Abstract
Background: China has some of the highest incidence rates for gastric adenocarcinoma (GA) and esophageal squamous cell carcinoma (ESCC) in the world. Prospective studies suggested that vitamin C may reduce risks; however, associations are unclear because of limited sample size.
Objective: The objective was to examine the relation between prediagnostic plasma vitamin C and the risk of GA and ESCC.
Design: A case-cohort study was used to assess the association between prediagnostic plasma vitamin C and incidence of GA (n = 467) and ESCC (n = 618) in the General Population Nutrition Intervention Trial. With the use of multivariate Cox proportional hazards models, we estimated the HRs and 95% CIs. We also conducted a meta-analysis of the literature up to 1 October 2012 on the relation between circulating vitamin C and gastric cancer incidence. Two cohort studies and the current study were included to assess the body of evidence.
Results: For GA, each 20-μmol/L increase in plasma vitamin C was associated with a 14% decrease in risk (HR: 0.86; 95% CI: 0.76, 0.96). Compared with individuals with low plasma vitamin C concentrations (≤28 μmol/L), those with normal concentrations (>28 μmol/L) had a 27% reduced risk of GA (HR: 0.73; 95% CI: 0.56, 0.94). No association between vitamin C concentrations and ESCC was seen. Meta-analysis showed that the risk of incident GA among those with the highest concentration of plasma vitamin C was 31% lower (random-effects-pooled-odds ratio 0.69; 95% CI: 0.54, 0.89) than those in the lowest category.
Conclusion: Our data provide evidence that higher circulating vitamin C was associated with a reduced risk of incident GA, but no association was seen for ESCC.
INTRODUCTION
Gastric and esophageal cancer represent the second and sixth most common causes of cancer death worldwide, respectively (1). China has high incidence rates for these cancers, and its population accounts for 42% of gastric and 50% of esophageal cancers in the world (2). Within China, the Linxian region has the highest rate of esophageal cancer and high rates of gastric adenocarcinoma (GA)4; of note, essentially all cases of esophageal cancers in this region are esophageal squamous cell carcinomas (ESCCs).
Vitamin C or ascorbic acid is vital to most living tissues. Humans lack the ability to synthesize vitamin C, and it must be obtained from the diet. Serum vitamin C concentrations >28 μmol/L are considered normal, 11–28 insufficient, and <11 deficient (3). The prevalence of vitamin C insufficiency is likely to be greater in nutritionally deficient areas of economically developing regions, such as Linxian (4).
Vitamin C may be particularly relevant for gastric cancer because of its interrelation with Helicobacter pylori (5). Infection with H. pylori is the leading etiologic factor for gastric cancer across all populations (6). H. pylori–infected patients typically have lower concentrations of vitamin C in their gastric juice, which may normalize after H. pylori eradication (7). Atrophic gastritis, a cancer-predisposing state caused by H. pylori, can lead to increased gastric juice pH, which impairs vitamin C secretion from the gastric mucosa (8). Vitamin C may possess antimicrobial activity against H. pylori (9), although the evidence is equivocal (10).
Linxian residents have low nutritional status, primarily because of the low consumption of fruit and vegetables (4) and the high prevalence of H. pylori infection (11). However, the role of nutritional deficiency in GA and ESCC is incompletely understood in Linxian. Some nutrients have shown strong association with risk (12, 13), whereas others have not (14, 15); results have also varied by cancer location within the stomach. Because of the known relation with H. pylori (5) and potential anticancer properties (16–18), vitamin C may contribute to the etiology of both cancers in this high-risk area.
Few observational epidemiologic studies have examined the association between circulating vitamin C concentrations and risk of incident gastric or esophageal cancers. There are no published prospective cohort studies of esophageal cancer and circulating vitamin C, and the 2 extant prospective cohort studies of gastric cancer had modest sample size (n ∼ 200) (19, 20). The only study to report risks separately by gastric subsites (cardia and noncardia) is the European Prospective Investigation into Cancer and Nutrition, which observed a significant inverse association with GA (20).
In the current study, we investigated the association between prediagnostic plasma vitamin C concentrations and risk of incident GA, including both anatomic subsites, and ESCC in a large population-based cohort study in China.
SUBJECTS AND METHODS
Study population
The Linxian General Population Nutrition Intervention Trial (NIT) was a randomized double-blind primary intervention trial (March 1986 through May 1991). The trial tested 4 regimens of vitamin-mineral combinations, including a combination of vitamin C and molybdenum, on the incidence of esophageal cancer and gastric cancer. Detailed descriptions of the trial design (21), results (22), and follow-up results (2) have been published. Briefly, 29,584 men and women aged 40–69 y were recruited from northern Linxian in the Henan province of central China. In 1985, participants were interviewed by baseline questionnaire on basic demographic characteristics and dietary intakes. In 1991, at the end of the trial, a questionnaire was administered to obtain selected characteristics, including BMI. Last, a brief questionnaire was administered in 1996 to obtain updated lifestyle information.
Since the conclusion of the trial in 1991, follow-up of all participants for vital status and cancer endpoints has continued via monthly checks of village doctors’ records and quarterly checks of the Linxian Cancer Registry.
Between 1999 and 2000, all living NIT participants were invited to participate in a blood collection survey, and ∼16,000 participated (∼80% of all eligible participants). Participants were requested to fast the previous night before blood collection. Immediately after collection, the blood was stored on ice for 3 to 6 h, separated by centrifugation, and then portioned into aliquots within 24 h An aliquot of plasma was mixed with 4 parts of 6% meta-phosphoric acid (MPA) specifically to enable future analysis of plasma vitamin C. These stabilized samples were then stored at −85°C until analyzed. In the current study, we used a case-cohort design to select subjects for plasma vitamin C assays from this subpopulation of NIT members.
