Abstract
Background
Over the last 25 years, donor source, conditioning, graft-versus-host disease prevention and supportive care for children undergoing hematopoeitic stem cell transplantation (HSCT) have changed dramatically. HSCT indications for acute lymphoblastic leukemia (ALL) now include high-risk patients in first and subsequent remission. There is a large burden of infectious and pre-HSCT morbidities, due to myelosuppressive therapy required for remission induction. We hypothesized that, despite these trends, overall survival (OS) had increased.
Procedure
A retrospective audit of allogeneic pediatric HSCT for ALL was performed in our institution over 25 years. Outcomes for 136 HSCTs were analyzed in three consecutive 8-year periods (Period 1: 1/1/1984–31/8/1992, Period 2: 1/9/1992–30/4/2001, Period 3: 1/5/2001–31/12/2009).
Results
Despite a significant increase in unrelated donor HSCT, event-free and OS over 25 years improved significantly. (EFS 31.6–64.8%, P = 0.0027; OS 41.8–78.9%, P < 0.0001) Concurrently, TRM dropped from 33% to 5% (P = 0.0004) whilst relapse rate was static (P = 0.07). TRM reduced significantly for matched sibling and unrelated cord blood transplantation (UCT) in Period 3 compared with earlier periods (P = 0.036, P = 0.0098, respectively). Factors leading to improved survival in patients undergoing UCT include better matching, higher total nucleated cell doses, and significantly faster neutrophil engraftment. Length of initial HSCT admission was similar over time.
Conclusion
EFS and OS have increased significantly despite heightened HSCT complexity. This survival gain was due to TRM reduction. Contemporary patients have benefited from refined donor selection and improved supportive care. Overall rates of leukemic relapse post-HSCT are unchanged, and remain the focus for improvement.
Keywords: hematopoeitic stem cell transplant, lymphoblastic leukemia, outcomes, pediatric acute survival, transplant-related mortality
INTRODUCTION
Refinements in pediatric acute lymphoblastic leukemia (ALL) treatment from collaborative clinical trials have achieved cure rates of 85% [1] and 5-year event-free survival (EFS) of 80% [2]. Relapsed leukemia remains the commonest cause of pediatric cancer death.
Allogeneic hematopoeitic stem cell transplantation (HSCT) in pediatric ALL is considered using clinical and laboratory features such as length of first remission in relapsed patients and minimal residual disease (MRD) in first remission [3]. MRD monitoring has facilitated risk stratification and treatment selection [3–8]. Allogeneic HSCT indications are evolving [9,10], as survival with chemotherapy-only treatment improves [3,4,9,11].
Donor sources for HSCT have changed over the last 25 years. A matched sibling donor (MSD) was historically preferred over unrelated donors, due to perceived transplant-related mortality (TRM) risk [12–14]. Limited MSD availability has prompted use of alternate donors, including family-related donors (FRD), matched unrelated donors (MUD), and matched unrelated cord blood transplants (UCT). UCT is increasingly used, due to factors including wider range of compatible donors and ease of availability [15]. In childhood ALL, alternate donor HSCTs comprise 61% of allografts (1999–2002) [14] whereas prior to 1996, MSD accounted for 70% of HSCT [14]. UCT now accounts for one-third of HSCT for acute leukemia [16].
With the advent of alternate donor HSCT, TRM risk has increased [17]. Audits of adult patients demonstrate this [17,18]. In addition, relapsed patients often require greater myelosuppression to achieve pre-HSCT remission [16,18–20], with consequences of increased pre-HSCT comorbidities and infective burden. Despite a higher recent predicted TRM, one study involving adults showed significant improvement in survival and reduction in overall mortality, including non-relapse and relapse mortality [18]. TRM figures for pediatric HSCT, including ALL patients, are 5–10% for MSD [3,21,22], 8–24% for MUD [3,6,21,22], and over 20% for mismatched donors [3,21,23]. Earlier TRM figures for unrelated donor HSCT in pediatric ALL were 16–42% [22,24,25].
Although there are general trends of improved overall survival (OS) and reduced TRM [17,26,27] following HSCT, few studies have discussed temporal trends in pediatric ALL HSCT [16,22,25]. We analyzed our experience with pediatric HSCT between 1984 and 2009 in ALL. We hypothesized that OS in our allogeneic HSCT cohort had improved, despite increase in unrelated donor HSCT.
PATIENTS AND METHODS
Data Collection
A retrospective review of 136 consecutive allogeneic HSCT for ALL at Sydney Children’s Hospital was conducted. Data from 1/1/1984 to 31/12/2009 were analyzed from the Cord & Marrow Transplant database at Sydney Children’s Hospital. Comprehensive data were collected prospectively in the Transplant database with internal and external audits to assess data accuracy. The data set was censored at 31/12/2010. Data for survivors were censored at date of last follow-up. This study received institutional ethics committee approval.
The study period was divided into three time frames (Period 1: 1/1/1984–31/8/1992, Period 2: 1/9/1992–30/4/2001, Period 3: 1/5/2001–31/12/2009) and HSCT outcomes analyzed. Time frames reflected changes in choice of alternate donors, with predominant use of FRD in Period 1, MUD in Period 2 and UCT in Period 3. Similar numbers of HSCT were performed in each period (Table I).
TABLE I.
