Abstract
Background:
The content of icaritin and genistein in herba is very low, preparation with relatively large quantities is an important issue for extensive pharmacological studies.
Objective:
This study focuses on preparing and enzymic hydrolysis of flavonoid glycosides /β-cyclodextrin inclusion complex to increase the hydrolysis rate.
Materials and Methods:
The physical property of newly prepared inclusion complex was tested by differential scanning calorimetry (DSC). The conditions of enzymatic hydrolysis were optimized for the bioconversion of flavonoid glycosides /β-cyclodextrin inclusion complex by mono-factor experimental design. The experiments are using the icariin and genistein as the model drugs.
Results:
The solubility of icariin and genistein were increased almost 17 times from 29.2 μg/ml to 513.5 μg/ml at 60°C and 28 times from 7.78 μg/ml to 221.46 μg/ml at 50°C, respectively, demonstrating that the inclusion complex could significantly increase the solubility of flavonoid glycosides. Under the optimal conditions, the reaction time of icariin and genistin decreased by 68% and 145%, when compared with that without β-CD inclusion. By using this enzymatic condition, 473 mg icaritin (with the purity of 99.34%) and 567 mg genistein(with the purity of 99.46%), which was finally determined by melt point, ESI-MS, UV, IR, 1H NMR and 13C NMR, was obtained eventually by transforming the inclusion complex(contains 1.0 g substrates).
Conclusion:
This study can clearly indicate a new attempt to improve the speed of enzyme-hydrolysis of poorly water-soluble flavonoid glycosides and find a more superior condition which is used to prepare icaritin and genistein.
Keywords: Cellulase, enzymatic hydrolysis, flavonoid glycosides, snailase, β-CD inclusion complex
INTRODUCTION
Comparing with extraction or chemical synthesis, the application of biological enzymolysis technology to preparing of active component of Chinese traditional medicine was one of the research hotspot in Chinese traditional medicine field due to its advantage of high sensitivity, nice selectivity, easier process and steadier yield.
Icaritin[1,2,3] and genistein[4,5,6,7] were the aglycones of flavonoid glycosides (icariin and genistin). Both of them have strong anlitumor activity. Thereby, they may be of a great use for cancer treatment. However, the content in herba is very low, preparation with relatively large quantities is an important issue for extensive pharmacological studies. The low solubility of icariin and genistin,[8] which also restricted the speed of enzymatic hydrolysis because it is hard to connect with enzymes adequately.
There are lots of methods used in improving the rate of enzyme-hydrolysis, such as promoting the activity of enzymes at the certain high pressure,[9] processing by immobllized biocatalysts.[10] However, the immobilized enzymes generally present mass transfer limitations.[11] High pressure added the process cost. Therefore, the above-mentioned methods has no significant effect on the poorly water-soluble substrates. So, to find an approach to increase conversion efficiency by enhancing the aqueous solubility of poorly water-soluble substrates seems necessary and urgent.
As we know, β-cyclodextrin (β-CD), as presented in Figure 1., linked by α - 1, 4-bonds. Hydrophilic outer tails and hydrophobic inner cavities of β-CDs make drugs easily to form inclusion complex with a diversity of molecules[12] increasing the solubility of drugs. In the pharmaceutical industry, β-CD has often been used to enhance the solubility such as isoproturon,[13] celecoxib[14] and so many others.[15,16] That gives us a conjecture that β-CD inclusion complex may improve solubility of substrates and consequently increase the enzymatic hydrolysis rate.
Figure 1.
Schematic representation of the β-cyclodextrin molecule
To the best of our knowledge, few papers have been reported on enzymatic hydrolysis of flavonoid glycosides/β-CD inclusion complex for its aglycone. The aim of this paper is to form inclusion complex increasing the solubility of icariin and genistin as the model drugs. And we made a primary test for preparation of icaritin and genistein through enzymatic hydrolysis of β-CD inclusion complex [Figure 2].
Figure 2.

The process of β-CD inclusion complex enzymatic hydrolysis
MATERIALS AND METHODS
Apparatus
The analysis was carried out by a Agilent 1100 HPLC system (American Agilent) which consists of an Auto Sample injection(G1313A), QuatPump(G1311A), Column Component(G1316A), Photodiode Array Detector (G1315A) and ZORBAX SB-C18 (4.6 × 250mm,5μm.Angilent). Agilent Chem Station Software (Rev.B.03.02) was used to deal with the data. Enzymatic hydrolysis was carried out by digital temperature air bath oscillator (Jintan Shuangjie Experimental Instrument Factory). A TGL-16H high-speed centrifuge (Shanghai Precision Instrument Factory Anping) was used to treat the samples.