Cases and subcohort
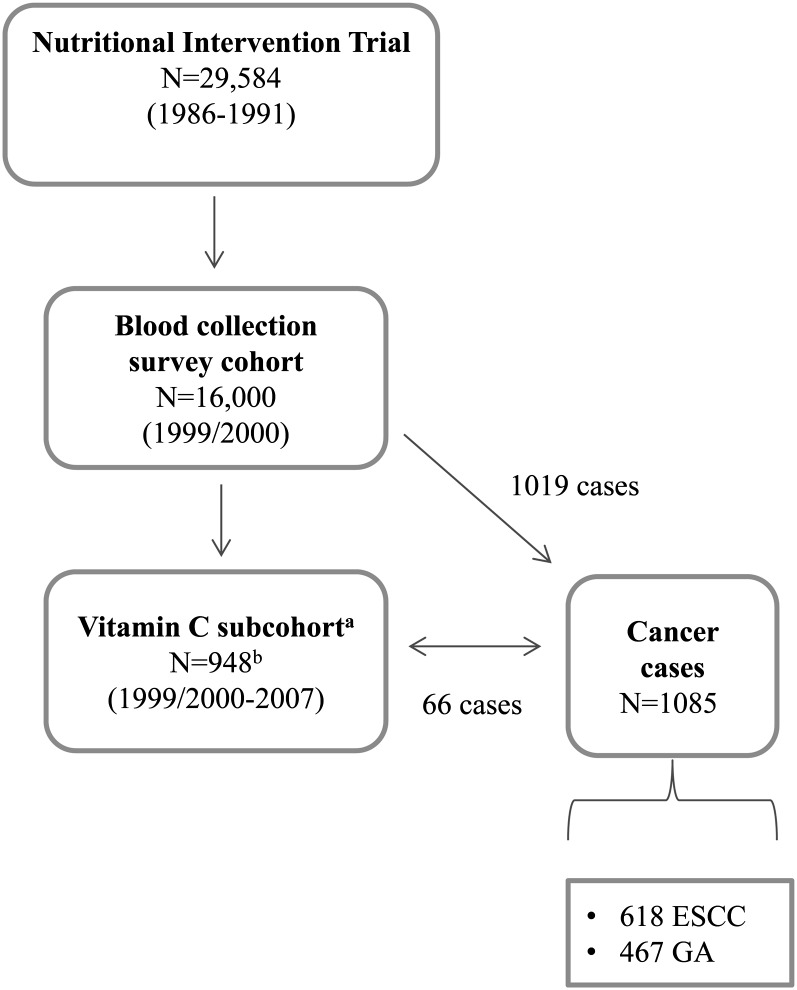
A schema illustrating the selection of the vitamin C subcohort and the origins of cancer cases is shown in Figure 1. From the NIT cohort members who participated in the blood collection survey, we selected an age- and sex-stratified random sample of 1000 subjects to serve as a reference subcohort for the current vitamin C study. Of these 1000 subcohort members, 52 were excluded because of inadequate MPA-preserved plasma and thus could not undergo vitamin C analysis. The final vitamin C subcohort consisted of 948 members.
FIGURE 1.
Schema of the vitamin C case-cohort study. aReference vitamin C subcohort. bFifty-two subcohort members from the original selected 1000 members did not have adequate meta-phosphoric acid–preserved plasma for vitamin C analyses and thus were excluded in the final subcohort. ESCC, esophageal squamous cell carcinoma; GA, gastric adenocarcinoma.
Cases were defined as a census of all subjects with a diagnosis of ESCC or GA after the 1999/2000 blood collection with follow-up through the end of 2007. Gastric tumors were further defined as cardia adenocarcinomas if they were centered in the most proximal 3 cm of the stomach and as noncardia adenocarcinomas if they were centered distal to this region. Per the definition of a case-cohort study, cases could originate from either the entire cohort (eg, the blood collection survey) or from the vitamin C subcohort. Sixty-six members of the vitamin C subcohort developed cancer during the course of follow-up; all other cases came from the entire cohort with available MPA-preserved plasma. The current study comprised a total of 618 ESCC and 467 GA (329 cardia and 138 noncardia) patients.
Laboratory analyses
MPA-preserved plasma samples were thawed, pipetted into aliquots, and immediately refrozen to −70°C at Fisher BioServices. Samples were shipped on dry ice overnight to Craft Technologies Inc. At Craft Technologies Inc, samples were stored at −70°C until processed for analysis. Plasma specimens were analyzed for vitamin C by HPLC by using methods similar to those used by NHANES for their vitamin C analysis (23).
Quality control (QC) for vitamin C measurements included 3 in-house QC samples with known concentrations (4.88, 23.3, and 164.4 μmol/L), whose target values had been verified against National Institute of Standards and Technology Reference Materials. In addition to the 3 in-house QC samples, we included QC samples from the pooled serum of 80 study subjects from our cohort drawn at baseline. The 3 in-house QC samples were included at the beginning and end of each batch. Each batch therefore consisted of 30 randomly selected samples (6 in-house QCs, 2 pooled QCs, and 22 pooled samples from the cohort). The overall CVs corresponding to the 3 in-house QC samples from the highest to the lowest concentrations were 9%, 4%, and 2%, respectively. The within-batch, between-batch, and total CVs for the pooled QC samples were 4%, 4%, and 6%, respectively. Laboratory personnel were blinded to subject case status and the existence of pooled QC samples.
Methods used to measure H. pylori and pepsinogens (I and II) were previously described (24). The H. pylori seropositivity cutoff was defined as absorbances of ≥1.0 for whole-cell antibodies (25). Pepsinogens were assessed by using an enzyme-linked immunosorbent assay (Biohit ELISA kit; Biohit).
Statistical analyses
Median values for plasma vitamin C were calculated in the vitamin C subcohort by using the sampling weights from the entire Linxian General Population NIT cohort, so that estimates represented the entire cohort, including those who later developed cancer. We compared vitamin C concentrations by selected characteristics with the use of Wilcoxon's Mann-Whitney U test/K-sample test for the equality of medians or the Jonckheere-Terpstra test for categorical variables. For all primary risk analyses, we used Cox proportional hazards models to estimate crude and adjusted HRs and 95% CIs. Models were fit by using the case-cohort Cox models available in Epicure Software (Hirosoft). We fit separate models for each cancer evaluated (ESCC, GA, cardia adenocarcinoma, and noncardia adenocarcinoma).
Plasma vitamin C concentration was analyzed in 3 ways: 1) as a continuous variable (with HRs estimated for risk related to each 20-μmol/L increase (half the IQR), which approximates the increment from one quartile to the next 2); in season-specific quartiles (based on the distributions of the entire subcohort); and 3) as a dichotomous variable by using a single cutoff based on the literature [normal (>28 μmol/L) compared with low (≤2 μmol/L), ie, individuals with either insufficient (11–28 μmol/L) or deficient (<11 μmol/L) circulating vitamin C concentrations]. Because circulating vitamin C varied by season of blood draw, all estimates were based on the models conditioned on season of blood draw with the cumulative HR presented. Multivariate models were adjusted for 6 sex and age group categories used in the subcohort selection, history of smoking (yes or no), BMI (in kg/m2; by using cutoffs appropriate for Asians: <18.5, 18.5–23.9, or ≥24), season of blood draw (winter or spring), and H. pylori seropositivity. Although we considered the contribution of the dietary intake (using data from the 1996 follow-up questionnaire), we decided that inclusion of the dietary data in the final models would not be informative because of the limited distribution of the dietary data (see Supplemental Table 1 under “Supplemental data” in the online issue) and the incomplete detailed capture of all sources of vitamin C (ie, we did not capture individual types of fruit) with plasma vitamin C concentration. In addition, further adjustment for alcohol consumption, assignment to NIT intervention treatment Factor C, serologic atrophic gastritis, fruit and vegetable intake, and meat intake did not materially change the associations between plasma vitamin C concentrations and cancer outcomes (data not shown).
