Abstract
Background:
Psoralea corylifolia (Bakuchi), a weed, which possesses a highly potent and medicinally important compound psoralen. P. corylifolia has been widely exploited since ages for its biological potential.
Materials and Methods:
Fifteen root nodulating bacteria as pure culture collection (PCC) were isolated from P. corylifolia in India. Further, these strains were evaluated for their effect on the psoralen content in P. corylifolia. High performance liquid chromatography (HPLC) method was used for the estimation of psoralen in P. corylifolia seed extracts. The effectiveness of these rhizobial strains was assessed on the basis of screening of various plant growth promoting attributes.
Results:
The 16S ribosomal RNA sequencing analysis revealed the identity of two most effective rhizobial isolates PCC2 and PCC7 as Rhizobium leguminosarum and Sinorhizobium meliloti, respectively. The R. leguminosarum PCC2 (JN546144) and Ensifer meliloti PCC7 (JN546145) strains showed solubilization of insoluble inorganic phosphate, secreted indole acetic acid (IAA), produced siderophore, showed ACC deaminase activity, and were positive for nodulation and nitrogen fixing genes. Seeds of P. corylifolia were bacterized with combination of R. leguminosarum PCC2 and Ensifer meliloti PCC7 along with their individual application that resulted in enhancement of various early vegetative and late reproduction parameters of plants in two consecutive field trials in the year 2009 and 2010. The psoralen content in the seeds of P. corylifolia was observed to be increased in the field trials where the combination of rhizobial strains PCC2 and PCC7 was used (2.79%) compared to control (1.91%).
Conclusion:
These findings indicate that rhizobial strains PCC2 and PCC7 showing good plant growth promoting attributes can be effective for increasing the psoralen content in the seeds of P. corylifolia to a certain level.
Keywords: Psoralea corylifolia, rhizobia, Rhizobium leguminosarum, Sinorhizobium meliloti, psoralen content
INTRODUCTION
Psoralea corylifolia is an annual weed growing throughout the plains of India, especially in the semi-arid region of Rajasthan and eastern districts of Punjab adjoining Uttar Pradesh and Uttarakhand states. The plant is of immense biological importance and it has been widely exploited since ages for its magical effects against several skin diseases such as psoriasis, leucoderma and leprosy.[1] Dry fruits of P. corylifolia are the most popular traditional Chinese medicines officially listed in Chinese Pharmacopeia.[2] In the Ayurvedic Pharmacopoeia of India, several reports revealed the presence of essential oil, coumarins, alkaloids, flavonoids and terpenoids like beneficiary compounds in the seeds of P. corylifolia. The literature also reveals therapeutic action of P. corylifolia against various diseases such as asthma, diarrhea, alopecia aretae,[2] impotence, menstrual disorder, uterine hemorrhage, as well as showing antitumor,[3] antiallergic,[4] antioxidant,[5] insecticidal and antimicrobial activities. P. corlifolia seed extracts have been reported to stimulate immune system in mice. Administration of P. corlifolia seed extract has been found to inhibit ehrlich ascites carcinoma (EAC) ascetic tumor growth and stimulates natural killer activity, antibody dependent cellular-cytotoxicity, antibody forming cells and the antibody complement mediated cytotoxicity during tumor development.[6,7]
Plant growth promoting rhizobacteria (PGPR) are the root nodulating bacteria in symbiotic association with leguminous plants.[8] Legume and their symbiotic bacteria make the maximum contribution of global nitrogen fixation. The Rhizobium legume symbiosis, because of its agricultural importance, has ensured continuing research support worldwide and is presently one of the best understood plant-microbe interactions.[9] PGPR are classified into various genera including Rhizobium, Bradyrhizobium, Mesorhizobium and Ensifer etc., Different PGP attributes including phosphate solubilization, indole acetic acid (IAA), 1-aminocyclopropane-1-carboxylate (ACC) deaminase, siderophore production,[10] lytic enzymes,[11] antibiotic resistant are reported to effect the growth of the plant. Glick et al.[12] reported the presence of direct and indirect mechanisms of PGP which affect the growth of the plant, yet further studies are needed for complete elucidation of the bacteria-plant association in respect to growth promotion.
Psoralen, a tricyclic furocoumarin with potent photosensitizing property is the major and the most active furocoumarin present in P. corylifolia, which promotes pigmentation.[13] Psoralen has been found to intercalate into DNA where it forms mono- and di-adducts in the presence of long wavelength UV lights which are used for the treatment of hypo-pigmented lesions of the skin like leucoderma and psoriasis.[14] Several reports revealed various methods for the estimation of psoralen using high performance liquid chromatography (HPLC), high performance thin layer chromatography (HPTLC) and spectrophotometery. Previously Ali et al.[7] proposed a method for the estimation of psoralen from P. corylifolia oil. Khusboo et al.[15] also developed a method for the determination of psoralen using HPTLC technique.
In our present study, we have reported the presence of two root nodulating, fast growing R. leguminosarum (PCC2) and E. meliloti (PCC7) rhizobacteria from the root nodules of P. corylifolia and their PGP activity on Psoralea corylifolia. HPLC estimation of psoralen content was also performed with the use of bacterized seeds which clearly showed the increasing level of psoralen content when compared to the control. It is the first report of the isolation of R. leguminosarum and E. meliloti from roots of P. corylifolia.
