Abstract
Background: Researchers have proposed biologically plausible mechanisms linking excessive gestational weight gain (GWG) to maternal metabolic and cardiovascular diseases later in life.
Objective: The objective was to determine the extent to which GWG was associated with abdominal adiposity and other cardiometabolic risk factors in a sample of women 4–12 y after delivery.
Design: We used data from The Women's and Infants’ Study of Healthy Hearts, a cohort of women who gave birth between 1997 and 2002 at Magee-Womens Hospital in Pittsburgh, PA. By design, women with small-for-gestational-age and preterm births were oversampled. Women with preeclampsia, prepregnancy hypertension, or diabetes were excluded. GWG was ascertained from prenatal records, and GWG adequacy was assessed according to 2009 Institute of Medicine/National Research Council guidelines. Abdominal obesity was defined as waist circumference (WC) >88 cm and weight change as current weight – prepregnancy weight.
Results: The prevalence of inadequate, adequate, and excessive GWG was 22% (107/478), 30% (145/478), and 47% (226/478), respectively. The analyses were adjusted for age at outcome assessment, prepregnancy BMI, marital status and insurance at delivery, race, smoking during target pregnancy, and current education, parity, and smoking. Associations between excessive GWG and blood pressure, lipids, glucose, insulin, and metabolic syndrome were null. However, women with excessive GWG had a 3.6-kg (1.5, 5.6) greater weight change, a 3.2-cm (1.2, 5.2) greater WC, and 3-fold greater odds of abdominal obesity (2.9; 1.6, 5.1) compared with women who gained weight as recommended.
Conclusion: Excessive GWG is associated with long-term maternal abdominal adiposity, which may increase a woman's risk of cardiovascular and metabolic disease.
INTRODUCTION
The prevalence of obesity is increasing worldwide, leading to rising rates of diabetes mellitus and cardiovascular disease. Obesity disproportionately affects women, both in terms of raw prevalence and burden of disease attributable to obesity (1, 2). Less than one-third of pregnant women gain within Institute of Medicine (IOM)4/National Research Council (NRC) guidelines, and most women gain above IOM/NRC recommendations (3). The data suggest that excessive gestational weight gain (GWG) is associated with postpartum weight retention and subsequent obesity in the short (up to 3 mo postpartum) (4–6), intermediate (3 mo–3 y) (4, 5, 7–10), and long term (up to 21 y postpartum) (11–14). Researchers have proposed biologically plausible mechanisms linking excessive GWG to maternal metabolic and cardiovascular diseases later in life.
During pregnancy, women experience an increase in visceral fat (15). When compared with women who remained nulliparous during a 5-y follow-up period, women who had a single pregnancy had greater increases in waist-to-hip ratio that were independent of weight gain (16). Another study reported increases in waist girth proportionately larger than increases in overall weight gain observed with more births during follow-up, which suggests that pregnancy promotes central adiposity (17). This hypothesis was further supported in a longitudinal study assessing changes in adiposity via computed tomography and dual-energy X-ray absorptiometry that reported a greater increase in visceral adipose tissue for similar gains in total body fat in women with an interim birth compared with no interim birth during a 5-y follow-up period (18). Visceral (or intraabdominal) fat is more metabolically active than fat depots in other body areas and is linked to a more adverse cardiometabolic profile (19–23). Visceral fat is associated with an increased risk of heart disease, diabetes, and the metabolic syndrome (19–23).
Higher GWG may contribute to excess later-life cardiometabolic risk. In a recent report, Fraser et al (24) observed associations between excessive GWG and waist circumference (WC; in cm) (β = 5.84; 95% CI: 4.15, 7.54) and risk of central adiposity (OR: 2.67; 95% CI: 1.78, 4.01) even after adjustment for many potential confounders, including parity, in mothers 16 y after pregnancy. It is unclear whether these associations are present in women earlier in their postpartum years. Moreover, to our knowledge, there have been no studies examining the potential associations between GWG and other measures of cardiometabolic risk, such as cholesterol, glucose, and the metabolic syndrome. The primary aim of this study was to determine the extent to which GWG was associated with BMI, weight change (current weight – prepregnancy weight), WC, and abdominal obesity in a sample of women 4–12 y after delivery. In exploratory analyses, we sought to determine the extent to which GWG was associated with additional cardiometabolic risk factors.
SUBJECTS AND METHODS
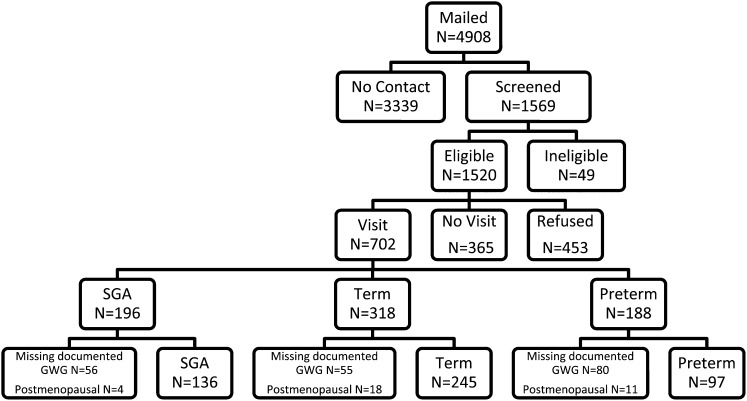
The Women's and Infants’ Study of Healthy Hearts (WISH) is a cohort study of cardiovascular disease risk factors assessed among women 4–12 y after the delivery of singleton infants who were either small for gestational age (SGA, <10th percentile for based on hospital nomograms), preterm (<37 wk of gestation), or term non-SGA births. By design, women with SGA and preterm births were oversampled. Eligible women were those who gave birth between 1997 and 2002 at Magee-Womens Hospital in Pittsburgh, PA, who did not have preeclampsia or prepregnancy hypertension or diabetes. Of the 4908 eligible women identified via a hospital electronic birth registry, 1569 (32%) were located via mail or phone and were screened (Figure 1). A total of 818 women (53.8%) declined participation, and an additional 49 women were ineligible because they were currently pregnant or reporting that they had preeclampsia or chronic hypertension before the target pregnancy. Of the women screened, 702 (45%) provided informed consent and were enrolled (318 term non-SGA births, 196 term SGA births, and 188 preterm births). The 702 enrolled women were more likely to be African American (28.6% compared with 24.4%; P = 0.02) and were slightly older (37.3 compared with 36.8 y; P < 0.01) compared with eligible women. Of the 702 women enrolled, 511 had data on documented GWG. We excluded women who were postmenopausal (n = 33). The Institutional Review Board of the University of Pittsburgh approved this study.
