Abstract
Background: Cardiovascular disease (CVD) and osteoporosis are 2 major public health problems that share common pathophysiological mechanisms. It is possible that strategies to reduce CVD risk may also benefit bone health.
Objective: We tested the hypothesis that adherence to the 2006 American Heart Association Diet and Lifestyle Recommendations (AHA-DLR) is associated with bone health.
Design: We previously developed a unique diet and lifestyle score (American Heart Association Diet and Lifestyle Score; AHA-DLS) to assess adherence to the AHA-DLR. In a cross-sectional study of 933 Puerto Ricans aged 47–79 y, we modified the AHA-DLS to test associations with bone health. Bone mineral density (BMD) at the femoral neck, trochanter, total hip, and lumbar spine (L2–L4) was measured by using dual-energy X-ray absorptiometry.
Results: For every 5-unit increase in the modified AHA-DLS, BMD at the femoral neck, trochanter, total hip, and lumbar spine (L2–L4) was associated with a 0.005–0.008-g/cm2 (P < 0.05) higher value. No component of the AHA-DLR alone was responsible for the observed positive associations. For every 5-unit increase in the modified AHA-DLS, the odds for osteoporosis or osteopenia at the trochanter, total hip, and lumbar spine (L2–L4) were lower by 14% (OR: 0.86; 95% CI: 0.79, 0.92), 17% (OR: 0.83; 95% CI: 0.76, 0.92), and 9% (OR: 0.91; 95% CI: 0.84, 0.99), respectively.
Conclusions: Dietary guidelines for CVD risk reduction may also benefit bone health in this Hispanic cohort. Synchronizing dietary guidelines for these 2 common diseases may provide a simplified public health message. This trial was registered at clinicaltrials.gov as NCT01231958.
INTRODUCTION
Cardiovascular disease (CVD)4 and osteoporosis are chronic conditions that result in a major public health burden but have remained understudied in minority populations. CVD is the leading cause of death among Hispanics. Among Mexican American adults aged ≥20 y, 28.5% of men and 34.5% of women have been reported to have CVD (1). In individuals aged ≥65 y, the prevalence of osteoporosis and related fractures in Hispanic Americans was 2 times that in African Americans (2). Furthermore, the prevalence of risk factors for CVD and osteoporosis—such as smoking, physical inactivity, and metabolic syndrome—remain high in the Hispanic population (1). Although CVD and osteoporosis have long been thought to be independent, chronic diseases coexisting in the aging population, recent evidence from basic science, and epidemiologic research have shown that these 2 chronic conditions are linked by several biological mechanisms, such as inflammation. This implies that strategies for CVD risk reduction may also reduce the risk of osteoporosis.
The most basic components for chronic disease prevention and risk reduction are diet and lifestyle. Recognizing this, the American Heart Association (AHA) periodically releases guidelines and recommendations intended to reduce CVD risk in Americans >2 y of age. The most recent diet and lifestyle recommendations, released in 2006 deserve special emphasis, as these guidelines are based on an overall healthy dietary pattern, making positive lifestyle choices, and provide multiple benefits for reduction of CVD risk factors (3). It has been suggested that these guidelines may also protect against other chronic diseases, such as type 2 diabetes and osteoporosis (4). However, no studies have evaluated the effect of adherence to CVD risk reduction recommendations on bone health. This is particularly important in the Puerto Rican population, a group with documented health disparities (5). Puerto Ricans, the second largest Hispanic group in the United States and the largest in the northeastern United States, have traditionally been underrepresented in research. Yet, they present with multiple comorbidities and have a high prevalence of CVD risk factors (6).
Understanding the potential benefit of dietary and lifestyle recommendations, originally intended for CVD risk reduction on bone health, may help synchronize guidelines for 2 major chronic conditions and provide foundational data for public health practice. Thus, the objective of the current study was to examine whether adherence to the 2006 AHA Diet and Lifestyle Recommendations (AHA-DLR) is beneficial to bone health in a population of older Puerto Ricans living in the greater Boston area.
SUBJECTS AND METHODS
Participants
Participants from this study were part of the Boston Puerto Rican Osteoporosis study—an ancillary study to the Boston Puerto Rican Health Study. The design of the Boston Puerto Rican Health Study was described in detail elsewhere (7). Recruitment began in 2004 by enrolling self-identified Puerto Ricans aged 45–75 y living in the greater Boston area. Baseline recruitment was completed in the year 2009; therefore, participants had baseline assessments at varying time points. At baseline and 2 y, home interviews were conducted by bilingual interviewers who administered questionnaires to obtain information on socioeconomic status, health and health behaviors, acculturation, stress, and usual diet. In addition, anthropometric and blood pressure measures were recorded. Biological samples, including saliva, urine, and 12-h fasting blood, were collected by the phlebotomist in the participants’ homes on the day after the interview or as soon as possible thereafter. The mean (±SD) follow-up time between the baseline and 2-y home visit was 2.2 ± 0.61 y. At the end of the 2-y home visit, participants reconsented to enrollment in the osteoporosis study. Between December 2006 and October 2012, 974 of the 1276 participants who completed the 2-y follow-up interview had visited the Metabolic Research Unit at the USDA Human Nutrition Research Center for Aging at Tufts University for measurement of bone mineral density (BMD), 12-h fasting blood sampling, and administration of a questionnaire to assess sun exposure, estrogen use, and history of falls and fracture. Every attempt was made to schedule the osteoporosis study visit within 1 mo of completion of the 2-y follow-up. Primary reasons for nonparticipation in the current study included not being interested (n = 207), scheduling problems (n = 52), loss to follow-up (n = 13), and moved out of Massachusetts (n = 10). Another 20 participants had died since their 2-y follow-up interview. Participants who declined participation were more likely to be older (58.6 compared with 56.5 y; P = 0.0001). At the time of analysis, complete BMD information was available for 974 participants (283 men and 691 women). All study protocols were approved by the Institutional Review Board of Tufts Medical Center.