Materials and reagents
Acetonitrile and Methanol were of chromatographic grade (TEDIA Company Inc., UN). All other reagents were of analytical grade. Ultra-pure water was prepared with Milli-Q water (Millipore, Bedford, MA). β-cyclodextrin (average Mw = 1135) was purchased from Shanghai Chemical Reagent Company of China Pharmaceutical Group. Snailase was purchased from Baier Di Biotechnology Co. Ltd. Standard icariin (purity > 98%), icaritin (purity > 98%), genistin (purity > 98%) and genistein (purity > 98%) were provided by the Laboratory of Pharmaceutical Preparation (Jiangsu Provincial Academy of Chinese Medicine, China).
Preparation of β-cyclodextrin inclusion complex
The inclusion complex of icariin (genistin) with β-cyclodextrin at 1:1 molar ratio was prepared using the dropping method. The accurately weighed β-cyclodextrin was dissolved in distilled water to get a saturated solution. Then icariin (genistin) was dissolved in 50% (v/v) ethanol which was added to β-CD drop by drop. The suspension was stirred at 60°C for 12h. Standing overnight in a cold storage, the suspension was filtered and the uncomplexed drug was washed from the residue using 50% (v/v) ethanol. Finally, the residue was dried, and the complexation of icariin (genistin) with β-CD was obtained.
Determination of solubility of substrates and their β-CD inclusion complex.
An excess amount of icariin (genistin) and icariin (genistin)/β-CD inclusion complex was added to the water, respectively. The solution was constantly stirred using a magnetic stirrer for 24 h at 60 ± 0.1°C. The stirrer was turned off to let the solution settle for 2h. Then the supernatant liquid was taken and filtered through a 0.45 μm membrane. The first 15% of the filtrate was abandoned to avoid any potential loss of the drug, because of absorption by the filter and the subsequent filtrate was collected. All procedures were conducted at the test temperature to avoid any precipitation of the drug.[17] And a 20μl aliquot of the resulting solution was analyzed by an HPLC system in triplicate.
Differential scanning calorimetry (DSC)
Samples were sealed in aluminum crimp cells and heated at a rate of 10°C/minfrom 30°C to 450°C in a nitrogen atmosphere (DSC-60, SHI-MADZU, JAPAN). The peak transition maximum temperatures of β-CD, pure icariin (genistin), the mixture of β-CD and pure icariin (genistin) and icariin (genistin) /β-CD inclusion compounds were compared using a Thermal Analyzer (TA-60WS, SHIMADZU, JAPAN).
Experimental Design and Analysis
Icariin (genistin) /β-CD inclusion complex was dissolve in 10ml distilled deionized water in a 10ml eppendorf tube with a constant speed of 100 r/min–1 in digital temperature air bath oscillator. The enzymatic hydrolysis was tested using unvariate experiment, including temperature, time, pH and the rate of icariin (genistin) /β-CD inclusion complexs. Three factors are the same, just one factor difference. As shown in Figures 3-6. All samples were tested in triplicate.
Figure 3.
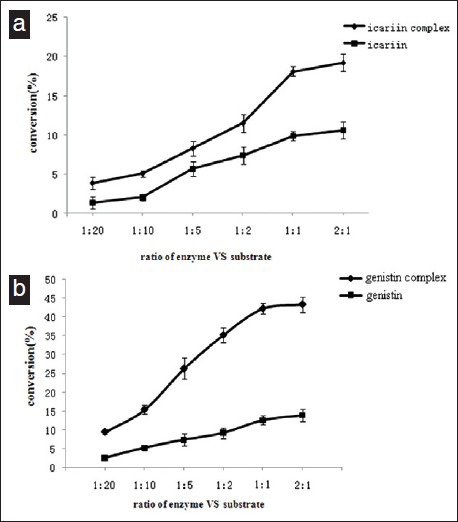
(a). Effect of proportion of icariin (icariin/β-CD inclusion compounds) on enzyme efficiency of snailase hydrolysis Substrate was mixed with 5, 10, 20, 50, 100 and 200mg snailase (substrates: enzyme = 20:1, 10:1, 5:1, 2:1, 1:1 and 1:2), respectively. Distilled deionized water was added to the mixture at a final volume of 10 ml. Then it was incubated in digital temperature air bath oscillator at 37°C for 3 h. (b). Effect of proportion of genistin (genistin /β-CD inclusion compounds) on enzyme efficiency of cellulase hydrolysis. Substrate was mixed with 5, 10, 20, 50, 100 and 200mg cellulase (substrates: enzyme = 20:1, 10:1, 5:1, 2:1, 1:1 and 1:2), respectively. Distilled deionized water was added to the mixture at a final volume of 10ml. Then it was incubated in digital temperature air bath oscillator at 37°C for 3 h. Values are expressed as mean ± SD, n = 3
Figure 6.
