Abstract
Background: The Estimated Average Requirement (EAR) and Recommended Dietary Allowance (RDA) for vitamin A are 1.7 and 2.4 μmol/d (500 and 700 μg retinol activity equivalents/d), respectively, for nonpregnant, nonlactating women aged >19 y. This intake is presumed to maintain a minimally acceptable liver concentration of 0.07 μmol (20 μg) retinol/g; however, liver reserves have not been evaluated with respect to vitamin A intake in women of any age group defined in the Dietary Reference Intakes.
Objective: This cross-sectional study examined vitamin A intake and liver reserves estimated by stable-isotope dilution testing.
Design: Forty nonpregnant, nonlactating women (mean ± SD age: 22.4 ± 2.3 y) completed a Harvard food-frequency questionnaire (FFQ) and 3-d diet record (3DDR) before undergoing vitamin A status assessment by using a [13C2]retinol stable-isotope dilution test.
Results: Vitamin A intake was 70% higher than the RDA by both dietary-assessment methods (P < 0.001). The mean (±SD) liver concentration of vitamin A was 0.45 ± 0.31 μmol/g (129 ± 89 μg/g) and ranged from 0.09 (26 μg/g) to 1.79 μmol/g (513 μg/g). Liver and total-body vitamin A were highly correlated with intake measured by FFQ (P ≤ 0.009), but 3DDR was not (P ≥ 0.22). Prediction equations were developed for 3- and 7-d data.
Conclusions: In this well-nourished population, vitamin A consumption was considerably higher than recommended, and liver reserves were consistent with intake. Because of their sensitivity, stable-isotope techniques can help to describe the vitamin A status and better characterize the intake needs of all groups defined in the Dietary Reference Intakes. Registration was not required for this trial.
INTRODUCTION
Vitamin A is an essential nutrient that is important for visual, reproductive, and immunological health (1). In the United States, an estimated average requirement (EAR)4 is the “average daily nutrient intake that is estimated to meet the nutrient needs of half of the healthy individuals in a life stage or gender group” and is calculated from published data (2). The Recommended Dietary Allowance (RDA) for a nutrient is a mathematically derived estimate that should meet the needs of 97% to 98% of the population and is calculated from the EAR.
For vitamin A, the EAR is the estimated daily intake required to meet physiologic needs and maintain “minimal acceptable liver reserves” (1, 3), which are estimated to be 0.07 μmol (20 μg) vitamin A (retinol)/g liver. This concentration is assumed to prevent clinical signs of deficiency, maintain adequate plasma vitamin A concentrations, allow biliary excretion of vitamin A, and protect an individual consuming a vitamin A–deficient diet against clinical signs of deficiency for 4 mo (1). The RDA for vitamin A is the EAR plus 2 times a CV of 20%. For women aged ≥19 y, the EAR and RDA for vitamin A are estimated to be 500 and 700 μg retinol activity equivalents (RAE)/d (1.7 and 2.4 μmol retinol/d), respectively (1). Liver reserves have not been assessed with respect to vitamin A intake in women of any age group defined in the Dietary Reference Intakes. Thus, it is unknown whether the EAR and RDA yield the intended liver reserves.
Measuring liver reserves of vitamin A is difficult because liver biopsy, the gold standard, is rarely justifiable or feasible. Stable-isotope-dilution methods are a less invasive alternative. Simply put, a small dose of vitamin A tracer labeled with stable deuterium (2H) or carbon (13C) can be administered orally and after mixing with the endogenous vitamin A pool, after which blood samples are obtained and analyzed for 2H- or [13C]retinol. The dilution of tracer can be used to calculate the total body pool (4). The amount of vitamin A stored in the liver is estimated to be 40–90% of the total body pool, depending on the size of the pool, which is positively correlated with hepatic storage (5, 6).
Previous researchers have shown that liver reserves calculated by using tetradeuterated vitamin A were highly correlated with actual hepatic reserves measured from liver biopsy samples obtained during abdominal surgery in Bangladeshi and American patients (r = 0.75 and 0.88, respectively) (7, 8). Liver reserves calculated with [13C]retinol isotope dilution were also highly correlated with liver reserves (r = 0.98) in rats given low and moderate doses of vitamin A (9) and in nonhuman primates with hypervitaminosis A (10). Our goal in this study was to evaluate the relation between self-reported vitamin A intake in young adult women aged 19–30 y with liver stores by using a minimally invasive and safe [13C2]retinol isotope dilution test.
SUBJECTS AND METHODS
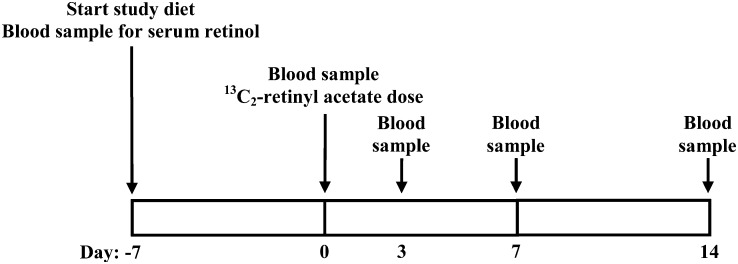
All aspects of the study were approved by the Health Sciences Institutional Review Board of the University of Wisconsin School of Medicine and Public Health before participant recruitment. This was a cross-sectional study of vitamin A intake and vitamin A status in women aged 19–30 y. The primary variables were vitamin A intake—as estimated with a 3-d diet record (3DDR) and a Harvard food-frequency questionnaire (FFQ)—and total and hepatic vitamin A—as assessed with a [13C2]retinol isotope dilution test. Secondary variables included BMI, body composition, and serum retinol. The study design is shown in Figure 1 and is described in detail in the following paragraphs.
FIGURE 1.
Study timeline. Rectangles represent 1 wk. Study days are enumerated underneath the rectangles, and study events are denoted by an arrow.