We performed separate analyses by sex, age (above or below median), smoking (yes or no), H. pylori seropositivity, Factor C intervention (which included vitamin C treatment assignment), and serologic atrophic gastritis status (defined as a serum pepsinogen I to II ratio ≤4). We based our selection on variables that either showed differential plasma vitamin C concentration or were hypothesized to be possible effect modifiers. Formal interaction testing was based on the likelihood ratio test by using plasma vitamin C concentrations as a dichotomous variable (≤28 or >28 μmol/L). The results did not materially change when we used plasma vitamin C as a continuous variable. We also examined the effect of time of follow-up by excluding cases diagnosed within the first year of follow-up.
Statistical analyses other than Cox models were completed by using SAS version 9.1 (SAS Institute Inc). We used 2-sided P values and considered P values <0.05 or CIs that excluded 1.0 as statistically significant. The proportional hazard assumption was met as examined by using graphic representations and formal testing with the use of time interaction terms.
Meta-analyses
To examine the totality of the evidence on prediagnostic plasma vitamin C and incidence of gastric cancer, we conducted a systematic review of the literature and meta-analysis of the published data and that of the present study.
Search strategy and data extraction
We searched PubMed using the following search terms: ((“plasma”[MeSH Terms] OR “plasma”[All Fields]) AND (“ascorbic acid”[MeSH Terms] OR (“ascorbic”[All Fields] AND “acid”[All Fields]) OR “ascorbic acid”[All Fields] OR “vitamin c”[All Fields])) OR (“plasma”[MeSH Terms] OR “plasma”[All Fields]) AND “ascorbic acid”[All Fields] OR (prediagnostic[All Fields] AND (“trace elements”[MeSH Terms] OR (“trace”[All Fields] AND “elements”[All Fields]) OR “trace elements”[All Fields] OR “micronutrient”[All Fields] OR “trace elements”[Pharmacologic Action] OR “micronutrients”[MeSH Terms] OR “micronutrients”[All Fields] OR “micronutrients”[Pharmacologic Action])) AND ((“stomach neoplasms”[MeSH Terms] OR (“stomach”[All Fields] AND “neoplasms”[All Fields]) OR “stomach neoplasms”[All Fields] OR (“gastric”[All Fields] AND “cancer”[All Fields]) OR “gastric cancer”[All Fields]) AND (“risk”[MeSH Terms] OR “risk”[All Fields])) AND “humans”[MeSH Terms]. The search included all studies published up to 1 October 2012. Only English articles were included. In addition, we hand-searched relevant references in review articles.
The following exclusion criteria were applied to the screening of articles: 1) no original data (reviews, editorials, meta-analyses, and systematic reviews); 2) studies not addressing the association between plasma or serum vitamin C/ascorbic acid and gastric cancer risk; 3) studies not in humans; 4) cross-sectional studies, case-control studies, case reports, and case series; and 5) studies of precancerous gastric lesions. The eligibility of each abstract or full-text article was assessed independently by 2 reviewers. For each eligible article, 2 reviewers abstracted the data into an Excel spreadsheet. The data abstraction was performed serially, and any disagreements between reviewers were resolved by consensus.
Statistical analysis
The primary quantitative analyses focused on prediagnostic circulating (plasma or serum) vitamin C (or ascorbic acid) and risk of incident gastric cancer. For all studies, risk estimates and their respective 95% CIs were abstracted. We used inverse-variance weights in random-effects models to obtain pooled RR estimates, comparing the highest with the lowest categories of circulating vitamin C. Statistical heterogeneity was assessed by using the DerSimonian and Laird's Q statistic and the I2 statistic. This meta-analysis was performed by using Stata version 11.1 (StataCorp).
RESULTS
Compared with the subcohort, subjects with GA were more commonly males and smokers with serologically defined atrophic gastritis, whereas subjects with esophageal cancer were more likely female, who tend not to smoke tobacco or drink alcohol (Table 1).
TABLE 1.
Selected characteristics of subcohort and cases of the Linxian General Population Nutritional Intervention Trial1
| Characteristics | Subcohort(n = 948) | ESCC(n = 618) | Gastric2(n = 467) |
| Age at 1999/2000 blood draw (y)3 | 63.9 ± 7.6 | 63.9 ± 6.9 | 64.1 ± 7.0 |
| Mean time between blood donation and diagnosis (y) | NA | 3.6 | 3.7 |
| Sex, female [n (%)] | 475 (50.1) | 330 (53.4) | 196 (41.9) |
| BMI (kg/m2)4 | 22.3 ± 2.6 | 22.1 ± 2.5 | 22.1 ± 2.5 |
| Smoking [n (%)] | 637 (67.5) | 440 (71.4) | 283 (60.6) |
| Alcohol consumption [n (%)] | 695 (73.6) | 479 (77.8) | 338 (72.3) |
| Pepsinogen I:II ratio | |||
| Median (IQR) | 8.6 (7.1) | 8.3 (6.6) | 6.6 (6.0) |
| Serologic atrophic gastritis (%)5 | 11.9 | 12.3 | 19.2 |
| Helicobacter pylori–positive subjects [n (%)] | 907 (95.6) | 596 (96.4) | 446 (95.5) |
Numbers may not add up to 100% because of missing data. ESCC, esophageal squamous cell carcinoma; NA, not available.
Gastric adenocarcinoma includes both cardia and noncardia cases.
Values are means ± SDs.
Values are medians ± SDs.
Defined as pepsinogen I/pepsinogen II <4.
Plasma vitamin C concentrations by selected characteristics are shown in Table 2. The median plasma vitamin C concentration in the subcohort was 32.7 μmol/L. Plasma vitamin C concentrations were significantly higher in females, in samples collected during the winter, and in never-smokers. The observed seasonal difference in plasma vitamin C is consistent with the previously reported lower consumption of fresh fruit and vegetables in the spring in this region (4, 26). There was no difference in plasma vitamin C between individuals with and without serologically defined atrophic gastritis (31.2 and 33.8 μmol/L, respectively; P = 0.75).
TABLE 2.