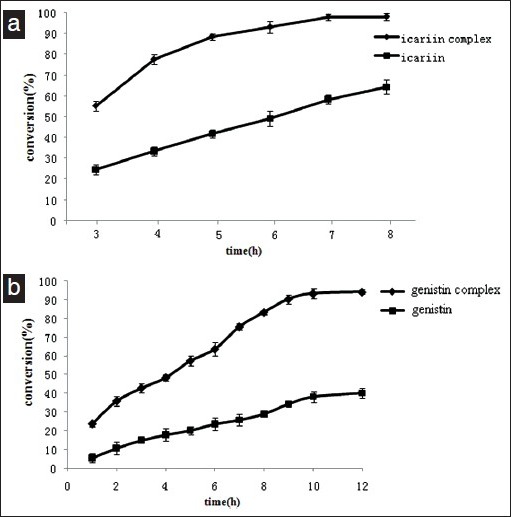
(a) An Effect of reaction time on icariin (icariin/β-CD inclusion compounds) hydrolysis Substrate, 100mg snailase and 10ml distilled deionized water were added in a 10ml eppendorf tube, incubated in digital temperature air bath oscillator at 60°C for 3h, 4h, 5h, 6h, 7h and 8h, respectively. b. Effect of reaction time on genistin (genistin /β-CD inclusion compounds) hydrolysis. Substrate, 100mg cellulase and 10ml distilled deionized water were added in a 10ml eppendorf tube, incubated in digital temperature air bath oscillator at 60°C for 1h, 2h, 3h, 4h,5h,6h, 7h, 8h, 9h, 10h and 12h, respectively. Values are expressed as mean ± SD, n = 3
Preparation of icaritin (genistin) under optimum condition.
The inclusion complex was transformed into target compound according to the optimized screening condition. Consequently, the maximum product of target compound was obtained individually. We chosed the product that achieved better transformation efficiency for the conduct of further purification. Finally, target compound was obtained through the reduced pressure recovery. The identification of produced target compound was carried out by melt point, ESI-MS, UV, IR, 1H NMR and 13C NMR.
Analytical method
Analytical method of icariin and icaritin
Separation was achieved on a Agilent ZORBAX SB-C18 (4.6 × 250mm, 5μm.) column maintained at 30°C. The mobile phase consisted of A (water) and B (acetonitrile) with linear radient elution: 0-7 min 38% B, 7-8 min 75% B, 8-20 min75% B and 20-30 min 38% B. The flow rate was 1.0ml/min. The wavelength of detector was set at 270nm.
Analytical method of genistin and genistein
Separation was achieved on an Agilent ZORBAX SB-C18 (4.6 × 250mm, 5μm.) column maintained at 30°C. The mobile phase consisted of A (water contains 0.2% H3PO4) and B (acetonitrile) with isocratic elution (A:B = 6:4). The flow rate was 1.0ml/min. The wavelength of detector was set at 260nm.
Due to the distinct variation in contents of icariin and icaritin (genistin and genistein) in the samples, the stock solutions of standards, were prepared and diluted with methanol solution to appropriate concentration for the establishment of calibration curves. Seven concentrations of icariin and icaritin (genistin and genistein) solution were injected in triplicate. And then the calibration curves were constructed by plotting the peak areas against the concentration of each analyte. Each aliquot (20μl) from samples were injected into HPLC, and the content of icariin and icaritin (genistin and genistein) were calculated using the calibration curves. Precision and accuracy also be tested.
Calculation conversion
After reaction, 100μl sample solution was extracted with 900μl methanol, vortexed 30s and then centrifuged at 11000 r/min for 15 min, 20μl supernatant was injected into HPLC. The conversion rate was calculated using following equation:
Conversion rate (%) = [CV/(m2 × M1/M2)] × 100% (1)
C—concentration of icaritin (genistein), V—volume of icaritin (genistein), M1 —moleculor weight of icaritin (genistein), M2 —moleculor weight of icariin (genistin), m2 —weight of icariin (genistin)
RESULTS AND DISCUSSION
Method validation
Method validation of icariin and icaritin
The analytical method was validated in terms of linearity, precision and accuracy. The calibration curves were as follows: Icariin A = 22 543C + 29.746, correlation coefficient 0.9997, linear range 15.25 - 1344.2μg/ml, Icaritin A = 22 602 C + 807.96, correlation coefficient 0.9994, linear range 12.31-1107.9μg/ ml. The precision and accuracy test showed that the HPLC method is precise and accurate enough for the quantitative determination of icariin and icaritin in samples.
Method validation of genistin and genistein
The analytical method was validated in terms of linearity, precision and accuracy, the calibration curves were as follows: Genistin A = 347146C - 84.56, correlation coefficient 0.9993, linear range 2.1-32.8μg/ml, Genistein A = 164562C - 88.781, correlation coefficient 0.9998, linear range 3.31-54.5μg/ml. The precision and accuracy test showed that the HPLC method is precise and accurate enough for the quantitative determination of genistin and genistein in samples.
The solubility of substrates and its β-CD inclusion compounds
The solubility of icariin and icariin/β-CD inclusion compounds
The water solubility of icariin/β-CD inclusion compounds was higher than that of free icariin. The solubility of icariin was increased almost 17 times from 29.2 μg/ml to 513.5 μg/ml at 60°C, demonstrating that the inclusion complex could significantly increased the solubility of icariin.