Characteristics of Each Period
| Period 1 1/1/1984–31/8/1992 | Period 2 1/9/1992–30/4/2001 | Period 3 1/5/2001–31/12/2009 | P-value | |
|---|---|---|---|---|
| Number of HSCTa | 41 | 48 | 47 | |
| Number of patients | 40 | 46 | 44 | |
| Baseline characteristics | ||||
| Males | 30 (73.2%) | 35 (72.9%) | 31 (66%) | 0.7 |
| Median age (months) at HSCT (range) | 117 (9–235) | 97 (10–206) | 108 (17–221) | 0.48 |
| Median time (months) from diagnosis to HSCT (range) | 36 (4–114) | 34 (5–122) | 23 (4–80) | <0.01 |
| Remission status | ||||
| CR1 | 4 (9.8%) | 4 (8.3%) | 15 (31.9%) | 0.003 |
| CR2 | 23 (56.1%) | 35 (72.9%) | 25 (53.2%) | 0.108 |
| CR3 | 12 (29.3%) | 7 (14.6%) | 5 (10.6%) | 0.058 |
| CR4 | 1 (2.4%) | 1 (2.1%) | 0 | 0.59 |
| Not documented | 0 | 0 | 2 (4.3%) | N/Ah |
| Persistent disease | 1 (2.4%) | 1 (2.1%) | 0 | N/Ah |
| Radiotherapy administered prior to HSCT conditioning | 12 (29.3%) | 11 (22.9%) | 1 (2.13%) | <0.01 |
| Graft type | ||||
| MSDb | 27 (65.9%) | 20 (41.7%) | 15 (31.9%) | 0.02 |
| UCTc | 0 | 8 (16.7%) | 21 (44.7%) | <0.01 |
| MUDd | 2 (4.9%) | 12 (25%) | 10 (21.3%) | 0.033 |
| FRDe (Haplo/MMSDf) | 12 (29.3%) | 8 (16.7%) | 1 (2.1%) | 0.002 |
| T cell depletion | 8 (19.51%) | 16 (33.3%) | 10 (21.3%) | 0.25 |
| Conditioning type | ||||
| TBI-based conditioning | 15 (36.6%) | 31 (64.6%) | 45 (95.7%) | <0.01 |
| Chemotherapy alone | 26 (63.4%) | 17 (35.4%) | 2 (2.1%) | <0.01 |
| CMV status (donor/recipient) | ||||
| D−/R−g | 16 (39%) | 14 (29.2%) | 25 (53.2%) | 0.06 |
| D−/R+ | 3 (7.3%) | 8 (16.7%) | 11 (23.4%) | 0.125 |
| D+/R+ | 10 (24.4%) | 16 (33.3%) | 5 (10.6%) | 0.03 |
| D+/R− | 9 (22%) | 9 (18.8%) | 6 (12.8%) | 0.52 |
| Not tested | 3 (7.3%) | 1 (2.1%) | 0 | N/Ah |
Hematopoeitic stem cell transplant
Matched sibling donor
Umbilical cord blood transplant
Matched unrelated donor
Family-related donor
Mismatched sibling donor
D−/R− denotes that pre-transplant CMV status is negative for donor, negative for recipient
P-value not generated as numbers for analysis are too small.
Patients who received HSCT in CR1 fulfilled high-risk or very high-risk features on ALL protocols used. Risk stratification was based on Berlin–Frankfurt–Munster (BFM) criteria, and incorporated MRD criteria in Period 3.
Conditioning regimens varied according to transplant period (Table I). There was a significant increase in TBI use (P < 0.01), with predominantly TBI-based conditioning in Period 3. Four reduced-intensity transplants were performed in Period 3, one upfront and three for post-HSCT relapse. In Period 3, 12.5% of patients who received TBI-based myeloablative conditioning also received a CNS boost. No CNS boost was administered in earlier periods. TBI was generally avoided in those aged under 2 years at time of HSCT. Overall, 89% of chemotherapy-alone conditioning contained cyclophosphamide and busulphan. In Period 1, a regimen of busulphan, cyclosphosphamide, and melphalan was used in 74% of HSCT episodes. The combination of busulphan, cyclosphamide, and etoposide was used in 53% of HSCT in Period 2. Busulphan levels were not obtained. Anti-thymocyte globulin (Pfizer) was used for UCT and MUD HSCT at 12 mg/kg/dose for three doses, including T-cell-depleted MUD HSCT. Anti-thymocyte globulin was prophylaxis for rejection and graft-versus-host disease (GVHD).
Graft characteristics are shown in Table I. The majority of patients who underwent MUD HSCT received bone marrow. Tissue typing and HLA matching were performed by the Australian Bone Marrow Donor Registry (ABMDR) using established international methods [28,29]. High-resolution typing for DRB1 has been used since the late 1990s and high-resolution typing for Class 1 allele (HLA-ABC) testing has been routine in recent years [22]. UCT were 4/6 matches or better (Table III). FRD were mostly one to two antigen mismatches, and included mismatched siblings, parents or relatives. In Period 1, one FRD was a haploidentical parent.
TABLE III.
UCT Matching, TNC, and Engraftment
| Period 1a | Period 2b | Period 3c | Total | ||
|---|---|---|---|---|---|
| Total allogeneic transplants | 41 | 48 | 47 | 136 | P-value |
| UCTd | 0 | 8 | 21 | 29 | <0.001 |
| 4/6 match | 4 (50%) | 2 (10%) | 6 (20.7%) | 0.028 | |
| 5/6 match | 2 (25%) | 11 (52.4%) | 13 (44.8%) | 0.23 | |
| 6/6 match | 2 (25%) | 4 (19%) | 6 (20.7%) | 0.75 | |
| Double cord transplante | 0 | 4 (19%) (3 × 5/6 match, 1 × 4/6) | 4 (13.8%) | ||
| Median TNCf (range) | 3.35 (0.6–5.0) | 4.7 (0.5–8.4) | 4.7 (0.5–8.4) | 0.08 | |
| Median neutrophil engraftment (days, range) | 25.5 (18–34) | 16 (6–33) | 20 (6–34) | 0.014 | |
| Median platelet engraftment (days, range) | 60.5 (19–84) | 40.5 (23–91) | 46 (19–91) | 0.64 | |
| Median hospital days (range) | 41 (29–87) | 45 (29–80) | 42 (29–87) | 0.16 |
Period 1: 1/1/1984–31/8/1992
Period 2: 1/9/1992–30/4/2001
Period 3:1/5/2001–31/12/2009
Umbilical cord blood transplant
Matching for double cord transplants was according to the least matched cord
Total nucleated cells × 107/kg.
Supportive care practices in our unit varied prior to 2004. We used graft support with colony-stimulating factor from 1991 for unrelated donor HSCT. Cyclosporine was the consistent backbone of GVHD prophylaxis. For patients receiving MSD HSCT after 2004, cyclosporine and methotrexate were given as GVHD prophylaxis. Patients transplanted using single UCT prior to 2004 received cyclosporine, methylprednisone, and methotrexate for GVHD prophylaxis, whereas only cyclosporine and methylprednisone were used after 2004. Cyclosporine and mycophenolate were used for GVHD prophylaxis in patients receiving double UCT. For patients undergoing MUD HSCT prior to 2000, cyclosporine and red cell E-rosette were used for GVHD prophylaxis, whilst cyclosporine and CD34 selection together were used after 2000. Penicillin prophylaxis was used in Period 3 for patients with GVHD.
In Period 3, patients who were CMV IgG positive received ganciclovir therapy (pre-HSCT and post-engraftment) and weekly CMV immunoglobulin to Day +100. Qualitative CMV PCR monitoring was routine from 2004 and quantitative PCR from 2008. PJP (Pneumocystis jirovecii pneumonia) prophylaxis included routine pentamidine (since 2004) and cotrimoxazole post-engraftment. Patients who were HSV IgG positive received aciclovir. Additional changes from 2004 were routine antifungal prophylaxis—fluconazole for standard risk and liposomal amphotericin or voriconazole for patients at high risk of fungal disease; routine ursodeoxycholic acid for veno-occlusive disease prophylaxis [30], with additional prophylactic defibrotide for high-risk patients [31]. Features indicating higher VOD risk included heavily pre-treated patients, prior HSCT, underlying liver dysfunction, hepatic iron overload, and causative drugs [32].
