Abstract
A rostrocaudal pathway connecting the temporal and parietal lobes was described in monkeys using autoradiography and was named the middle longitudinal fasciculus (MdLF). Recently, the use of diffusion tensor tractography has allowed it to be depicted in human volunteers. In the present study, a technique of fiber dissection was used in 18 cadaveric human brains to investigate the presence of this fasciculus and to detail its anatomical relationships. On the basis of our findings, fiber dissection provides evidence for a long horizontal bundle medial to the arcuate fasciculus and extending to the superior temporal gyrus. Its fibers occupy the lateral-most layer of the upper portion of the stratum sagittale and partially cover the inferior fronto-occipital fasciculus, which is situated deeper and slightly inferiorly. Whereas MdLF fibers continue on a relatively superficial level to reach the superior temporal gyrus, the inferior fronto-occipital fasciculus penetrates the deep temporal white matter and crosses the insular lobe. Although diffusion tensor imaging suggests that the MdLF terminates in the angular gyrus, this was not confirmed by the present study. These long association fibers continue onward posteriorly into upper portions of the occipital lobe. Further studies are needed to understand the role of the MdLF in brain function.
Keywords: anatomy, connectivity, fiber pathways, fiber dissection, white matter
Introduction
A rostrocaudal fiber bundle directly connecting the anterior portion of the temporal lobe to the parietal lobe was described for the first time in the 1980s (Seltzer & Pandya, 1984). Identified in the Rhesus monkey by means of an in vivo technique using radioisotope injections, this pathway was named the middle longitudinal fasciculus (MdLF). More recently, the use of diffusion tensor imaging (DTI) tractography allowed the MdLF to be depicted in healthy human volunteers (Makris et al., 2008). It has been shown to originate near the caudal portion of the angular gyrus (AG) and to extend anteriorly into the superior temporal gyrus. However, DTI is an indirect method with inherent limitations of studying white matter anatomy (Mori, 2007).
Fiber dissection has been performed for more than three centuries, but after significant progress on the technique (Ludwig & Klingler, 1956) and especially after the advent of DTI (Filler et al., 1993; Basser et al., 1994), the study of the three-dimensional organization of the white matter has regained the interest of anatomists, surgeons and radiologists. However, to our knowledge, the MdLF has never been studied with fiber dissection using human post-mortem brains and two major questions need to be answered. First, despite similarities between monkey and human brains, there might be major differences on bundle trajectory and terminations, which are not always clearly seen with DTI. Secondly, it is necessary to characterize this fascicle precisely and to assess whether it is independent from adjacent bundles, such as the arcuate fasciculus (AF) and the inferior fronto-occipital fasciculus (iFOF).
In the present study, we propose the use of a technique of fiber dissection to investigate the presence of the MdLF and to detail its anatomical relationships in cadaveric human brains. These findings may have important implications both in clinical practice and in fundamental research, including for the modeling of the neural basis of superior functions, such as language in the dominant hemisphere.
Materials and methods
The technique used in the present study was based on one originally described by Klingler (Klingler, 1935; Ludwig & Klingler, 1956) and later used by several authors for fiber dissection (Ture et al., 2000; De Castro et al., 2005; Fernandez-Miranda et al., 2008; Martino et al., 2009, 2010). Eighteen human brain hemispheres (nine left, nine right) obtained from subjects enrolled in a body donation program were fixed in formalin. The pia-mater, arachnoid membrane and vascular structures were carefully removed and the hemispheres were frozen at −20° C for a 2–4 weeks and then slowly defrosted. As described by the authors of the original technique, the crystallization of the water molecules and formalin inside the cerebral parenchyma disrupts the structure of gray matter, enabling the cortex to be removed easily from the brain. Freezing also slightly spreads groups of white matter fibers, rendering tracts more visible and dissectible.
After studying and photographing sulci and gyri, hand-made wooden spatulas of various sizes were used to peel away both the cortex and the short ‘U’ association (intergyral) fibers. The dissection was performed gradually from the lateral to the medial surface of the hemisphere, with simultaneous detailed photographic documentation (D90, Nikon Corporation, Tokyo, Japan; Tamron SP 90 mm f/2,8 Di Macro Lens, Tamron Corporation Ltd., Saitama, Japan). Surgical magnifying glasses or an operating microscope (OPMI 6-F, Carl Zeiss Meditec, Jena, Germany) were used when necessary for delicate dissection of both deeper short ‘U’ association fibers and deeper bundles.
