Abstract
Obectives
Remote ischemic preconditioning (RIPC) is a powerful endogenous mechanism whereby a brief period of ischemia is capable of protecting remote tissues from subsequent ischemic insult. While this phenomenon has been extensively studied in the heart and brain in animal models, little work has been done to explore the effects of RIPC in human patients with acute cerebral ischemia. This study investigates whether chronic peripheral hypoperfusion, in the form of pre-existing arterial peripheral vascular disease (PVD) that has not been surgically treated, is capable of inducing neuroprotective effects for acute ischemic stroke.
Methods
Individuals with PVD who had not undergone prior surgical treatment were identified from a registry of stroke patients. A control group within the same database was identified by matching patient’s demographics and risk factors. The two groups were compared in terms of outcome by NIH Stroke Scale (NIHSS), modified Rankin Scale (mRS), mortality, and volume of infarcted tissue at presentation and at discharge.
Results
The matching algorithm identified 26 pairs of PVD-control patients (9 pairs were female and 17 pairs were male). Age range was 20 to 93 years (mean 73). The PVD group was found to have significantly lower NIHSS scores at admission (NIHSS ≤ 4: PVD 47.1%, Control 4.35%, p < 0.003), significantly more favorable outcomes at discharge (mRS ≤ 2: PVD 30.8%, Control 3.84%, p < 0.012), and a significantly lower mortality rate (PVD 26.9%, Control 57.7% p=0.024). Mean acute stroke volume at admission and at discharge were significantly lower for the PVD group (Admission: PVD 39.6mL, Control 148.3mL, p < 0.005 and Discharge: PVD 111.7mL, Control 275mL, p < 0.001).
Conclusion
Chronic limb hypoperfusion induced by PVD can potentially produce a neuroprotective effect in acute ischemic stroke. This effect resembles the neuroprotection induced by RIPC in preclinical models.
2. Introduction
Ischemic stroke is a leading cause of disability worldwide and the fourth leading cause of mortality in adults [1, 2]. There is a growing volume of literature that has demonstrated the powerful effect of ischemic preconditioning as an endogenous neuroprotective mechanism against the effects of cerebral ischemia both in animal models and in early clinical studies [3–7]. However, clinical application of ischemic preconditioning techniques for acute ischemic stroke can be difficult due to the sudden and largely unpredictable nature of acute cerebral ischemia.
Ischemic preconditioning has typically been elicited in both experimental and clinical studies by brief periods of transient, complete ischemia (total suppression of blood flow) to an organ or tissue for a sub-lethal period of time. It has not been previously investigated in a clinical setting whether chronic remote hypoperfusion is as effective at initiating these neuroprotective effects. To address this question, we hypothesized that a pre-existing condition that causes non-cerebral hypoperfusion would have a protective effect in those patients who subsequently suffer a cerebral infarction. Peripheral vascular disease (PVD) represents just such a condition. We evaluated whether PVD produces a neuroprotective effect, as measured by clinical outcome and infarct volume, by comparing acute ischemic stroke patients with pre-existing, untreated PVD with matched controls with no prior history of PVD.
2. Materials and Methods
2.1. Patient Selection
Patients with a history of PVD were retrospectively identified from a prospectively collected institutional registry of acute ischemic stroke cases. Only individuals who had not undergone any prior surgical intervention for PVD were included. For each PVD patient, a control was identified using a risk-factor matching algorithm. This algorithm matched patients demographically based on age, sex, ethnicity, and race, followed by past medical history for at least two of the following conditions: hypertension, diabetes mellitus, or tobacco use, stroke and transient ischemic attacks (TIA).
2.2. Clinical Measurements
NIH Stroke Scale (NIHSS) at presentation and functional outcome at discharge as indicated by modified Rankin Scale (mRS) were collected as clinical measurements of stroke severity and clinical outcome. The NIHSS scores were stratified into categories for comparison between groups, as described in Table 1 [8], and the mRS were graded as favorable (mRS 0–2) or unfavorable (mRS 3–6).
Table 1.
The NIHSS Score ranges that were used to classify each patient
| NIH Stroke Score Ranges | |
|---|---|
| Normal/near normal examination | 0 |
| Minor stroke | 1–4 |
| Moderate stroke | 5–15 |
| Moderate/severe stroke | 16–20 |
| Severe stroke | >20 |
2.3. Infarct Volume
As an objective measurement of stroke magnitude, the acute stroke volume was measured at presentation and discharge. Acute stroke volume at presentation was evaluated with diffusion-weighted MRI (DWI). Acute ischemic lesions were identified as hyperintense regions consistent with restricted diffusion on b=1,000 s/mm2 DWIs upon initial hospital admission.
