Abstract
Background: Twin and family studies that estimated the heritability of daily physical activity have been limited by poor measurement quality and a small sample size.
Objective: We examined the heritability of daily physical activity and sedentary behavior assessed objectively by using combined heart rate and movement sensing in a large twin study.
Design: Physical activity traits were assessed in daily life for a mean (±SD) 6.7 ± 1.1 d in 1654 twins from 420 monozygotic and 352 dizygotic same-sex twin pairs aged 56.3 ± 10.4 y with body mass index (in kg/m2) of 26.1 ± 4.8. We estimated the average daily movement, physical activity energy expenditure, and time spent in moderate-to-vigorous intensity physical activity and sedentary behavior from heart rate and acceleration data. We used structural equation modeling to examine the contribution of additive genetic, shared environmental, and unique environmental factors to between-individual variation in traits.
Results: Additive genetic factors (ie, heritability) explained 47% of the variance in physical activity energy expenditure (95% CI: 23%, 53%) and time spent in moderate-to-vigorous intensity physical activity (95% CI: 29%, 54%), 35% of the variance in acceleration of the trunk (95% CI: 0%, 44%), and 31% of the variance in the time spent in sedentary behavior (95% CI: 9%, 51%). The remaining variance was predominantly explained by unique environmental factors and random error, whereas shared environmental factors played only a marginal role for all traits with a range of 0–15%.
Conclusions: The between-individual variation in daily physical activity and sedentary behavior is mainly a result of environmental influences. Nevertheless, genetic factors explain up to one-half of the variance, suggesting that innate biological processes may be driving some of our daily physical activity.
INTRODUCTION
Physical activity energy expenditure (PAEE)5 is the most variable component of total energy expenditure (1), and both PAEE and body movement are important determinants of cardiometabolic health in adults (2–4) and children (5, 6). In addition, the time spent in moderate-to-vigorous intensity physical activity and sedentary behavior has been associated with metabolic risk (7–9). Although it is clear that environmental factors play a major role in the determination of physical activity levels at the population level (10), the role of intrinsic, biological factors in the regulation of physical activity levels within individuals has not received much attention in humans. Such biological factors consist of the interaction of proteins and peptides with receptors, the function of which is largely determined by their structure that, in turn, is encoded by the sequence of the respective gene. Epigenetic processes that modify the genome without altering the genetic sequence may also play a role by affecting gene expression.
Since the early work of Rundquist (11) on the inheritance of spontaneous activity in rats, a body of literature has provided evidence for a role of genetic factors in spontaneous physical activity in rodent models. In humans, family and twin studies have suggested that genetic factors may contribute to the between-individual variation in daily physical activity and sedentary behavior, but heritability (h2) estimates that have been reported vary widely, ranging from 0% to 57% in family studies (12–16) and 0% to 78% in twin studies (17–24). These wide ranges of h2 estimates may partly reflect differences in the way that physical activity was assessed. Most of the reported family and twin studies have relied on a subjective assessment of physical activity [eg, the use of use questionnaires (13–19, 23, 24)]. Such methods are prone to recall bias and measurement error, some of which stems from the difficulty in translating answers to quantitative estimates of overall physical activity and, therefore, may result in an imprecise reflection of daily physical activity (25). Few twin studies have used more-objective methods to estimate the daily physical activity (ie, indirectly, by using a combination of doubly labeled water and indirect calorimetry and/or directly by using accelerometry). Such studies have been limited by small sample sizes (n < 120 twin pairs) (20–22). Because of the limitations of previous studies, we performed heritability analyses of daily physical activity and sedentary behavior assessed objectively by using combined heart rate and movement sensing in a large twin study.
SUBJECTS AND METHODS
Participants
We recruited twins from the TwinsUK registry, which consists of a national twin volunteer population of >12,000 adult twins aged 18–103 y of whom 83% are women. Monozygotic twins make up 51% of the cohort and dizygotic twins make up 49% of the cohort (26).
For the current study, we contacted 1842 twins, mainly from the ongoing Healthy Ageing Twin Study, which aims to address how closely changes at several organs or tissues correlate with the overall physiologic decline over time (26). Of those individuals, 1659 twins of 775 complete pairs agreed to participate (90% response rate). On average, twins who agreed to participate were younger (aged 56 compared with 60 y; P = 3.2 × 10−6) and leaner (BMI 26.1 compared with 27.5 kg/m2; P = 1.2 × 10−3) than twins who did not accept our invitation. A total of 1296 twins were fit with a combined heart rate and movement sensor (Actiheart; CamNtech) (27) while visiting the clinic in St Thomas’ Hospital Campus, London (78% of responders). The remaining 363 twins (22% of responders), who had either recently completed or were not part of the Healthy Ageing Twin Study, received the sensor by mail, accompanied by instructions on how to attach the device.
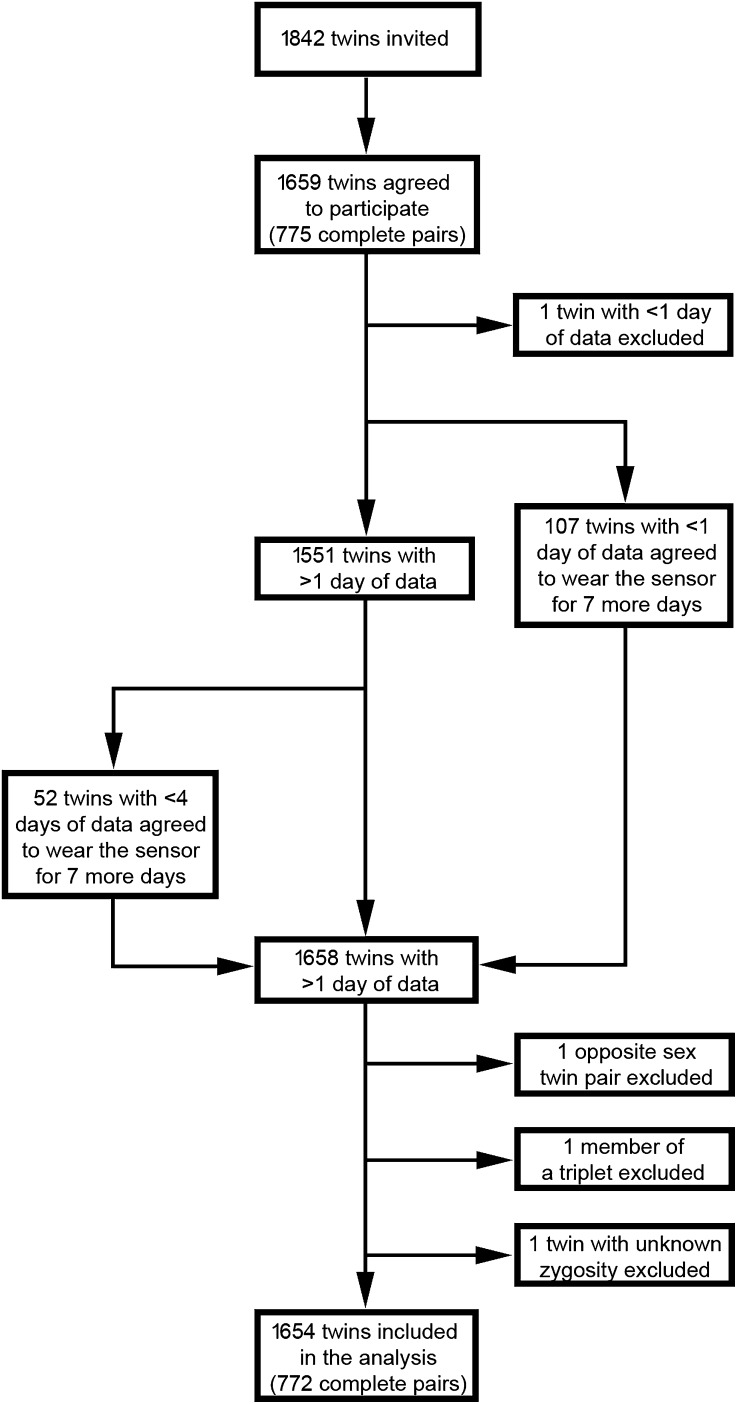
We instructed twins to wear the sensor continuously for 7 d data collection in daily life and derived ≥1 d of physical activity data for 1551 of 1659 twins (93%), 1233 of whom had the sensor fit in the clinic (74% of participants) and 318 of whom received it by mail (19%). To maximize the quality of our data, we reinvited 107 twins for whom no physical activity data were acquired during the first measurement period and 87 twins with <4 d of physical activity data to wear the sensor for a second period of 7 d, to which 159 twins agreed. These twins received the sensor for a second period of physical activity assessment by mail. The second measurement period resulted in the acquisition of good-quality data in all 159 participating twins, which resulted in availability of ≥1 d of physical activity data in 1658 of 1659 twins who participated (Figure 1). There was no difference between monozygotic and dizygotic twins in the proportion of twins who received the monitor in the clinic or by mail [P = 0.27 (chi-square test)]. Data from one opposite-sex dizygotic pair, one twin of unknown zygosity, and one member of a dizygotic triplet were excluded from the analysis, which resulted in a total study population of 1654 twins. All physical activity measurements were performed between January 2008 and November 2010.