Plasma vitamin C concentrations in a subcohort of the Linxian General Population Nutrition Intervention Trial by selected characteristics1
| Percentile of vitamin C concentrations |
||||||||
| Characteristic | No. of subjects | Median vitamin C concentration | IQR | 5th | 25th | 75th | 95th | P value |
| μmol/L | ||||||||
| Subcohort | 948 | 32.7 | 41.6 | 5.0 | 13.5 | 55.2 | 72.6 | |
| Season of blood draw | ||||||||
| Winter, 1999 | 457 | 51.5 | 27.5 | 11.4 | 34.8 | 62.3 | 76.4 | |
| Spring, 2000 | 487 | 16.8 | 23.0 | 3.7 | 9.1 | 32.2 | 64.9 | <0.00012 |
| Sex | ||||||||
| Female | 475 | 37.3 | 41.0 | 6.0 | 16.4 | 57.4 | 75.3 | |
| Male | 473 | 26.7 | 39.2 | 3.6 | 10.7 | 50.0 | 70.2 | <0.00012 |
| Age at blood draw | ||||||||
| <60 y | 302 | 35.3 | 36.7 | 5.3 | 16.2 | 53.0 | 71.7 | |
| ≥60 y | 642 | 31.9 | 43.8 | 4.8 | 12.5 | 56.3 | 72.8 | 0.202 |
| BMI3 | ||||||||
| <18.5 kg/m2 | 41 | 35.9 | 52.6 | 3.8 | 12.0 | 64.6 | 90.7 | |
| 18.5–23.9 kg/m2 | 545 | 31.2 | 40.1 | 4.8 | 14.2 | 54.3 | 74.4 | |
| ≥24 kg/m2 | 176 | 34.1 | 42.6 | 5.3 | 12.9 | 55.6 | 69.0 | 0.0504 |
| Smoking | ||||||||
| No | 637 | 35.3 | 41.0 | 5.4 | 15.4 | 56.5 | 73.5 | |
| Yes | 307 | 25.9 | 37.7 | 3.6 | 5.5 | 48.0 | 71.4 | 0.000302 |
| Alcohol consumption | ||||||||
| No | 695 | 34.5 | 42.1 | 5.2 | 14.0 | 56.1 | 73.5 | |
| Yes | 249 | 29.3 | 39.9 | 4.8 | 12.4 | 52.4 | 70.7 | 0.102 |
| Serologic atrophic gastritis5 | ||||||||
| No | 772 | 31.2 | 41.6 | 5.0 | 12.9 | 54.5 | 72.2 | |
| Yes | 113 | 33.8 | 43.3 | 3.6 | 11.6 | 54.9 | 74.0 | 0.802 |
Plasma vitamin C concentrations are weighted, accounting for age and sex, to the original cohort.
Wilcoxon's-Mann-Whitney test/K-sample test on the equality of medians.
BMI (in kg/m2; on the basis of cutoffs appropriate for Asians: <18.5, 18.5–23.9, or ≥24).
Jonckheere-Terpstra test.
No = pepsinogen I/pepsinogen II ≥4; yes = pepsinogen I/pepsinogen II <4.
We found a significant inverse association between higher plasma vitamin C concentrations and risk of gastric cancer. The HR (95% CI) associated with each 20-μmol/L increase of plasma vitamin C was 0.86 (0.76, 0.97) for GA (Table 3). The inverse associations remained when season-specific quartiles were used (Table 2). Similarly, when we used the established cutoff for low (≤28 μmol/L) compared with normal (>28 μmol/L) plasma concentrations, individuals with normal plasma vitamin C concentrations had a 27% reduced risk of gastric cancer compared with those with low concentrations (HR>28 vs ≤28 μmol/L: 0.73; 95% CI: 0.56, 0.97; P = 0.03; Table 4). In analyses by anatomic subsites, we found borderline statistically significant inverse association for cardia (HR>28 vs ≤28 μmol/L: 0.73; 95% CI: 0.52, 1.00; P = 0.05) and no statistical association for noncardia (HR>28 vs ≤28 μmol/L: 0.74; 95% CI: 0.48, 1.14; P = 0.18. Although the median concentrations were quite different, season did not modify the association observed between plasma vitamin C concentration and risk of gastric cancer (P-interaction > 0.05; Table 4).
TABLE 3.
HRs and 95% CIs for the association between plasma vitamin C concentrations and cancers in the Linxian General Population Nutrition Intervention Trial cohort1
| Plasma vitamin C2 |
|||||||||
| Crude3 |
Adjusted4 |
Continuous, per 20-μmol/L increase3 |
|||||||
| Cancer type | No. of cases | HR | 95% CI | P-trend | HR | 95% CI | P-trend | HR | 95% CI |
| ESCC | |||||||||
| Q1 | 151 | 1.00 | Ref | 1.00 | Ref | 0.97 | 0.86, 1.09 | ||
| Q2 | 161 | 0.99 | 0.74, 1.33 | 1.00 | 0.74, 1.35 | ||||
| Q3 | 151 | 0.89 | 0.67, 1.19 | 0.91 | 0.67, 1.23 | ||||
| Q4 | 155 | 0.89 | 0.67, 1.19 | 0.33 | 0.89 | 0.66, 1.20 | 0.35 | ||
| Gastric adenocarcinoma | |||||||||
| Q1 | 132 | 1.00 | Ref | 1.00 | Ref | 0.86 | 0.76, 0.97 | ||
| Q2 | 124 | 0.88 | 0.63, 1.20 | 0.96 | 0.70, 1.32 | ||||
| Q3 | 109 | 0.73 | 0.52, 1.00 | 0.81 | 0.58, 1.12 | ||||
| Q4 | 102 | 0.67 | 0.48, 0.92 | 0.007 | 0.77 | 0.54, 1.04 | 0.070 | ||
| Cardia adenocarcinoma | |||||||||
| Q1 | 98 | 1.00 | Ref | 1.00 | Ref | 0.85 | 0.74, 0.98 | ||
| Q2 | 89 | 0.85 | 0.58, 1.19 | 0.93 | 0.65, 1.32 | ||||
| Q3 | 64 | 0.58 | 0.39, 0.83 | 0.64 | 0.44, 0.94 | ||||
| Q4 | 78 | 0.69 | 0.49, 0.98 | 0.0090 | 0.80 | 0.55, 1.15 | 0.080 | ||
| Noncardia adenocarcinoma | |||||||||
| Q1 | 34 | 1.00 | Ref | 1.00 | Ref | 0.88 | 0.72, 1.08 | ||
| Q2 | 35 | 0.97 | 0.58, 1.61 | 1.06 | 0.61, 1.77 | ||||
| Q3 | 45 | 1.17 | 0.71, 1.90 | 1.28 | 0.77, 2.11 | ||||
| Q4 | 24 | 0.61 | 0.36, 1.07 | 0.19 | 0.68 | 0.35, 1.21 | 0.38 | ||
HRs were derived from Cox proportional hazard models. Multivariate models were fit by using the case-cohort Cox models available in Epicure Software (Hirosoft). ESCC, esophageal squamous adenocarcinome; Q1–Q4, quartile 1 to quartile 4 (season-specific based on the vitamin C subcohort); Ref, reference.
Median plasma vitamin C concentrations (in μmol/L) of the subcohort per season-specific quartile (winter/spring): Q1: 20.0/5.5; Q2: 43.4/11.7; Q3: 56.3/20.4; Q4: 68.5/47.8.
Conditioned on season of blood draw (winter/spring).