MATERIALS AND METHODS
Collection of plant materials
The seeds of P. corylifolia were collected from different provinces of India viz., Uttrakhand, Rajasthan and Uttar Pradesh, in the year 2008, and stored at room temperature for further use.
Isolation of bacteria from root nodules
Fifteen root nodulating bacteria were isolated from nodules of P. corylifolia plants according to the previously develop method and were subjected to growth and were maintained on yeast extract mannitol agar (YEMA). These isolates were further subjected to preliminary investigation including physiological, morphological and biochemical characterization according to Bergey's Manual of Determination Bacteriology.[16]
Characterization of bacterial strains on the basis of phylogenetic analysis 16S ribosomal DNA sequencing
Based on the wide array of PGP attributes, out of 15 isolates, two isolates PCC2 and PCC7 were subjected to further phylogenetic analysis with the help of 16S rDNA sequencing. Full 16S rDNA gene sequencing was performed after PCR amplification with primer fD1 (50-CGAATTCGTCGACAACAGAGTTTGATCCTG GCTCAG-30) and rD1 (50-CCCGGGATCCAAGCTT AAGGAGGTGATCCA GCC-30). The sequences were analyzed against the NCBI database. The sequencing revealed that both the strains belonged to rhizobial group, R. leguminosarum (PCC2) and E. meliloti (PCC7).
PGP attributes
Various direct and indirect plant growth promoting attributes were examined both qualitatively and quantitavely which included IAA production that was observed in exponentially grown cultures (108 cells/ml) of both the strains R. leguminosarum PCC2 and E. meliloti PCC7, when incubated in yeast extract mannitol (YEM) broth supplemented with tryptophan (0.01%) and without tryptophan for 24 h at 150 rpm and at 28°C.[17] Siderophore production was determined on Chrome- azural S (CAS) medium, whereas phosphate solubilization was detected by the formation of transparent zones surrounding bacterial colonies on Pikovaskya agar.[18] ACC deaminase activity[12] and intrinsic antibiotic resistance were carried out according to the methods proposed by Miller and May.[19] For the screening of ACC deaminase (1-aminocyclopropane-1-carboxylate), the bacterial isolates were grown in YEM broth, and the suspension after incubation was centrifuged at 8000 g for 10 min. Sterile distilled water was used to wash the pellets and was resuspended in 1 ml of sterile distilled water. On the Petri plates with minimal medium containing ACC, the suspension was spot inoculated. Negative controls were taken with plates containing minimal medium without ACC. The plates with minimal medium with (NH4)2SO4 served as positive controls. (NH4)2SO4 was used as N2 source, and further the plates were incubate for 3-4 days at 28 ± 1°C.
Breaking of seed dormancy
Dormancy of the seeds of P. corylifolia is a significant problem which needs to be minimized so as to increase the yield of this important medicinal legume. Earlier a method has been proposed by Mitter et al.,[20] where H2SO4 (98%) treatment was given to seeds for 10 min. However, in our studies, this treatment resulted in more than 80% immortality due to burnt seeds and therefore, ineffective for breaking the dormancy. Hence, a modification was done in the above method by reducing the time of treatment of H2SO4 (98%) to 5 min and washing the seeds with sterile distilled water, several times. These treated seeds were soaked in water for 48 h with changing of water after every 6 h. These soaked seeds were tied in a wet cotton cloth for next 48 h with frequent spraying of water for the emergence of seedlings.
Root hair curling
The treated seeds were spread on N2 free medium and incubated at 28 ± 1°C in dark for 2 days. Log phase culture of rhizobial suspension (2 ml) was inoculated on the Petri-plates containing 2 days old germinating seeds. The plates were further incubated for 4 days in dark and the roots were then removed and washed with sterile distilled water, cut into 1 cm long pieces. The root pieces were stained with methylene blue (0.01%) w/v d.wt. for 15 min, and observed under microscope.[8]
Microbial consortium preparation
Antagonistic behaviors of both the bacterial strains PCC2 and PCC7 were tested against each other for the development of effective bacterial combination following the method of Kumar et al.[18] YEM broth was inoculated with E. meliloti PCC7 and R. leguminosarum PCC2 separately and incubated at 28°C under shaking for 24 h. After incubation, a 5 μl of each culture was spotted on potato dextrose agar (PDA) plates, incubated for 24 h. These incubated plates were spread with a 24 h culture of a single strain using chromatography sprayer and further incubated at for 24 h at 28°C. The zone of inhibition was measured, if present. The entire experiment was performed twice and each treatment was replicated thrice.
Seed bacterization
For seed bacterization, the method of Weller and Cook[21] was adapted. Bacterial cultures were grown by inoculating in YEM medium at 28 ± 1°C for 48 h in a 500 ml flask. The isolates taken for the study were R. leguminosarum (PCC2), E. meliloti (PCC7) and their combination separately. The overnight grown cultures were centrifuged at 7100 rpm at 4°C for 15 min. The cultures supernants were discarded and pellets were washed and resuspended in sterile distilled water (SDW) to get final bacterial population (1 × 108 cells/ml). The cell suspensions of the bacterial strains were mixed with 1% carboxymethyl cellulose (CMC) solution in a ratio of 1:0.5 separately to form slurry coated on the surface of seeds according to the procedure of Gupta et al.[22] P. corylifolia seeds coated with 1% CMC slurry without bacterial culture served as positive controls.