Women aged 19–30 y who had a normal BMI (in kg/m2; 18.5–24.9) by self-report and were nonsmokers, not pregnant or trying to become pregnant, not lactating, and living in the greater Madison, WI, area were invited to participate via posted flyers. An E-mail advertisement was also sent to female students at the University of Wisconsin, Madison. Recruitment occurred during February and March of 2008. Exclusion criteria included a weight loss of 4.5 kg (10 lb) or more during the 3 mo before recruitment, actively trying to lose weight, an inability to refrain from drinking alcohol when requested, an inability to follow the study diet, amenorrhea, acute or chronic illness including hepatitis, and current or previous history of anorexia or bulimia. Participants were invited to participate consecutively until the study was full. Participants gave written informed consent after receiving verbal and written information about the study and having all questions answered by a study investigator.
Baseline nutrient intakes were quantified by using a 3DDR and the Harvard FFQ 2007 Booklet, which are standard methods for estimating both short- (11) and long-term (over a 1- to 4-y period) (12, 13) nutrient intakes, respectively. Harvard FFQs were completed privately and collected by the study investigator immediately after completion. The Harvard School of Public Health quantified the FFQs, and the results were not shared with the participants. For the 3DDR, participants were asked to record all foods and beverages, including vitamins and supplements, for 2 weekdays and 1 weekend day before starting the study diet. As an incentive to complete the 3DDR, study participants were given copies of the results of their dietary analysis.
3DDRs were analyzed by using Nutritionist Pro (version 4.3.0; Axxya Systems), which uses nutrient analysis from the USDA food-composition database among others. For any foods not found in Nutritionist Pro, nutrient composition was estimated by nutritional information provided on food labels, by restaurants, or by recipe analysis with the use of Nutritionist Pro. Vitamin A intake was quantified in RAE, which include contributions from preformed vitamin A and provitamin A carotenoids with the use of the Institute of Medicine 2001 conversion factors (1), where 1 RAE = 1 μg all-trans-retinol, 2 μg supplemental all-trans-β-carotene, 12 μg dietary all-trans-β-carotene, or 24 μg of all other provitamin A carotenoids (ie, α-carotene and β-cryptoxanthin).
Fat and fat-free body mass were estimated by using air-displacement plethysmography and whole-body densitometry in a BOD POD (Life Measurements Inc)—a Food and Drug Administration–approved medical device for estimating body composition. The Siri equation, where % body fat = (4.95/body density – 4.5) × 100, was used (14). One certified BOD POD user (ARV) performed all of the measurements. Body weight was measured on the BOD POD electronic scale, which was calibrated before each participant. Height was measured by using a stadiometer, and BMI was calculated [(weight in kg)/(height in m squared)]. After the baseline measurements were made, participants were weighed weekly with a floor scale to monitor weight while consuming the study diet.
Study diet
Participants were instructed to avoid all vitamin A–containing foods and supplements for the duration of the 21-d study. The study diet, provided free-of-charge, contained 1900–2200 kcal/d with 15–30% of kcal from protein, 20–30% from fat, and the remainder from carbohydrates. The diet supplied ∼100% of the Adequate Intake for calcium (1000 mg) and 25% of the RDA for vitamin A. Participants started the study diet 7 d before the isotope dilution test was performed.
We provided all foods and limited vitamin A intake throughout the study to maintain a consistent intake of vitamin A. We instructed all participants to discontinue any supplements during the study. The contents of multivitamins and supplements are not regulated by the Food and Drug Administration and therefore contain unknown and variable amounts of vitamin A, despite labeling claims. An inconsistent influx of vitamin A would affect isotope dilution kinetics. Participants were given daily food logs, which listed the calorie, fat, protein, and carbohydrate contents of all study foods. The logs also indicated which foods contained vitamin A. Participants were instructed to eat the full portion of all foods containing vitamin A and as much or as little of any other foods they wished. Participants were asked to indicate the amount of the vitamin A–containing foods they consumed in their food logs and to record any nonstudy foods they added, so that vitamin A intake during the study could be more accurately estimated.
Sample collection
Before baseline (day −7), participants provided one venous blood sample in the morning after an 8-h fast to quantify serum retinol. Immediately after the blood draw, they started the study diet. A fasting blood sample was collected again 7 d later (baseline, day 0) immediately after which participants were given an oral dose of 2 μmol [13C2]retinyl acetate (15) dissolved in corn oil measured in a medical 1-mL syringe. Participants were required to eat peanut butter on either a cracker or banana immediately after dosing to provide a fat source for adequate dose absorption. A fasting blood sample was collected again 14 d after dosing (day 14) to estimate total-body and liver retinol reserves. The equilibrium period was chosen based on animal studies. In rats with medium to high vitamin A stores, equilibrium of labeled vitamin A between serum and liver was observed after 8 d (9), whereas in rhesus monkeys with high vitamin A stores it was achieved between 7 and 14 d (10). Blood samples were also collected at 3 and 7 d after dosing (days 3 and 7, respectively) to determine whether these earlier time points could predict the 14-d value. Participants were asked to abstain from drinking alcohol for 2 d before each blood draw and on the day of [13C2]retinyl acetate dosing. Participants were paid $25 for the completion of paperwork and blood draws.
Trained phlebotomists collected blood samples into serum separator tubes with clot activator (Becton Dickinson). After clotting at room temperature in the dark to avoid photolysis of vitamin A, the samples were centrifuged at 2200 × g for 10 min at 4°C. Serum was collected and placed into vials of 1- or 2-mL aliquots. Nitrogen was blown into the vials to displace oxygen before storage at –70°C until analysis.
Carbon isotope composition of serum retinol and estimation of vitamin A stores
Retinol was purified from 1–2-mL serum samples as described previously (16) with minor modifications: a GraceSmart RP18 (150 × 4.6 mm, 5 μm, 120 Å) column was used for the first purification and a GraceSmart RP18 (250 × 4.6 mm, 5 μm, 120 Å) column for the second. Reconstituted purified retinol from the serum was injected into the same gas chromatograph–combustion–isotope ratio mass spectrometer system as previously published (16) and run under the same conditions used by Escaron et al (10). The gas chromatograph–combustion–isotope ratio mass spectrometer generates a value based on the enrichment of the carbon pool with 13C or atom percent 13C (At% 13C).