Measurement of bone mineral density and osteoporosis
BMD of the femur and lumbar spine (L2-L4) was measured by dual-energy X-ray absorptiometry by using a GE-Lunar model Prodigy scanner in the Bone Metabolism Laboratory at the Human Nutrition Research Center on Aging. The root mean square precision of these measurements in our laboratory is 1.31% BMD at the femoral neck, 1.03% at the trochanter, 0.65% at the total femur, and 1.04% at the lumbar spine (8). In all analyses, we made an a priori decision to include BMD measurements at the femoral neck, total femur, and posterior-anterior lumbar spine (L2-L4), based on recommendations from the International Society for Clinical Densitometry (9), and at the trochanter to provide a complete picture of the total femur. We used the WHO definition of osteoporosis and osteopenia as T-score thresholds of ≥2.5 or 1.0 SD, respectively, below the healthy young adult mean at each respective bone site. All scans with T-scores >4.0 were reviewed by an academic endocrinologist (BD-H) to check for extraskeletal calcification or for the presence of nonanatomical parts in the dual-energy X-ray absorptiometry scan region. For the current analyses, we excluded one poor-quality femur scan.
Dietary assessment and AHA Diet and Lifestyle Score
At the baseline and 2-y follow-up interview, usual dietary intake was assessed by using a semiquantitative food-frequency questionnaire that was designed for and validated with the Puerto Rican population (10). Reported food intakes were converted into gram amounts. Serving sizes were calculated by dividing the gram amount of the food with the reference serving amounts from the USDA Food Guide Pyramid. Nutrient intakes were calculated by using the Nutrition Data System for Research software (version 2007), developed by the Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN.
At baseline and 2 y, we constructed a unique diet and lifestyle score based on the 2006 AHA-DLR for CVD risk reduction (3). A higher score indicates greater adherence to the AHA-DLR. The development and validation of the AHA Diet and Lifestyle Score (AHA-DLS) has been described in detail elsewhere (11). Briefly, a total of 9 recommendations have been put forth by the AHA for CVD risk reduction. These include the following: balance calorie intake and physical activity to achieve or maintain a healthy body weight; consume a diet rich in fruit and vegetables; choose whole-grain, high-fiber foods; consume fish, especially oily fish, at least twice a week; limit your intake of saturated and trans fat and cholesterol; minimize your intake of beverages and foods with added sugars; choose and prepare foods with little or no salt; if you consume alcohol, do so in moderation; and when you eat food that is prepared outside of the home, follow the AHA-DLR. The AHA-DLS was based on the first 8 recommendations. Participants received scores for each of the 8 components, with scores ranging from a minimum of zero to maximums of 4, 6, or 10 for the various subcomponents. The total possible score on the AHA-DLS was 110. Foods, nutrients, and lifestyle variables [BMI (in kg/m2) and physical activity] were included in the development of the AHA-DLS. Greater adherence to the AHA-DLR was associated with a higher HDL concentration and lower waist circumference, 10-y coronary heart disease risk (assessed by the Framingham risk score, among women), and serum glucose (only in those with BMI <25), insulin, and C-reactive protein (CRP) concentrations (11).
We used 2 subcomponents, BMI and physical activity, to represent adherence to the recommendation to balance calorie intake and physical activity to achieve or maintain a healthy body weight. Because greater body weight is a strong protective factor for BMD (12), we modified the AHA-DLS by removing the BMI subcomponent. Because the total possible score for the BMI subcomponent was 10 points, total points possible on the modified AHA-DLS were reduced to 100. To control for potential confounding due to BMI, this variable was adjusted for in all statistical analyses.
Because diet is known to be relatively stable (13) and to better control for random within-person variation in dietary data, we used the cumulative average of baseline and 2-y dietary data. Complete information on bone health and the modified AHA-DLS was available on 933 participants (male, n = 254; female, n = 679).
Assessment of covariates
We calculated age by subtracting the date of the participant's osteoporosis study visit from the participant's date of birth. Standing height was measured with a stadiometer (Seca). Weight was measured with a digital scale (model Alpha; Seca). BMI was calculated by dividing weight (kg) by height (m)2. At the osteoporosis study visit, we administered a short questionnaire to collect information on prescription medication use for treatment of osteoporosis (yes or no), defined as current use of bisphosphonates, calcitonin, calcium, vitamin D, or cod liver oil. Because season is known to affect BMD in the New England area (14, 15), we created a 4-level categorical variable for season of BMD measurement as follows: July to September was coded as summer, October to December as fall, January to March as winter, and April to June as spring. Plasma 25-hydroxyvitamin D (ng/mL) was measured by using a 125I radioimmunoassay kit procedure (DiaSorin Inc). The intra- and interassay CVs for this analyte are 10.8% and 9.4%, respectively.
Information on the educational status of the participant was captured at the baseline home visit and categorized as <9th grade, 9th–12th grade/General Education Development, or college/some graduate school. At the 2-y follow-up interview, we collected information on total household income (US$/y) and current smoking (yes or no) status. Multivitamin supplement use was identified by asking participants to show us the containers for any prescription or over-the-counter supplements used. This was treated as a binary variable (yes or no) in the current analysis. At baseline and 2 y, acculturation was assessed as reported preference of language use in various everyday activities (16) and the Spanish version of the perceived stress scale was administered (17). Usual intakes of calcium (mg/d) and total energy (kcal/d) were assessed by food-frequency questionnaire (10). For all continuous covariates (except age), we used the average of the baseline and 2-y measures.
Statistical analyses
All statistical analyses were completed by using SAS statistical software (version 9.3; SAS Institute). Formal hypothesis testing was 2-sided, with a nominal type I error rate of 0.05. We examined the distributions of the primary analytic variables and other descriptive data. The modified AHA-DLS was treated as a continuous variable and was also divided into energy-adjusted quartiles by using the residual method (18). We assessed the stability of the diet over 2 y by comparing baseline measures of the modified AHA-DLS with the 2-y follow-up visit values. We calculated the age- and sex-adjusted least-squares means by using general linear models for the sociodemographic characteristics, lifestyle behaviors, and biologic measures across energy-adjusted quartiles of the modified AHA-DLS. Dietary characteristics were additionally adjusted for energy intake. We assessed statistical significance across quartiles of the modified AHA-DLS using linear (for continuous variables) and logistic (for categorical variables) regression.