Since MdLF is hypothesized to run just medial to the AF to horizontally connect temporal and parietal lobes, the following steps were undertaken. We first dissected the lateral aspect of the AF (Fernandez-Miranda et al., 2008; Ture et al., 2000) while preserving the superior temporal gyrus (T1) white matter and posterior parietal cortex as much as possible. Secondly, the AF was divided near the parietal operculum and groups of C-shaped arcuate fibers were followed posteriorly around the circular sulcus of the insula and progressively peeled away. This was repeated layer by layer until horizontal fibers started to appear in the stratum sagittale (SS), in contact with the medial aspect of SLF/AF complex. Then, groups of horizontal fibers were followed anteriorly and posteriorly as far as possible to search for any penetration into the superior temporal gyrus or the posterior parietal cortex. Finally, to avoid misinterpretation with fibers from the temporal portion of the inferior fronto-occipital fasciculus (iFOF) as it enters the SS, a subsequent step of the dissection consisted of further exposing this bundle from the SS to the external capsula.
Results
A detailed description of the gradual dissection of the temporo-parietal white matter is provided in the figure legends. After the removal of arachnoid membrane and vascular elements, the first step in the dissection was the study of sulci and gyri of the lateral aspect of the hemisphere (Fig.1).
Fig 1.
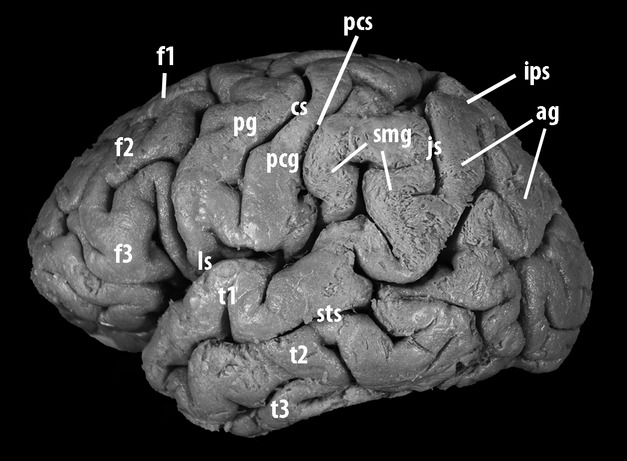
Lateral aspect of the left hemisphere of a human brain prepared for fiber dissection using an adaptation of the Klingler technique. Before approaching the cerebral parenchyma, the procedure requires removal of all meningeal and vascular structures from the brain surface and the study of the individual anatomy of sulci and gyri. The inferior parietal lobule is limited anteriorly and superiorly by the postcentral-intraparietal sulcus complex. The sulcus intermedius primus (Jensen's sulcus) runs caudally from the intraparietal sulcus and splits the inferior parietal lobule into two horse-shaped plis de passage: the supramarginal gyrus, anteriorly located, contours the posterior tip of the lateral fissure, whereas the angular gyrus surmounts the posterior extremity of the superior temporal sulcus. The lateral surface of the temporal lobe, below the lateral sulcus, is divided by the superior and inferior temporal sulci into three parallel gyri: the superior, middle and inferior temporal gyri. The superior aspect of the superior temporal gyrus (planum polare, transverse temporal gyrus and sulcus, and planum temporale) borders the lateral fissure inferiorly, whereas its lateral aspect is continuous posteriorly with the supramarginal and angular gyri. The middle temporal gyrus is continuous with the angular gyrus. Cortical bridges linking the middle and the inferior temporal gyri are often observed, as in the case of this specimen. ag, angular gyrus; cs, central sulcus; f1, superior frontal gyrus; f2, middle frontal gyrus; f3, inferior frontal gyrus; ls, lateral sulcus; js, Jensen's sulcus; t1, superior temporal gyrus; t2, middle temporal gyrus, t3, inferior temporal gyrus; sts, superior temporal sulcus; pg, pre-central gyrus; pcg, post-central gyrus.
Cerebral sulci and gyri
Although a relatively constant sulcal organization was observed in the temporal and parietal lobes we studied, some variants were recorded. The lateral aspect of the temporal lobe was typically divided by the superior and inferior temporal sulci into three parallel temporal gyri: superior (T1), middle (T2) and inferior (T3). However, T1 was interrupted by a short vertical sulcus in four hemispheres (22.2%), T2 in two (11.1%) and T3 in four (22.2%). Cortical bridges linking T1 to T2 were observed in four of the specimens (22.2%), and linking the T2 to T3 in the other 14 cases (77.8%). In four cases, the supramarginal gyrus (SMG) presented with a variant in which a narrow gyrus was intercalated either between the AG and the typically horse-shaped circumvolution around the lateral sulcus (n = 3) or between this circumvolution and the postcentral gyrus (n = 1).