Total-body reserves (TBRs) of vitamin A were estimated by applying the standard mass-balance equation (17) to 13C in retinol:
where a is μmol retinol absorbed from the dose, estimated to be 100% of the administered dose for those with normal to excessive stores (10), b is the TBR in μmol vitamin A at baseline, and c is the TBR in μmol of vitamin A after dosing (c = a +b). Fa is the fraction of the dose that is labeled with 13C, which is equal to 0.100 because [13C2]retinyl acetate is converted to [13C2]retinol in vivo at the time of absorption, 2 of the 20 carbons were labeled, and F = R/(R + 1), where R is 13C/12C. Fb is the At% 13C in retinol at baseline in decimal form, and Fc is the decimal form of At% 13C after dosing.
Equation 1 is solved for b, the TBR of retinol at baseline. The baseline TBR of vitamin A can then be corrected for catabolism of the dose of labeled vitamin A, which is assumed to be equivalent to the half-life of unlabeled vitamin A (140 d in adults) and independent of vitamin A status and time (8, 18) using Equation 2.
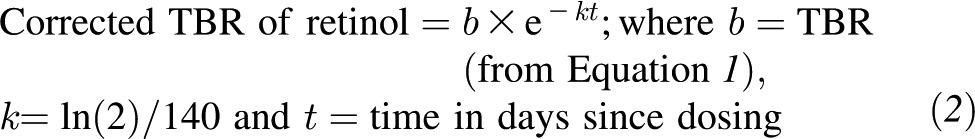 |
Previous studies have shown that liver storage is positively correlated with total body status and can range from 40% to 90% (1, 19) of total-body vitamin A. In rhesus monkeys with hypervitaminosis A, a liver reserve of 80% of TBR of vitamin A underestimated actual liver storage (10). Thus, to estimate hepatic stores of vitamin A, we assumed that we were studying a well-nourished population and estimated that 80% of TBR was contained in the liver (ie, total hepatic vitamin A = corrected TBR × 0.8). Finally, liver vitamin A was expressed per gram, assuming that liver weight is 2.4% of body weight in adults (3). Serum was analyzed for retinol by HPLC as published previously (16).
Statistics
Two-tailed t tests, paired and unpaired, were applied to compare means as appropriate. Pearson correlation, simple linear regression, and multiple linear regression were used to evaluate associations between variables and for prediction equations. Data are presented as means with measurement uncertainty described with SDs, unless otherwise indicated. Participants with missing data were included in the analyses. All analyses were performed with SPSS version 20 (IBM). P < 0.05 was considered statistically significant unless a Bonferroni correction was applied (noted in text).
RESULTS
Forty women participated in the study. Baseline characteristics of the study participants are presented in Table 1. All women completed FFQs, 33 returned 3DDRs, and 39 were successfully analyzed by the 13C2-RID test to assess vitamin A status. Twenty-five women (64%) reported using oral contraceptives. Post hoc analyses were performed to compare baseline characteristics between women who reported the use of oral contraceptives (Table 1). One woman did not disclose her use or nonuse of oral contraceptives; thus, n = 39. A more conservative P value (P < 0.01) was used for the post hoc analyses to account for multiple comparisons. No differences in baseline characteristics were found between groups.
TABLE 1.
Baseline characteristics of the study participants and subgroup analysis of OC use1
| All participants | OC user (n = 25) | No OC (n = 14) | P value | |
| Age (y) | 22.4 ± 2.3 (19–27)2 | 22.2 ± 2.2 | 23.0 ± 2.5 | 0.29 |
| Weight (kg) | 61.2 ± 7.2 (45.6–77.3) | 60.4 ± 6.4 | 62.7 ± 8.3 | 0.35 |
| BMI (kg/m2) | 22.3 ± 2.2 (18.6–26.0) | 22.0 ± 2.1 | 22.6 ± 2.3 | 0.39 |
| Fat mass (%) | 26.6 ± 6.1 (13.2–41.9) | 26.3 ± 4.9 | 26.8 ± 8.1 | 0.84 |
| Energy intake (kcal/d)3 | 1768 ± 578 (555–3600) | 1648 ± 486 | 2034 ± 650 | 0.042 |
| Protein intake (g/d)3 | 73 ± 26 (30–151) | 67 ± 21 | 85 ± 30 | 0.031 |
| Total fat intake (g/d)3 | 57 ± 23 (19–123) | 54 ± 20 | 64 ± 27 | 0.22 |
| Vitamin A intake including supplements, by FFQ (RAE/d)3 | 1213 ± 778 (378–3890) | 1035 ± 588 | 1479 ± 999 | 0.088 |
| Vitamin A intake excluding supplements, by FFQ (RAE/d)3 | 811 ± 405 (259–2190) | 706 ± 281 | 1026 ± 514 | 0.046 |
| Vitamin A intake, by 3DDR (RAE/d)4 | 1180 ± 705 (78–3020) | 1009 ± 591 | 1478 ± 850 | 0.077 |
| Reporting supplement use on FFQ [n (%)] | 40 (16) | 40 (10) | 43 (6) | 0.67 |
| Reporting supplement use on 3DDR [n (%)]5 | 9 (3) | 5 (1) | 18 (2) | 0.43 |
| Serum retinol (μmol/L)5 | 2.68 ± 0.88 (0.81–5.89) | 2.68 ± 0.69 | 2.69 ± 1.3 | 0.96 |
| Total-body vitamin A (μmol)6 | 816.5 ± 537.4 (141.5–3116) | 854.8 ± 639.5 | 731.2 ± 289.7 | 0.51 |
| Hepatic vitamin A (μmol/g)7 | 0.45 ± 0.31 (0.09–1.79) | 0.48 ± 0.37 | 0.40 ± 0.18 | 0.48 |
n = 40, except where indicated. P < 0.01 was considered significant (multiple post hoc comparisons; t tests or chi-square tests were used to compare groups). FFQ, food-frequency questionnaire; OC, oral contraceptive; RAE, retinol activity equivalents [where 1 RAE = 1 μg all-trans-retinol, 2 μg supplemental all-trans-β-carotene, 12 μg dietary all-trans-β-carotene, or 24 μg of all other provitamin A carotenoids (ie, α-carotene and β-cryptoxanthin)]; 3DDR, 3-d diet record.