In the consecutive years 2009 and 2010, field trials were carried out in two different locations. Field trial in year 2009 was carried out in sandy loam soil (79.8% sand, 7.3% silt, 7.1% clay, total organic C 0.0798%) at pH 6.07% with water holding capacity of 70%. Both the strains and their combination were tested for their ability to enhance the overall growth of P. corylifolia under the field conditions. Four treatments were prepared viz. treatment 1 (T1): R. leguminosarum PCC2; treatment (T2): E. meliloti PCC7 and treatment 3 (T3): (R. leguminosarum PCC2 + E. meliloti PCC7) along with treatment 4 (T4): As a control, which was composed of the seeds without bacterization. The experiments were conducted using random block design (RBD) with three replications. After 15 days of sowing, seed germination (%) was noted in each of the treatments. From each plot, 10 plants were randomly selected for recording data after every 30 days; frequent irrigation of the plants was done throughout the growth period of the crop. After every 30 days of sowing (DOS) the early vegetative and reproductive growth parameters were recorded till 150 days. The data was analyzed by using analysis of variance (ANOVA) for individual parameter on the basis of mean values to find out the significance at the 1% and 5% significance levels.
Root colonization by root nodulating bacteria
Antibiotic resistant markers of both R. leguminosarum PCC2 and E. meliloti PCC7 were raised to study the root colonization.[19] Cotrimoxazole (100 μg/ml) and nitrofurantoin (100 μg/ml) supplemented medium was used to assess the population of E. meliloti PCC7 and R. leguminosarum PCC2 in the rhizosphere of the plant, respectively. Bacterization of seed was performed with the desired strain and according to the treatment and further at the interval of every 30 days after sowing, the samples were collected from the root nodules of each treatment. With the help of a shovel, the plants were carefully uprooted and adhering soil particles to root surface were gently removed. For enumeration of the bacterial population on the roots, the roots were cut into 1 cm long segments and 1 g of root fragments was dipped into 5 ml of sterilize distilled water, and vortexed 4-5 times to release the rhizosphere bacteria into water. This bacterial suspension was further diluted up to 10-6 dilution and was poured down on YEM agar medium containing cotrimaxozole (100 mg/l) and nitrofurantoin (100 mg/l) for the enumeration of the introducing strains E. meliloti PCC7(Cm+) and R. leguminosarum PCC2(Nf+). The suspension was also poured separately on nutrient agar medium to evaluate the population of indigenous bacteria. With the help of colony counter (Harrison), the colony forming units (CFUs) were counted after 24 h of incubation at 28 ± 1°C.
Extraction of plant material
Classical Soxhlet assembly technique was used for the solvent extraction of active ingredients from the seed matrices of P. corylifolia plant. The plant material (40 g) was placed in a thimble holder, and filled with condensed fresh solvent from a distillation flask. Seeds were extracted with 240 ml each of the following solvents: petroleum ether, benzene, chloroform, acetone, ethanol, methanol and water. Each time before extracting with next solvent, the soxhlet assembly was cleaned and dried properly. When the liquid reaches the overflow level, a siphon aspirates the solution of the thimble holder and unloads it back into the distillation flask, carrying extracted solutes into the bulk liquid. In the solvent flask, solute is separated from the solvent using distillation. Solute is left in the flask and fresh solvent passes back into the plant solid bed. The operation is repeated until complete extraction is achieved. After complete extraction with the respective solvent, the residue was evaporated under a rotary vacuum evaporator and the dry extract thus obtained was stored at 4°C in air tight glass bottle for further estimation of psoralen content. Percent extractive value was calculated by following formula:
Percent yield = weight of dried extract/weight of dried plant material × 100
HPTLC analysis of extracted plant material
HPTLC was performed on 20 cm × 10 cm thin layer chromatographic (TLC) aluminium plates coated with 200-μm layer thickness of silica gel 60F 254 (E. Merck, Germany). Samples were applied as 6 mm width bands using Camag 100 micro-liter sample syringe (Hamilton, Switzerland) with a Camag Linomat 5 applicator (Camag, Switzerland). A constant application rate of 150 nl/s was used. Linear ascending development with toluene–ethyl acetate 7.5:2.5 (v/v) as mobile phase was carried out in a twin trough glass chamber (Camag) (20 × 10 cm) previously saturated with mobile phase vapor for 20 min (optimized chamber saturation time) at room temperature (25 ± 2°C). The development distance was 80 mm. After development, the plates were air-dried. Scanning was performed using Camag TLC scanner 3 at 299 nm in the absorbance mode and operated by WinCATS software (version 1.4.1). The source of radiation was a deuterium lamp emitting a continuous UV spectrum in the range 190-400 nm. The slit dimensions were 5 mm × 0.45 mm and the scanning speed was 100 mm/s.
Estimation of psoralen content
For the estimation of psoralen, method proposed by Murali and Anand[23] was adopted. Estimation of psoralen was carried out by HPLC using the Perkin Elmer 3B model liquid chromatography equipped with L-75 auto control UV detector and with a sigma 10/2 data station. A Merck 250 mm long column diameter 4.0 mm packed with RP-8 (10μ) was employed under operating condition with water-acetonitrile (79:21) solvent system with flow rate of 2 ml minute-1 and the detection wavelength was 300 nm.
Pure psoralen was used as standard to optimize separation condition to obtain the detention temperature. A calibration curve was plotted by injecting 5 μl of standard solution of known and variable concentration of psoralen ranging from 0.02 to 0.05 μg/ml. A straight line was obtained by plotting concentration versus peak area whose slope and intercept were determined by the least square method. By using the obtained equation, the psoralen in the methanol extract of P. corylifolia seeds was determined.
