth muscles layers. Uptake of PE-GNRs by the endothelium and not smooth muscles could be explained by an anatomical argument. Endothelial cells, the most inner surface of blood vessel, are in direct contact with nanorods whereas smooth muscle cells are separated from the arterial lumen by the endothelium and sub-endothelial connective tissue.
Despite the significant cellular uptake of PAA- and PAH-GNRs into the endothelium, PEG-GNRs neither entered the endothelium nor the smooth muscles. The differential uptake of polyelectrolytes-coated and PEGylated gold nanorods may be explained by the fact that proteins in culture media could adsorb to the surface of PAH-or PAA-GNRs and not to PEG-GNRs (media contains 10% serum proteins). The adsorption of protein molecules to the surface of PE-GNRs and not PEG-GNRs was confirmed by significant loss of proteins from incubation media upon mixing with PE-GNRs but not PEG-GNRs (see SI for details and Figure S6). It is known that adsorbed proteins from culture media can induce a receptor-mediated endocytosis for nanoparticles into cells.[18] Moreover, PEGylation of nanoparticles is a surface chemistry known to prevent protein adsorption to the surface of nanoparticles and to prevent their cellular uptake.[19] Our results agree with previous reports where PAA- and PAH-GNRs have been shown to enter cells by receptor-mediated endocytosis, where PEGylation found to prevent the cellular uptake of gold nanorods into several cultured cells.[8b, 19] Quantitative analysis of the endothelium uptake of gold nanorods with different surface chemistries and the uptake mechanism is a subject for future communication. However, our qualitative TEM results suggest that PEGylation is a powerful tool to prevent cellular uptake of nanoparticles into vascular endothelium in blood vessel after systemic injections.
4. Conclusions
In summary, we have shown that polyelectrolyte-coated gold nanorod solution can induce toxicity in vascular endothelial cells of exposed blood vessels, which result in impaired vascular function. The toxicity of these nanoparticles rises from their surface-capping agent (CTAB), which could be prevented by displacing CTAB with “safer” molecules (PEG) to prevent endothelium injury. Interestingly, PEGylated nanoparticles did not show significant toxicity or uptake in the vascular endothelium of aortic rings. These results highlight the importance of surface modifications in preventing toxicity and cellular uptake of nanoparticles to vascular endothelium in blood vessels.
5. Experimental Section
Gold nanorods synthesis and surface modifications (CTAB-GNRs, PAA-GNRs, PAH-GNRs, and PEG-GNRs)
Gold nanorods were prepared using wet chemical seed-mediated method as described previously using cetyltrimethylammonium bromide (CTAB) as a shape-directing agent.[6] The prepared gold nanorods are capped with a positively charged bilayer of CTAB. CTAB-capped gold nanorods (CATB-GNRs) were over coated with negatively charged polyelectrolytes (poly(acrylic acid), PAA) to prepare anionic nanorods (PAA-GNRs) as described previously.[8a] Positively charged polyelectrolytes (poly(allylamine hydrochloride), PAH) was used to over coat PAA-GNRs and thus to prepare cationic gold nanorods (PAH-GNRs). To prepare polyethylene glycol capped gold nanorods (PEG-GNRs), we exchanged the CTAB molecules on the surface of CTAB-GNRs with thiolated-PEG.[11b] Details on the synthesis, purification, surface modifications, and nanoparticles characterization can be found in the Supplementary Materials.
In vitro toxicity evaluation
Cell viability was assessed using the WST-1 assay (water soluble tetrazolium salt, Roche Applied Science) and bovine aortic endothelial cells (BAEC). BAEC were plated in 96-well flat bottom plate at a concentration of 4×103 cells/well (200 μL/well) and incubated at 37 °C and 5% CO2. After allowing 24 hours for cell attachment, gold nanorods were added to the growth media (M199 media contains 10% serum proteins) from concentrated stock solution. The media was not changed during the incubation period (8 hours). After incubation, wells were aspired and washed three times with fresh media (100 μL/well). WST-1 reagent (10 μL/well) was added and incubated for four hours at 37 oC and 5% CO2. Absorbance readings at 400 and 660 nm (signal and background readings, respectively) were measured using plate reader (PowerWave X52, Bio-Tek instruments Inc). Cell viability was calculated as a percentage compared to untreated control cells.