Mean ± SD; range in parentheses (all such values).
Baseline dietary intake evaluated by using the Harvard FFQ.
Baseline dietary intake evaluated by using the 3DDR, n = 33. Includes supplements/vitamin pills.
n = 33.
Calculated by using Equations 1 and 2 (see Subjects and Methods for details), n = 39.
Calculated by assuming that 80% total-body vitamin A is stored in the liver and a liver weight of 2.4% body weight (see Subjects and Methods for additional details), n = 39.
Baseline daily intake of vitamin A was significantly higher than the RDA as estimated by both the FFQ and 3DDR (P < 0.001). Approximately 33% of daily vitamin A intake over the prior year was from supplements or multivitamins, as assessed by FFQ. On the basis of FFQ data, bivariate correlations showed that vitamin A intake including supplements was not correlated with calorie intake (Pearson correlation coefficient = 0.29, P = 0.066). However, when supplements were excluded, vitamin A intake was positively correlated with calories (Pearson correlation coefficient = 0.74, P < 0.001). Vitamin A intake including supplements by FFQ was not correlated with intake by 3DDR (Pearson correlation coefficient = 0.31, P = 0.084); however, when supplements were excluded from the FFQ analysis, a significant correlation with the 3DDR was found (Pearson correlation coefficient = 0.48, P = 0.005). Daily vitamin A intake on the study diet was 155 ± 29 μg RAE (∼22% of the RDA), and body weight did not change (P > 0.05; data not shown).
The mean liver concentration (Table 1) was >0.07—the current cutoff for deficiency (P < 0.0001, t test). None of the women had deficient stores. However, a higher cutoff for deficiency was recently proposed at 0.1 μmol/g (20), and one woman had liver stores below this cutoff. Two women had stores above the proposed upper limit of normal (1.05 μmol/g liver) (4).
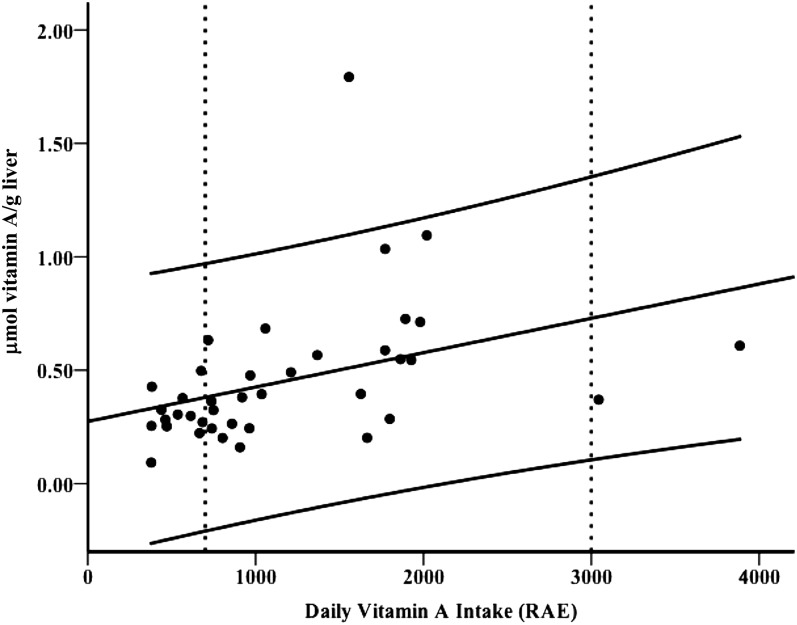
Simple linear regression showed that liver and TBR vitamin A were dependent on intake as assessed by FFQ when supplements were included: Pearson correlation coefficient = 0.41 (P = 0.009; Figure 2) and 0.40 (P = 0.011), respectively; however, no association was found when supplements were excluded (P ≥ 0.48). TBR and hepatic vitamin A were not correlated with intake by 3DDR (P ≥ 0.22). TBR and liver vitamin A were not correlated with FFQ total energy, fat, protein, or carbohydrate intake (P ≥ 0.86). TBR and liver reserves were negatively correlated with percentage fat mass (Pearson correlation coefficient = −0.34, P ≤ 0.034) but not with BMI or body weight (P ≥ 0.24). Fat mass was not correlated with vitamin A intake by FFQ (P = 0.13). The linear regression model improved when both vitamin A intake by FFQ and percentage fat mass were included as independent predictors of hepatic liver reserves (R2 = 0.25, P = 0.005). Finally, removal of an outlier made only a marginal improvement in the model (R2 = 0.27, P = 0.004). No association was found between serum retinol and liver reserves (P = 0.18).
FIGURE 2.
Liver reserves of vitamin A in young adult women (n = 39) calculated by using the [13C2]retinol isotope dilution test were correlated with vitamin A intake, including supplements and vitamins, as assessed with a Harvard food-frequency questionnaire in RAE (Pearson correlation coefficient = 0.41, P = 0.009). 1 RAE = 1 μg all-trans-retinol, 2 μg supplemental all-trans-β-carotene, 12 μg dietary all-trans-β-carotene, or 24 μg of all other provitamin A carotenoids (ie, α-carotene and β-cryptoxanthin). The center solid line is the linear regression line (liver reserves = 0.0001 × daily intake + 0.23, P = 0.009 and 0.017 for the slope and intercept, respectively) with 95% CIs. Vertical dashed lines represent the Recommended Dietary Allowance (700 μg/d) and the Tolerable Upper Intake Level (3000 μg/d) for women aged ≥19 y. RAE, retinol activity equivalents.