RESULTS
Isolation and characterization of bacteria isolated from P. corylifolia roots
From the roots of P. corylifolia, a total of 15 bacterial isolates were isolated. On the basis of morphological, physiological and biochemical and molecular techniques, isolate PCC2 was identified as R. leguminosarum whereas isolate PCC7 was identified as E. meliloti. Both PCC2 and PCC7 were Gram-negative, non-spore formers, non-capsulated, motile and found as semi translucent rounded like structures showing smooth mucoid colonies with 2-4 mm in diameter. When the generation time was assessed, both the strains were found to be fast growers with an average generation time of 1.5 h. These were catalase and oxidase positive and tolerated 8% KNO3 and 2% NaCl. Both the isolates failed to grow on GPA but were able to grow on heterotrophic aerobic bacteria media (HAB) and tolerated 8% KNO3 and 2% NaCl. E. meliloti (MTCC100), R. leguminosarum (MTCC99), M. loti (MTCC2378) were taken as the standard rhizobial cultures for comparing the characteristics of the isolated strains of PCC2 and PCC7, and the isolates PCC2 and PCC7 given similar results to their respective standard strains. The sequences of both the strains were submitted to NCBI database and accession number for both the strains E. meliloti PCC7 and R. leguminosarum PCC2 were obtained as (JN546145) and (JN546144), respectively [Figure 1].
Figure 1.
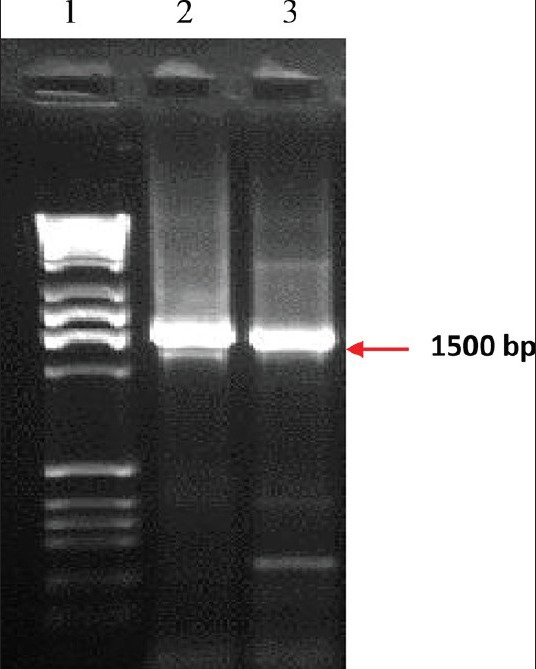
Amplified 16S ribosomal DNA fragment of root nodule isolates of P. corylifolia L. (Lane 1: marker with 2kb ladder [2000 bp]; L2: R. leguminosarum PCC2 [JN546144]; L3: Ensifer meliloti PCC7 [JN546145])
PGP attributes
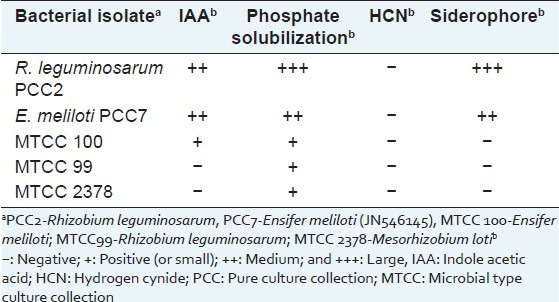
Phosphate solubilization was observed in Pikovskaya agar, the haloes were of yellow color due to the lowering of pH of the medium which in turn changed the color of indicator. Siderophore production was confirmed by the orange halo zones formed around the colonies on CAS agar. R. leguminosarum PCC2 and E. meliloti PCC7 were also found to secrete IAA which was confirmed by forming pink colored of the culture filtrates when added with the reagent. It was interesting to note that all the isolates produced ACC deaminase as confirmed by growth on ACC minimal medium [Table 1].
Table 1.
Plant growth promoting properties of rhizobia isolated from the roots of Psoralea corylifolia
Interaction of R. leguminosarum PCC2 and E. meliloti PCC7
In vitro microbe-microbe interaction was checked for studying if any inhibitory effect exists among the isolates, so as to use them for consortium bioinoculant preparation, after the screening on solid agar plate. R. leguminosarum PCC2 and E. meliloti PCC7 showed synergistic growth among each other during the test when the supernatants of two bacteria were placed in pre-seeded plates, no inhibition zone was observed.