Conditioned on season of blood draw (winter/spring) and adjusted for 6 sex and age categories, BMI (in kg/m2; on the basis of cutoffs appropriate for Asians: <18.5, 15.5–23.9, or ≥24), smoking, and Helicobacter pylori seropositivity.
TABLE 4.
HRs and 95% CIs for the association between plasma vitamin C concentrations and risk of upper gastrointestinal cancers in the Linxian General Population Nutrition Intervention Trial cohort overall and by season1
| All |
Winter, 1999 |
Spring, 2000 |
|||||||||||
| Cancer type Vitamin C concentration | No. of cases | HR2 | 95% CI | P value | No. of cases | HR3 | 95% CI | P value | No. of cases | HR3 | 95% CI | P value | P-interaction |
| ESCC | |||||||||||||
| ≤28 umol/L | 275 | 1.00 | Ref | 46 | 1.00 | Ref | 120 | 1.00 | Ref | ||||
| >28 umol/L | 343 | 0.89 | 0.69, 1.14 | 0.37 | 268 | 0.90 | 0.61, 1.33 | 0.50 | 75 | 0.84 | 0.60, 1.18 | 0.33 | 0.89 |
| Gastric adenocarcinoma | |||||||||||||
| ≤28 umol/L | 234 | 1.00 | Ref | 43 | 1.00 | Ref | 86 | 1.00 | Ref | ||||
| >28 umol/L | 233 | 0.73 | 0.56, 0.97 | 0.030 | 171 | 0.66 | 0.44, 1.00 | 0.050 | 50 | 0.77 | 0.53, 1.13 | 0.19 | 0.43 |
| Cardia adenocarcinoma | |||||||||||||
| ≤28 umol/L | 159 | 1.00 | Ref | 34 | 1.00 | Ref | 57 | 1.00 | Ref | ||||
| >28 umol/L | 170 | 0.73 | 0.52, 1.00 | 0.050 | 133 | 0.64 | 0.41, 1.00 | 0.053 | 37 | 0.82 | 0.53, 1.27 | 0.38 | 0.16 |
| Noncardia adenocarcinoma | |||||||||||||
| ≤28 umol/L | 63 | 1.00 | Ref | 10 | 1.00 | Ref | 34 | 1.00 | Ref | ||||
| >28 umol/L | 75 | 0.74 | 0.48, 1.14 | 0.18 | 46 | 0.75 | 0.37, 1.51 | 0.42 | 17 | 0.70 | 0.38, 1.26 | 0.23 | 0.46 |
Plasma vitamin C concentrations: low, ≤28 μmol/L; normal, >28 μmol/L. ESCC, esophageal squamous cell carcinoma; Ref, reference.
HRs were derived from Cox proportional hazard models and adjusted for 6 sex and age group categories, BMI, smoking, and Helicobacter pylori.
HRs were derived from Cox proportional hazard models and conditioned on season of blood draw (winter/spring) and adjusted for 6 sex and age group categories, BMI, smoking, and H. pylori seropositivity.
For ESCC, we found no significant evidence of an association with plasma vitamin C concentrations (HR>28 vs ≤28 μmol/L: 0.89; 95% CI: 0.69, 1.14; P = 0.37), regardless of the metric used to model the exposure (Tables 3 and 4).
We examined the associations between plasma vitamin C concentrations and risk of each cancer separately by selected characteristics. No evidence of effect modification by sex, age, smoking, serologically defined atrophic gastritis, or Factor C intervention group assignment were found for either gastric cancer or ESCC (see Supplemental Figure 1 under “Supplemental data” in the online issue). The results of the analyses that excluded individuals with a diagnosis made within 1 y after the blood draw showed similar risk estimates (data not shown).
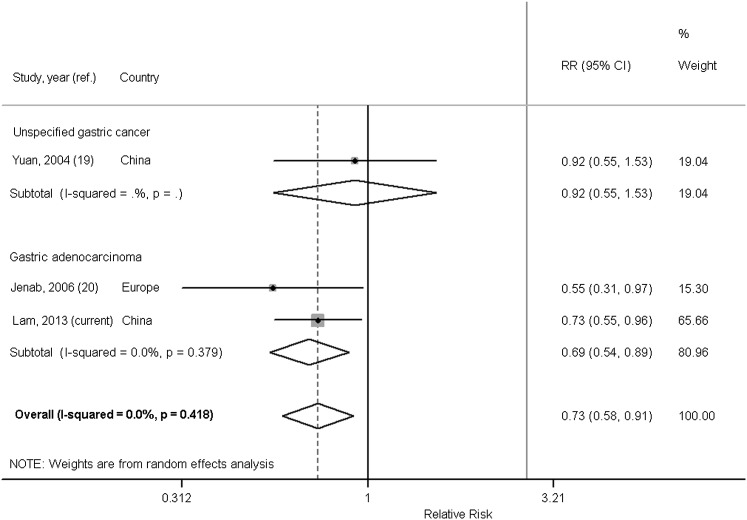
In our systematic review, we identified 3 prospective cohort studies (19, 20, 27) that quantified the association between circulating vitamin C and gastric cancer. Of these, we excluded the Basel Study (27) because results were presented only stratified by age such that we were unable to calculate the overall RR (95% CI) from the published data. Selected characteristics of the included studies and ours are presented in Table 5. The 2 included studies were nested case-control studies of comparable size. The study in Chinese men (19) found no relation with the incidence of unspecified gastric cancer (no. of cases = 191; ORQ4vsQ1: 0.92; 95% CI: 0.55, 1.55), whereas the European study found that plasma vitamin C concentrations were inversely associated with the risk of incident GA (no. of cases = 199; ORQ4vsQ1: 0.55; 95% CI: 0.31, 0.97) (20). The latter study also reported insignificant inverse associations for gastric noncardia (no. of cases = 113; ORQ4vsQ1: 0.28, 1.42; 95% CI: 0.52, 1.00) and gastric cardia (no. of cases = 56; OR Q4vsQ1: 0.36; 95% CI: 0.10, 1.33). Similar to the current study, the cases in the European study (20) were identified as GA, whereas cases in the study by Yuan et al (19) were not specified. A meta-analysis of these 2 studies and the current study showed an overall random-effect pooled RRhighest vs lowest of 0.73 (95% CI: 0.58, 0.91, P-heterogeneity = 0.42; I2 = 0%), Figure 2. When we pooled the results from Jenab et al (20) and the current study to examine the association between plasma vitamin C and GA only, the magnitude of the inverse association was stronger (random-effect pooled RRhighest vs lowest: 0.69; 95% CI: 0.54, 0.89; P-heterogeneity = 0.38; I2 = 0%).
TABLE 5.