Statistical Analysis and Definitions
OS, EFS, leukemia-free survival (LFS), TRM, and cumulative incidence of relapse were analyzed from time of HSCT. Kaplan–Meier survival curves were constructed [18,33] and sub-analyses performed to compare survival according to graft type, period of HSCT, and remission status. Survival outcomes for Periods 1 and 2 were not statistically different and were combined for analysis. Each HSCT episode was analyzed separately for survival and TRM.
An event was defined as relapse, TRM, death from any cause or second malignancy. TRM was defined as death due to complication (other than relapse) following HSCT. LFS was defined as alive, without evidence of leukemia, or leukemia-free death. A second malignancy was defined as malignancy other than the primary leukemia, occurring post-HSCT.
Viral, bacterial and fungal infections were analyzed as recorded on the Transplant database. Neutrophil engraftment was defined as the first of three consecutive days of ANC ≥0.5 × 109/L. Platelet engraftment was defined as unsupported platelet count >20 × 109/L. Acute GVHD (aGVHD) was graded according to the Glucksberg severity scale [34]. Chronic GVHD was GVHD occurring after Day +100 [34].
Statistical analysis was performed using PRISM (Prism 5, GraphPad Software, Inc. 2005–2010). Two-sided P values (Mantel–Cox Log Rank) were significant if below 0.05 and 95% confidence intervals used. PASW Version 18 software was used for variance analysis (ANOVA, t tests, Kruskall Wallis chi-squared tests, Cox-regression).
RESULTS
Patient Population
An overview of HSCT characteristics and outcomes is shown in Tables I and II, respectively. The groups had similar pre-HSCT characteristics, apart from remission status and time from diagnosis to HSCT. There was a significant increase in patients transplanted in CR1 in Period 3 (P = 0.003). This increase reflected use of HSCT for patients with high-risk disease based on MRD. The time from diagnosis to transplant was shorter in Period 3 compared to earlier periods (P < 0.01). There was no difference in time from diagnosis to HSCT for patients transplanted in ≥CR2 over 25 years (P = 0.07). There was no increase in patients being transplanted with persistent disease.
TABLE II.
Outcomes
| Period 1b | Period 2c | Period 3d | ||
|---|---|---|---|---|
| Number of HSCTa | 41 | 48 | 47 | P-valuee |
| 5-Year OS (%) | 35.70% | 46.80% | 78.90% | <0.0001 |
| Median survival (months) post-HSCT in months (range) | 19 (1–312) | 20 (0–198) | 29 (2–108) | 0.04 |
| TRM | 13 (31.7%) | 15 (31.3%) | 2 (4.3%) | 0.0004 |
| Relapses post-HSCT | 16 (39%) | 16 (33.3%) | 12 (25.5%) | 0.207 |
| Median time (months) to relapse (range) | 7 (3–47) | 6 (1–42) | 15 (4–30) | 0.61 |
| Second malignancy | 1 (2.4%) | 2 (4.2%) | 1 (2.1%) | 0.69 |
| 5-Year LFSf | 49.60% | 56.60% | 68.20% | 0.07 |
| 5-Year EFS | 31.60% | 40.40% | 64.80% | 0.0005 |
| Acute GVHD | ||||
| Grade 2–4 | 16 (39.0%) | 11 (22.95%) | 18 (38.3%) | 0.35 |
| Grade 3–4 | 10 (24.4%) | 7 (14.6%) | 9 (19.1%) | 0.99 |
| Grade 4 | 3 (7.3%) | 3 (6.3%) | 3 (6.4%) | 0.94 |
| Chronic GVHD | 5 (12.2%) | 5 (10.4%) | 6 (12.8%) | 0.79 |
| Engraftment (days) | ||||
| Median neutrophil engraftment (range) | 17 (8–28) | 20 (10–42) | 18 (6–35) | 0.68 |
| Median platelet engraftment (range) | 22 (11–75) | 26 (12–95) | 29 (15–91) | 0.30 |
| Hospital inpatient days (range) | 30 (16–137) | 30 (14–87) | 34 (25–80) | 0.92 |
Haematopoietic stem cell transplant
Period 1: 1/1/1984–31/8/1992
Period 2: 1/9/1992–30/4/2001
Period 3:1/5/2001–31/12/2009
P value is for Period 1 and 2 combined as compared to Period 3
Leukemia-free survival.
Overall median follow-up time for survivors was 75 months (range 4–312 months). Median follow-up for Period 1 was 191 months (range 15–312 months), Period 2 was 133 months (58–198 months), Period 3 was 45 months (4–108 months). Those who died had a median survival of 9 months (range 1–97 months) in Period 1, 4 months (range 0–45 months) in Period 2, and 21 months (range 2–27 months) for Period 3.
HSCT Characteristics
There were 41 allogeneic HSCT for 40 patients in Period 1. One patient received a second allogeneic HSCT, following relapse post-HSCT. There were 48 HSCT episodes for 46 patients in Period 2. This included two patients who received a second allogeneic HSCT, one for graft failure and the other for relapse post-HSCT. In Period 3, there were 47 HSCT episodes and 44 patients. Subsequent transplants in Period 3 were for relapse post-HSCT. Where a patient received a subsequent HSCT, this was performed in the same period as first HSCT. There were three graft failures, all in patients receiving FRD HSCT (one in Period 1, two in Period 2).
Donor selection changed over time with significant decrease in MSD (P = 0.02) and FRD grafts (P = 0.002), and significant increase in UCT (P < 0.01) and MUD HSCT (P = 0.033). In Period 3, mostly UCT or MSD HSCT were performed (Table I). Four double-cord UCT were performed, all in Period 3 (Table III). There were fewer CMV D+/R+ pairs over time (P = 0.03) and a trend towards more CMV D−/R− pairs in Period 3 (Table I).
There was no difference in median neutrophil and platelet engraftment between periods for the entire group (Table II). Graft subanalysis showed median neutrophil engraftment for UCT in Period 3 was significantly faster than Period 2 (Table III). UCT matching improved over time, with a greater percentage of ≥5/6 matched cords between Period 2 and 3 (Table III). In Period 3 UCT, there was a trend towards higher median total nucleated cell doses, although numbers are small (Table III).
There was no significant difference in speed to engraftment for other grafts over time (Table IV). For the 25-year period, median platelet engraftment was significantly longer for UCT (46 days, range 19–91 days) compared to other grafts (P < 0.001). Engraftment times are listed in Table III (UCT) and Table IV (other grafts).
TABLE IV.