Animals and tissue harvest
Protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the Georgia Health Sciences University. Experiments were performed with Spraque-Dawley male rats (10 weeks in age, 340–350 g in weight). Rats were anesthetized with intraperitoneal injections of ketamine HCl and xylazine HCl (100 and 10 mg/Kg body weight respectively). After rapidly opening the chest, the aorta was harvested, placed immediately in ice-cold Krebs-Henselet buffer, and cleaned from connective tissue for immediate use.
Vascular function studies
Rat aorta was harvested, placed immediately in ice-cold Krebs-Henselet buffer, cleaned from connective tissue and cut into 2–3 mm segments. Aortic rings were incubated with or without gold nanorods for 8 hours in M199 media at 37 °C and 5% CO2. Aortic rings were mounted in an oxygenated wire myograph chamber (Danish Myo Technology). Tissues were allowed to equilibrate at a resting tension of 10 mN for one hour with buffer changes. Following precontraction to phenylephrine (1 μM), relaxation curves were performed using progressive doses of the endothelium-independent vasorelaxant sodium nitroprusside (SNP) and the endothelium-dependent vasorelaxant acetylcholine (ACh). Changes in tension were measured by force transducers. A one-hour equilibration was performed between subsequent relaxation curves. Vasorelaxation responses are calculated as percent of phenylephrine-induced contraction.
Nitric oxide measurements
Nitrite (NO2), the stable breakdown product of NO, was analyzed using NO-specific chemiluminescence method. Aortic rings were incubated with or without gold nanorods (0–1.0 nM) for 8 hours in M199 media at 37 °C and 5% CO2, medium was removed and aortic rings were washed three times with Krebs-Henselet buffer containing 100 μM L-arginine. Aortic rings were incubated with Krebs-Henselet buffer containing 100 μM L-arginine and 1 μM calcium ionophore ionomycin (Sigma Aldrich, St. Louis, MO) for two hours and then samples from the incubation buffer were collected for NO analysis. In brief, samples were injected in reaction vessel contains glacial acetic acid and sodium iodide where NO2 is quantitatively reduced to NO. A chemiluminescence detector was used to quantify produced NO after reaction with ozone in a NO analyzer (Sievers, Boulder, CO). The amount of NO generated is calculated based on a calibration curve using sodium nitrite (NaNO2, Fisher Scientific) dissolved in Krebs-Henselet buffer.
Fluorescence confocal microscopy imaging of aortic rings
Aortic rings were incubated with or without gold nanorods (0–1.0 nM) for 8 hours in M199 media at 37 °C and 5% CO2 and then medium was removed and aortic rings were washed three times with Krebs-Henselet buffer. Aortic rings were immediately incubated in oxygenated Krebs-Henselet buffer containing calcein AM and ethidium homodimer-1 (4 and 16 μM respectively, Live/Dead Kit from Molecular Probes) at 37 °C for 30 minutes. Aortic rings (cylindrical in shape) were washed three times with buffer, cut in their longitudinal planes, unfolded into rectangles, and placed on 35 mm Glass Bottom Culture Dish (MatTek Corporation, USA) where the endothelium is facing the microscope lenses. Positive controls were prepared by soaking aortic rings in 70% ethanol for 10 minutes. Fluorescence images were taken using a Zeiss LSM 510 Axioplan 2 Confocal Laser Scanning Microscope with an IR-Achroplan water dipping 40x objective. Z-stack images were taken along the z-axis of the imaged tissue to examine both endothelial and smooth muscle layers with optical slices of 2.4 micrometers.
Transmission electron microscopy (TEM) imaging of gold nanorods in aortic rings
Aortic rings were incubated with gold nanorods for 8 hours in M199 media at 37 °C and 5% CO2, washed three times with Krebs-Henselet buffer, and other three times with heparin sodium solution (100 USP unit/mL) to get rid of nanoparticles that electrostatically-adsorbed to the tissue. The washed aortic rings were fixed in 2% glutaraldehyde in 0.1 M sodium cacodylate (NaCac) buffer (pH 7.4), postfixed in 2% osmium tetroxide in NaCac, stained en bloc with 2% uranyl acetate, dehydrated with a graded ethanol series and embedded in Epon-Araldite resin. Thin sections (65 nm) were cut with a diamond knife on a Leica EM UC6 ultramicrotome (Leica Microsystems, Inc, Bannockburn, IL), collected on copper grids and stained with uranyl acetate and lead citrate. Specimens were observed in a JEM 1230 transmission electron microscope (JEOL USA Inc., Peabody, MA) at 110 kV and imaged with an UltraScan 4000 CCD camera & First Light Digital Camera Controller (Gatan Inc., Pleasanton, CA).