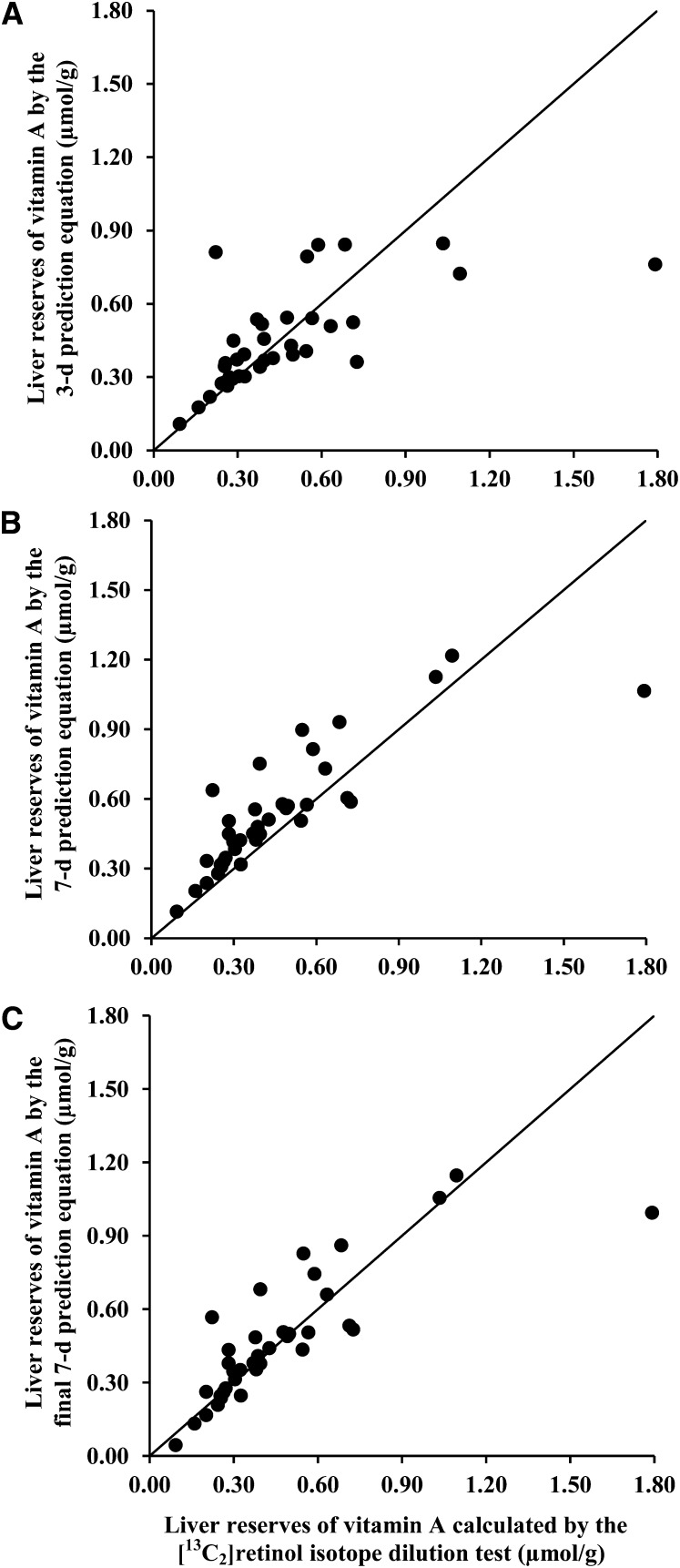
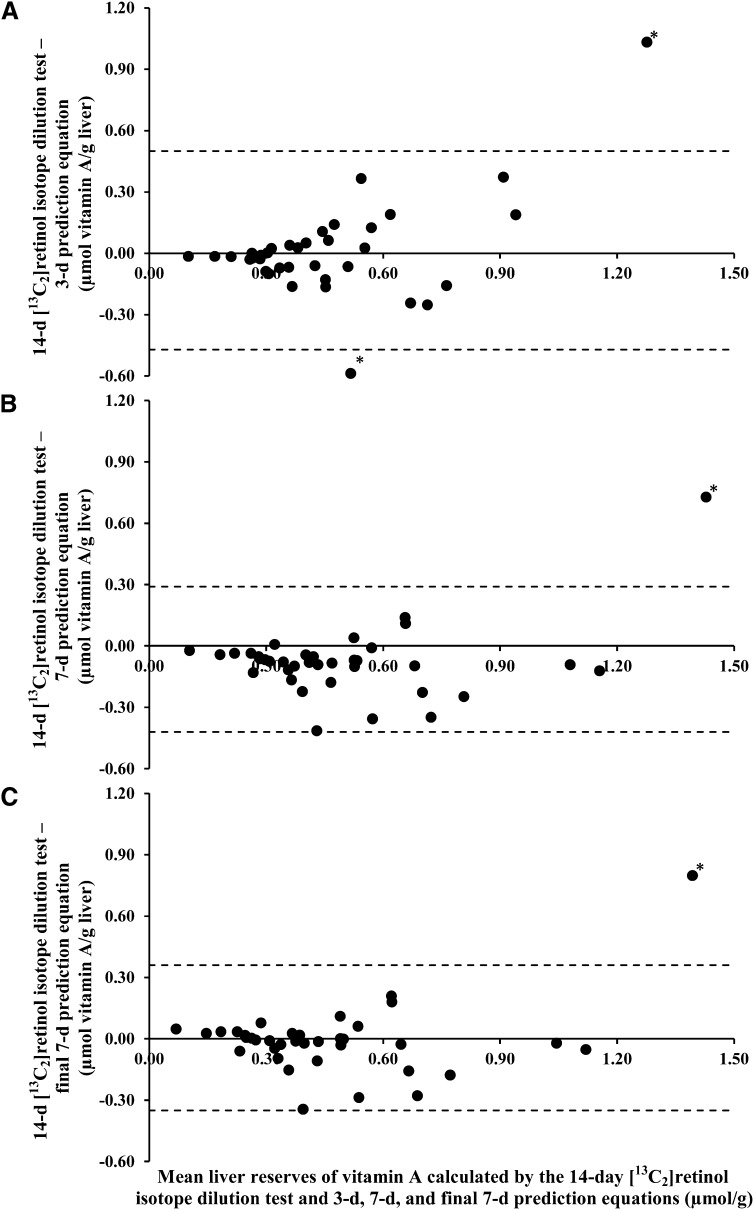
Liver stores of vitamin A calculated by using the 3- and 7-d %At 13C values were positively correlated with (P < 0.0001 for all), but were significantly different from (P < 0.0001, paired t tests with Bonferroni correction), the 14-d estimate (Pearson correlation coefficient = 0.62 and 0.87, for 3- and 7-d data, respectively). Linear regression equations were calculated for the 14-d value as a function of the 3- or 7-d data. For the 3-d data, the slope was 3.53 (P < 0.0001), and the intercept was 0.009 (P = 0.93). For the 7-d data, the slope and intercept were 1.72 (P < 0.0001) and –0.083 (P = 0.22), respectively. For simplicity, the prediction equations were rounded as follows: 14-d predicted liver reserves = 3.5 × (3-d estimate) and = 1.7 × (7-d estimate). With the use of these equations, predicted liver reserves were calculated and plotted against the actual 14-d liver reserve in Figure 3 (A and B). Bland-Altman plots are shown in Figure 4 (A and B). The difference between actual 14-d values and predicted 14-d values using the 3-d equation was not different from zero (P = 0.76). A significant difference was found between actual 14-d values and predicted 14-d values using the 7-d prediction equation, where the 7-d prediction equation overestimated liver reserves by 0.07 μmol/g (P = 0.026). Thus, an intercept was included in the final 7-d prediction equation such that 14-d predicted liver reserves = 1.7 × (7-d estimate) – 0.07 (Pearson correlation coefficient = 0.81, P < 0.0001). The relation between the final 7-d prediction equation and actual 14-d values is shown in Figure 3C, and the respective Bland-Altman plot is shown in Figure 4C.