Associations between the modified AHA-DLS, both as a continuous (5-unit increase) and categorical (energy-adjusted quartiles) measure, and BMD (continuous) of the femoral neck, trochanter, total femur, and lumbar spine were examined by using the general linear models procedure. For each association, 3 multivariable models were constructed. First, we adjusted for sociodemographic characteristics and osteoporosis risk factors, including age (y), sex (male or female), BMI (kg/m2), height (m), current smoking status (yes or no), educational status (≤9th grade, 9th–12th grade/General Education Development, or college/some graduate school), season of BMD measurement (summer, fall, winter, or spring), plasma vitamin D concentration (ng/mL), and intakes of total energy (kcal/d) and calcium (mg/d). In our second model, we controlled for confounding by indication by including current use of prescription osteoporosis medications (yes or no) and multivitamin supplements (yes or no). Because the diet of Hispanic elders is known to be associated with acculturation (19), we included this variable in model 3. Finally, to consider the effect of potential confounding due to stress, we also adjusted for perceived stress score in model 3. Adjusted mean BMD at each bone site was calculated across energy-adjusted quartiles of the modified AHA-DLS. All analyses were adjusted for multiple comparisons by using Dunnett's adjustment with the lowest quartile as the reference group.
To determine the importance of each of the AHA recommendations on BMD, we included scores for each subcomponent as an independent variable one at a time in the regression model. The association with BMD was calculated by a 1-unit increase in each subcomponent. Each regression model was adjusted for covariates and the total modified AHA-DLS minus the subcomponent being investigated. We applied Bonferroni adjustment by multiplying the alpha with the total number of comparisons at each bone site (P = 0.004). For all linear regression models, we tested the assumptions of normality, linearity, and homogeneity by examining the plots of residuals compared with predicted values and normal probability plots of the residuals.
We used logistic regression to model the odds of osteoporosis or osteopenia for a 5-unit increase in the modified AHA-DLS, sequentially adjusting for the covariates used in the linear regression models. In all analyses, we tested for potential effect modification due to sex by including a cross-product term with the modified AHA-DLS score. Tests for linear trend were conducted by assigning each participant the median value of the modified AHA-DLS for each quartile category and treating this as a continuous variable in regression analyses.
RESULTS
The AHA-DLS was normally distributed in our sample. The means (±SDs) of the modified AHA-DLS at baseline and 2-y follow-up visit were 29.9 ± 10.8 and 30.1 ± 10.6, respectively. Only 2.5% (n = 23) of study participants had a baseline-modified AHA-DLS that was 2.0-SD away from their mean 2-y modified AHA-DLS. Adherence to the AHA-DLS was low. Fewer than 3% (n = 29) of the participants received a score of more than half of the total possible score. No significant interaction was observed with sex (P > 0.05) in any of our analyses. Thus, data from men and women were analyzed together, and sex was included as a covariate in regression models.
The median modified AHA-DLS score in the highest quartile (42.2) was about twice that of the median score in the lowest quartile (18.8). Those in the highest compared with the lowest quartile were more likely to be older, be more physically active, have a higher calcium intake, have a higher total household income, and have a higher educational status. Highest (compared with lowest) quartile participants were also more likely to have higher acculturation scores and to report lower perceived stress (Table 1). After adjustment for sociodemographic characteristics and osteoporosis risk factors (model 1), every 5-unit increase in the modified AHA-DLS was associated with a 0.005–0.008-g/cm2 higher BMD at all 4 bone sites (P < 0.05 for each) (Table 2). Further adjustment for medication use, multivitamin use, acculturation, and perceived stress did not appreciably change the results.
TABLE 1.
Characteristics of Puerto Ricans by energy-adjusted quartiles of the modified AHA-DLS1
| Quartiles of AHA-DLS |
|||||
| 1(n = 233) | 2(n = 233) | 3(n = 234) | 4(n = 233) | ||
| Characteristic | 18.8 (5.7–22.9)2 | 26.0 (23.0–28.9) | 32.4 (29.0–36.2) | 42.2 (36.3–60.6) | P-trend |
| Age (y)3 | 59.1 ± 0.54 | 59.7 ± 0.5 | 60.7 ± 0.5 | 61.2 ± 0.55 | 0.002 |
| BMI (kg/m2)6 | 31.2 ± 0.4 | 31.9 ± 0.4 | 32.0 ± 0.4 | 31.3 ± 0.4 | 0.99 |
| Height (m)6 | 1.60 ± 0.01 | 1.61 ± 0.01 | 1.61 ± 0.01 | 1.61 ± 0.01 | 0.25 |
| Current smoker (%)6 | 27.9 | 26.7 | 26.4 | 23.5 | 0.27 |
| Physical activity score6 | 30.4 ± 0.4 | 31.0 ± 0.4 | 31.6 ± 0.45 | 32.8 ± 0.47 | <0.0001 |
| Total energy intake (kcal/d)6 | 2045 ± 51 | 2066 ± 51 | 2164 ± 51 | 2077 ± 51 | 0.47 |
| Calcium intake (mg/d)8 | 870 ± 27 | 935 ± 27 | 935 ± 27 | 1061 ± 276 | <0.0001 |
| Plasma vitamin D (ng/mL)6 | 19.6 ± 0.5 | 19.4 ± 0.5 | 19.2 ± 0.5 | 19.7 ± 0.5 | 0.93 |
| Diabetes (%)6 | 48.1 | 41.0 | 39.3 | 46.7 | 0.99 |
| Total household income (US$/y)6 | 14822 ± 1323 | 16984 ± 1334 | 19777 ± 13365 | 24382 ± 13267 | <0.0001 |
| Education (%)6 | |||||
| <9th grade | 52.1 | 52.5 | 46.7 | 33.07 | <0.0001 |
| 9th–12th grade/GED | 40.7 | 35.7 | 39.1 | 40.1 | 0.72 |
| College/some graduate school | 7.1 | 11.3 | 14.2 | 26.77 | <0.0001 |
| Multivitamin use (%)6 | 51.9 | 54.8 | 58.1 | 58.2 | 0.14 |
| Osteoporosis medication use (%)6 | 29.1 | 33.6 | 33.0 | 40.15 | 0.01 |
| Season of BMD measurement (%)6 | |||||
| Winter | 24.3 | 28.0 | 23.6 | 20.6 | 0.21 |
| Spring | 31.0 | 30.2 | 29.1 | 31.5 | 0.94 |
| Summer | 28.2 | 24.3 | 31.9 | 28.9 | 0.52 |
| Fall | 16.0 | 17.4 | 15.4 | 18.5 | 0.58 |
| Acculturation6 | 21.5 ± 1.4 | 23.5 ± 1.4 | 27.2 ± 1.49 | 31.2 ± 1.47 | <0.0001 |
| Perceived stress score6 | 25.1 ± 0.5 | 23.2 ± 0.65 | 22.6 ± 0.59 | 20.8 ± 0.67 | <0.0001 |
AHA-DLS, American Heart Association Diet and Lifestyle Score; BMD, bone mineral density; GED, General Education Development.