FIGURE 1.
Flowchart showing the process of participant recruitment and quality control.
The 1654 twins represented 772 complete, same-sex twin pairs and 110 twins for whom data from the co-twin was not available. The 1654 twins had a mean (±SD) age of 56 ± 10 y (range: 17–82 y) and BMI of 26.1 ± 4.8 kg/m2 (range: 15.5–48.2 kg/m2) (Table 1; see Supplementary Table 1 under “Supplemental data” in the online issue). Monozygotic twins were leaner than dizygotic twins (BMI: 25.8 compared with 26.4 kg/m2; P = 0.03) and had a higher variance in age (10.8 compared with 10.0 y; P = 0.03). The proportion of men was also higher in monozygotic twins [3.3 compared with 0.7%; P = 1.7 × 10−4 (chi-square test)]. Of 1654 twins, 1570 twins (95%) reported to be of European origin. The remaining twins were of African (n = 8), Asian (n = 5), mixed (n = 15), or unknown (n = 56) origin. The proportion of twins from non-European descent was higher in monozygotic than dizygotic twins [2.5% compared with 0.8%; P = 9.9 × 10−3 (chi-square test)]. We confirmed zygosity by using genotyping in 1219 twins from 571 complete pairs, whereas self-reported zygosity was used in 435 twins from 201 complete pairs. Unpublished observations in TwinsUK data suggested that self-reported zygosity compared with the use of genotyping data resulted in a misclassification of zygosity in ∼2.5% of twins. The majority of the 772 complete twin pairs lived in separate households at the time of participation (Table 1). The zygosity status did not affect the likelihood of twin pairs living together [P = 0.50 (chi-square test)]. Seventy-nine twins from 32 complete monozygotic and 36 complete dizygotic pairs reported a disability that restricted their daily physical activity (eg, asthma or arthritis). The prevalence of such disabilities was not dissimilar in complete monozygotic and dizygotic pairs [P = 0.20 (chi-square test)].
TABLE 1.
Descriptive information1
| Monozygotic twins | Dizygotic twins | |
| Individuals (n) | 899 | 755 |
| Women (n) | 869 | 750 |
| Complete pairs (n) | 420 | 352 |
| Complete pairs living apart [n (%)] | 371 (88) | 325 (92) |
| Complete pairs living together [n (%)] | 28 (7) | 21 (6) |
| Complete pairs with unknown residential status [n (%)] | 21 (5) | 7 (2) |
| Complete pairs with a self-reported disability [n (%)] | 32 (8) | 36 (10) |
| Age (y) | 56 ± 112 | 57 ± 10 |
| BMI (kg/m2) | 25.8 ± 4.6 | 26.3 ± 4.9 |
| PAEE (kJ/d) | 2517 (1906, 3110)3 | 2318 (1821, 3022) |
| PAEE (kJ · kg-1 · d-1) | 37.5 (29.2, 46.7) | 35.1 (27.1, 44.2) |
| Acceleration (m/s2)4 | 0.113 ± 0.050 | 0.105 ± 0.044 |
| MVPA (min/d)5 | 37 (18, 61) | 30 (15, 54) |
| Sedentary (min/d)6 | 1039 ± 127 | 1055 ± 129 |
| Wearing time (d) | 6.7 ± 1.2 | 6.7 ± 1.1 |
MVPA, moderate-to-vigorous intensity physical activity; PAEE, physical activity energy expenditure.
Mean ± SD (all such values) (for normally distributed traits).
Median; IQR in parentheses (all such values) (for skewed distributions).
Average acceleration of the trunk along the vertical axis of the body.
Time spent in MVPA (>3 metabolic equivalent of task).
Time spent in sedentary behavior (≤1.5 metabolic equivalent of task).
The study conformed to the standards set by the Declaration of Helsinki of 1975 as revised in 1983, and the local ethics committee approved the study. All participants provided written informed consent before participating in the study.
Objective methods of physical activity assessment
We initialized the combined heart rate and movement sensor for long-term recording, by summarizing the data into 1-min epochs. We aimed to start the assessment of daily physical activity on the same day for co-twins from the same twin pair, in which we succeeded for 598 of 772 complete pairs (77%). For 49 of the remaining pairs, the difference in the start date was 1–7 d (6%). The remainder could mainly be explained by the reassessment of physical activity in twin pairs with <4 d data during the first measurement period. The within-pair difference in the start date did not differ by zygosity status [difference (chi-square test): >0 d, P = 0.07; >7 d, P = 0.08; >14 d, P = 0.12; >31 d, P = 0.07]. Monozygotic twins were monitored more frequently in the summer than were dizygotic twins [25.1% compared with 19.9%, P = 1.2 × 10−2 (chi-square test)] and less frequently in the winter [17.9% compared with 22.3%; P = 7.4 × 10−3 (chi-square test)].
We downloaded data collected during free living to a personal computer and processed the heart rate trace by using a robust Gaussian process regression method to handle potential measurement noise (28). Periods of nonwear time were inferred from a combination of nonphysiologically plausible heart rate and prolonged periods of inactivity as indicated by the sensor's accelerometer; this classification was verified by inspection of time-series plots for all participants. All 1654 twins from 772 complete pairs had ≥1 d (24 h wear) physical activity data in both twins; 1581 twins had ≥4 d data (95.6%), which was shown previously to achieve a reliability of physical activity levels >80% (29, 30) (see Supplementary Table 2 under “Supplemental data” in the online issue).