Infections (All Grafts) and Engraftment (Other Than Cords)
| Period 1b | Period 2c | Period 3d | Total | ||
|---|---|---|---|---|---|
| Total HSCTa | 41 | 48 | 47 | 136 | P-value |
| Confirmed infectionse (total number, % of HSCT) | |||||
| Bacterial | 13 (31.7%) | 15 (31.3%) | 30 (63.8%) | 58 (42.7%) | 0.001 |
| Viral (except for CMV) | 11 (26.8%) | 8 (16.7%) | 19 (40.4%) | 38 (27.9%) | 0.035 |
| CMV | 5 (12.2%) | 7 (14.5%) | 7 (15%) | 19 (14%) | 0.927 |
| Fungal | 3 (7.3%) | 6 (12.5%) | 8 (17%) | 17 (12.5%) | 0.395 |
| PJPf | 0 | 3 (6.3%) | 1 (2.1%) | 4 (2.9%) | 0.206 |
| Outcomes by Graft type (days) | Period 1 | Period 2 | Period 3 | Total | P-value |
|---|---|---|---|---|---|
| MSDg | n = 27 | n = 20 | n = 15 | n = 62 | |
| Median neutrophil engraftment (range) | 17 (8–28) | 20 (13–42) | 19 (12–35) | 19 (8–42) | 0.14 |
| Median platelet engraftment (range) | 21 (12–75) | 24 (15–90) | 20.5 (17–34) | 21 (12–90) | 0.63 |
| Median hospital days (range) | 27 (14–147) | 27 (16–147) | 27 (14–87) | 27 (14–147) | 0.969 |
| FRDh | n = 12 | n = 8 | n = 1 | n = 21 | |
| Median neutrophil engraftment (range) | 16 (8–28) | 15 (13–22) | N/A | 16 (8–28) | 1 |
| Median platelet engraftment (range) | 26.5(21–56) | 25 (24–33) | N/A | 25.5 (17–56) | 1 |
| Median hospital days (range) | 62 (19–93) | 30 (23–78) | N/A | 38 (19–93) | 0.052 |
| MUDi | n = 2 | n = 12 | n = 10 | n = 24 | |
| Median neutrophil engraftment (range) | 13.5 (11–16) | 20(10–25) | 12.5(10–28) | 15.5 (10–28) | 0.231 |
| Median platelet engraftment (range) | 11.5 (11–12) | 23 (12–95) | 17 (15–34) | 19 (11–95) | 0.22 |
| Median hospital days (range) | 40 (33–46) | 30 (21–67) | 34 (25–48) | 33 (21–67) | 0.45 |
Hematopoeitic stem cell transplant
Period 1: 1/1/1984–31/8/1992
Period 2: 1/9/1992–30/4/2001
Period 3:1/5/2001–31/12/2009
Several infections were often documented in the one patient
Pneumocystis jirovecii pneumonia
Matched sibling donor
Family-related donor
Matched unrelated donor.
Clinical Outcomes
Survival for 25-year period
Overall, 5-year EFS was 45.8%, 5-year OS 53.9%, and 5-year LFS 58.8% (Supplementary Fig 1). TRM was 22.1%. Corresponding survival figures according to HSCT period are listed in Table II. Survival outcomes over 25 years, stratified for remission status, are depicted in Supplementary Fig 1. There was no significant difference in 5-year OS between patients transplanted in CR1 and those transplanted in ≥CR2 (66% vs. 51.2%, P = 0.25). TRM for patients in CR1 was not significantly different over 25 years compared to ≥CR2 (9% vs. 26.2%, P = 0.14).
Figure 1.
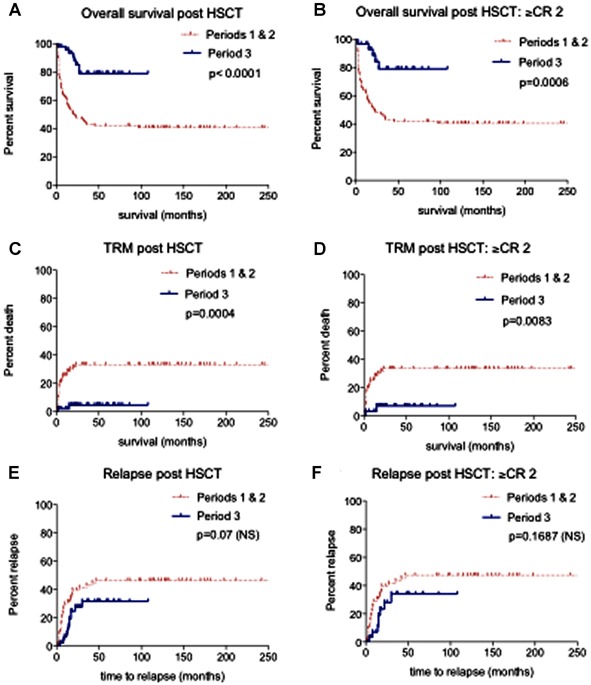
Significant recent improvement in survival and reduced TRM. Periods 1 and 2: 1/1/1984–30/4/2001 (n = 89), Period 3: 1/5/2001–31/12/2009 (n = 47). A and B: Overall survival post HSCT for Periods 1 and 2 compared to Period 3, for all patients (A) and for patients in ≥CR2 (B); C and D: TRM post HSCT for Period 1 and 2 compared to Period 3, for all patients (C) and for patients in ≥CR2 (D); E and F: Relapse post HSCT for Period 1 and 2 compared to Period 3, for all patients (E) and for patients in ≥CR2 (F).
Survival
Significant improvements in survival were observed when Periods 1 and 2 combined (1/1/1984–30/4/2001) were compared to Period 3 (1/5/2001–31/12/2009).
EFS
5-year EFS improved significantly over time, from 36.5% (Periods 1 and 2) to 64.8% (Period 3; P = 0.0005). When stratified for remission status, 5-year EFS significantly improved for patients in ≥CR2 over time, increasing from 35.9% to 60.6% (P = 0.0093). There was a trend towards improved EFS for patients transplanted in CR2 (P = 0.0598).
OS
There was a significant increase in 5-year OS from 41.8% (Periods 1 and 2) to 79% (Period 3; P < 0.0001). This improvement remained when stratified for remission status. 5-year OS for patients transplanted in CR2 increased from 42.7% (Periods 1 and 2) to 75.4% (Period 3; P = 0.0052) and from 41.7% to 79%, respectively for ≥CR2 (P = 0.0006; Fig 1).
Survival for graft type over 25-year period
Over 25 years, 5-year OS was 53.4% for MSD, 42.9% for FRD, 50.6% for MUD, and 68.4% for UCT. TRM was highest for FRD (39.2%) and ranged between 15% and 25% for other graft types.