T2-weighted or fluid attenuated inversion recovery (FLAIR) imaging and non-contrast CT were used to determine final stroke volume at time of discharge [10, 11]. Final stroke lesion volume was defined as hyperintense regions on FLAIR images or hypodense areas on CT.
Lesion volumes were calculated with the freely-available Analysis of Functional NeuroImages software package (AFNI; afni.nimh.nih.gov/afni) using a semi-automated process consisting of manually defining the relative region of the lesion, then thresholding the CT, DWI or FLAIR images based on an empirical intensity-based threshold, and manually editing the resulting contours to exclude obvious errors. Lesion volumes were estimated by multiplying the voxel resolution by the number of voxels retained within the individual contours.
2.4. Statistics
Categorical variables, including stratified NIHSS and mRS, were compared between groups using the Fisher Exact Test for 2×2 contingency tables. The mortality rates between the two groups were compared using an odds ratio. Imaging stroke volumes were compared using a paired t-test. Statistical analysis was performed using R: A Language and Environment for Statistical Computing, version 2.15.1 (2012-06-22).
3. Results
3.1. Patient Selection
The matching algorithm identified 26 pairs of PVD-control patients. Of these, 9 pairs were female and 17 pairs were male (34.6% and 65.4%). The age range was 20 to 93 years of age with a mean of 73 years for both groups and comparable standard deviations (PVD 13.6 years, Control 12.3 years). The mean age difference between each case and their matched control was 2.5 years. In addition to the clinical risk factors considered in the matching algorithm, the history of atrial fibrillation was compared between the two groups, and found to be no significantly different (38.5% of PVD patients and 30.8% of controls, p-value > 0.75). Table 2 details the demographics and risk factors after the matching algorithm was applied.
Table 2.
Summary of the demographic and risk factors
| Demographics and Risk Factors |
PVD Group | Control Group | P-Value | |
|---|---|---|---|---|
| Number of Patients | 26 | 26 | ||
| Mean age ± | 73±13.6 | 73±12.3 | ||
| Gender | ||||
| Female | 9 (34.6%) | 9 (34.6%) | ||
| Male | 17 (65.4%) | 17 (65.4%) | ||
| Ethnicity | ||||
| White | 21(80.7%) | 21(80.7%) | ||
| Black | 3 (11.5%) | 3 (11.5%) | ||
| Asian | 1 (3.9%) | 1 (3.9%) | ||
| Unspecified | 1 (3.9%) | 1 (3.9%) | ||
| Past Medical History | ||||
| Hypertension | 23 (88.5%) | 11 (42.3%) | 0.001 | |
| Diabetes | 11 (42.3%) | 22 (84.6%) | 0.003 | |
| Previous Stroke | 8 (30.8%) | 6 (23.1%) | 0.755 | |
| Previous TIA | 3 (11.5%) | 2 (7.7%) | 1.000 | |
| Smoker | 3 (11.5%) | 2 (7.7%) | 1.000 | |
| Atrial Fibrilation | 10 (38.5%) | 8 (30.8%) | 0.771 | |
3.2. Clinical Measurements
As a clinical measure of stroke severity, twenty PVD-control pairs had NIHSS scores recorded during their acute hospitalization. The distribution of scores is detailed in Figure 1. Among patients with PVD, there were a greater number with minor neurological deficits at presentation (NIHSS 1–4) than in control patients (47.1% vs. 3.9%, respectively, p-value < 0.003). In terms of clinical outcome, mRS scores were available for all patients. There was a larger proportion of good outcomes in patients with PVD compared to controls, as indicated by an mRS score less than 3 (30.8% vs. 38.4%, respectively, p-value < 0.012) (Figure 2). Furthermore, there were significantly fewer mortalities in the PVD group (7 patients, 26.9%) compared to controls (15 patients, 57.7%, Odds Ratio of mortality for PVD group 0.28, CI 0.082–0.885, p-value < 0.03).
Figure 1.
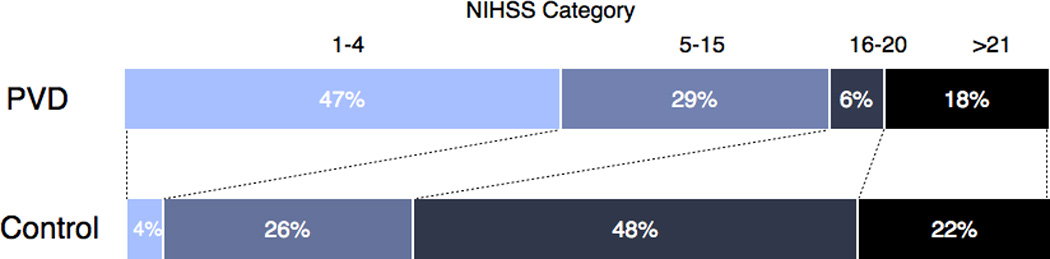
The distribution of NIHSS score categories between untreated PVD and control patients
Figure 2.