The solubility of genistin and genistin /β-CD inclusion compounds.
The water solubility of genistin /β-CD inclusion compounds was higher than that of free icariin. The solubility of genistin was increased almost 28 times from 7.78 μg/ml to 221.46 μg/ml at 50°C, demonstrating that the inclusion complex could significantly increased the solubility of genistin.
Differential scanning calorimetry (DSC)
Differential scanning calorimetry (DSC) of icariin/β-CD inclusion compounds
Differential scanning calorimetry is a rapid and reliable way to screen icariin and β-CD compatibility. And it provides maximum absorption about possible interactions. Figure 7 shows the DSC curves of icariin (a), β-CD (b), physical mixture (c) and icariin/β-CD inclusion compounds (d).[18] Thermogram of icariin exhibited a characteristic peaks (242.1°C). And β-CD exhibited a clearly maximum absorption peaks is 315.4°C. Physical mixture of icariin and β-CD shows two peaks. The former has the same onset temperature (242.6°C) of the icariin and the later has nearly the same onset temperature (310.3°C) of β-CD. DSC of icariin/β-CD inclusion compounds showed the endothermal peak of icariin is disappeared. It could indicate that it was inclusion complex instead of the new compound or physical mixture.
Figure 7.
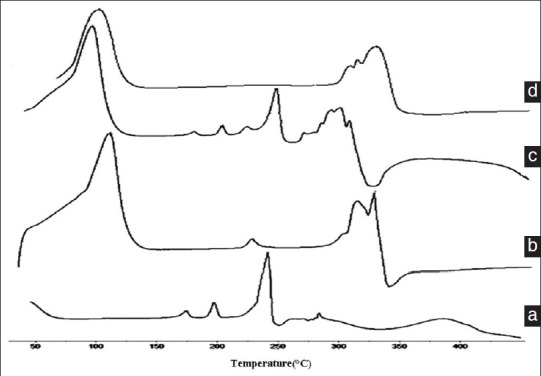
DSC thermograms of icariin (a), β-CD (b), physical mixture (c) and icariin/β-CD inclusion compounds (d)
Differential scanning calorimetry (DSC) of genistin /β-CD inclusion compounds.
Differential scanning calorimetry is a rapid and reliable way to screen genistin and β-CD compatibility. And it provides maximum absorption about possible interactions. Figure 8 shows the DSC curves of genistin (a), β-CD (b), physical mixture (c) and genistin /β-CD inclusion compounds (d).[19] Thermogram of genistin exhibited a characteristic peaks (286.5°C). And β-CD exhibited a clearly maximum absorption peaks is 315.4°C. Physical mixture of genistin and β-CD shows two peaks. The former has the same onset temperature (286.6°C) of the genistin and the later has nearly the same onset temperature (310.3°C) of β-CD. DSC of genistin/β-CD inclusion compounds showed the endothermal peak of genistin is disappeared. And the new peak (216.4°C) appears. It could indicate that it was inclusion complex instead of the new compound or physical mixture.
Figure 8.
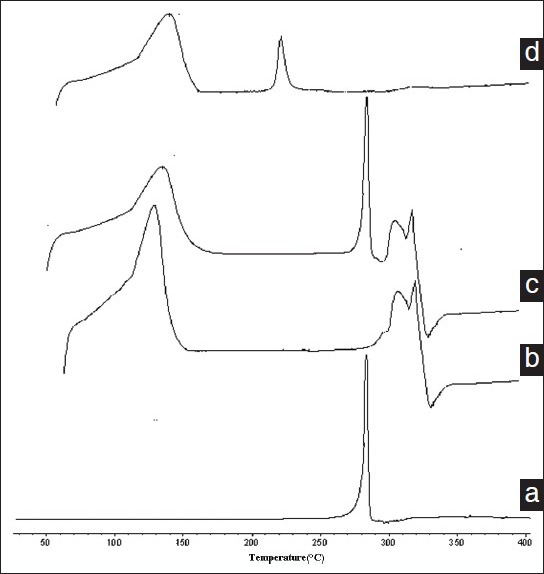
DSC thermograms of genistin (a), β-CD (b), physical mixture (c) and genistin/β-CD inclusion compounds (d)
Optimal conditions for enzymatic hydrolysis:
Effect of proportion of substrate on enzyme hydrolysis.
Effect of proportion of icariin (icariin/β-CD inclusion compounds) on enzyme hydrolysis
Hydrolysis of icariin was performed with different concentrations of snailase in Figure 3a. And the hydrolysis increased both with and without inclusion. Forming the inclusion would get higher conversion at low enzyme concentration. At the ratio of substrate/enzyme (1:1), the increase in hydrolysis was 18.1% compared to a 9.9% increase with inclusion. At all enzyme concentrations, the conversion of icaritin was lower when did not form the inclusion complex. However, an increased percentage of icaritin was observed with increased enzyme concentration when form the icariin inclusion complex. For the ratio of substrate/enzyme, the content of icaritin increased with the rising ratio of icariin/snailase in the mixture and reached its maximum at 1:1. Therefore, the ratio of substrate/enzyme (1:1) was chosen as the best proportion.