Graft survival per period
There was a significant improvement in 5-year OS for patients receiving MSD and UCT over time (Fig 2). Five-year OS for MSD in Periods 1 and 2 was 44.1% versus 85.7% in Period 3 (P = 0.0093). Five-year OS for UCT was 37.5% for Period 2 and 79.3% in Period 3 (P = 0.0030). Significant TRM reduction was seen only in MSD and UCT groups. TRM for patients undergoing MSD HSCT for Periods 1 and 2 was 26.9%, and 0% for Period 3 (P = 0.036). TRM for patients undergoing UCT in Period 2 was 40% and 6.25% for Period 3 (P = 0.0098). In Period 3, there was no significant difference in OS (P = 0.80) and TRM (P = 0.3329) between recipients of MSD and UCT. Comparative 5-year OS for MUD HSCT was 41% for Periods 1 and 2 and 65.6% for Period 3 (P = 0.17).
Figure 2.
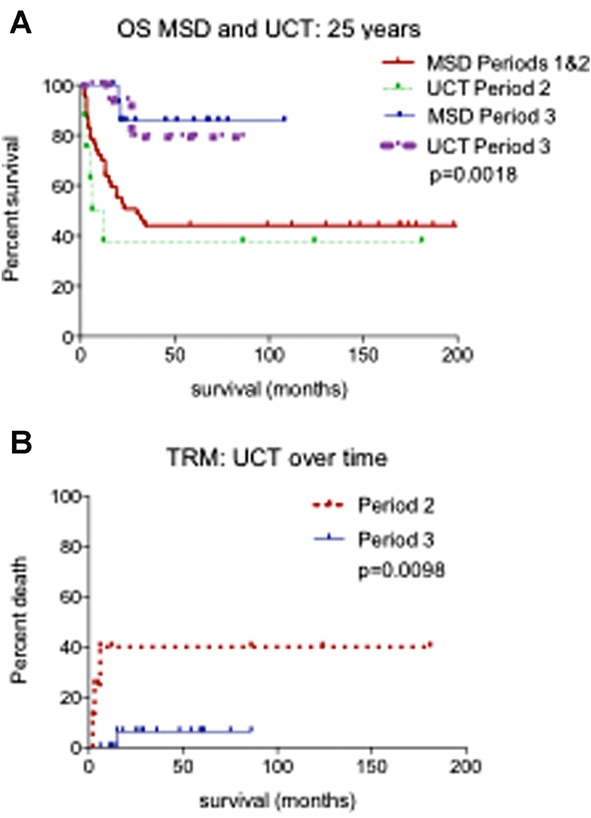
Significant recent survival benefit for MSD and UCT. Periods 1 and 2: 1/1/1984–30/4/2001, Period 3: 1/5/2001–31/12/2009. MSD (matched sibling donor), UCT (umbilical cord blood transplant). A: Overall survival for MSD and UCT cohorts, for Periods 1 and 2 compared to Period 3; B: TRM post UCT for Period 2 compared to Period 3.
TRM
TRM significantly decreased from 33% (Periods 1 and 2) to 5% (Period 3; P = 0.0004). TRM for patients transplanted in CR2 dropped from 31.2% (Periods 1 and 2) to 9.3% for Period 3 (P = 0.0417) and for ≥CR2 from 33.6% to 7.3% (P = 0.0083) (Fig 1).
TRM causes
Deaths from interstitial pneumonitis (IP), infection and GVHD decreased over time. Causes of TRM in Periods 1 and 2 were predominantly IP and GVHD. In Period 1, there were 15 cases of IP, including two CMV and 13 cases with no organism identified (idiopathic). Of these, seven patients died, including both patients with documented CMV. Period 2 also had 15 cases of IP, with five CMV, three PJP, and seven idiopathic. Of these, 10 patients died, including four CMV and three patients with PJP and three idiopathic cases. One patient died from IP in Period 3 (PJP).
GVHD claimed four patients in Period 1 and two patients in Period 2. In Period 1, three patients had multi-organ failure, compared to none in later periods. There were only two TRM deaths in Period 3. There were no deaths from VOD. Where TRM was multi-factorial (five patients in Period 1, three patients in Period 2 and one in Period 3), the main factors were GVHD and infection.
Relapse
The 5-year cumulative incidence of relapse did not vary over time (Periods 1 and 2 vs. Period 3: 46.5% vs. 31.6%, P = 0.07) nor when stratified for remission status. Five-year cumulative incidence of relapse for patients transplanted in CR2 was 50.1% in Periods 1 and 2 compared to 40.8% in Period 3 (P = 0.3229) and for ≥CR2 was 47.2% and 34.1%, respectively (P = 0.1687; Fig 1). There was no difference in LFS stratified by graft type (Supplementary Appendix I).
HSCT-related complications
There were more bacterial and viral (non-CMV) infections post-HSCT in Period 3 (P = 0.001, P = 0.035, respectively; Table IV). CMV and fungal infection rates were unchanged at 15% and 17%, respectively in Period 3 (Table IV).
Despite increased use of unrelated donor HSCT, there was no overall increase in GVHD (Table II). Following UCT, there was significantly more Grade 2–4 aGVHD compared to MUD (P = 0.018) and MSD HSCT (P = 0.046). Incidence of Grade 3–4 aGVHD for UCT was 24.1%, and Grade 2–4 was 51.7%. Equivalent aGVHD figures for other grafts were: MUD Grade 3–4: 12.5%, Grade 2–4: 20.89%; MSD 17.7%, 29%; FRD 23.8%, 33.3%, respectively. Chronic GVHD rates were similar over time (Table II).
Length of stay
There was no difference in length of hospital stay over periods (Tables II and III) or between graft types (P = 0.54).
DISCUSSION
Our data show significant improvement over time in EFS and OS post-HSCT for pediatric ALL, despite significant increase in unrelated donor HSCT. Rates of relapse and major HSCT-related complications, such as severe GVHD, remained steady. The major contributor to improved survival was significant TRM reduction.
We have shown greatest survival and TRM improvements for patients undergoing MSD and UCT. UCT TRM and OS trends over time have not been the focus of previous papers [16,22,35,36]. We observed a significant survival advantage in Period 3 UCT. There are several contributors to this, including better graft selection, with significantly smaller degree of mismatch and trend towards a higher median TNC. This resulted in significantly faster median neutrophil engraftment for Period 3 UCT. Survival benefits of choosing better matched UCT units, with TNC >3 × 107/kg are documented [35]. Total nucleated cell dose alone did not account for the survival benefit in UCT in our cohort, as there was no difference between cell doses for survivors and those who died (median 4.7 × 107/kg vs. 3.9 × 107/kg, P = NS).
Our HSCT outcomes for ALL compare favorably with national and international studies [12,16,22,37–41]. Despite a significant shift towards alternate donor HSCT, overall engraftment times and length of initial hospital stay were unchanged. There was a significant increase in bacterial and viral (non-CMV) infections post-HSCT over time, but a decrease in IP. Discharge criteria remained the same over time. This suggests we are managing treatment-related toxicities with well-directed supportive care strategies. The increased incidence of bacterial and viral (non-CMV) infections probably reflects increased use of immunosuppressive therapies in Period 3, and improved testing modalities. CMV, PJP, and fungal infection incidence is unchanged over time, possibly reflecting disease prophylaxis in Period 3.