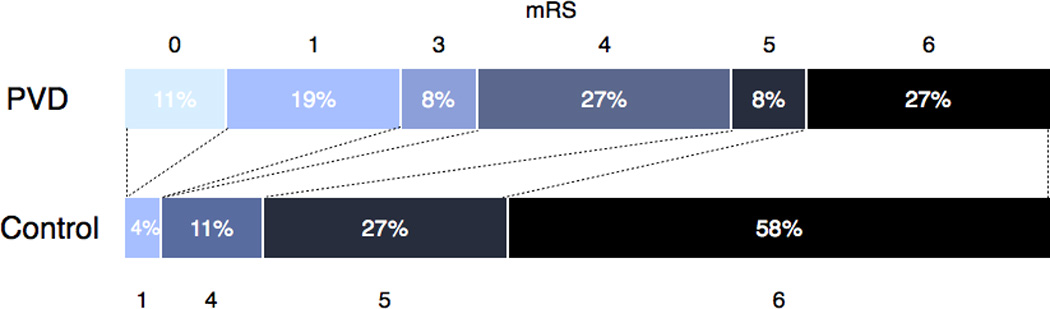
The distribution of mRS scores between the untreated PVD and control patients
3.3. Infarct Volume
Ten case-control pairs had DWI imaging at presentation available for review (Figure 3). The calculated volumes of restricted diffusion were significantly smaller for the PVD (39.6mL, SD 39.4mL) group than for the control group (148.3mL, SD 91.5mL, p-value < 0.005) (Figure 4). Additionally, seventeen case-control pairs had imaging at discharge (10 FLAIR and 7 CT), with an average post-stroke time of 4.8 days for the PVD group and 5.2 days for the control group (Figures 5). The calculated volume of completed infarct was significantly lower for the PVD group (111.8mL, SD 128.63mL) than for the control group (275.0mL, SD 141.58mL, p-value < 0.001) (Figure 6).
Figure 3.
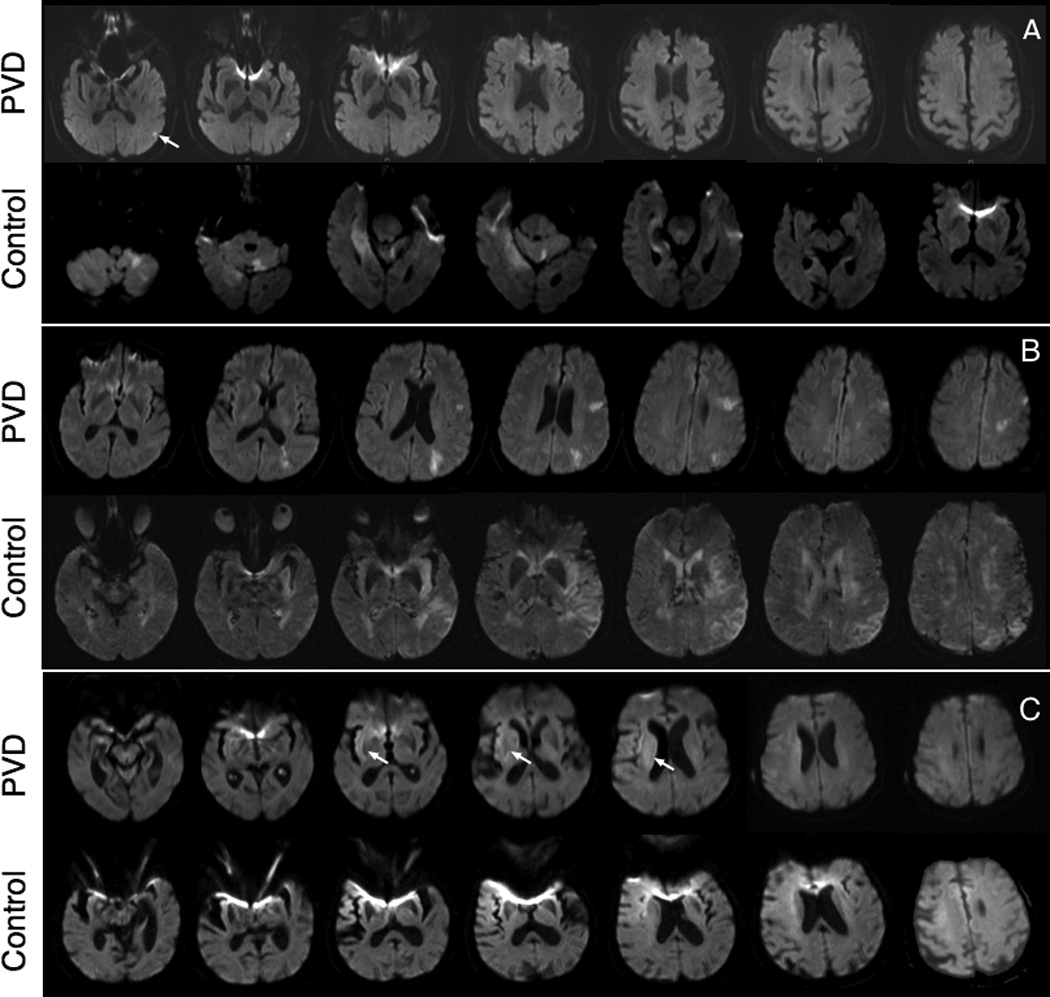