Lateral aspect of the arcuate fasciculus
The freezing process made the cortical gray matter fragile and friable so that its removal could be achieved by delicate, repetitive peeling. At the subcortical level, short ‘U’ association fibers were encountered and exposed without any technical difficulty on the lateral surface of the temporal lobe and the inferior parietal lobule (IPL). Progressive removal of short association fibers allowed exposition of the lateral aspect of the AF in the postero-superior portion of the temporal lobe and deep to the SMG. At this stage of the dissection, the superior temporal gyrus as well as the AG white matter were kept in place and preserved as much as possible (Fig.2A).
Fig 2.
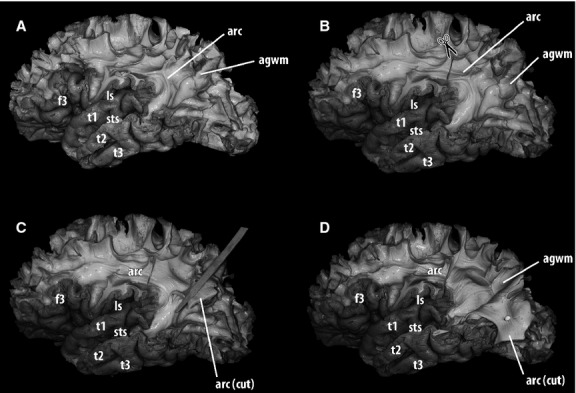
Gradual dissection of the white matter of the lateral aspect of the left hemisphere using a fiber-dissection technique: a subsequent stage to Fig. 1. The next step was the removal of the cortex and subcortical ‘U’ association fibers from the area of study, so that longer association tracts could be exposed. (A) Progressing from lateral to medial, the first long association bundle encountered in the temporo-parietal junction was the superior longitudinal/arcuate complex. Longer arcuate fibers directly linked the frontal and temporal lobes, running around the circular sulcus of the insula. In this specimen, the pre-central, post-central and the majority of the supramarginal gyri were removed. A small portion of the anterior part of the angular gyrus, where it is continuous with the superior temporal gyrus, was also removed to expose arcuate fibers. The rest of the white matter of the angular gyrus was preserved. (B) Exploration of the association fibers located medial to the arcuate fasciculus began with the section of the arcuate fasciculus near the parietal operculum. (C) After the arcuate fasciculus was divided, groups of fibers were elevated, so that the fasciculus could be progressively peeled away. Care was taken not to damage horizontal association fibers situated at a deeper level. (D) When the arcuate fasciculus was completely ventrally retracted, the plane between the arcuate fasciculus and deeper horizontal association fibers was exposed. With this procedure, the dissection exposed the contact surfaces between the medial aspect of the arcuate fasciculus and the most lateral layer of the stratum sagittale (continues in Fig.3). agwm, angular gyrus white matter; arc, arcuate fasciculus; f1, superior frontal gyrus; f2, middle frontal gyrus; f3, inferior frontal gyrus; ls, lateral sulcus; t1, superior temporal gyrus; t2, middle temporal gyrus, t3, inferior temporal gyrus; sts, superior temporal sulcus.
Elevation of the arcuate fasciculus
After completing the dissection of its lateral aspect, the AF was divided in its postero-superior portion (deep to the parietal operculum) and ventrally reflected (Fig.2B,D). With this procedure, the most lateral fibers of the SS were exposed, in contact with the medial aspect of long arcuate fibers. In this layer, it was possible to identify a group of horizontally oriented fibers running into the white matter of the base of the temporal operculum (Fig.3). These fibers could be followed further anteriorly up to the caudal portion of the anterior third of T1. Occasionally, additional groups of fibers entered T1 at a different point, which could be exposed by gentle upward retraction of that operculum (Fig.3B). Posteriorly, they ran deeper and were covered by a thick contingent of roughly vertically oriented fibers of the AF.
Fig 3.