Characteristics of cohort studies reporting RRs and 95% CIs for the association between prediagnostic plasma vitamin C concentrations (highest compared with lowest category) and gastric cancer incidence1
| Author | Year | Cohort name | Region | Sex | Study design | Measurement for vitamin C | GC cases, type | OR/HR (95% CI) | Factors adjusted for |
| Yuan et al (19) | 2004 | Shanghai Cohort Study | China | M | Nested case-control | HPLC | 197, unspecified | 0.92 (0.55, 1.55) | Age, date of biospecimen collection, neighborhood of residence at recruitment, smoking, alcohol, urinary epigallocatechin, and Helicobacter pylori |
| Jenab et al (20) | 2006 | EPIC | European | M and F | Nested case-control | Fluorometric | 199, adenocarcinoma | 0.55 (0.31, 0.97) | H. pylori, smoking, sex, age, center, and date of blood draw |
| Lam et al (current study) | 2013 | NIT | China | M and F | Case-cohort | HPLC | 467, adenocarcinoma | 0.73 (0.56, 0.97) | Season of blood drawn, age, sex, BMI, smoking, and H. pylori |
EPIC, European Prospective Investigation in Cancer and Nutrition cohort; GC, gastric cancer; NIT, Nutritional Intervention Trial.
FIGURE 2.
Forest plot of the highest compared with the lowest category of circulating plasma vitamin C and gastric adenocarcinoma and gastric cancer risk in prospective studies. We used inverse-variance weights in random-effects models to obtain pooled RR estimates, comparing the highest with the lowest categories of circulating vitamin C. Analyses were performed by using Stata version 11.1. ref., reference.
DISCUSSION
In the current study, we found an inverse association between higher prediagnostic concentrations of plasma vitamin C and subsequent risk of incident GA in a high-risk Chinese population, but little evidence of a relation with risk of incident ESCC. This study was the largest prospective cohort study on the association between circulating vitamin C and GA risk to date and the first prospective evaluation of the relation with ESCC. Results based on our meta-analysis of prospective cohort studies suggest that plasma vitamin C is inversely associated with GA incidence.
To our knowledge, there are no published cohort studies relating prediagnostic plasma vitamin C to ESCC risk. The World Cancer Research Fund review concluded that foods containing vitamin C probably protect against esophageal cancer (28). Case-controls studies suggest that dietary vitamin C is inversely associated with ESCC (29, 30). Fresh fruit consumption was inversely associated with risk of ESCC in Linxian (31). Nevertheless, in the current study, we found little evidence of an association between circulating vitamin C concentrations and ESCC.
Two previous prospective studies (19, 20) reported on circulating prediagnostic vitamin C and gastric cancer incidence, and one prospective study (27) reported on gastric cancer mortality. Our meta-analysis of gastric cancer incidence showed that persons in the highest concentration of prediagnostic plasma vitamin C had a significant 27% and 31% lower risk of incident gastric cancer and GA, respectively, compared with those with the lowest concentration. The gastric cancer mortality study (no. of cases = 20) from Switzerland showed a benefit of lower mortality from gastric cancer with higher plasma vitamin C only among older men (>60 y), but the effect was no longer significant when cases diagnosed within the first 2 y were excluded (27).
Although vitamin C has been included as a component of multivitamin supplements in randomized controlled trials (RCTs), it has not been investigated as a single intervention agent with respect to gastric cancer incidence. Its effects on precancerous gastric lesions, however, were investigated in several RCTs, and the results were largely null (22, 32–35). Correa et al (32) observed a beneficial effect of vitamin C supplement and increased rates of regression for precancerous gastric lesions; however, a subsequent analysis after 12 y of follow-up showed no effect (33). You et al (34) and Ma et al (36) found no beneficial effect of a regimen of vitamins C and E and selenium on precancerous gastric lesions.
The Linxian General Population NIT, from which participants in the current study were selected, investigated the effect of vitamin C supplementation on cancer incidence and disease-specific mortality. Trial participants randomly assigned to Factor C received vitamin C (120 mg, ∼1.5 times the US Recommended Dietary Allowance) and molybdenum (30 μg) daily for 5.25 y, but no effect was seen for Factor C on gastric cancer mortality or incidence at the end of the intervention period (22) or through the subsequent 10-y postintervention follow-up (2).
Contrary to the RCTs, results from observational studies have shown beneficial effects associated with vitamin C concentrations. Higher serum vitamin C concentrations were associated with a reduced prevalence of intestinal metaplasia (37) and reduced progression to dysplasia and gastric cancer (38). Notably, the results of the current study differ from those of the NIT intervention (2, 22). There are numerous reasons why the results of observational studies and RCTs of nutritional agents might not agree, particularly when RCTs are null. Most prominent among these are the selection of an inappropriate intervention dose (too high or too low), duration (too short), timing (too late in life), or study population (already has adequate nutrition) (39).
Several biologically plausible mechanisms support the role of vitamin C in the etiology of gastric cancer. Because infection of H. pylori is linked to the development of gastric cancer, the interrelation between vitamin C and H. pylori presents a compelling hypothesis for vitamin C's role as an inverse factor in gastric carcinogenesis. As an antioxidant, vitamin C could protect cells against oxidative DNA damage (40), inhibit the formation of N-nitroso compounds (41), and/or neutralize the toxicity of reactive oxidative species caused by H. pylori infection (42). Vitamin C may also inhibit H. pylori growth (9, 43). There is also evidence that vitamin C may inhibit cell proliferation (16, 18) and promote H. pylori–associated apoptosis (10). A possible explanation for the lack of association observed for ESCC in our study might be that H. pylori is not a risk factor for ESCC (44); thus, vitamin C does not confer strong protection against it in this population. Nevertheless, this is speculative at best because the antioxidative properties attributed to vitamin C should also apply regardless of cancer sites.
The strengths of the current study include its prospective design, large sample size, and detailed modeling of the associations using a variety of strategies. Limitations include the fact that assessment of vitamin C occurred at only one point before diagnosis and that follow-up was relatively short (average ∼6.7 y). A single measurement of vitamin C might not reflect true exposure because plasma vitamin C varies seasonally. However, we observed similar inverse associations for vitamin C concentrations and GA when we stratified by season, which suggests that season of blood draw did not modify the association. Whereas the loss of vitamin C because of long-term storage was a possibility, a previous study showed no degradation in human plasma preserved at −80°C following stabilization by MPA for 5 y (45). Furthermore, any differences should be nondifferential with regard to case status. We assessed follow-up time as a potential factor influencing our findings by excluding individuals who received a diagnosis of cancer within 1 y of their blood draw; we found similar results, which suggests that preclinical disease did not explain our findings. Finally, because the study population in Linxian was deficient in several essential nutrients and had a high prevalence of H. pylori seropositivity, our results may differ from Western populations with lower H. pylori rates and better nutritional status. Nevertheless, our findings are similar to those of the European Prospective Investigation into Cancer and Nutrition study, whose population has adequate nutrition.
In conclusion, we found evidence that higher plasma vitamin C concentrations were associated with protection against the development of GA but no evidence of an association between circulating vitamin C and ESCC.