Effect of proportion of genistin (genistin /β-CD inclusion compounds) on enzyme hydrolysis
Hydrolysis of genistin was performed with different concentrations of cellulase in Figure 3b. And the hydrolysis increased both with and without inclusion. Forming the inclusion would get higher conversion at low enzyme concentration. At the ratio of substrate/enzyme (1:1), the increase in hydrolysis was 42.19% compared to a 12.58% increase with inclusion. At all enzyme concentrations, the conversion of genistin was lower when did not form the inclusion complex. However, an increased percentage of genistin was observed with increased enzyme concentration when form the genistin inclusion complex. For the ratio of substrate/enzyme, the content of icaritin increased with the rising ratio of genistin /cellulase in the mixture and reached its maximum at 1:1. Therefore, the ratio of substrate/enzyme (1:1) was chosen as the best proportion.
Effect of pH on efficiency of substrate hydrolysis
Effect of pH on efficiency of icariin (icariin/β-CD inclusion compounds) hydrolysis
Figure 4a showed that for icariin, the opimal pH was 7.0. It is not the same as hydrolyzing arctiin and chitosan.[20,21] That may because snailase is a compound enzyme system. It has cellulase, pectinase, amylase, protease and the forth. And pH 7.0 was not the best pH for the enzymes contained within the snailase. It also showed that when form the inclusion, the conversion of icaritin would increase remarkably. And when the buffer pH was at 7.0, the conversion of icaritin, which form the inclusion, would reach maximum value 20.1% reacting for 3 h. The transformation rate had grown more than 100%. That may because after form the inclusion it could significantly enhance the dissolubility of icariin so that the transformation rate increased.
Figure 4.

(a). Effect of pH on efficiency of icariin (icariin/β-CD inclusion compounds) hydrolysis The reaction was performed by adding icariin/β-CD inclusion compounds(contains 100mg icariin) or 100mg icariin, 100mg snailase and 10ml glacial acetic acid and sodium acetate anhydrous buffer (pH 4.0, 4.5, 5.0, 5.5, 6.0, 6.5 and 7.0 respectively) at 37°C in digital temperature air bath oscillator for 3h. (b). Effect of pH on efficiency of genistin (genistin /β-CD inclusion compounds) hydrolysis. The reaction was performed by adding genistin/β-CD inclusion compounds (contains 100mg genistin) or 100mg genistin, 100mg cellulase and 10ml glacial acetic acid and sodium acetate anhydrous buffer (pH 4.0, 4.5, 5.0, 5.5, 6.0, 6.5 and 7.0 respectively) at 37°C in digital temperature air bath oscillator for 3h. Values are expressed as mean ± SD, n = 3
Effect of pH on efficiency of genistin (genistin/β-CD inclusion compounds) hydrolysis
Figure 4b showed that for genistin, the optimal pH was 5.0. It is the same as hydrolyzing grapefruit peel.[22] It showed that when form the inclusion, the conversion of genistein would increase remarkably. And when the buffer pH was at 5.0, the conversion of genistin, which form the inclusion, would reach value 41.28 % reacting for 3h. The transformation rate had grown more than 3.16 fold. That may because after form the inclusion it could significantly enhance the dissolubility of genistin so that the transformation rate increased.
Effect of the enzymatic hydrolysis of substrate by temperature:
Effect of the enzymatic hydrolysis of icariin (icariin/β-CD inclusion compounds) by temperature
Snailase hydrolyze different substrates had not the same optimized temperature. It hydrolyzed ginsenoside - Rb1 and chitin at 40°C,[23] 25°C,[24] respectively. However, in this experiment, 60°C was most suitable for reaction as demonstrated in Figure 5a. The conversion of icaritin increased remarkably with the reaction temperature increasing from 25°C and reached its maximum at 60°C, then decreased slowly with the temperature increasing. And at 60°C was the optimum temperature both for snailase with or without inclusion. At 60°C, the activity of the snailase with inclusion was significantly superior to the snailase without inclusion. The conversion was increased by almost 125% (from 55.2 to 24.5%)
Figure 5.