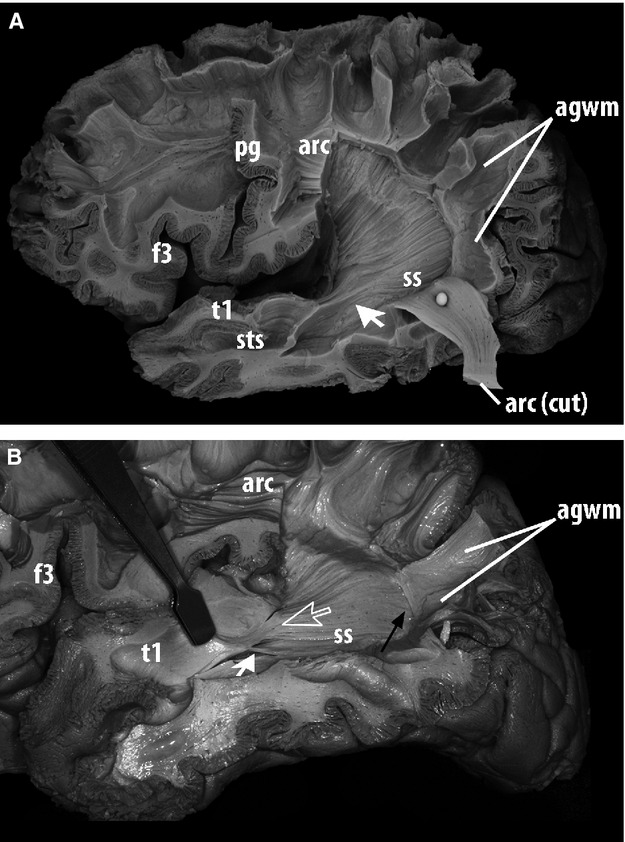
Lateral view of the dissection of the deep white matter of the left hemisphere of a human brain, a subsequent stage to Fig.2. After the arcuate fasciculus was caudally retracted, the plane between the arcuate fasciculus and deeper horizontal association fibers was the interface between the medial aspect of the arcuate fasciculus and the lateral layer of the sagittal stratum. (A) Horizontal fibers of this layer were followed throughout the dissection to assess whether they entered the superior temporal gyrus, which is exhibited in this specimen (arrow). (B) Another specimen, in which the parieto-temporal portion of the arcuate fasciculus was elevated and removed. Delicate groups of fibers from the most lateral layer of the stratum sagittale did not submerge in deep portions of the temporal lobe but remained relatively superficial on entering the superior temporal gyrus (large blank arrow). Additional groups of fibers entering the superior temporal gyrus at different points (small white arrow) could occasionally be exposed by gentle upward retraction of the temporal operculum. Although these fibers were encountered in the posterior and middle-thirds of T1/PT, the procedure failed to follow them for a long trajectory up to the temporal pole. (A,B) Posteriorly, these fibers stayed deep to the white matter of the angular gyrus without any fiber group shifting to its cortex: association fibers of the posterior bank of the angular gyrus were continuous with those of the arcuate fasciculus but discontinuous with fibers of the superficial layer of the stratum sagittale (black arrow). agwm, angular gyrus white matter; arc, arcuate fasciculus; f3, inferior frontal gyrus; ls, lateral sulcus; pg, pre-central gyrus; t1, superior temporal gyrus; sts, superior temporal sulcus, ss, stratum sagittale.
Posterior terminations
As described above, the previously identified horizontal fibers entering T1 occupied the most lateral layer of the SS. The progression of the dissection showed that this layer continued further posteriorly, beyond the caudal limits of the IPL. It finally reached the cerebral cortex next to the postero-superior border of the lateral aspect of the occipital lobe (O1) and next to the inferior lip of the parieto-occipital arcus (Fig.4). In 16 of the 18 specimens, no SS fibers reflecting laterally and entering the AG were observed. Instead, they were covered by the medial aspect of the SLF/AF complex. The white matter of the AG receives fibers coming from the SLF/AF, which are numerous even in its posterior portions. In the two remaining (left-sided) hemispheres, no definitive conclusion could be made, because the consistency of the preparation was not optimal in one, and because of ruptured fibers in the other.
Fig 4.
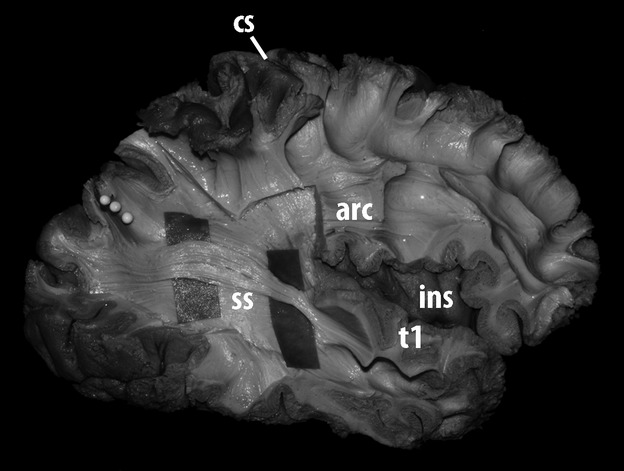
Lateral view of the dissection of the most external layer of the stratum sagittale of the right hemisphere of a human brain. The horizontal association fibers entering the region of the superior temporal gyrus were further dissected and exposed. In this process, most of the arcuate fasciculus was removed. Following those fibers posteriorly showed that they were directed to upper portions of the occipital lobe, next to the postero-superior border of the hemisphere and the parieto-occipital arcus. The level of the parieto-occipital sulcus was marked with three white pins. arc, arcuate; cs, central sulcus; ins, insula; t1, superior temporal gyrus; ss, stratum sagittale.