Examples of diffusion weighted images (DWI) of case-control patient pairs at presentation of stroke. A) PVD: 80 year-old male with restricted diffusion within the left cortical parietal lobe (white arrows, hyperintense volume: 0.3852mL). Control: 83 year-old male with restricted diffusion in left cerebellum, right temporal and right occipital lobe (21.36mL). B) PVD: 70 year-old female with restricted diffusion in the posterior right MCA territory (121.9mL). Control: 70 year-old female with restricted diffusion in the left MCA, caudate and semiovale (201.1mL). C) PVD: 99 year-old female, restricted diffusion in the right parietal lobe (67.96mL). Control: 87 year-old female, restricted diffusion of the right frontal cortex, right insular cortex, and extending to anterolateral right frontal lobe cortex and corona radiata (12.09mL).
Figure 4.
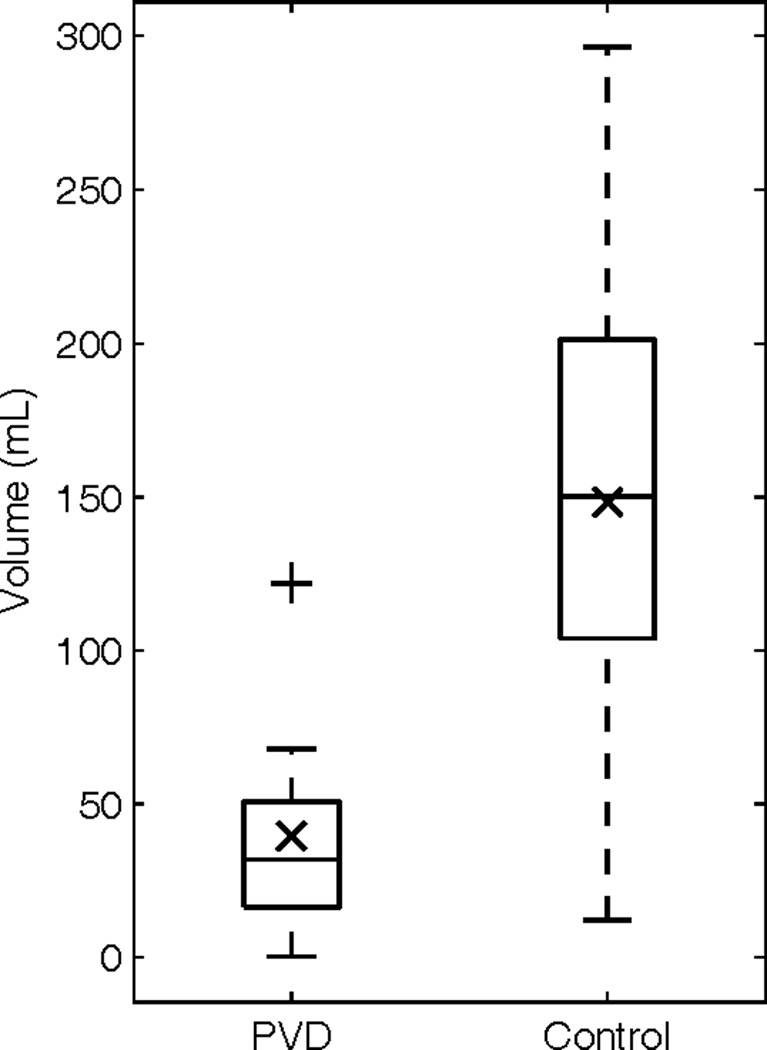
Box plot of the calculated hyperintesity volumes on DWI taken at admission (PVD mean 39.59mL sd. 39.36mL, Control mean 148.3mL sd. 91.5mL, p-value < 0.005) (× mean, + outlier).
Figure 5.