Effect of seed bacterization
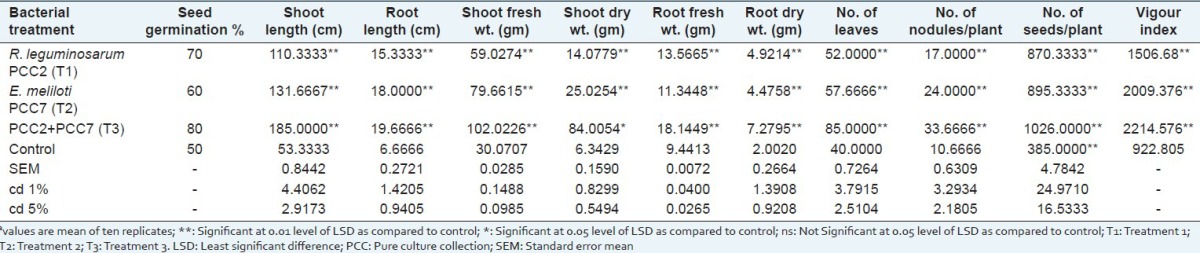
R. leguminosarum PCC2 and E. meliloti PCC7 were selected for seed bacterization on the basis of strong PGP attributes. After sowing the bacterized seeds into the field, various parameters such as early vegetation and late reproduction were checked to find out if any vegetative parameters such as number of seeds per plant, number of nodules, root length, shoot length, shoot wt., root wt., (dry/fresh) were found to be significantly enhanced when compared to the control after 120 days of sowing. Individual inoculation on the seeds was comparable to the combination and observed that combination showed enhanced seed germination as well as other parameters when compared to individual seed inoculation. The R. leguminosarum PCC2 + E. meliloti PCC7 (treatment 3) resulted in 80% seed germination whereas E. meliloti PCC7 (treatment 2) and R. leguminosarum PCC2 (treatment 1) resulted in 70% and 60% seed germination, respectively. As compared to individual seed bacterization as well as control seeds, maximum vigor index was noted in the combination PCC2 + PCC7 [Table 2].
Table 2.
Effect of bacterial treatment on the growth of Psoralea corylifia 120 days after sowinga
Intrinsic antibiotic activity for studying root colonization
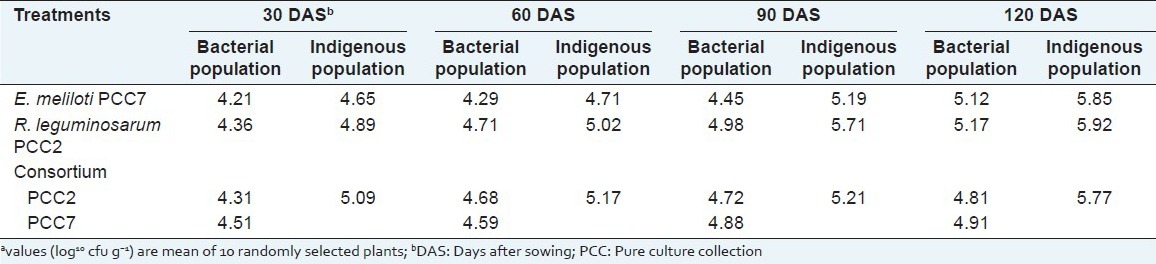
Estimation of indigenous resistant bacteria and antibiotic resistant isolates PCC2Cm+ and PPC7Nf+ was done in the rhizosphere of P. corylifolia in the field. Population of R. leguminosarum PCC2 and E. meliloti PCC7 increased slightly from their initial inoculation density after 30, 60, 90 and 120 days after sowing in combination as well as individually [Table 3]. When compared single inoculation control and combination, plant growth parameters for the treatment T3 (PCC2 + PCC7) showed better results. Significant root colonization was observed in all the treatments and it can be correlated with the enhanced growth parameters.
Table 3.
Average root colonization of Psoralea corylifolia inoculated with Ensifer meliloti and Rhizobium leguminosaruma
Extraction and estimation of psoralen in the seeds of P. corylifolia after field trial
HPTLC analysis of psoralen from the methanolic extract of P. corylifolia revealed a well defined peak at Rf value of 0.54 in the seeds of treatment 3 field plants when compared to the peak of standard psoralen, showing similar Rf value (0.54 Rf). Treatment 1 and 2 also showed the peak at 0.55 and 0.53, respectively [Figure 2]. The amount of psoralen in dry methanolic extract of P. corylifolia seeds was found to be 1.97% and 1.93% (w/w) in treatment T1 and T2, respectively. However, a maximum percentage was seen in the methanolic seed extract of treatment T3 where the value was found to be 2.97% (w/w). These results were comparable to the control where only 1.91% of psoralen content was found [Figure 3].
Figure 2.
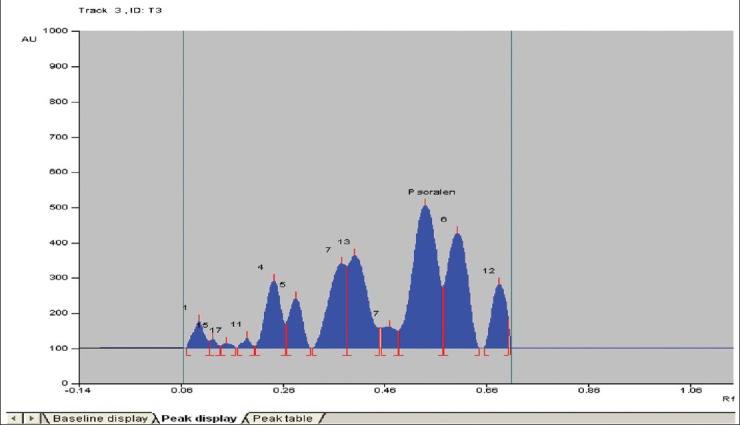
Two-dimensional high performance thin layer chromatographic (HPTLC) chromatogram of psoralen from different samples. The mobile phase used was toluene–ethyl acetate 7.5:2.5 (v/v). The thin layer chromatographic (TLC) plate was scanned at 299 nm through HPTLC scanner
Figure 3.