FIGURE 1.
Flowchart of participant recruitment and data analysis (final analytic sample, n = 478). GWG, gestational weight gain; SGA, small for gestational age.
GWG
Medical records for each woman's target pregnancy were reviewed to obtain pregnancy, labor, and delivery data. Prepregnancy weight, recalled at the first prenatal visit, and admission weight at delivery (either measured or self-reported) were abstracted from medical records. GWG was calculated by subtracting the prepregnancy weight from weight at delivery admission. At the WISH study visit, height (in cm) was measured with a stadiometer calibrated daily and current weight (in kg) was measured on a standard balance beam scale that was certified yearly by Allegheny County. Measurements took place in the Bellefield Clinic, which serves as the clinical assessment facility in numerous nationally funded studies conducted through the University of Pittsburgh Department of Epidemiology. Prepregnancy BMI (in kg/m2) [recalled pregravid weight (kg)/measured height (m)2] and current BMI [measured weight (kg)/measured height (m)2] were categorized as underweight (BMI <18.5), normal weight (18.5–24.9), overweight (25.0–29.9), or obese (≥30.0). We defined the adequacy of GWG as the ratio of observed GWG to expected (recommended) GWG at the gestational age of delivery multiplied by 100, as described previously (25–27). Expected GWG was defined as 100% of the 2009 IOM/NRC recommendations at the gestational age of delivery (3). The percentage weight gain recommendations met were classified as inadequate, adequate, or excessive based on ranges of IOM/NRC-recommended weight gains (3, 25, 26).
Outcome measures
Weight change was calculated as current weight (kg) – prepregnancy weight (kg). WC was measured with a metal tape measure marked in centimeters and placed at the level of the umbilicus. Abdominal obesity was defined as WC >88 cm. Fasting (12 h) blood samples were collected by trained technicians, and all assays were completed at the Nutrition Laboratory in the Department of Epidemiology at the University of Pittsburgh, which is Clinical Laboratory Improvement Amendments–certified and participates in the CDC–National Heart, Lung and Blood Institute Lipid Standardization and College of American Pathologists’ Proficiency Programs. Blood for the lipid, glucose, and insulin assays was collected in a 10.0-mL standard red-top evacuated tube. The blood was placed upright at room temperature for 30 to 40 min and placed in the refrigerator if not processed within 45 min from the time of collection. It was not to be stored in the refrigerator for >1 h. The tubes were then centrifuged at 4°C for 15 min at 1500 relative centrifugal force. The blood was transferred to cryovials and refrigerated with delivery to the laboratory on the day of collection. Total cholesterol, HDL, and triglycerides were measured in duplicate by using standard enzymatic procedures (28–30). LDL was estimated by using the Friedewald calculation (31), and women with triglycerides >400 mg/dL were excluded from LDL specific analyses (n = 3). The CV ranged from 1.3% to 6.5%. Glucose was determined by an enzymatic determination (32), and the CV was 1.8%. Insulin was measured by using a radioimunnoassay procedure developed by Linco Research Inc (CV: 2.6%). HOMA was calculated per Matthews et al (33). Blood pressure was evaluated as the mean of 3 measurements after a 10-min rest with a standard mercury sphygmomanometer. The metabolic syndrome was defined by using Joint Interim Statement criteria, which requires the presence of 3 of 5 possible risk factors (34). These risk factors include the following: fasting blood glucose of ≥100 mg/dL or glucose medication use, triglycerides ≥150 mg/dL or triglyceride medication use, HDL cholesterol <50 mg/dL or HDL medication use, blood pressure ≥130/85 or blood pressure medication use, and WC >88 cm.
Covariates
Delivery characteristics were abstracted from hospital birth records. Birth outcomes were categorized as being preterm, SGA, or term non-SGA infants. Women were categorized as having delivered preterm (<37 wk of gestation) or term. SGA infants were those less than the 10th percentile based on hospital-specific nomograms accounting for gestational age, infant sex, and maternal race (35). In addition, select variables were abstracted from the Magee Maternal and Infant database, including marital status at delivery (married or marriage-like or unmarried), type of insurance at delivery (private or other), and smoking during target pregnancy (yes or no). At the WISH participant visit, data on race-ethnicity (black, White, Asian/Pacific Islander, Native American, or non-Hispanic compared with Hispanic) was ascertained. Because of the small numbers of Asian/Pacific Islander, Native American, and Hispanic participants and because GWG patterns were similar between these groups and whites, the participants were categorized into 2 groups: non-Hispanic black and other. Additional covariates collected at the WISH participant visit included the following: maternal education (≤high school or ≥some college), current smoking (yes or no), and parity. Duration of lactation of the target pregnancy was assessed at the WISH participant visit by asking women, “How old was your child when you stopped nursing?” Physical activity was assessed with the Pfaffenberger Physical Activity Scale and is reported as metabolic equivalent hours/wk (36).
Statistical methods
Characteristics of women with excessive, adequate, and inadequate GWG were compared by using chi-square and ANOVA tests. For characteristics that differed significantly at P < 0.05, we completed pairwise comparisons, comparing both those who gained inadequately and excessively with those who gained adequately; for the pairwise comparisons, a P value of 0.025 was considered significant to account for multiple comparisons. The distributions of BMI, triglycerides, glucose, and insulin were skewed and therefore were natural log-transformed for analysis. Weight change, WC, systolic blood pressure, diastolic blood pressure, HDL, LDL, and total cholesterol were normally distributed. We also examined the distribution of GWG by prepregnancy BMI by using ANOVA.