Supplementary Material
Acknowledgments
We thank the residents of Linxian for their generous participation in our study.
The authors’ responsibilities were as follows—TKL: study concept and design, data acquisition, statistical analysis, study management, data analysis and interpretation, and manuscript writing; NDF and PRT: study concept and design, data acquisition, data interpretation, and manuscript preparation; J-HF and SMD: manuscript preparation; Y-LQ: data acquisition and manuscript preparation; and CCA: study concept and design, data acquisition, funding, technical support, manuscript preparation, and final approval of the manuscript. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: ESCC, esophageal squamous cell carcinoma; GA, gastric adenocarcinoma; MPA, meta-phosphoric acid; NIT, Nutrition Intervention Trial; QC, quality control; RCT, randomized controlled trial.
REFERENCES
- 1.Chen WQ, Zhang S, Zou X, Zhoa P. Cancer incidence and mortality in China, 2006. Chin J Cancer Res 2011;23:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qiao YL, Dawsey SM, Kamangar F, Fan JH, Abnet CC, Sun XD, Johnson LL, Gail MH, Dong ZW, Yu B, et al. Total and cancer mortality after supplementation with vitamins and minerals: follow-up of the Linxian General Population Nutrition Intervention Trial. J Natl Cancer Inst 2009;101:507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loria CM, Whelton PK, Caulfield LE, Szklo M, Klag MJ. Agreement among indicators of vitamin C status. Am J Epidemiol 1998;147:587–96. [DOI] [PubMed] [Google Scholar]
- 4.Zou XN, Taylor PR, Mark SD, Chao A, Wang W, Dawsey SM, Wu YP, Qiao YL, Zheng SF. Seasonal variation of food consumption and selected nutrient intake in Linxian, a high risk area for esophageal cancer in China. Int J Vitam Nutr Res 2002;72:375–82. [DOI] [PubMed] [Google Scholar]
- 5.Zhang ZW, Farthing MJ. The roles of vitamin C in Helicobacter pylori associated gastric carcinogenesis. Chin J Dig Dis 2005;6:53–8. [DOI] [PubMed] [Google Scholar]
- 6.Dawsey SM, Mark SD, Taylor PR, Limburg PJ. Gastric cancer and H pylori. Gut 2002;51:457–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang ZW, Patchett SE, Perrett D, Katelaris PH, Domizio P, Farthing MJ. The relation between gastric vitamin C concentrations, mucosal histology, and CagA seropositivity in the human stomach. Gut 1998;43:322–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawaguchi H, Haruma K, Komoto K, Yoshihara M, Sumii K, Kajiyama G. Helicobacter pylori infection is the major risk factor for atrophic gastritis. Am J Gastroenterol 1996;91:959–62. [PubMed] [Google Scholar]
- 9.Zhang HM, Wakisaka N, Maeda O, Yamamoto T. Vitamin C inhibits the growth of a bacterial risk factor for gastric carcinoma: Helicobacter pylori. Cancer 1997;80:1897–903. [PubMed] [Google Scholar]
- 10.Zhang ZW, Abdullahi M, Farthing MJ. Effect of physiological concentrations of vitamin C on gastric cancer cells and Helicobacter pylori. Gut 2002;50:165–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamangar F, Qiao YL, Blaser MJ, Sun XD, Katki H, Fan JH, Perez-Perez GI, Abnet CC, Zhao P, Mark SD, et al. Helicobacter pylori and oesophageal and gastric cancers in a prospective study in China. Br J Cancer 2007;96:172–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mark SD, Qiao YL, Dawsey SM, Wu YP, Katki H, Gunter EW, Fraumeni JF, Jr, Blot WJ, Dong ZW, Taylor PR. Prospective study of serum selenium levels and incident esophageal and gastric cancers. J Natl Cancer Inst 2000;92:1753–63. [DOI] [PubMed] [Google Scholar]
- 13.Taylor PR, Qiao YL, Abnet CC, Dawsey SM, Yang CS, Gunter EW, Wang W, Blot WJ, Dong ZW, Mark SD. Prospective study of serum vitamin E levels and esophageal and gastric cancers. J Natl Cancer Inst 2003;95:1414–6. [DOI] [PubMed] [Google Scholar]
- 14.Abnet CC, Qiao YL, Dawsey SM, Buckman DW, Yang CS, Blot WJ, Dong ZW, Taylor PR, Mark SD. Prospective study of serum retinol, beta-carotene, beta-cryptoxanthin, and lutein/zeaxanthin and esophageal and gastric cancers in China. Cancer Causes Control 2003;14:645–55. [DOI] [PubMed] [Google Scholar]
- 15.Chen W, Dawsey SM, Qiao YL, Mark SD, Dong ZW, Taylor PR, Zhao P, Abnet CC. Prospective study of serum 25(OH)-vitamin D concentration and risk of oesophageal and gastric cancers. Br J Cancer 2007;97:123–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brigelius-Flohé R, Flohé L. Ascorbic acid, cell proliferation, and cell differentiation in culture. Subcell Biochem 1996;25:83–107. [DOI] [PubMed] [Google Scholar]
- 17.Halliwell B. Vitamin C and genomic stability. Mutat Res 2001;475:29–35. [DOI] [PubMed] [Google Scholar]
- 18.Campbell JD, Cole M, Bunditrutavorn B, Vella AT. Ascorbic acid is a potent inhibitor of various forms of T cell apoptosis. Cell Immunol 1999;194:1–5. [DOI] [PubMed] [Google Scholar]
- 19.Yuan JM, Ross RK, Gao YT, Qu YH, Chu XD, Yu MC. Prediagnostic levels of serum micronutrients in relation to risk of gastric cancer in Shanghai, China. Cancer Epidemiol Biomarkers Prev 2004;13:1772–80. [PubMed] [Google Scholar]
- 20.Jenab M, Riboli E, Ferrari P, Sabate J, Slimani N, Norat T, Friesen M, Tjonneland A, Olsen A, Overvad K, et al. Plasma and dietary vitamin C levels and risk of gastric cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST). Carcinogenesis 2006;27:2250–7. [DOI] [PubMed] [Google Scholar]
- 21.Li B, Taylor PR, Li JY, Dawsey SM, Wang W, Tangrea JA, Liu BQ, Ershow AG, Zheng SF, Fraumeni JF, Jr, et al. Linxian nutrition intervention trials. Design, methods, participant characteristics, and compliance. Ann Epidemiol 1993;3:577–85. [DOI] [PubMed] [Google Scholar]
- 22.Blot WJ, Li JY, Taylor PR, Guo W, Dawsey S, Wang GQ, Yang CS, Zheng SF, Gail M, Li GY, et al. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst 1993;85:1483–92. [DOI] [PubMed] [Google Scholar]