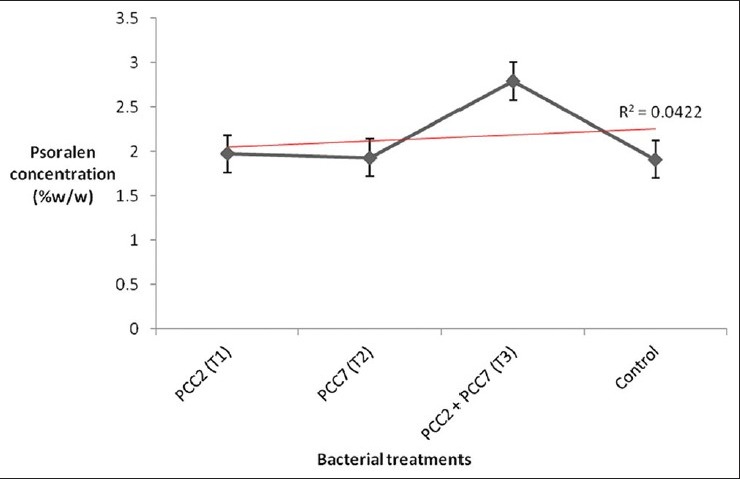
Estimation of psoralen content from the methanolic seed extract of P. corylifolia with different treatments. T1 = R. leguminosarum PCC2 (JN546144); T2 = E. meliloti PCC7 (JN546145); T3 = PCC2 + PCC7; T4 (Control) = seeds without bacterial treatment
DISCUSSION
All the root-nodulating bacteria isolated from P. corylifolia were Gram-negative in nature, non-spore forming, non capsulated and motile. When compared the morphological, physiological, biochemical and molecular characteristics of PCC2 and PCC7 with other standard strains E. meliloti (MTCC100), R. leguminosarum (MTCC99), the isolates PCC7 and PCC2 showed similar pattern, respectively.[16] Other reports of bacteria from same plant have been reported by others[24] who isolated Mesorhizobium sp. and Bradyrhizobium spp. from another species of Psoralea (P. bituminosa). However, in this study, we isolated Rhizobium leguminosarum and Ensifer meliloti species from the roots of P. corylifolia which is not only the first report but also indicates that multiple species of same genera can possess specific rhizobia as also noted by Kumar et al.[25] These findings also validate the general views regarding rhizobial specificity and few natural rhizobial populations have ever been reported for wider cross nodulation.
Plant growth promoting attributes, play a very important role in the rhizosphere which in turn affect the growth of plant health as well as influence the activity of other deleterious microflora. In our studies, it was found that both the strains were able to solubilize inorganic phosphate which must have influenced the overall vegetative and reproductive parameters of plant health.[26,27] Siderophore production was also evident in our strains, which is a positive indication of iron chelating activity of the bacteria, and these results were in strong agreement to the other reports.[28,29] In addition, earlier reports described the presence of hydroxamate type of siderophore in rhizobia as also seen in our studies.[30] Ethylene inhibits nodulation in various legumes; however, through the action of ACC deaminase which is present in our rhizobial strains, ethylene biosynthesis in the plants can be reduced. Both the isolates PCC2 and PCC7 from P. corylifolia were found to produce ACC deaminase which was in accordance with earlier reports.[12] It has been noted that many rhizobia which contain 1-aminocyclopropane-1-carboxylate (ACC) deaminase cleave the ethylene precursor ACC 2-α keto-butyrate and ammonia thereby reducing the level of ethylene in developing plants or stressed plants whereas mutant strains failed to do so.[31] Root hair curling was observed when P. corylifolia seeds were treated with plant growth promoting strains of E. meliloti PCC7 and R. leguminosarum PCC2. This is well established character of pre-nodulation stage and is in accordance with the hypothesis of attachment of rhizobia to root hairs and proves the process of root hair curling as a host specific process. Plant growth promoting rhizobacteria and their potential is achieved to the maximum when there is a better understanding of the factors controlling the ecology and establishment within the root system in the form of nodule as also observed in our studies.[25]
Moreover, when the field trials were performed with these two root nodulating and plant growth promoting strains, the bacterial consortium effectively enhanced the vegetative and reproductive growth of P. corylifolia resulting in increased vigour index by 63.27%, 117.7% and 139.9% for R. leguminosarum PCC2, E. meliloti PCC7 and PCC2 + PCC7, respectively when compared to the control. Arora et al.,[32] also observed the enhanced seed germination, seedling biomass, confirming that rhizobia are able to produce plant growth regulators through plant roots.