Median; range in parentheses (all such values).
Adjusted for sex by ANOVA (PROC GLM; SAS Institute).
Mean ± SEM (all such values).
Significantly different from quartile 1 (Dunnett's adjustment): 5P < 0.05, 7P < 0.0001, 9P < 0.01.
Adjusted for age and sex by ANOVA (PROC GLM; SAS Institute).
Adjusted for age, sex, and energy intake by ANOVA (PROC GLM; SAS Institute).
TABLE 2.
Cross-sectional associations between energy-adjusted American Heart Association Diet and Lifestyle Score (11) and bone mineral density (g/cm2)
| Femoral neck |
Trochanter |
Total femur |
Lumbar spine (L2-L4) |
|||||
| No. of subjects | β (95% CI)1 | No. of subjects | β (95% CI)1 | No. of subjects | β (95% CI)1 | No. of subjects | β (95% CI)1 | |
| Model 12 | 896 | 0.005 (0.001, 0.009) | 896 | 0.008 (0.004, 0.013) | 887 | 0.008 (0.003, 0.013) | 895 | 0.006 (0.001, 0.012) |
| Model 23 | 896 | 0.005 (0.001, 0.010) | 896 | 0.009 (0.005, 0.013) | 887 | 0.008 (0.003, 0.013) | 895 | 0.007 (0.001, 0.013) |
| Model 34 | 896 | 0.005 (0.001, 0.009) | 896 | 0.008 (0.004, 0.013) | 887 | 0.008 (0.003, 0.012) | 895 | 0.007 (0.001, 0.013) |
For every 5-unit increase in the American Heart Association Diet and Lifestyle Score.
Adjusted for age (y), sex, BMI (kg/m2), height (m), current smoking (yes or no), educational status (<9th grade, 9th–12th grade, or college/some graduate school), season of bone mineral density measurement (winter, spring, summer, or fall), plasma vitamin D status (ng/mL), and intakes of total energy (kcal/d) and calcium (mg/d).
Adjusted as for model 1 plus osteoporosis medication use (yes or no) and multivitamin use (yes or no).
Adjusted as for model 2 plus acculturation and perceived stress score.
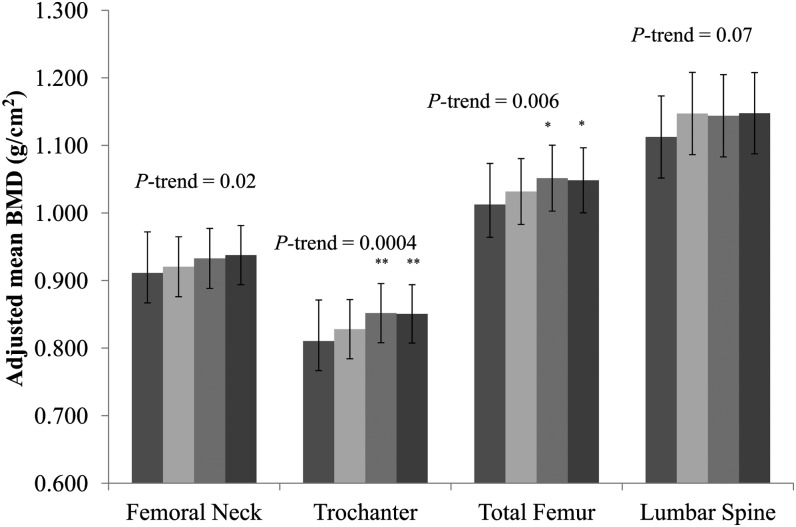
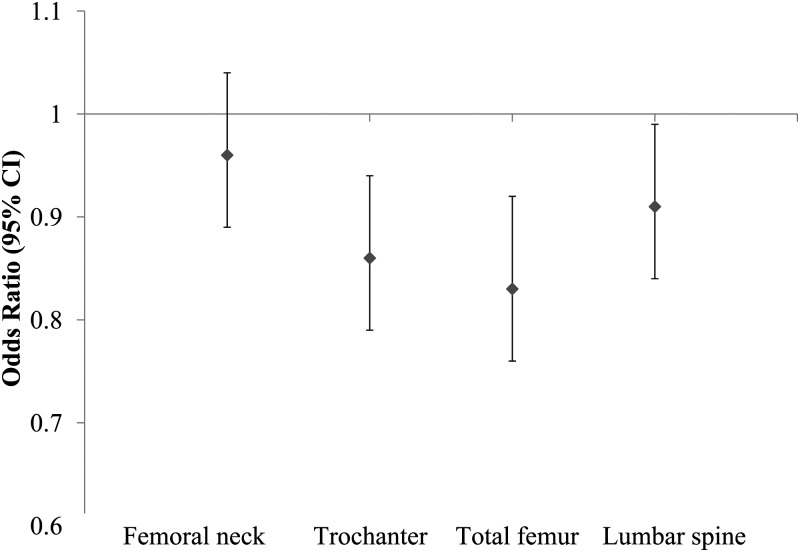
At all 3 femur sites, a significant and positive linear trend was observed for BMD across increasing quartiles of the modified AHA-DLS (P-trend <0.05 for each). We found a nonsignificantly higher lumbar spine BMD with a higher modified AHA-DLS (Figure 1). The contribution of each subcomponent of the modified AHA-DLS to BMD was assessed by adjusting for the remaining portion of the modified AHA-DLS and for covariates included in model 3 (Table 3). After a Bonferroni adjustment for multiple comparisons was applied, none of the individual subcomponents were significantly associated with BMD at any of the 4 bone sites (P > 0.004). After multivariate adjustment, the modified AHA-DLS was associated with a lower risk of osteoporosis or osteopenia at the trochanter, total femur, and lumbar spine (Figure 2). For each 5-unit increase in the modified AHA-DLS, the multiple adjusted ORs (95% CIs) for osteoporosis/osteopenia were 0.96 (0.89, 1.04), 0.86 (0.79, 0.92), 0.83 (0.76, 0.92), and 0.91 (0.84, 0.99) for the femoral neck, trochanter, total femur, and lumbar spine, respectively.