The calibration equation of the uniaxial trunk acceleration to whole-body workload was the same for all participants (31). The relation of the heart rate with workload was determined on an individual level according to age, sex, sleeping heart rate, and β-blocker use modeled in an independent calibration study in which 1941 healthy, middle-aged participants each performed two 8-min ramped-step tests (31, 32). At each time point, we subsequently used the heart rate and acceleration to estimate the physical activity intensity (in J · min−1 · kg−1) by using a branched equation framework (33). We collapsed the acquired physical activity intensity into the average time that participants spent in moderate-to-vigorous intensity physical activity and sedentary behavior (in min/d). Moderate-to-vigorous intensity physical activity has an intensity of >3 times resting metabolic rate, which is commonly referred to as the metabolic equivalent of task (MET) and sedentary behavior of ≤1.5 METs. We used the standard definition of activity intensity, whereby 1 MET equaled an oxygen uptake of 3.5 mL O2 · min−1 · kg body mass−1. In addition, we analyzed data by using the Oxford resting metabolic rate equations (34), which yielded similar results. We summarized all individual time series into daily physical activity traits, while minimizing the diurnal information bias caused by potential nonwear. We multiplied body mass–specific PAEE by individual body mass to compute the average daily PAEE (in kJ/d). Finally, information acquired by the sensor's uniaxial accelerometer was summarized in the average daily acceleration of the trunk along the vertical axis of the body (in m/s2). Acceleration of the trunk provides the purest reflection of whole-body movements but is limited by the types of activity that people partake in because accelerometers are more sensitive to weight bearing than non–weight bearing activities (35, 36).
Anthropometric measurements
In twins who visited the clinic, height and weight were measured by using standard procedures. Body mass was measured with twins dressed in light clothing and without footwear to the nearest 0.1 kg by using a Seca Alpha Weight scale (Seca), and height was measured to the nearest 0.1 cm by using a Leicester Height Measure (Seca). BMI was calculated as body mass divided by height squared. For twins who received the sensor by mail, we used height and body mass as measured during the most recent clinic visit a median of 7.5 mo earlier (IQR: 2.3–14.4 mo).
Statistical analysis
PAEE and time spent in moderate-to-vigorous intensity physical activity were inverse-normally transformed to normalize distributions. We subsequently created residuals of daily physical activity traits adjusted for sex, age, and age-squared. For reasons provided in Heritability Analyses, we created residuals of physical activity traits and sedentary behavior with and without additional adjustment for BMI.
Classical twin studies assume that means and variances of monozygotic and dizygotic twins are the same, which we tested by using 2-tailed, unpaired t tests. Monozygotic twins were more physically active and spent less time in sedentary behavior than did dizygotic twins and showed a higher variance in acceleration of the trunk (P < 0.05; see Supplementary Table 1 under “Supplemental data” in the online issue). However, these differences were attenuated and no longer reached significance after adjustment for sex, age, BMI, ethnicity, and seasonality after adjustment for multiple testing (see Supplementary Table 1 under “Supplemental data” in the online issue). Differences in the mean and variance of acceleration of the trunk were additionally driven by the 9 monozygotic twins with the largest acceleration and did not reach significance after their exclusion from the analysis (P-mean = 0.06, P-variance = 0.11 after exclusion). Additional adjustment of heritability analyses for ethnicity and seasonality or exclusion of 9 monozygotic twins with the largest acceleration of the trunk did not affect results. Hence, we performed heritability analyses as planned.
Heritability analyses
Twins raised together share part of their environment and this sharing is assumed to be the same for monozygotic and dizygotic twins (19). Monozygotic twins are genetically identical, whereas dizygotic twins share on average 50% of genotypes. This implies that a higher resemblance in daily physical activity and sedentary behavior in monozygotic compared with dizygotic pairs is indicative of a role for genetic factors.
We first assessed the nature of genetic and environmental contributions to the variance in daily physical activity and sedentary behavior by comparing Pearson's intrapair correlation coefficients (ICCs) for residuals of the 4 traits after adjustment for covariates in monozygotic and dizygotic pairs by using data from all 1654 available twins. Next, we performed structural equation modeling to estimate the contribution of additive genetic factors (A), shared or common environmental factors (C), and unique or nonshared environmental factors (E) to the observed phenotypic variance by using OpenMx software, version 1.3 (37). We a priori hypothesized that a model in which all phenotypic variance is explained by a combination of A, C, and E (ACE model) is the biologically most plausible model, and we fit this model to the residuals for all traits first.
ICCs that are >2-fold higher in monozygotic than dizygotic twins suggest that a nonadditive or dominant genetic factors (D) may also play a role. Hence, we fit ADE models to the data for traits that adhered to this criterion.
Alternative nested models were fit by constraining variance component(s) to zero, which resulted in AE, CE, DE (where appropriate), and E models. Nested models were compared with ACE and ADE models (where appropriate) by using a maximum likelihood approach with the accompanying Akaike's information criterion (38). The model with the lowest Akaike's information criterion is generally considered the best compromise between the goodness of fit and parsimony. We applied a bootstrapping approach to obtain 95% CIs of variance component estimates by using the percentile method (39) after resampling the appropriate number of twins 10,000 times from the original data set for all traits and (nested) models.
Compared with lean individuals, obese individuals were shown previously to have a similar absolute PAEE but a lower level of objectively assessed daily body movement (40). The latter was already observed in the early 1960s (41). Because increased levels of adiposity may prevent a physically active lifestyle, and adiposity is highly heritable itself (42), the heritability of unadjusted daily physical activity traits may, at least partly, reflect the heritability of adiposity. Therefore, we estimated the heritability of daily physical activity and sedentary behavior by using BMI-adjusted residuals and subsequently compared h2 estimates for all traits calculated by using residuals with and without adjustment for BMI.
We performed sensitivity analyses to examine if the inclusion of twin pairs with a potentially suboptimal data quality affected results. First, we examined if the exclusion of the 73 twins with <4 d data changed estimates. Second, we examined if a within-pair difference in start date of physical activity assessment influenced results by excluding the 174 twin pairs for whom the measurement period started on a different day in both co-twins, which was followed by an analysis in which the 125 twin pairs for whom the start date differed by ≥1 w were excluded. Because self-reported information on zygosity may result in underestimates of h2 (42), we next examined whether the exclusion of the 435 twins with self-reported zygosity influenced results. We also repeated the structural equation modeling after the exclusion of the 79 twins with a self-reported disability that seriously restricted daily physical activity because such impairments may override the normal pattern of genetic and environmental factors that influence daily physical activity. Finally, we performed a sensitivity analysis in which the 35 male twins were excluded from the analyses to examine if the combination of data from men and women affected results.
The results are presented as means ± SDs for normally distributed variables and medians (IQRs) for traits with skewed distributions. Statistical analyses were performed with SAS version 9.2 for Windows software (SAS Institute) and OpenMX software, version 1.3 (37).