Multivariable linear and logistic regression were used to determine whether excessive or inadequate GWG was associated with BMI, weight change, WC, and risk of abdominal obesity and each additional measure of cardiometabolic risk compared with adequate GWG. Potential confounders were selected a priori. After initial adjustment for time from target pregnancy, maternal age at outcome assessment, prepregnancy BMI, offspring sex, socioeconomic variables (marital status and type of insurance at delivery of target pregnancy), race, and smoking during target pregnancy; subsequent models were additionally adjusted for current parity, education, and smoking. To be consistent, and thus comparable with existing literature, we additionally adjusted for variables that may be on the causal pathway linking GWG to maternal cardiometabolic risk. These covariates included birth outcome of target pregnancy, gestational age of target pregnancy, mode of delivery, duration of lactation after target pregnancy, and current physical activity. In this set of analyses, to account for multiple comparisons (ie, numerous outcomes) while accounting for the modest sample size, a P value of 0.01 was used to determine statistical significance.
Subjects with missing covariate data were dropped from analyses involving that covariate; all covariates were present for >99% of participants. Potential issues of collinearity were examined by using the tolerance statistic, with ≤0.10 indicative of collinearity. Effect modification by prepregnancy BMI (as a continuous variable) was assessed by using a likelihood ratio test (α = 0.10). For variables that were natural log transformed, estimates were back transformed by using the following formula: 100 × [exp(estimate) −1]. Analyses were performed with SAS (version 9.2; SAS Institute, Inc).
RESULTS
The final analytic sample was 478. Women included in the study sample were similar to women excluded on a variety of sociodemographic and lifestyle characteristics (see Supplementary Table S1 under “Supplemental data” in the online issue). However, women excluded from the sample were more likely to have had a preterm birth (41% compared with 20%); less likely to be married (47% compared with 57%); less likely to have completed some college (49% compared with 58%); less likely to have a higher WC (mean ± SD: 92.92 ± 16 compared with 90.14 ± 15), systolic blood pressure (108.77 ± 13 compared with 106.71 ± 12), diastolic blood pressure (71.03 ± 9 compared with 69.51 ± 9), and LDL cholesterol (117.36 ± 32 compared with 109.48 ± 33); and less likely to have a lower HDL cholesterol (54.40 ± 14 compared with 58.31 ± 14) than women included in the study sample (all P < 0.05).
GWG among participating mothers was classified as inadequate (22%), adequate (30%), and excessive (47%) according to the 2009 IOM/NRC gestational weight gain guidelines (Table 1). Women who gained excessively were more likely to have had a preterm birth, were less likely to be married, and were less likely to have a college education than were women who gained as recommended.
TABLE 1.
Participant characteristics by Institute of Medicine/National Research Council category of GWG (n = 478)1
| No. of subjects | All participants | Inadequate GWG(n = 107; 22%) | Adequate GWG(n = 145; 30%) | Excessive GWG(n = 226; 47%) | P2 | |
| Prepregnancy BMI (kg/m2) | 478 | 24.17 ± 53 | 23.91 ± 6 | 23.49 ± 4 | 24.73 ± 5 | 0.05 |
| Prepregnancy BMI [n (%)] | 478 | <0.01 | ||||
| Underweight | 18 (4) | 9 (8) | 4 (3) | 5 (2) | ||
| Normal weight | 304 (64) | 70 (65) | 102 (70) | 132 (58) | ||
| Overweight | 104 (22) | 15 (14) | 31 (21) | 58 (26) | ||
| Obese | 52 (11) | 13 (12) | 8 (6) | 31 (14) | ||
| Target pregnancy outcome [n (%)] | 478 | <0.014 | ||||
| Preterm | 97 (20) | 15 (14) | 21 (14) | 61 (27) | ||
| SGA | 136 (28) | 47 (44) | 41 (28) | 48 (21) | ||
| Term non-SGA | 245 (51) | 45 (42) | 83 (57) | 117 (52) | ||
| Gestational age of target pregnancy (wk) | 478 | 39 (37–40)5 | 39 (38–40) | 39 (38–40) | 39 (36–40) | 0.044 |
| Mode of delivery [n (%)] | 478 | 0.35 | ||||
| Vaginal | 401 (84) | 91 (85) | 126 (87) | 184 (81) | ||
| Cesarean | 77 (16) | 16 (15) | 19 (13) | 42 (19) | ||
| Offspring sex, target pregnancy [n (%)] | 477 | 0.85 | ||||
| Male | 230 (48) | 50 (47) | 68 (47) | 112 (50) | ||
| Female | 247 (52) | 57 (53) | 76 (53) | 114 (50) | ||
| Duration of lactation, target pregnancy (mo) | 478 | 1 (0–7) | 0 (0–6) | 3 (0–8) | 1 (0–6) | 0.31 |
| Marital status at delivery [n (%)] | 478 | 0.044 | ||||
| Unmarried | 204 (43) | 44 (41) | 51 (35) | 109 (48) | ||
| Married/married-like | 274 (57) | 63 (59) | 94 (65) | 117 (52) | ||
| Insurance at delivery [n (%)] | 478 | 0.06 | ||||
| Private | 319 (67) | 63 (59) | 106 (73) | 150 (66) | ||
| Other | 159 (33) | 44 (41) | 39 (27) | 76 (34) | ||
| Smoked during target pregnancy [n (%)] | 477 | 106 (22) | 29 (27) | 28 (19) | 49 (22) | 0.31 |
| Age at time of outcome assessment (y) | 478 | 37.40 ± 7 | 38.04 ± 7 | 37.37 ± 7 | 37.11 ± 7 | 0.54 |
| Time since target pregnancy (y) | 478 | 8.19 ± 2 | 8.16 ± 2 | 8.05 ± 2 | 8.30 ± 2 | 0.41 |
| Race-ethnicity [n (%)] | 478 | 0.07 | ||||
| Non-Hispanic black | 127 (27) | 36 (34) | 30 (21) | 61 (27) | ||
| Other6 | 351 (73) | 71 (66) | 115 (79) | 165 (73) | ||
| Maternal education [n (%)] | 478 | 0.044 | ||||
| ≤High school | 151 (32) | 33 (31) | 35 (24) | 83 (37) | ||
| ≥Some college | 327 (68) | 74 (69) | 110 (76) | 143 (63) | ||
| Current smoker [n (%)] | 478 | 138 (29) | 32 (30) | 28 (19) | 78 (35) | <0.01 |
| Physical activity (MET-h/wk) | 477 | 8.75 (4–17) | 8.25 (3–17) | 8.50 (3–16) | 9.17 (4–17) | 0.16 |
| Parity [n (%)] | 478 | 0.38 | ||||
| 1 | 70 (15) | 16 (15) | 24 (17) | 30 (13) | ||
| 2 | 217 (45) | 53 (50) | 69 (48) | 95 (42) | ||
| ≥3 | 191 (40) | 38 (36) | 52 (36) | 101 (45) |
GWG, gestational weight gain; MET, metabolic equivalent task; SGA, small for gestational age.