In this study, when the combinations of bacterial strains were used in the field trials, effective enhancement of vegetative and reproductive growth of P. corylifolia plants was seen. All the plant growth parameters were found to be enhanced when compared with the control. For monitoring the root colonization of P. corylifolia roots, the antibiotic resistant marker strains of R. leguminosarum PCC2(Nf+) and E. meliloti PCC7(Cm+) were used at different time intervals for the successful analysis of PGPR associated with promotion of plant growth and yield. As confirmed previously, the effective root colonization is a pre-requisite attribute,[33] which is an important strain specific rate of rhizobacteria.[34] An antibiotic resistant marker has been frequently used because of its simple and cost effective identification technique. The positive root colonization ability of PCC2(Cm+) R. leguminosarum, and PCC7(Nf+) E. meliloti as a consortium lies in it being the successful colonizer of the spermosphere, increased seed emergence and its establishment in the rhizosphere of the plant resulting in enhanced yield. Several workers have exploited the intrinsic resistant of rhizobacteria towards antibiotic to observe survival of introduced rhizobia in rhizosphere. Arora et al.,[32] used 100 μl/ml of ampicillin to the isolate the rhizobia RMP1(Amp+) from the uninoculated rhizosphere. Further successful recovery of rhizobial antibiotic marker was obtained even after its 20 years of field inoculation.[34]
In addition, psoralen present in the P. corylifolia seeds is one of the important compounds used for the treatment of psoriasis, leucoderma and leprosy. Several methods have been developed for the standardization of psoralen in dried seed powder extracts of P. corylifolia such as spectroflurometry[15] which can also quantify the psoralen content. However, in this study, HPTLC method for the determination of psoralen in P. corylifolia seeds was developed for the quantification of psoralen content in the methanolic seed extract of P. corylifolia.[15] A single spot at Rf 0.54 was observed in the methanolic seed extract P. corylifolia which corroborated with the finding of Khushboo et al.[15] Earlier, the extraction of bioactive compounds from the seeds of P. corylifolia has also been carried out using the high pressure super critical CO2 (SCCO2) system on different pressure bars at temperature of 40°C.[35] As a novel finding, psoralen content in the seeds of P. corylifolia was significantly increased in this study by different bacterial treatments which was observed using HPLC technique.
These findings conclude that rhizobial strains showing important plant growth promoting traits proved to be very efficient in promoting the early vegetative and late reproductive growth parameters of the medicinal legume P. corylifolia with excellent root colonization ability. Hence, isolation of beneficial rhizobia that were used in this study as successful inoculants for promoting plant growth can be used to increase the level of certain important phyto-components that may be utilized in pharmaceutical industries. Apart from it, various ayurvedic medicines which use psoralen can be benefitted by increased psoralen content.
ACKNOWLEDGEMENTS
Financial support from Uttarakhand State Council for Science and Technology (UCOST) (Dehradun), University Grant Commission (UGC) (New Delhi) and Council for Scientific and Industrial Research (CSIR) (New Delhi), India is gratefully acknowledged. Laboratory facilities provided by Patanjali Ayurved Ltd., Haridwar (Uttarakhand, India) are also deeply acknowledged.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kirtikar KR, Basu BD. 2nd ed. Vol. 1. Dehradun, India: International Book Publisher; 1990. Indian Medicinal Plants; p. 536. [Google Scholar]
- 2.Qiao CF, Han QB, Song JZ, Mo SF, Kong LD, Kung HF, et al. Chemical fingerprint and quantitative analysis of Fructus Psoraleae by HPLC. J Sep Sci. 2007;30:813–8. doi: 10.1002/jssc.200600339. [DOI] [PubMed] [Google Scholar]
- 3.Liu R, Aifeng LI, Sun A, Kong L. Preparative isolation and purification from Psoralea corylifolia by high speed counter current chromatography. J Chromatogr. 2004;1057:225–8. doi: 10.1016/j.chroma.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 4.Hisashi M, Sachie K, Sachiko S, Shin A, Seikou N, Masayuki Y, et al. Bioactive constituents from Chinese natural medicines X ×. Inhibitors of antigen induced de-granulation in RBL-2H3 cells from the seeds of Psoralea corylifolia. Chem Pharm Bull. 2007;55:106–10. doi: 10.1248/cpb.55.106. [DOI] [PubMed] [Google Scholar]
- 5.Guo J, Weng X, Wu H, Li Q, Bi K. Antioxidants from Chinese medicinal herb-Psoralea corylifolia L. Food Chem. 2005;91:287–92. [Google Scholar]
- 6.Latha PG, Panikkar KR. Inhibition of chemical carcinogenesis by Psoralea corylifolia seeds. J Ethnopharmacol. 1999;68:295–8. doi: 10.1016/s0378-8741(99)00062-8. [DOI] [PubMed] [Google Scholar]
- 7.Ali J, Akhtar N, Sultana Y, Baboota S, Ahmad S. Thin-layer chromatographic analysis of psoralen in babchi (Psoralea corylifolia) oil. Acta Chromatogr. 2008;20:277–82. [Google Scholar]
- 8.Chandra S, Choure K, Dubey RC, Maheshwari DK. Rhizosphere competent Mesorhizobium loti MP6 induces root hair curling inhibits Sclerotinia sclerotiorum and enhances growth of Indian mustard (Brassica campestris) Braz J Microbiol. 2007;38:124–30. [Google Scholar]