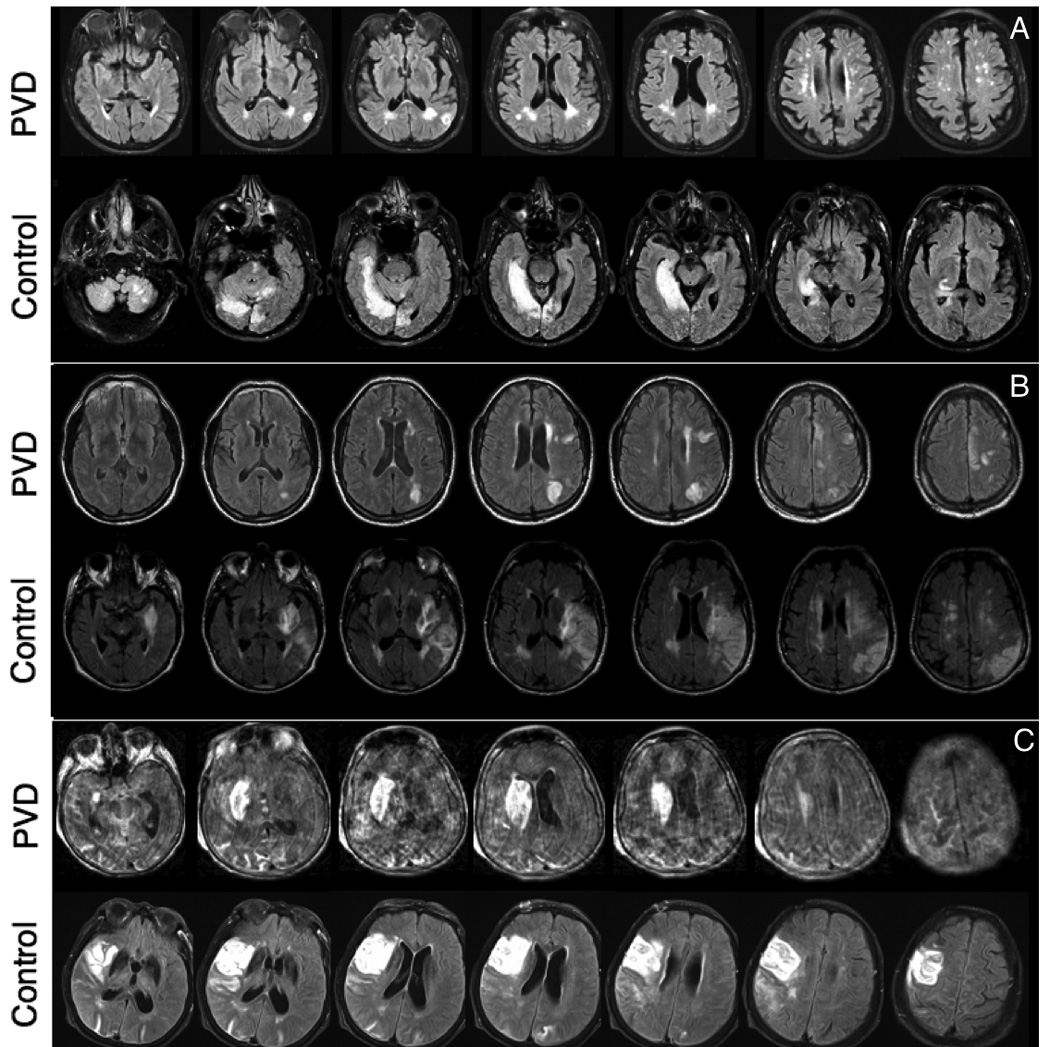
Examples of Fluid Attenuated Inversion Recovery (FLAIR) images for final stroke volume at discharge of the case-control pairs from Figure 3. A) PVD: 80 year-old male with confluent periventricular and scattered subcortical white matter hyperintensities (3.1mL). Control: 83 year-old male with hyperintensity in left cerebellum, right temporal and right occipital lobe (62.4mL). B) PVD: 70 year-old female with hyperintensity in the posterior portion of the right MCA territory (200.7mL). Control: 70 year-old female with hyperintensity in the left cortical MCA and medial left globus pallidus (348.4mL). C) PVD: 99 year-old female with hyperintensity of the right basal ganglia (39.3mL). Control: 87 year-old female with hyperintensity of right MCA territory, right postcentral gyrus and in the posterior right temporal lobe white matter (128.4.3mL).
Figure 6.