FIGURE 3.
Liver reserves in young adult women were calculated by using the 3-d (A), 7-d (B), and final 7-d (C) prediction equations compared with liver reserves calculated by using the 14-d [13C2]retinol isotope dilution test. The prediction equations were as follows: 3-d prediction equation, liver reserves (μmol/g) = 3.5 × (3-d [13C2]retinol isotope dilution test value); 7-d prediction equation, liver reserves (μmol/g) = 1.7 × (7-d [13C2]retinol isotope dilution test value); and final 7-d prediction equation, liver reserves (μmol/g) = 1.7 × (7-d [13C2]retinol isotope dilution test value) − 0.07. The [13C2]retinol isotope dilution test value was obtained for each time point by solving the following equation for b: (Fa × a) + (Fb × b) = (Fc × c), where a is μmol retinol absorbed from the 2-μmol dose, estimated to be 100%; b is the total body reserves in μmol vitamin A at baseline; and c = a + b. Fa is the fraction of the dose that is labeled with 13C (0.100 because 2 of the 20 carbons were labeled with 13C). Fb is atom percent 13C (At% 13C) in serum retinol at baseline in decimal form, and Fc is the decimal form of At% 13C in serum retinol 3, 7, or 14 d after dosing. Variable b was corrected for catabolism of the dose [b × e−kt; where k = ln(2)/140 and t = time in days since dosing]. n = 36, 38, and 38 for A, B, and C, respectively.
FIGURE 4.
Bland-Altman plots of the 14-d [13C2]retinol isotope dilution test results and 3-d (A), 7-d (B), and final 7-d (C) prediction equations in young adult women. Liver reserves were calculated once by using the 14-d [13C2]retinol isotope dilution test and the 3-d, 7-d, and final 7-d prediction equations, where estimated liver reserves = 3.5 × (3-d [13C2]retinol isotope dilution test value), 1.7 × (7-d [13C2]retinol isotope dilution test value), or 1.7 × (7-d [13C2]retinol isotope dilution test value) + 0.07, respectively. The difference and mean of the 14-d [13C2]retinol isotope dilution test and each prediction equation were calculated and plotted. Limits of agreement (mean difference ± 2 SDs) are shown by the dashed lines. Possible outliers are denoted with an asterisk. The 3-d prediction equation appears to have increasing variability from the 14-d [13C2]retinol isotope dilution test result as liver reserves increase. The 7-d prediction equation systematically overestimated liver reserves compared with the 14-d [13C2]retinol isotope dilution test by 0.07 μmol/g (t test, P = 0.026). The mean difference between the final 7-d prediction equation and the 14-d [13C2]retinol isotope dilution test result was not different from zero (t test, P = 0.96). n = 36, 38, and 38 for A, B, and C, respectively.
Because mean vitamin A intake was considerably higher than the EAR and RDA, we performed 2 post hoc analyses to examine liver reserves among women who consumed vitamin A in amounts closer to the EAR and/or RDA. We arbitrarily chose a range of vitamin A consumption of 400 to 1000 RAE (ie, the RDA ± 300) and 300 to 700 RAE (ie, the EAR ± 200) by FFQ and calculated the average liver reserves of women whose intake was within these ranges. Nineteen women consumed between 400 and 1000 RAE/d (mean = 709 ± 167 RAE/d) and had mean liver reserves of 0.32 ± 0.11 μmol/g. Twelve women consumed 300–700 RAE/d (mean = 521 ± 119 RAE/d) and had mean liver reserves of 0.30 ± 0.10 μmol/g.
DISCUSSION
Our study contributes several new findings on vitamin A intake and status in young adult women in the United States. We provide evidence that vitamin A intake among this group is higher than the RDA and show a correlation between reported intakes by using FFQ and estimated hepatic concentrations of vitamin A by using a stable-isotope-dilution technique. Furthermore, our data suggest that at these higher-than-recommended intakes, liver reserves remain within normal limits for most women. In addition, our study was the first to provide rudimentary evidence that the current RDA results in higher liver storage than expected. Finally, these data suggest that the use of earlier time points to predict liver reserves is likely feasible with appropriate corrections, as has been suggested in previous literature on vitamin A status assessments using isotope-dilution techniques (21, 22).