Results were derived from chi-square tests or ANOVA.
Mean ± SD (all such values).
P value for the comparison of excessive with adequate <0.025 (chi-square test, t test, or Wilcoxon's rank-sum test). There were no significant differences between the inadequate and adequate groups.
Median; IQR in parentheses (all such values).
Other races include white, Asian/Pacific Islander, Native American, and Hispanic.
Women who were obese before pregnancy were most likely to gain excessively; however, on average, they gained less weight during pregnancy than did normal-weight women (underweight: 13.28 ± 6 kg; normal weight: 14.51 ± 5 kg; overweight: 13.27 ± 7 kg; obese: 9.73 ± 8 kg; P < 0.01). Thus, heavier women do not necessarily gain more weight in pregnancy; however, they are more readily categorized as excessive because the guidelines recommend lower weight gain thresholds for this group.
Women were assessed, on average, at 8 y postpartum (8.19 ± 2 y). In unadjusted analyses, women who gained excessively had the greatest BMI, weight change, WC, and rate of abdominal obesity, followed by women who gained adequately, and then those who gained inadequately (Table 2). Similarly, women who gained excessively had the highest rate of metabolic syndrome and had the lowest HDL followed by women who gained adequately, and then women who gained inadequately.
TABLE 2.
Participant measures of cardiometabolic risk by Institute of Medicine/National Research Council category of GWG (n = 478)1
| Inadequate GWG(n = 107; 22%) | Adequate GWG(n = 145; 30%) | Excessive GWG(n = 226; 47%) | P2 | |
| BMI (kg/m2) | 25.00 ± 73 | 25.68 ± 6 | 28.45 ± 7 | <0.01 |
| Weight change, current weight − prepregnancy weight (kg) | 2.91 ± 7 | 5.86 ± 9 | 10.06 ± 11 | <0.01 |
| Waist circumference (cm) | 86.20 ± 15 | 87.37 ± 13 | 93.78 ± 15 | <0.01 |
| Abdominal obesity, waist circumference >88 cm [n (%)] | 42 (17) | 57 (24) | 142 (59) | <0.01 |
| SBP (mm Hg) | 107.31 ± 13 | 106.46 ± 11 | 107.07 ± 10 | 0.81 |
| DBP (mm Hg) | 69.84 ± 9 | 69.33 ± 7 | 69.77 ± 8 | 0.84 |
| Triglycerides (mg/dL) | 85 (58–124)4 | 88 (65–128) | 86.5 (66–131) | 0.24 |
| HDL (mg/dL) | 60.77 ± 15 | 60.06 ± 14 | 56.00 ± 14 | <0.01 |
| LDL (mg/dL) | 109.58 ± 35 | 105.48 ± 33 | 112.08 ± 32 | 0.17 |
| Total cholesterol (mg/dL) | 189.07 ± 38 | 185.79 ± 39 | 189.93 ± 39 | 0.59 |
| Glucose (mg/dL) | 93 (88–98) | 92 (88–99) | 93 (89–98) | 0.61 |
| Insulin (IU/mL) | 8.60 (7–11) | 9.45 (7–12) | 9.90 (7–13) | 0.15 |
| Metabolic syndrome | 14 (17) | 18 (22) | 49 (60) | 0.03 |
| Components of the metabolic syndrome [n (%)] | ||||
| Fasting blood glucose ≥100 mg/dL5 | 24 (24) | 31 (31) | 26 (46) | 0.91 |
| Blood pressure ≥130/85 mm Hg5 | 10 (28) | 8 (22) | 18 (50) | 0.49 |
| Triglycerides ≥150 mg/dL5 | 13 (16) | 25 (32) | 41 (52) | 0.37 |
| HDL cholesterol <50 mg/dL5 | 31 (20) | 36 (24) | 86 (56) | 0.02 |
| Waist circumference >88 cm | 42 (17) | 57 (24) | 142 (59) | <0.01 |
DBP, diastolic blood pressure; GWG, gestational weight gain; SBP, systolic blood pressure.
Results were derived from chi-square tests or ANOVA.
Mean ± SD (all such values).
Median; IQR in parentheses (all such values).
Either the condition for each metabolic criterion was met or the individual was taking medication for the specific condition (eg, blood pressure).