RESULTS
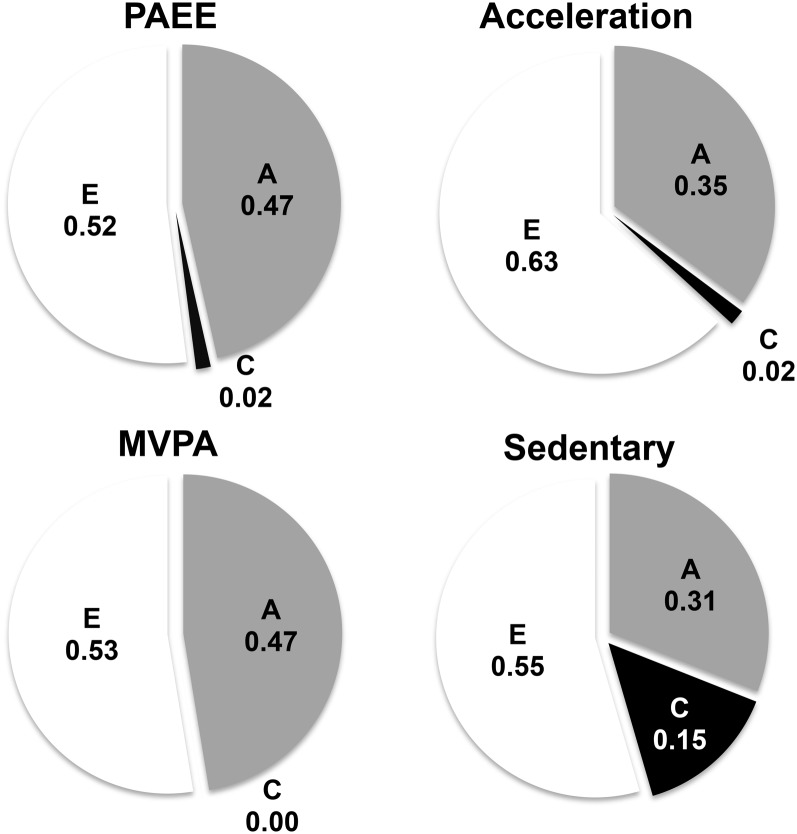
Pearson's ICCs were higher in monozygotic than dizygotic pairs for all daily physical activity traits and sedentary behavior, which suggested a role for genetic factors (Table 2). The structural equation modeling in data from all 1654 twins showed that h2 estimates ranged from 31% (95% CI: 9.4%, 50.5%) for the time spent in sedentary behavior to 47.4% (95% CI: 28.9%, 53.8%) for PAEE under the ACE model and reached significance for all traits except for bodily movements as reflected by acceleration of the trunk (95% CI: 0.0%, 44.2%). The variance that remained unexplained by genetic factors (A) was largely explained by environmental factors that were unique to each twin and random (measurement) error (E) (Figure 2, Table 3). The contribution of shared environmental factors (C) was small and nonsignificant for all traits and ranged from 0.0% (95% CI: 0.0%, 16.6%) for the time spent in moderate-to-vigorous intensity physical activity to 14.5% (95% CI: 0.0%, 32.1%) for the time spent in sedentary behavior (Figure 2, Table 3).
TABLE 2.
ICCs for daily physical activity traits and sedentary behavior1
| Monozygotic |
Dizygotic |
|||
| Trait | ICC | No. of pairs | ICC | No. of pairs |
| PAEE (kJ/d)2 | 0.464 | 420 | 0.247 | 352 |
| Acceleration (m/s2)3 | 0.407 | 420 | 0.150 | 352 |
| MVPA (min/d)24 | 0.485 | 419 | 0.186 | 351 |
| Sedentary (min/d)5 | 0.449 | 419 | 0.298 | 351 |
Pearson's ICCs were calculated by using sex, age, age-squared, and BMI-adjusted residuals. ICC, intrapair correlation coefficient; MET, metabolic equivalent of task; MVPA, moderate-to-vigorous intensity physical activity; PAEE, physical activity energy expenditure.
Trait was analyzed after inverse-normal transformation.
Average acceleration of the trunk along the vertical axis of the body.
Time spent in MVPA (>3 METs).
Time spent in sedentary behavior (≤1.5 METs).
FIGURE 2.
Variance component estimates of the ACE model for daily physical activity traits and sedentary behavior. Variance component estimates as acquired by using structural equation modeling are shown for A, C, and E for PAEE, average daily acceleration of the trunk along the vertical axis of the body (Acceleration), and time spent in MVPA (>3 METs) and sedentary behavior (≤1.5 METs). A, additive genetic factors; C, common or shared environmental factors; E, unique or nonshared environmental factors; MET, metabolic equivalent of task; MVPA, moderate-to-vigorous intensity physical activity; PAEE, physical activity energy expenditure.
TABLE 3.
Variance component estimates (95% CIs) for daily physical activity traits and sedentary behavior1
| Variance components |
Model fit |
Compared with ACE and ADE models |
|||||||||
| Model | A | C | D | E | h2 | −2lL | df | AIC | Δ−2lL | Δdf | P |
| Physical activity energy expenditure (kJ/d)2 | |||||||||||
| ACE | 0.465 (0.234, 0.534) | 0.015 (0.000, 0.211) | — | 0.521 (0.459, 0.595) | 0.465 | 4265.6 | 1650 | 965.6 | — | — | — |
| AE | 0.481 (0.413, 0.545) | — | — | 0.519 (0.455, 0.587) | 0.481 | 4265.6 | 1651 | 963.6 | 0.0 | 1 | 0.89 |
| CE | — | 0.369 (0.306, 0.428) | — | 0.631 (0.572, 0.694) | — | 4281.2 | 1651 | 979.2 | 15.6 | 1 | 0 |
| E | — | — | — | 1.000 (—) | — | 4390.8 | 1652 | 1086.8 | 12.2 | 2 | 0 |
| Acceleration of the trunk along the vertical axis of the body (m/s2) | |||||||||||
| ACE | 0.352 (0.000, 0.442) | 0.016 (0.000, 0.320) | — | 0.632 (0.554, 0.716) | 0.352 | −5675.9 | 1650 | −8975.9 | — | — | — |
| ADE | 0.370 (0.000, 0.433) | — | 0.000 (0.000,0.416) | 0.630 (0.542, 0.706) | 0.370 | −5675.9 | 1650 | −8975.9 | — | — | — |
| AE | 0.370 (0.289, 0.449) | — | — | 0.630 (0.551, 0.711) | 0.370 | −5675.9 | 1651 | −8977.9 | 0.1 | 1 | 0.9 |
| CE | — | 0.311 (0.239, 0.378) | — | 0.689 (0.622, 0.761) | — | −5669.2 | 1651 | −8971.2 | 6.7 | 1 | 0.01 |
| DE | — | — | 0.376 (0.294,0.463) | 0.624 (0.537, 0.706) | 0.376 | −5673.4 | 1651 | −8975.4 | 2.5 | 1 | 0.11 |
| E | — | — | — | 1.000 (—) | — | −5591.8 | 1652 | −8895.8 | 84.1 | 2 | 0 |
| Time spent in moderate-to-vigorous intensity physical activity (>3 METs) (min/d)2 | |||||||||||
| ACE | 0.474 (0.289, 0.538) | 0.000 (0.000, 0.166) | — | 0.526 (0.460, 0.603) | 0.474 | 4280.3 | 1648 | 984.3 | — | — | — |
| ADE | 0.312 (0.000, 0.521) | — | 0.170 (0.000,0.515) | 0.519 (0.449, 0.591) | 0.482 | 4279.7 | 1649 | 983.7 | — | — | — |
| AE | 0.474 (0.400, 0.541) | — | — | 0.526 (0.459, 0.600) | 0.474 | 4280.3 | 1649 | 982.3 | 0.0 | 1 | 1 |
| CE | — | 0.361 (0.289, 0.426) | — | 0.639 (0.574, 0.711) | — | 4301.2 | 1649 | 1003.2 | 21.0 | 1 | 0 |
| DE | — | — | 0.488 (0.414,0.555) | 0.512 (0.445, 0.586) | 0.488 | 4281.7 | 1649 | 983.7 | 2.0 | 1 | 0.16 |
| E | — | — | — | 1.000 (—) | — | 4405.3 | 1650 | 1105.3 | 125.0 | 2 | 0 |
| Time spent in sedentary behavior (≤1.5 METs) (min/d) | |||||||||||
| ACE | 0.310 (0.094, 0.505) | 0.145 (0.000, 0.321) | — | 0.545 (0.478, 0.618) | 0.310 | 20,389.2 | 1648 | 17,093.2 | — | — | — |
| AE | 0.470 (0.404, 0.532) | — | — | 0.530 (0.468, 0.596) | 0.470 | 20,391.2 | 1649 | 17,093.2 | 2.0 | 1 | 0.16 |
| CE | — | 0.384 (0.322, 0.437) | — | 0.616 (0.563, 0.678) | — | 20,396.3 | 1649 | 17,098.3 | 7.1 | 1 | 0.01 |
| E | — | — | — | 1.000 (—) | — | 20,516.9 | 1650 | 17,216.9 | 127.7 | 2 | 0 |
Variance explained by A, C, D, and E. Estimates (95% CIs) of variance components were acquired by using structural equation modeling and adjusted for sex, age, age-squared, and BMI. Results from the ACE model were preferred for all traits over those of the most parsimonious model. Δ−2lL and P values show comparisons with the ACE model for the AE, CE, and E models and with the ADE model for the DE model. A, additive genetic factors; AIC, Akaike's information criterion; C, common or shared environmental factors; D, nonadditive or dominant genetic factors; E, unique or nonshared environmental factors; MET, metabolic equivalent of task; −2lL, −2 log-likelihood.