- 23.McCoy LF, Bowen MB, Xu M, Chen H, Schleicher RL. Improved HPLC assay for measuring serum vitamin C with 1-methyluric acid used as an electrochemically active internal standard. Clin Chem 2005;51:1062–4. [DOI] [PubMed] [Google Scholar]
- 24.Abnet CC, Zheng W, Ye W, Kamangar F, Ji BT, Persson C, Yang G, Li HL, Rothman N, Shu XO, et al. Plasma pepsinogens, antibodies against Helicobacter pylori, and risk of gastric cancer in the Shanghai Women's Health Study Cohort. Br J Cancer 2011;104:1511–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren JS, Kamangar F, Qiao YL, Taylor PR, Liang H, Dawsey SM, Liu B, Fan JH, Abnet CC. Serum pepsinogens and risk of gastric and oesophageal cancers in the General Population Nutrition Intervention Trial cohort. Gut 2009;58:636–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng SF, Ershow AG, Yang CS, Li GY, Li RS, Li H, Zou XL, Liu XF, Song LH, Qing QS, et al. Nutritional status in Linxian, China: effects of season and supplementation. Int J Vitam Nutr Res 1989;59:190–9. [PubMed] [Google Scholar]
- 27.Stähelin HB, Gey KF, Eichholzer M, Ludin E, Bernasconi F, Thurneysen J, Brubacher G. Plasma antioxidant vitamins and subsequent cancer mortality in the 12-year follow-up of the prospective Basel Study. Am J Epidemiol 1991;133:766–75. [DOI] [PubMed] [Google Scholar]
- 28.World Cancer Research Fund, American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington, DC: AICR, 2007. [Google Scholar]
- 29.Franceschi S, Bidoli E, Negri E, Zambon P, Talamini R, Ruol A, Parpinel M, Levi F, Simonato L, La Vecchia C. Role of macronutrients, vitamins and minerals in the aetiology of squamous-cell carcinoma of the oesophagus. Int J Cancer 2000;86:626–31. [DOI] [PubMed] [Google Scholar]
- 30.Mayne ST, Risch HA, Dubrow R, Chow WH, Gammon MD, Vaughan TL, Farrow DC, Schoenberg JB, Stanford JL, Ahsan H, et al. Nutrient intake and risk of subtypes of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev 2001;10:1055–62. [PubMed] [Google Scholar]
- 31.Gao Y, Hu N, Han XY, Ding T, Giffen C, Goldstein AM, Taylor PR. Risk factors for esophageal and gastric cancers in Shanxi Province, China: a case-control study. Cancer Epidemiol 2011;35(6):e91–e9. [DOI] [PMC free article] [PubMed]
- 32.Correa P, Fontham ET, Bravo JC, Bravo LE, Ruiz B, Zarama G, Realpe JL, Malcom GT, Li D, Johnson WD, et al. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-helicobacter pylori therapy. J Natl Cancer Inst 2000;92:1881–8. [DOI] [PubMed] [Google Scholar]
- 33.Mera R, Fontham ET, Bravo LE, Bravo JC, Piazuelo MB, Camargo MC, Correa P. Long term follow up of patients treated for Helicobacter pylori infection. Gut 2005;54:1536–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.You WC, Brown LM, Zhang L, Li JY, Jin ML, Chang YS, Ma JL, Pan KF, Liu WD, Hu Y, et al. Randomized double-blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions. J Natl Cancer Inst 2006;98:974–83. [DOI] [PubMed] [Google Scholar]
- 35.Plummer M, Vivas J, Lopez G, Bravo JC, Peraza S, Carillo E, Cano E, Castro D, Andrade O, Sanchez V, et al. Chemoprevention of precancerous gastric lesions with antioxidant vitamin supplementation: a randomized trial in a high-risk population. J Natl Cancer Inst 2007;99:137–46. [DOI] [PubMed] [Google Scholar]
- 36.Ma JL, Zhang L, Brown LM, Li JY, Shen L, Pan KF, Liu WD, Hu Y, Han ZX, Crystal-Mansour S, et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J Natl Cancer Inst 2012;104:488–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang L, Blot WJ, You WC, Chang YS, Liu XQ, Kneller RW, Zhao L, Liu WD, Li JY, Jin ML, et al. Serum micronutrients in relation to pre-cancerous gastric lesions. Int J Cancer 1994;56:650–4. [DOI] [PubMed] [Google Scholar]
- 38.You WC, Zhang L, Gail MH, Chang YS, Liu WD, Ma JL, Li JY, Jin ML, Hu YR, Yang CS, et al. Gastric dysplasia and gastric cancer: Helicobacter pylori, serum vitamin C, and other risk factors. J Natl Cancer Inst 2000;92:1607–12. [DOI] [PubMed] [Google Scholar]
- 39.Kristal AR. Are clinical trials the “gold standard” for cancer prevention research? Cancer Epidemiol Biomarkers Prev 2008;17:3289–91. [DOI] [PubMed] [Google Scholar]
- 40.Correa P, Malcom G, Schmidt B, Fontham E, Ruiz B, Bravo JC, Bravo LE, Zarama G, Realpe JL. Review article: antioxidant micronutrients and gastric cancer. Aliment Pharmacol Ther 1998;12(suppl 1):73–82. [DOI] [PubMed] [Google Scholar]
- 41.Tannenbaum SR, Wishnok JS, Leaf CD. Inhibition of nitrosamine formation by ascorbic acid. Am J Clin Nutr 1991;53(suppl):247S–50S. [DOI] [PubMed] [Google Scholar]
- 42.Drake IM, Davies MJ, Mapstone NP, Dixon MF, Schorah CJ, White KL, Chalmers DM, Axon AT. Ascorbic acid may protect against human gastric cancer by scavenging mucosal oxygen radicals. Carcinogenesis 1996;17:559–62. [DOI] [PubMed] [Google Scholar]
- 43.Jarosz M, Dzieniszewski J, Dabrowska-Ufniarz E, Wartanowicz M, Ziemlanski S, Reed PI. Effects of high dose vitamin C treatment on Helicobacter pylori infection and total vitamin C concentration in gastric juice. Eur J Cancer Prev 1998;7:449–54. [DOI] [PubMed] [Google Scholar]
- 44.Whiteman DC, Parmar P, Fahey P, Moore SP, Stark M, Zhao ZZ, Montgomery GW, Green AC, Hayward NK, Webb PM. Association of Helicobacter pylori infection with reduced risk for esophageal cancer is independent of environmental and genetic modifiers. Gastroenterology 2010;139(1):73–83; quiz e11–e12. [DOI] [PubMed]
- 45.Lykkesfeldt J. Ascorbate and dehydroascorbic acid as reliable biomarkers of oxidative stress: analytical reproducibility and long-term stability of plasma samples subjected to acidic deproteinization. Cancer Epidemiol Biomarkers Prev 2007;16:2513–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.