FIGURE 1.
Adjusted mean (±SEM) BMD across energy-adjusted quartiles of modified AHA-DLS. The bars from left to right are quartiles 1, 2, 3, and 4 of energy-adjusted modified AHA-DLS. Data were adjusted for age (y), sex, BMI (kg/m2), height (m), current smoking (yes or no), educational status (<9th grade, 9th–12th grade, or college/some graduate school), season of bone mineral density measurement (summer, spring, fall, or winter), plasma vitamin D status (ng/mL), intakes of total energy (kcal/d) and calcium (mg/d), osteoporosis medication use (yes or no), multivitamin supplement use (yes or no), acculturation (%), and perceived stress score based on ANCOVA (PROC GLM; SAS Institute). Adjustment for multiple comparisons was performed by using Dunnett's adjustment. Median (range): quartile 1, 18.8 (5.7–22.9), n = 233; quartile 2, 26.0 (23.0–28.9), n = 233; quartile 3, 32.4 (29.0–36.2), n = 234; quartile 4, 42.2 (36.3–60.6), n = 233. *P < 0.05 and **P < 0.01 compared with quartile 1 (Dunnett's adjustment). AHA-DLS, American Heart Association Diet and Lifestyle Score; BMD, bone mineral density.
TABLE 3.
Associations between subcomponents of the modified American Heart Association Diet and Lifestyle Score (11) and bone mineral density (g/cm2)
| β (99.6% CI)2 |
||||
| Subcomponent1 | Femoral neck(n = 896) | Trochanter(n = 896) | Total hip(n = 887) | Lumbar spine (L2-L4)(n = 895) |
| Physical activity | 0.0002 (−0.006, 0.007) | 0.002 (−0.004, 0.008) | 0.001 (−0.006, 0.008) | −0.001 (−0.009, 0.008) |
| Fruit and vegetable intake | 0.001 (−0.003, 0.006) | 0.0001 (−0.004, 0.005) | 0.001 (−0.005, 0.006) | 0.003 (−0.004, 0.009) |
| Fruit and vegetable variety | 0.001 (−0.003, 0.006) | 0.003 (−0.001, 0.007) | 0.003 (−0.002, 0.007) | −0.001 (−0.006, 0.005) |
| Whole grain intake | 0.004 (−0.002, 0.010) | 0.003 (−0.004, 0.009) | 0.003 (−0.004, 0.010) | 0.007 (−0.001, 0.016) |
| Fish intake | −0.0001 (−0.004, 0.004) | 0.002 (−0.002, 0.006) | 0.002 (−0.003, 0.007) | −0.0001 (−0.006, 0.005) |
| Saturated fat | 0.002 (−0.015, 0.018) | −0.003 (−0.019, 0.013) | −0.003 (−0.021, 0.015) | 0.006 (−0.017, 0.028) |
| trans Fat | 0.005 (−0.008, 0.019) | 0.003 (−0.010, 0.016) | 0.001 (−0.013, 0.016) | 0.007 (−0.011, 0.026) |
| Cholesterol | 0.001 (−0.011, 0.012) | 0.006 (−0.006, 0.017) | 0.004 (−0.009, 0.017) | 0.006 (−0.010, 0.021) |
| Percentage fat | −0.004 (−0.012, 0.004) | −0.005 (−0.012, 0.003) | −0.005 (−0.014, 0.003) | 0.0001 (−0.011, 0.011) |
| Sodium intake | 0.001 (−0.005, 0.007) | 0.002 (−0.004, 0.008) | 0.002 (−0.005, 0.008) | −0.001 (−0.009, 0.007) |
| Added sugar intake | 0.002 (−0.005, 0.008) | 0.002 (−0.004, 0.009) | 0.003 (−0.004, 0.011) | 0.007 (−0.002, 0.016) |
| Alcohol intake | 0.001 (−0.003, 0.006) | 0.002 (−0.003, 0.006) | 0.001 (−0.004, 0.006) | 0.001 (−0.007, 0.005) |
Adjusted for age (y), sex, BMI (kg/m2), height (m), current smoking (yes or no), educational status (<9th grade, 9th–12th grade, or college/some graduate school), season of bone mineral density measurement (winter, spring, summer, or fall), plasma vitamin D status (ng/mL), intakes of total energy (kcal/d) and calcium (mg/d), osteoporosis medication use (yes or no), multivitamin use (yes or no), acculturation (%), perceived stress score, and total modified American Heart Association Diet and Lifestyle Score minus the component being studied.
β Coefficients (99.6% CIs) were calculated by using ANCOVA (PROC GLM; SAS Institute).
FIGURE 2.
ORs (95% CIs) of osteoporosis or osteopenia for every 5-unit increase in the modified American Heart Association Diet and Lifestyle score. Data were adjusted for age (y), sex, BMI (kg/m2), height (m), current smoking (yes or no), educational status (<9th grade, 9th–12th grade, or college/some graduate school), season of bone mineral density measurement (summer, spring, fall, or winter), plasma vitamin D status (ng/mL), intakes of total energy (kcal/d) and calcium (mg/d), osteoporosis medication use (yes or no), multivitamin supplement use (yes or no), acculturation (%), and perceived stress score based on logistic regression (PROC LOGISTIC; SAS Institute). Femoral neck, n = 896; trochanter, n = 896; total femur, n = 887; lumbar spine, n = 894.