Statistics
Data are given as mean ± SEM. Statistical analysis was performed by one-way analysis of variance (ANOVA) with the Bonferroni post-test. All statistical analysis was performed with GraphPad Prism version 4.03 (San Diego, CA). Results were considered significant when p<0.05.
Supplementary Material
Acknowledgments
The authors thank Mr. Robert Smith from the Electron Microscopy and Histology Core, Department of Cellular Biology and Anatomy (Georgia Health Sciences University) for assistance in TEM imaging; Prof. Paul L. McNeil and Dr. Amber C. Howard for their valuable scientific discussion; Prof. Catherine J. Murphy and Stefano P. Boulos from the University of Illinois for zeta potential analysis. This work was supported by the National Institute of Health grants RO1 HL-70215 and RO1 EY-11766.
Footnotes
Supporting Information is available on the WWW under http://www.small-journal.com.
Contributor Information
Dr. Alaaldin M. Alkilany, Department of Pharmacology and Toxicology, Georgia Health Sciences University, Augusta, Georgia 30912 (USA)
Dr. Alia Shatanawi, Department of Pharmacology and Toxicology, Georgia Health Sciences University, Augusta, Georgia 30912 (USA)
Timothy Kurtz, Cell Imaging Laboratory, Georgia Health Sciences University, Augusta, Georgia 30912 (USA).
Prof. Ruth B. Caldwell, Center and Department of Cell Biology & Anatomy, Georgia Health Sciences University, Augusta, Georgia 30912 (USA)
Prof. R. William Caldwell, Email: WCALDWEL@georgiahealth.edu, Department of Pharmacology and Toxicology, Georgia Health Sciences University, Augusta, Georgia 30912 (USA), Phone: (706) 721-3383, Fax: (706) 721-2347
References
- 1.a) Kennedy LC, Bickford LR, Lewinski NA, Coughlin AJ, Hu Y, Day ES, West JL, Drezek RA. Small. 2011;7:169–183. doi: 10.1002/smll.201000134. [DOI] [PubMed] [Google Scholar]; b) Alkilany AM, Thompson LB, Boulos SP, Sisco PN, Murphy CJ. Adv Drug Delivery Rev. 2011 doi: 10.1016/j.addr.2011.03.005. In press. [DOI] [PubMed] [Google Scholar]
- 2.Khlebtsov N, Dykman L. Chem Soc Rev. 2011;40:1647–1671. doi: 10.1039/c0cs00018c. [DOI] [PubMed] [Google Scholar]
- 3.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, Barnathan ES, McCrae KR, Hug BA, Schmidt AM, Stern DM. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- 4.Cai H, Harrison DG. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 5.a) Brook RD, Rajagopalan S, Pope CA, 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC, Jr, Whitsel L, Kaufman JD. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]; b) Brook RD, Brook JR, Urch B, Vincent R, Rajagopalan S, Silverman F. Circulation. 2002;105:1534–1536. doi: 10.1161/01.cir.0000013838.94747.64. [DOI] [PubMed] [Google Scholar]; c) LeBlanc AJ, Moseley AM, Chen BT, Frazer D, Castranova V, Nurkiewicz TR. Cardiovasc Toxicol. 2010;10:27–36. doi: 10.1007/s12012-009-9060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Nurkiewicz TR, Porter DW, Barger M, Castranova V, Boegehold MA. Environ Health Perspect. 2004;112:1299–1306. doi: 10.1289/ehp.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Courtois A, Andujar P, Ladeiro Y, Baudrimont I, Delannoy E, Leblais V, Begueret H, Galland MAB, Brochard P, Marano F, Marthan R, Muller B. Environ Health Perspect. 2008;116:1294–1299. doi: 10.1289/ehp.11021. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Miller MR, Borthwick SJ, Shaw CA, McLean SG, McClure D, Mills NL, Duffin R, Donaldson K, Megson IL, Hadokel PWF, Newby DE. Environ Health Perspect. 2009;117:611–616. doi: 10.1289/ehp.0800235. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Rosas-Hernandez H, Jimenez-Badillo S, Martinez-Cuevas PP, Gracia-Espino E, Terrones H, Terrones M, Hussain SM, Ali SF, Gonzalez C. Toxicol Lett. 2009;191:305–313. doi: 10.1016/j.toxlet.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Sau TK, Murphy CJ. Langmuir. 2004;20:6414–6420. doi: 10.1021/la049463z. [DOI] [PubMed] [Google Scholar]
- 7.Nikoobakht B, El-Sayed MA. Langmuir. 2001;17:6368–6374. [Google Scholar]
- 8.a) Gole A, Murphy CJ. Chem Mater. 2005;17:1325–1330. [Google Scholar]; b) Alkilany AM, Nagaria PK, Hexel CR, Shaw TJ, Murphy CJ, Wyatt MD. Small. 2009;5:701–708. doi: 10.1002/smll.200801546. [DOI] [PubMed] [Google Scholar]; c) Hauck TS, Ghazani AA, Chan WCW. Small. 2008;4:153–159. doi: 10.1002/smll.200700217. [DOI] [PubMed] [Google Scholar]
- 9.Cook JA, Mitchell JB. Anal Biochem. 1989;179:1–7. doi: 10.1016/0003-2697(89)90191-7. [DOI] [PubMed] [Google Scholar]
- 10.Monette R, Small DL, Mealing G, Morley P. Brain Res Protoc. 1998;2:99–108. doi: 10.1016/s1385-299x(97)00020-2. [DOI] [PubMed] [Google Scholar]
- 11.a) Niidome T, Yamagata M, Okamoto Y, Akiyama Y, Takahashi H, Kawano T, Katayama Y, Niidome Y. J Controlled Release. 2006;114:343–347. doi: 10.1016/j.jconrel.2006.06.017. [DOI] [PubMed] [Google Scholar]; b) von Maltzahn G, Park JH, Agrawal A, Bandaru NK, Das SK, Sailor MJ, Bhatia SN. Cancer Res. 2009;69:3892–3900. doi: 10.1158/0008-5472.CAN-08-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.a) Alkilany AM, Murphy CJ. J Nanopart Res. 2010;12:2313–2333. doi: 10.1007/s11051-010-9911-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lewinski N, Colvin V, Drezek R. Small. 2008;4:26–49. doi: 10.1002/smll.200700595. [DOI] [PubMed] [Google Scholar]
- 13.a) Tong L, He W, Zhang Y, Zheng W, Cheng JX. Langmuir. 2009;25:12454–12459. doi: 10.1021/la902992w. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Huang X, Peng X, Wang Y, Shin DM, El-Sayed MA, Nie S. ACS Nano. 2010;4:5887–5896. doi: 10.1021/nn102055s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spector S, Fleisch JH, Maling HM, Brodie BB. Science. 1969;166:1300–1301. doi: 10.1126/science.166.3910.1300. [DOI] [PubMed] [Google Scholar]
- 15.Karakoti AS, Das S, Thevuthasan S, Seal S. Angew Chem, Int Ed Engl. 2011;50:1980–1994. doi: 10.1002/anie.201002969. [DOI] [PubMed] [Google Scholar]
- 16.Alkilany AM, Thompson LB, Murphy CJ. ACS Appl Mater Interfaces. 2010;2:3417–3421. doi: 10.1021/am100879j. [DOI] [PubMed] [Google Scholar]
- 17.Kelly KL, Coronado E, Zhao LL, Schatz GC. J Phys Chem B. 2003;107:668–677. [Google Scholar]
- 18.a) Lynch I, Dawson KA. Nano Today. 2008;3:40–47. [Google Scholar]; b) Lynch I, Cedervall T, Lundqvist M, Cabaleiro-Lago C, Linse S, Dawson KA. Adv Colloid Interface Sci. 2007;134–35:167–174. doi: 10.1016/j.cis.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 19.Huff TB, Hansen MN, Zhao Y, Cheng JX, Wei A. Langmuir. 2007;23:1596–1599. doi: 10.1021/la062642r. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.