Traits were analyzed after inverse normal transformation.
ICCs were 2–3 times higher in monozygotic than dizygotic pairs for acceleration of the trunk and time spent in moderate-to-vigorous intensity physical activity, which suggested that dominant genetic factors may play a role for these traits. Structural equation modeling subsequently showed that approximately one-third of the heritability of the time spent in moderate-to-vigorous intensity physical activity consisted of dominant genetic factors, whereas dominant genetic factors did not play a role in acceleration of the trunk. ACE and ADE models fit the data equally well for both traits and resulted in similar h2 estimates (Table 3). Hence, we selected results from the a priori hypothesized ACE model for additional analyses to ensure the comparability of results across traits.
The AE model provided the most parsimonious fit for all traits (Table 3). Heritability estimates of the AE model deviated <2.0% from estimates acquired by using the ACE model for all traits, except for the time spent in sedentary behavior, for which the AE model provided a 16% higher h2 estimate. Because constraining variance components to zero may inflate h2 estimates (42), and the identification of a small contribution of shared environmental factors is still informative, we preferred the conservative h2 estimates acquired by using the ACE model.
Adjustment of the analyses for BMI did not substantially affect h2 estimates, with estimates that were between 3.9% higher for PAEE and 2.4% lower for acceleration of the trunk in unadjusted compared with adjusted analysis (see Supplementary Table 3 under “Supplemental data” in the online issue). The exclusion of twins with <4 d data, a different start date of physical activity assessment, a self-reported disability, or of male pairs did not materially change the results either, although the h2 estimate of acceleration of the trunk did reach significance after the exclusion of twins with self-reported disabilities (95% CI: 0.8%, 45.6%). The inclusion of data from twin pairs with self-reported zygosity may have resulted in an underestimation of the heritability of the time spent in sedentary behavior but did not affect estimates for the other traits (see Supplementary Table 4 under “Supplemental data” in the online issue). Taken together, sensitivity analyses showed that h2 estimates of daily physical activity traits and sedentary behavior were robust and mostly unaffected by the inclusion of data of potentially suboptimal quality.
DISCUSSION
In the largest twin study with objective measurements of physical activity, we showed that daily physical activity and sedentary behavior were moderately heritable. Our h2 estimates of 35–47% for different physical activity traits and 31% for the time spent in sedentary behavior suggested that biological processes influence daily physical activity levels. h2 estimates were robust and largely unaffected by adjustment for BMI or the inclusion of twins with potentially suboptimal data quality. Besides the moderate contribution of genetic factors, most of the variance in daily physical activity and sedentary behavior was explained by a combination of environmental factors that were unique to each twin and random (measurement) error. Environmental factors that were shared between twins within a pair played a marginal role at most.
PAEE and bodily movements as reflected by acceleration of the trunk have both been used in epidemiologic studies as estimates of the latent and unobservable phenomenon of habitual physical activity. When assessed by combined heart rate and movement sensing, there are intrinsic differences between the 2 traits that should not be dismissed. The sensor's accelerometer provides a better reflection of some activities, such as walking, compared with activities that involve little movement of the upper body along the vertical axis of the body, such as cycling. The latter activities are recognized more accurately when information from the sensor's accelerometer is integrated with that of its heart rate sensor. Combined sensing also discriminates better between walking with or without an external load as well as between walking on a flat or sloping surface compared with acceleration alone. Thus, PAEE provides a more comprehensive estimate of daily physical activity than acceleration of the trunk alone. However, PAEE has limitations when acquired by a combined heart rate and movement sensor in large epidemiologic data sets, in which the individual calibration of the association between the heart rate and workload during a graded exercise test is not feasible. We used the results of a calibration study in an independent sample, which leads to slightly less precise estimates of PAEE (31). As such, our h2 estimates for PAEE and bodily movements are likely conservative.
Combined heart rate and movement sensing was also used to objectively assess the time spent in sedentary behavior, which should be interpreted as the absence of physical activity. Although piezoelectric accelerometers such as the one in our sensor have often been used to quantify sedentary behavior objectively, other devices such as inclinometers, piezoresistant accelerometers, or piezocapacitive triaxial accelerometers are able to distinguish between lying, sitting, and standing (43, 44) and, therefore, may be better suited to assess sedentary behavior depending on one's definition of sedentary behavior.