Isolation from the inferior fronto-occipital-fasciculus
This stage of the dissection assessed whether the previously dissected fibers were dependent on an adjacent bundle: the iFOF. During this approach, the white matter deep to T1 was explored while the attachment of the superior temporal gyrus to the SS was still preserved. Fiber dissection allowed the identification of the uncinate fasciculus (UNC) posterior to the limen insulae, the iFOF posterior to the UNC, and claustro-fugal fibers in the postero-superior portion of the external capsula.
The results of this procedure showed that the iFOF was located deeper to the fibers entering T1. While the fibers of the SS forming the iFOF inclined medially and went deeper to cross the insula, those connecting T1 did not incline but maintained a relatively superficial level and a straighter trajectory. In the upper SS, iFOF fibers occupied a more medial layer (partially covered by the fibers entering T1) and were situated in a more ventral location. As a consequence, before the iFOF could be adequately exposed, the previously dissected bundle had to be elevated. In addition, T1 white matter and at least part of the cortex of the insula had to be removed (Fig.5).
Fig 5.
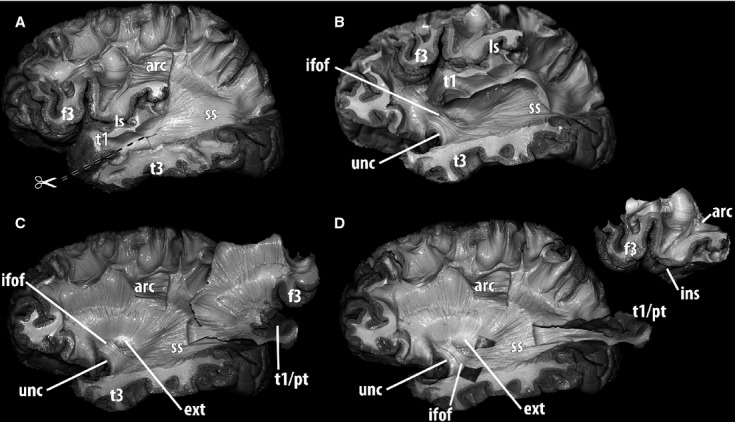
Further dissection of the deep white matter along the stratum sagittale and the anterior temporal lobe in a human left hemisphere, lateral view. The fibers coming from the inferior fronto-occipital fasciculus need to be identified to assess whether they are distinguishable from those entering the superior temporal gyrus and to expose their relationships. (A) Fibers from the lateral layer of the stratum sagittale ran deep to the arcuate fasciculus and were dissected up to their posterior terminations in the occipital lobe. Anteriorly, they entered the superior temporal gyrus. In order to advance to the depth of the temporal lobe and insula, an incision was performed at the level of the superior temporal sulcus. (B) After the superior temporal gyrus including the planum temporale was detached from the deep temporal white matter, a plane was developed by passing through the insula, lateral to the external capsula. To dissect deeper structures, the superior temporal gyrus, the insular cortex and the inferior frontal gyrus were removed completely. The attachment of the superior temporal gyrus to the stratum sagittale was preserved. (C)The whole block comprising insular cortex and frontal and temporal opercula was fully elevated laterally, providing access to the structures in the external capsula. At this level, fiber dissection allowed identification of the uncinate fasciculus posterior to the limen insulae, and the inferior fronto-occipital fasciculus, located postero-superior to the uncinate fasciculus and running from the frontal lobe to the stratum sagittale. With this procedure, claustro-fugal fibers were also exposed in the postero-superior portion of the external capsula. (D) The insular cortex and the frontal operculum were detached from the temporal operculum. A divider was introduced deep to the fibers of the inferior fronto-occipital fasciculus, which were further dissected. This procedure showed that the iFOF was medial to fibers of the stratum sagittale reaching the superior temporal gyrus. Whereas the fibers of the stratum sagittale that form the iFOF inclinated medially and went deeper to reach the external capsula, those connecting the superior temporal gyrus did not incline and remained relatively superficial with a straighter trajectory. Also, in the stratum sagittale, iFOF fibers were more medial and slightly inferiorly situated. arc, arcuate fasciculus; ext, external capsula; f3, inferior frontal gyrus; ifof, inferior fronto-occipital fasciculus; ins, insula; ls, lateral sulcus; pt, planum temporale; t1, superior temporal gyrus; t3, inferior temporal gyrus; unc, uncinate fasciculus; ss, sagittal stratum.