Our data show that contemporary HSCT outcomes are significantly improved and that outcomes for patients undergoing MSD and UCT are equivalent. This is consistent with previous literature [13]. Retrospective studies showed equivalent outcomes for MUD and UCT [35,42]. One recent study suggested MSD might be superior to alternate donor HSCT; however UCT data were not analyzed [21].
We postulate that improved OS by way of reduced TRM is due to multiple factors. These are likely to include selection of better matched UCT units with higher TNC doses [43] resulting in faster neutrophil engraftment times, a shorter time to transplant [27], better supportive care practices, availability of broader antimicrobials, improved prevention of and monitoring for CMV infection [44] and aggregate experience caring for HSCT patients [26]. Improvements in HSCT care have resulted in survival benefits for contemporary patients undergoing HSCT for high-risk ALL [45]. Units subjected to external audit have improved post-HSCT outcomes [46]. We expected to see improvement in MUD outcomes, due to introduction of high-resolution typing [3,14,28,47], but small numbers may have limited analysis.
The significant increase in patients transplanted in CR1 in Period 3 is not responsible for change in survival and TRM outcomes, as these significant improvements were also observed for patients transplanted in ≥CR2. Pre-HSCT status of patients transplanted in ≥CR2 was probably similar over time, as their median time to HSCT was unchanged over time.
Published studies, not confined to ALL in children, show reduced TRM over time [17,18,26,27]. Results are conflicting regarding TRM trends over time in pediatric ALL HSCT. Two publications reported significant improvement in OS and reduced TRM [16,22]. Another found no difference over time for OS post-unrelated HSCT, but did not discuss TRM trends for year of transplant [25].
This study was a single-center, retrospective analysis, with relatively small patient numbers. A strength of the study is uniform transplant practice since 2004, which represents the majority of Period 3. There was considerable heterogeneity in transplant practices in Periods 1 and 2. Uniform reporting of pre-HSCT performance scores would have provided additional comparison, although a surrogate marker, such as time from diagnosis to HSCT, has shown no change over time for patients transplanted in ≥CR2.
The incidence of aGVHD (Grades 2–4) was equivalent to retrospective series that included pediatric ALL [22,40,48]. Rates of aGHVD (Grade 3–4; 19.9%) were consistent with one prospective UCT study [43], but higher than another of MSD and MUD HSCT [3]. The higher proportion of UCT in our study likely contributed to this result. Higher aGVHD rates in UCT, as compared to other donor sources in our series, did not increase TRM or reduce relapse rates.
Post-HSCT relapse rates in our study, consistent with published ranges (18–37%) [3] were stable. Median follow-up times, including for Period 3 (median 45 months) were sufficient for detection of most relapses [20,27]. Although there was significant increase in TBI use in Period 3, this did not result in improved leukemic control, as rates of relapse were unchanged. Some pediatric ALL studies demonstrate reduction in post-HSCT relapse [27], while others do not [16,20,22,25,49]. The impact of increased numbers of patients in CR1 being transplanted on relapse rates is unclear. Taken together, this indicates that strategies to reduce post-HSCT relapse remain a priority.
Further studies using MRD may help determine extent of pre-HSCT cytoreduction [16], and help assess if contemporary patients in CR2 have more treatment-resistant disease [16,25] or increased risk of post-HSCT relapse [20,49]. Pre-transplant MRD >10−3 and disease status at transplant are the strongest predictors of relapse [4–8,13]. Additional factors may lead to increased relapse [42,43,50], including novel single nucleotide polymorphisms [51] and CMV D−/R− status [48].
Discovery of new cytoreductive agents [52,53] and establishing therapeutic pathways for post-HSCT MRD monitoring [4] are important. Prospective randomized pediatric HSCT studies need to compare UCT with both MSD and MUD outcomes, and HSCT versus chemotherapy-alone regimens. Validated pre-HSCT algorithms that incorporate performance scores and estimate TRM are required to assist in physician decision-making [17,25,54].
We found that contemporary outcomes for HSCT for pediatric ALL are significantly improved, due to several mechanisms, despite increased use of unrelated donors. TRM decreased significantly over 25 years. Relapse rates are static. International collaboration is required to target and develop novel treatment strategies for patients at high risk of relapse post-HSCT.
Acknowledgments
Dr. Marion Mateos completed a Clinical Haematology/Oncology Fellowship between 2010 and 2012 funded by the Sydney Children’s Hospital Foundation. The authors would like to acknowledge Professor Marcus Vowel’s previous clinical leadership, Transplant Clinical Nurse Consultants Lucy Maurice and Anne Senner, patients and families, and all team members who provided care for these patients.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site.
Survival over 25 years stratified for remission status.