Our h2 estimates were similar to those reported previously in relatively large twin studies with self-reported physical activity traits (17–19, 23, 24) but differed from estimates obtained in small twin studies with objectively assessed physical activity (20–22). We cannot exclude the possibility of true population differences in the relative contribution of genetic and environmental factors because heritability estimates are, by definition, population and time specific. Nevertheless, the difference between our h2 estimates and those reported previously in twin studies with objectively assessed physical activity may partly reflect the small sample size of previous efforts (22), a potentially higher level of heritability in adults compared with that in children and adolescents (20, 21, 45), a more accurate quantification of some activities by triaxial compared with uniaxial accelerometers (46), and the inclusion of same-sex, non-twin siblings in dizygotic twin pairs by other authors (22).
h2 estimates shown in our twin study were higher than estimates reported previously in family studies with questionnaire-derived physical activity (13–16). Although twin studies typically result in higher h2 estimates of complex traits than family studies (42), the difference in h2 estimates for physical activity likely reflected the use of self-reported relatedness in family studies with self-reported physical activity because our estimates were similar to those of a large family study with objectively assessed physical activity in which relatedness was assessed by using a gene-based method (12). Although a range of factors complicates a straightforward comparison of our results with those of previous studies, to our knowledge, our study is the largest twin study with objective measurements of daily physical activity and confirms a role for genetic factors in physical activity regulation.
Our findings have important consequences for public health initiatives, because adherence to a physical activity intervention program is likely more challenging for individuals who lack a biological drive to be active, and might even experience adverse effects in response to being physically active (47), than for individuals with a strong genetic predisposition to being physically active. Thus, such a predisposition might explain in part why some individuals respond better to physical activity intervention programs than others.
Thus far, 2 genes have been identified as playing a role in physical activity regulation on the basis of evidence from ≥4 independent lines of research, all of which were animal studies (48). DRD1 encodes a dopamine receptor and likely influences physical activity via the reward system (49, 50), whereas NHLH2 encodes nescient helix loop helix 2, which presumably exerts its effect by affecting β-endorphin production and interacting with the melanocortin-4 receptor (MC4R) (51, 52). Equally convincing evidence from human data is lacking. Some candidate-gene studies have provided promising leads (53–57), but few associations have been replicated in subsequent efforts (58). This situation likely reflects a poor choice of candidate genes, resulting from limited insights from biology, poor coverage of genetic variation, the small scale of studies, and the complexity of the trait for which small effect sizes are anticipated. Hypothesis-free approaches (ie, linkage) (12, 59, 60) and genome-wide association studies (61) have not identified loci that are robustly associated with physical activity traits in humans either. Larger efforts with more-detailed phenotypic information will likely clarify biological pathways that are relevant for daily physical activity and sedentary behavior in humans, because large-scale genome-wide association studies have previously identified loci that are associated with other cardiovascular risk factors of comparable heritability (62–64). Besides aerobic capacity and sensitivity to internal and external rewarding cues, such pathways may relate to susceptibility to fatigue, physical discomfort after physical activity, perception of ability, and triggering of food intake when physical activity is used as a weight-loss strategy.
A limitation of the current study is that our sample consisted almost exclusively of women. However, our heritability estimates were comparable with those of a family study with objectively assessed daily physical activity in data from men and women combined, as well as those of large twin studies with self-reported physical activity traits in both sexes, some of which reported higher h2 estimates in men than women (17, 18). Another limitation related to the difference in age and BMI between twins who agreed to participate and those who declined. This healthy participant bias, whereby participants in epidemiologic studies present with fewer risk factors than those who decided not to participate, has been described previously and does not necessarily affect the representativeness of results for the general population (65, 66). Importantly for our effort, there was no difference in the response rate between monozygotic and dizygotic twins.
In conclusion, to our knowledge, our study is the first in which heritability estimates are based on results from a large number of twins with objectively assessed physical activity and shows that daily physical activity and sedentary behavior are moderately heritable in adults. This result implies that physical activity regulation is influenced by biological factors.
Supplementary Material
Acknowledgments
We appreciate the efforts associated with recruiting twins by TwinsUK as well as the efforts required for processing heart rate and movement sensor (Actiheart; CamNtech) data by the physical activity technical team at the Medical Research Council Epidemiology Unit. We also thank the twins participating in the study for their time and efforts.
The authors’ responsibilities were as follows—MdH, SB, UE, TDS, NJW, and RJFL: designed the research; SB, UE, TDS, NJW, and RJFL: provided essential materials; MdH and KW: analyzed data; MdH and JHZ: performed statistical analyses; MdH, SB, JHZ, KW, AN, UE, TDS, and NJW: wrote the manuscript; and MdH: had primary responsibility for the final content of the manuscript. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: A, additive genetic factors; C, common or shared environmental factors; D, nonadditive or dominant genetic factors; E, unique or nonshared environmental factors; ICC, intrapair correlation coefficient; MET, metabolic equivalent of task; PAEE, physical activity energy expenditure.
REFERENCES
- 1.Ravussin E, Swinburn BA. Pathophysiology of obesity. Lancet 1992;340:404–8. [DOI] [PubMed] [Google Scholar]
- 2.Ekelund U, Brage S, Franks PW, Hennings S, Emms S, Wareham NJ. Physical activity energy expenditure predicts progression toward the metabolic syndrome independently of aerobic fitness in middle-aged healthy Caucasians: the Medical Research Council Ely Study. Diabetes Care 2005;28:1195–200. [DOI] [PubMed] [Google Scholar]
- 3.Ekelund U, Brage S, Franks PW, Hennings S, Emms S, Wong MY, Wareham NJ. Physical activity energy expenditure predicts changes in body composition in middle-aged healthy whites: effect modification by age. Am J Clin Nutr 2005;81:964–9. [DOI] [PubMed] [Google Scholar]