Adequacy of GWG was independently associated with measures of adiposity in the years after pregnancy (Table 3). After adjustment for prepregnancy BMI, offspring sex, marital status at delivery of target pregnancy, type of insurance at delivery of target pregnancy, race, smoking during target pregnancy, time from target pregnancy, age at outcome assessment, and current education, parity, and smoking, women with inadequate GWG by IOM/NRC recommendations had a 5.4% (2.5%, 8.3%) lower BMI and a 3.4 kg (0.99, 5.7 kg) lower weight change than did women who gained weight as recommended (model 2). Women with excessive GWG had a 4.9% (2.2%, 7.6%) higher BMI, a 3.6-kg (1.5, 5.6 kg) greater weight change, a 3.2-cm (1.2, 5.2) greater WC, and a 3-fold greater odds of abdominal obesity (OR: 2.9; 95% CI: 1.6, 5.1) than did women who gained weight as recommended (model 2). Additional adjustment for covariates on the potential pathway linking GWG to maternal cardiometabolic health had a minimal effect on these estimates (model 3). We did not observe significant associations between inadequate or excessive GWG and blood pressure, triglycerides, LDL, total cholesterol, glucose, insulin, or the metabolic syndrome.
TABLE 3.
Relations between Institute of Medicine/National Research Council category of GWG and established measures of cardiometabolic risk1
| Outcome and category | No. of subjects | Model 12 | Model 23 | Model 34 |
| BMI (kg/m2)5 | 477 | |||
| Inadequate | −5.49 (−8.34, −2.55)6 | −5.43 (−8.30, −2.48)6 | −5.14 (−8.02, −2.16)6 | |
| Adequate | Reference | Reference | Reference | |
| Excessive | 4.62 (1.99, 7.32)6 | 4.85 (2.16, 7.61)6 | 5.24 (2.52, 8.04)6 | |
| Weight change (kg)7 | 477 | |||
| Inadequate | −3.41 (−5.77, −1.04)6 | −3.36 (−5.73, −0.99)6 | −3.13 (−5.51, −0.75) | |
| Adequate | Reference | Reference | Reference | |
| Excessive | 3.40 (1.43, 5.36)6 | 3.55 (1.54, 5.55)6 | 3.88 (1.86, 5.90)6 | |
| Waist circumference (cm)7 | 477 | |||
| Inadequate | −2.82 (−5.16, −0.46) | −2.81 (−5.17, −0.45) | −2.43 (−4.78, −0.069) | |
| Adequate | Reference | Reference | Reference | |
| Excessive | 3.32 (1.27, 5.28)6 | 3.21 (1.21, 5.21)6 | 3.52 (1.52, 5.52)6 | |
| Abdominal obesity, waist circumference >88 cm8 | 477 | |||
| Inadequate | 0.66 (0.32, 1.36) | 0.66 (0.32, 1.36) | 0.74 (0.35, 1.57) | |
| Adequate | Reference | Reference | Reference | |
| Excessive | 3.00 (1.70, 5.30)6 | 2.87 (1.61, 5.12)6 | 3.34 (1.80, 6.17)6 | |
| Systolic blood pressure (mm Hg)7 | 476 | |||
| Inadequate | −0.68 (−3.32, 1.95) | −0.71 (−3.35, 1.94) | −0.51 (−3.17, 2.16) | |
| Adequate | Reference | Reference | Reference | |
| Excessive | −0.61 (−2.80, 1.59) | −0.67 (−2.91, 1.56) | −0.79 (−3.06, 1.47) | |
| Diastolic blood pressure (mm Hg)7 | 476 | |||
| Inadequate | −0.41 (−2.36, 1.55) | −0.37 (−2.33, 1.59) | −0.36 (−2.34, 1.62) | |
| Adequate | Reference | Reference | Reference | |
| Excessive | −0.27 (−1.90, 1.35) | −0.31 (−1.96, 1.35) | −0.35 (−2.03, 1.33) | |
| Triglycerides (mg/dL)5 | 471 | |||
| Inadequate | −5.04 (−15.98, 7.33) | −6.27 (−16.92, 5.74) | −5.50 (−16.23, 6.61) | |
| Adequate | Reference | Reference | Reference | |
| Excessive | 3.80 (−6.22, 14.89) | 1.02 (−8.73, 11.82) | −0.034 (−9.73, 10.71) | |
| HDL (mg/dL)7 | 471 | |||
| Inadequate | 1.49 (−1.81, 4.79) | 1.52 (−1.78, 4.81) | 1.46 (−1.88, 4.79) | |
| Adequate | Reference | Reference | Reference | |
| Excessive | −2.89 (−5.63, −0.16) | −2.23 (−5.00, 0.54) | −2.51 (−5.33, 0.31) | |
| LDL (mg/dL)7 | 468 | |||
| Inadequate | 3.97 (−4.19, 12.14) | 3.81 (−4.39, 12.01) | 3.65 (−4.59, 11.90) | |
| Adequate | Reference | Reference | Reference | |
| Excessive | 6.23 (−0.54, 13.00) | 6.07 (−0.84, 12.97) | 4.97 (−2.01, 11.94) | |
| Total cholesterol (mg/dL)7 | 471 | |||
| Inadequate | 4.13 (−5.24, 13.49) | 3.73 (−5.67, 13.13) | 3.65 (−5.76, 13.05) | |
| Adequate | Reference | Reference | Reference | |
| Excessive | 4.72 (−3.05, 12.49) | 4.60 (−3.31, 12.52) | 2.85 (−5.12, 10.81) | |
| Glucose (mg/dL)5 | 471 | |||
| Inadequate | 0.46 (−2.26, 3.26) | 0.41 (−2.32, 3.22) | 0.49 (−2.28, 3.34) | |
| Adequate | Reference | Reference | Reference | |
| Excessive | 0.58 (−1.68, 2.90) | 0.66 (−1.65, 3.03) | 0.54 (−1.81, 2.95) | |
| Insulin (IU/mL)5 | 467 | |||
| Inadequate | −8.23 (−18.34, 3.13) | −8.29 (−18.45, 3.13) | −8.82 (−18.98, 2.61) | |
| Adequate | Reference | Reference | Reference | |
| Excessive | −0.75 (−9.84, 9.25) | −0.31 (−9.62, 9.96) | 0.26 (−9.20, 10.72) | |
| HOMA5 | ||||
| Inadequate | 467 | −10.78 (−22.28, 2.43) | −10.78 (−22.35, 2.52) | −11.43 (−22.97, 1.83) |
| Adequate | Reference | Reference | Reference | |
| Excessive | −0.33 (−11.04, 11.65) | 0.28 (−10.70, 12.62) | 1.29 (−9.91, 13.88) | |
| Metabolic syndrome8 | 477 | |||
| Inadequate | 0.72 (0.31, 1.65) | 0.69 (0.30, 1.62) | 0.70 (0.30, 1.65) | |
| Adequate | Reference | Reference | Reference | |
| Excessive | 1.54 (0.81, 2.93) | 1.40 (0.72, 2.71) | 1.39 (0.71, 2.71) |
Results were derived from linear or logistic regression models. 95% CIs in parentheses. GWG, gestational weight gain.