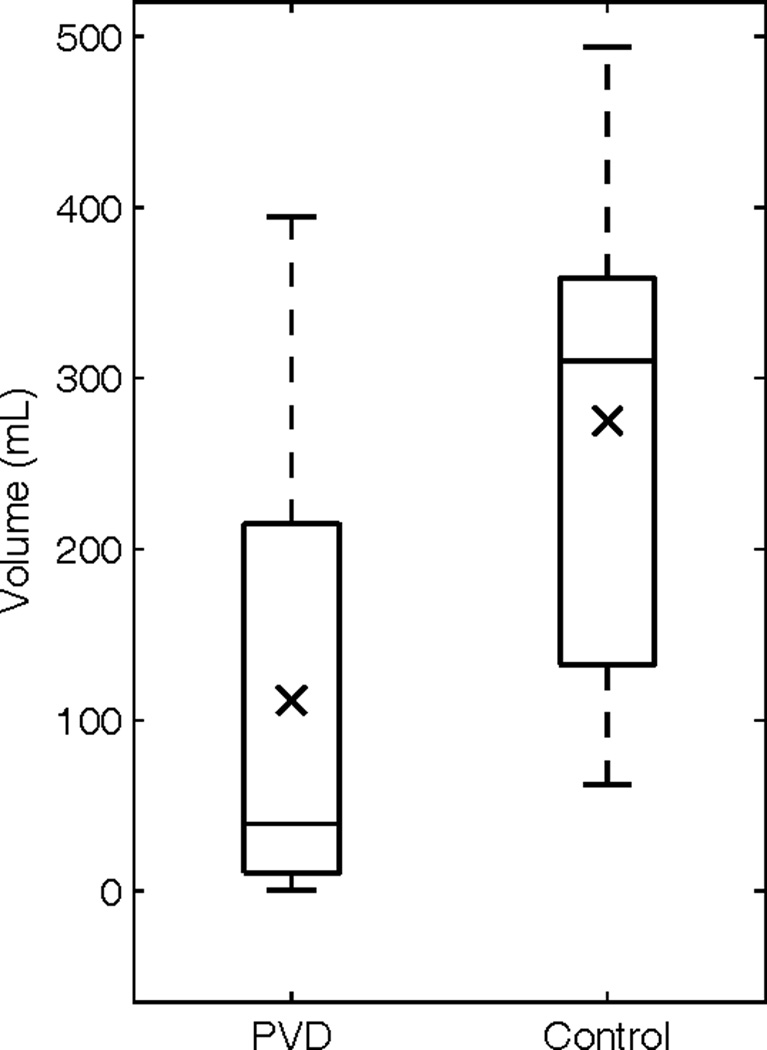
Box plot of the calculated hyperintesity volumes on FLAIR taken at discharge (PVD mean 111.8mL sd. 128.63mL, and Control mean 275.0mL sd. 141.58mL, p-value < 0.001) (× mean).
4. Discussion
4.1 Clinical Application of Ischemic Preconditioning to the Nervous System in the Literature
Remote preconditioning is a well-documented mechanism by which ischemia in a distant organ induces a protective effect against subsequent acute ischemic insult in other tissues or organs such as the brain or heart [3, 12–15]. Experiments in animal models of RIPC have shown that a sub-lethal ischemia to the hind leg will reduce the neurological deficit scores after a complete cerebral artery occlusion [12, 16].
Early clinical studies have assessed the feasibility and effect of applying RIPC in humans. A study by Koch et al. induced RIPC by using repeated cycles of ischemia and reperfusion in the limbs of patients with subarachnoid hemorrhage and found that the procedure was both safe and well tolerated [5]. This is further supported by our own findings in a clinical trial of RIPC in patients with aneurysmal subarachnoid hemorrhage. Muscle microdialysis data from these patients demonstrated that transient limb ischemia by inflation of a blood pressure cuff above systolic blood pressure induced a decrease in lactate-pyruvate ratio consistent with muscle ischemia. However, the ischemia produced by the preconditioning stimulus was at a safe, sub-lethal level, as indicated by the absence of change in glycerol levels, which indicates that cellular membranes were preserved [17]. In terms of the end-organ effects of preconditioning in these patients, we found that during the RIPC procedures the intracranial pressure and transcranial Doppler waveforms exhibited changes consistent with vasodilation. Moreover, brain microdialysis showed a reduction in the lactate/pyruvate (L/P) ratio and glycerol which persisted for 25–54 hours after the last RIPC session [7].
A trial by Meng et al. to assess the effects of RIPC on patients with intracranial arterial stenosis showed that twice-daily bilateral upper-limb ischemia for 300 days significantly reduced the incidence of stroke compared to a control group [6]. In addition to the growing evidence in support of a neuroprotective effect from remote forms of preconditioning, several studies have addressed the effects of endogenous direct ischemic preconditioning, in the form of transient ischemic attacks preceding acute ischemic stroke. A retrospective study by Weih et al. found that patients who had TIAs before stroke had less severe strokes on admission and significantly better outcomes on follow-up compared to those who first presented with an ischemic stroke [18]. A multicenter study by Wegner et al. also found that patients with prodromal TIAs tended to have smaller initial MRI DWI lesions and significantly smaller final volumes of infarcted tissue [19].