Although we did not document significant effect modification by sex (P-interaction > 0.30), we evaluated sex-specific associations between the AHA-DLS and our outcome variables in sensitivity analyses. In general, associations were stronger in women than in men, in whom, after multivariate adjustment, every 5-unit increase in the modified AHA-DLS was associated with a 0.012–0.019-g/cm2 greater BMD (P < 0.05 at all 4 bone sites). Effect sizes in men were comparable with those in women for trochanter, total femur, and lumbar spine but were not significant (see Supplemental Table 1 under “Supplemental data” in the online issue). In women, we found a significant positive trend across tertiles of the AHA-DLS at all femur sites. However, in men, a significant positive trend was observed only at the trochanter (see Supplemental Figure 1 under “Supplemental data” in the online issue). The multiple-adjusted OR and the 95% CI for osteoporosis/osteopenia at the femoral neck was significant in men (OR: 0.85; 95% CI: 0.72, 0.99) but not in women (OR: 0.98; 95% CI: 0.89, 1.08). On the other hand, a 5-unit higher AHA-DLS was associated with 23% lower odds (95% CI: 14%, 32%) of osteoporosis/osteopenia at the total femur in women but not in men (see Supplemental Figure 2 under “Supplemental data” in the online issue).
DISCUSSION
In this cross-sectional study of older Puerto Rican adults, adherence to the 2006 AHA-DLR was associated with a higher BMD at the femoral neck, trochanter, total hip, and lumbar spine. Although our associations were cross-sectional in nature, the effect sizes (0.005–0.009 g/cm2) observed for a 5-unit increase in the modified AHA-DLS are comparable with the annual age-related bone loss in older men and perimenopausal women (20, 21). An interesting observation was that no single recommendation alone was responsible for the positive associations between the modified AHA-DLS and BMD. It is possible that there was a synergistic action between the various components of the AHA-DLR such that the effect of the sum is greater than its parts on bone health. These results reinforce the importance of patterns of healthy behavior, rather than single dietary or lifestyle choices in reducing chronic disease risk.
Accumulating evidence supports a biological association between CVD and osteoporosis. In addition to age, other risk factors for CVD, including inflammation (22, 23), oxidative stress (24), hypercholesterolemia (25), and abdominal adiposity (26), have all been associated with osteoporosis. The presence of these unifying mechanisms makes it plausible that strategies that reduce CVD risk also prevent bone loss. In fact, several dietary patterns based on the principles of the AHA dietary recommendations were shown to be associated with these common mechanisms. For example, in the Multi-Ethnic Study of Atherosclerosis, a Comprehensive Healthy Dietary Pattern was associated with lower concentrations of CRP, IL-6, fibrinogen, triacylglycerol, and insulin (27). In the Nurses’ Health Study, a pattern characterized by foods that the AHA recommends limiting, including sugar-sweetened beverages, refined grains, diet soft drinks, and processed meat, was positively correlated with several inflammatory biomarkers (28). In the Health, Aging, and Body Composition Study, a dietary pattern high in low-fat dairy products, fruit, whole grains, poultry, fish, and vegetables was associated with a lower concentration of IL-6 (29). Several components of the Mediterranean dietary pattern, such as fruit, cereals, virgin olive oil, and nuts, were differentially but inversely associated with lower serum concentrations of vascular cell adhesion molecule-1, intercellular adhesion molecule, IL-6, and CRP in participants at high risk of CVD (30). Most recently, adherence to a Mediterranean diet was associated with a 7% lower hip fracture incidence in a European cohort (31). Similarly, in a cohort of Japanese men and women, greater adherence to a healthy dietary pattern characterized by fruit and vegetables was associated with lower serum CRP (32). In addition to markers of systemic inflammation, a Western dietary pattern loading heavily on refined bread and butter was associated with higher total and LDL cholesterol, whereas a pattern with high factor loadings on fruit, yellow and orange vegetables, other vegetables, and fish was associated with higher HDL cholesterol in a group of Japanese adults (33).
Central obesity is another significant risk factor for CVD (26). We previously showed that centrally located body fat was associated with lower BMD at the femur and lumbar spine and with higher odds for osteoporosis and osteopenia at the femoral neck (34). In the Framingham Offspring cohort, a wine and moderate eating dietary pattern was associated with 66% lower odds (95% CI: 0.13, 0.89) for incident abdominal obesity, compared with those reporting an “empty calorie” pattern (35). Furthermore, in the Jackson Heart Study, higher “southern” and “fast food” pattern scores were associated with higher odds for high abdominal visceral adipose tissue (36).
Although no data have directly assessed the effect of adhering to the AHA-DLR on bone health, our findings agree with several studies that have tested associations between dietary patterns based on the principles of the AHA-DLR, or individual components of the AHA-DLR, and BMD. An example of an eating pattern consistent with the AHA-DLR is the Dietary Approaches to Stop Hypertension diet. Consumption of this diet has been reported to reduce bone turnover (37). Our data support earlier observations that a dietary pattern based on fruit, vegetables, and cereal or whole grain is associated with a higher BMD (38, 39). Likewise, consuming a “healthy” pattern defined by high intakes of green and dark-yellow vegetables, mushrooms, fish and shellfish, fruit, and processed fish by premenopausal Japanese farm women was reported to be positively correlated with forearm BMD. Consistent with the AHA recommendations for dietary fat type and cholesterol, these authors noted that a “Western” pattern tended to be inversely associated with BMD (40). Langsetmo et al (41) found that a “nutrient-dense” pattern characterized by high intakes of fruit, vegetables, and whole grains was associated with a reduced risk of fracture in both men and postmenopausal women aged ≥50 y. Importantly, these associations were independent of BMI, BMD, falls, and demographic variables.