Adjusted for time from target pregnancy, age at outcome assessment, prepregnancy BMI, marital status at delivery, insurance at delivery, race, and smoking during target pregnancy.
Adjusted as for model 1 plus current education, parity, and smoking.
Adjusted as for model 1 plus gestational age of target pregnancy, target pregnancy outcome, mode of delivery, duration of lactation after target pregnancy, and current physical activity.
Values are percentage differences. For variables that were natural log transformed, estimates were back transformed by using the following formula: 100 × [exp(estimate) − 1].
P ≤ 0.01.
Values are β.
Values are ORs.
Interactions between adequacy of GWG and prepregnancy BMI were evaluated for each measure of adiposity and cardiometabolic risk examined by entering the cross product of GWG and prepregnancy BMI into each model. No significant interactions between adequacy of GWG and prepregnancy BMI were found for any of the measures of adiposity or cardiometabolic risk factors examined.
DISCUSSION
In this analysis, we showed that when compared with women who gained weight during pregnancy as recommended by the IOM/NRC, women who gained an excessive amount of weight had significantly greater BMI, weight change, larger WC, and a higher odds of abdominal obesity at 8 y postpartum. These relations persisted after adjustment for many socioeconomic and lifestyle factors. These results suggest that excessive GWG is associated with overall adiposity, and specifically with fat deposition in the abdominal region. These results build on previous work reporting associations of both parity and GWG with postpartum weight retention and BMI, showing that excessive GWG is not only associated with long-term maternal weight and overall body fat, but perhaps specifically with abdominal fat.
Our finding of greater BMI at 8 y with excessive GWG is consistent with previous studies (4–10). However, few studies have assessed the effect of GWG on BMI beyond 3 y postpartum (11–14). A potential mechanism linking excessive GWG to long-term adiposity is postpartum weight retention; indeed, we observed a significant independent association between excessive GWG and weight change. However, these relations could also be explained by behaviors that persisted, or began in pregnancy, that promote greater adiposity, which continue into later life or may simply reflect the tracking of weight throughout the life span.
We also showed that women who gained more than recommended have greater abdominal adiposity, as indicated by WC and abdominal obesity, when compared with women who gained as recommended. The relation between excessive GWG and greater abdominal adiposity is supported by findings of increased visceral fat with parity (16–18, 37–40). To date, we are only aware of one study that has examined the association between GWG and maternal abdominal adiposity. Fraser et al (24) reported associations between excessive GWG and WC (cm) (β = 5.84 (95% CI: 4.15, 7.54) and risk of central adiposity (OR: 2.67; 95% CI: 1.78, 4.01) even after adjustment for many potential confounders, including parity, in mothers 16 y after pregnancy. Our results further support the hypothesis that excessive GWG may contribute to increased abdominal fat gained with pregnancy. However, because our study was observational in nature, we do not know whether higher amounts of adiposity, both overall and abdominal, indicate actual weight retained or postpartum weight gained.
In contrast with results reported by Fraser et al (24), we did not observe significant associations between GWG and blood pressure. Differences in our results may have been due to differences in population characteristics, such as ethnic composition. Furthermore, the average follow-up time in our study was only 8 y compared with the 16-y average reported by Fraser et al; thus, it is possible that associations of GWG with blood pressure, or other metabolic variables, may emerge with greater follow-up time. We did, however, observe an association between excessive GWG and HDL cholesterol that was attenuated somewhat by adjustment for parity, education, and smoking. Although the association between excessive GWG and higher blood pressure suggests a link through vascular mechanisms, our observation of an increased risk of low HDL with excessive GWG suggests that further exploration into the metabolic effects of GWG may be warranted.
Our findings must be interpreted with the understanding that all observational studies may be subject to residual confounding. This analysis was limited to women who agreed to enroll in the WISH study and had complete data for the analyses. Women excluded from these analyses were more likely to deliver preterm infants and were less likely to be married or have completed some college at the time of delivery of target pregnancy and had a greater WC, blood pressure, HDL cholesterol, and LDL cholesterol than did women included in the study sample. Each of these factors introduces potential for selection bias. These results also may not be generalizable to other populations because of the recruitment of a convenience sample of women enriched for the delivery of SGA or preterm births at a single, high-risk referral hospital in Pittsburgh, PA.
Prepregnancy weight, recalled at the first prenatal visit, and admission weight at delivery (either measured or self-reported) were abstracted from medical records. This method of ascertainment introduces a potential for bias into the calculation of total GWG. Data suggest that both self-reported delivery weight and recalled pregravid weight are underreported on average, but may vary widely among individuals (41–43). Future research, including physical measures of both pregravid and delivery weights, are warranted to determine the true accuracy of GWG documented in medical records.
Because prepregnancy BMI is known to affect the adequacy of GWG and would be associated with long-term adiposity, we were particularly interested in examining effect modification by prepregnancy BMI. However, our efforts were limited by the small number of women who were overweight or obese before pregnancy. Therefore, studies with larger samples of women who were overweight and obese before pregnancy remain needed to parse out the extent to which the associations between GWG and adiposity vary by prepregnancy BMI.
Furthermore, although we adjusted for birth outcome, future studies of the relation between GWG and cardiometabolic health in larger samples of women with prior preterm and SGA births may aid in describing the relations between GWG and cardiometabolic health in these high-risk populations. It is important to note that when we limited our analyses to women with uncomplicated pregnancies, results were similar but precision was compromised (see Supplementary Table S2 under “Supplemental data” in the online issue). In addition, our estimates in uncomplicated pregnancies were similar to those reported in Fraser et al's sample of term deliveries.