- 9.Somasegaran P, Hoben HJ. Handbook for Rhizobia. New York, USA: Springer-Verlag; 1994. Methods in legume-rhizobium technology. [Google Scholar]
- 10.Kumar H, Dubey RC, Maheshwari DK. Effect of plant growth promoting rhizobia on seed germination, growth promotion and suppression of Fusarium wilt of fenugreek (Trigonella foenum-graecum L.) Crop Prot. 2011;30:1396–403. [Google Scholar]
- 11.Antoun H, Bordeleau LM, Gagnon C. Antagonism between Rhizobium meliloti and Fusarium oxysporum in relation to symbiotic effectiveness. Can J Plant Sci. 1978;58:75–8. [Google Scholar]
- 12.Glick BR, Penrose DM, Li J. A mode for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J Theor Biol. 1998;190:63–8. doi: 10.1006/jtbi.1997.0532. [DOI] [PubMed] [Google Scholar]
- 13.Sebastien P. New York: Elsevier Health Science; 2006. Ayurvedic Medicine: The principles of traditional practice. Part 2; pp. 135–6. [Google Scholar]
- 14.Vaidya AD. Reverse Pharmacological correlates of Ayurvedic drug actions. Indian J Pharmacol. 2006;38:311–5. [Google Scholar]
- 15.Khushboo PS, Jadhav VM, Kadam VJ. Development and validation of a HPTLC method for determination of psoralen in Psoralea corylifolia (Bavachi) Int J Pharm Tech Res. 2009;1:1122–8. [Google Scholar]
- 16.Holt JG, Kreig NR, Sneath PH, Staley JT, Williams ST. 9th ed. Baltimore, USA: Williams and Wilkins; 1994. Bergey's Manual of determinative bacteriology. [Google Scholar]
- 17.Bric JM, Bustock RM, Silversone SE. Rapid in situ assay for indole acetic acid production by bacteria immobilization on a nitrocellulose membrane. Appl Environ Microbiol. 1991;57:535–8. doi: 10.1128/aem.57.2.535-538.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar H, Bajpai VK, Dubey RC, Maheshwari DK, Kang SC. Wilt disease management and enhancement of growth and yield of Cajanus cajan (L) var. Manak by bacterial consortia amended with chemical fertilizer. Crop Prot. 2010;30:1396–403. [Google Scholar]
- 19.Miller RH, May S. Legume inoculation: successes and failures. In: Keister DL, Cregan PB, editors. The rhizosphere and Plant growth. Dordrecht The Netherlands: Kluwer Academic Publishers; 1991. pp. 123–34. [Google Scholar]
- 20.Mitter V, Srinivasan K, Singh BM. Overcoming hard seededness on Psorlea corylifolia. Seed Res. 1993;21:31–4. [Google Scholar]
- 21.Weller DM, Cook RJ. Suppression of take - all of wheat by seed treatments with fluorescent pseudomonads. Phytopathology. 1983;73:463–9. [Google Scholar]
- 22.Gupta CP, Dubey RC, Maheshwari DK. Plant growth enhancement and suppression of Macrophomina phaseolina causing charcoal rot of peanut by fluorescent Pseudomonas. Biol Fert Soils. 2002;35:399–405. [Google Scholar]
- 23.Murali B, Anand MS, Venkatraman BV. An HPLC method for simultaneous estimation of psoralen, bakuchicin and bakuchiol in Psoralea corylifolia. J Nat Remedies. 2002;2:76–9. [Google Scholar]
- 24.Muresu R, Polone E, Sulas L, Baldan B, Tondello A, Delogu G, et al. Coexistence of predominantly non-culturable rhizobia with diverse endophytic bacterial taxa within nodules of wild legumes. FEMS Microbiol Ecol. 2008;63:383–400. doi: 10.1111/j.1574-6941.2007.00424.x. [DOI] [PubMed] [Google Scholar]
- 25.Kumar H, Dubey RC, Maheshwari DK. Effect of plant growth promoting rhizobia on seed germination, growth promotion and suppression of Fusarium wilt of fenugreek (Trigonella foenum-graecum L.) Crop Prot. 2011;30:1396–403. [Google Scholar]
- 26.Hayat R, Ali S, Amara U, Khalid R, Ahmed I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann Microbiol. 2010;60:579–98. [Google Scholar]
- 27.Osorio NW. Berlin, Heidelberg: Springer; 2011. Effectiveness of phosphate solubilizing microorganism in increasing plant phosphate uptake and growth in tropical soils sulfur-oxidizing bacteria: A novel bioinoculant for sulfur; pp. 65–81. [Google Scholar]
- 28.Carson KC, Holliday S, Glenn AR, Dilworth MJ. Siderophore and organic acid production in root nodule bacteria. Arch Microbiol. 1995;157:264–71. doi: 10.1007/BF00245160. [DOI] [PubMed] [Google Scholar]
- 29.Rajendran G, Sing F, Desai AJ, Archana G. Enhanced growth and nodulation of pigeon pea by co-inoculation of Bacillus strains with Rhizobium spp. Biores Technol. 2008;99:4544–50. doi: 10.1016/j.biortech.2007.06.057. [DOI] [PubMed] [Google Scholar]
- 30.Gupta CP, Sharma A, Dubey RC, Maheshwari DK. Effect of metal ions on the growth of Pseudomonas aeruginosa and siderophore and protein production. Indian J Exp Biol. 2001;39:1318–21. [PubMed] [Google Scholar]
- 31.Penrose DM, Glick BR. Biochemical and genetic characteristics of ACC deaminase. Indian J Microbial. 2001;52:457–62. [Google Scholar]
- 32.Arora NK, Kang SC, Maheshwari DK. Isolation of siderophore producing strains of Rhizobium meliloti and their biocontrol potential against Macrophomina phaseolina that causes charcoal rot of groundnut. Curr Sci. 2001;81:673–7. [Google Scholar]
- 33.Kloepper JW, Lifshitz R, Zablotowich RK. Free living bacterial inocula for enhancing crop productivity trends. Biotechnology. 1989;7:39–43. [Google Scholar]
- 34.Obaton M, Bouniols A, Piva G, Vadez V. Are Bradyrhizobium japonicum stable during a long stay in soil. Plant Soil. 2002;245:315–26. [Google Scholar]
- 35.Manohar BK, Sankar U. Extraction modeling and characterization of bioactive components from Psoralea corylifolia L. obtained by supercritical carbon dioxide. J Food Process Technol. 2012;3:1–8. [Google Scholar]