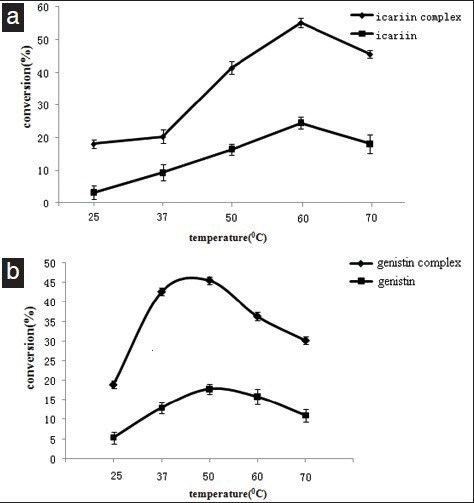
(a). Effect of the enzymatic hydrolysis of icariin (icariin/β-CD inclusion compounds) by temperature. Icariin/β-CD inclusion compounds(contains 100mg icariin) or 100mg icariin, 100mg snailase and 10ml distilled deionized water were added in a 10ml eppendorf tube, incubated in digital temperature air bath oscillator at 25°C, 37°C, 50°C, 60°C and 70°C for 3h, respectively. (b). Effect of the enzymatic hydrolysis of genistin (genistin /β-CD inclusion compounds) by temperature.
Genistin/β-CD inclusion compounds(contains 100mg genistin) or 100mg genistin, 100mg cellulase and 10ml distilled deionized water were added in a 10ml eppendorf tube, incubated in digital temperature air bath oscillator at 25°C, 37°C, 50°C, 60°C and 70°C for 3 h, respectively. Values are expressed as mean ± SD, n = 3
Effect of the enzymatic hydrolysis of genistin (genistin /β-CD inclusion compounds) by temperature
In this experiment, 50°C was most suitable for reaction as demonstrated in Figure 5b. It is the same as the literatures reports.[25,26] The conversion of genistein increased remarkably with the reaction temperature increased from 25°C and reached its maximum at 50°C, then decreased slowly with the temperature increasing. And at 50°C was the optimum temperature both for cellulase with or without inclusion. At 50°C, the activity of the cellulase with inclusion was significantly superior to the snailase without inclusion. The conversion was increased by almost 156% (from 17.75 to 45.58%).
Effect of reaction time on hydrolysis:
Effect of reaction time on icariin (icariin/β-CD inclusion compounds) hydrolysis
Icariin was hydrolyzed with the snailase and the time course of hydrolysis was determined with and without inclusion. A substantial increase in the conversion, measured as when form the inclusion to the hydrolysis icariin. Its effect was relatively constant during the entire 7h hydrolysis. After 3h, the conversion was increased by almost 125% (from 55.2 to 24.5%) and after 7h, the increase was. 68% (from 58.4 to 97.9%) And the conversion of icariside II increased with the reaction time going on and reached its maximum at 7h - 8h [Figure 6a]. Therefore, the best reaction time is 7h. It can significantly shorten the reaction of icariin hydrolysize into icaritin.
Effect of reaction time on genistin (genistin /β-CD inclusion compounds) hydrolysis
Genistin was hydrolyzed with the cellulase and the time course of hydrolysis was determined with and without inclusion. A substantial increase in the conversion, measured as when form the inclusion to the hydrolysis genistin. Its effect was relatively constant during the entire 10h hydrolysis. After 3h, the conversion was increased by almost 156% (from 17.75 to 45.58%) and after 10h, the increase was 145% (from 38.14 to 93.46%). And the conversion of genistein increased with the reaction time going on and reached its maximum at 10-12h [Figure 6b]. Therefore, the best reaction time is 10h. It can significantly shorten the reaction time of genistin.
Preparation by optimum condition
Preparation of icaritin by optimum condition
The reaction time has averagely decreased by 85.4% as compared with that in literature.[27] And the conversion of icaritin was up to 97.91%. It can observably reduce the reaction time and increase the transformation rate.
The inclusion complex of icariin containing 1.013 g icariin, was mixed with 1.024 g snailase in a 100 ml water. The buffer was subsequently incubated in a HH-4 digital constant temperature water bath at 60°C for 7h. The final reaction mixture was extracted with ethyl acetate three times. The combined ethyl acetate layer was evaporated to dryness under reduced pressure. HPLC analysis showed that 97.9% of the inclusion complexe of icariin was transformed into icaritin. To isolate icaritin, the residue was dissolved in a 10ml methanol and subjected to silica gel column chromatography using CHCl3–MeOH system as elution. Finally, 473mg icaritin was obtained with the purity of 99.3%. The identification of produced icaritin was carried out by melt point, ESI-MS, UV, IR, 1H NMR and 13CNMR and compared with the data given in references.[28] The test results are as follows: yellow needle crystal, molecular formula, C21H20O6; mp 205–207°C; ESIMS, m/z (%): 369 [M + H]+; UVmax: 270nm; IR(KBr)νmaxcm–1: 3319, 1655, 1603, 1511, 1461, 1385, 1257, 1227, 838; 13CNMR(DMSO - d6, 100MHz): d 146.65(C2), 136.39(C3), 176.70(C4), 160.95(C5), 98.30(C6), 161.72(C7), 106.09(C8), 153.99(C9), 103.54(C10), 122.96(C1´), 129.64(C2´), 114.56(C3´), 158.77(C4´), 114.56(C5´), 129.64(C6´), 55.85(C - OMe) 21.67(Cl´´), 124.05(C2´´), 131.47(C3´´), 25.90(C4´´), 18.30(C5´´); 1H NMR(DMSO-d6, 400 MHz): d 12.374(1H, s, C5 - OH), 10.737(1H,s, C7 - OH), 9.475(1H, s, C3 - OH), 8.10(2H, d, J = 8.7Hz, H- 2´,6´), 7.10(2H, d, J = 5.7Hz, H-3´,5´), 6.306(1H, s, H - 6), 5.183(1H, t, J = 6.7Hz, H - 2´), 3.851(3H, s, 4´- OMe), 3.433(2H, d, J = 6.7Hz, H-1´´), 1.684(3H, s, H - 4´´), 1.61(3H, s, H - 5´´).