Furthermore, studies of the effects of GWG on maternal health, including those assessing the relation between GWG and postpartum weight retention, can be criticized for focusing on a single pregnancy. Indeed, in our analysis we were unable to adjust for characteristics of intervening reproductive events. To better understand the relation between GWG and long-term maternal health, longitudinal data covering each of a woman's pregnancies are needed (44). Finally, our approach in model 3, which adjusts for potential mediators, may lead to collider stratification bias (45).
The strengths of our study include a large number of women with pregnancy data abstracted from medical records and physical measurements, fasting blood samples, and interview data collected at 8 y postpartum. GWG guidelines were recently updated to more adequately balance risks between mothers and their offspring (3). However, the 2009 IOM/NRC report called for increased research into the effects of GWG on long-term maternal health outcomes because of the lack of research in this area. Our results further support initiatives to examine the potential long-term effects of inadequate and excessive GWG on long-term maternal health because of the link between abdominal adiposity and chronic disease risk.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—CKM, JMC, RN, and LMB: designed the research; JMC and RN: conducted the research; CKM: analyzed the data and had primary responsibility for the final content; and CKM and JMC: wrote the manuscript. All authors read and approved the final manuscript. None of the authors had any potential conflicts of interest to disclose.
Footnotes
Abbreviations used: GWG, gestational weight gain; IOM, Institute of Medicine; NRC, National Research Council; SGA, small for gestational age; WC, waist circumference; WISH, Women's and Infants’ Study of Healthy Hearts.
REFERENCES
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 2012;307:491–7. [DOI] [PubMed] [Google Scholar]
- 2.Muennig P, Lubetkin E, Jia H, Franks P. Gender and the burden of disease attributable to obesity. Am J Public Health 2006;96:1662–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Institute of Medicine, National Research Council. Reexamining the guidelines: weight gain during pregnancy. Washington, DC: The National Academies Press, 2009. [PubMed] [Google Scholar]
- 4.Krause KM, Lovelady CA, Peterson BL, Chowdhury N, Ostbye T. Effect of breast-feeding on weight retention at 3 and 6 months postpartum: data from the North Carolina WIC Programme. Public Health Nutr 2010;13:2019–26. [DOI] [PubMed] [Google Scholar]
- 5.Kac G, Benicio MH, Velasquez-Melendez G, Valente JG. Nine months postpartum weight retention predictors for Brazilian women. Public Health Nutr 2004;7:621–8. [DOI] [PubMed] [Google Scholar]
- 6.Walker LO, Timmerman GM, Sterling BS, Kim M, Dickson P. Do low-income women attain their pre-pregnant weight by the 6th week of postpartum? Ethn Dis 2004;14:119–26. [PubMed] [Google Scholar]
- 7.Lowell H, Miller DC. Weight gain during pregnancy: adherence to Health Canadaaposs guidelines. Health reports/Statistics Canada. Ottawa, Canada: Canadian Centre for Health Information, 2010;21(2):31–6. [PubMed]
- 8.Maddah M, Nikooyeh B. Weight retention from early pregnancy to three years postpartum: a study in Iranian women. Midwifery 2009;25:731–7. [DOI] [PubMed] [Google Scholar]
- 9.Østbye T, Krause KM, Swamy GK, Lovelady CA. Effect of breastfeeding on weight retention from one pregnancy to the next: results from the North Carolina WIC program. Prev Med 2010;51:368–72. [DOI] [PubMed] [Google Scholar]
- 10.Rode L, Kjaergaard H, Ottesen B, Damm P, Hegaard HK. Association between gestational weight gain according to body mass index and postpartum weight in a large cohort of Danish women. Matern Child Health J 2012;16:406–13. [DOI] [PubMed] [Google Scholar]
- 11.Rooney BL, Schauberger CW, Mathiason MA. Impact of perinatal weight change on long-term obesity and obesity-related illnesses. Obstet Gynecol 2005;106:1349–56. [DOI] [PubMed] [Google Scholar]
- 12.Mamun AA, Kinarivala M, O'Callaghan MJ, Williams GM, Najman JM, Callaway LK. Associations of excess weight gain during pregnancy with long-term maternal overweight and obesity: evidence from 21 y postpartum follow-up. Am J Clin Nutr 2010;91:1336–41. [DOI] [PubMed] [Google Scholar]
- 13.Amorim AR, Rossner S, Neovius M, Lourenco PM, Linne Y. Does excess pregnancy weight gain constitute a major risk for increasing long-term BMI? Obesity (Silver Spring) 2007;15:1278–86. [DOI] [PubMed] [Google Scholar]
- 14.Linne Y, Dye L, Barkeling B, Rossner S. Weight development over time in parous women—the SPAWN study—15 years follow-up. Int J Obes Relat Metab Disord 2003;27(12):1516–22. [DOI] [PubMed] [Google Scholar]
- 15.Kinoshita T, Itoh M. Longitudinal variance of fat mass deposition during pregnancy evaluated by ultrasonography: the ratio of visceral fat to subcutaneous fat in the abdomen. Gynecol Obstet Invest 2006;61:115–8. [DOI] [PubMed] [Google Scholar]
- 16.Smith DE, Lewis CE, Caveny JL, Perkins LL, Burke GL, Bild DE. Longitudinal changes in adiposity associated with pregnancy. The CARDIA Study. Coronary Artery Risk Development in Young Adults Study. JAMA 1994;271:1747–51. [PubMed] [Google Scholar]
- 17.Gunderson EP, Murtaugh MA, Lewis CE, Quesenberry CP, West DS, Sidney S. Excess gains in weight and waist circumference associated with childbearing: the Coronary Artery Risk Development in Young Adults Study (CARDIA). Int J Obes Relat Metab Disord. 2004;28(4):525–35. [DOI] [PMC free article] [PubMed]