REFERENCES
- 1.Hunger S, Raetz E, Loh M, et al. Improving outcomes for high-risk ALL: Translating new discoveries into clinical care. Pediatr Blood Cancer. 2011;56:984–993. doi: 10.1002/pbc.22996. [DOI] [PubMed] [Google Scholar]
- 2.Pui C, Carroll W, Meshinchi S, et al. Biology, risk stratification, and therapy of pediatric acute leukemias: An update. J Clin Oncol. 2011;29:551–565. doi: 10.1200/JCO.2010.30.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pulsipher M, Peters C, Pui C. High-risk pediatric acute lymphoblastic leukemia. To transplant or not to transplant. Biol Blood Marrow Transplant. 2011;17:s137–s148. doi: 10.1016/j.bbmt.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pulsipher M, Bader P, Klingebiel T, et al. Allogeneic transplantation for pediatric acute lymphoblastic leukemia: The emerging role of peritransplantation minimal residual disease chimerism monitoring and novel chemotherapeutic molecular, and immune approaches aimed at preventing relapse. Biol Blood Marrow Transplant. 2009;15:62–71. doi: 10.1016/j.bbmt.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Bader P, Kreyenberg H, Henze G, et al. Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia; The ALL-REZ BFM Study Group. J Clin Oncol. 2009;27:377–384. doi: 10.1200/JCO.2008.17.6065. [DOI] [PubMed] [Google Scholar]
- 6.Bader P, Hancock J, Kreyenberg H, et al. Minimal residual disease (MRD) status prior to allogeneic stem cell transplantation is a powerful predictor for post-transplant outcome in children with ALL. Leukemia. 2002;16:1668–1672. doi: 10.1038/sj.leu.2402552. [DOI] [PubMed] [Google Scholar]
- 7.Knechtli C, Goulden N, Hancock J, et al. Minimal residual disease status before allogeneic bone marrow transplantation is an important determinant of successful outcome for children and adolescents with acute lymphoblastic leukemia. Blood. 1998;92:4072–4079. [PubMed] [Google Scholar]
- 8.Szczepanski T, Orfao A, van der Velden V, et al. Minimal residual disease in leukaemia patients. Lancet Oncol. 2001;2:409–417. doi: 10.1016/s1470-2045(00)00418-6. [DOI] [PubMed] [Google Scholar]
- 9.Parker C, Waters R, Leighton C, et al. Effect of mitoxantrone on outcome of children with first relapse of acute lymphoblastic leukaemia (ALL R3): An open-label randomised trial. Lancet. 2010;376:2009–2017. doi: 10.1016/S0140-6736(10)62002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tallen G, Ratei R, Mann G, et al. Long-term outcome in children with relapsed acute lymphoblastic leukemia after time-point and site-of-relapse stratification and intensified short-course multidrug chemotherapy: Results of the trial ALL-REZ BFM 9. J Clin Oncol. 2010;28:2339–2347. doi: 10.1200/JCO.2009.25.1983. [DOI] [PubMed] [Google Scholar]
- 11.Schultz KR, Bowman WP, Aledo A, et al. Improved early event-free survival with imatinib in Philadelphia chromosome positive acute lymphoblastic leukemia: A Children’s Oncology Group Study. J Clin Oncol. 2009;27:5175–5181. doi: 10.1200/JCO.2008.21.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peters C, Schrauder A, Schrappe M, et al. Allogeneic haematopoeitic stem cell transplantation in children with acute lymphoblastic leukaemia: The BFM/IBFM/EBMT concepts. Bone Marrow Transplant. 2005;35:s9–s11. doi: 10.1038/sj.bmt.1704835. [DOI] [PubMed] [Google Scholar]
- 13.Eapen M, Rubinstein P, Zhang M, et al. Comparable long-term survival after unrelated and HLA-matched sibling donor hematopoeitic stem cell transplantations for acute leukemia in children younger than 18 months. J Clin Oncol. 2006;24:145–151. doi: 10.1200/JCO.2005.02.4612. [DOI] [PubMed] [Google Scholar]
- 14.Lanino E, Sacchi N, Peters C, et al. Strategies of the donor search for children with second CR ALL lacking a matched sibling donor. Bone Marrow Transplant. 2008;41:S75–S79. doi: 10.1038/bmt.2008.59. [DOI] [PubMed] [Google Scholar]
- 15.Rocha V, Locatelli F. Searching for alternative hematopoeitic stem cell donors for pediatric patients. Bone Marrow Transplant. 2008;41:207–214. doi: 10.1038/sj.bmt.1705963. [DOI] [PubMed] [Google Scholar]
- 16.MacMillan M, Davies S, Nelson G, et al. Twenty years of unrelated donor bone marrow transplantation for pediatric acute leukemia facilitated by the National Marrow Donor Program. Biol Blood Marrow Transplant. 2008;14:16–22. doi: 10.1016/j.bbmt.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 17.Gratwohl A, Stern M, Brand R, et al. Risk score for outcome after allogeneic hematopoeitic stem cell transplantation: A retrospective analysis. Cancer. 2009;115:4715–4726. doi: 10.1002/cncr.24531. [DOI] [PubMed] [Google Scholar]
- 18.Gooley T, Chien J, Pergam S, et al. Reduced mortality after allogeneic hematopoeitic-cell transplantation. N Engl J Med. 2010;363:2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slats A, Egeler R, van der Does-van denBerg A, et al. Causes of death—Other than progressive leukemia—In childhood acute lymphoblastic leukemia (ALL) and myeloid leukemia (AML): The Dutch Childhood Oncology Group experience. Leukemia. 2005;19:537–544. doi: 10.1038/sj.leu.2403665. [DOI] [PubMed] [Google Scholar]
- 20.Woolfrey A, Anasetti C, Storer B, et al. Factors associated with outcome after unrelated marrow transplantation for treatment of acute lymphoblastic leukemia in children. Blood. 2002;99:2002–2008. doi: 10.1182/blood.v99.6.2002. [DOI] [PubMed] [Google Scholar]
- 21.Shaw P, Kan F, Ahn K, et al. Outcomes of pediatric bone marrow transplantation for leukemia and myelodysplasia using matched sibling, mismatched related, or matched unrelated donors. Blood. 2010;116:4007–4015. doi: 10.1182/blood-2010-01-261958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Locatelli F, Zecca M, Messina C, et al. Improvement over time in outcome for children with acute lymphoblastic leukemia in second remission given hematopoeitic stem cell transplantation from unrelated donors. Leukemia. 2002;16:2228–2237. doi: 10.1038/sj.leu.2402690. [DOI] [PubMed] [Google Scholar]
- 23.Pession A, Rondelli R, Locatelli F, et al. Role of a hematopoeitic stem cell transplant registry in childhood: The experience of the Associazione Italiana Ematologia Oncologia Pediatrica (AIEOP) Haematologica. 2002;87:42–46. [PubMed] [Google Scholar]
- 24.Borgmann A, von Stackelberg A, Hartmann R, et al. Unrelated donor stem cell transplantation compared with chemotherapy for children with acute lymphoblastic leukemia in a second remission: A matched-pair analysis. Blood. 2003;101:3835–3839. doi: 10.1182/blood.V101.10.3835. [DOI] [PubMed] [Google Scholar]
- 25.Bunin N, Carston M, Wall D, et al. Unrelated marrow transplantation for children with acute lymphoblastic leukemia in second remission. Blood. 2002;99:3151–3157. doi: 10.1182/blood.v99.9.3151. [DOI] [PubMed] [Google Scholar]
- 26.Miano M, Labopin M, Hartmann O, et al. Haematopoietic stem cell transplantation trends in children over the last three decades: A survey by the paediatric diseases working party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2007;39:89–99. doi: 10.1038/sj.bmt.1705550. [DOI] [PubMed] [Google Scholar]
- 27.Dini G, Cancedda R, Giorgiani G, et al. Unrelated donor marrow transplantation in childhood: A report from the Associazione Italiana Ematologia e Oncologia Pediatrica (AEIOP) and the Gruppo Italiano per il Trapianto Midollo Osseo (GITMO) Haematologica. 2002;87:51–57. [PubMed] [Google Scholar]