Preparation of genistein by optimum condition
The inclusion complex of icariin containing 1.053g genistin, was mixed with 1.044 g cellulase in a 100ml glacial acetic acid and sodium acetate anhydrous buffer (pH 5.0). The buffer was subsequently incubated in a HH-4 digital constant temperature water bath at 50°C for 12h. The final reaction mixture was filtrated and dissolved by anhydrous ethyl alcohol. Then, filtrate the solution; evaporate under reduced pressure, and automatic crystallization. Using anhydrous ethyl alcohol to wash the crystal, yellow needle crystal was obtained. The identification of produced genistein was carried out by melt point, ESI - MS, UV, IR, 1H NMR and 13CNMR and compared with the data given in references.[29,30]
CONCLUSIONS
The purpose of the present study was to increase hydrolysis rate, and shorten the reaction time significantly. Hydrophilic outer tails and hydrophobic inner cavities of β-CDs could increase the solubility of flavonoid glycosides and has no influence on glycosidic bond. The solubility of icariin and genistin were increased almost 35 times from 14.6μg/ml to 513.5 μg/ml at 60°C and 28 times from 7.78 μg/ml to 221.46 μg/ml at 50°C, respectively, demonstrating that the inclusion complex could significantly increased the solubility of flavonoid glycosides. Under the optimum conditions, reaction time of icariin and genistin decreased by 68% and 145%, when compared with that without β-CD inclusion. And this study can also clearly indicate a new attempt to improve the speed of enzyme-hydrolysis of poorly water-soluble flavonoid glycosides and find a more superior condition which prepare icaritin and genistein.
ACKNOWLEDGMENTS
The authors report no conflicts of interest in this work. This work was supported by the National Natural Science Foundation of China (Nos., 30572372 and 30973944).
Footnotes
Source of Support: Nil
Conflict of Interest: No.
REFERENCES
- 1.Yap SP, Shen P, Butler MS, Gong Y, Loy CJ, Yong EL. New estrogenic prenylflavone from Epimedium brevicornum inhibits the Growth of Breast Cancer Cells. Planta Med. 2005;71:114. doi: 10.1055/s-2005-837776. [DOI] [PubMed] [Google Scholar]
- 2.Huang X, Zhu DY, Lou YJ. A novel anticancer agent, icaritin, induced cell growth inhibition, G1 arrest and mitochondrial transmembrane potential drop in human prostate carcinoma PC-3 cells. Eur J Pharmacol. 2007;564:26–36. doi: 10.1016/j.ejphar.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 3.Wang ZQ, Lou YJ. Proliferation-stimulating effects of icaritin and desmethylicaritin in MCF-7 cells. Eur J Pharmacol. 2004;504:147–53. doi: 10.1016/j.ejphar.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Liao CH, Pan SL, Guh JH, Teng CM. Genistein inversely affects tubulin-binding agent-induced apoptosis in human breast cancer cells. Biochem Pharmacol. 2004;67:2031–8. doi: 10.1016/j.bcp.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Huang YH, Yuan P, Zhang QH, Xin XY. Genistein sensitizes ovarian carcinoma cells to chemotherapy by switching the cell cycle progression in vitro. J Med Coll PLA. 2009;24:125–35. [Google Scholar]
- 6.Yang SH, Zhou Q, Yang XH. Caspase-3 status is a determinant of the differential responses to genistein between MDA-MB-231 and MCF-7 breast cancer cells. Biochim Biophys Acta. 2007;177:3903–11. doi: 10.1016/j.bbamcr.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Fan YJ, Huang NS, Xia L. Genistein synergizes with RNA interference inhibiting survivin for inducing DU-145 of prostate cancer cells to apoptosis. Cancer Lett. 2009;284:189–97. doi: 10.1016/j.canlet.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 8.Wang YL, Jia XB, Chen Y, Li X. Determination of equilibrium solubility of icariin and its apparent oil/water partition coefficient. Chin J Tradit Chin Med Pharm. 2008;23:777–9. [Google Scholar]
- 9.Vila Real HJ, Alfaia AJ, Calada AR, Ribeiro MH. High pressure-temperature effects on enzymatic activity: Naringin bioconversion. Food Chem. 2007;102:565–70. [Google Scholar]