- 4.Ekelund U, Franks PW, Sharp S, Brage S, Wareham NJ. Increase in physical activity energy expenditure is associated with reduced metabolic risk independent of change in fatness and fitness. Diabetes Care 2007;30:2101–6. [DOI] [PubMed] [Google Scholar]
- 5.Ekelund U, Luan J, Sherar LB, Esliger DW, Griew P, Cooper A. International Children's Accelerometry Database C. Moderate to vigorous physical activity and sedentary time and cardiometabolic risk factors in children and adolescents. JAMA 2012;307:704–12 (Published erratum appears in JAMA 2012;307:1915. ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owen CG, Nightingale CM, Rudnicka AR, Sattar N, Cook DG, Ekelund U, Whincup PH. Physical activity, obesity and cardiometabolic risk factors in 9- to 10-year-old UK children of white European, South Asian and black African-Caribbean origin: the Child Heart And health Study in England (CHASE). Diabetologia 2010;53:1620–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ekelund U, Griffin SJ, Wareham NJ. Physical activity and metabolic risk in individuals with a family history of type 2 diabetes. Diabetes Care 2007;30:337–42. [DOI] [PubMed] [Google Scholar]
- 8.Højbjerre L, Sonne MP, Alibegovic AC, Dela F, Vaag A, Meldgaard JB, Christensen KB, Stallknecht B. Impact of physical inactivity on subcutaneous adipose tissue metabolism in healthy young male offspring of patients with type 2 diabetes. Diabetes 2010;59:2790–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipman RL, Raskin P, Love T, Triebwasser J, Lecocq FR, Schnure JJ. Glucose intolerance during decreased physical activity in man. Diabetes 1972;21:101–7. [DOI] [PubMed] [Google Scholar]
- 10.Bauman AE, Reis RS, Sallis JF, Wells JC, Loos RJ, Martin BW. Correlates of physical activity: why are some people physically active and others not? Lancet 2012;380:258–71. [DOI] [PubMed] [Google Scholar]
- 11.Rundquist EA. Inheritance of spontaneous activity in rats. J Comp Psychol 1933;16:415–38. [Google Scholar]
- 12.Cai G, Cole SA, Butte N, Bacino C, Diego V, Tan K, Goring HH, O'Rahilly S, Farooqi IS, Comuzzie AG. A quantitative trait locus on chromosome 18q for physical activity and dietary intake in Hispanic children. Obesity (Silver Spring) 2006;14:1596–604. [DOI] [PubMed] [Google Scholar]
- 13.Choh AC, Demerath EW, Lee M, Williams KD, Towne B, Siervogel RM, Cole SA, Czerwinski SA. Genetic analysis of self-reported physical activity and adiposity: the Southwest Ohio Family Study. Public Health Nutr 2009;12:1052–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell BD, Rainwater DL, Hsueh WC, Kennedy AJ, Stern MP, Maccluer JW. Familial aggregation of nutrient intake and physical activity: results from the San Antonio Family Heart Study. Ann Epidemiol 2003;13:128–35. [DOI] [PubMed] [Google Scholar]
- 15.Pérusse L, Tremblay A, Leblanc C, Bouchard C. Genetic and environmental influences on level of habitual physical activity and exercise participation. Am J Epidemiol 1989;129:1012–22. [DOI] [PubMed] [Google Scholar]
- 16.Simonen RL, Pérusse L, Rankinen T, Rice T, Rao DC, Bouchard C. Familial aggregation of physical activity levels in the Quebec Family Study. Med Sci Sports Exerc 2002;34:1137–42. [DOI] [PubMed] [Google Scholar]
- 17.Aaltonen S, Ortega-Alonso A, Kujala UM, Kaprio J. A longitudinal study on genetic and environmental influences on leisure time physical activity in the Finnish Twin Cohort. Twin Res Hum Genet 2010;13:475–81. [DOI] [PubMed] [Google Scholar]
- 18.Carlsson S, Andersson T, Lichtenstein P, Michaelsson K, Ahlbom A. Genetic effects on physical activity: results from the Swedish Twin Registry. Med Sci Sports Exerc 2006;38:1396–401. [DOI] [PubMed] [Google Scholar]
- 19.Eriksson M, Rasmussen F, Tynelius P. Genetic factors in physical activity and the equal environment assumption–the Swedish young male twins study. Behav Genet 2006;36:238–47. [DOI] [PubMed] [Google Scholar]
- 20.Fisher A, van Jaarsveld CH, Llewellyn CH, Wardle J. Environmental influences on children's physical activity: quantitative estimates using a twin design. PLoS ONE 2010;5:e10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franks PW, Ravussin E, Hanson RL, Harper IT, Allison DB, Knowler WC, Tataranni PA, Salbe AD. Habitual physical activity in children: the role of genes and the environment. Am J Clin Nutr 2005;82:901–8. [DOI] [PubMed] [Google Scholar]
- 22.Joosen AM, Gielen M, Vlietinck R, Westerterp KR. Genetic analysis of physical activity in twins. Am J Clin Nutr 2005;82:1253–9. [DOI] [PubMed] [Google Scholar]
- 23.Maia JA, Thomis M, Beunen G. Genetic factors in physical activity levels: a twin study. Am J Prev Med 2002;23(suppl):87–91. [DOI] [PubMed] [Google Scholar]
- 24.Stubbe JH, Boomsma DI, Vink JM, Cornes BK, Martin NG, Skytthe A, Kyvik KO, Rose RJ, Kujala UM, Kaprio J, et al. Genetic influences on exercise participation in 37,051 twin pairs from seven countries. PLoS ONE 2006;1:e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helmerhorst HJ, Brage S, Warren J, Besson H, Ekelund U. A systematic review of reliability and objective criterion-related validity of physical activity questionnaires. Int J Behav Nutr Phys Act 2012;9:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moayyeri A, Hammond CJ, Valdes AM, Spector TD. Cohort profile: TwinsUK and healthy ageing twin study. Int J Epidemiol 2013;42:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brage S, Brage N, Franks PW, Ekelund U, Wareham NJ. Reliability and validity of the combined heart rate and movement sensor Actiheart. Eur J Clin Nutr 2005;59:561–70. [DOI] [PubMed] [Google Scholar]
- 28.Stegle O, Fallert SV, MacKay DJ, Brage S. Gaussian process robust regression for noisy heart rate data. IEEE Trans Biomed Eng 2008;55:2143–51. [DOI] [PubMed] [Google Scholar]
- 29.Levin S, Jacobs DR, Jr, Ainsworth BE, Richardson MT, Leon AS. Intra-individual variation and estimates of usual physical activity. Ann Epidemiol 1999;9:481–8. [DOI] [PubMed] [Google Scholar]
- 30.Matthews CE, Ainsworth BE, Thompson RW, Bassett DR., Jr Sources of variance in daily physical activity levels as measured by an accelerometer. Med Sci Sports Exerc 2002;34:1376–81. [DOI] [PubMed] [Google Scholar]
- 31.Brage S, Ekelund U, Brage N, Hennings MA, Froberg K, Franks PW, Wareham NJ. Hierarchy of individual calibration levels for heart rate and accelerometry to measure physical activity. J Appl Physiol 2007;103:682–92. [DOI] [PubMed] [Google Scholar]
- 32.The Interact Consortium. Validity of a short questionnaire to assess physical activity in 10 European countries. Eur J Epidemiol 2012;27:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brage S, Brage N, Franks PW, Ekelund U, Wong MY, Andersen LB, Froberg K, Wareham NJ. Branched equation modeling of simultaneous accelerometry and heart rate monitoring improves estimate of directly measured physical activity energy expenditure. J Appl Physiol 2004;96:343–51. [DOI] [PubMed] [Google Scholar]
- 34.Henry CJ. Basal metabolic rate studies in humans: measurement and development of new equations. Public Health Nutr 2005;8:1133–52. [DOI] [PubMed] [Google Scholar]
- 35.Brandes M, VAN Hees VT, Hannöver V, Brage S. Estimating energy expenditure from raw accelerometry in three types of locomotion. Med Sci Sports Exerc 2012;44:2235–42. [DOI] [PubMed] [Google Scholar]