- 28.Kamani N, Spellman S, Hurley C, et al. State of the art review: HLA matching and outcome of unrelated donor umbilical cord blood transplants. Biol Blood Marrow Transplant. 2008;14:1–6. doi: 10.1016/j.bbmt.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Hurley C, Lowe L, Logan B, et al. National marrow donor program HLA matching guidelines for unrelated marrow transplants. Biol Blood Marrow Transplant. 2003;9:610–615. doi: 10.1016/j.bbmt.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Ohashi K, Tanabe J, Watanabe R, et al. The Japanese Multicenter Open Randomized Trial of ursodeoxycholic acid prophylaxis for hepatic veno-occlusive disease after stem cell transplantation. Am J Hematol. 2000;64:32–38. doi: 10.1002/(sici)1096-8652(200005)64:1<32::aid-ajh6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 31.Qureshi A, Marshall L, Lancaster D. Defibrotide in the prevention and treatment of veno-occlusive disease in autologous and allogeneic stem cell transplantation in children. Pediatr Blood Cancer. 2008;50:831–832. doi: 10.1002/pbc.21425. [DOI] [PubMed] [Google Scholar]
- 32.Cheuk D, Wang P, Lee T, et al. Risk factors and mortality predictors of hepatic veno-occlusive disease after pediatric hematopoeitic stem cell transplantation. Bone Marrow Transplant. 2007;40:935–944. doi: 10.1038/sj.bmt.1705835. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 34.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Eapen M, Rubinstein P, Zhang M, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: A comparison study. Lancet. 2007;369:1947–1954. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 36.Rocha V, Kabbara N, Ionescu I, et al. Pediatric related and unrelated cord blood transplantation for malignant diseases. Bone Marrow Transplant. 2009;44:653–659. doi: 10.1038/bmt.2009.291. [DOI] [PubMed] [Google Scholar]
- 37.Barrett A, Horowitz M, Pollock B, et al. Bone marrow transplants from HLA-identical siblings as compared with chemotherapy for children with acute lymphoblastic leukemia in a second remission. N Engl J Med. 1994;331:1253–1258. doi: 10.1056/NEJM199411103311902. [DOI] [PubMed] [Google Scholar]
- 38.Uderzo C, Valsecchi M, Balduzzi A, et al. Allogeneic bone marrow transplantation versus chemotherapy in high-risk childhood acute lymphoblastic leukaemia in first remission. Br J Haematol. 1997;96:387–394. doi: 10.1046/j.1365-2141.1997.d01-2033.x. [DOI] [PubMed] [Google Scholar]
- 39.Saarinen-Pihkala U, Gustafsson G, Ringden O, et al. No disadvantages in outcome of using matched unrelated donors as compared with matched sibling donors for bone marrow transplantation in children with acute lymphoblastic leukemia in second remission. J Clin Oncol. 2001;19:3406–3414. doi: 10.1200/JCO.2001.19.14.3406. [DOI] [PubMed] [Google Scholar]
- 40.Locatelli F, Rocha V, Chastang C, et al. Factors associated with outcome after cord blood transplantation in children with acute leukemia. Blood. 1999;93:3662–3671. [PubMed] [Google Scholar]
- 41.Moore A, Shaw P, Hallahan A, et al. Haemotopoietic stem cell transplantation for children in Australia and New Zealand,1998–2006: A report on behalf of the Australasian Bone Marrow Transplant Recipient Registry and the Australian and New Zealand Children’s Haematology Oncology Group. Med J Aust. 2009;190:121–125. doi: 10.5694/j.1326-5377.2009.tb02309.x. [DOI] [PubMed] [Google Scholar]
- 42.Rocha V, Cornish J, Sievers E, et al. Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants in children with acute leukemia. Blood. 2001;97:2962–2971. doi: 10.1182/blood.v97.10.2962. [DOI] [PubMed] [Google Scholar]
- 43.Kurtzberg J, Prasad V, Carter S, et al. Results of the Cord Blood Transplantation Study (COBLT): Clinical outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with hematologic malignancies. Blood. 2008;112:4318–4327. doi: 10.1182/blood-2007-06-098020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ljungman P, Aschan J, Lewensohn-Fuchs I, et al. Results of different strategies for reducing cytomegalovirus-associated mortality in allogeneic stem cell transplant recipients. Transplantation. 1998;66:1330–1334. doi: 10.1097/00007890-199811270-00012. [DOI] [PubMed] [Google Scholar]
- 45.Leung W, Pui C, Coustan-Smith E, et al. Detectable minimal residual disease before hematopoeitic cell transplantation is prognostic but does not preclude cure for children with very-high-risk leukemia. Blood. 2012;120:468–472. doi: 10.1182/blood-2012-02-409813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gratwohl A, Brand R, Niederwieser D, et al. Introduction of a quality management system and outcome after hematopoeitic stem-cell transplantation. J Clin Oncol. 2011;29:1980–1986. doi: 10.1200/JCO.2010.30.4121. [DOI] [PubMed] [Google Scholar]
- 47.Lee S, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching continues to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 48.Behrendt C, Rosenthal J, Bolotin E, et al. Donor and recipient CMV serostatus and outcome of pediatric allogeneic HSCT for acute leukemia in the era of CMV-preemptive therapy. Biol Blood Marrow Transplant. 2009;15:54–60. doi: 10.1016/j.bbmt.2008.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lausen B, Heilmann C, Vindelov L, et al. Outcome of acute lymphoblastic leukaemia in Danish children after allogeneic bone marrow transplantation. Superior survival following transplantation with matched unrelated donor grafts. Bone Marrow Transplant. 1998;22:325–330. doi: 10.1038/sj.bmt.1701351. [DOI] [PubMed] [Google Scholar]
- 50.Boeckh M, Nichols W. The impact of cytomegalovirus serostatus of donor and recipient before hematopoeitic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004;104:2003–2008. doi: 10.1182/blood-2003-10-3616. [DOI] [PubMed] [Google Scholar]
- 51.Mayor N, Shaw B, Hughes D, et al. Single nucleotide polymorphisms in the NOD2/CARD15 gene are associated with an increased risk of relapse and death for patients with acute leukemia after hematopoeitic stem-cell transplantation with unrelated donors. J Clin Oncol. 2007;25:4262–4269. doi: 10.1200/JCO.2007.12.1897. [DOI] [PubMed] [Google Scholar]
- 52.Ko R, Ji L, Barnette P, et al. Outcome of patients treated for relapsed or refractory acute lymphoblastic leukemia: A Therapeutic Advances in Childhood Leukemia Consortium study. J Clin Oncol. 2010;28:648–654. doi: 10.1200/JCO.2009.22.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carol H, Boehm I, Reynolds C, et al. Efficacy and pharmacokinetic/pharmacodynamic evaluation of the Aurora kinase A inhibitor MLN8237 against preclinical models of pediatric cancer. Cancer Chemother Pharmacol. 2011;68:1291–1304. doi: 10.1007/s00280-011-1618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diaz M, Gonzalez-Vicent M, Gonzalez M, et al. Long-term outcome of allogeneic PBSC transplantation in pediatric patients with hematological malignancies: A report of the Spanish Working Party for Blood and Marrow Transplantation in Children (GETMON) and the Spanish Group for Allogeneic Peripheral Blood Transplantation (GETH) Bone Marrow Transplant. 2005;36:781–785. doi: 10.1038/sj.bmt.1705135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Survival over 25 years stratified for remission status.