- 10.Ribeiro IA, Ribeiro MH. Kinetic modelling of naringin hydrolysis using a bitter sweet alfa-rhamnopyranosidase immobilized in k-carrageenan. J Mol Catal B Enzym. 2008;51:10–8. [Google Scholar]
- 11.Cao AP, Ai HH, Ding YL, Dai CY, Fei JJ. Biocompatible hybrid film of β-cyclodextrin and ionic liquids: A novel platform for electrochemical biosensing. Sens Actuators B Chem. 2011;2:632–8. [Google Scholar]
- 12.Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins, Drug solubilization and stabilization. J Pharm Sci. 1996;85:1017–25. doi: 10.1021/js950534b. [DOI] [PubMed] [Google Scholar]
- 13.Manolikar MK, Sawant MR. Study of solubility of isoproturon by its complexation with β-cyclodextrin. Chemosphere. 2003;51:811–6. doi: 10.1016/S0045-6535(03)00099-7. [DOI] [PubMed] [Google Scholar]
- 14.Rawat S, Jain S. Solubility enhancement of celecoxib using β-cyclodextrin inclusion complexes. Eur Pharm Biopharm. 2004;57:263–7. doi: 10.1016/j.ejpb.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Zhao YM, Guo CY, Zhang SM, Liu CL, Zhang DS, et al. Ultrasound-assisted extraction of total flavonoids from Inula helenium. Pharmacogn Mag. 2012;30:166–70. doi: 10.4103/0973-1296.96581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen YJ, Wang J, Wan DR. Determination of total flavonoids in three Sedum crude drugs by UV-Vis spectrophotometry. Pharmacogn Mag. 2010;24:259–63. doi: 10.4103/0973-1296.71784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higuchi T, Connors KA. Phase solubility techniques. Adv J Anal Chem Instrum. 1965;4:117–212. [Google Scholar]
- 18.Wang JH, Cai ZS. Investigation of inclusion complex of miconazole nitrate with β-cyclodextrin. Carbohydr Polym. 2008;72:255–60. [Google Scholar]
- 19.Ficarra R, Tommasini S, Raneri D, Calabro ML, Di Bella MR, Rustichelli C, et al. Study of flavonoids/β-cyclodextrins inclusion complexes by NMR, FT-IR, DSC, X-ray investigation. J Pharm Biomed Anal. 2002;29:1005–14. doi: 10.1016/s0731-7085(02)00141-3. [DOI] [PubMed] [Google Scholar]
- 20.Hu YJ, Fan YH, Xiao MX, Zhou J, Lu YY, Yang ZF, et al. Preparation of Arctigenin by Enzymolysis of Arctiin with Snail Hydrolase. J Guangzhou Univ Tradit Chin Med. 2004;21:473–5. [Google Scholar]
- 21.Zhang T, Wu CY, Zhang YM, Wang L. Study on chitooligosaccharide production by chitosan hydrolysis with snailase. West China J Pharm Sci. 2010;25:313–5. [Google Scholar]
- 22.Wilkins MR, Widmer WW, Grohmann K, Cameron RG. Hydrolysis of grapefruit peel waste with cellulase and pectinase enzymes. Bioresour Technol. 2007;98:1596–601. doi: 10.1016/j.biortech.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 23.Liu X, Cui Y, Yang L, Yang SL. Purification of a Ginsenoside-Rb₁ hydrolase from Heli nailase. Chin J Biotechnol. 2005;6:929–33. [PubMed] [Google Scholar]
- 24.Zhang J, Jin YJ, Wu ZL. Hydrolysis of Chitosan with Chitin Immobilized Snailase. Polym Mater Sci Eng. 2009;21:142–5. [Google Scholar]
- 25.Lu J, Reye J, Banerjee S. Temperature dependence of cellulase hydrolysis of paper fiber. Biomass Bioenerg. 2010;34:1973–7. [Google Scholar]
- 26.Xu F, Ding HS, Osborn D, Tejirian A, Brown K, Albano W, et al. Partition of enzymes between the solvent and insoluble substrate during the hydrolysis of lignocellulose by cellulases. J Mol Catal B Enzym. 2008;51:42–8. [Google Scholar]
- 27.Jia DS, Jia XB, Xue J, Shi F, Sun E, Chen LL, et al. Preparation of icaritin by enzymolysis of icariin with snailase. China J Chin Mater Med. 2010;35:857–60. doi: 10.4268/cjcmm20100711. [DOI] [PubMed] [Google Scholar]
- 28.Mizuno M, Iinuma M, Tanaka T. Flavonol glyeosides in the roots of Epimedium diphyllum. Phytochemistry. 1988;27:36–45. [Google Scholar]
- 29.Ma Q, Lei HM, Zhou YX. Studies on chemical constituents of Trifolium pratense. Chin Pham J. 2005;40:1057–9. [Google Scholar]
- 30.Zhang L, Zhang YK, Chen Y. Isoflavones in Leaves of Belamcanda chinensis. Nat Prod Res Dev. 2011;23:69–71. [Google Scholar]