Discussion
The present study assessed the existence of a middle longitudinal fasciculus in humans using an adaptation of a classic fiber dissection technique initially developed by Ludwig & Klingler (1956) and widely used in anatomical studies to study the three-dimensional organization of fiber pathways of the brain. The exploration of the parietal and temporal white matter using a specific protocol showed that delicate groups of roughly horizontal association fibers, running deep to the arcuate fasciculus, left the most superficial layer of the SS and joined the white matter of the superior temporal gyrus. The dissection of the posterior prolongation of these fibers did not lead to the IPL, as previously observed in the Rhesus monkey (Seltzer & Pandya, 1984) and suggested by DTI studies in humans (Makris et al., 2008). Instead, they were directed to the upper portions of the lateral aspect of the occipital cortex just posterior to the IPL, near the postero-superior edge of the hemisphere.
Methodological considerations
The protocol for approaching the temporo-parietal white matter presented here has been specifically developed for the present study. Evolving from previous variants, it showed itself to be the most adequate for this region. Direct exploration of T1 white matter after careful removal of the short ‘U’ association fibers between T1 and T2 was also considered in order to detect delicate horizontal fibers in the base of the posterior portion of T1. However, they would have been rapidly peeled away and fractured during the dissection progress deep to the non-removed AF.
As a consequence, such a ‘direct approach’ has limited usefulness in the exploration of the horizontal association pathway joining the SS. In contrast, the elevation of previously exposed SLF/AF is advantageous, since the interface between the temporal portion of the AF and the SS can be easily defined. Since the directions of their fibers are roughly orthogonal, it is possible to gradually remove the entire thickness of the SLF/AF without any damage to the SS. The operator is then able to search for horizontal fibers directed to T1, progressing from posterior to anterior, and to start working where they were most resistant, and not where they would easily fracture, as is the case in the direct exploration of T1 white matter. For the same reason, we also consider that it is preferable to section the AF at a point above the lateral sulcus and to retract it downwards, rather than in the opposite direction.
MdLF trajectory
The MdLF presents an overall organization in three well-defined segments. The posterior one is the longest and widest in the sagittal plan. In this segment, fibers are distributed in a narrow layer immediately medial to the AF. In the middle segment, the MdLF leaves the SS to enter the deep posterior portion of T1. Here, the fibers are relatively compacted, giving a constricted appearance to this segment. Finally, the anterior segment of the MdLF runs inside the white matter of the temporal operculum.
After removal of short ‘U’ association fibers, the white matter of T1 is made up of a layer located between two gyral banks: the opercular (bounding the lateral fissure) and the sulcal (superior bank of the superior temporal sulcus). Therefore, the orientation of this core white matter is perpendicular to the overall organization of the SS. The anterior terminations of the MdLF are also constrained by this pattern; as a consequence, its middle segment reveals a 60–90o lateral rotation (Fig.6).
Fig 6.

The middle longitudinal fasciculus. The fibers of this association bundle run medial to the superior longitudinal/arcuate fasciculus complex, and penetrate the white matter of the superior temporal gyrus. As a consequence, these fibers run in two nearly perpendicular planes: that of the stratum sagittale and that of the superior temporal gyrus white matter.
Anatomical relationships between the MdLF and other fiber bundles
DTI tractography was used to differentiate the MdLF from adjacent bundles such as SLF-II and AF (Makris et al., 2008). SLF-II is the major segment of the horizontal portion of the superior longitudinal fasciculus, which runs horizontally in the centrum semiovale. As the majority of the trajectory of the SLF-II and MdLF is widely separated by the lateral sulcus and the opercular structures, we considered that the exposure of both bundles in the same specimens was not necessary. However, the iFOF presents a similar trajectory in the temporal lobe and needs to be isolated from MdLF fibers.
Progressive lateral to medial exploration of the white matter permitted clear differentiation of fibers coming from the SLF/AF, the MdLF and the iFOF. Whereas MdLF leaves the SS at a relatively lateral level to enter the temporal operculum, the iFOF has a medial curve in the deep white matter of the temporal lobe which crosses the insula and the external capsula, posterior to the uncinate fasciculus. A full view of this portion of the iFOF is only possible after the removal of at least part of the anterior portions of the temporal lobe and insula.
Other authors have studied the lateral aspect of the SS and, although this was not the aim of their investigations, fibers penetrating the superior temporal gyrus were exposed. In 2004, Sincoff et al. (2004) published a study on optical radiations and their surgical importance in approaching the temporal horn of the lateral ventricle. In an illustration in which the SS was exposed, one can see fibers entering the temporal operculum. In another figure, in which the temporal cortex was completely removed and the iFOF was exposed together with the Meyer loop, one can see that the MdLF was ruptured by the dissection. In 2008, Lawes et al. (2008) published a study on the correlations between gross anatomy and images of tractography. An unidentified temporo-parietal-occipital pathway entering the superior temporal gyrus was observed in the right hemisphere. However, the piece of parenchyma that illustrates their study seems to contain MdLF fibers (and possibly some AF and external capsule fibers).