The current study was unique in that it was the first to investigate the association between a validated diet and lifestyle score, originally intended for CVD risk reduction, and bone health. However, our study had several limitations. First, the cross-sectional nature of our associations limited our ability to draw inferences about causality. Longitudinal data are needed to assess the effect of diet and lifestyle on bone loss. It is possible that participants with osteoporosis may make healthier lifestyle and dietary choices. However, if this were true, our associations would be biased toward the null. Second, whereas we adjusted for important potential confounders, residual and unmeasured confounding always remains a possibility. However, our data allowed for adjustment of a comprehensive set of covariates, based on current knowledge of potential confounders and underlying biological mechanisms. Finally, our study was limited to the Puerto Rican population, and, although it is likely that these findings can be applied to the general population, they should be replicated in other groups.
In conclusion, dietary and lifestyle recommendations for CVD risk reduction appear to benefit bone health. Our findings have important public health implications. Synchronizing guidelines, both dietary and lifestyle, provides a holistic approach to the prevention of 2 major diseases that contribute significantly to the morbidity and mortality of the aging population.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—SNB and KLT: study design; SNB: data analysis, data interpretation, and manuscript writing; KLT: study oversight; and BD-H, MTH, and AHL: data interpretation and critical revision of the manuscript. The authors had no conflicts of interest.
Footnotes
Abbreviations used: AHA, American Heart Association; AHA-DLR, AHA Diet and Lifestyle Recommendations; AHA-DLS, AHA Diet and Lifestyle Score; BMD, bone mineral density; CRP, C-reactive protein; CVD, cardiovascular disease.
REFERENCES
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, et al. Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation 2010;121:e46–215 (Published erratum appears in Circulation 2011;124:e425.) [DOI] [PubMed] [Google Scholar]
- 2.Cheng H, Gary LC, Curtis JR, Saag KG, Kilgore ML, Morrisey MA, Matthews R, Smith W, Yun H, Delzell E. Estimated prevalence and patterns of presumed osteoporosis among older Americans based on Medicare data. Osteoporos Int 2009;20:1507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation 2006;114:82–96. [DOI] [PubMed] [Google Scholar]
- 4.Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckelbaum RJ, Erdman JW, Jr, Kris-Etherton P, Goldberg IJ, Kotchen TA, et al. AHA Dietary Guidelines: revision 2000: A statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation 2000;102:2284–99. [DOI] [PubMed] [Google Scholar]
- 5.Tucker KL, Falcon LM, Bianchi LA, Cacho E, Bermudez OI. Self-reported prevalence and health correlates of functional limitation among Massachusetts elderly Puerto Ricans, Dominicans, and non-Hispanic white neighborhood comparison group. J Gerontol A Biol Sci Med Sci 2000;55:M90–7. [DOI] [PubMed] [Google Scholar]
- 6.Lin H, Bermudez OI, Falcon LM, Tucker KL. Hypertension among Hispanic elders of a Caribbean origin in Massachusetts. Ethn Dis 2002;12:499–507. [PubMed] [Google Scholar]
- 7.Tucker KL, Mattei J, Noel SE, Collado BM, Mendez J, Nelson J, Griffith J, Ordovas JM, Falcon LM. The Boston Puerto Rican Health Study, a longitudinal cohort study on health disparities in Puerto Rican adults: challenges and opportunities. BMC Public Health 2010;10:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White J, Harris SS, Dallal GE, Dawson-Hughes B. Precision of single vs bilateral hip bone mineral density scans. J Clin Densitom 2003;6:159–62. [DOI] [PubMed] [Google Scholar]
- 9.Densitometry TISfC. Official positions of the International Society for Clinical Densitometry. West Hartford, CT: International Society for Clinical Densitometry, 2007:4–5.
- 10.Tucker KL, Bianchi LA, Maras J, Bermudez OI. Adaptation of a food frequency questionnaire to assess diets of Puerto Rican and non-Hispanic adults. Am J Epidemiol 1998;148:507–18. [DOI] [PubMed] [Google Scholar]
- 11.Bhupathiraju SN, Lichtenstein AH, Dawson-Hughes B, Tucker KL. Adherence index based on the AHA 2006 diet and lifestyle recommendations is associated with select cardiovascular disease risk factors in older Puerto Ricans. J Nutr 2011;141:460–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castro JP, Joseph LA, Shin JJ, Arora SK, Nicasio J, Shatzkes J, Raklyar I, Erlikh I, Pantone V, Bahtiyar G, et al. Differential effect of obesity on bone mineral density in white, Hispanic and African American women: a cross sectional study. Nutr Metab (Lond) 2005;2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newby PK, Weismayer C, Akesson A, Tucker KL, Wolk A. Long-term stability of food patterns identified by use of factor analysis among Swedish women. J Nutr 2006;136:626–33. [DOI] [PubMed] [Google Scholar]
- 14.Dawson-Hughes B, Dallal GE, Krall EA, Harris S, Sokoll LJ, Falconer G. Effect of vitamin D supplementation on wintertime and overall bone loss in healthy postmenopausal women. Ann Intern Med 1991;115:505–12. [DOI] [PubMed] [Google Scholar]
- 15.Krall EA, Sahyoun N, Tannenbaum S, Dallal GE, Dawson-Hughes B. Effect of vitamin D intake on seasonal variations in parathyroid hormone secretion in postmenopausal women. N Engl J Med 1989;321:1777–83. [DOI] [PubMed] [Google Scholar]
- 16.Marin G, Gamba RJ. A new measurement of acculturation for Hispanics: the Bidimensional Acculturation Scale for Hispanics (BAS). Hisp J Behav Sci 1996;18:297–316. [Google Scholar]
- 17.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385–96. [PubMed] [Google Scholar]
- 18.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65(suppl):1220S–8S; discussion 9S–31S. [DOI] [PubMed]