- 18.Gunderson EP, Sternfeld B, Wellons MF, Whitmer RA, Chiang V, Quesenberry CP, Jr, Lewis CE, Sidney S. Childbearing may increase visceral adipose tissue independent of overall increase in body fat. Obesity (Silver Spring) 2008;16:1078–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pascot A, Lemieux S, Lemieux I, Prud'homme D, Tremblay A, Bouchard C, Nadeau A, Couillard C, Tchernof A, Bergeron J, et al. Age-related increase in visceral adipose tissue and body fat and the metabolic risk profile of premenopausal women. Diabetes Care 1999;22:1471–8. [DOI] [PubMed] [Google Scholar]
- 20.Van Pelt RE, Evans EM, Schechtman KB, Ehsani AA, Kohrt WM. Contributions of total and regional fat mass to risk for cardiovascular disease in older women. Am J Physiol Endocrinol Metab 2002;282:E1023–8.. [DOI] [PubMed] [Google Scholar]
- 21.Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, O'Donnell CJ, Fox CS. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation 2008;117:605–13. [DOI] [PubMed] [Google Scholar]
- 22.Jensen MD. Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab 2008;93(Suppl 1):S57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fox CS, Hwang SJ, Massaro JM, Lieb K, Vasan RS, O'Donnell CJ, Hoffmann U. Relation of subcutaneous and visceral adipose tissue to coronary and abdominal aortic calcium (from the Framingham Heart Study). Am J Cardiol 2009;104:543–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fraser A, Tilling K, Macdonald-Wallis C, Hughes R, Sattar N, Nelson SM, Lawlor DA. Associations of gestational weight gain with maternal body mass index, waist circumference, and blood pressure measured 16 y after pregnancy: the Avon Longitudinal Study of Parents and Children (ALSPAC). Am J Clin Nutr 2011;93:1285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bodnar LM, Siega-Riz AM, Arab L, Chantala K, McDonald T. Predictors of pregnancy and postpartum haemoglobin concentrations in low-income women. Public Health Nutr 2004;7:701–11. [DOI] [PubMed] [Google Scholar]
- 26.Siega-Riz AM, Adair LS, Hobel CJ. Maternal underweight status and inadequate rate of weight gain during the third trimester of pregnancy increases the risk of preterm delivery. J Nutr 1996;126:146–53. [DOI] [PubMed] [Google Scholar]
- 27.Bodnar LM, Siega-Riz AM, Simhan HN, Himes KP, Abrams B. Severe obesity, gestational weight gain, and adverse birth outcomes. Am J Clin Nutr 2010;91:1642–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bucolo G, David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem 1973;19:476–82. [PubMed] [Google Scholar]
- 29.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem 1974;20:470–5. [PubMed] [Google Scholar]
- 30.Warnick GR, Albers JJ. A comprehensive evaluation of the heparin-manganese precipitation procedure for estimating high density lipoprotein cholesterol. J Lipid Res 1978;19:65–76. [PubMed] [Google Scholar]
- 31.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502. [PubMed] [Google Scholar]
- 32.Bondar RJ, Mead DC. Evaluation of glucose-6-phosphate dehydrogenase from Leuconostoc mesenteroides in the hexokinase method for determining glucose in serum. Clin Chem 1974;20:586–90. [PubMed] [Google Scholar]
- 33.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- 34.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC., Jr Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–5. [DOI] [PubMed] [Google Scholar]
- 35.Goldenberg RL, Cliver SP. Small for gestational age and intrauterine growth restriction: definitions and standards. Clin Obstet Gynecol 1997;40:704–14. [DOI] [PubMed] [Google Scholar]
- 36.Paffenbarger RS, Jr, Blair SN, Lee IM, Hyde RT. Measurement of physical activity to assess health effects in free-living populations. Med Sci Sports Exerc 1993;25:60–70. [DOI] [PubMed] [Google Scholar]
- 37.Troisi RJ, Wolf AM, Mason JE, Klingler KM, Colditz GA. Relation of body fat distribution to reproductive factors in pre- and postmenopausal women. Obes Res 1995;3:143–51. [PubMed] [Google Scholar]
- 38.den Tonkelaar I, Seidell JC, van Noord PA, Baanders-van Halewijn EA, Ouwehand IJ. Fat distribution in relation to age, degree of obesity, smoking habits, parity and estrogen use: a cross-sectional study in 11,825 Dutch women participating in the DOM-project. Int J Obes 1990;14:753–61. [PubMed] [Google Scholar]
- 39.Kaye SA, Folsom AR, Prineas RJ, Potter JD, Gapstur SM. The association of body fat distribution with lifestyle and reproductive factors in a population study of postmenopausal women. Int J Obes 1990;14:583–91. [PubMed] [Google Scholar]
- 40.Mansour AA, Ajeel NA. Parity is associated with increased waist circumference and other anthropometric indices of obesity. Eating and weight disorders. Eat Weight Disord 2009;14:e50–5. [DOI] [PubMed] [Google Scholar]
- 41.Schieve LA, Perry GS, Cogswell ME, Scanion KS, Rosenberg D, Carmichael S, Ferre C. Validity of self-reported pregnancy delivery weight: an analysis of the 1988 National Maternal and Infant Health Survey. NMIHS Collaborative Working Group. Am J Epidemiol 1999;150:947–56. [DOI] [PubMed] [Google Scholar]
- 42.Yu SM, Nagey DA. Validity of self-reported pregravid weight. Ann Epidemiol 1992;2:715–21. [DOI] [PubMed] [Google Scholar]
- 43.Stevens-Simon C, McAnarney ER. Adolescent pregnancy. Gestational weight gain and maternal and infant outcomes. Am J Dis Child 1992;146:1359–64. [DOI] [PubMed] [Google Scholar]
- 44.Rasmussen KM, Abrams B. Gestational weight gain and later maternal health: are they related? Am J Clin Nutr 2011;93:1186–7. [DOI] [PubMed] [Google Scholar]
- 45.Hernán MA, Hernandez-Diaz S, Werler MM, Mitchell AA. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol 2002;155:176–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.