Agreements and disagreements between diffusion tensor imaging and fiber dissection
Our study presents results that are partially concordant with the previous evidence provided by DTI in humans. The location of the bundle, the point where it penetrates the temporal operculum, and its relation to the medial aspect of the SLF/AF (in contact, but completely independent), all agree with the previous evidence. In addition, although it is suggested that the MdLF may extend to the temporal pole, most profiles obtained in the pioneer study by Makris et al. (2008) using DTI showed the anterior extremity at the level of the anterior third of T1. MdLF fibers naturally tend to be less numerous and less organized as a single bundle as they approach the temporal pole. This increases the technical difficulty of using both DTI and fiber dissection.
Nevertheless, results provided here present differences that are worth mentioning. First, the volume of the MdLF inside T1 seems higher with DTI, and the fibers seem more widely present in the thickness of this gyrus, and sometimes even in the superficial white matter. The explanation for this minor dissimilarity may be an overestimation of these fibers by DTI fiber tracking, the limitations of the fiber dissection technique, or a combination of both factors. As previously mentioned, short transverse sulci, variable in depth, may be present at the level of T1, giving the gyrus a partially discontinuous appearance (Fig.1). Deep to those sulci, short ‘U’ association fibers have been encountered. Since DTI detects the preferential direction of the diffusion of water molecules, sites of antero-posterior diffusion in the superficial white matter may be depicted on the eigenvector maps and contaminate the image of the adjacent long association bundle.
The major disagreement between our results and those obtained with DTI is the posterior termination of the MdLF. While DTI suggests that it connects the temporal lobe to the posterior portion of the AG, the present study did not provide evidence for that in humans. However, the white matter of the AG received fibers coming from the SLF/FA, which are numerous even in its most posterior portions. The horizontal fibers running deep to SLF/AF did not change their trajectory at that point to enter the AG. Instead, they continued further posteriorly and reached the cerebral cortex just caudal to the IPL, at the level of both the dorsal portions of the occipital lobe and the inferior lip of the parieto-occipital arcus.
The explanation for this may involve the particular three-dimensional organization of fiber groups in this area. The presence of a highly developed and thick SLF/AF complex in humans may have shifted caudally the point where horizontal fibers incline laterally. In addition, the performance of DTI is limited by a decrease in anisotropy as a given fascicle becomes close to the cerebral cortex. Thus, its fibers can hardly be followed up to the cortical gray matter, reflecting the relative lack of resolution currently available in the majority of MRI machines. As a consequence, superposition of the extremity of a tracked bundle with a given cerebral gyrus does not necessarily imply that its cortical destination is being depicted.
Functional considerations
On the basis of the location and trajectory described with DTI, it was suggested that MdLF might participate in attention-processing and be the principal conduit of linguistic information in the dominant hemisphere (Makris et al., 2008). Based on our findings, we may suppose that if it does have any role in language-processing implicating the AG, this will probably imply short association pathways between the MdLF terminations and the IPL.
However, in a recent study using intraoperative subcortical electrostimulation in the language dominant hemisphere (De Witt et al., 2010), no language interference could be induced in the topography of the anterior segment of the MdLF. In addition, despite resection of a large anterior portion of the MdLF in eight patients, no permanent language deficits were produced. As a consequence, it was postulated that the MdLF may participate but does not seem essential for language processing, and at least its anterior portion may be safely removed. In such a case, language processing would still be adequately assured by other association tracts such as the SLF/AF complex and the iFOF.
Current methodological difficulties in the study of fiber bundles in the human brain
Both fiber dissection and DTI present limitations. Very sparse and delicate groups of fibers may be difficult to dissect and be partially destroyed when the neighboring white matter is removed. Imaging techniques are not affected by this problem. However, they are susceptible to noise, resolution problems, contamination from adjacent bundles, and abrupt changes in fiber direction. More information may come from further studies and from other techniques such as direct subcortical stimulation, and from the advancement of neuroimaging methods. We consider that these techniques are highly complementary and together have the potential to help neuroscientists understand the hodological organization of cerebral white matter.
Conclusions
On the basis of our findings, the fiber dissection technique provides evidence of the presence of a long horizontal association bundle located in the white matter immediately medial to the arcuate fasciculus and extending to the superior temporal gyrus. At this level, these fibers occupy the most lateral layer of the stratum sagittale. They partially cover the inferior fronto-occipital fasciculus, which is situated deeper and slightly inferiorly. While MdLF fibers continue in a relatively superficial level to reach anterior portions of the temporal lobe, the inferior fronto-occipital fasciculus penetrates the deep temporal white matter to reach the insular lobe.