In this study, vitamin A intake in women with a mean age of 22.4 y was ∼70% higher than the RDA for their age and sex groups. Nearly one-third of dietary vitamin A was obtained from supplements over the long term. Vitamin A intake, as assessed by FFQ when supplements were included, was highly correlated with liver concentration as assessed by the [13C2]retinol isotope dilution test. The high intake in this age group was not surprising given the low cost of supplements, the availability of vitamin A–fortified foods, and the general encouragement by health care professionals to take a daily multivitamin. Concerns have been raised about the ease of inadvertent overconsumption of vitamin A (23) and a possible association between high intake as an older adult and an increased risk of osteoporosis (24). It is possible that a chronic high intake of vitamin A, starting at a young age, may have a greater effect on osteoporosis risk than do high intakes for only a few years at an older age. Although intake in this study was higher than recommended, it was less than the Tolerable Upper Intake Level, and liver reserves reflected an adequate but not excessive vitamin A status in most women.
The results of this study allow for a rudimentary evaluation of the RDA for women aged 19–30 y. The EAR, from which the RDA is derived, was set by calculating the amount of daily vitamin A intake that would maintain normal physiologic function plus 4 mo of protection during times of deficient vitamin A intake by using the following equation:
where A is the percentage of body vitamin A stores lost per day during ingestion of a vitamin A–free diet = 0.5%, B is the minimum acceptable liver vitamin A reserve = 0.07 μmol/g liver (ie, 20 μg/g), C is the ratio of liver to body weight = 0.03, D is the reference weight of a specific age group and sex (ie, 61 kg for adult women), E is the ratio of total body to liver vitamin A reserves = 1.1, and F is the efficiency of storage of ingested vitamin A = 0.5 (1, 3).
We examined a subset of women with a mean daily vitamin A intake similar to the EAR (mean ± SD = 521 ± 119 RAE/d) and found an average liver reserve of 0.30 ± 0.10 μmol/g, which is >4 times the minimum acceptable reserve. There are many possible explanations for this finding. First, variable F in Equation 3 may underestimate efficiency of hepatic vitamin A storage when total-body vitamin A is high. Alternatively, variable E may also vary with the total body pool of vitamin A. Finally, an additional factor or factors that account for the duration of adequate intake or status may be required to adjust the EAR for alterations in vitamin A metabolism and storage during periods of adequacy or excess.
Our study also contributes to the literature on isotope dilution testing for vitamin A status. Using the standard mass–balance equation, liver reserves calculated by using the 3- and 7-d %At 13C values were different from but highly correlated with those calculated by using the 14-d value. Using linear regression, we were able to calculate a simple prediction equation for liver reserves for each of the earlier time points. There is mounting evidence that a prediction equation could be developed to estimate liver reserves based on a 3-d blood sample (21, 22, 25–27). For our study, the Bland-Altman plot shows that as liver reserves increase, the liver reserves estimated by the 3-d prediction equation become less similar to the 14-d calculated values. Prior research has shown that 3 d is too short for the test dose to equilibrate with the body pool of vitamin A (7, 9, 10, 26). The 3-d sampling may be best suited for a qualitative, rather than quantitative, description of vitamin A status. Our final prediction equation using the 7-d data were highly correlated with and without any systematic deviation from the 14-d calculated liver reserves and appears to be a fairly good predictor of liver reserves in our study population. We caution against the routine use of either of the prediction equations that we calculated in this study, because these are preliminary data based on a homogeneous study population. Using earlier time points to predict liver reserves must be investigated across a wider range of vitamin A statuses as well as after an intervention to be confident of their accuracy.
Our study was limited by the homogeneous sample that consisted primarily of white university students in their early twenties. This group of women likely represents a higher socioeconomic demographic than all women aged 19–30 y living in the United States. Thus, our study may have been confounded by a higher nutritional and educational status, which contributed to a greater intake of vitamin A. We do not know the magnitude of this potential sampling bias, although our results are consistent with other studies showing that high intakes are correlated with normal to high liver stores in both animals and humans. Given our limited sample, this study may not be generalizable to all American women aged 19–30 y. However, we believe that our findings offer a foundation from which to base and compare future investigations.
Additional limitations of this and other isotope dilution tests are the multiple assumptions that are applied when calculating vitamin A liver reserves. We assumed a liver storage of 80% TBR based on prior research. This storage percentage may be highly variable, both among different populations and individuals, and there are no data to suggest that 80% is the best value to use in this group of women. Furthermore, we estimated liver size. Perhaps vitamin A status should be redefined in terms of TBR, as opposed to hepatic storage, to avoid multiple assumptions. A difficulty of defining vitamin A status in terms of TBR is the effect of body habitus. We found that vitamin A status was negatively correlated with body fat in our population of normal-weight women. Were vitamin A status to be defined in terms of TBR, a correction for body fat, weight, or BMI may be necessary.
In summary, vitamin A intake is higher than recommended among our sample of American women in their early twenties, who are a defined subgroup for the RDA. Whereas liver storage is generally within normal limits, 2 women had liver reserves considered hypervitaminotic by some researchers, although the clinical significance of this definition is currently unknown. In a subset of women consuming an average EAR for vitamin A, liver reserves were 4 times greater than the minimally acceptable cutoff from which the EAR was originally calculated. It is unknown whether liver stores of this size in young adulthood pose any clinical concerns. The EAR for well-nourished populations needs to be reevaluated with regard to vitamin A balance, and healthy vitamin A status needs to be better defined to avoid potential long-term consequences of high intake. Stable-isotope technology is a promising approach for addressing these issues (20).
Acknowledgments
The authors’ responsibilities were as follows—ARV and SAT: designed the research and wrote the manuscript; ARV and CRD: conducted the research; ARV: analyzed the data; and SAT: had primary responsibility for the final content. The authors had no conflicts of interest with the content of this manuscript.
Footnotes
Abbreviations used: At%, atom percent; EAR, estimated average requirement; FFQ, food-frequency questionnaire; RAE, retinol activity equivalents; RDA, Recommended Dietary Allowance; TBR, total body reserve; 3DDR, 3-d diet record.