- 36.Woodward MI, Cunningham JL. Skeletal accelerations measured during different exercises. Proc Inst Mech Eng H 1993;207:79–85. [DOI] [PubMed] [Google Scholar]
- 37.Boker S, Neale M, Maes H, Wilde M, Spiegel M, Brick T, Spies J, Estabrook R, Kenny S, Bates T, et al. OpenMx: an open source extended structural equation modeling framework. Psychometrika 2011;76:306–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neale MCCL. Methodology for genetic studies of twins and families. Dordrecht, Netherlands: Kluwer Academic, 1992. [Google Scholar]
- 39.Peng Q, Zhao J, Xue F. PCA-based bootstrap confidence interval tests for gene-disease association involving multiple SNPs. BMC Genet 2010;11:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ekelund U, Aman J, Yngve A, Renman C, Westerterp K, Sjostrom M. Physical activity but not energy expenditure is reduced in obese adolescents: a case-control study. Am J Clin Nutr 2002;76:935–41. [DOI] [PubMed] [Google Scholar]
- 41.Chirico AM, Stunkard AJ. Physical activity an human obesity. N Engl J Med 1960;263:935–40. [DOI] [PubMed] [Google Scholar]
- 42.Elks CE, den Hoed M, Zhao JH, Sharp SJ, Wareham NJ, Loos RJF, Ong KK. Variability in the heritability of body masss index: a systematic review and meta-regression. Front Endocrinol (Lausanne) 2012;3:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonomi AG, Goris AH, Yin B, Westerterp KR. Detection of type, duration, and intensity of physical activity using an accelerometer. Med Sci Sports Exerc 2009;41:1770–7. [DOI] [PubMed] [Google Scholar]
- 44.Carr LJ, Mahar MT. Accuracy of intensity and inclinometer output of three activity monitors for identification of sedentary behavior and light-intensity activity. J Obes 2012;2012:460271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stubbe JH, Boomsma DI, De Geus EJ. Sports participation during adolescence: a shift from environmental to genetic factors. Med Sci Sports Exerc 2005;37:563–70. [DOI] [PubMed] [Google Scholar]
- 46.Plasqui G, Joosen AM, Kester AD, Goris AH, Westerterp KR. Measuring free-living energy expenditure and physical activity with triaxial accelerometry. Obes Res 2005;13:1363–9. [DOI] [PubMed] [Google Scholar]
- 47.Bouchard C, Blair SN, Church TS, Earnest CP, Hagberg JM, Hakkinen K, Jenkins NT, Karavirta L, Kraus WE, Leon AS, et al. Adverse metabolic response to regular exercise: is it a rare or common occurrence? PLoS ONE 2012;7:e37887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lightfoot JT. Current understanding of the genetic basis for physical activity. J Nutr 2011;141:526–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knab AM, Bowen RS, Hamilton AT, Gulledge AA, Lightfoot JT. Altered dopaminergic profiles: implications for the regulation of voluntary physical activity. Behav Brain Res 2009;204:147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rhodes JS, Garland T. Differential sensitivity to acute administration of Ritalin, apomorphine, SCH 23390, but not raclopride in mice selectively bred for hyperactive wheel-running behavior. Psychopharmacology (Berl) 2003;167:242–50. [DOI] [PubMed] [Google Scholar]
- 51.Fox DL, Vella KR, Good DJ. Energy balance pathways converging on the Nhlh2 transcription factor. Front Biosci. 2007;12:3983–93. [DOI] [PubMed] [Google Scholar]
- 52.Good DJ, Coyle CA, Fox DL. Nhlh2: a basic helix-loop-helix transcription factor controlling physical activity. Exerc Sport Sci Rev 2008;36:187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loos RJ, Rankinen T, Tremblay A, Pérusse L, Chagnon Y, Bouchard C. Melanocortin-4 receptor gene and physical activity in the Quebec Family Study. Int J Obes (Lond) 2005;29:420–8. [DOI] [PubMed] [Google Scholar]
- 54.Lorentzon M, Lorentzon R, Lerner UH, Nordstrom P. Calcium sensing receptor gene polymorphism, circulating calcium concentrations and bone mineral density in healthy adolescent girls. Eur J Endocrinol 2001;144:257–61. [DOI] [PubMed] [Google Scholar]
- 55.Richert L, Chevalley T, Manen D, Bonjour JP, Rizzoli R, Ferrari S. Bone mass in prepubertal boys is associated with a Gln223Arg amino acid substitution in the leptin receptor. J Clin Endocrinol Metab 2007;92:4380–6. [DOI] [PubMed] [Google Scholar]
- 56.Stefan N, Vozarova B, Del Parigi A, Ossowski V, Thompson DB, Hanson RL, Ravussin E, Tataranni PA. The Gln223Arg polymorphism of the leptin receptor in Pima Indians: influence on energy expenditure, physical activity and lipid metabolism. Int J Obes Relat Metab Disord 2002;26:1629–32. [DOI] [PubMed] [Google Scholar]
- 57.Winnicki M, Accurso V, Hoffmann M, Pawlowski R, Dorigatti F, Santonastaso M, Longo D, Krupa-Wojciechowska B, Jeunemaitre X, Pessina AC, et al. Physical activity and angiotensin-converting enzyme gene polymorphism in mild hypertensives. Am J Med Genet A 2004;125A:38–44. [DOI] [PubMed] [Google Scholar]
- 58.de Vilhena e Santos DM, Katzmarzyk PT, Seabra AF, Maia JA. Genetics of physical activity and physical inactivity in humans. Behav Genet 2012;42:559–78. [DOI] [PubMed] [Google Scholar]
- 59.De Moor MH, Posthuma D, Hottenga JJ, Willemsen G, Boomsma DI, De Geus EJ. Genome-wide linkage scan for exercise participation in Dutch sibling pairs. European journal of human genetics. Eur J Hum Genet 2007;15:1252–9. [DOI] [PubMed] [Google Scholar]
- 60.Simonen RL, Rankinen T, Pérusse L, Rice T, Rao DC, Chagnon Y, Bouchard C. Genome-wide linkage scan for physical activity levels in the Quebec Family study. Med Sci Sports Exerc 2003;35:1355–9. [DOI] [PubMed] [Google Scholar]
- 61.De Moor MH, Liu YJ, Boomsma DI, Li J, Hamilton JJ, Hottenga JJ, Levy S, Liu XG, Pei YF, Posthuma D, et al. Genome-wide association study of exercise behavior in Dutch and American adults. Med Sci Sports Exerc 2009;41:1887–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Newton-Cheh C, Eijgelsheim M, Rice KM, de Bakker PI, Yin X, Estrada K, Bis JC, Marciante K, Rivadeneira F, Noseworthy PA, et al. Common variants at ten loci influence QT interval duration in the QTGEN Study. Nat Genet 2009;41:399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sotoodehnia N, Isaacs A, de Bakker PI, Dorr M, Newton-Cheh C, Nolte IM, van der Harst P, Muller M, Eijgelsheim M, Alonso A, et al. Common variants in 22 loci are associated with QRS duration and cardiac ventricular conduction. Nat Genet 2010;42:1068–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dastani Z, Hivert MF, Timpson N, Perry JR, Yuan X, Scott RA, Henneman P, Heid IM, Kizer JR, Lyytikainen LP, et al. Novel loci for adiponectin levels and their influence on type 2 diabetes and metabolic traits: a multi-ethnic meta-analysis of 45,891 individuals. PLoS Genet 2012;8:e1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Klesges RC, Williamson JE, Somes GW, Talcott GW, Lando HA, Haddock CK. A population comparison of participants and nonparticipants in a health survey. Am J Public Health 1999;89:1228–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lynch WD, Golaszewski TJ, Clearie A, Vickery DM. Characteristics of self-selected responders to a health risk appraisal: generalizability of corporate health assessments. Am J Public Health 1989;79:887–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.