4.2 Peripheral Vascular Disease as Remote Ischemic Preconditioning
Based on these prior findings, we hypothesized that a condition of chronic blood flow reduction to a limb can act as a form of endogenous remote preconditioning. In this study, we performed a retrospective matched case-control analysis comparing patients with and without PVD who have suffered an ischemic stroke. Our results demonstrate that the group with PVD had significantly better clinical outcomes, smaller infarct volumes, and a lower mortality rate. By using a matching algorithm to pair patient demographics, past medical history and risk factors, and by accounting for stroke etiology in terms of history of atrial fibrillation and a history of TIAs, as it is a potential mechanism of preconditioning, PVD appears to be the only factor responsible for these observed differences. While this study design cannot definitively prove a cause-andeffect relationship between PVD and better clinical and imaging outcomes, the findings suggest that PVD may be acting as a form of remote ischemic preconditioning which exerts a chronic neuroprotective effect.
The results of this study also lend support to the hypothesis that hypoperfusion induced by peripheral vascular disease on the lower limb tissues is a suitable stimulus to induce remote preconditioning. The majority of preconditioning models utilize a brief period of transient, complete (zero blood flow) ischemia as a preconditioning stimulus. However, as evidenced by the improved outcomes and reduced infarct volumes in the PVD group, it may be possible that chronically reduced, but not absent, blood flow relative to the need of a tissue or organ is capable of producing similar neuroprotective effects as seen in transient, complete ischemia preconditioning models.
The correlation between PVD and acute cerebral ischemia, owing to their shared underlying risk factors and atherosclerotic vascular pathology, has been well explored in the literature. Heald et al. conducted a systematic literature review of studies comparing a low ankle-brachial index (ABI), a non-invasive measure of PVD in the lower limbs, with cardiovascular disease [9]. They found that patients with an ABI < 0.9 were at greater risk for both fatal and non-fatal stroke. Another study by Manzano et al. reported a higher prevalence of extracranial carotid disease and one year stroke rate in patients with ABI < 0.8 who were admitted for acute cerebral infarction [10]. While these results point to an expected increased occurrence of stroke in patients with PVD, they do not assess the difference in the severity of outcomes once a stroke has occurred.
In contrast to our findings, Kim et al. found that a low ABI was associated with worse functional outcomes (mRS > 2) in patients with a first-ever acute cerebral infarction [11]. This difference in results may be due to several factors. Kim’s study was performed in an exclusively Asian population while ours included a more heterogeneous racial background. Also, Kim’s study included only individuals with ABI below 0.9, while our inclusion criteria was only a history of PVD that had not been treated surgically. We excluded patients that had undergone revascularization for PVD, whom are presumably those with more severe underlying PVD, and hence lower ABIs, to begin with. Therefore, our study may have selected patients with less severe PVD and less profoundly abnormal ABIs. In addition, the severe limb vascular insufficiency of the patients in Kim’s study may have had a profound impact on those patients’ recovery potential and thus impacted their final mRS. A limitation of our study is that ABI information was not available, and therefore we cannot conclude if there is level of low ABI below which the harmful effects of limb ischemia outweigh the potentially protective effects of preconditioning in terms of overall functional outcome. However, the measurements of infarct volumes we have performed have not been analyzed in prior studies on this subject. This volumetric analysis provides an additional quantitative evaluation of stroke severity that is unaffected by PVD-related limb symptoms and resulting effects on functional status. Final infarction volume is a useful surrogate endpoint when exploring the effects of RIPC on acute stroke as confounding factors, such as limb impairment, may affect functional outcomes.
Our study is limited by its retrospective design. However, we make efforts to overcome this limitation by using a matching algorithm to allow evaluation against a control group of patients without PVD with comparable demographics and risk factors. However, the retrospective nature of the study made it impossible to control for quantitative measures of PVD severity. There is also limited availability of diagnostic images for all cases, which reduced the number of matched pairs in which stroke volume assessments were made.
5. Conclusion
This retrospective matched case-control study demonstrates that patients with PVD have better outcomes than patients without PVD following acute cerebral ischemia, in terms of NIHSS and mRS scores, infarct volume, and mortality. This suggests that chronic hypoperfusion of the lower extremities, such as that caused by PVD, may be a sufficient stimulus to induce the neuroprotective mechanisms of RIPC. Furthermore, this study supports the hypothesis that RIPC could have a protective effect in human ischemic stroke. Although the mechanisms involved in RIPC are still not completely elucidated, the association we are reporting and the potential neuroprotective effects of remote ischemic preconditioning should be further evaluated in prospective, randomized, controlled trials.