- 19.Lin H, Bermudez OI, Tucker KL. Dietary patterns of Hispanic elders are associated with acculturation and obesity. J Nutr 2003;133:3651–7. [DOI] [PubMed] [Google Scholar]
- 20.Finkelstein JS, Brockwell SE, Mehta V, Greendale GA, Sowers MR, Ettinger B, Lo JC, Johnston JM, Cauley JA, Danielson ME, et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab 2008;93:861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warming L, Hassager C, Christiansen C. Changes in bone mineral density with age in men and women: a longitudinal study. Osteoporos Int 2002;13:105–12. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura K, Saito T, Kobayashi R, Oshiki R, Oyama M, Nishiwaki T, Nashimoto M, Tsuchiya Y. C-reactive protein predicts incident fracture in community-dwelling elderly Japanese women: the Muramatsu study. Osteoporos Int 2011;22:2145–50. [DOI] [PubMed] [Google Scholar]
- 23.Koh JM, Khang YH, Jung CH, Bae S, Kim DJ, Chung YE, Kim GS. Higher circulating hsCRP levels are associated with lower bone mineral density in healthy pre- and postmenopausal women: evidence for a link between systemic inflammation and osteoporosis. Osteoporos Int 2005;16:1263–71. [DOI] [PubMed] [Google Scholar]
- 24.Basu S, Michaelsson K, Olofsson H, Johansson S, Melhus H. Association between oxidative stress and bone mineral density. Biochem Biophys Res Commun 2001;288:275–9. [DOI] [PubMed] [Google Scholar]
- 25.Tarakida A, Iino K, Abe K, Taniguchi R, Higuchi T, Mizunuma H, Nakaji S. Hypercholesterolemia accelerates bone loss in postmenopausal women. Climacteric 2011;14:105–11. [DOI] [PubMed] [Google Scholar]
- 26.Despres JP, Arsenault BJ, Cote M, Cartier A, Lemieux I. Abdominal obesity: the cholesterol of the 21st century? Can J Cardiol 2008;24(suppl):7D–12D. [DOI] [PMC free article] [PubMed]
- 27.Nettleton JA, Schulze MB, Jiang R, Jenny NS, Burke GL, Jacobs DR., Jr A priori-defined dietary patterns and markers of cardiovascular disease risk in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 2008;88:185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schulze MB, Hoffmann K, Manson JE, Willett WC, Meigs JB, Weikert C, Heidemann C, Colditz GA, Hu FB. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am J Clin Nutr 2005;82:675–84, quiz 714–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson AL, Harris TB, Tylavsky FA, Perry SE, Houston DK, Lee JS, Kanaya AM, Sahyoun NR. Dietary patterns, insulin sensitivity and inflammation in older adults. Eur J Clin Nutr 2012;66:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salas-Salvadó J, Garcia-Arellano A, Estruch R, Marquez-Sandoval F, Corella D, Fiol M, Gomez-Gracia E, Vinoles E, Aros F, Herrera C, et al. Components of the Mediterranean-type food pattern and serum inflammatory markers among patients at high risk for cardiovascular disease. Eur J Clin Nutr 2008;62:651–9. [DOI] [PubMed] [Google Scholar]
- 31.Benetou V, Orfanos P, Pettersson-Kymmer U, Bergstrom U, Svensson O, Johansson I, Berrino F, Tumino R, Borch KB, Lund E, et al. Mediterranean diet and incidence of hip fractures in a European cohort. Osteoporos Int 2013;24:1587–98. [DOI] [PubMed] [Google Scholar]
- 32.Nanri H, Nakamura K, Hara M, Higaki Y, Imaizumi T, Taguchi N, Sakamoto T, Horita M, Shinchi K, Tanaka K. Association between dietary pattern and serum C-reactive protein in Japanese men and women. J Epidemiol 2011;21:122–31. [DOI] [PMC free article] [PubMed]
- 33.Sadakane A, Tsutsumi A, Gotoh T, Ishikawa S, Ojima T, Kario K, Nakamura Y, Kayaba K. Dietary patterns and levels of blood pressure and serum lipids in a Japanese population. J Epidemiol 2008;18:58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhupathiraju SN, Dawson-Hughes B, Hannan MT, Lichtenstein AH, Tucker KL. Centrally located body fat is associated with lower bone mineral density in older Puerto Rican adults. Am J Clin Nutr 2011;94:1063–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimokoti RW, Gona P, Zhu L, Newby PK, Millen BE, Brown LS, D'Agostino RB, Fung TT. Dietary patterns of women are associated with incident abdominal obesity but not metabolic syndrome. J Nutr 2012;142:1720–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J, Hickson DA, Musani SK, Talegawkar SA, Carithers TC, Tucker KL, Fox CS, Taylor HA. Dietary patterns, abdominal visceral adipose tissue and cardiometabolic risk factors in African Americans: the Jackson Heart Study. Obesity (Silver Spring) 2013;21(3):644–51. [DOI] [PMC free article] [PubMed]
- 37.Lin PH, Ginty F, Appel LJ, Aickin M, Bohannon A, Garnero P, Barclay D, Svetkey LP. The DASH diet and sodium reduction improve markers of bone turnover and calcium metabolism in adults. J Nutr 2003;133:3130–6. [DOI] [PubMed] [Google Scholar]
- 38.Tucker KL, Chen H, Hannan MT, Cupples LA, Wilson PW, Felson D, Kiel DP. Bone mineral density and dietary patterns in older adults: the Framingham Osteoporosis Study. Am J Clin Nutr 2002;76:245–52. [DOI] [PubMed] [Google Scholar]
- 39.Langsetmo L, Poliquin S, Hanley DA, Prior JC, Barr S, Anastassiades T, Towheed T, Goltzman D, Kreiger N. Dietary patterns in Canadian men and women ages 25 and older: relationship to demographics, body mass index, and bone mineral density. BMC Musculoskelet Disord 2010;11:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okubo H, Sasaki S, Horiguchi H, Oguma E, Miyamoto K, Hosoi Y, Kim MK, Kayama F. Dietary patterns associated with bone mineral density in premenopausal Japanese farmwomen. Am J Clin Nutr 2006;83:1185–92. [DOI] [PubMed] [Google Scholar]
- 41.Langsetmo L, Hanley DA, Prior JC, Barr SI, Anastassiades T, Towheed T, Goltzman D, Morin S, Poliquin S, Kreiger N. Dietary patterns and incident low-trauma fractures in postmenopausal women and men aged >/= 50 y: a population-based cohort study. Am J Clin Nutr 2011;93:192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.