REFERENCES
- 1.Institute of Medicine Food and Nutrition Board. Vitamin A. In: Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc Washington, DC: National Academy Press, 2001:82–161. [Google Scholar]
- 2.Institute of Medicine. Introduction to the dietary reference intakes Otten JJ, Hellwig JP, Meyers LD. eds. Dietary reference intakes: the essential guide to nutrient requirements Washington, DC: National Academies Press, 2006:5–17. [Google Scholar]
- 3.Olson JA. Recommended dietary intakes (RDI) of vitamin A in humans. Am J Clin Nutr 1987;45:704–16. [DOI] [PubMed] [Google Scholar]
- 4.Haskell MJ, Ribaya-Mercado JD. Washington, DC: HarvestPlus, 2005. [Google Scholar]
- 5.Rietz P, Wiss O, Weber F. Metabolism of vitamin A and the determination of vitamin A status. Vitam Horm 1974;32:237–49. [DOI] [PubMed] [Google Scholar]
- 6.Bausch J, Rietz P. Method for the assessment of vitamin A liver stores. Acta Vitaminol Enzymol 1977;31:99–112. [PubMed] [Google Scholar]
- 7.Haskell MJ, Handelman GJ, Peerson JM, Jones AD, Rabbi MA, Awal MA, Wahed MA, Mahalanabis D, Brown KH. Assessment of vitamin A status by the deuterated-retinol-dilution technique and comparison with hepatic vitamin A concentration in Bangladeshi surgical patients. Am J Clin Nutr 1997;66:67–74. [DOI] [PubMed] [Google Scholar]
- 8.Furr HC, Amedee-Manesme O, Clifford AJ, Bergen HR, Jones AD, Anderson DP, Olson JA. Vitamin A concentrations in liver determined by isotope dilution assay with tetradeuterated vitamin A and by biopsy in generally healthy adult humans. Am J Clin Nutr 1989;49:713–6. [DOI] [PubMed] [Google Scholar]
- 9.Tanumihardjo SA. Vitamin A status assessment in rats with 13C4-retinyl acetate and gas chromatography/combustion/isotope ratio mass spectrometry. J Nutr 2000;130:2844–9. [DOI] [PubMed] [Google Scholar]
- 10.Escaron AL, Green MH, Howe JA, Tanumihardjo SA. Mathematical modeling of serum 13C-retinol in captive rhesus monkeys provides new insights on hypervitaminosis A. J Nutr 2009;139:2000–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammond K. Assessment: dietary and clinical data Escott-Stump S, Krause MV, Mahan LK. eds. St Louis, MO: Elsevier Saunders, 2008:383–410. [Google Scholar]
- 12.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- 13.Willett WC, Sampson L, Browne ML, Stampfer MJ, Rosner B, Hennekens CH, Spelzer FE. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol 1988;127:188–99. [DOI] [PubMed] [Google Scholar]
- 14.Siri WE. Body composition from fluid spaces and density: analysis of methods. 1961. Nutrition 1993;9:480–91. [PubMed] [Google Scholar]
- 15.Tanumihardjo SA. Synthesis of 10,11,14,15-13C4 and 14,15-13C2 retinyl acetate. J Labelled Comp Radiopharm 2001;44:365–72. [Google Scholar]
- 16.Howe JA, Valentine AR, Hull AK, Tanumihardjo SA. 13C natural abundance in serum retinol acts as a biomarker for increases in dietary provitamin A. Exp Biol Med (Maywood) 2009;234:140–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodman KJ, Brenna JT. High sensitivity tracer detection using high-precision gas chromatography-combustion isotope ratio mass spectrometry and highly enriched [U-13C]-labeled precursors. Anal Chem 1992;64:1088–95. [DOI] [PubMed] [Google Scholar]
- 18.Sauberlich HE, Hodges RE, Wallace DL, Kolder H, Canham JE, Hood H, Raica N, Jr, Lowry LK. Vitamin A metabolism and requirements in the human studied with the use of labeled retinol. Vitam Horm 1974;32:251–75. [DOI] [PubMed] [Google Scholar]
- 19.Raica N, Jr, Scott J, Lowry L, Sauberlich HE. Vitamin A concentration in human tissues collected from five areas in the United States. Am J Clin Nutr 1972;25:291–6. [DOI] [PubMed] [Google Scholar]
- 20.Tanumihardjo SA. Vitamin A: biomarkers of nutrition for development. Am J Clin Nutr 2011;94(suppl):658S–65S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang G, Qin J, Hao LY, Yin SA, Russell RM. Use of a short-term isotope-dilution method for determining the vitamin A status of children. Am J Clin Nutr 2002;76:413–8. [DOI] [PubMed] [Google Scholar]
- 22.Ribaya-Mercado JD, Solon FS, Dallal GE, Solomons NW, Fermin LS, Mazariegos M, Dolnikowski GG, Russell RM. Quantitative assessment of total body stores of vitamin A in adults with the use of a 3-d deuterated-retinol-dilution procedure. Am J Clin Nutr 2003;77:694–9. [DOI] [PubMed] [Google Scholar]
- 23.Penniston KL, Tanumihardjo SA. Vitamin A in dietary supplements and fortified foods: too much of a good thing? J Am Diet Assoc 2003;103:1185–7. [DOI] [PubMed] [Google Scholar]
- 24.Penniston KL, Tanumihardjo SA. The acute and chronic toxic effects of vitamin A. Am J Clin Nutr 2006;83:191–201. [DOI] [PubMed] [Google Scholar]
- 25.Adams WR, Green MH. Prediction of liver vitamin A in rats by an oral isotope dilution technique. J Nutr 1994;124:1265–70. [DOI] [PubMed] [Google Scholar]
- 26.Ribaya-Mercado JD, Mazariegos M, Tang G, Romero-Abal ME, Mena I, Solomons NW, Russell RM. Assessment of total body stores of vitamin A in Guatemalan elderly by the deuterated-retinol-dilution method. Am J Clin Nutr 1999;69:278–84. [DOI] [PubMed] [Google Scholar]
- 27.Ribaya-Mercado JD, Solon FS, Fermin LS, Perfecto CS, Solon JA, Dolnikowski GG, Russell RM. Dietary vitamin A intakes of Filipino elders with adequate or low liver vitamin A concentrations as assessed by the deuterated-retinol-dilution method: implications for dietary requirements. Am J Clin Nutr 2004;79:633–41. [DOI] [PubMed] [Google Scholar]