Acknowledgements
Nestor Gonzalez, M.D. Research is supported by grant 1K23NS079477-0181 from the National Institute of Neurologic Disorders and Stroke – National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.(CDC), C.f.D.C.a.P. Prevalence and most common causes of disability among adults–United States. MMWR Morb Mortal Wkly Rep. 2009;(58–426):421–426. [PubMed] [Google Scholar]
- 2.Roger VL, et al. Heart Disease and Stroke Statistics - 2012 Update. pp. e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dave KR, et al. Remote organ ischemic preconditioning protect brain from ischemic damage following asphyxial cardiac arrest. Neurosci Lett. 2006;404(1–2):170–175. doi: 10.1016/j.neulet.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 4.Koch S, Sacco RL, Perez-Pinzon MA. Preconditioning the Brain. Stroke. 2012;43(6):1455–1457. doi: 10.1161/STROKEAHA.111.646919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koch S, et al. Remote Ischemic Limb Preconditioning After Subarachnoid Hemorrhage. Stroke. 2011;42(5):1387–1391. doi: 10.1161/STROKEAHA.110.605840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meng R, et al. Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology. 2013 doi: 10.1212/WNL.0b013e318271f76a. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez NR, et al. Cerebral Hemodynamic and Metabolic Effects of Remote Ischemic Preconditioning in Patients with Subarachnoid Hemorrhage. In: Zuccarello M, et al., editors. Cerebral Vasospasm: Neurovascular Events After Subarachnoid Hemorrhage. Vienna: Springer; 2013. pp. 193–198. [DOI] [PubMed] [Google Scholar]
- 8.Mack W, et al. Principles of Endovascular Therapy. In: Robert MD, Daroff B, editors. Bradley's Neurology in Clinical Practice. Philadelphia: Elsevier Saunders; 2012. pp. 828–850. [Google Scholar]
- 9.Heald CL, et al. Risk of mortality and cardiovascular disease associated with the ankle-brachial index: Systematic review. Atherosclerosis. 2006;189(1):61–69. doi: 10.1016/j.atherosclerosis.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Manzano JJF, et al. Associations of ankle-brachial index (ABI) with cerebral arterial disease and vascular events following ischemic stroke. Atherosclerosis. 2012;223(1):219–222. doi: 10.1016/j.atherosclerosis.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Kim J, et al. Low ankle-brachial index is an independent predictor of poor functional outcome in acute cerebral infarction. Atherosclerosis. 2012;224(1):113–117. doi: 10.1016/j.atherosclerosis.2012.06.058. [DOI] [PubMed] [Google Scholar]
- 12.Birnbaum Y, Hale SL, Kloner RA. Ischemic Preconditioning at a Distance. Circulation. 1997;96(5):1641–1646. doi: 10.1161/01.cir.96.5.1641. [DOI] [PubMed] [Google Scholar]
- 13.Ren C, et al. Limb remote-preconditioning protects against focal ischemia in rats and contradicts the dogma of therapeutic time windows for preconditioning. Neuroscience. 2008;151(4):1099–1103. doi: 10.1016/j.neuroscience.2007.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen HA, et al. Remote Ischemic Preconditioning Protects the Brain Against Injury After Hypothermic Circulatory Arrest. Circulation. 2011;123(7):714–721. doi: 10.1161/CIRCULATIONAHA.110.986497. [DOI] [PubMed] [Google Scholar]
- 15.Vlasov TD, Korzhevskii DE, Polyakova EA . Ischemic Preconditioning of the Rat Brain as a Method of Endothelial Protection from Ischemic/Repercussion Injury. Neurosci Behav Physiol. 2005;35(6):567–572. doi: 10.1007/s11055-005-0095-0. [DOI] [PubMed] [Google Scholar]
- 16.Kharbanda RK, et al. Transient Limb Ischemia Induces Remote Ischemic Preconditioning In Vivo. Circulation. 2002;106(23):2881–2883. doi: 10.1161/01.cir.0000043806.51912.9b. [DOI] [PubMed] [Google Scholar]
- 17.Bilgin-Freiert A, et al. Muscle Microdialysis to Confirm Sublethal Ischemia in the Induction of Remote Ischemic Preconditioning. Transl Stroke Res. 2012;3(2):266–272. doi: 10.1007/s12975-012-0153-1. [DOI] [PubMed] [Google Scholar]
- 18.Weih M, et al. Attenuated Stroke Severity After Prodromal TIA. Stroke. 1999;30(9):1851–1854. doi: 10.1161/01.str.30.9.1851. [DOI] [PubMed] [Google Scholar]
- 19.Wegener S, et al. Transient Ischemic Attacks Before Ischemic Stroke: Preconditioning the Human Brain? Stroke. 2004;35(3):616–621. doi: 10.1161/01.STR.0000115767.17923.6A. [DOI] [PubMed] [Google Scholar]