Although diffusion tensor imaging suggests a posterior termination in the caudal portion of the angular gyrus, this was not confirmed by the present study. These long association fibers continue further posteriorly to upper portions of the occipital lobe. Further studies are needed to confirm these findings and to better understand the exact role of these fibers in brain function.
Acknowledgements
We would like to express our heartfelt thanks to the individuals who donated their bodies to education and research at the Faculté de Médecine de l'Université Montpellier I and the Faculté de Médecine de Université François Rabelais de Tours. We acknowledge Professor François Bonnel and Dr. Guillaume Captier, from the Laboratory of Human Anatomy at Montpellier, for their constant and enthusiastic motivation, and Professor François Canovas for his administrative assistance. We also would like to thank Frank Meyer and Jean Marc Gory for their help in preparing the anatomic specimens.
Conflict of interest
The authors declare that they do not have any conflict of interest.
References
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Castro I, Cristoph DH, Santos DP, et al. Internal structure of the cerebral hemispheres. An introduction of fiber dissection technique. Arq Neuropsiquiatr. 2005;63:252–258. doi: 10.1590/s0004-282x2005000200011. [DOI] [PubMed] [Google Scholar]
- De Witt HamerP, Moritz-Gasser S, Gatignol P, et al. Is the human left middle longitudinal fascicle essential for language? A brain electrostimulation study. Hum Brain Mapp. 2010;32:962–973. doi: 10.1002/hbm.21082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Miranda JC, Rhoton AL, Alvarez-Linera J, Kakizawa Y, Choi C, de OliveiraEP. Three-dimensional microsurgical and tractographic anatomy of the white matter of the human brain. Neurosurgery. 2008;62:SHC989–1026. doi: 10.1227/01.neu.0000333767.05328.49. Jr ; discussion SHC1026-8. [DOI] [PubMed] [Google Scholar]
- Filler AG, Howe FA, Richards TL, et al. World Intellectual Property Organization. IB Image Neurography and Diffusion Anisotropy Imaging. Seattle: University of Washington; 1993. [Google Scholar]
- Klingler J. Erleichterung der makroskopischen Praeparation des Gehirns durch den Gefrierprozess. Schweiz Arch Neurol Psychiatr. 1935;36:247–256. [Google Scholar]
- Lawes IN, Barrick TR, Murugam V, et al. Atlas-based segmentation of white matter tracts of the human brain using diffusion tensor tractography and comparison with classical dissection. Neuroimage. 2008;39:62–79. doi: 10.1016/j.neuroimage.2007.06.041. [DOI] [PubMed] [Google Scholar]
- Ludwig E, Klingler J. 1956. Atlas cerebri humani. Der innere Bau des Gehirns dargestellt auf Grund makroskopischer Präparate. Anatomisches Institut der Universität Basel. Basel: S. Karger.
- Makris N, Papadimitriou GM, Kaiser JR, et al. Delineation of the middle longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2008;19:777–785. doi: 10.1093/cercor/bhn124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino J, Brogna C, Robles SG, et al. Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex. 2009;46:691–699. doi: 10.1016/j.cortex.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Martino J, Vergani F, Gil Robles S, et al. New insights into the anatomic dissection of the temporal stem with special emphasis on the inferior fronto-occipital fasciculus: implications in surgical approach to left mesiotemporal and temporoinsular structures. Neurosurgery. 2010;66:4–12. doi: 10.1227/01.NEU.0000348564.28415.FA. [DOI] [PubMed] [Google Scholar]
- Mori S. Introduction to Diffusion Tensor Imaging. Elsevier: Boston; 2007. [Google Scholar]
- Seltzer B, Pandya DN. Further observations on parieto-temporal connections in the rhesus monkey. Exp Brain Res. 1984;55:301–312. doi: 10.1007/BF00237280. [DOI] [PubMed] [Google Scholar]
- Sincoff EH, Tan Y, Abdulrauf SI. White matter fiber dissection of the optic radiations of the temporal lobe and implications for surgical approaches to the temporal horn. J Neurosurg. 2004;101:739–746. doi: 10.3171/jns.2004.101.5.0739. [DOI] [PubMed] [Google Scholar]
- Ture U, Yasargil MG, Friedman AH, et al. Fiber dissection technique: lateral aspect of the brain. Neurosurgery. 2000;47:417–426. doi: 10.1097/00006123-200008000-00028. discussion 426–7. [DOI] [PubMed] [Google Scholar]


